Abstract
Aims:
Although lower urinary tract symptoms (LUTS) may occur at different periods during the life course of women, little research on LUTS has adopted a life course perspective. The purpose of this conceptual paper is to demonstrate how Life Course Theory and life course epidemiology can be applied to study bladder health and LUTS trajectories. We highlight conceptual work from the Prevention of Lower Urinary Tract Symptoms (PLUS) Research Consortium to enhance understanding of life course concepts.
Methods:
Consortium members worked in transdisciplinary teams to generate examples of how life course concepts may be applied to research on bladder health and LUTS in eight prioritized areas: (1) biopsychosocial ecology of stress and brain health; (2) toileting environment, access, habits, and techniques; (3) pregnancy and childbirth; (4) physical health and medical conditions; (5) musculoskeletal health; (6) lifestyle behaviors; (7) infections and microbiome; (8) hormonal status across the life span.
Results:
Life course concepts guided consortium members’ conceptualization of how potential risk and protective factors may influence women’s health. For example, intrapartum interventions across multiple pregnancies may influence trajectories of bladder health and LUTS, illustrating the Principle of Life Span Development. Consortium members also identified and summarized methodologic and practical considerations in designing life course research.
Conclusions:
This paper may assist researchers from a variety of disciplines to design and implement research identifying key risk and protective factors for LUTS and bladder health across the life course of women. Results from life course research may inform health promotion programs, policies, and practices.
Keywords: lower urinary tract symptoms, bladder health, girls, women, prevention, life course, theory, epidemiology, transdisciplinary
Introduction
Lower urinary tract symptoms (LUTS) and associated conditions comprise a variety of bothersome bladder complaints. Some examples are bothersome frequent and/or urgent urination, urgency urinary incontinence (UUI; strong urge “to go” with urine loss before reaching a toilet), stress urinary incontinence (SUI; urine loss with physical activity or increases in abdominal pressure such as a cough or sneeze), nocturnal enuresis (urine loss while asleep), difficulty urinating, dribbling after urination, and bladder or urethral pain before, during, or after urination.(1, 2)
LUTS have a substantial impact on the health and quality of life of girls and women across the life course. LUTS predominantly include UUI and nocturnal enuresis during early childhood, with an increase in urinary tract infections (UTIs) during adolescence, particularly with sexual debut.(3) In a population-based birth cohort, the prevalence of any daytime or nighttime urinary incontinence (UI) was roughly 10% and 14%, respectively, among girls at 6.5 years.(4, 5) Corresponding percentages declined to 5% and 6% by 9.5 years,(4, 5) and to 4% and 2% by 14 years.(6) In adulthood, LUTS predominantly include SUI during the reproductive years, UUI and mixed UI (combined UUI and SUI) with increasing age,(7) and recurrent UTIs after menopause.(8) Estimates of UI in women range from 10% to 50%, depending on the definition of incontinence and age of the study population.(9, 10) In addition to impacting physical health, LUTS can negatively impact the emotional well-being of girls and women. Children and adolescents with LUTS often experience shame, embarrassment, and other forms of psychological distress. More than 50% of women with LUTS report anxiety or depression,(11, 12) and social isolation is common.(13, 14)
Although LUTS are known to occur at different periods over the life course, little research on LUTS has adopted a life course perspective. Potential explanations for this gap include a historical focus on the identification and treatment of LUTS rather than consideration of risk and protective factors that can influence bladder health and be targeted through prevention.(18) In addition, LUTS researchers may be unfamiliar with life course concepts and how to incorporate them into etiologic studies. A life course perspective allows for the investigation of social ecological and biological exposures that begin early in life and evolve throughout the life course. These exposures may confer risk for or protection from LUTS and worsening bladder health. Results from life course research can inform when and how health professionals and policy makers intervene to prevent or ameliorate LUTS and promote bladder health. Early prevention is particularly important, as early experiences of LUTS may predispose girls and women to repeated episodes of LUTS or chronic LUTS.(8, 19)
The purpose of this paper is to demonstrate how concepts of Life Course Theory and life course epidemiology can be applied to the study of bladder health and LUTS among girls and women. Conceptual work from the Prevention of Lower Urinary Tract Symptoms (PLUS) Research Consortium (PLUS) is highlighted to enhance understanding of life course concepts. The PLUS Research Consortium is a transdisciplinary scientific network established by the National Institute of Diabetes and Digestive and Kidney Diseases in 2015. The goal is to expand research beyond the detection and treatment of LUTS to also encompass LUTS prevention and the promotion and preservation of bladder health among girls and women.(15) Consistent with the World Health Organization’s definition of health,(16) the PLUS Research Consortium conceptualizes bladder health as “a complete state of physical, mental, and social well-being related to bladder function, and not merely the absence of LUTS,” with function that “permits daily activities, adapts to short term physical or environmental stressors, and allows optimal well-being (e.g., travel, exercise, social, occupational, or other activities).”(17) Through the present manuscript, our intent is to educate readers and stimulate their own interest in conducting life course research to advance the field of bladder health. In the sections that follow, we introduce prevention science and life course concepts and provide an overview of how life course concepts may be applied to research on the etiology of poor bladder health and LUTS. We close by summarizing key points and calling upon our colleagues across diverse disciplines to incorporate a life course perspective into their research on bladder health.
Prevention Science.
Prevention science involves the systematic study of potential precursors to human dysfunction and health, termed risk and protective factors, respectively. (20, 21) Risk factors are attributes, characteristics, or exposures of an individual that increase the likelihood of developing a disease or dysfunction. In contrast, protective factors enhance health and lessen the likelihood that disease or dysfunction will occur in response to risk factors. More recently, protective factors have been termed “promoting factors” when they exert direct, positive effects on health.(22) Prevention scientists conduct etiologic studies to identify risk and protective factors across different levels of biology within individuals (e.g., genes, cells, tissues, organs, multi-organ systems), as well as across different levels of social ecology surrounding individuals (e.g., family, peer group, school or workplace, health care system, neighborhood, community, society).(20, 21, 23, 24) Risk and protective factors identified through etiologic research become candidates for preventive intervention studies. These studies aim to modify identified factors with the goal of promoting health and preventing major dysfunction and disease.(20, 21) Preventive interventions may include health promotion programs and changes to practices and policies within institutions and governments.(24, 25) Evidence accumulated over time will point to effective prevention programs, practices, and policies that can be implemented to foster health promoting behaviors among individuals and health promoting features of the environment.
Life Course Concepts.
Prevention science is enhanced by the application of a life course developmental perspective to etiologic research. In their recently published Handbook on Life Course Health Development, Halfon and Forrest describe how prevailing conceptualizations of public health problems have evolved from biomedical to biopsychosocial to life course health development.(26) Precipitating influences include David Barker’s observation that under-nutrition during pregnancy can lead to “fetal metabolic programming” that predisposes individuals to cardiovascular disease decades later in adulthood.(27) This finding and others spurred a large body of research over the past quarter century, which has informed conceptual frameworks emphasizing the fetal and developmental origins of health and disease.(28) Subsequent frameworks have emphasized the life course as a means of understanding health disparities and inequities.(24, 29) Two resources within the life course literature, highlighted in Table 1, describe principles and concepts that can be tested in etiologic research investigating how social ecology and biology may influence bladder health and LUTS over time. The first resource, Life Course Theory, is summarized through five general principles developed by Elder Jr. and colleagues to explain a variety of sociological outcomes.(30) The second resource, written by Kuh and colleagues, is a life course approach specifically developed to guide research on health, human development, and aging. These authors define life course epidemiology as the study of long term effects on later health or disease risk of physical or social exposures during gestation, childhood, adolescence, young adulthood, and later adult life.(31) The intent of life course epidemiology is to understand biological, behavioral, and psychosocial processes that operate across an individual’s life course, or across generations, to influence the development of disease.(31) Thus, a life course approach emphasizes exposures to risk and protective factors across an individual’s life span.
Table 1.
