Abstract
Purpose of review
Alzheimer’s disease (AD) is a progressive neurodegenerative disease without effective pharmacological treatment. Noninvasive brain stimulation (NIBS) techniques, such as repetitive transcranial magnetic stimulation (rTMS) and transcranial electrical stimulation (tES), are increasingly being investigated for their potential to ameliorate the symptoms of AD and related dementias (ADRD).
Recent findings
A comprehensive literature review for primary research reports that investigated the ability of TMS/tES to improve cognition in ADRD patients yielded a total of 20 reports since 2016. Eight studies used rTMS and twelve used transcranial direct current stimulation (tDCS), the most common form of tES. Eight of the studies combined NIBS with cognitive training. Promising results should encourage continued investigation, however there is presently insufficient evidence to support widespread adoption of NIBS-based clinical treatments for ADRD.
Summary
NIBS remains an active area of investigation for treatment of ADRD, though the predominance of small, heterogeneous, proof-of-principle studies precludes definitive conclusions. We propose the establishment of a consortium to achieve the benefits of large-scale, controlled studies using biomarker-based diagnostic characterization of participants, development of neurophysiological markers to verify target engagement, and standardization of parameters.
Keywords: Repetitive transcranial magnetic stimulation, transcranial electrical stimulation, noninvasive brain stimulation, Alzheimer’s disease, mild cognitive impairment
INTRODUCTION
Alzheimer’s Disease (AD) is the most common cause of dementia worldwide [1]. With the growth of the aging population, the prevalence of AD in the United States alone is projected to rise from 5.5 million to 13.8 million by 2050 unless new treatments to prevent, slow, or reverse the disease are developed [2]. Currently available medications for AD may offer some symptomatic relief [3,4], but do not alter the underlying disease process or pathology. Recent drug trial failures for AD and related dementias (ADRD) have left the field with a lack of disease-modifying therapies [5,6]. In this context, non-pharmacological interventions including lifestyle modifications, physical activity, cognitive training, and non-invasive brain stimulation (NIBS) have been increasingly investigated as potential treatments or symptomatic therapies for AD-related cognitive decline [7–10]. This review will focus on the two most widely studied NIBS techniques to-date, transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (tES). However, we want to emphasize that given the complex pathophysiologic nature of ADRD, a single therapeutic intervention is unlikely to be a satisfactory response, and that combination of various interventions is probably critical. NIBS has the appeal that is can be easily combined with pharmacologic and behavioral interventions, and may play a useful role in future multimodality treatment approaches that are likely to be needed in ADRD.
TMS is a means of inducing brief pulses of intracranial electrical currents with a powerful, rapidly fluctuating, handheld electromagnet [11]. A single pulse of TMS can depolarize neuronal membranes leading to action potentials. TMS of the primary motor cortex (M1) can evoke descending corticospinal volleys, which can give rise to activations of contralateral muscles. These can be recorded as motor evoked potentials (MEPs) via electromyography (EMG). TMS to motor or non-motor regions can also elicit intracranial TMS-evoked potentials (TEPs) that can be recorded via electroencephalography (EEG) and are presumed to be the results of activation of cortical neural elements. Delivering trains of TMS pulses at a specified frequency and intensity, termed repetitive TMS (rTMS), can induce changes in brain excitability that can persist for some time after the period of stimulation [12]. The immediate aftereffects of a single rTMS application are typically measured as changes in the performance of a behavioral task or some measure of cortical excitability, such as average MEP or TEP amplitude. Daily sessions of rTMS are thought to yield a cumulative effect and form the basis for the stimulation protocols of the U.S. Food and Drug Administration (FDA)-cleared devices for clinical treatment of patients with medication-resistant for major depression [13] and obsessive-compulsive disorder [14].
In ADRD, several small pilot studies have shown promise using rTMS protocols to improve global cognition or language function [15–17], either using rTMS alone or combined with cognitive training. One example is the NeuroAD protocol (Neuronix, Ltd., Yoqneam, Israel), in which rTMS to 6 brain regions is delivered paired with interleaved cognitive training of the function of the targeted brain region [18]. There have been several early proof-of-principle studies using the NeuroAD protocol [15,16]. In 2016, a large multisite clinical trial (ClinicalTrials.gov: NCT01825330) was completed and awaits a final declaration by the U.S. FDA.
The other major form of NIBS is tES, which involves passing weak electrical current between two or more electrodes placed on the scalp [19,20]. The most common form of tES is transcranial direct current stimulation (tDCS), in which a constant current (typically 1–2 mA) is applied to create electrical gradients, which are thought to modulate cortical excitability indirectly by increasing (depolarizing) or decreasing (hyperpolarizing) the resting membrane potentials of neural elements in the vicinity of the anode or the cathode, respectively [21,22].
In ADRD, tDCS has been studied as a therapeutic tool in several pilot studies, and has shown promise in improving memory performance [23–25]. Other forms of tES include transcranial alternating current stimulation (tACS), in which the current is rapidly alternated at a specific frequency to entrain cortical oscillations, and transcranial random noise stimulation (tRNS), in which a full-band current spectrum is applied to boost endogenous rhythms by means of stochastic resonance [26]. While there have not been many studies using tACS in ADRD to date, it is an appealing approach given evidence of abnormal brain oscillations in AD [27]. Similarly, although there have not been any published reports investigating the potential therapeutic benefit of tRNS in ADRD, it has been shown to improve fluid intelligence in healthy adults when paired with adaptive cognitive training [28]. Future studies may explore the potential of these and other new NIBS techniques for ADRD.
The purpose of the present review is to assess recent developments in the investigation of NIBS as treatment for ADRD. While preliminary studies of TMS and tDCS have shown evidence of improving specific cognitive domains AD, there is at present no clear consensus about which NIBS paradigms are the most promising for treatment of ADRD, and which, if any, might be disease-modifying versus simply symptomatic. Given the rapidly changing state of the field, this review includes only recent studies from 2016–2018 and focuses on those investigations into the clinical benefit of NIBS to treat AD. For state of the field before 2016, we refer to a prior review by Gonsalvez and colleagues [7]. Since 2016, there have been a number of studies investigating the diagnostic [29,30] or prognostic [31] potential of NIBS for ADRD, or to better understand its pathophysiology [32,33], but these are outside the scope of this review. We will discuss commonalities and discrepancies across interventional studies and point out areas where further investigation is needed. Finally, we will discuss future directions, including opportunities offered by novel technologies in NIBS.
METHODS
A literature search was performed in PubMed using the following Boolean combinations of terms related to ADRD (“Alzheimer’s,” “mild cognitive impairment,” “dementia”) plus those related to NIBS (“noninvasive brain stimulation,” “non-invasive brain stimulation,” “transcranial magnetic stimulation,” “repetitive transcranial magnetic stimulation,” “theta burst stimulation,” “transcranial electrical stimulation,” “transcranial current stimulation,” “transcranial direct current stimulation,” “transcranial alternating current stimulation,” “transcranial random noise stimulation”). Articles with a publication date prior to 01/01/2016 were excluded as they were reviewed and discussed in Gonsalvez et al. [7]. Abstracts were reviewed and selected for inclusion if they represented a case study, case series, pilot or proof-of-principle study, or randomized control study for the use of NIBS as a treatment for AD or MCI, with a primary aim of improving cognitive function. Studies focusing primarily on other disease pathologies or other diagnostic groupings were not included.
