Abstract
Heart failure is one of the leading causes of death worldwide. New therapeutic concepts are urgently required to lower the burden of heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), the two major forms of heart failure. Lipolytic processes are induced during the development of heart failure and occur in adipose tissue and multiple organs, including the heart. Increasing evidence suggests that cellular lipolysis, in particular, adipose triglyceride lipase (ATGL) activity, has an important function in cardiac (patho)physiology. This review summarizes the crucial role of cellular lipolysis for normal cardiac function and for the development of HFrEF and HFpEF. We discuss the most relevant pre-clinical studies and elaborate on the cardiac consequences of non-myocardial and myocardial lipolysis modulation. Finally, we critically analyze the therapeutic importance of pharmacological ATGL inhibition as a potential treatment option for HFrEF and/or HFpEF in the future.
Cytosolic lipolysis is induced during heart failure, and adipose triglyceride lipase (ATGL) regulates cardiac function. Kintscher et al. discuss how the deletion or inhibition of adipose tissue ATGL improves cardiac function in pre-clinical heart failure models. Pharmacological inhibition of adipose tissue ATGL may provide a therapeutic intervention direction for heart failure.
Main Text
Chronic heart failure (HF) is still one of the leading causes of death worldwide.1, 2, 3, 4 Despite extensive (non-) pharmacological therapies, the 5-year mortality rate of up to 75% remains very high and resembles the rate observed in various types of cancer.4 Therefore, new therapeutic concepts are required to lower the burden of this disease.3,5
According to recent guidelines, HF has been defined as a complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood.3,5 HF is characterized by typical symptoms (e.g., dyspnea, fatigue) that may be accompanied by clinical signs such as elevated jugular venous pressure, pulmonary crackles, and peripheral edema.3,5 The two major types include HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF).3,5 In HFrEF, patients present with an EF below 40%, whereas in HFpEF an EF ≥50% is preserved and diastolic dysfunction occurs.3
Considering the pathogenesis of both forms, major differences are noticeable. HFrEF is commonly evoked by intrinsic cardiac damage and a loss of functional myocardium caused, for example, by myocardial infarction, ischemia, or genetic defects.6, 7, 8 This leads to ventricular remodeling, dilatation, and a reduction in EF.6,7 HFpEF is likely to be caused by extracardiac comorbidities such as hypertension, obesity, metabolic syndrome, or diabetes.7, 8, 9 These comorbidities drive the pathophysiology of the disease by low-grade systemic inflammation, which impairs cardiac nitric oxide bioavailability, ultimately leading to increased cardiomyocyte stiffness, extracellular matrix deposition, fibrosis, and impaired diastolic filling.10,11 The different underlying pathophysiological processes have resulted in the development of disparate preclinical models for HFrEF versus HFpEF.12,13 All of these models exhibit certain limitations and do not reflect the complete clinical pictures of HFrEF or HFpEF. When discussing the role of lipolysis in HF, we name the applied HFrEF or HFpEF model, where appropriate.
The prevalence of HF is strongly age dependent. While only 1%–2% of the total adult population is affected, this number increases to ≥10% in individuals aged 70 years or older.3,14, 15, 16, 17 The latest reports show that among patients with chronic HF, one-third suffer from HFrEF and approximately two-thirds from HFpEF.18 Despite recent advances in management, the prognosis of patients with HF is still very poor and resembles that of common cancers.4,19
Targeting metabolic processes in the heart may represent a promising way to develop new therapeutic approaches for HF.20 Normal cardiac function relies on the continuous supply of the main energy substrates glucose, fatty acids (FAs), ketone bodies, or lactate.21 Quantitatively, FAs provide >70% of “fuel” for the heart.22 Exogenous non-esterified FAs, as cardiac energy fuel, are derived either from adipose tissue triacylglycerol (TAG) mobilization or from the hydrolysis of TAGs from TAG-rich lipoproteins by lipoprotein lipase.23 During fasting, the liver additionally converts adipose tissue-derived FAs to ketone bodies, which, after their secretion, represent an additional energy substrate for cardiomyocytes.21 In cardiomyocytes, exogenously delivered FAs can be immediately oxidized or reesterified to TAGs for transient storage and release upon later demand.
The enzymatic pathway to release FAs from stored TAGs in adipocytes and non-adipocytes (e.g., cardiomyocytes) is called lipolysis. Intracellular lipolysis occurs in two variants, cytosolic lipolysis and lysosomal lipolysis, depending on whether lipolytic enzymes act at neutral or acidic pH, respectively.24 In adipocytes and cardiomyocytes, neutral lipolysis is predominant and the main topic of this review. The major enzymes catalyzing cytosolic lipolysis are adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), and monoacylglycerol lipase (MGL), which sequentially hydrolyze TAGs, diacylglycerols, and monoacylglycerols to eventually generate glycerol and FAs as the end products of lipolysis.24
During the development of HF lipolysis is induced in multiple organs, including the heart and adipose tissue.25,26 Increasing evidence suggests that ATGL activity and cytosolic lipolysis both in cardiac muscle and adipose tissue affect cardiac function and HF development.27, 28, 29, 30 Recent data obtained from experiments with mutant mouse lines overexpressing or lacking ATGL, or with the application of the recently developed small-molecule ATGL inhibitor Atglistatin,31 suggest that the inhibition of ATGL in adipose tissue or the activation of ATGL in cardiomyocytes represents potential pharmacological targets for the management of HF.29,32 This review summarizes the current evidence for a role of ATGL in the regulation of cardiac function and the pathogenesis of HF. We briefly summarize the molecular basics of lipolysis and discuss the most relevant pre-clinical studies, elaborating on the cardiac consequences of myocardial and non-myocardial ATGL modulation. We further review the clinical evidence for the significance of lipolysis in HF. Finally, we critically analyze the therapeutic potential of pharmacological ATGL inhibition as a new treatment option for HF.
Neutral Lipid Hydrolases and Their Regulation
In principle, cytosolic lipolysis occurs to some degree in all cell types and tissues. The highest lipolytic activities are observed in white and brown adipose tissue, followed by oxidative tissues such as cardiac muscle, skeletal muscle, and the liver. ATGL, HSL, and MGL are also highly expressed in white and brown adipose tissue and at lower levels in other metabolically important organs.33 The catalytic domain of ATGL (patatin domain) is localized in the N-terminal part of the protein. The C-terminal part of the enzyme possesses a hydrophobic structure, which enables its binding to lipid droplets.34,35 The activity of ATGL and concomitantly the initiation of TAG hydrolysis are markedly controlled by ATGL-specific cofactors, which bind to the patatin domain. Those cofactors include, among others, the co-activator CGI-58 (comparative gene identification-58, α/β hydrolase domain 5 (ABHD5),36,37 the co-repressor G0S2 (G0/G1 switch gene 2),38,39 and perilipins (PLINs).40, 41, 42 HSL is the second lipolytic enzyme, predominantly catalyzing the breakdown of diacylglycerol (DAG) to monoacylglycerol (MAG) and FAs.23,43 Unlike ATGL, HSL has a broad substrate specificity hydrolyzing TAGs, retinylesters, and cholesterylesters in addition to DAGs. Protein kinase A-dependent phosphorylation of HSL on several Ser residues affects its binding affinity to lipid droplets and significantly increases its lipolytic activity.43, 44, 45 The final step in the lipolytic cascade is the hydrolysis of MAGs catalyzed by MGL.43 In addition to its role in intracellular neutral lipolysis, MGL is involved in the catabolism of the important endocannabinoid, 2-arachidonylglycerol, which explains the high expression levels of MGL in the brain.46 In addition to ATGL, HSL, and MGL, other enzymes may contribute to TAG hydrolysis in various tissues. They include members of the carboxylester and the a/b hydrolase domain families of hydrolases, and may contribute to neutral TAG and MAG hydrolysis, respectively.47 Their quantitative contribution to bulk lipolysis is insufficiently understood.
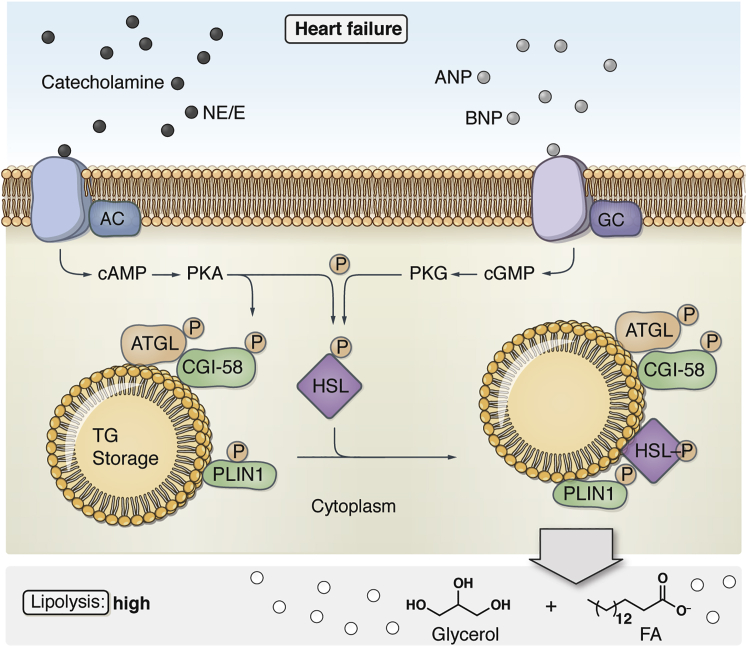
Several hormones such as catecholamines and natriuretic peptides (atrial natriuretic peptide or brain natriuretic peptide), which control cardiac function and are dysregulated in HF, are also crucial regulators of lipolysis.48, 49, 50 Catecholamines induce lipolysis by activating α- and β-adrenergic receptors48,50 (Figure 1). Adrenergic receptor-mediated stimulation of adenylyl cyclase leads to the formation of cyclic adenosine monophosphate and the subsequent activation of protein kinase A.50 In adipose tissue, activated protein kinase A phosphorylates lipid droplet-bound PLIN1, CGI-58, and HSL.50 This leads to the translocation of HSL to the surface of lipid droplets and the dissociation of CGI-58 from PLIN1. CGI-58 is then able to interact with ATGL to stimulate its enzymatic activity (Figure 1). The activation process of lipolysis may differ in oxidative tissues (e.g., muscle, liver) because they express PLIN2 and PLIN5 instead of PLIN1. Both represent a barrier for TAG hydrolysis because their cardiac-specific overexpression in mice causes lipid droplet accumulation and steatosis.51,52 However, the molecular mechanism underlying this observation remains unclear. PLIN5 is able to bind ATGL, HSL, and CGI-58, and this interaction activates40,53 or inhibits54,55 lipolysis. In addition, PLIN5 links lipolysis to mitochondrial function by bridging lipid droplets to the mitochondrial membrane.41
Figure 1.