Key Concepts of Life Course Theory and Life Course Epidemiology: Sources, Descriptions, and Possible Research Applications
| Key Concept | Source and Description | Possible Research Application |
|---|---|---|
| Life Course Theory (30) | Elder, Jr., Kirkpatrick Johnson, and Crosnoe (2003) | |
| Principle of Life Span Development | Human development and aging are lifelong processes that require the study of lives over substantial periods of time, including potentially changing environments (e.g., relationships, workplaces, schools, and communities). |
PLUS Research Theme: Pregnancy
& Childbirth Application: Intrapartum interventions across multiple pregnancies may influence trajectories of bladder health and LUTS. Women’s environmental context may influence selection of and responses to specific intrapartum interventions. |
| Principle of Agency | Individuals construct their own life course through the choices and actions they take within the opportunities and constraints of history and social circumstance. |
PLUS Research Theme: Infections
& Microbiome Application: Patients may request and receive antimicrobial medications based on information gleaned through social networks or media, as well as history of use. |
| Principle of Time and Place | The life course of individuals is embedded and shaped by the historical times and places they experience over their lifetime. The same historical event or change may affect individuals in different ways across different regions or nations. (consistent with birth cohort effects) |
PLUS Research Theme: Lifestyle
Behaviors Application: Messaging about the types and amounts of fluids that one should consume are driven by prevailing medical views, health and diet fads, marketing campaigns (e.g., bottled water, sugar-sweetened beverages), and historical policy decisions that affect water safety in specific geographic regions (e.g., Flint, Michigan water supply change, local fracking spills). |
| Principle of Timing | The developmental antecedents and consequences of life transitions, events, and behavioral patterns vary according to their timing in a person’s life. The same events or experiences may affect individuals in different ways depending on when they occur in the life course. (consistent with critical and sensitive periods) |
PLUS Research Theme: Physical
Health & Medical Conditions Application: Nutrient-poor foods may be readily available and consumed during key periods when weight gain is particularly likely to occur, such as menarche, pregnancy, and menopause. |
| Principle of Linked Lives | Lives are lived interdependently and socio-historical influences are expressed through networks of shared relationships. Transitions in one individual’s life often result in transitions for others (e.g., caregiver and child). |
PLUS Research Themes:
Musculoskeletal Health Application: Social networks may influence physical activity participation and choices by adolescent (94, 95) and adult women.(96) |
| Life Course Epidemiology (31) | Kuh, Ben-Shlomo, Lynch, Hallqvist, and Power (2003) | |
| Birth cohort effects | Differences in adult disease that result from the location of an individual in historical time, as indexed by year of birth. Birth cohorts may experience social and environmental changes. They may also vary in their exposure to risk factors. (consistent with the Principle of Time and Place) |
PLUS Research Theme: Infections
& Microbiome Application: Best practices for the use of antimicrobial therapy have varied as new antimicrobial agents have been developed, resistance to other agents has emerged, and the importance of antimicrobial stewardship has been recognized.(73, 97) |
| Context | The location of an individual by time and place. Place refers to both geographical location and to group membership arising from the social and economic structure of society (e.g., family, friends, gender, ethnicity, class, residence). |
PLUS Research Theme: Hormonal Use
across the Life Span Application: Premenopausal and postmenopausal hormone use may be influenced by prevailing norms at a particular period of time and geographic location, as well as within one’s community. |
| Accumulation of risk over time | Life course exposures or insults gradually accumulate through episodes of illness and injury, adverse environmental conditions, and health damaging behaviors. Cumulative damage to biological systems occurs as the number, duration, or severity of exposures increase, and as body systems age and become less able to repair damage. |
PLUS Research Theme:
Musculoskeletal Health, Pregnancy &
Childbirth Application: Accumulation of several musculoskeletal injuries may increase risk of LUTS (e.g., anterior cruciate ligament (ACL) tear prenatally, pelvic girdle pain during pregnancy, levator ani tear at delivery). |
| Chains of risk | A sequence of linked exposures that raise disease risk because one adverse experience or exposure leads to another, and then another. Social, psychological, and biological chains of risk are possible. Risk is probabilistic rather than deterministic. |
PLUS Research Theme: Toileting
Environment, Access, Habits, &
Techniques Application: Restricted bathroom access in schools may lead to restricted fluid intake, infrequent urination, and dysfunctional voiding (i.e., habitual contraction of the pelvic floor, leading to incomplete bladder emptying),(38, 98) which may cause UTIs in childhood and adolescence. Early UTIs may predispose women to experience UTIs across the life course.(41, 99) |
| Critical and sensitive periods | A critical period model of disease causation occurs when an exposure during a specific period of development has adverse effects on the structure or function of organs, tissues, or body systems; structure or function is not modified in any dramatic way by exposure outside of the critical period. A sensitive period occurs when an exposure has a stronger effect on development and subsequent disease risk than it would at other times. (consistent with the Principle of Timing) |
PLUS Research Theme:
Musculoskeletal Health, sensitive
period Application: Participation in high-impact physical activity may be more likely to serve as a risk factor for LUTS when the female musculoskeletal system undergoes dynamic changes (e.g., normal growth, hormonal changes related to menarche, pregnancy, childbirth, and menopause.) |
| Embodiment | Describes how extrinsic factors experienced at different life stages are inscribed into an individual’s body structures or functions. |
PLUS Research Theme:
Biopsychosocial Ecology of Stress & Brain
Health Application: Adverse childhood experiences are associated with increased central nervous system dysregulation and executive functioning difficulties during early childhood,(32, 36, 100) which in turn may impact a child’s ability to control the bladder.(33) |
| Vulnerability and resilience to adversity | Negative or positive adaptation in the face of adversity, respectively. Behavioral and physiological adaptation is shaped by factors extrinsic and intrinsic to the individual. |
PLUS Research Theme:
Biopsychosocial Ecology of Stress & Brain Health,
resilience Application: Girls and women exposed to ACEs may reduce the likelihood of developing LUTS during different periods of the life course by engaging in adaptive coping strategies and seeking support from others, including professional therapy and medical treatment. |
Materials and Methods
The PLUS Research Consortium previously developed a conceptual framework to organize potential risk and protective factors for LUTS and bladder health by level of social ecology and biology, as well as the developmental period during which a factor is most likely to confer risk or protection.(18) The Consortium also grouped potential risk and protective factors into eight prioritized areas of study to guide future prevention science research (see Table 2). For the present project, the lead author solicited the involvement of PLUS Consortium members whose collective expertise represented different periods of the life span (e.g., infancy; childhood; adolescence; early, middle, and older adulthood), each of the eight PLUS prioritized areas of study, life course concepts, and study design and methodology. Co-authors joined small, transdisciplinary writing teams to develop different sections of this paper. This approach matched expertise to tasks, while also facilitating the generation of research questions about bladder health and LUTS that expanded beyond each member’s individual training and research experience. The lead author engaged with small writing teams to clarify and suggest methods for collaborating on specific tasks, synthesized sections, and solicited feedback on the paper as a whole.
Table 2.
Applying Concepts of Life Course Theory and Life Course Epidemiology to the Study of BH and LUTS among Girls and Womena
| Life Course Theory Principle | Life Course
Epidemiology Key Concept |
Initial Period(s) of Study | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLUS Research Theme and Example Life Course Question |
(1) Principle of Life Span Development | (2) Principle of Agency | (3) Principle of Time and Place | (4) Principle of Timing | (5) Principle of Linked Lives | (a) Birth cohort effects | (b) Context | (c) Accumulation of risk over time | (d) Chains of risk | (e) Critical and sensitive periods | (f) Embodiment | (g) Vulnerability and resilience to adversity | In Utero - Childhood | Adolescence | Younger Adulthood | Midlife Adulthood | Older Adulthood |
|
1. Biopsychosocial Ecology of Stress
& Brain Health How do the timing and nature of adverse childhood experiences (ACEs) impact toilet training, age of daytime and nighttime bladder control, bladder health, and LUTS development at different periods of the life course? |
X | X | X | X | X | X | X | X | |||||||||
|
2. Toileting Environment, Access,
Habits, & Techniques What factors contribute to restricted bathroom access in school settings among girls, and how might restricted access cumulatively affect the bladder health of girls and women across the life course? |
X | X | X | X | X | X | |||||||||||
|
3. Pregnancy &
Childbirth How does management of the intrapartum period across lifetime pregnancies influence bladder health and LUTS across the life course? |
X | X | X | X | X | ||||||||||||
|
4. Musculoskeletal
Health How might engagement in physical activity affect bladder health and the incidence and severity of LUTS across the life course? |
X | X | X | X | X | X | X | X | |||||||||
|
5. Lifestyle
Behaviors What messages do women receive about fluid intake over their life course, and how do these messages affect fluid intake and bladder health? |
X | X | X | X | X | X | |||||||||||
|
6. Physical Health & Medical
Conditions Is lifetime cardiovascular health associated with bladder health across the life course? If so, what mechanisms may account for this association? |
X | X | X | X | X | X | X | X | X | ||||||||
|
7. Infections &
Microbiome Through what mechanisms might life course exposure to antimicrobial medications increase the risk of poor bladder health? |
X | X | X | X | X | X | X | X | X | X | X | X | |||||
|
8. Hormone Use across the Life
Span How might life course exposure to exogenous hormones influence bladder health and LUTS? |
X | X | X | X | X | X | X | X | X | X | X | ||||||
This table shows the life course principles and concepts that have been applied across the 8 proposed research questions in this paper (also see results, right column of Table 1, and last row of Table 3). It is possible to apply a greater number of principles and concepts to each research question than what has been discussed in this paper.
BH=Bladder Health; LUTS=Lower Urinary Tract Symptoms
In the Results section, research questions are proposed for each of the eight research areas prioritized by PLUS. Some research questions reflect past consortium-wide activities to identify and prioritize research questions, while other questions were newly generated by writing team members. All research questions were further developed by writing team members to reflect a life course perspective. Proposed research questions are intended to serve as examples; they illustrate what is possible to investigate through life course research, and not necessarily what the PLUS Research Consortium will choose to investigate over a specific period of time. An example is provided for each of the eight research areas, prioritized by PLUS, to stimulate interest in life course research across the broad readership of Neurourology and Urodynamics. It is not feasible for a single study to investigate all the research questions provided as examples. Table 2 identifies general principles of Life Course Theory (30) and key concepts from life course epidemiology (31) illustrated by each research question. Life course principles and concepts are discussed in greater detail below, followed by possible study designs and methodological approaches that could be used to test proposed research questions. Research teams that are interested in generating and refining life course research questions can consider the three questions that PLUS investigators considered as they developed the examples below (see Figure 1).
Figure 1.