RESULTS
Figure 1 shows a flow diagram of the PubMed search. The literature search yielded 39 studies focused on treatment of neurodegenerative disorders using NIBS techniques from 2016–2018; 20 of these focused on the treatment of cognition in AD or mild cognitive impairment (MCI), and were included in this review. The additional 19 studies investigated NIBS treatments for other neurodegenerative pathologies, and included primary progressive aphasia (PPA), fronto-temporal dementia (FTD), MCI due to Parkinson’s disease (PD), Lewy body disease (LBD), and other conditions outside of the scope of the current review.
Figure 1: Investigations of NIBS for treatment of ADRD since 2016.
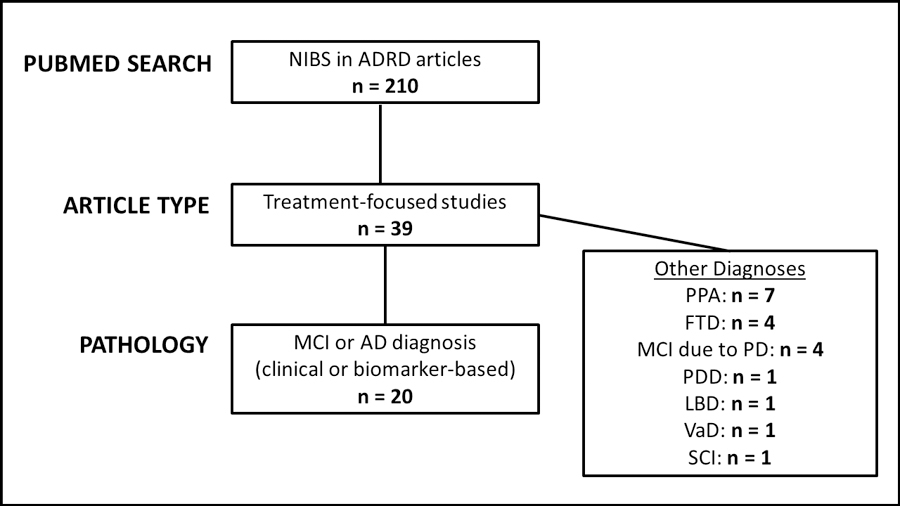
Flow diagram of literature search. Abbreviations: NIBS = noninvasive brain stimulation; AD = Alzheimer’s disease; MCI = mild cognitive impairment; PPA = primary progressive aphasia; FTD = frontotemporal dementia; PD = Parkinson’s disease; PDD = Parkinson’s disease dementia; LBD = Lewy Body disease; VaD = vascular dementia; SCI = subjective cognitive impairment.
Trials Using rTMS in ADRD
Table 1 lists the eight articles focusing on rTMS treatment of AD that were included in the review. Six of the eight studies focused on patients meeting criteria for AD dementia [34–39], while two studies focused on early-stage AD (prodromal AD or MCI) [40,41]. Determination of MCI or AD status was primarily based on clinical diagnostic criteria with one study used CSF biomarkers to confirm the diagnosis [40].
Table 1: Studies investigating rTMS as a therapeutic tool in ADRD.
Studies investigating TMS for treatment of ADRD using clinical or biomarker diagnostic criteria. Age is shown as Mean±SD or Mean (SEM). Several studies followed the Neuro AD protocol, targeting 6 brain regions: R prefrontal, L prefrontal, R parietal, L parietal, Broca’s area, Wernicke’s area. ADAS-Cog = Alzheimer’s Disease Assessment Scale-cognitive; BDS = Blessed Dementia Scale; MMSE = Mini-Mental State Examination; CGIC = Clinical Global Impression of Change; GDS = Geriatric Depression Scale; MOCA = Montreal Cognitive Assessment; AVLT = Auditory-Verbal Learning Test; FAB = Frontal Assessment Battery; DSST = Digit Symbol Substitution Test; NPI = Neuropsychiatric Inventory; IDDD = Interview for Deterioration in Daily Living Activities in Dementia; CGI = Clinical Global Impression; 3MS = Modified Mini-Mental Status Exam; AES = Apathy Evaluation Scale; TMT = Trail Making Test; EXIT-25 = Executive Interview; ADLs = Activities of Daily Living; I-ADLS = Instrumental Activities of Daily Living; ZBS = Zarit Burden Scale; ACE = Addenbrooke Cognitive Examination.
| TMS Studies 2016–2018 | Criteria for AD/MCI and disease stage | No. of participants | Sham/Control | Age | Target area; localization method | Interleaved Cognitive Training | Intensity (%RMT) | TMS frequency and pattern | Stimulation duration; number of TMS trains; number of pulses/day | Length of Intervention; Number of Sessions | Cognitive Domain | Neuropsychological Tests – Primary Outcome | Neuropsychological Tests – Secondary Outcomes | Main Significant Neuropsychological Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pilot Studies and Randomized Controlled Trials | ||||||||||||||
| Lee, et al., 2016 | Probable AD based on DSM-IV criteria, CDR 1–2, MMSE 18–26 (mild-moderate AD) | 26 | 2:1 Treatment:Sham | Treatment group age=71.2±7.6, Sham group age=70.3±4.8 | 6 brain regions; MRI guided | Yes | 90–110% RMT | 10 Hz rTMS; 20 trains applied with 2 seconds on, 20–40 seconds off; with interleaved cognitive task | 1 hour session/day; 3 brain regions/day; 1200 pulses/day | 6 weeks; 30 sessions | Global Cognition | ADAS-Cog | MMSE; CGIC; GDS | There was a significant improvement after the intervention in ADAS-Cog in the treatment group, but the between-group difference compared with sham was not significant. In both treatment and sham, the largest improvement was seen in mild AD compared to moderate AD. |
| Zhao, et al.; 2017. | Probable AD based on DSM-IV criteria, CDR 1–2, MMSE 18–26 (mild-moderate AD) | 30 | 17:13 Treatment:Sham | Treatment group Age=69.3±5.8, Sham group Age=71.4±5.2 | Treatment areas not clearly specified, but included parietal P3/P4, posterior temporal T5/T6; 10–20 system | Yes | Not Specified | 20 Hz rTMS; 20 trains applied with 10 seconds on, 20 seconds off; with interleaved cognitive tasks | 1 hour session/day; 3 brain regions/day; 12000 pulses | 6 weeks; 30 sessions | Global Cognition & Verbal Memory | Not specified | ADAS-Cog, MMSE, MOCA, AVLT | There was a significant improvement in ADAS-Cog, MMSE, and AVLT in the treatment group, but there was no between-group difference compared with sham. Mild AD showed a larger improvement compared with moderate AD. |
| Nguyen, et al.; 2017 | Probable AD by clinical diagnosis, severity ranging from MCI to moderate-to-severe AD | 10 | None | Age=70.3 (7.2) | 6 brain areas plus L DLPFC and R DLPFC; MRI guided | Yes | 100% RMT | 6 Brain Region Treatment: 10 Hz rTMS; 20 trains applied with 2 seconds on over 10 minutes; with interleaved cognitive training. DLPFC Treatment: 10 Hz rTMS; 5 trains applied with 2 seconds on over 2.5 minutes; with interleaved cognitive training. | 1 hour session/day; 4 brain regions/day; up to 1300 pulses/day | 5 weeks; 25 sessions | Global Cognition | ADAS-Cog | performance on interleaved cognitive training tasks, MMSE, Dubois score, FAB, Stroop test, locomotor score, apathy score, caregiver burden interview, dependence score | Immediately after the treatment procedure, there was improvement in the ADAS-Cog, locomotor score, apathy score, and dependence score. Six months later, ADAS-Cog scores had returned to baseline, but apathy and dependence scores showed continued improvement. |