Lipolytic Pathways in Adipocytes
Heart failure induces high rates of lipolysis. Catecholamines (norepinephrine [NE] and epinephrine [E]) and natriuretic peptides (atrial natriuretic peptide [ANP] and brain natriuretic peptide [BNP]) induce lipolysis in adipocytes using two different signaling pathways. NE and E activats β-adrenergic receptors, which leads to the subsequent activation of adenylyl cyclase (AC), increased cyclic adenosine monophosphate (cAMP) production, and activation of protein kinase A (PKA). PKA mediates the phosphorylation and activation of adipose triglyceride lipase (ATGL) and comparative gene identification-58 (CGI-58), as well as hormone-sensitive lipase (HSL) and perilipin 1 (PLIN1). This leads to the formation of the ATGL-CGI-58 complexes on the lipid droplet and interaction between PLIN1 and HSL. ANP and BNP activate natriuretic peptide receptor-A, which leads to guanylyl cyclase (GC)-dependent cyclic guanosine monophosphate (cGMP) synthesis and triggers the activity of protein kinase G (PKG). When activated, PKG phosphorylates and activates HSL and PLIN1 in adipocytes, consequently inducing lipolytic activity in an ATGL-independent manner. FA, fatty acid.
In addition to catecholamines, atrial-, brain-, and C-type natriuretic peptides, also regulate lipolytic activity. Most studies focused on their regulation of HSL. Natriuretic peptides regulate lipolytic activity by binding to specific G-protein-coupled receptors: natriuretic peptide receptor A, B, and C. Both natriuretic peptide receptors A and B, when stimulated, induce cyclic guanosine monophosphate synthesis via guanylyl cyclase and trigger the activity of the protein kinase G56,57 (Figure 1). When activated, protein kinase G phosphorylates and activates HSL and PLINs, subsequently inducing lipolytic activity. Although it has not been shown yet, it would be predicted that PLIN phosphorylation by protein kinase G enables ATGL activation by CGI-58 in a similar manner as PLIN1 phosphorylation by protein kinase A. In summary, lipolytic TAG breakdown in the cytoplasm of adipose and oxidative tissues such as the heart occurs in a highly coordinated fashion involving an enzymatic cascade with ATGL, HSL, and MGL. The activity of these enzymes is tightly controlled by multiple mechanisms, including the sympathetic nervous and natriuretic peptide systems, both of which are activated in HF.
Cardiac and Non-cardiac Lipolysis in HF—Clinical Data
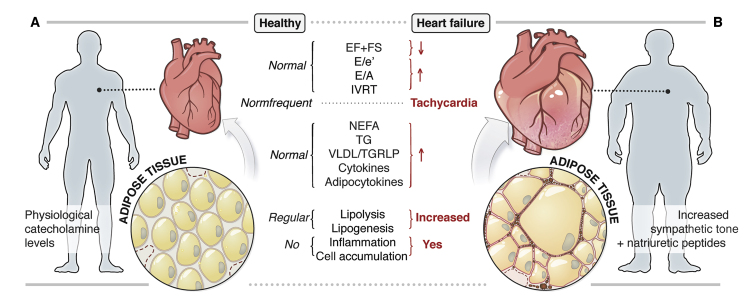
A common pathophysiological event in HFrEF and HFpEF comprises a chronic activation of the sympathetic nervous system.58, 59, 60, 61, 62 Notably in HFrEF, sympathetic nervous system activation crucially determines outcomes.59 In a compensatory manner, the adrenergic system is stimulated to maintain cardiac output by increasing heart rate. However, this systemic chronic adrenergic drive does not only affect cardiac function but it also stimulates other processes such as non-cardiac lipolysis primarily in adipose tissue. In addition, lipolytic processes in adipose tissue during HF are further stimulated by high levels of natriuretic peptides63 (Figure 2). HFrEF and HFpEF are often accompanied by insulin resistance and hyperinsulinemia.64,65 Since insulin is a major regulator of lipolysis and lipogenesis, it is very likely that the impairment of insulin signaling contributes to non-cardiac lipolysis during HF.66 In contrast, cardiac lipolysis, in particular in cardiomyocytes, seems to be attenuated, at least in the presence of distinct comorbidities such as diabetes or obesity.
Figure 2.
Pathophysiological Changes in the Heart and Adipose Tissue during the Development of Chronic Heart Failure
(A) Healthy state, with regular cardiac function (systolic and diastolic), normal circulating lipid and cytokine levels, and regular adipose tissue function.
(B) Heart failure, with increased sympathetic tone and natriuretic peptide levels, systolic and diastolic cardiac dysfunction, elevated circulating lipids and cytokines, and adipose tissue dysfunction.
e′, tissue Doppler-passive LV filling; EF, ejection fraction; E-wave, early passive LV filling; FS, fractional shortening; IVRT, isovolumic relaxation time; LV, left ventricular; NEFA, non-esterified fatty acid; TAG, triacylglycerol; TGRLP, triglyceride-rich lipoprotein; VLDL, very-low-density lipoprotein.
Cardiac Lipolysis in HFrEF and HFpEF
Cardiac lipid metabolism is subject to close-meshed control processes to respond in an adaptive and fast manner to changes in cardiac energy demand. As mentioned above, exogenously imported FAs can be either oxidized or stored in cardiomyocytes as TAGs. A precisely regulated balance between FA uptake, TAG synthesis, TAG hydrolysis, and FA oxidation is the prerequisite for effective cardiac FA and energy metabolism and proper heart function.67 The imbalance of these processes, as commonly observed in obese and diabetic patients, leads to cardiac steatosis and cardiac dysfunction, likely resulting from non-cardiac and cardiac insulin resistance.66 Sharma and colleagues described high intramyocardial lipid content in LV tissue sections from patients with advanced non-ischemic HFrEF, with the highest content detected in patients with HFrEF and diabetes.68 Along this line, myocardial TAG content correlated inversely with regional systolic function in healthy volunteers using 1H-magnetic resonance spectroscopy.69 Furthermore, prominent lipid droplets were also detected in the border zone of an acute myocardial infarct and in hypertrophic cardiomyopathies.70,71 Two recent studies in HFpEF patients demonstrated significantly enhanced myocardial TAG accumulation compared to controls assessed by 1H-cardiac magnetic resonance spectroscopy.72,73 In addition to TAG accumulation, increased abundance of toxic lipid species such as ceramides and DAGs have been described as important drivers of HF development.67,74 These data support a link between increased cardiac lipid content and cardiac dysfunction in HFrEF and HFpEF.
Cardiac lipid accumulation can result either from increased TAG synthesis or defective TAG hydrolysis. The importance of impaired TAG hydrolysis in humans became evident in patients carrying mutations in the patatin-like phospholipase domain containing protein 2 gene coding for ATGL.75 These patients develop neutral lipid storage disease with myopathy. The majority of affected individuals present with cardiac steatosis, and 50% develop cardiomyopathy.33 The degree of cardiac dysfunction in homozygous ATGL-deficient patients varies, but in many patients HF becomes so severe that they require heart transplantation.33,76 It is unfortunate that most studies did not discriminate between HFrEF and HFpEF. However, in two studies with reported EF, two patients showed a rEF and two patients had a pEF.77,78
In summary, there is clinical evidence for cardiac lipid accumulation in HFrEF and HFpEF. Studies in patients with mutations in the human gene encoding ATGL suggest that impaired lipolysis may contribute to cardiac steatosis. However, whether reduced lipolysis and cardiac steatosis represents a more general mechanism of pathogenesis in HFrEF and HFpEF patients requires better validation.
Non-cardiac Lipolysis in HFrEF and HFpEF
Non-cardiac lipolytic processes are markedly augmented in HF. More than 20 years ago, Lommi and colleagues identified significantly increased serum levels of free FAs in a small group of patients with HFrEF (n = 5) and HFpEF (n = 2) compared to controls (n = 7), indicating enhanced lipolysis in white adipose tissue.25 Consistent with this observation, Polak and colleagues directly showed enhanced adipose tissue lipolysis in HFrEF patients by using local microdialysis.26 Increased lipolytic rates in adipose tissue closely correlated with plasma atrial natriuretic peptide levels in HFrEF patients, suggesting the involvement of natriuretic peptides in this process.79 These authors even hypothesize that enhanced adipose tissue lipolysis during HF results in adipose tissue wasting contributing to cardiac cachexia. Given the established interaction between insulin resistance and adipose tissue lipolysis, as well as the observation that HFrEF and HFpEF are often associated with insulin resistance, it appears very likely that systemic insulin signaling is involved in the regulation of lipolysis during HF.65,66 The involvement of other lipolytic hormones, such as glucagon, in these processes is unknown. Despite documented cardiac actions of glucagon in HF,80 the impact of this hormone on metabolic alterations, in particular on lipolysis, during HF has not been investigated.
Data on lipolytic activity in other non-adipose organs such as skeletal muscle are very limited. Twenty years ago, Tikunov and colleagues provided indirect evidence of stimulated lipolysis and FA oxidation in the diaphragmatic muscle by showing a significantly increased activity of β-hydroxyacyl-coenzyme A dehydrogenase in patients with advanced HFrEF (mean EF: 18% ± 8%).81 In contrast, other groups demonstrated excess intracellular lipid accumulation in quadriceps biopsies from HFrEF patients and increased intramyocellular lipid content in tibialis anterior muscles studied by 1H-magnetic resonance spectroscopy, indicative of decreased TAG turnover.82,83
Robust evidence exists for an enhanced, non-cardiac systemic lipolysis during HF development (Figure 2), with most studies performed in HFrEF patients. Based on the available clinical data, it seems most likely that adipose tissue lipolysis is the strongest contributor to this process (Figure 2). However, despite the evidence of increased adipose tissue lipolysis in HF patients, the impact of this exacerbation for cardiac metabolism and function is incompletely understood. In contrast to non-cardiac lipolysis, cardiac lipolytic processes seem to be attenuated, resulting in cardiac lipid accumulation. These differences in cardiac versus non-cardiac lipolysis will likely play an important role in the development of lipolysis-based pharmacological interventions.