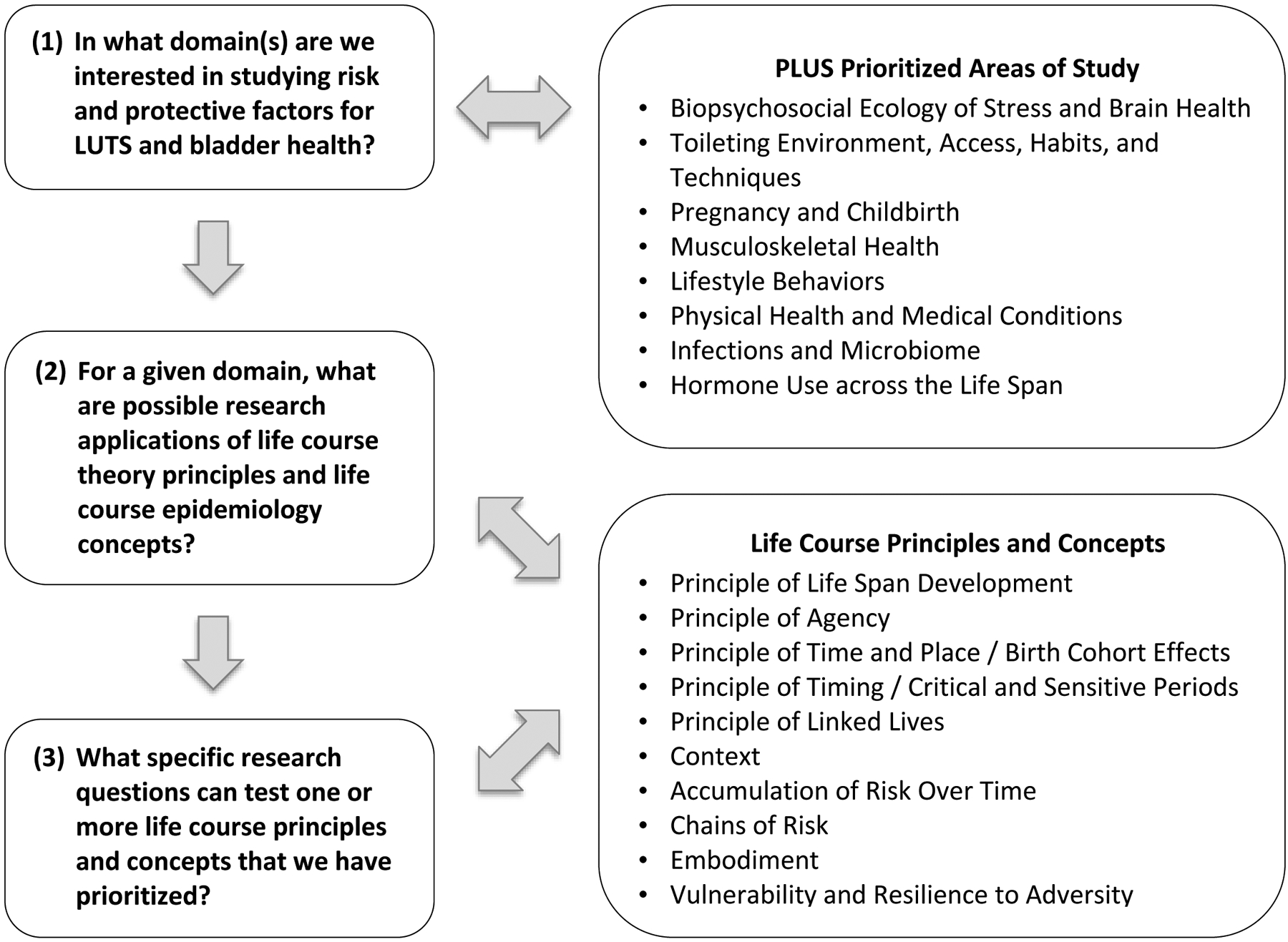
Generating and Refining Life Course Research Questions: Three Key Considerations.
Results
(1). Biopsychosocial Ecology of Stress and Brain Health:
Adverse childhood experiences (ACEs) are harmful, early life experiences that affect children directly (e.g., abuse and neglect) and indirectly through the child’s living environment (e.g., parental conflict, substance use, or mental illness).(32) ACEs have been linked to delayed attainment of bladder control among children and the emergence of LUTS later in life.(33, 34) We ask, “How do the timing and nature of ACEs impact toilet training, age of daytime and nighttime bladder control, bladder health, and LUTS development at different periods of the life course?”
Consistent with the Principle of Life Span Development, this question examines the age at which bladder control is attained during childhood, as well as the development of LUTS during childhood, adolescence, and later life periods. Continence requires central nervous system maturation and adequate functioning throughout the life course, including recognition and interpretation of the physical sensation to urinate, distinction between different degrees of bladder fullness and urge, planning and execution of toileting behaviors (e.g., requesting permission to use a restroom), and suppression of urge until a toilet is available.(35) Consistent with the Principle of Timing and critical and sensitive periods, the consequences of ACEs may differ depending on their timing in relation to (1) development of brain regions that govern executive control,(36) (2) sensitive periods during which the central nervous system is particularly vulnerable to stressors,(36) and (3) establishment of daytime and nighttime bladder control.(33) Thus, exposure to ACEs should be assessed prenatally and during infancy, as well as during childhood and adolescence. This would permit the identification of associations that may be specific to a critical or sensitive period during which ACEs occur. A life course approach to research is also ideal for assessing the potential influence of stressor chronicity. The accumulation of ACEs over time (defined by number, duration, and severity) could increase risk for abnormal timing of toilet training, regression of bladder control, and development of subsequent LUTS.
(2). Toileting Environment, Access, Habits, and Techniques:
While toilet training typically occurs at home, toddlers continue to develop toileting habits in daycare and pre-school, and girls can refine habits in primary and secondary school.(37) We ask, “What factors contribute to restricted bathroom access in school settings among girls, and how might restricted access cumulatively affect the bladder health of girls and women across the life course?”
Girls’ restricted access to toilets in the school context illustrates the Principle of Linked Lives. School systems may not have standardized policies regarding student bathroom access, leaving such determinations to classroom teachers, who may lack knowledge or training in bladder health. Indeed, teachers may not question their own restricted access to bathrooms (i.e., accepting a norm or requirement that teachers not leave the classroom unattended). Teachers may limit students’ bathroom access for many reasons, including concern for misbehavior in the bathroom (e.g., vandalism, bullying) or missed classroom time.(38) Girls’ behavioral responses to limited bathroom access illustrate the Principle of Agency. Agency to void may be compromised by rules restricting bathroom access, location of bathrooms, limited time to void, and behaviors of peers that impact the cleanliness or safety of the toileting environment.(39) In addition, subsets of students may avoid school bathrooms when gendered bathroom spaces do not align with their gender identity.(40) Students may restrict fluids or delay voiding despite urge;(39) these compensatory behaviors may become habits impacting future bladder health trajectories.(41) By investigating the Principles of Linked Lives and Agency in relation to restricted bathroom access and subsequent bladder health, life course researchers will be well positioned to inform policies and practices governing bathroom access within schools.
(3). Pregnancy and Childbirth.
Bladder health status may change across pregnancy and childbirth experiences.(42) Pregnancy and vaginal birth are known risk factors for UI secondary to pelvic floor musculature and nerve injury.(43–46) We ask, “How does management of the intrapartum period across lifetime pregnancies influence bladder health and LUTS across the life course?”
Not all childbirth experiences damage pelvic floor musculature. Several factors may enhance vulnerability to pelvic floor muscular damage during childbirth, including older age at delivery, mode of delivery (i.e., vaginal versus cesarean), longer duration of labor, and management of second stage labor, such as intervention exposure (e.g., forceps, episiotomy),(44, 47) method of pushing (i.e., Valsalva versus open glottis), duration of pushing, and maternal position for birth (e.g., supine versus lateral position). Pelvic floor muscular tears across pregnancies, as well as number of childbirth episodes and associated interventions, may contribute to accumulated risk for LUTS and poorer bladder health. Researchers who assess intrapartum management characteristics of a first childbirth, or only the most recent childbirth, may misrepresent the risk associated with different management characteristics.
Labor management practices are context specific and may be dictated by local protocols and practices (e.g., American College of Obstetricians and Gynecologists [ACOG] and American College of Nurse-Midwives [ACNM] guidelines), provider type (e.g., obstetrician; midwife), location (e.g., hospital, birth center, home), and insurance (e.g., private or public).(48, 49) By examining the effects of labor management practices on urologic outcomes across lifetime pregnancies, life course researchers will be well positioned to inform policies and practices governing maternity care. Findings could potentially be used to tailor recommended care based on age, parity, and mode of delivery, among other factors.
(4). Musculoskeletal Health:
The importance of physical activity for overall health is well established.(50) We ask, “How might engagement in physical activity affect bladder health and the incidence and severity of LUTS across the life course?”
Consistent with the Principle of Life Span Development, this question considers how type, intensity, and duration of different physical activities may promote bladder health and serve as protective or risk factors for LUTS development across the life course. A key distinction is between low-to-moderate and high-impact activities. Participation in high-impact physical activity during adolescence (e.g., gymnastics) is associated with SUI during activity performance,(51, 52) as well as SUI in middle age.(53) Participation in high-impact physical activity during adulthood (e.g., long-distance running) is associated with UI after first pregnancy and delivery, while participation in low-impact activity pre-pregnancy (e.g., walking) is associated with lower prevalence of postpartum UI.(54) A recent systematic review suggests that prenatal exercise (pelvic floor muscle training with or without aerobic exercise) can prevent UI.(55)
Greater vulnerability to LUTS as women age may be explained by different biological chains of risk that could be ameliorated through low-to-moderate physical activity. For example, menopause leads to decreased endogenous estrogen, which can alter lower urinary tract tissue in a manner that causes new onset urgency and frequency.(56) Another example involves strength and coordination; aging women may experience decreased muscle strength and coordination, resulting in decreased pelvic floor support and bladder dysfunction.(57) In each case, low-to-moderate intensity physical activity may be helpful in offsetting biological changes and preventing LUTS among aging women.
Researchers who adopt a life course approach to examining the effects of physical activity on bladder health and LUTS will be well positioned to inform guidelines for physical activity at different ages and to potentially tailor guidelines based on an individual’s profile of risk and protective factors.
(5). Lifestyle Behaviors:
Adequate fluid intake is essential to homeostasis.(58) Water plays a major role in nutrient delivery, toxin removal, thermoregulation, and tissue hydration.(59, 60) Adolescent and adult women often receive the message from healthcare providers, educators, family members, and peers that they should stay well hydrated. However, little is known about the impact of this message on women’s behavior and bladder health. We ask, “What messages do women receive about fluid intake over their life course, and how do these messages affect fluid intake and bladder health?”