| Koch, et al.; 2018 | prodromal AD by Dubois, 2016 criteria with positive CSF biomarker | 14 | Crossover design, with participants receiving both treatment and sham stimulation | Age=70.0±5.1 | Precuneus; MRI guided, stimulation site confirmed with source localization | No | 100% RMT | 20 Hz rTMS; 40 trains applied with 2 seconds on, 28 seconds off. | 20 minutes of rTMS; 1600 pulses/day. | 2 weeks; 10 sessions | Verbal Memory, EEG, and TMS-EEG | Not specified | RAVLT, MMSE, FAB, DSST | RAVLT Delayed Recall showed a significant improvement after treatment compared with sham; other tests showed no main effect of treatment. |
| Alcalá-Lozano, et al.; 2018. | diagnosis of AD by DSM-V, MMSE >= 15, GDS-Reisberg level 2–4 | 19 | 1:1 randomization into 2 active treatment groups: “simple” vs “complex” stimulation protocol | Simple Group Age=73.3±6.0; Complex Group Age=71±4.3 | Simple Protocol: singlesite DPLFC stimulation. Complex Protocol: 6 brain regions; 10–20 system | No | 100% RMT | 5 Hz rTMS; 30 trains applied with 10 seconds off, 60 seconds off. | In the Simple Protocol, single-site stimulation was applied to DLPFC daily, in the Complex Protocol, 3 brain regions were treated daily; 1500 pulses/day | 3 weeks; 15 sessions | Global Cognition | ADAS-Cog | MMSE, NPI, GDS, IDDD, CGI | Both treatment groups showed an improvement in ADAS-Cog, MMSE, IDDD, NPI immediately after treatment, which persisted one month later. There was no significant difference between the two treatment groups. |
| Padala, et al.; 2018. | MCI diagnosis by Peterson’s criteria, MMSE >=23, with apathy (AES-C >=30). | 8 | Double-blind, randomized, Crossover design, with participants receiving both treatment and sham | Group 1 Age=68.0±10.0; Group 2 Age=64.0±9.0 | L DLPFC; 5.5 cm anterior to motor hotspot location | No | 120% RMT | 10 Hz rTMS; 75 trains applied with 3 seconds on, 26 seconds off. | 37.5 minutes of rTMS; 3000 pulses/day | 2 weeks; 10 sessions | Apathy | AES-C | 3MS, MMSE, TMT B, TMT A, EXIT-25, CGI, I-ADLS, ADLS, ZBS | There was a significant improvement in AES-C after the active treatment compared to the sham condition. There was also significant improvement in 3MS, MMSE, TMT A, and CGI-I in the treatment group compared with sham. |
| Case Reports and Clinical Case Series | ||||||||||||||
| Avirame, et al.; | moderate-severe AD by clinical diagnosis | 11 | None | Age=76±7 | Bilateral Prefrontal Cortex using deep TMS; 6 cm anterior to motor hotspot location | No | 120% RMT | 10 Hz deep TMS, 42 trains applied with 2 seconds on, 20 seconds off | 1 20-minute session/day, 2–3 times per week, with a minimum interval of 1 day between sessions | 20 sessions | Global Cognition | n/a | Mindstreams and ACE | 60% of patients improved on Mindstreams, and 77% showed improvement on the ACE compared to baseline. Treatment with deep TMS significantly improved ACE scores in a subset of the most progressed patients |
| Rabey and Dobronevsky; 2016. | mild-to-moderate AD clinical diagnosis | 30 | None; Patients treated in 2 private clinics offering commercial NeuroAD treatments | not reported | 6 brain regions; MRI guided | Yes | 90–110% RMT | 3/4 Paradigms: 10 Hz rTMS; 20 trains applied with 2 seconds on over 10 minutes; with interleaved cognitive training. 1/4 Paradigm: 10 Hz rTMS; 5 trains applied with 2 seconds on over 2.5 minutes; with interleaved cognitive training. | 1 hour session/day; 3 brain regions/day; 1300 pulses/day | 6 weeks; 30 sessions | Global Cognition | n/a | ADAS-Cog and MMSE | ADAS-Cog and MMSE both improved after treatment compared to baseline scores. |
Parameters of rTMS stimulation (including intensity, frequency, duration, and number of sessions) varied considerable across protocols. Half of the studies used MRI-guided neuronavigation [34,35,39,40]. Brain regions targeted included the precuneus, prefrontal cortex, and a multi-site 6-ROI protocol adapted from NeuroAD. Interleaved cognitive training was included in four of the rTMS studies following the NeuroAD approach [34–36,39]. Two studies employed a sham control [35,36], two studies employed a crossover design with participants receiving both sham and treatment conditions sequentially [40,41], and one study compared two different stimulation paradigms [37].
The primary cognitive outcome measures studied included global cognition, verbal memory, and apathy. Overall, results suggested a potential for improvement in cognitive measures after rTMS treatments, but results were mixed as to whether rTMS was significantly more effective than sham.
Trials Using tES in ADRD
Table 2 lists the 12 trials using tES as a treatment in AD that were included in the review. AD and MCI diagnoses were mostly made clinically [42–52], aside from one case report of posterior cortical atrophy [53] which confirmed AD biomarker positivity using CSF. Five studies focused on MCI [43–47]. One case series examined the use of tES for treatment of auditory hallucinations in AD and LBD [51], and another case report examined tES for treatment of language dysfunction in AD [52].
Table 2: Studies investigating tES as a therapeutic tool in ADRD.
Studies investigating tES for treatment of ADRD using clinical or biomarker diagnostic criteria. Age is shown as Mean±SD. LBD = Lewy body dementia. CVLT = California Verbal Learning Test; TMT = Trail Making Test; MMSE = Mini-Mental State Examination; MMQ = Multifactorial Memory Questionnaire; PMIT = Picture Memory Impairment Test; MOCA = Montreal Cognitive Assessment; ADAS-Cog = Alzheimer’s Disease Assessment Scale-cognitive; BDS = Blessed Dementia Scale; DAD = Disability Assessment for Dementia; D-KEFS = Delis-Kaplan Executive Function System; WMS = Wechsler Memory Scale; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; BADA = Battery for the Analysis of the Aphasic Deficit; AHRS = Auditory Hallucinations Rating Scale.