Adipogenesis and Lipogenesis and Cardiac Function
In addition to lipolytic processes, adipogenesis and lipogenesis have been recently discussed as being involved in the pathogenesis of HF. Notably in obese patients with HFpEF, enhanced adipogenesis and lipogenesis in epicardial adipose tissue has been documented.84 As a result of chronic systemic inflammation in obesity, adipogenesis is particularly stimulated in the epicardium.84, 85, 86 The enlarged epicardial adipose tissue depot has been suggested to induce systemic inflammation in the myocardium, thereby aggravating cardiac inflammatory and fibrotic processes.84,87,88 In addition, the amount of epicardial adipose tissue is associated with impaired myocardial microcirculation, a central feature of HFpEF.89 Increased adipogenesis in atrial epicardial adipose tissue may be involved in the development of atrial fibrillation, a common arrhythmia in HFpEF and HFrEF.90,91 These data indicate that, in addition to lipolysis, adipogenesis and lipogenesis are involved in the pathogenesis of HF. Adipogenic and lipogenic processes seem to be predominantly relevant in the epicardium and in the obese phenotype of HFpEF, whereas alterations of lipolysis likely occur in the myocardium and/or non-cardiac tissues.
The Role of ATGL in HF
Cardiac ATGL in Cardiac Function and HF
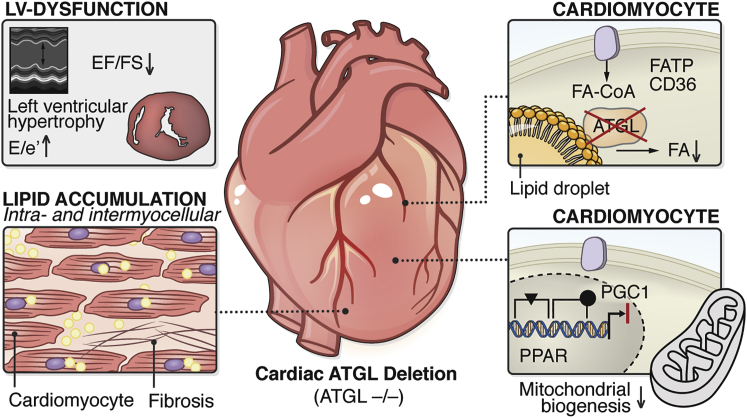
A series of preclinical studies using gain- or loss-of-function approaches investigated the role of ATGL in the heart and in different HF models. Cardiomyocyte-specific overexpression of ATGL reduces cardiac TAG content and improves systolic LV function.92 Similar results were reported in diabetic and obese mice, in which the cardiomyocyte-specific overexpression of ATGL reduced intramyocardial TAG levels, reduced lipotoxicity, and improved systolic and diastolic functional parameters, including EF, E/A ratio, or isovolumic relaxation time.93,94 ATGL deletion in mice led to an even more severe cardiac phenotype. Constitutive ATGL knockout mice showed massive lipid accumulation in the heart with a 20 times higher cardiac TAG content compared to wild-type (WT) mice27 (Figure 3). TAGs were predominantly stored as intracellular lipid droplets in cardiomyocytes. Cardiac TAG accumulation in ATGL−/− mice was accompanied by a robust impairment of cardiac function with decreased LVEF in ATGL−/− mice, which resulted in a markedly higher mortality rate27 (Figure 3). Further investigations revealed that defective lipolysis in ATGL−/− mice led to dysfunctional cardiac peroxisome proliferator-activated receptor (PPAR)α and PPARδ signaling, resulting in a reduced expression of cardiac PPARγ coactivator 1α and 1β and the respective target genes28 (Figure 3). That in turn impaired mitochondrial function and oxidative phosphorylation in cardiac tissue, causing decreased substrate utilization, increased deposition of lipids, and cardiac dysfunction.28 Defective PPARα signaling was restored by the administration of PPARα agonists in that model, which resulted in extended survival, arguing for a causative relation between impaired PPAR signaling and cardiac failure. Inducible cardiomyocyte-specific deletion of ATGL in mice corroborated the cardiac phenotype observed by constitutive deletion, including increased myocardial TAG content, cardiac hypertrophy, and impaired cardiac function.95 Vice versa, the cardiac phenotype of the whole-body ATGL knockout mice could be rescued by a cardiomyocyte-specific overexpression of ATGL in ATGL−/− mice.96 The restoration of cardiac lipolysis in these mice resulted in a complete reversion of cardiac TAG deposition, recovery of cardiac mitochondrial function, and prolonged lifespan. Thus, the cardiac phenotype in ATGL−/− mice exclusively results from the absence of cardiac ATGL and is not due to the lack of ATGL in other organs or defects in in utero development. These data show that cardiac ATGL is essential for normal cardiac and mitochondrial function, as well as for cardiac energy substrate metabolism (Figure 3). Thus, therapeutic interventions that lead to a complete inhibition of cardiac ATGL must be avoided.
Figure 3.
Consequences of ATGL Deletion and Inhibition in the Heart
Cardiac-specific deletion and inhibition of ATGL results in systolic and diastolic LV dysfunction, LV hypertrophy (upper left box), cardiac lipid accumulation (lower left box), reduced cardiomyocytic lipolysis with lower cellular FA levels (upper right box), decreased ligand (FA)-induced PPARα/δ activation/reduced PGC-1 expression, and reduced mitochondrial biogenesis/mitochondrial dysfunction (lower right box).
CD36, cluster of differentiation 36 (also known as fatty acid translocase); e′, tissue Doppler-passive LV filling; E-wave (early passive LV filling); EF, ejection fraction; FA-CoA, fatty acyl-CoA ester; FATP, fatty acid transport protein; FS, fractional shortening; LV, left ventricular; PGC1, PPARγ coactivator-1; PPAR, peroxisome proliferator-activated receptor.
Non-cardiac ATGL in Cardiac Function and HF
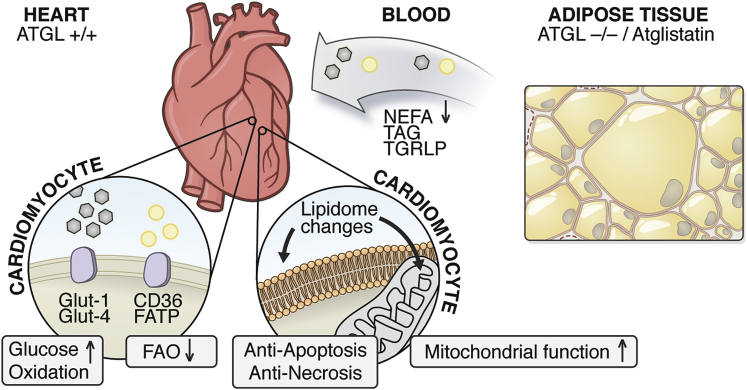
A series of publications in recent years has investigated the importance of non-cardiac ATGL for cardiac function and for the development of HF. The main focus of these investigations was on the analysis of adipose tissue and its interaction with the heart (Figure 4).
Figure 4.
Potential Cardioprotective Actions of ATGL Deletion and Inhibition in Adipose Tissue
Adipose tissue-specific deletion and pharmacological inhibition (Atglistatin) of ATGL in the presence of normal cardiac ATGL expression level results in adipose tissue mass expansion with larger adipocytes (right box); reduced circulating NEFA, TAG, and TGRLP levels; enhanced cardiac glucose oxidation and decreased cardiac FAO; cardiac lipidomic changes, cardiomyocytic anti-apoptosis/ necrosis, improved mitochondrial function.
CD36, cluster of differentiation 36 (also known as fatty acid translocase); FAO, fatty acid oxidation; FATP, fatty acid transport protein; Glut1/2, glucose transporter 1/2; NEFA, non-esterified fatty acid; TAG, triacylglycerol; TGRLP: triglyceride-rich lipoprotein.
The first study on adipose tissue-specific deletion of ATGL (ATGLfl/fl-aP2-Cre) was published in 2011, with the primary focus on the metabolic phenotype of these mice.97 Similar to ATGL−/− mice, ATGLfl/fl-aP2-Cre mice had increased body weight due to the enlargement of adipose tissue depots, including brown adipose tissue.97 However, despite increased adiposity, ATGLfl/fl-aP2-Cre mice on a high-fat diet exhibited improved systemic glucose and insulin tolerance.97 This may have resulted from the retention of lipids in fat depots and the subsequent protection of non-adipose organs, in particular, the liver, against lipid overload.97 Consistent with this conclusion, the lack of ATGL in adipose tissue resulted in a reduction in FA release from fat, accompanied by lower circulating TAG and non-esterified FAs97,98 (Figure 4). Schoiswohl and colleagues confirmed and extended these data by studying ATGLfl/fl-Adipoq-Cre mice.98 They also found reduced basal and stimulated adipose tissue lipolysis, improved systemic insulin and glucose metabolism, and improved hepatic insulin sensitivity as a result of diminished liver steatosis. In addition, the absence of adipose ATGL resulted in a marked reduction in hepatic inflammation. Apparently, adipose tissue-specific deficiency of ATGL results in lower circulating lipid levels and protects the liver against lipid overload and lipotoxicity, arguing for lipolysis-driven metabolic interorgan crosstalk.
To assess whether crosstalk also exists between adipose tissue and the heart, studies were first performed in ATGLfl/fl-aP2-Cre mice under conditions of exercise-induced lipolysis.99 When subjected to chronic exercise, mice with adipose-specific ATGL deletion had impaired adipose tissue lipolysis accompanied by the absence of exercise-induced FA appearance in the circulation and by diminished systemic lipid oxidation.99 Similar results were found in ATGLfl/fl-Adipoq-Cre mice challenged with exhaustive submaximal endurance training.100 When we studied the cardiac response to exercise in these mice, positron emission tomography (PET) analysis revealed reduced cardiac FA uptake and instead enhanced glucose uptake. Notably, this substrate switch was associated with an attenuation of the cardiac hypertrophic response to exercise, highlighting the relevance of adipose tissue lipolysis in the regulation of cardiac metabolism and function.99 Two recent studies investigating the impact of adipose tissue ATGL on cardiac function in an HFrEF model of pressure-induced cardiac failure by transverse aortic constriction (TAC) substantiated this conclusion.29,30 Adipose tissue lipolysis is enhanced during the development of LV failure in the TAC model.101 In mice with adipose tissue ATGL deletion, either in ATGLfl/fl-aP2-Cre or ATGLfl/fl-Adipoq-Cre mice, a marked improvement in systolic LV function during pressure overload was found.29,30 The lack of ATGL in adipose tissue protected mice against pressure-induced cardiac hypertrophy, LV enlargement, and cardiac fibrosis, and almost completely preserved EF to levels observed in sham-operated mice.