Messaging for infants and children are directed toward parents and other caretakers, while adolescents may receive direct messaging. Familial intergenerational messages may exert a powerful influence on the frequency and types of fluids provided and consumed, illustrating the Principle of Linked Lives. Likewise, a caregiver may influence fluid consumption in an older care recipient by assisting with fluid management. Adolescent and adult women may make independent choices about fluid intake after hearing messages, illustrating the Principle of Agency. It remains to be demonstrated through research, however, whether compliance with the message to stay well hydrated confers risk or protection with respect to LUTS. Researchers who adopt a life course approach to studying the messages women receive about fluid intake, as well as their compliance with messages and resulting health status, will be well positioned to inform lay discourse messaging about bladder health habits (e.g., “drink water when thirsty” versus “drink water continuously throughout the day”).
(6). Physical Health and Medical Conditions:
Known protective factors for cardiovascular health include (1) blood pressure management, (2) cholesterol control, (3) blood glucose reduction, (4) physical activity, (5) healthy diet, (6) weight loss, and (7) smoking cessation.(61–63) These behavioral factors are strongly associated with medical and physical health conditions that influence bladder health, including obesity and diabetes. We ask, “Is lifetime cardiovascular health associated with bladder health across the life course? If so, what mechanisms may account for this association?”
Routine engagement in low-to-moderate intensity physical activity is protective against LUTS.(64) Modest amounts of weight loss among overweight and obese women may improve LUTS, specifically UI.(65, 66) Links between LUTS and blood pressure, cholesterol, and smoking have not been supported or are inconsistent(67, 68). Mechanisms linking cardiovascular health protective behaviors to bladder health are unclear (e.g., direct links versus indirect links through medical conditions such as obesity and diabetes). Potential mechanisms underlying an association between poor cardiovascular and bladder health include decreases in bladder function and sensation and poor pelvic floor muscle function secondary to autonomic nervous system dysfunction, peripheral neuropathy, impaired peripheral vasculature, or musculoskeletal disease.(69, 70) These potential mechanisms illustrate biological chains of risk.
Researchers who adopt a life course approach will be well positioned to examine mechanisms that unfold over time, as well as the degree to which different environments may impact women’s bladder health. Women’s context (e.g., geography, community and culture, socioeconomic circumstances) may facilitate or constrain their agency to make behavioral choices (e.g., physical activity, diet, smoking). The Principle of Time and Place and birth cohort effects are illustrated by changes in the prevalence of obesogenic environments. For example, historical changes in the availability of high fructose corn syrup and high saturated fat foods (i.e., processed foods) have contributed to increased body weight and obesity rates in women, particularly among individuals with limited resources for making healthy choices.(71, 72) These life course concepts highlight the importance of prevention strategies that target obesogenic environments and structural inequities that constrain women’s agency to make healthy choices.
(7). Infections and Microbiome:
Antimicrobial medications (i.e., “antibiotics”) are prescribed to treat diagnosed and suspected bacterial infections, and prophylactically to minimize risk of acquiring infection.(73, 74) A broad spectrum of antimicrobial agents is in common use today, many with variable doses and recommended durations of therapy.(73) Untoward effects of antimicrobials include alterations in women and girls’ genitourinary and gastrointestinal microbiomes.(75) We ask, “Through what mechanisms might life course exposure to antimicrobial medications increase the risk of poor bladder health?”
Some evidence exists to suggest that alterations in genitourinary and gastrointestinal microbiomes may lead to LUTS such as overactive bladder, urgency UI, and recurrent lower UTIs,(76) although the direction of associations has not been established. Consistent with the Principle of Life Span Development and accumulation of risk over time, this question examines varying and cumulative exposure to anti-microbial medications in relation to bladder health and LUTS over the life course. The Principle of Timing and critical and sensitive periods are also relevant. It is possible that risk of LUTS may be dependent on the period during which antimicrobial exposure occurs. Birth, infancy, early childhood, adolescence, sexual debut, pregnancy, menopause, and older age are periods during which girls and women may be more sensitive to the effects of antimicrobial exposure, due to age-related physiological states of organ systems, as well as endogenous hormonal effects on the genitourinary tract.(75) For example, following menopause women experience a rise in vaginal pH and a decrease in protective Lactobacilli that normally inhabit the vagina, which in turn allows more pathologic organisms to colonize the vagina, potentially leading to urogenital infection.(77) Chains of risk are particularly relevant to antimicrobial exposure, as initial exposure to antimicrobial agents may alter host defenses, which in turn may lead to greater susceptibility to infection, additional antimicrobial exposure, and even further altered host defenses.(75) Researchers who adopt a life course approach to examining the effects of antimicrobial medications will be well positioned to inform health care policies, guidelines, and practices, which may be tailored based on age and previous trajectories of health.
(8). Hormone Use across the Life Span:
The most common exogenous hormones are oral contraceptives and replacement estrogens, with over 25% of women aged 15–49 years and 3–13% of women aged 45–64 years using hormones for birth control, menstrual regulation, and control of perimenopausal or menopausal symptoms.(78, 79) Exogenous medications that influence hormones include selective estrogen receptor modulators (e.g., tamoxifen), used for the prevention and treatment of breast cancer,(80) and medications such as clomiphene, used to induce ovulation in women with ovulatory infertility.(81) We ask, “How might life course exposure to exogenous hormones influence bladder health and LUTS?”
Estrogen deficiency has been linked with LUTS,(82) potentially through a decline in lactobacillus or other alterations of the microbiome.(77) Exogenous hormone use may favorably influence pelvic floor function through improvement of pelvic muscle tissue quality (e.g., ability to detect bladder sensations and apply urethral pressure).(83) In contrast to a large literature on menopausal estrogen replacement, few studies have assessed the potentially beneficial impact of oral contraceptive use on pre- and postmenopausal LUTS. A recent systematic review supported an association between oral contraceptive use and bladder pain syndrome (BPS),(84) but this association could be due to use of oral contraceptives to address symptoms that are often comorbid with BPS (i.e., reverse causation). The review identified too few high quality and prospective studies to assess other LUTS.(84)
Consistent with the Principle of Life Span Development and accumulation of risk (or in this case, protection), this question examines cumulative premenopausal and postmenopausal exposure to exogenous hormones, including first use of contraception early during a woman’s reproductive years, and potential changes in exposure. The Principles of Agency and Linked Lives apply, as women typically choose contraceptive practices, while also being influenced by members of their social networks, including family members, peers, partners, and providers. The Principle of Time and Place and birth cohort effects are illustrated by changing hormonal preparations (e.g., low dose formulations), patterns of use, and public sentiment towards oral contraceptives and other exogenous hormones (e.g., hormone replacement therapy) over time.(85) Researchers who adopt a life course approach may inform health care guidelines governing the use of exogenous hormones at different ages, as well as health messaging directed towards women. Women may advocate for specific contraceptives or estrogen replacement therapies (agency), potentially as a function of what others recommend (linked lives).
Life Course Study Designs and Methodological Approaches.
Factors that contribute to the maintenance of bladder health and etiology of LUTS can be examined through cohort, case-control, and cross-sectional study designs. Table 3 highlights four example study designs and methodological approaches for exploring risk and protective factors for LUTS and bladder health across the life course: (1) prospective cohort, (2) retrospective cohort, (3) ambidirectional cohort, and (4) retrospective case-control or cross-sectional study of women with and without LUTS, with recalled information about potential risk and protective factors. Each of these designs has strengths and weaknesses. From a methodological perspective, the three cohort study approaches allow for valid assessment of temporality. A prospective or retrospective cohort study in which early-life exposures are measured close to their time of occurrence is best for assessing the impact of these factors on bladder health and LUTS later in life, due to greater accuracy of exposure assessment. Studies with assessment of recalled early-life exposures among LUTS-free individuals and follow-up assessment of LUTS (i.e., ambidirectional designs) tend to be better than retrospective case-control and cross-sectional studies of participants with and without LUTS that rely upon participants’ recall of early life events, as recall may be influenced by current health status. From a practical perspective, however, researchers may not have the time, ability, or funding to follow a cohort from early to later life. Researchers often rely upon retrospective case-control or cross-sectional studies because of their greater efficiency and lower cost. In addition, case-control studies are better suited for assessing outcomes that are rare, such as recurrent UTIs or interstitial cystitis, because they avoid following hundreds, if not thousands, of participants prospectively, only to see very few develop the outcome of interest.
Table 3.