| Electrical Stimulation Studies 2016–2018 | Criteria for AD/MCI and disease stage | No. of Participants | Type of Stimulation | Sham/Control | Interleaved Cognitive Stimulation | Age | Target area; localization method | Scalp Electrode 1 | Scalp Electrode 2 | Scalp Electrode size (cm2) | Extracranial Electrode and size (cm2) | Current | Duration (min) | Total Number of Sessions; Length of Intervention | Cognitive Domain | Neuropsychological Tests – Primary Outcome | Neuropsychological Tests – Secondary Outcomes | Main Significant Neuropsychological Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pilot Studies and RCTs | ||||||||||||||||||
| Bystad, et al.; 2016. | Probable AD with increased level of certainty by NINCDS-ADRDA; MMSE > 18 | 25 | tDCS; awake | 1:1 Treatment:Sham | No | Treatment group age=70.0±8.0; Sham group age=75.0±8.7 | L temporal lobe; 10–20 system | Anode = T3 | Cathode = Fp2 | 35 | None | 2 mA | 30 | 6 sessions; 10 days | Verbal Memory | CVLT-II | MMSE, TMT A, TMT B, clock-drawing test | No significant differences in CVLT-II, MMSE, TMT A, TMT B, or clock drawing test were seen between the treatment and sham group. |
| Yun, et al,; 2016. | MCI diagnosis by Peterson criteria | 16 | tDCS; awake | 1:1 Treatment:Sham | No | Treatment group Age = 74.8±7.5; Sham group Age = 73.1±4.2 | Bilateral DLPFC; 10–20 system | Anode = F3 | Cathode = F4 | 25 | None | 2 mA | 30 | 6 sessions; 10 days | Subjective Memory Complaint Scale from Participants, FDG-PET | MMQ | n/a | The treatment group showed improvement in subjective memory scores on the MMQ-A (ability) and MMQ-C (contentment) subscores compared to sham. |
| Ladenbauer; et al.; 2017. | Amnestic MCI (single or multidomain) by Mayo criteria, with objective cognitive decline with scores < 1 SD below norms on memory tests; MMSE >=24 | 16 | Slow oscillatory tDCS; delivered during a daytime nap | Balanced crossover design, each participant received 1 treatment and 1 sham session, at least 2 weeks apart | No | Age=71±9 | Bifrontal stimulation, using anodal current with sinusoidal oscillations at a frequency of 0.75 Hz; 10–20 system | F3 | F4 | 8 mm diameter | Bilateral Mastoids; 0.64 | 0.522 mA/cm2 | 15–25 (5 minute blocks of stimulation given during stage 2,3, or 4 NREM sleep, for a total of 3–5 blocks) | 1 session; 1 day | Visual recognition memory, EEG | Visuospatial Memory Task comprised on neutral pictures taken from the International Affective Picture System | Procedural fingertapping task, verbal memory task, location memory task | There was an improvement in visual recognition memory in the treatment group compared to sham when controlling for sleepiness. There was no effect of treatment on procedural memory, verbal memory, or location memory. |
| Murugaraja, et al.; 2017 | MCI diagnosis by NIA-AA criteria, CDR=0.5 | 11 | tDCS; awake | None | No | Age=59.6 | L DLPFC; 10–20 system | Anode = F3-FP1 | Cathode = R supra-orbital region | 35 | None | 2 mA | 20 | 5 sessions; 5 days | Visual Memory | PMIT | n/a | Improved immediate and delayed recall on the PMIT immediately after conclusion of the treatment. Improvement on delayed recall PMIT persisted 1 month later |
| Cruz Gonzalez, et al.; 2018 | MCI diagnosis by modified Peterson’s criteria, MOCA 19–26, CDR <=0.5 | 5 | tDCS; awake | A-B-C-A protocol; anodal tDCS + CS, sham tDCS + CS, and CS only | Yes | Age=72.8±6.6 | L DLPFC; 10–20 system | Anode = L DLPFC | n/a | 35 | R deltoid; 35 | 2 mA | 30 | 1–5 sessions total of active tDCS + cognitive stimulation | Not Specified | Not specified | Performance on Cognitive Stimulation tasks from Neuron Up, MOCA, Digit Span, TMT | There was an improvement on the Neuron Up task in three subjects who received three or more tDCS sessions |
| Manenti, et al.; 2017. | Amnestic MCI by Petersen criteria, MMSE 24–30, CDR=0.5 | 18 | tDCS; awake | 1:1 Treatment:Sham | Yes | Treatment group Age=75.3±4.8; Sham group Age=75.3±2.2 | L lateral prefrontal cortex; 10–20 system | Anode = F3 | Cathode = Fp2 | 35 | n/a | 1.5 mA | 15 | Day 1 = Initial Learning Session only, Day 2 = Memory Reactivation + active tDCS or Sham Session, Day 3 and Day 30 = Retrieval Session only | Verbal Memory | Experimental Memory Task (Learning, Reactivation, Free Recall, and Recognition) | n/a | Active tDCS treatment enhanced Memory Recognition scores compared to Sham. |
| Case Reports and Clinical Case Series | ||||||||||||||||||
| Andrade, et al.; 2016. | AD diagnosis by NINCDSADRDA criteria, CDR = 1 | 1 | tDCS; awake | None | No | Age=73 | L DLPFC; 10–20 system | Anode = F3 | Cathode = R supraorbital region | 35 | n/a | 2 mA | 30 | 10 sessions; 2 weeks | Global Cognition | ADAS-Cog | NPI, BDS, DAD | After treatment, ADAS-Cog, NPI, BDS, and DAD showed improvement compared to baseline |
| Bystad, et al.; 2016. | Early AD diagnosis, criteria not specified | 1 | tDCS; awake | None | No | Age=59 | L temporal lobe; 10–20 system | Anode = T3 | Cathode = FP2 | not specified | n/a | 2 mA | 30 | 12 sessions; 6 days | Verbal Memory | CVLT-II, EEG | MMSE, TMT A, D-KEFS Word Fluency, WMS Attention Span, Clock-drawing test | After treatment, the CVLT-II and MMSE showed improvement compared to baseline. |
| Bystad, et al.; 2017. | Early-onset AD, Dubois criteria. | 1 | tDCS; awake, applied at home with help from family | None | No | Age=60 | L temporal lobe; 10–20 system | Anode = T3 | Cathode = FP2 | not specified | n/a | 2 mA | 30 | daily for 8 months | Memory, Visuospatial, Language, and Attention | RBANS | Overall the patient’s cognitive function remained stable over 8 months, with improvement in memory (immediate and delayed recall), and decline in visuospatial function. | |
| Costa, et al.; 2017. | Possible AD diagnosis by NINCDS-ADRDA criteria, MMSE = 14.27 | 1 | tDCS; awake | 1 week of Sham was followed by treatment intervention | Yes | Age=67 | R angular and supramarginal gyrus; 10–20 system | Anode = P6-CP6 | Cathode = L supraorbital region | 35 | n/a | 2 mA | 30 | 5 sessions; 1 week | Language | BADA | After treatment there was a significant improvement in comprehension of verbs, compared to sham. This persisted for 2 weeks post-stimulation. | |
| Gramegna, et al.; 2018. | Posterior cortical atrophy with AD diagnosis via CSF tau and a-beta | 1 | tDCS; awake | None | Cognitive rehab therapy preformed prior to initiation of tDCS | Age=58 | L DLPFC; 10–20 system | Anode = F3 | n/a | not specified | R shoulder | 2 mA | 20 | 20 sessions; 4 weeks; repeated for 2 separate cycles, total of 40 tDCS sessions | Executive function, fMRI | Stroop task while in the fMRI scanner | Complete NPS evaluation | The patient showed improvement on the Stroop task after cognitive training, which was maintained after the first and second tDCS cycle. |
| Mukku, et al.; 2018. | 1 patient with an AD diagnosis, 1 patient with a LBD diagnosis | 2 | High Definition tDCS; awake | None | No | AD patient Age =; LBD patient Age=68 | 10–10 system | High Definition tDCS used five ring electrodes arranged on the scalp around the central Cathode | Cathode = CP5 | ring electrode with outer radius 12 mm, inner radius 6 mm | n/a | 2 mA | 20 | 2 sessions per day, 10–20 sessions total; 5–10 days of treatment | Auditory Hallucinations | AHRS | Both the AD and LBD patient showed decrease frequency of auditory hallucinations on the AHRS and decreased acting out behavior. | |
Most tDCS studies applied stimulation to patients while they were awake, but one study examined slow oscillatory tDCS delivered during a daytime nap [44]. Four of the tDCS studies included cognitive training either before or during brain stimulation, with the intent to use brain stimulation to potentiate the effects of task-specific learning [46,47,52,53]. Out of 12 studies, three employed a separate sham control [42,43,47], and three employed sham in a crossover design [44,46,52]. Electrode localization exclusively used scalp landmarks; no studies used neuronavigation or modeling to target stimulation. Brain regions targeted included either bilateral or unilateral prefrontal cortex or temporal lobe.
A variety of neuropsychiatric outcomes were measured across studies, including global cognition, verbal memory, visual memory, subjective memory, and language. Overall, results suggested a potential for boosting cognitive function using tES, but results were mixed as to whether tES demonstrated statistically significantly superiority compared to sham.