Pharmacological Inhibition of ATGL
In 2013, Rolf Breinbauer and colleagues succeeded in developing the first small-molecule inhibitor of ATGL, called Atglistatin.31 Atglistatin is a potent, highly specific, and competitive inhibitor of ATGL that does not affect the activity of other TAG hydrolases such as HSL, MGL, pancreatic lipase, or lipoprotein lipase. In a high-fat diet-induced obesity mouse model, Atglistatin potently but transiently blocked white adipose tissue lipolysis, reduced plasma FA levels, improved insulin sensitivity and glucose tolerance, and led to a reduction in body weight, at least in part mediated by lower food intake and reduced intestinal fat absorption.32 Bioavailability studies and tissue-specific lipolysis assay revealed that Atglistatin inhibits ATGL in white and brown adipose tissue but not in non-adipose tissues. Consistent with specifically targeting adipose ATGL, FA delivery to and TAG accumulation in non-adipose tissues such as heart, skeletal muscle, and liver decreased in Atglistatin-treated mice.32 This finding inspired the Dyck laboratory to assess the effect of Atglistatin-mediated ATGL inhibition on heart function in a murine model of HFrEF.29 Atglistatin treatment markedly improved LV systolic function, reduced LV hypertrophy, and prevented pulmonary congestion in pressure-mediated HF. The tissue-selective action of Atglistatin suggested that the beneficial actions of Atglistatin are mediated by its effects in white adipose tissue. It is unfortunate that Atglistatin does not inhibit human ATGL activity, disqualifying it for (pre-) clinical drug development.31,32,102
The underlying molecular mechanisms by which adipose tissue ATGL regulates cardiac function are incompletely understood. Cardiac energy substrate metabolism undergoes profound changes during the development of HF.21 The healthy heart is able to use multiple energy substrates, including FAs, glucose, lactate, ketones, and amino acids, to maintain adequate ATP production.21 It is well established that in HF, this metabolic flexibility is reduced and various metabolic substrate changes occur, one of which is the increase in cardiac glucose metabolism.21 This increase has been considered beneficial in a hypoxic or failing heart since glucose has higher oxygen efficiency (ATP produced/oxygen consumed) for ATP synthesis compared to FAs. As pointed out earlier, ATGL deletion in adipose tissue reduces overall lipid oxidation and increases glucose metabolism.97, 98, 99 Cardiac glucose uptake is increased and FA uptake is decreased in adipose tissue-specific ATGL-deficient mice.99 Thus, changes in cardiac energy substrate availability, uptake, and metabolism may be a potential beneficial mechanism in mice with ATGL blockade in fat (Figure 4). In addition to the beneficial regulation of cardiac energy substrate utilization, ATGL deletion in adipose tissue resulted in dramatic changes in the circulating lipid profile, which subsequently led to robust changes in cardiac lipid composition30 (Figure 4). The most prominent alteration in cardiac lipid species was an upregulation of phosphatidylethanolamine (PE), along with a decreased phosphatidylcholine (PC):PE ratio in failing WT hearts, which was completely prevented by the perturbation of ATGL-mediated adipose tissue lipolysis.30 The ratio of PC:PE has been identified as an important determinant of cell membrane integrity, and a decreased ratio is often associated with cell damage.103 Thus, the maintenance of a physiological cardiac PC:PE ratio by ATGL inhibition in adipose tissue may result in the preservation of “healthier” cellular lipid membranes in cardiomyocytes. Whether these processes occur in plasma membranes or membranes of other subcellular organelles, such as mitochondria, requires further investigation.
The inhibition of adipose tissue ATGL activity improves cardiac function. The underlying mechanisms of the beneficial actions by adipose tissue ATGL inhibition in HF need to be fully elucidated, and it seems likely that they will involve changes in cardiac energy substrate utilization and/or alterations in cardiac membrane lipid composition.
ATGL Cofactors and HF
ATGL activity is tightly regulated by multiple cofactors. In addition to the above-mentioned CGI-58, G0S2, and PLINs, a number of proteins additionally affect ATGL enzyme function, including hypoxia-inducible lipid droplet-associated protein, the ubiquitin-like domain containing protein-8, fat-specific protein-27, and pigment epithelium-derived factor.24 Whether these factors are involved in cardiac lipolysis and metabolism, thereby affecting cardiac function, awaits further investigation.
Deletion of the ATGL coactivator CGI-58 exclusively in cardiomyocytes and skeletal muscle, using CGI-58fl/fl-muscle-specific creatine kinase-Cre mice, resulted in a comparable although less severe cardiac phenotype as observed in cardiac ATGL-deficient mice, including impaired cardiac lipolysis, massive cardiac TAG accumulation, impaired FA oxidation, and decreased related gene expression levels, as well as reduced oxygen consumption rates.104,105 In addition, LV systolic function was markedly impaired, accompanied by LV hypertrophy.104,105 These functional alterations in the heart occurred in the presence of prominently higher ATGL protein levels, thus supporting the importance of CGI-58 as an activating cofactor of ATGL activity in the heart, at least in mice.105 This crucial role of CGI-58 in cardiac lipolysis and function should be critically considered during future developments of ATGL-based therapies for cardiac disease. Whether forced changes in CGI-58 expression in adipose tissue affect cardiac function is unknown. Published studies that either overexpressed or knocked down CGI-58 in adipose tissue did not report cardiac function or a heart phenotype.106, 107, 108
G0S2 is a potent peptide inhibitor of the lipolytic activity of ATGL.39 In consonance, mice overexpressing G0S2 under the control of α-myosin heavy-chain promoter specifically in the heart exhibit massive cardiac TAG accumulation, accompanied by significantly reduced cardiac TAG hydrolase activity.109 However, the phenotype is less severe than in ATGL−/− mice. PPARα signaling and FA oxidation is normal in G0S2−/− mice, and the expression of inflammatory and pro-fibrotic genes is much lower than in ATGL−/− mice.109 Echocardiographic analysis of cardiomyocyte-specific G0S2 transgenic mice demonstrated LV hypertrophy, including increased LV mass and wall thickening. However, normal fractional shortening in G0S2 transgenic mice indicated normal LV systolic function.109 This is in sharp contrast to the pronounced impairment of LV systolic function in ATGL-deficient mice, and it suggests different cardiac responses, depending on manipulations of the enzyme or its co-regulators. Whether these differences involve the phospholipase activity of ATGL, as suspected by Kanno and colleagues,110 requires further elucidation.
As pointed out above, PLINs are important regulators of ATGL. In particular, PLIN5 is highly expressed in the heart, and its impact on cardiac function has been studied in overexpression as well as deletion mouse models. Cardiomyocyte-specific overexpression of PLIN5 led to severe cardiac steatosis and reduced lipid droplet-associated hydrolase activity.51 It appears that PLIN5 blocks ATGL activity by impeding the access of the enzyme to the lipid droplet surface, forming a lipolytic barrier.51 This observation contradicts the finding that the binding of ATGL to PLIN5 is required for normal ATGL function40 and highlights apparent mechanistic deficiencies in our understanding of cardiac lipolysis. Unlike ATGL deficiency, cardiac-specific PLIN5 overexpression did not affect diastolic or systolic cardiac function.41 Cardiomyocyte morphology, however, was markedly different in PLIN5 overexpressing mice compared to ATGL−/− mice. While the deletion of ATGL led to a heterogenous pattern of lipid droplets, including giant and small droplets associated with an atrophic cellular architecture, PLIN5-overexpressing cardiomyocytes exhibited uniform hypertrophied lipid droplets tightly attached to mitochondria.51 Mitochondrial respiration was maintained or only mildly impaired in PLIN5-overexpressing cardiomyocytes compared to a stronger defect in ATGL-deficient hearts.28,41,111 The differences in cardiac function between PLIN5 transgenic- and ATGL-deficient hearts in the presence of similar cardiac TAG accumulation have been explained by the induction of the antioxidative pathway mediated by nuclear factor-E2-related factor 2 in PLIN5 mice.41 In addition, recently, PLIN5 has been shown to protect mitochondrial function via the reduction of mitochondrial fission.111 Consistent with the proposed barrier function, the constitutive deletion of PLIN5 strongly reduced cardiac TAG content. PLIN5 deficiency resulted in a significant reduction in systolic LV function under dobutamine stress.112 The cause for this impact on cardiac function remains to be elucidated, but it may involve the proposed function of PLIN5 to “bridge” lipolysis with mitochondrial FA oxidation.41,111
HSL and MGL in HF
In addition to ATGL, HSL and MGL complete the process of sequential intracellular neutral lipolysis. HSL expression in cardiomyocytes was first described in the 1980s.113, 114, 115 Subsequently, the role of HSL in the regulation of cardiac lipid metabolism was investigated by using genetic mouse models. Since severe cardiac defects were absent in these mice, a further detailed analysis of cardiac function was not performed in these studies. Transgenic mice with a heart-specific overexpression of HSL prevented fasting- and diabetes-induced cardiac lipid accumulation,116,117 whereas constitutive deletion of HSL resulted in DAG accumulation in the heart.118,119 HSL-deficient mice were protected against high-fat diet-induced cardiac insulin resistance, which may be linked to the lower cardiac TAG content.119 To our knowledge, adipose tissue-specific HSL-deficient mice have not yet been phenotyped regarding cardiac function,120 and it may be a worthwhile model to study. Similar to mice, no cardiac phenotype was reported in the few human individuals that are affected by HSL deficiency.121,122
Studies on the role of MGL in the heart are limited. Severson and Hee-Cheong described MGL activity in isolated rat cardiomyocytes in 1988.123 Pharmacological inhibition of MGL with JZL184 resulted in enhanced cardiac inflammation after myocardial infarction associated with worsened cardiac function.124 In this study, augmented cardiac inflammation and impairment of cardiac function by MGL inhibition was accompanied by increased levels of 2-arachidonoylglyerol pointing toward the role of MGL in endocannabinoid catabolism. However, data on cardiac function in murine models of MGL deficiency were not reported.125,126 The available data are not sufficient for a definitive conclusion on the significance of cardiac and non-cardiac HSL and MGL in the development of HF.
Future Therapeutic Options
Recent experimental evidence suggests that lipolysis and lipolysis-dependent metabolic processes are important for normal cardiac function and may, when dysfunctional, contribute to the pathogenesis of HFrEF and HFpEF. Therefore, lipolysis may represent a suitable target for the treatment of these diseases. In particular, the inhibition of ATGL in adipose tissue appears to be promising. However, its pharmacological inhibition requires unconditional organ specificity because ATGL blockade in non-adipose organs such as the heart is likely to exert deleterious side effects. Atglistatin fulfills this requirement by inhibiting ATGL predominantly in adipose tissue. However, the inhibitor acts only on murine and rat ATGL and does not target the human enzyme.32 To address this limitation and to improve pharmacodynamic-pharmacokinetic properties, the search for potent and safe small-molecule inhibitors of human ATGL is actively pursued.127, 128, 129 Nevertheless, the work so far has provided the proof-of-principle that pharmacological inhibition of ATGL in an organ-specific manner is possible and may be beneficial for the treatment of cardiac disease.