Methodologic Considerations, Practical Considerations, and Examples, Presented for Key Life Course Study Designs (86–90)
| Life Course Study Design | ||||
|---|---|---|---|---|
| Cohort approaches | (4) Retrospective case-control or cross-sectional study (recalled early life information) |
|||
| (1) Prospective cohort study (participants followed from early life)a |
(2) Retrospectively-assembled cohort study (use of existing early life records)a |
(3) Ambidirectional cohort study (recalled early life information and follow-up for later outcomes)a |
||
| Methodologic considerations | ||||
| Ability to establish a temporal relation | ⬤ | ⬤ | ⬤ | ○ |
| Accuracy of information: | ||||
| - Early-life risk and protective factors | ⬤ | ●b/⬤ | ● | ○ |
| - Bladder health/LUTS | ●c/⬤d | ●c/⬤d | ●c/⬤d | ●c/⬤d |
| - Confounders | ⬤ | ●b/⬤ | ● | ● |
| Protection from bias: | ||||
| - Differential recall bias by bladder health/LUTS status | ⬤ | ⬤ | ⬤ | ○ |
| - Selection bias | ⬤ | ● | ● | ○ |
| Practical considerations | ||||
| Time to perform the study | ○ | ●e/⬤ | ● | ⬤ |
| Cost to perform the study | ○ | ●f/⬤ | ● | ⬤ |
| Other practical considerations | May not be feasible in the lifetime of an investigator | May be difficult to identify existing early-life records | Useful to study rare outcomes and/or preliminary hypotheses | |
| Examples | ||||
| Infections & Microbiome Life Course Question: Through what mechanisms might life course exposure to antimicrobial medications increase the risk of poor bladder health?g | In a birth cohort of girls with annual assessment of antibiotic exposure in the past year; urine and stool specimen collection during childhood; and urine, vaginal, and stool specimen collection during adolescence and early adulthood, the possible influence of antibiotic exposure at different sensitive periods (birth, infancy, early childhood, and adolescence) can be studied in relation to urogenital and gastrointestinal microbiomes. | In a cohort study retrospectively assembled in a geographic region with uniform health care coverage and centralized electronic medical records, the possible influence of cumulative lifetime antibiotic exposure can be studied in relation to subsequent diagnosis of LUTS that are most prevalent during the age period of the cohort (e.g., UTIs among women aged 15–35 years; overactive bladder, urgency, and UTIs among women aged 50–70 years). | Breastfeeding is believed to reduce susceptibility to infection and, consequently, reduce exposure to antibiotics among children. In a cohort study of young women with recalled information about having been breastfed and exposed to antibiotics, as well as available medical record data, the possible influence of exclusive bottle feeding (versus breastfeeding) and antibiotic exposure in childhood can be studied as a chain of risk in relation to future development of poor bladder health. | In a case-control study of women with recurrent UTIs and age-matched controls without recurrent UTIs, the possible influence of agency and linked lives with respect to antibiotic use can be examined. Women can indicate the frequency with which they were diagnosed with different medical conditions during specific periods of adolescence and adulthood, the frequency with which they were prescribed antibiotics for each condition, and who advocated for or against antibiotic use (i.e., caregiver, self, provider). |
⬤ Major strength of the study design; ● Moderate strength; ○ Minor strength or weakness
Includes nested case-control and case-cohort studies conducted within an established cohort study.
Moderate rather than a major strength in some instances, because the accuracy of early-life risk and protective factor information may not be as high as for prospective cohort studies. Early-life data are typically not collected for the purpose of the retrospective cohort studies.
Moderate strength when the outcome is a risk factor or intermediate marker of LUTS/worse bladder health.
Major strength when the outcome is the onset of LUTS or worsening of bladder health.
Identifying and extracting data from existing, older records may be time-consuming.
Identifying and extracting data from existing, older records may be expensive.
Examples were designed such that research questions could be tested within an initial 5-year funding period. With prospective cohort studies, researchers can pursue additional funding to continue data collection across participants’ life course.
Table 3 provides examples to illustrate different approaches for addressing the question, “Through what mechanisms might life course exposure to antimicrobial medications increase the risk of poor bladder health?” In addition, Figure 2 illustrates the timing of assessments that could be made at different life periods for each study design and methodological approach. Several texts are available to provide a more thorough grounding in different study designs, methods, and analytic techniques that may be applied to life course research. (86–90)
Figure 2.
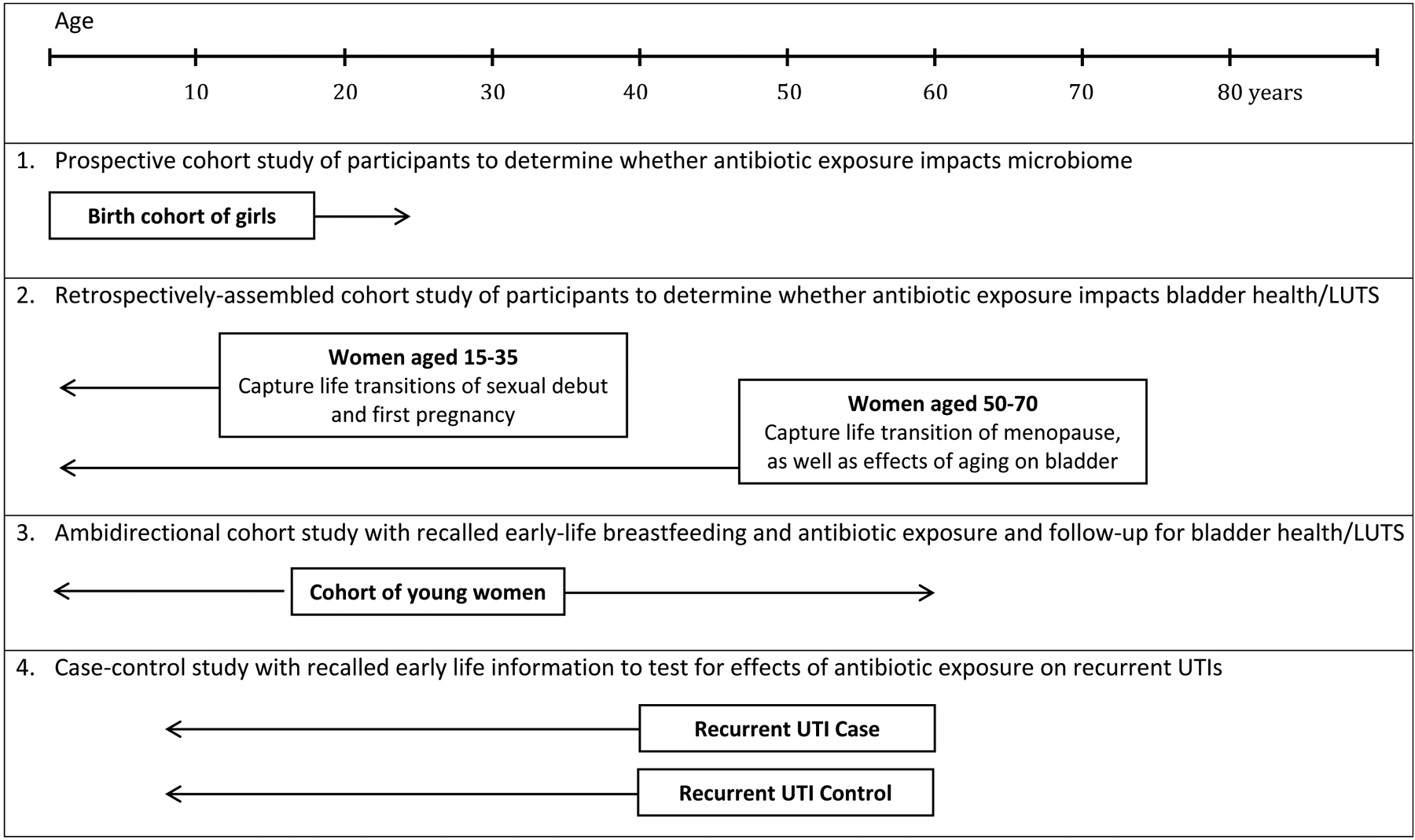
Illustration of Key Life Course Study Designs for Infections & Microbiome Examples in Table 3.
Discussion
Prevention science can expand research and practice beyond the detection and treatment of LUTS to bladder health promotion and LUTS prevention among girls and women. This conceptual paper may assist researchers from a variety of disciplinary backgrounds to develop and implement research that identifies key risk and protective factors for LUTS and bladder health across the life course of girls and women. Studies may be designed to address a single research question or multiple questions, particularly when the mechanisms influencing trajectories of bladder health and LUTS may overlap across different risk and protective factors. For the present paper, PLUS Research Consortium members worked in small, transdisciplinary teams to propose a single research question for each of the eight areas of study prioritized by PLUS. Key proposed mechanisms overlapped across questions, highlighting the efficiency of investigating multiple priority areas at once. For example, a study could be designed to investigate the impact of physical activity, exogenous hormone use, lifetime pregnancies, and intrapartum management strategies on pelvic floor function, a key mechanism proposed to link physical activity, hormone use, pregnancy, and childbirth experiences to trajectories of bladder health and LUTS. Another study could be designed to examine life course concepts that apply across multiple priority areas. For example, the influence of linked lives (e.g., women’s relationships with family members, peers, partners, and providers) could be examined in relation to women’s toileting habits and techniques; fluid intake; use of antimicrobial medicines; and exogenous hormone use. It is also possible to combine different prioritized areas of study to answer a specific question. For example, both physical health conditions (e.g., obesity, diabetes) and psychiatric health conditions (e.g., depression, anxiety) may explain associations between ecological stressors and trajectories of bladder health and LUTS.
While it is possible to focus on a specific period of the life span when applying a prevention science approach to etiologic research, such an approach would provide a limited understanding of how trajectories of dysfunction or health unfold over extended periods of time. Such an approach also would provide limited information about the development and persistence of potential socioeconomic inequities in health.(91, 92) A life course developmental perspective is essential to gaining a more precise understanding of how bladder health is preserved and LUTS develop among girls and women. Research findings can, in turn, be used to develop programs, practices, and policies that promote bladder health and prevent LUTS in diverse settings and at different periods across the life course.(26, 28, 93)
Conclusions
We call upon our colleagues across diverse disciplines to focus on prevention and incorporate a life course perspective in their research on LUTS and bladder health. Contributions are needed from the fields of urology, obstetrics and gynecology, midwifery, female pelvic medicine and reconstructive surgery, nursing, infectious disease, epidemiology and other public health specialties, sociology, pediatrics, adolescent medicine, and geriatrics, among other clinical and social science fields. To the extent possible, transdisciplinary approaches are encouraged. While it may not be possible to include investigators representing the wide array of disciplines highlighted above in a single research study, partnering with one or more colleagues with expertise in life course theory or life course epidemiology is sufficient to begin a program of research that incorporates life course concepts. It also is possible to investigate life course research questions by conducting secondary analyses of existing longitudinal datasets or adding new measures to ongoing longitudinal cohort studies.