DISCUSSION
This review found an ongoing, robust interest in the application of NIBS to ADRD, spanning a range of disease severity. Since our previous review capturing data until 2016 [7], there have been 12 new randomized-controlled trials or proof-of-principle studies, and 8 new case reports or clinical case series, representing a combined 244 ADRD patients studied. Results were encouraging for the use of NIBS to improve global cognition and memory measures in patients with a clinical diagnosis of AD. However, widespread adoption of NIBS as a standard course of treatment remains hindered by a number of methodological challenges, including the lack of clear consensus regarding optimal stimulation parameters, with variability seen in the type, intensity, frequency, location, and duration of stimulation. In the future, studies with larger numbers of participants, rigorous blinding and sham procedures, and biomarker-confirmation of AD diagnosis are needed to validate whether NIBS techniques are useful as primary or adjunct treatments for ADRD. In the following paragraphs we summarize and discuss the strengths and limitations of the state-of-the-field in several key areas.
Patient characterization
Great strides have been made in developing in vivo biomarkers of AD pathophysiology, chiefly, tests for beta-amyloid and tau proteins in the cerebral spinal fluid (CSF) or on positron emission tomography (PET) imaging. The recent NIA-AA research framework proposed by Jack and colleagues [54] promotes a biomarker-based definition of AD in vivo, allowing for standardization of diagnostic criteria for use in interventional research and biomarker studies. Whether due to cost, risk, limited access, or a combination of these factors, only a few studies in our review confirmed AD pathology using available biomarkers, and none demonstrated alteration of underlying disease pathogenesis. Instead, most studied relied on probable diagnostic criteria based on clinical and neuropsychological evaluations. The lack of thorough characterization of patients invites unknown heterogeneity, which in turn increases the risk of Type II (or false-negative) errors. Improvements in diagnostic characterization of patients will also facilitate the search for interventions for different variants of AD, dementias of non-AD etiologies, and preclinical/prodromal populations (for recent meta-analyses, see [55,56]). Attempts have been made to improve information about and access to AD biomarker test, including the recently-completed IDEAS (Imaging Dementia—Evidence for Amyloid Scanning) study (ClinicalTrials.gov: NCT02420756). In the future, we recommend a biomarker-based approach to subject inclusion in NIBS treatment trials, to confirm disease pathology and assure translatability to clinical populations.
Study design and use of sham/placebo
Small pilot studies were the most common encountered in the literature, followed by clinical reports. Publications of large, randomized, double-blinded, placebo-controlled clinical trials were lacking. The majority of studies approached NIBS as a symptomatic treatment, aimed at boosting specific domains of cognitive function. More than a third of studies employed interleaved cognitive training or used NIBS to boost or extend the effects of previously performed cognitive rehabilitation.
Our review found no large-scale studies demonstrating superiority of NIBS treatments compared to sham stimulation. Recently there has been a resurgence of interest in the placebo effect and its implications for clinical research (for a review, see [57]). This is particularly relevant to NIBS, where appropriate blinding is difficult to obtain due to the occurrence of robust peripheral (auditory, somatosensory, motor) effects that accompany TMS pulses or the ramping of tES currents. Crossover designs offer additional challenges given potential carry-over and long-lasting effects, as well as intra-individual variability of NIBS [58] coupled with inter-individual or disease-specific differences in expectation and memory, which can results in effects that are difficult to interpret. These challenges may be especially problematic in ADRD given that patients may not spontaneously report or recall prior experiences making assessment of blinding success and expected outcomes difficult. In the future, we recommend rigorous sham-control procedures without a crossover design, inclusion of only NIBS-naïve participants, and post-study assessment of blinding by both AD participants and their study partners (who may be providing information regarding functional patient outcomes).
Identification of target(s)
With the opportunity to target specific brain regions and networks, NIBS show potential for symptomatic treatment of AD-related cognitive decline in global cognition or within specific domains such as memory, language, attention, or motivation. Although brain stimulation sites varied across studies, the rationale for target sites was generally based on neuroanatomical correlates of cognitive dysfunction in AD. Studies using TMS were able to target cortical regions with greater focality and using MRI guidance, and frequently stimulated brain targets known to be strongly involved in AD pathogenesis, including the 6 brain regions adapted from the NeuroAD trial. Knowledge of distributed resting state networks also played a role in the choice of stimulation site, with one study using the precuneus as a TMS target due to connectivity with the default mode network. Several studies used tES to target symptoms of AD such as memory, apathy, language dysfunction, or auditory hallucinations. Another tES application used slow oscillatory tDCS during a daytime nap, which aimed to increase the power of sleep related slow oscillations and sleep spindles to improve memory consolidation. While the use of structural and functional neuroimaging can improve the selection of targets for TMS and tES, a major limitation common to all reviewed studies is the lack of an appropriate neurophysiological markers to gauge target engagement and monitor response. Modeling of the induced electrical field can help bridge this gap, though the future will undoubtedly require the combination of NIBS with concurrent electroencephalography (EEG), MRI, or PET imaging. While a few basic research studies highlight the potential and feasibility of these combined approaches [59–61], they have yet to be applied to clinical trials for ADRD and there remain critical questions about methodology, analysis, and interpretation.
Temporal interference
A commonality across the NIBS techniques included in this review is that their targets are largely restricted to superficial regions of cortex. Exceptions to this rule do exist, namely that the effects of TMS are polysynaptic and stimulation of deeper regions (such as the cingulate cortex) is possible with certain coils such as the double-cone [62] or H-Coil [63]. However, the physics of electromagnetic induction stipulate that deeper permeation comes at the expense of reduced focality. Likewise, some models of tDCS do suggest the induced electrical field extends beyond superficial layers, though the effects are always strongest directly adjacent to the electrodes [64]. Given the prominent role of the hippocampal formation and adjacent structures in AD pathology (or the basal ganglia in Parkinson’s and Huntington’s diseases), the ability to directly and selectively target deeper structures has long been a challenging, aspirational goal for researchers and practitioners of NIBS. This may change with a tACS-based approach of temporally interfering electrical fields, or “temporal interference” (TI) [65]. The principles of TI bear some resemblance to those of confocal microscopy, wherein two half-strength photons are directed to collide and thus summate to excite a deeper structure. In T1, two ultra-high frequency oscillations with small difference (e.g., 10,000 Hz and 10,010 Hz) are directed into the brain from opposing areas such that they “collide” in some deep structure such as the hippocampus. While the individual frequencies are too high to affect neural tissue, they summate by subtraction, resulting in a stimulating frequency of the difference (e.g., 10 Hz). To date, TI has moved beyond modeling to animal studies, confirming the ability to selectively stimulate deeper structures such as the hippocampus in rodents [65]. In the future, TI may be translated to humans who have or are at risk of developing ADRD [66], which would allow for improved focality of stimulation on deep cortical targets, including medial limbic structures.
Gamma oscillations
While the studies to date have focused on the use of NIBS to enhance neural activity related to cognition, there is preliminary evidence to suggest tACS may be able to decrease amyloid deposits the brain. Working with a mouse model of AD, Iaccarino and colleagues [67] demonstrated that using optogenetics to entrain fast-spiking parvalbumin-positive interneurons at 40 Hz (i.e., gamma frequency) reduced levels of amyloid-β (Aβ)1–40 and Aβ1–42 isoforms. In theory, tACS could achieve a similar effect in humans. Indeed, there is an ongoing open-label proof-of-principle study to test the efficacy of daily 1-hour sessions of 40 Hz tACS (ClinicalTrials.gov: NCT03290326). Further study is needed to determine whether this approach can lead to a lasting alteration of electrographic cortical rhythms, interact with proteins involved in neurodegeneration, or lead to meaningful clinical improvement in ADRD.