In contrast to the direct inhibition of ATGL by means of small-molecule inhibitors, other approaches to pharmacologically modulate ATGL-mediated lipolysis are conceivable. These alternative avenues include strategies to interfere with the interaction of ATGL with activating or inhibitory cofactors. Based on the identification of a core peptide from G0S2, Cerk and colleagues developed a synthetic peptide that inhibits ATGL activity in a non-competitive manner in the nanomolar range.130 Along this line, CGI-58/ABDH5 has been targeted recently by synthetic ligands modifying the CGI-58/ABDH5-PLIN interaction and subsequently adipocyte and muscle lipolysis.131 Although this study focused on the activation of lipolysis by endogenous and synthetic ABDH5 ligands, further development of this concept toward the inhibition of white adipose tissue lipolysis seems conceivable.
Conclusions
Robust clinical evidence argues for an important role of lipolytic processes in the regulation of cardiac function and in the development of HF. Preclinical data have clearly shown that the modulation of ATGL activity strongly affects cardiac morphology and function, and, more important, that the inhibition of non-cardiac ATGL has been beneficial in pre-clinical heart failure models. Thus, in view of the urgent need for new treatment strategies for HFrEF and HfpEF, the development of pharmacological inhibitors that target human ATGL in adipose tissue seems to be a promising track to follow.
Acknowledgments
The authors thank Johannes Richers for excellent work in creating all of the illustrations and figures. R.Z. is an Einstein BIH Visiting Fellow supported by the Foundation Charité (EVF-BIH-2018-440), and is funded by the Fondation Leducq Transatlantic Network grant 12CVD04, the Louis-Jeantet Prize for Medicine 2015, the Austrian Science Fund (FWF) SFB Lipid Hydrolisis F-40, and the European Research Council (ERC) Grant Agreement 340896. U.K. is supported by the German Centre for Cardiovascular Research (DZHK; BER 5.4 PR), the Deutsche Forschungsgemeinschaft (DFG – KI 712/10-1), the BMBF (German Ministry of Education and Research) (BfR1328-564), and the Einstein Foundation/Foundation Charité (EVF-BIH-2018-440).
Author Contributions
All of the authors wrote, edited, and reviewed the manuscript.
Declaration of Interests
R.Z. holds a patent for Atglistatin.
References
- 1.Crespo-Leiro M.G., Anker S.D., Maggioni A.P., Coats A.J., Filippatos G., Ruschitzka F., Ferrari R., Piepoli M.F., Delgado Jimenez J.F., Metra M., Heart Failure Association (HFA) of the European Society of Cardiology (ESC) European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur. J. Heart Fail. 2016;18:613–625. doi: 10.1002/ejhf.566. [DOI] [PubMed] [Google Scholar]
- 2.Maggioni A.P., Dahlström U., Filippatos G., Chioncel O., Crespo Leiro M., Drozdz J., Fruhwald F., Gullestad L., Logeart D., Fabbri G., Heart Failure Association of the European Society of Cardiology (HFA) EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot) Eur. J. Heart Fail. 2013;15:808–817. doi: 10.1093/eurjhf/hft050. [DOI] [PubMed] [Google Scholar]
- 3.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., Falk V., González-Juanatey J.R., Harjola V.P., Jankowska E.A., ESC Scientific Document Group 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 4.Shah K.S., Xu H., Matsouaka R.A., Bhatt D.L., Heidenreich P.A., Hernandez A.F., Devore A.D., Yancy C.W., Fonarow G.C. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J. Am. Coll. Cardiol. 2017;70:2476–2486. doi: 10.1016/j.jacc.2017.08.074. [DOI] [PubMed] [Google Scholar]
- 5.Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Jr., Drazner M.H., Fonarow G.C., Geraci S.A., Horwich T., Januzzi J.L., American College of Cardiology Foundation. American Heart Association Task Force on Practice Guidelines 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Katz A.M., Rolett E.L. Heart failure: when form fails to follow function. Eur. Heart J. 2016;37:449–454. doi: 10.1093/eurheartj/ehv548. [DOI] [PubMed] [Google Scholar]
- 7.Triposkiadis F., Giamouzis G., Parissis J., Starling R.C., Boudoulas H., Skoularigis J., Butler J., Filippatos G. Reframing the association and significance of co-morbidities in heart failure. Eur. J. Heart Fail. 2016;18:744–758. doi: 10.1002/ejhf.600. [DOI] [PubMed] [Google Scholar]
- 8.Wolsk E., Claggett B., Køber L., Pocock S., Yusuf S., Swedberg K., McMurray J.J.V., Granger C.B., Pfeffer M.A., Solomon S.D. Contribution of cardiac and extra-cardiac disease burden to risk of cardiovascular outcomes varies by ejection fraction in heart failure. Eur. J. Heart Fail. 2018;20:504–510. doi: 10.1002/ejhf.1073. [DOI] [PubMed] [Google Scholar]
- 9.Iorio A., Senni M., Barbati G., Greene S.J., Poli S., Zambon E., Di Nora C., Cioffi G., Tarantini L., Gavazzi A. Prevalence and prognostic impact of non-cardiac co-morbidities in heart failure outpatients with preserved and reduced ejection fraction: a community-based study. Eur. J. Heart Fail. 2018;20:1257–1266. doi: 10.1002/ejhf.1202. [DOI] [PubMed] [Google Scholar]
- 10.Paulus W.J., Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 11.Pfeffer M.A., Shah A.M., Borlaug B.A. Heart Failure With Preserved Ejection Fraction In Perspective. Circ. Res. 2019;124:1598–1617. doi: 10.1161/CIRCRESAHA.119.313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houser S.R., Margulies K.B., Murphy A.M., Spinale F.G., Francis G.S., Prabhu S.D., Rockman H.A., Kass D.A., Molkentin J.D., Sussman M.A., Koch W.J., American Heart Association Council on Basic Cardiovascular Sciences, Council on Clinical Cardiology, and Council on Functional Genomics and Translational Biology Animal models of heart failure: a scientific statement from the American Heart Association. Circ. Res. 2012;111:131–150. doi: 10.1161/RES.0b013e3182582523. [DOI] [PubMed] [Google Scholar]
- 13.Valero-Muñoz M., Backman W., Sam F. Murine Models of Heart Failure with Preserved Ejection Fraction: a “Fishing Expedition”. JACC Basic Transl. Sci. 2017;2:770–789. doi: 10.1016/j.jacbts.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bleumink G.S., Knetsch A.M., Sturkenboom M.C., Straus S.M., Hofman A., Deckers J.W., Witteman J.C., Stricker B.H. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur. Heart J. 2004;25:1614–1619. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 15.Ceia F., Fonseca C., Mota T., Morais H., Matias F., de Sousa A., Oliveira A., EPICA Investigators Prevalence of chronic heart failure in Southwestern Europe: the EPICA study. Eur. J. Heart Fail. 2002;4:531–539. doi: 10.1016/s1388-9842(02)00034-x. [DOI] [PubMed] [Google Scholar]
- 16.Mosterd A., Hoes A.W. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redfield M.M., Jacobsen S.J., Burnett J.C., Jr., Mahoney D.W., Bailey K.R., Rodeheffer R.J. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 18.Vasan R.S., Xanthakis V., Lyass A., Andersson C., Tsao C., Cheng S., Aragam J., Benjamin E.J., Larson M.G. Epidemiology of Left Ventricular Systolic Dysfunction and Heart Failure in the Framingham Study: An Echocardiographic Study Over 3 Decades. JACC Cardiovasc. Imaging. 2018;11:1–11. doi: 10.1016/j.jcmg.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mamas M.A., Sperrin M., Watson M.C., Coutts A., Wilde K., Burton C., Kadam U.T., Kwok C.S., Clark A.B., Murchie P. Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland. Eur. J. Heart Fail. 2017;19:1095–1104. doi: 10.1002/ejhf.822. [DOI] [PubMed] [Google Scholar]
- 20.Bertero E., Maack C. Metabolic remodelling in heart failure. Nat. Rev. Cardiol. 2018;15:457–470. doi: 10.1038/s41569-018-0044-6. [DOI] [PubMed] [Google Scholar]
- 21.Kolwicz S.C., Jr., Purohit S., Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ. Res. 2013;113:603–616. doi: 10.1161/CIRCRESAHA.113.302095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doenst T., Nguyen T.D., Abel E.D. Cardiac metabolism in heart failure: implications beyond ATP production. Circ. Res. 2013;113:709–724. doi: 10.1161/CIRCRESAHA.113.300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young S.G., Zechner R. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes Dev. 2013;27:459–484. doi: 10.1101/gad.209296.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zechner R., Madeo F., Kratky D. Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat. Rev. Mol. Cell Biol. 2017;18:671–684. doi: 10.1038/nrm.2017.76. [DOI] [PubMed] [Google Scholar]
- 25.Lommi J., Kupari M., Yki-Järvinen H. Free fatty acid kinetics and oxidation in congestive heart failure. Am. J. Cardiol. 1998;81:45–50. doi: 10.1016/s0002-9149(97)00804-7. [DOI] [PubMed] [Google Scholar]
- 26.Polak J., Kotrc M., Wedellova Z., Jabor A., Malek I., Kautzner J., Kazdova L., Melenovsky V. Lipolytic effects of B-type natriuretic peptide 1-32 in adipose tissue of heart failure patients compared with healthy controls. J. Am. Coll. Cardiol. 2011;58:1119–1125. doi: 10.1016/j.jacc.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 27.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 28.Haemmerle G., Moustafa T., Woelkart G., Büttner S., Schmidt A., van de Weijer T., Hesselink M., Jaeger D., Kienesberger P.C., Zierler K. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat. Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parajuli N., Takahara S., Matsumura N., Kim T.T., Ferdaoussi M., Migglautsch A.K., Zechner R., Breinbauer R., Kershaw E.E., Dyck J.R.B. Atglistatin ameliorates functional decline in heart failure via adipocyte-specific inhibition of adipose triglyceride lipase. Am. J. Physiol. Heart Circ. Physiol. 2018;315:H879–H884. doi: 10.1152/ajpheart.00308.2018. [DOI] [PubMed] [Google Scholar]