The PLUS Research Consortium is currently applying Life Course Theory and life course epidemiology to the design of etiologic research that will identify key risk and protective factors for LUTS and bladder health among adolescent and adult women. The present paper may assist researchers who are interested or engaged in similar work. This paper can also be applied to better understand life course influences on a variety of other health outcomes. Results from life course research may inform health promotion programs, policies, and practices, which in turn may enhance the likelihood that individuals will experience optimal health at different life periods and across the life course.
Acknowledgements
The Prevention of Lower Urinary Tract Symptoms (PLUS) Research Consortium is supported by the National Institutes of Health (NIH) through cooperative agreements (grants U01DK106786, U01DK106853, U01DK106858, U01DK106898, U01DK106893, U01DK106827, U01DK106908, U01DK106892). Additional support is provided by the National Institute on Aging, NIH Office of Research on Women’s Health, and NIH Office of Behavioral and Social Sciences Research. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Appendix
Participating research centers at the time of this writing are as follows:
Loyola University Chicago - 2160 S. 1st Avenue, Maywood, Il 60153-3328
Linda Brubaker, MD, MS, Multi-PI; Elizabeth R. Mueller, MD, MSME, Multi-PI; Colleen M. Fitzgerald, MD, MS, Investigator; Cecilia T. Hardacker, RN, MSN, Investigator; Jeni Hebert-Beirne, PhD, MPH, Investigator; Missy Lavender, MBA, Investigator; David A. Shoham, PhD, Investigator
University of Alabama at Birmingham - 1720 2nd Ave South, Birmingham, AL 35294
Kathryn L. Burgio, PhD, PI; Cora E. Lewis, MD, MSPH, Investigator; Alayne D. Markland, DO, MSc, Investigator; Gerald McGwin, PhD, Investigator; Camille P. Vaughan, MD, MS, Investigator; Beverly Williams, PhD, Investigator
University of California San Diego - 9500 Gilman Drive, La Jolla, CA 92093-0021
Emily S. Lukacz, MD, PI; Sheila Gahagan, MD, MPH, Investigator; D. Yvette LaCoursiere, MD, MPH, Investigator; Jesse N. Nodora, DrPH, Investigator
University of Michigan - 500 S. State Street, Ann Arbor, MI 48109
Janis M. Miller, PhD, MSN, PI; Lawrence Chin-I An, MD, Investigator; Lisa Kane Low, PhD, CNM, Investigator
University of Minnesota, Scientific and Data Coordinating Center (SDCC) - 3 Morrill Hall, 100 Church St. S.E., Minneapolis MN 55455
Bernard L. Harlow, PhD, Multi-PI; Kyle Rudser, PhD, Multi-PI; Sonya S. Brady, PhD, Investigator; John Connett, PhD, Investigator; Haitao Chu, MD, PhD, Investigator; Cynthia S. Fok, MD, MPH, Investigator; Todd Rockwood, PhD, Investigator; Melissa Constantine, PhD, MPAff, Investigator
University of Pennsylvania – Urology, 3rd FL West, Perelman Bldg, 34th & Spruce St, Philadelphia, PA 19104
Diane K. Newman, DNP, ANP-BC, FAAN PI; Amanda Berry, PhD, CRNP, Investigator; C. Neill Epperson, MD, Investigator; Kathryn H. Schmitz, PhD, MPH, FACSM, FTOS, Investigator; Ariana L. Smith, MD, Investigator; Ann E. Stapleton, MD, FIDSA, FACP, Investigator; Jean F. Wyman, PhD, RN, FAAN, Investigator
Washington University in St. Louis - One Brookings Drive, St. Louis, MO 63130
Siobhan Sutcliffe, PhD, PI; Aimee James, PhD, MPH, Investigator; Jerry Lowder, MD, MSc, Investigator
Yale University - PO Box 208058 New Haven, CT 06520-8058
Leslie Rickey, MD, PI; Deepa Camenga, MD, MHS, Investigator; Shayna D. Cunningham, PhD, Investigator; Toby Chai, MD, Investigator; Jessica B. Lewis, PhD, MFT, Investigator
Steering Committee Chair: Mary H. Palmer, PhD
NIH Program Office: National Institute of Diabetes and Digestive and Kidney Diseases, Division of Kidney, Urologic, and Hematologic Diseases, Bethesda, MD
NIH Project Scientist: Tamara Bavendam MD, MS; Project Officer: Ziya Kirkali, MD; Scientific Advisors: Chris Mullins, PhD and Jenna Norton, MPH
Footnotes
Conflict of Interest Statement
There are no reported financial conflicts directly related to this work. The Prevention of Lower Urinary Symptoms (PLUS) Research Consortium is supported by the National Institutes of Health (NIH) through cooperative agreements (U01DK106786, U01DK106853, U01DK106858, U01DK106898, U01DK106893, U01DK106827, U01DK106908, U01DK106892).
Financial Disclosures
Sonya S. Brady: None
Amanda Berry: None
Deepa Camenga: None
Colleen M. Fitzgerald: None
Sheila Gahagan: None
Cecilia T. Hardacker: None
Bernard L. Harlow: None
Jeni Hebert-Beirne: None
D. Yvette LaCoursiere: None
Jessica B. Lewis: None
Lisa Kane Low: None
Jerry L. Lowder: None
Alayne D. Markland: None
Gerald McGwin: None
Diane K. Newman: Editor, Digital Science Press
Mary H. Palmer: None
David A. Shoham: None
Ariana L. Smith: None
Ann Stapleton: Previous service on advisory boards related to urinary tract infection, GSK, and Paratek.
Siobhan Sutcliffe: None
Beverly Williams: None
Contributor Information
Sonya S. Brady, Division of Epidemiology & Community Health, University of Minnesota School of Public Health, Minneapolis, MN.
Amanda Berry, Division of Urology, Children’s Hospital of Philadelphia, Philadelphia, PA.
Deepa R. Camenga, Department of Pediatrics, Yale School of Medicine, New Haven, CT.
Colleen M. Fitzgerald, Department of Obstetrics and Gynecology, Loyola University Chicago Stritch School of Medicine, Chicago, IL.
Sheila Gahagan, Division of Academic General Pediatrics, University of California San Diego School of Medicine, San Diego, CA.
Cecilia T. Hardacker, Howard Brown Health, Chicago, IL.
Bernard L. Harlow, Boston University School of Public Health, Boston, MA.
Jeni Hebert-Beirne, University of Illinois at Chicago School of Public Health, Chicago, IL.
D. Yvette LaCoursiere, Department of Obstetrics, Gynecology, & Reproductive Sciences, UC San Diego School of Medicine, San Diego, CA.
Jessica B. Lewis, Yale School of Public Health, New Haven, CT.
Lisa Kane Low, Department of Health Behavior and Biological Sciences, University of Michigan School of Nursing, Ann Arbor, MI.
Jerry L. Lowder, Division of Female Pelvic Medicine and Reconstructive Surgery, Washington University in St. Louis School of Medicine, St. Louis, MO
Alayne D. Markland, Division of Gerontology, Geriatrics, and Palliative Care, University of Alabama at Birmingham School of Medicine and Birmingham VA Medical Center, Birmingham, AL.
Gerald McGwin, Department of Epidemiology, University of Alabama at Birmingham School of Public Health, Birmingham, AL.
Diane K. Newman, Department of Surgery, Division of Urology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
Mary H. Palmer, University of North Carolina at Chapel Hill School of Nursing, Chapel Hill, NC.
David A. Shoham, Department of Public Health Sciences, Loyola University Chicago, Maywood, IL.
Ariana L. Smith, Department of Surgery, Division of Urology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
Ann Stapleton, Department of Medicine, Division of Allergy and Infectious Disease, University of Washington, Seattle, WA.
Beverly Rosa Williams, Department of Medicine, Division of Gerontology, Geriatrics and Palliative Care, University of Alabama at Birmingham.
Siobhan Sutcliffe, Department of Surgery, Division of Public Health Sciences, Washington University School of Medicine, St. Louis, MO.