CONCLUSIONS
NIBS remains an active area of investigation for treatment of ADRD, though the predominance of small, heterogeneous, proof-of-principle studies precludes definitive conclusions. There is presently insufficient evidence to support widespread adoption of NIBS-based clinical treatments for ADRD, but promising results should encourage continued investigation. The future of NIBS as a therapeutic intervention for ADRD will depend on overcoming two major obstacles: (1) the standardization of NIBS stimulation parameters and confirmation of target engagement, and (2) the recruitment of large, well-characterized cohorts with a biomarker-confirmed diagnosis with sufficient longitudinal follow-up. Addressing both of these challenges is a high bar to cross for any individual research laboratory or center, though a failure to do so will keep the field mired in small, heterogeneous, proof-of-principle studies and case reports lacking in scientific rigor. We therefor propose the establishment of a large-scale, possibly international, consortium, with collaboration between academia and industry. Based on the successful model of the Alzheimer’s Disease Neuroimaging Initiative [68], methodological parameters should be published in advance and data collected from this consortium should be placed in a repository and made available to independent researchers.
KEY POINTS.
Noninvasive brain stimulation (NIBS) with or without cognitive training has the potential to improve cognition in Alzheimer’s disease and related dementias (ADRD).
A paucity of large-scale trials and a lack of consistency in treatment parameters precludes definitive conclusions.
The use of available biomarkers would greatly improve diagnostic characterization of ADRD patients.
Neurophysiological or modeling-based indicators are needed to confirm the engagement of cortical targets and monitor stimulation efficacy.
The field would benefit from a consortium or other multi-site coordinated efforts.
Acknowledgements
Financial support and sponsorship
This work was primarily supported by grants from the National Institutes of Health (NIH; 3R01MH115949-01S1 and R21AG051846). S.S.B. was further supported by the Sidney R. Baer Jr. Foundation (01028951) and NeuroNEXT. A.P.L. was also supported by the Sidney R. Baer, Jr. Foundation, Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758), the Football Players Health Study at Harvard University, and by the Defense Advanced Research Projects Agency (DARPA) via HR001117S0030. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Institutes of Health, NeuroNEXT, the Sidney R. Baer Jr. Foundation, The Football Platers Health Study, or DARPA.
Footnotes
Conflicts of Interest
A.P.L. serves on the scientific advisory boards for Starlab Neuroscience, Neuroelectrics, Neosync, NovaVision, and Cognito; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging. The authors declare no competing interests.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.World Alzheimer Report 2015, The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. 2015,
- 2.Alzheimer’s Association: 2018 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2018, 13:367–429. [Google Scholar]
- 3.Di Santo SG, Prinelli F, Adorni F, Caltagirone C, Musicco M: A meta-analysis of the efficacy of donepezil, rivastigmine, galantamine, and memantine in relation to severity of Alzheimer’s disease. J Alzheimers Dis 2013, 35:349–361. [DOI] [PubMed] [Google Scholar]
- 4.Zhu CW, Livote EE, Scarmeas N, Albert M, Brandt J, Blacker D, Sano M, Stern Y: Long-term associations between cholinesterase inhibitors and memantine use and health outcomes among patients with Alzheimer’s disease. Alzheimers Dement 2013, 9:733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, et al. : Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 2014, 370:322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, Raman R, Sun X, Aisen PS, et al. : Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med 2014, 370:311–321. [DOI] [PubMed] [Google Scholar]
- 7.Gonsalvez I, Baror R, Fried P, Santarnecchi E, Pascual-Leone A: Therapeutic Noninvasive Brain Stimulation in Alzheimer’s Disease. Curr Alzheimer Res 2017, 14:362–376. [DOI] [PubMed] [Google Scholar]
- 8.Cammisuli DM, Innocenti A, Franzoni F, Pruneti C: Aerobic exercise effects upon cognition in Mild Cognitive Impairment: A systematic review of randomized controlled trials. Arch Ital Biol 2017, 155:54–62. [DOI] [PubMed] [Google Scholar]
- 9.Maliszewska-Cyna E, Lynch M, Oore JJ, Nagy PM, Aubert I: The Benefits of Exercise and Metabolic Interventions for the Prevention and Early Treatment of Alzheimer’s Disease. Curr Alzheimer Res 2017, 14:47–60. [DOI] [PubMed] [Google Scholar]
- 10.Imtiaz B, Tolppanen A-M, Kivipelto M, Soininen H: Future directions in Alzheimer’s disease from risk factors to prevention. Biochem Pharmacol 2014, 88:661–670. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi M, Pascual-Leone A: Transcranial magnetic stimulation in neurology. Lancet Neurol 2003, 2:145–156. [DOI] [PubMed] [Google Scholar]
- 12.Klomjai W, Katz R, Lackmy-Vallée A: Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann Phys Rehabil Med 2015, 58:208–213. [DOI] [PubMed] [Google Scholar]
- 13.Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A, Wirecki TS: The Clinical TMS Society Consensus Review and Treatment Recommendations for TMS Therapy for Major Depressive Disorder. Brain Stimul 2016, 9:336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar S, Singh S, Chadda RK, Verma R, Kumar N: The Effect of Low-Frequency Repetitive Transcranial Magnetic Stimulation at Orbitofrontal Cortex in the Treatment of Patients With Medication-Refractory Obsessive-Compulsive Disorder: A Retrospective Open Study. J ECT 2018, 34:e16–e19. [DOI] [PubMed] [Google Scholar]
- 15.Rabey JM, Dobronevsky E, Aichenbaum S, Gonen O, Marton RG, Khaigrekht M: Repetitive transcranial magnetic stimulation combined with cognitive training is a safe and effective modality for the treatment of Alzheimer’s disease: a randomized, double-blind study. J Neural Transm (Vienna) 2013, 120:813–819. [DOI] [PubMed] [Google Scholar]
- 16.Bentwich J, Dobronevsky E, Aichenbaum S, Shorer R, Peretz R, Khaigrekht M, Marton RG, Rabey JM: Beneficial effect of repetitive transcranial magnetic stimulation combined with cognitive training for the treatment of Alzheimer’s disease: a proof of concept study. J Neural Transm 2011, 118:463–471. [DOI] [PubMed] [Google Scholar]
- 17.Cotelli M, Manenti R, Cappa SF, Zanetti O, Miniussi C: Transcranial magnetic stimulation improves naming in Alzheimer disease patients at different stages of cognitive decline. Eur J Neurol 2008, 15:1286–1292. [DOI] [PubMed] [Google Scholar]
- 18.Andrade SM, de Oliveira EA, Alves NT, Dos Santos ACG, de Mendonça CTPL, Sampaio DDA, da Silva EEQC, da Fonsêca ÉKG, de Almeida Rodrigues ET, de Lima GNS, et al. : Neurostimulation Combined With Cognitive Intervention in Alzheimer’s Disease (NeuroAD): Study Protocol of Double-Blind, Randomized, Factorial Clinical Trial. Front Aging Neurosci 2018, 10:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bestmann S, Walsh V: Transcranial electrical stimulation. Curr Biol 2017, 27:R1258–R1262. [DOI] [PubMed] [Google Scholar]