- 30.Salatzki J., Foryst-Ludwig A., Bentele K., Blumrich A., Smeir E., Ban Z., Brix S., Grune J., Beyhoff N., Klopfleisch R. Adipose tissue ATGL modifies the cardiac lipidome in pressure-overload-induced left ventricular failure. PLoS Genet. 2018;14:e1007171. doi: 10.1371/journal.pgen.1007171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayer N., Schweiger M., Romauch M., Grabner G.F., Eichmann T.O., Fuchs E., Ivkovic J., Heier C., Mrak I., Lass A. Development of small-molecule inhibitors targeting adipose triglyceride lipase. Nat. Chem. Biol. 2013;9:785–787. doi: 10.1038/nchembio.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schweiger M., Romauch M., Schreiber R., Grabner G.F., Hütter S., Kotzbeck P., Benedikt P., Eichmann T.O., Yamada S., Knittelfelder O. Pharmacological inhibition of adipose triglyceride lipase corrects high-fat diet-induced insulin resistance and hepatosteatosis in mice. Nat. Commun. 2017;8:14859. doi: 10.1038/ncomms14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schreiber R., Xie H., Schweiger M. Of mice and men: the physiological role of adipose triglyceride lipase (ATGL) Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019;1864:880–899. doi: 10.1016/j.bbalip.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duncan R.E., Wang Y., Ahmadian M., Lu J., Sarkadi-Nagy E., Sul H.S. Characterization of desnutrin functional domains: critical residues for triacylglycerol hydrolysis in cultured cells. J. Lipid Res. 2010;51:309–317. doi: 10.1194/jlr.M000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schweiger M., Schoiswohl G., Lass A., Radner F.P., Haemmerle G., Malli R., Graier W., Cornaciu I., Oberer M., Salvayre R. The C-terminal region of human adipose triglyceride lipase affects enzyme activity and lipid droplet binding. J. Biol. Chem. 2008;283:17211–17220. doi: 10.1074/jbc.M710566200. [DOI] [PubMed] [Google Scholar]
- 36.Hofer P., Boeszoermenyi A., Jaeger D., Feiler U., Arthanari H., Mayer N., Zehender F., Rechberger G., Oberer M., Zimmermann R. Fatty Acid-binding Proteins Interact with Comparative Gene Identification-58 Linking Lipolysis with Lipid Ligand Shuttling. J. Biol. Chem. 2015;290:18438–18453. doi: 10.1074/jbc.M114.628958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lass A., Zimmermann R., Haemmerle G., Riederer M., Schoiswohl G., Schweiger M., Kienesberger P., Strauss J.G., Gorkiewicz G., Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Schweiger M., Paar M., Eder C., Brandis J., Moser E., Gorkiewicz G., Grond S., Radner F.P., Cerk I., Cornaciu I. G0/G1 switch gene-2 regulates human adipocyte lipolysis by affecting activity and localization of adipose triglyceride lipase. J. Lipid Res. 2012;53:2307–2317. doi: 10.1194/jlr.M027409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X., Lu X., Lombès M., Rha G.B., Chi Y.I., Guerin T.M., Smart E.J., Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010;11:194–205. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Granneman J.G., Moore H.P., Mottillo E.P., Zhu Z., Zhou L. Interactions of perilipin-5 (Plin5) with adipose triglyceride lipase. J. Biol. Chem. 2011;286:5126–5135. doi: 10.1074/jbc.M110.180711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H., Sreenivasan U., Gong D.W., O’Connell K.A., Dabkowski E.R., Hecker P.A., Ionica N., Konig M., Mahurkar A., Sun Y. Cardiomyocyte-specific perilipin 5 overexpression leads to myocardial steatosis and modest cardiac dysfunction. J. Lipid Res. 2013;54:953–965. doi: 10.1194/jlr.M032466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolins N.E., Quaynor B.K., Skinner J.R., Tzekov A., Croce M.A., Gropler M.C., Varma V., Yao-Borengasser A., Rasouli N., Kern P.A. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes. 2006;55:3418–3428. doi: 10.2337/db06-0399. [DOI] [PubMed] [Google Scholar]
- 43.Vaughan M., Berger J.E., Steinberg D. Hormone-Sensitive Lipase and Monoglyceride Lipase Activities in Adipose Tissue. J. Biol. Chem. 1964;239:401–409. [PubMed] [Google Scholar]
- 44.Anthonsen M.W., Rönnstrand L., Wernstedt C., Degerman E., Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J. Biol. Chem. 1998;273:215–221. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- 45.Strålfors P., Belfrage P. Phosphorylation of hormone-sensitive lipase by cyclic AMP-dependent protein kinase. J. Biol. Chem. 1983;258:15146–15152. [PubMed] [Google Scholar]
- 46.Dinh T.P., Carpenter D., Leslie F.M., Freund T.F., Katona I., Sensi S.L., Kathuria S., Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl. Acad. Sci. USA. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lian J., Nelson R., Lehner R. Carboxylesterases in lipid metabolism: from mouse to human. Protein Cell. 2018;9:178–195. doi: 10.1007/s13238-017-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lafontan M., Berlan M. Fat cell adrenergic receptors and the control of white and brown fat cell function. J. Lipid Res. 1993;34:1057–1091. [PubMed] [Google Scholar]
- 49.Vaughan C.H., Zarebidaki E., Ehlen J.C., Bartness T.J. Analysis and measurement of the sympathetic and sensory innervation of white and brown adipose tissue. Methods Enzymol. 2014;537:199–225. doi: 10.1016/B978-0-12-411619-1.00011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zechner R., Zimmermann R., Eichmann T.O., Kohlwein S.D., Haemmerle G., Lass A., Madeo F. FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15:279–291. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pollak N.M., Schweiger M., Jaeger D., Kolb D., Kumari M., Schreiber R., Kolleritsch S., Markolin P., Grabner G.F., Heier C. Cardiac-specific overexpression of perilipin 5 provokes severe cardiac steatosis via the formation of a lipolytic barrier. J. Lipid Res. 2013;54:1092–1102. doi: 10.1194/jlr.M034710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ueno M., Suzuki J., Hirose M., Sato S., Imagawa M., Zenimaru Y., Takahashi S., Ikuyama S., Koizumi T., Konoshita T. Cardiac overexpression of perilipin 2 induces dynamic steatosis: prevention by hormone-sensitive lipase. Am. J. Physiol. Endocrinol. Metab. 2017;313:E699–E709. doi: 10.1152/ajpendo.00098.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Granneman J.G., Moore H.P., Krishnamoorthy R., Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl) J. Biol. Chem. 2009;284:34538–34544. doi: 10.1074/jbc.M109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C., Zhao Y., Gao X., Li L., Yuan Y., Liu F., Zhang L., Wu J., Hu P., Zhang X. Perilipin 5 improves hepatic lipotoxicity by inhibiting lipolysis. Hepatology. 2015;61:870–882. doi: 10.1002/hep.27409. [DOI] [PubMed] [Google Scholar]
- 55.Wang H., Bell M., Sreenivasan U., Hu H., Liu J., Dalen K., Londos C., Yamaguchi T., Rizzo M.A., Coleman R. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J. Biol. Chem. 2011;286:15707–15715. doi: 10.1074/jbc.M110.207779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song D.L., Kohse K.P., Murad F. Brain natriuretic factor. Augmentation of cellular cyclic GMP, activation of particulate guanylate cyclase and receptor binding. FEBS Lett. 1988;232:125–129. doi: 10.1016/0014-5793(88)80400-9. [DOI] [PubMed] [Google Scholar]
- 57.Waldman S.A., Rapoport R.M., Murad F. Atrial natriuretic factor selectively activates particulate guanylate cyclase and elevates cyclic GMP in rat tissues. J. Biol. Chem. 1984;259:14332–14334. [PubMed] [Google Scholar]
- 58.Arora R., Krummerman A., Vijayaraman P., Rosengarten M., Suryadevara V., Lejemtel T., Ferrick K.J. Heart rate variability and diastolic heart failure. Pacing Clin. Electrophysiol. 2004;27:299–303. doi: 10.1111/j.1540-8159.2004.00431.x. [DOI] [PubMed] [Google Scholar]
- 59.Cohn J.N., Levine T.B., Olivari M.T., Garberg V., Lura D., Francis G.S., Simon A.B., Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N. Engl. J. Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 60.de Souza S.B., Rocha J.A., Cuoco M.A., Guerra G.M., Ferreira-Filho J.C., Borile S., Krieger E.M., Bortolotto L.A., Consolim-Colombo F.M. High muscle sympathetic nerve activity is associated with left ventricular dysfunction in treated hypertensive patients. Am. J. Hypertens. 2013;26:912–917. doi: 10.1093/ajh/hpt032. [DOI] [PubMed] [Google Scholar]
- 61.Leimbach W.N., Jr., Wallin B.G., Victor R.G., Aylward P.E., Sundlöf G., Mark A.L. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation. 1986;73:913–919. doi: 10.1161/01.cir.73.5.913. [DOI] [PubMed] [Google Scholar]
- 62.Verloop W.L., Beeftink M.M., Santema B.T., Bots M.L., Blankestijn P.J., Cramer M.J., Doevendans P.A., Voskuil M. A systematic review concerning the relation between the sympathetic nervous system and heart failure with preserved left ventricular ejection fraction. PLoS One. 2015;10:e0117332. doi: 10.1371/journal.pone.0117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sengenès C., Berlan M., De Glisezinski I., Lafontan M., Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000;14:1345–1351. [PubMed] [Google Scholar]
- 64.Anker S.D., Chua T.P., Ponikowski P., Harrington D., Swan J.W., Kox W.J., Poole-Wilson P.A., Coats A.J. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96:526–534. doi: 10.1161/01.cir.96.2.526. [DOI] [PubMed] [Google Scholar]
- 65.Scherbakov N., Bauer M., Sandek A., Szabó T., Töpper A., Jankowska E.A., Springer J., von Haehling S., Anker S.D., Lainscak M. Insulin resistance in heart failure: differences between patients with reduced and preserved left ventricular ejection fraction. Eur. J. Heart Fail. 2015;17:1015–1021. doi: 10.1002/ejhf.317. [DOI] [PubMed] [Google Scholar]
- 66.Riehle C., Abel E.D. Insulin Signaling and Heart Failure. Circ. Res. 2016;118:1151–1169. doi: 10.1161/CIRCRESAHA.116.306206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goldberg I.J., Trent C.M., Schulze P.C. Lipid metabolism and toxicity in the heart. Cell Metab. 2012;15:805–812. doi: 10.1016/j.cmet.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma S., Adrogue J.V., Golfman L., Uray I., Lemm J., Youker K., Noon G.P., Frazier O.H., Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 69.Szczepaniak L.S., Dobbins R.L., Metzger G.J., Sartoni-D’Ambrosia G., Arbique D., Vongpatanasin W., Unger R., Victor R.G. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn. Reson. Med. 2003;49:417–423. doi: 10.1002/mrm.10372. [DOI] [PubMed] [Google Scholar]