References
- 1.Abrams P, Andersson KE, Birder L, et al. Fourth International Consultation on Incontinence Recommendations of the International Scientific Committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol Urodyn. 2010;29(1):213–240. [DOI] [PubMed] [Google Scholar]
- 2.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4–20. [DOI] [PubMed] [Google Scholar]
- 3.Horowitz M, Cohen J. Review of adolescent urinary tract infection. Curr Urol Rep. July 2007;8(4):319–323. [DOI] [PubMed] [Google Scholar]
- 4.Butler RJ, Heron J. The prevalence of infrequent bedwetting and nocturnal enuresis in childhood. A large British cohort. Scand J Urol Nephrol. 2008;42(3):257–264. [DOI] [PubMed] [Google Scholar]
- 5.Swithinbank LV, Heron J, von Gontard A, Abrams P. The natural history of daytime urinary incontinence in children: a large British cohort. Acta Paediatr. July 2010;99(7):1031–1036. [DOI] [PubMed] [Google Scholar]
- 6.Heron J, Grzeda MT, von Gontard A, Wright A, Joinson C. Trajectories of urinary incontinence in childhood and bladder and bowel symptoms in adolescence: prospective cohort study. BMJ Open. 2017;7(3):e014238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minassian VA, Bazi T, Stewart WF. Clinical epidemiological insights into urinary incontinence. Int Urogynecol J. May 2017;28(5):687–696. [DOI] [PubMed] [Google Scholar]
- 8.Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. January 2000;30(1):152–156. [DOI] [PubMed] [Google Scholar]
- 9.Aoki Y, Brown HW, Brubaker L, Cornu JN, Daly JO, Cartwright R. Urinary incontinence in women. Nat Rev Dis Primers. July 6 2017;3:17042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu JM, Matthews CA, Vaughan CP, Markland AD. Urinary, fecal, and dual incontinence in older U.S. Adults. J Am Geriatr Soc. May 2015;63(5):947–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warzak WJ. Psychosocial implications of nocturnal enuresis. Clin Pediatr (Phila). July 1993;Spec No:38–40. [DOI] [PubMed] [Google Scholar]
- 12.Grzeda MT, Heron J, von Gontard A, Joinson C. Effects of urinary incontinence on psychosocial outcomes in adolescence. Eur Child Adolesc Psychiatry. June 2017;26(6):649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coyne KS, Wein AJ, Tubaro A, et al. The burden of lower urinary tract symptoms: evaluating the effect of LUTS on health-related quality of life, anxiety and depression: EpiLUTS. BJU Int. April 2009;103 Suppl 3:4–11. [DOI] [PubMed] [Google Scholar]
- 14.Grimby A, Milsom I, Molander U, Wiklund I, Ekelund P. The influence of urinary incontinence on the quality of life of elderly women. Age Ageing. March 1993;22(2):82–89. [DOI] [PubMed] [Google Scholar]
- 15.Harlow BL, Bavendam TG, Palmer MH, et al. The Prevention of Lower Urinary Tract Symptoms (PLUS) Research Consortium: a transdisciplinary approach toward promoting bladder health and preventing lower urinary tract symptoms in women across the life course. J Womens Health. 2018;27(3):283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Constitution of the World Health Organization-Basic Documents, Supplement, October 2006. Geneva, Switzerland: WHO; 2006. [Google Scholar]
- 17.Lukacz ES, Bavendam TG, Berry A, et al. A Novel Research Definition of Bladder Health in Women and Girls: Implications for Research and Public Health Promotion. J Womens Health (Larchmt). August 2018;27(8):974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brady SS, Bavendam TG, Berry A, et al. The Prevention of Lower Urinary Tract Symptoms (PLUS) in girls and women: Developing a conceptual framework for a prevention research agenda. Neurourol Urodyn. November 2018;37(8):2951–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coyne KS, Kaplan SA, Chapple CR, et al. Risk factors and comorbid conditions associated with lower urinary tract symptoms: EpiLUTS. BJU Int. April 2009;103 Suppl 3:24–32. [DOI] [PubMed] [Google Scholar]
- 20.Coie JD, Watt NF, West SG, et al. The science of prevention. A conceptual framework and some directions for a national research program. Am Psychol. October 1993;48(10):1013–1022. [DOI] [PubMed] [Google Scholar]
- 21.Heller K. Coming of age of prevention science. Comments on the 1994 National Institute of Mental Health-Institute of Medicine Prevention reports. Am Psychol. November 1996;51(11):1123–1127. [DOI] [PubMed] [Google Scholar]
- 22.Kia-Keating M, Dowdy E, Morgan ML, Noam GG. Protecting and promoting: an integrative conceptual model for healthy development of adolescents. J Adolesc Health. March 2011;48(3):220–228. [DOI] [PubMed] [Google Scholar]
- 23.McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. Winter 1988;15(4):351–377. [DOI] [PubMed] [Google Scholar]
- 24.Glass TA, McAtee MJ. Behavioral science at the crossroads in public health: extending horizons, envisioning the future. Soc Sci Med. April 2006;62(7):1650–1671. [DOI] [PubMed] [Google Scholar]
- 25.Sallis J, Owen N. Health Behavior: Theory, Research, and Practice Ecological Models of Health Behavior. 5th Edition 5th ed. San Francisco: Jossey Bass; 2015. [Google Scholar]
- 26.Halfon N, Forrest CB. The emerging theoretical framework of life course health development In: Halfon N, Forrest CB, Lerner RM, Faustman EM, eds. Handbook of life course health development. Cham Switzerland: Springer; 2018:463–497. [PubMed] [Google Scholar]
- 27.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. April 10 1993;341(8850):938–941. [DOI] [PubMed] [Google Scholar]
- 28.Halfon N, Larson K, Lu M, Tullis E, Russ S. Lifecourse health development: past, present and future. Matern Child Health J. February 2014;18(2):344–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krieger N. Proximal, distal, and the politics of causation: what’s level got to do with it? Am J Public Health. February 2008;98(2):221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elder GH, Johnson MK, Crosnoe R. The emergence and development of life course theory In: T MJ, Shanahan M, eds. Handbook of the life course. New York, NY: Kluwer Academic/Plenum Publishers; 2003:3–19. [Google Scholar]
- 31.Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C. Life course epidemiology. Journal of epidemiology and community health. 2003;57(10):778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes K, Bellis MA, Hardcastle KA, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. August 2017;2(8):e356–e366. [DOI] [PubMed] [Google Scholar]
- 33.Jansson UB, Sillen U, Hellstrom AL. Life events and their impact on bladder control in children. J Pediatr Urol. June 2007;3(3):171–177. [DOI] [PubMed] [Google Scholar]
- 34.Link CL, Lutfey KE, Steers WD, McKinlay JB. Is abuse causally related to urologic symptoms? Results from the Boston Area Community Health (BACH) Survey. Eur Urol. August 2007;52(2):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jansson UB, Hanson M, Sillen U, Hellstrom AL. Voiding pattern and acquisition of bladder control from birth to age 6 years--a longitudinal study. J Urol. July 2005;174(1):289–293. [DOI] [PubMed] [Google Scholar]
- 36.Herzog JI, Schmahl C. Adverse Childhood Experiences and the Consequences on Neurobiological, Psychosocial, and Somatic Conditions Across the Lifespan. Front Psychiatry. 2018;9:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundblad B, Hellstrom AL. Perceptions of school toilets as a cause for irregular toilet habits among schoolchildren aged 6 to 16 years. J Sch Health. April 2005;75(4):125–128. [DOI] [PubMed] [Google Scholar]
- 38.Ko LN, Chuang KW, Champeau A, Allen IE, Copp HL. Lower Urinary Tract Dysfunction in Elementary School Children: Results of a Cross-Sectional Teacher Survey. J Urol. April 2016;195(4 Pt 2):1232–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norling M, Stenzelius K, Ekman N, Wennick A. High School Students’ Experiences in School Toilets or Restrooms. J Sch Nurs. June 2016;32(3):164–171. [DOI] [PubMed] [Google Scholar]
- 40.Kosciw JG, Greytak EA, Giga NM, Villenas C, Danischewski DJ. The 2015 National School Climate Survey: The Experiences of Lesbian, Gay, Bisexual, Transgender, and Queer Youth in Our Nation’s Schools. Available at https://www.glsen.org/sites/default/files/2015National GLSEN 2015 National School Climate Survey %28NSCS%29 - Full Report_0.pdf. New York: GLSEN; 2016. [Google Scholar]
- 41.Costantini E, Illiano E, Giannitsas K, et al. Urological dysfunction in young women: an inheritance of childhood? BJU Int. March 2018;121(3):453–457. [DOI] [PubMed] [Google Scholar]
- 42.Delancey JO, Kane Low L, Miller JM, Patel DA, Tumbarello JA. Graphic integration of causal factors of pelvic floor disorders: an integrated life span model. Am J Obstet Gynecol. December 2008;199(6):610.e611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Handa VL, Blomquist JL, Knoepp LR, Hoskey KA, McDermott KC, Munoz A. Pelvic floor disorders 5–10 years after vaginal or cesarean childbirth. Obstet Gynecol. October 2011;118(4):777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shek KL, Dietz HP. Intrapartum risk factors for levator trauma. Bjog. November 2010;117(12):1485–1492. [DOI] [PubMed] [Google Scholar]
- 45.Miller JM, Low LK, Zielinski R, Smith AR, DeLancey JO, Brandon C. Evaluating maternal recovery from labor and delivery: bone and levator ani injuries. Am J Obstet Gynecol. August 2015;213(2):188.e181–188.e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laterza RM, Schrutka L, Umek W, Albrich S, Koelbl H. Pelvic floor dysfunction after levator trauma 1-year postpartum: a prospective case-control study. Int Urogynecol J. January 2015;26(1):41–47. [DOI] [PubMed] [Google Scholar]
- 47.Low LK, Zielinski R, Tao Y, Galecki A, Brandon CJ, Miller JM. Predicting Birth-Related Levator Ani Tear Severity in Primiparous Women: Evaluating Maternal Recovery from Labor and Delivery (EMRLD Study). Open J Obstet Gynecol. April 1 2014;4(6):266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Committee Opinion No. 697: Planned Home Birth. Obstet Gynecol. April 2017;129(4):e117–e122. [DOI] [PubMed] [Google Scholar]
- 49.Supporting healthy and normal physiologic childbirth: a consensus statement by the American College of Nurse-Midwives, Midwives Alliance of North America, and the National Association of Certified Professional Midwives. J Midwifery Womens Health. Sep-Oct 2012;57(5):529–532. [DOI] [PubMed] [Google Scholar]
- 50.U. S. Department of Health and Human Services. Physical activity and health: A report of the Surgeon General. In: U.S. Department of Health and Human Services CfDCaP, National Center for Chronic Disease Prevention and Health Promotion,, ed Atlanta, GA: 1996. [Google Scholar]
- 51.de Mattos Lourenco TR, Matsuoka PK, Baracat EC, Haddad JM. Urinary incontinence in female athletes: a systematic review. Int Urogynecol J. December 2018;29(12):1757–1763. [DOI] [PubMed] [Google Scholar]