- 20.Kar K, Duijnhouwer J, Krekelberg B: Transcranial Alternating Current Stimulation Attenuates Neuronal Adaptation. J Neurosci 2017, 37:2325–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W: Modulation of cortical excitability by weak direct current stimulation--technical, safety and functional aspects. Suppl Clin Neurophysiol 2003, 56:255–276. [DOI] [PubMed] [Google Scholar]
- 22.Nitsche MA, Paulus W: Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol (Lond) 2000, 527 Pt 3:633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrucci R, Mameli F, Guidi I, Mrakic-Sposta S, Vergari M, Marceglia S, Cogiamanian F, Barbieri S, Scarpini E, Priori A: Transcranial direct current stimulation improves recognition memory in Alzheimer disease. Neurology 2008, 71:493–498. [DOI] [PubMed] [Google Scholar]
- 24.Boggio PS, Khoury LP, Martins DCS, Martins OEMS, de Macedo EC, Fregni F: Temporal cortex direct current stimulation enhances performance on a visual recognition memory task in Alzheimer disease. J Neurol Neurosurg Psychiatry 2009, 80:444–447. [DOI] [PubMed] [Google Scholar]
- 25.Boggio PS, Ferrucci R, Mameli F, Martins D, Martins O, Vergari M, Tadini L, Scarpini E, Fregni F, Priori A: Prolonged visual memory enhancement after direct current stimulation in Alzheimer’s disease. Brain Stimul 2012, 5:223–230. [DOI] [PubMed] [Google Scholar]
- 26.Antal A, Herrmann CS: Transcranial Alternating Current and Random Noise Stimulation: Possible Mechanisms. Neural Plast 2016, 2016:3616807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babiloni C, Lizio R, Marzano N, Capotosto P, Soricelli A, Triggiani AI, Cordone S, Gesualdo L, Del Percio C: Brain neural synchronization and functional coupling in Alzheimer’s disease as revealed by resting state EEG rhythms. Int J Psychophysiol 2016, 103:88–102. [DOI] [PubMed] [Google Scholar]
- 28.Brem A-K, Almquist JN-F, Mansfield K, Plessow F, Sella F, Santarnecchi E, Orhan U, McKanna J, Pavel M, Mathan S, et al. : Modulating fluid intelligence performance through combined cognitive training and brain stimulation. Neuropsychologia 2018, 118:107–114. [DOI] [PubMed] [Google Scholar]
- 29.Benussi A, Di Lorenzo F, Dell’Era V, Cosseddu M, Alberici A, Caratozzolo S, Cotelli MS, Micheli A, Rozzini L, Depari A, et al. : Transcranial magnetic stimulation distinguishes Alzheimer disease from frontotemporal dementia. Neurology 2017, doi: 10.1212/WNL.0000000000004232. [DOI] [PubMed] [Google Scholar]
- 30.Padovani A, Benussi A, Cantoni V, Dell’Era V, Cotelli MS, Caratozzolo S, Turrone R, Rozzini L, Alberici A, Altomare D, et al. : Diagnosis of Mild Cognitive Impairment Due to Alzheimer’s Disease with Transcranial Magnetic Stimulation. J Alzheimers Dis 2018, 65:221–230. [DOI] [PubMed] [Google Scholar]
- 31.Motta C, Lorenzo FD, Ponzo V, Pellicciari MC, Bonnì S, Picazio S, Mercuri NB, Caltagirone C, Martorana A, Koch G: Transcranial magnetic stimulation predicts cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2018, doi: 10.1136/jnnp-2017-317879. [DOI] [PubMed] [Google Scholar]
- 32.Koch G, Di Lorenzo F, Bonnì S, Ponzo V, Caltagirone C, Martorana A: Impaired LTP- but not LTD-like cortical plasticity in Alzheimer’s disease patients. J Alzheimers Dis 2012, 31:593–599. [DOI] [PubMed] [Google Scholar]
- 33.Di Lorenzo F, Ponzo V, Bonnì S, Motta C, Negrão Serra PC, Bozzali M, Caltagirone C, Martorana A, Koch G: LTP-like cortical plasticity is disrupted in Alzheimer’s disease patients independently from age of onset. Ann Neurol 2016, doi: 10.1002/ana.24695. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen J-P, Suarez A, Kemoun G, Meignier M, Le Saout E, Damier P, Nizard J, Lefaucheur J-P: Repetitive transcranial magnetic stimulation combined with cognitive training for the treatment of Alzheimer’s disease. Neurophysiol Clin 2017, 47:47–53.• This study used interleaved cognitive training with a multisite rTMS adapted from the NeuroAD, and is notable in that it showed an improvement in the primary outcome of global cognition immediately after treatment, but not persisting at 6 month follow up.
- 35.Lee J, Choi BH, Oh E, Sohn EH, Lee AY: Treatment of Alzheimer’s Disease with Repetitive Transcranial Magnetic Stimulation Combined with Cognitive Training: A Prospective, Randomized, Double-Blind, Placebo-Controlled Study. J Clin Neurol 2016, 12:57–64.•• This study is notable as it is one of the few high-quality randomized control trials, including a sham control without crossover design and MRI guidance to target brain stimulation sites. This study illustrates the ongoing interest in validating commonly used protocols verses sham controls, which is important given the issues with blinding and placebo effect inherent in NIBS.
- 36.Zhao J, Li Z, Cong Y, Zhang J, Tan M, Zhang H, Geng N, Li M, Yu W, Shan P: Repetitive transcranial magnetic stimulation improves cognitive function of Alzheimer’s disease patients. Oncotarget 2017, 8:33864–33871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alcalá-Lozano R, Morelos-Santana E, Cortés-Sotres JF, Garza-Villarreal EA, Sosa-Ortiz AL, González-Olvera JJ: Similar clinical improvement and maintenance after rTMS at 5 Hz using a simple vs. complex protocol in Alzheimer’s disease. Brain Stimul 2018, 11:625–627.• This study is notable for comparing two different TMS treatment protocols, which is important to begin to standardize and draw comparisons across stimulation procedures.
- 38.Avirame K, Stehberg J, Todder D: Benefits of Deep Transcranial Magnetic Stimulation in Alzheimer Disease: Case Series. J ECT 2016, 32:127–133.• This study was the only in this review which used deep TMS to target the prefrontal cortex, which allows for deeper tissue penetration but reduced focality of stimulation.
- 39.Rabey JM, Dobronevsky E: Repetitive transcranial magnetic stimulation (rTMS) combined with cognitive training is a safe and effective modality for the treatment of Alzheimer’s disease: clinical experience. J Neural Transm (Vienna) 2016, 123:1449–1455.• This report of clinical experiences in a private clinic offering commercial NeuroAD treatments shows the ongoing interest in the off-label use of TMS treatments in ADRD.
- 40.Koch G, Bonnì S, Pellicciari MC, Casula EP, Mancini M, Esposito R, Ponzo V, Picazio S, Di Lorenzo F, Serra L, et al. : Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage 2018, 169:302–311.•• This study is particularly notable because it is one of the few that used available biomarkers to confirm the diagnosis of prodromal AD and attempted to show a neurophysiological effect of the treatment.
- 41.Padala PR, Padala KP, Lensing SY, Jackson AN, Hunter CR, Parkes CM, Dennis RA, Bopp MM, Caceda R, Mennemeier MS, et al. : Repetitive transcranial magnetic stimulation for apathy in mild cognitive impairment: A double-blind, randomized, sham-controlled, cross-over pilot study. Psychiatry Res 2018, 261:312–318.• This study used a stimulation paradigm similar to rTMS treatment for depression to target apathy in MCI, showing the importance of choosing stimulation targets based on known neuroanatomical correlates of cognitive symptoms.
- 42.Bystad M, Grønli O, Rasmussen ID, Gundersen N, Nordvang L, Wang-Iversen H, Aslaksen PM: Transcranial direct current stimulation as a memory enhancer in patients with Alzheimer’s disease: a randomized, placebo-controlled trial. Alzheimers Res Ther 2016, 8:13.•• This study is notable as it was well designed and executed and reported a null finding on the effect of tDCS on Verbal memory in Alzheimer’s disease.