- 70.Hashani M., Witzel H.R., Pawella L.M., Lehmann-Koch J., Schumacher J., Mechtersheimer G., Schnölzer M., Schirmacher P., Roth W., Straub B.K. Widespread expression of perilipin 5 in normal human tissues and in diseases is restricted to distinct lipid droplet subpopulations. Cell Tissue Res. 2018;374:121–136. doi: 10.1007/s00441-018-2845-7. [DOI] [PubMed] [Google Scholar]
- 71.Straub B.K., Gyoengyoesi B., Koenig M., Hashani M., Pawella L.M., Herpel E., Mueller W., Macher-Goeppinger S., Heid H., Schirmacher P. Adipophilin/perilipin-2 as a lipid droplet-specific marker for metabolically active cells and diseases associated with metabolic dysregulation. Histopathology. 2013;62:617–631. doi: 10.1111/his.12038. [DOI] [PubMed] [Google Scholar]
- 72.Mahmod M., Pal N., Rayner J., Holloway C., Raman B., Dass S., Levelt E., Ariga R., Ferreira V., Banerjee R. The interplay between metabolic alterations, diastolic strain rate and exercise capacity in mild heart failure with preserved ejection fraction: a cardiovascular magnetic resonance study. J. Cardiovasc. Magn. Reson. 2018;20:88. doi: 10.1186/s12968-018-0511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei J., Nelson M.D., Szczepaniak E.W., Smith L., Mehta P.K., Thomson L.E., Berman D.S., Li D., Bairey Merz C.N., Szczepaniak L.S. Myocardial steatosis as a possible mechanistic link between diastolic dysfunction and coronary microvascular dysfunction in women. Am. J. Physiol. Heart Circ. Physiol. 2016;310:H14–H19. doi: 10.1152/ajpheart.00612.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chokshi A., Drosatos K., Cheema F.H., Ji R., Khawaja T., Yu S., Kato T., Khan R., Takayama H., Knöll R. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation. 2012;125:2844–2853. doi: 10.1161/CIRCULATIONAHA.111.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fischer J., Lefèvre C., Morava E., Mussini J.M., Laforêt P., Negre-Salvayre A., Lathrop M., Salvayre R. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat. Genet. 2007;39:28–30. doi: 10.1038/ng1951. [DOI] [PubMed] [Google Scholar]
- 76.Hirano K., Tanaka T., Ikeda Y., Yamaguchi S., Zaima N., Kobayashi K., Suzuki A., Sakata Y., Sakata Y., Kobayashi K. Genetic mutations in adipose triglyceride lipase and myocardial up-regulation of peroxisome proliferated activated receptor-γ in patients with triglyceride deposit cardiomyovasculopathy. Biochem. Biophys. Res. Commun. 2014;443:574–579. doi: 10.1016/j.bbrc.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 77.Natali A., Gastaldelli A., Camastra S., Baldi S., Quagliarini F., Minicocci I., Bruno C., Pennisi E., Arca M. Metabolic consequences of adipose triglyceride lipase deficiency in humans: an in vivo study in patients with neutral lipid storage disease with myopathy. J. Clin. Endocrinol. Metab. 2013;98:E1540–E1548. doi: 10.1210/jc.2013-1444. [DOI] [PubMed] [Google Scholar]
- 78.Pasanisi M.B., Missaglia S., Cassandrini D., Salerno F., Farina S., Andreini D., Agostoni P., Morandi L., Mora M., Tavian D. Severe cardiomyopathy in a young patient with complete deficiency of adipose triglyceride lipase due to a novel mutation in PNPLA2 gene. Int. J. Cardiol. 2016;207:165–167. doi: 10.1016/j.ijcard.2016.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Szabó T., Postrach E., Mähler A., Kung T., Turhan G., von Haehling S., Anker S.D., Boschmann M., Doehner W. Increased catabolic activity in adipose tissue of patients with chronic heart failure. Eur. J. Heart Fail. 2013;15:1131–1137. doi: 10.1093/eurjhf/hft067. [DOI] [PubMed] [Google Scholar]
- 80.Petersen K.M., Bøgevig S., Holst J.J., Knop F.K., Christensen M.B. Hemodynamic Effects of Glucagon: A Literature Review. J. Clin. Endocrinol. Metab. 2018;103:1804–1812. doi: 10.1210/jc.2018-00050. [DOI] [PubMed] [Google Scholar]
- 81.Tikunov B., Levine S., Mancini D. Chronic congestive heart failure elicits adaptations of endurance exercise in diaphragmatic muscle. Circulation. 1997;95:910–916. doi: 10.1161/01.cir.95.4.910. [DOI] [PubMed] [Google Scholar]
- 82.Hirabayashi K., Kinugawa S., Yokota T., Takada S., Fukushima A., Suga T., Takahashi M., Ono T., Morita N., Omokawa M. Intramyocellular lipid is increased in the skeletal muscle of patients with dilated cardiomyopathy with lowered exercise capacity. Int. J. Cardiol. 2014;176:1110–1112. doi: 10.1016/j.ijcard.2014.07.113. [DOI] [PubMed] [Google Scholar]
- 83.Lipkin D.P., Jones D.A., Round J.M., Poole-Wilson P.A. Abnormalities of skeletal muscle in patients with chronic heart failure. Int. J. Cardiol. 1988;18:187–195. doi: 10.1016/0167-5273(88)90164-7. [DOI] [PubMed] [Google Scholar]
- 84.Packer M. Epicardial Adipose Tissue May Mediate Deleterious Effects of Obesity and Inflammation on the Myocardium. J. Am. Coll. Cardiol. 2018;71:2360–2372. doi: 10.1016/j.jacc.2018.03.509. [DOI] [PubMed] [Google Scholar]
- 85.Marchington J.M., Pond C.M. Site-specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int. J. Obes. 1990;14:1013–1022. [PubMed] [Google Scholar]
- 86.Wernstedt Asterholm I., Tao C., Morley T.S., Wang Q.A., Delgado-Lopez F., Wang Z.V., Scherer P.E. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20:103–118. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lai Y.H., Yun C.H., Yang F.S., Liu C.C., Wu Y.J., Kuo J.Y., Yeh H.I., Lin T.Y., Bezerra H.G., Shih S.C. Epicardial adipose tissue relating to anthropometrics, metabolic derangements and fatty liver disease independently contributes to serum high-sensitivity C-reactive protein beyond body fat composition: a study validated with computed tomography. J. Am. Soc. Echocardiogr. 2012;25:234–241. doi: 10.1016/j.echo.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 88.Wu C.K., Tsai H.Y., Su M.M., Wu Y.F., Hwang J.J., Lin J.L., Lin L.Y., Chen J.J. Evolutional change in epicardial fat and its correlation with myocardial diffuse fibrosis in heart failure patients. J. Clin. Lipidol. 2017;11:1421–1431. doi: 10.1016/j.jacl.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 89.Nakanishi K., Fukuda S., Tanaka A., Otsuka K., Taguchi H., Shimada K. Relationships Between Periventricular Epicardial Adipose Tissue Accumulation, Coronary Microcirculation, and Left Ventricular Diastolic Dysfunction. Can. J. Cardiol. 2017;33:1489–1497. doi: 10.1016/j.cjca.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 90.Haemers P., Hamdi H., Guedj K., Suffee N., Farahmand P., Popovic N., Claus P., LePrince P., Nicoletti A., Jalife J. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur. Heart J. 2017;38:53–61. doi: 10.1093/eurheartj/ehv625. [DOI] [PubMed] [Google Scholar]
- 91.Suffee N., Moore-Morris T., Farahmand P., Rücker-Martin C., Dilanian G., Fradet M., Sawaki D., Derumeaux G., LePrince P., Clément K. Atrial natriuretic peptide regulates adipose tissue accumulation in adult atria. Proc. Natl. Acad. Sci. USA. 2017;114:E771–E780. doi: 10.1073/pnas.1610968114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kienesberger P.C., Pulinilkunnil T., Sung M.M., Nagendran J., Haemmerle G., Kershaw E.E., Young M.E., Light P.E., Oudit G.Y., Zechner R., Dyck J.R. Myocardial ATGL overexpression decreases the reliance on fatty acid oxidation and protects against pressure overload-induced cardiac dysfunction. Mol. Cell. Biol. 2012;32:740–750. doi: 10.1128/MCB.06470-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pulinilkunnil T., Kienesberger P.C., Nagendran J., Sharma N., Young M.E., Dyck J.R. Cardiac-specific adipose triglyceride lipase overexpression protects from cardiac steatosis and dilated cardiomyopathy following diet-induced obesity. Int. J. Obes. 2014;38:205–215. doi: 10.1038/ijo.2013.103. [DOI] [PubMed] [Google Scholar]
- 94.Pulinilkunnil T., Kienesberger P.C., Nagendran J., Waller T.J., Young M.E., Kershaw E.E., Korbutt G., Haemmerle G., Zechner R., Dyck J.R. Myocardial adipose triglyceride lipase overexpression protects diabetic mice from the development of lipotoxic cardiomyopathy. Diabetes. 2013;62:1464–1477. doi: 10.2337/db12-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kienesberger P.C., Pulinilkunnil T., Nagendran J., Young M.E., Bogner-Strauss J.G., Hackl H., Khadour R., Heydari E., Haemmerle G., Zechner R. Early structural and metabolic cardiac remodelling in response to inducible adipose triglyceride lipase ablation. Cardiovasc. Res. 2013;99:442–451. doi: 10.1093/cvr/cvt124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schoiswohl G., Schweiger M., Schreiber R., Gorkiewicz G., Preiss-Landl K., Taschler U., Zierler K.A., Radner F.P., Eichmann T.O., Kienesberger P.C. Adipose triglyceride lipase plays a key role in the supply of the working muscle with fatty acids. J. Lipid Res. 2010;51:490–499. doi: 10.1194/jlr.M001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ahmadian M., Abbott M.J., Tang T., Hudak C.S., Kim Y., Bruss M., Hellerstein M.K., Lee H.Y., Samuel V.T., Shulman G.I. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 2011;13:739–748. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schoiswohl G., Stefanovic-Racic M., Menke M.N., Wills R.C., Surlow B.A., Basantani M.K., Sitnick M.T., Cai L., Yazbeck C.F., Stolz D.B. Impact of Reduced ATGL-Mediated Adipocyte Lipolysis on Obesity-Associated Insulin Resistance and Inflammation in Male Mice. Endocrinology. 