- 52.Almousa S, Bandin Van Loon A. The prevalence of urinary incontinence in nulliparous female sportswomen: A systematic review. J Sports Sci. July 2019;37(14):1663–1672. [DOI] [PubMed] [Google Scholar]
- 53.Nygaard IE, Shaw JM, Bardsley T, Egger MJ. Lifetime physical activity and female stress urinary incontinence. Am J Obstet Gynecol. July 2015;213(1):40.e41–40.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eliasson K, Nordlander I, Larson B, Hammarstrom M, Mattsson E. Influence of physical activity on urinary leakage in primiparous women. Scand J Med Sci Sports. April 2005;15(2):87–94. [DOI] [PubMed] [Google Scholar]
- 55.Davenport MH, Nagpal TS, Mottola MF, et al. Prenatal exercise (including but not limited to pelvic floor muscle training) and urinary incontinence during and following pregnancy: a systematic review and meta-analysis. Br J Sports Med. November 2018;52(21):1397–1404. [DOI] [PubMed] [Google Scholar]
- 56.Waetjen LE, Ye J, Feng WY, et al. Association between menopausal transition stages and developing urinary incontinence. Obstet Gynecol. November 2009;114(5):989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Varella LR, Torres VB, Angelo PH, et al. Influence of parity, type of delivery, and physical activity level on pelvic floor muscles in postmenopausal women. J Phys Ther Sci March 2016;28(3):824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rondon-Berrios H, Berl T. Physiology and Pathophysiology of Water Homeostasis Disorders of Fluid and Electrolyte Metabolism. Vol 52: Karger Publishers; 2019:8–23. [DOI] [PubMed] [Google Scholar]
- 59.Benelam B, Wyness L. Hydration and health: a review. Nutrition Bulletin. 2010;35(1):3–25. [Google Scholar]
- 60.Bennett VA, Vidouris A, Cecconi M. Effects of Fluids on the Macro-and Microcirculations. Critical Care. 2018;22(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goff DC Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. July 1 2014;63(25 Pt B):2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karmali KN, Goff DC Jr., Ning H, Lloyd-Jones DM. A systematic examination of the 2013 ACC/AHA pooled cohort risk assessment tool for atherosclerotic cardiovascular disease. J Am Coll Cardiol. September 9 2014;64(10):959–968. [DOI] [PubMed] [Google Scholar]
- 63.Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. Jama. April 9 2014;311(14):1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nygaard IE, Shaw JM. Physical activity and the pelvic floor. Am J Obstet Gynecol. February 2016;214(2):164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Breyer BN, Creasman JM, Richter HE, et al. A Behavioral Weight Loss Program and Nonurinary Incontinence Lower Urinary Tract Symptoms in Overweight and Obese Women with Urinary Incontinence: A Secondary Data Analysis of PRIDE. J Urol. January 2018;199(1):215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Subak LL, Wing R, West DS, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. January 29 2009;360(5):481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bradley CS, Erickson BA, Messersmith EE, et al. Evidence of the impact of diet, fluid intake, caffeine, alcohol and tobacco on lower urinary tract symptoms: a systematic review. The Journal of urology. 2017;198(5):1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Litman HJ, Steers WD, Wei JT, Kupelian V, Link CL, McKinlay JB. Relationship of lifestyle and clinical factors to lower urinary tract symptoms: results from Boston Area Community Health survey. Urology. November 2007;70(5):916–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin WY, Andersson KE, Lin CL, Kao CH, Wu HC. Association of lower urinary tract syndrome with peripheral arterial occlusive disease. PLoS One. 2017;12(3):e0170288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sha K, Palmer MH, Yeo S. Yoga’s Biophysiological Effects on Lower Urinary Tract Symptoms: A Scoping Review. J Altern Complement Med. March 2019;25(3):279–287. [DOI] [PubMed] [Google Scholar]
- 71.Dubowitz T, Zenk SN, Ghosh-Dastidar B, et al. Healthy food access for urban food desert residents: examination of the food environment, food purchasing practices, diet and BMI. Public Health Nutr. August 2015;18(12):2220–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang MC, Kim S, Gonzalez AA, MacLeod KE, Winkleby MA. Socioeconomic and food-related physical characteristics of the neighbourhood environment are associated with body mass index. J Epidemiol Community Health. June 2007;61(6):491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Centers for Disease Control. Core Elements of Hospital Antibiotic Stewardship Programs. Atlanta, GA: US Department of Health and Human Services; In: US Department of Health and Human Services C, edAtlanta, GA 2014. [Google Scholar]
- 74.Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clinical Infectious Diseases. 2016;62(10):e51–e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science. April 29 2016;352(6285):544–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Govender Y, Gabriel I, Minassian V, Fichorova R. The Current Evidence on the Association Between the Urinary Microbiome and Urinary Incontinence in Women. Front Cell Infect Microbiol. 2019;9:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stapleton AE. The Vaginal Microbiota and Urinary Tract Infection. Microbiol Spectr. December 2016;4(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Daniels K, Abma JC. Current contraceptive status among women aged 15–49: United States, 2015–2017. NCHS Data Brief, no 327 In: US Department of Health and Human Services CfDCaP, ed Hyattsville, MD: National Center for Health Statistics; 2018. [Google Scholar]
- 79.Jewett PI, Gangnon RE, Trentham-Dietz A, Sprague BL. Trends of postmenopausal estrogen plus progestin prevalence in the United States between 1970 and 2010. Obstetrics and gynecology. 2014;124(4):727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. November 16 2005;97(22):1652–1662. [DOI] [PubMed] [Google Scholar]
- 81.Palomba S, Pasquali R, Orio F Jr., Nestler JE. Clomiphene citrate, metformin or both as first-step approach in treating anovulatory infertility in patients with polycystic ovary syndrome (PCOS): a systematic review of head-to-head randomized controlled studies and meta-analysis. Clin Endocrinol (Oxf). February 2009;70(2):311–321. [DOI] [PubMed] [Google Scholar]
- 82.Robinson D, Cardozo LD. The role of estrogens in female lower urinary tract dysfunction. Urology. October 2003;62(4 Suppl 1):45–51. [DOI] [PubMed] [Google Scholar]
- 83.Abdelbary AM, El-Dessoukey AA, Massoud AM, et al. Combined Vaginal Pelvic Floor Electrical Stimulation (PFS) and Local Vaginal Estrogen for Treatment of Overactive Bladder (OAB) in Perimenopausal Females. Randomized Controlled Trial (RCT). Urology. September 2015;86(3):482–486. [DOI] [PubMed] [Google Scholar]
- 84.Champaneria R, D’Andrea RM, Latthe PM. Hormonal contraception and pelvic floor function: a systematic review. Int Urogynecol J. May 2016;27(5):709–722. [DOI] [PubMed] [Google Scholar]
- 85.de Villiers TJ, Pines A, Panay N, et al. Updated 2013 International Menopause Society recommendations on menopausal hormone therapy and preventive strategies for midlife health. Climacteric. June 2013;16(3):316–337. [DOI] [PubMed] [Google Scholar]
- 86.Gordis L. Epidemiology. 5th ed. Philadelphia, PA: W. B. Saunders Company; 2013. [Google Scholar]
- 87.Szklo M, Nieto FJ. Epidemiology: beyond the basics. 4th ed. Burlington, MA: Jones & Bartlett Publishers; 2019. [Google Scholar]
- 88.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Vol 3: Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia; 2008. [Google Scholar]
- 89.Buka SL, Rosenthal SR, Lacy ME. Epidemiological study designs: Traditional and novel approaches to advance life course health development research In: Halfon N, Forrest CB, Lerner RM, Faustman EM, eds. Handbook of life course health development. Cham, Switzerland: Springer; 2018:541–560. [PubMed] [Google Scholar]
- 90.LIttle T. Core principles in life course health development methodology and analytics In: Halfon N, Forrest CB, Lerner RM, Faustman EM, eds. Handbook of life course health development. Cham, Switzerland: Springer; 2018:523–540. [PubMed] [Google Scholar]
- 91.Smith GD, Lynch J. Life course approaches to socioeconomic differentials in health In: Kuh D, Ben-Schlomo Y, eds. A life course approach to chronic disease epidemiology. 2nd ed: Oxford: University Press; 2004:77–115. [Google Scholar]
- 92.Kim P, Evans GW, Chen E, Miller G, Seeman T. How socioeconomic disadvantages get under the skin and into the brain to influence health development across the lifespan In: Halfon N, Forrest CB, Lerner RM, Faustman EM, eds. Handbook of life course health development. Cham, Switzerland: Springer; 2018:463–497. [PubMed] [Google Scholar]
- 93.Forrest CB, Riley AW. Childhood origins of adult health: a basis for life-course health policy. Health Aff (Millwood). Sep-Oct 2004;23(5):155–164. [DOI] [PubMed] [Google Scholar]
- 94.Marks J, de la Haye K, Barnett LM, Allender S. Friendship Network Characteristics Are Associated with Physical Activity and Sedentary Behavior in Early Adolescence. PLoS One. 2015;10(12):e0145344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shoham DA, Tong L, Lamberson PJ, et al. An actor-based model of social network influence on adolescent body size, screen time, and playing sports. PLoS One. 2012;7(6):e39795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hawkley LC, Thisted RA, Cacioppo JT. Loneliness predicts reduced physical activity: cross-sectional & longitudinal analyses. Health Psychol. May 2009;28(3):354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Norris AH, Shrestha NK, Allison GM, et al. 2018 Infectious Diseases Society of America Clinical Practice Guideline for the Management of Outpatient Parenteral Antimicrobial Therapy. Clin Infect Dis. January 1 2019;68(1):1–4. [DOI] [PubMed] [Google Scholar]
- 98.Jackson EC. Urinary tract infections in children: knowledge updates and a salute to the future. Pediatr Rev. April 2015;36(4):153–164; quiz 165–156. [DOI] [PubMed] [Google Scholar]
- 99.Fitzgerald MP, Thom DH, Wassel-Fyr C, et al. Childhood urinary symptoms predict adult overactive bladder symptoms. J Urol. March 2006;175(3 Pt 1):989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bright MA, Knapp C, Hinojosa MS, Alford S, Bonner B. The Comorbidity of Physical, Mental, and Developmental Conditions Associated with Childhood Adversity: A Population Based Study. Matern Child Health J. April 2016;20(4):843–853. [DOI] [PubMed] [Google Scholar]