- 43.Yun K, Song I-U, Chung Y-A: Changes in cerebral glucose metabolism after 3 weeks of noninvasive electrical stimulation of mild cognitive impairment patients. Alzheimers Res Ther 2016, 8:49.• This study used tDCS vs sham control to target subjective memory, reflecting memory satisfaction and memory strategies of participants.
- 44.Ladenbauer J, Ladenbauer J, Külzow N, de Boor R, Avramova E, Grittner U, Flöel A: Promoting Sleep Oscillations and Their Functional Coupling by Transcranial Stimulation Enhances Memory Consolidation in Mild Cognitive Impairment. J Neurosci 2017, 37:7111–7124.•• This study is notable as it investigated a unique application of slow-oscillatory tDCS during a daytime nap to improve memory consolidation.
- 45.Murugaraja V, Shivakumar V, Sivakumar PT, Sinha P, Venkatasubramanian G: Clinical utility and tolerability of transcranial direct current stimulation in mild cognitive impairment. Asian J Psychiatr 2017, 30:135–140.This study investigated the Picture Memory Impairment Test (PMIT), which is a memory instrument with low educational bias; further investigations using this test could increase the translatability of NIBS techniques into a wider diversity of clinical settings.
- 46.Cruz Gonzalez P, Fong KNK, Brown T: The Effects of Transcranial Direct Current Stimulation on the Cognitive Functions in Older Adults with Mild Cognitive Impairment: A Pilot Study. Behav Neurol 2018, 2018:5971385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manenti R, Sandrini M, Gobbi E, Binetti G, Cotelli M: Effects of transcranial direct current stimulation on episodic memory in amnestic mild cognitive impairment: A pilot study. J Gerontol B Psychol Sci Soc Sci 2018, doi: 10.1093/geronb/gby134.•• This study measured whether tDCS was superior to sham in enhancing memory consolidation, and showed an improvement specifically in Recognition scores in the treatment group, suggesting improvement in amnestic memory deficits related to AD.
- 48.Andrade SM, de Mendonça CTPL, Pereira TCL, Fernandez-Calvo B, Araújo RCN, Alves NT: Adjuvant transcranial direct current stimulation for treating Alzheimer’s disease: A case study. Dement Neuropsychol 2016, 10:156–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bystad M, Rasmussen ID, Abeler K, Aslaksen PM: Accelerated Transcranial Direct Current Stimulation in Alzheimer’s Disease: A Case Study. Brain Stimul 2016, 9:634–635. [DOI] [PubMed] [Google Scholar]
- 50.Bystad M, Rasmussen ID, Grønli O, Aslaksen PM: Can 8 months of daily tDCS application slow the cognitive decline in Alzheimer’s disease? A case study. Neurocase 2017, 23:146–148.• This case study used at-home treatment of tDCS applied by the patient with assistance from family members over a course of 8 months, suggesting that at-home therapy may be feasible and tolerable.
- 51.Mukku SSR, Selvaraj S, Parlikar R, Damodharan D, Sivakumar PT, Nagaraj C, Mangalore S, Venkatasubramanian G, Varghese M: High-Definition Transcranial direct current stimulation (HD-tDCS) for auditory hallucinations in dementia-A case series. Asian J Psychiatr 2018, 37:102–105.• This case study investigated the use of high definition tDCS using 5 anodal electrodes arranged around a central cathode to treat auditory hallucinations.
- 52.Costa V, Brighina F, Piccoli T, Realmuto S, Fierro B: Anodal transcranial direct current stimulation over the right hemisphere improves auditory comprehension in a case of dementia. NeuroRehabilitation 2017, 41:567–575.• This case study targeted language impairment in a patient with a diagnosis of AD, and reflects the growing interest in using NIBS to target specific domains of cognitive function.
- 53.Gramegna LL, Evangelisti S, Testa C, Baiardi S, Mitolo M, Capellari S, Stracciari A, Poda R, Di Stasi V, Cretella L, et al. : Cognitive Rehabilitation and Transcranial Direct Current Stimulation in a Patient with Posterior Cortical Atrophy: An fMRI Study. Am J Case Rep 2018, 19:729–733.• This case study investigated tDCS treatment in posterior cortical atrophy, an AD variant, using CSF biomarkers to confirm the diagnosis.
- 54.Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, et al. : NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018, 14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu Y, Qiu Z, Zhu J, Liu J, Wu J, Tao J, Chen L: The modulation effect of non-invasive brain stimulation on cognitive function in patients with mild cognitive impairment: a systematic review and meta-analysis of randomized controlled trials. BMC Neuroscience 2019, 20:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lawrence BJ, Gasson N, Bucks RS, Troeung L, Loftus AM: Cognitive Training and Noninvasive Brain Stimulation for Cognition in Parkinson’s Disease: A Meta-analysis. Neurorehabil Neural Repair 2017, 31:597–608. [DOI] [PubMed] [Google Scholar]
- 57.Burke MJ, Kaptchuk TJ, Pascual-Leone A: Challenges of Differential Placebo Effects in Contemporary Medicine: The Example of Brain Stimulation. Ann Neurol 2018, doi: 10.1002/ana.25387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fried PJ, Jannati A, Davila-Pérez P, Pascual-Leone A: Reproducibility of Single-Pulse, Paired-Pulse, and Intermittent Theta-Burst TMS Measures in Healthy Aging, Type-2 Diabetes, and Alzheimer’s Disease. Front Aging Neurosci 2017, 9:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Canali P, Sferrazza Papa G, Casali AG, Schiena G, Fecchio M, Pigorini A, Smeraldi E, Colombo C, Benedetti F: Changes of cortical excitability as markers of antidepressant response in bipolar depression: preliminary data obtained by combining transcranial magnetic stimulation (TMS) and electroencephalography (EEG). Bipolar Disord 2014, 16:809–819. [DOI] [PubMed] [Google Scholar]
- 60.Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, Bashir S, Vernet M, Shafi M, Westover B, et al. : Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI. Brain Topogr 2011, 24:302–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J: BOLD MRI responses to repetitive TMS over human dorsal premotor cortex. Neuroimage 2005, 28:22–29. [DOI] [PubMed] [Google Scholar]
- 62.Cai W, George JS, Chambers CD, Stokes MG, Verbruggen F, Aron AR: Stimulating deep cortical structures with the batwing coil: how to determine the intensity for transcranial magnetic stimulation using coil-cortex distance. J Neurosci Methods 2012, 204:238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roth Y, Pell GS, Chistyakov AV, Sinai A, Zangen A, Zaaroor M: Motor cortex activation by H-coil and figure-8 coil at different depths. Combined motor threshold and electric field distribution study. Clin Neurophysiol 2014, 125:336–343. [DOI] [PubMed] [Google Scholar]
- 64.Huang Y, Parra LC: Can transcranial electric stimulation with multiple electrodes reach deep targets? Brain Stimulation 2019, 12:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grossman N, Bono D, Dedic N, Kodandaramaiah SB, Rudenko A, Suk H-J, Cassara AM, Neufeld E, Kuster N, Tsai L-H, et al. : Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 2017, 169:1029–1041.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grossman N, Okun MS, Boyden ES: Translating Temporal Interference Brain Stimulation to Treat Neurological and Psychiatric Conditions. JAMA Neurol 2018, 75:1307–1308. [DOI] [PubMed] [Google Scholar]
- 67.Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, Mathys H, Seo J, Kritskiy O, Abdurrob F, et al. : Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jack CR, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, L Whitwell J, Ward C, et al. : The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 2008, 27:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]