2015;156:3610–3624. doi: 10.1210/en.2015-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Foryst-Ludwig A., Kreissl M.C., Benz V., Brix S., Smeir E., Ban Z., Januszewicz E., Salatzki J., Grune J., Schwanstecher A.K. Adipose Tissue Lipolysis Promotes Exercise-induced Cardiac Hypertrophy Involving the Lipokine C16:1n7-Palmitoleate. J. Biol. Chem. 2015;290:23603–23615. doi: 10.1074/jbc.M115.645341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dubé J.J., Sitnick M.T., Schoiswohl G., Wills R.C., Basantani M.K., Cai L., Pulinilkunnil T., Kershaw E.E. Adipose triglyceride lipase deletion from adipocytes, but not skeletal myocytes, impairs acute exercise performance in mice. Am. J. Physiol. Endocrinol. Metab. 2015;308:E879–E890. doi: 10.1152/ajpendo.00530.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shimizu I., Yoshida Y., Katsuno T., Tateno K., Okada S., Moriya J., Yokoyama M., Nojima A., Ito T., Zechner R. p53-induced adipose tissue inflammation is critically involved in the development of insulin resistance in heart failure. Cell Metab. 2012;15:51–64. doi: 10.1016/j.cmet.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 102.Iglesias J., Lamontagne J., Erb H., Gezzar S., Zhao S., Joly E., Truong V.L., Skorey K., Crane S., Madiraju S.R., Prentki M. Simplified assays of lipolysis enzymes for drug discovery and specificity assessment of known inhibitors. J. Lipid Res. 2016;57:131–141. doi: 10.1194/jlr.D058438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Z., Agellon L.B., Allen T.M., Umeda M., Jewell L., Mason A., Vance D.E. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 2006;3:321–331. doi: 10.1016/j.cmet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 104.Xie P., Kadegowda A.K., Ma Y., Guo F., Han X., Wang M., Groban L., Xue B., Shi H., Li H., Yu L. Muscle-specific deletion of comparative gene identification-58 (CGI-58) causes muscle steatosis but improves insulin sensitivity in male mice. Endocrinology. 2015;156:1648–1658. doi: 10.1210/en.2014-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zierler K.A., Jaeger D., Pollak N.M., Eder S., Rechberger G.N., Radner F.P., Woelkart G., Kolb D., Schmidt A., Kumari M. Functional cardiac lipolysis in mice critically depends on comparative gene identification-58. J. Biol. Chem. 2013;288:9892–9904. doi: 10.1074/jbc.M112.420620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brown J.M., Betters J.L., Lord C., Ma Y., Han X., Yang K., Alger H.M., Melchior J., Sawyer J., Shah R. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J. Lipid Res. 2010;51:3306–3315. doi: 10.1194/jlr.M010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cantley J.L., Yoshimura T., Camporez J.P., Zhang D., Jornayvaz F.R., Kumashiro N., Guebre-Egziabher F., Jurczak M.J., Kahn M., Guigni B.A. CGI-58 knockdown sequesters diacylglycerols in lipid droplets/ER-preventing diacylglycerol-mediated hepatic insulin resistance. Proc. Natl. Acad. Sci. USA. 2013;110:1869–1874. doi: 10.1073/pnas.1219456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Caviglia J.M., Betters J.L., Dapito D.H., Lord C.C., Sullivan S., Chua S., Yin T., Sekowski A., Mu H., Shapiro L. Adipose-selective overexpression of ABHD5/CGI-58 does not increase lipolysis or protect against diet-induced obesity. J. Lipid Res. 2011;52:2032–2042. doi: 10.1194/jlr.M019117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heier C., Radner F.P., Moustafa T., Schreiber R., Grond S., Eichmann T.O., Schweiger M., Schmidt A., Cerk I.K., Oberer M. G0/G1 Switch Gene 2 Regulates Cardiac Lipolysis. J. Biol. Chem. 2015;290:26141–26150. doi: 10.1074/jbc.M115.671842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kanno Y., Kawashita E., Kokado A., Okada K., Ueshima S., Matsuo O., Matsuno H. Alpha2-antiplasmin regulates the development of dermal fibrosis in mice by prostaglandin F(2α) synthesis through adipose triglyceride lipase/calcium-independent phospholipase A(2) Arthritis Rheum. 2013;65:492–502. doi: 10.1002/art.37767. [DOI] [PubMed] [Google Scholar]
- 111.Kolleritsch S., Kien B., Schoiswohl G., Diwoky C., Schreiber R., Heier C. Low cardiac lipolysis reduces mitochondrial fission and prevents lipotoxic heart dysfunction in Perilipin 5 mutant mice. Cardiovasc. Res. 2020;116:339–352. doi: 10.1093/cvr/cvz119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Drevinge C., Dalen K.T., Mannila M.N., Täng M.S., Ståhlman M., Klevstig M., Lundqvist A., Mardani I., Haugen F., Fogelstrand P. Perilipin 5 is protective in the ischemic heart. Int. J. Cardiol. 2016;219:446–454. doi: 10.1016/j.ijcard.2016.06.037. [DOI] [PubMed] [Google Scholar]
- 113.Miller W.C., Oscai L.B. Relationship between type L hormone-sensitive lipase and endogenous triacylglycerol in rat heart. Am. J. Physiol. 1984;247:R621–R625. doi: 10.1152/ajpregu.1984.247.4.R621. [DOI] [PubMed] [Google Scholar]
- 114.Palmer W.K., Kane T.A. Hormone-stimulated lipolysis in cardiac myocytes. Biochem. J. 1983;216:241–243. doi: 10.1042/bj2160241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Small C.A., Garton A.J., Yeaman S.J. The presence and role of hormone-sensitive lipase in heart muscle. Biochem. J. 1989;258:67–72. doi: 10.1042/bj2580067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Suzuki J., Shen W.J., Nelson B.D., Patel S., Veerkamp J.H., Selwood S.P., Murphy G.M., Jr., Reaven E., Kraemer F.B. Absence of cardiac lipid accumulation in transgenic mice with heart-specific HSL overexpression. Am. J. Physiol. Endocrinol. Metab. 2001;281:E857–E866. doi: 10.1152/ajpendo.2001.281.4.E857. [DOI] [PubMed] [Google Scholar]
- 117.Ueno M., Suzuki J., Zenimaru Y., Takahashi S., Koizumi T., Noriki S., Yamaguchi O., Otsu K., Shen W.J., Kraemer F.B., Miyamori I. Cardiac overexpression of hormone-sensitive lipase inhibits myocardial steatosis and fibrosis in streptozotocin diabetic mice. Am. J. Physiol. Endocrinol. Metab. 2008;294:E1109–E1118. doi: 10.1152/ajpendo.00016.2008. [DOI] [PubMed] [Google Scholar]
- 118.Haemmerle G., Zimmermann R., Hayn M., Theussl C., Waeg G., Wagner E., Sattler W., Magin T.M., Wagner E.F., Zechner R. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J. Biol. Chem. 2002;277:4806–4815. doi: 10.1074/jbc.M110355200. [DOI] [PubMed] [Google Scholar]
- 119.Park S.Y., Kim H.J., Wang S., Higashimori T., Dong J., Kim Y.J., Cline G., Li H., Prentki M., Shulman G.I. Hormone-sensitive lipase knockout mice have increased hepatic insulin sensitivity and are protected from short-term diet-induced insulin resistance in skeletal muscle and heart. Am. J. Physiol. Endocrinol. Metab. 2005;289:E30–E39. doi: 10.1152/ajpendo.00251.2004. [DOI] [PubMed] [Google Scholar]
- 120.Xia B., Cai G.H., Yang H., Wang S.P., Mitchell G.A., Wu J.W. Adipose tissue deficiency of hormone-sensitive lipase causes fatty liver in mice. PLoS Genet. 2017;13:e1007110. doi: 10.1371/journal.pgen.1007110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Albert J.S., Yerges-Armstrong L.M., Horenstein R.B., Pollin T.I., Sreenivasan U.T., Chai S., Blaner W.S., Snitker S., O’Connell J.R., Gong D.W. Null mutation in hormone-sensitive lipase gene and risk of type 2 diabetes. N. Engl. J. Med. 2014;370:2307–2315. doi: 10.1056/NEJMoa1315496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Farhan S.M., Robinson J.F., McIntyre A.D., Marrosu M.G., Ticca A.F., Loddo S., Carboni N., Brancati F., Hegele R.A. A novel LIPE nonsense mutation found using exome sequencing in siblings with late-onset familial partial lipodystrophy. Can. J. Cardiol. 2014;30:1649–1654. doi: 10.1016/j.cjca.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 123.Severson D.L., Hee-Cheong M. Monoacylglycerol lipase activity in cardiac myocytes. Biochem. Cell Biol. 1988;66:1013–1018. doi: 10.1139/o88-116. [DOI] [PubMed] [Google Scholar]
- 124.Schloss M.J., Horckmans M., Guillamat-Prats R., Hering D., Lauer E., Lenglet S., Weber C., Thomas A., Steffens S. 2-Arachidonoylglycerol mobilizes myeloid cells and worsens heart function after acute myocardial infarction. Cardiovasc. Res. 2019;115:602–613. doi: 10.1093/cvr/cvy242. [DOI] [PubMed] [Google Scholar]
- 125.Schlosburg J.E., Blankman J.L., Long J.Z., Nomura D.K., Pan B., Kinsey S.G., Nguyen P.T., Ramesh D., Booker L., Burston J.J. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat. Neurosci. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Taschler U., Radner F.P., Heier C., Schreiber R., Schweiger M., Schoiswohl G., Preiss-Landl K., Jaeger D., Reiter B., Koefeler H.C. Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance. J. Biol. Chem. 2011;286:17467–17477. doi: 10.1074/jbc.M110.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jin J., Huang S., Wang L., Leng Y., Lu W. Design and synthesis of Atglistatin derivatives as adipose triglyceride lipase inhibitors. Chem. Biol. Drug Des. 2017;90:1122–1133. doi: 10.1111/cbdd.13029. [DOI] [PubMed] [Google Scholar]
- 128.Mayer N., Schweiger M., Melcher M.C., Fledelius C., Zechner R., Zimmermann R., Breinbauer R. Structure-activity studies in the development of a hydrazone based inhibitor of adipose-triglyceride lipase (ATGL) Bioorg. Med. Chem. 2015;23:2904–2916. doi: 10.1016/j.bmc.2015.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Roy P.P., D’Souza K., Cuperlovic-Culf M., Kienesberger P.C., Touaibia M. New Atglistatin closely related analogues: synthesis and structure-activity relationship towards adipose triglyceride lipase inhibition. Eur. J. Med. Chem. 2016;118:290–298. doi: 10.1016/j.ejmech.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 130.Cerk I.K., Salzburger B., Boeszoermenyi A., Heier C., Pillip C., Romauch M., Schweiger M., Cornaciu I., Lass A., Zimmermann R. A peptide derived from G0/G1 switch gene 2 acts as noncompetitive inhibitor of adipose triglyceride lipase. J. Biol. Chem. 2014;289:32559–32570. doi: 10.1074/jbc.M114.602599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sanders M.A., Madoux F., Mladenovic L., Zhang H., Ye X., Angrish M., Mottillo E.P., Caruso J.A., Halvorsen G., Roush W.R. Endogenous and Synthetic ABHD5 Ligands Regulate ABHD5-Perilipin Interactions and Lipolysis in Fat and Muscle. Cell Metab. 2015;22:851–860. doi: 10.1016/j.cmet.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]