Summary
Individuals with obesity due to pathogenic heterozygous melanocortin 4 receptor (MC4R) mutations can be treated efficiently with the glucagon-like peptide-1 receptor agonist (GLP-1 RA) liraglutide. Here, we report the effect of 16 weeks of liraglutide 3 mg/day treatment in a woman with morbid obesity and type 2 diabetes (T2D) due to homozygous pathogenic MC4R mutation. The body weight loss was 9.7 kg, similar to weight loss in heterozygous MC4R mutation carriers and common obesity. In addition, the treatment led to clinically relevant decreases in fasting glucose, triglycerides, systolic blood pressure, and normalization of glucose tolerance. We conclude that liraglutide reduces body weight and blood glucose levels in hetero- and homozygous MC4R mutation carriers. This serves as proof-of-concept that MC4Rs are not required for the body weight and glucose lowering effects of GLP-1 RAs and that liraglutide may be used as part of the treatment of obesity and T2D due to MC4R mutations.
Keywords: Glucagon-like peptide-1 receptor agonists, GLP-1 RA, liraglutide, MC4R mutation, obesity, weight loss
Graphical Abstract
Highlights
Liraglutide induces weight loss in a woman homozygous for pathogenic MC4R mutation
Glucose tolerance normalized and fasting glucose and triglyceride levels reduced
MC4R is not required for GLP-1 RA-mediated weight loss
Liraglutide is an effective treatment for the most common form of monogenic obesity
In this case report, Iepsen et al. show that the GLP-1 RA liraglutide induces a weight loss of 10 kg and normalization of glucose tolerance in a woman homozygous for pathogenic MC4R mutation. Thus, the appetite-reducing effects of liraglutide are preserved in MC4R causal obesity and independent of the MC4R pathway.
Introduction
Heterozygous mutations in the melanocortin 4 receptor (MC4R) gene are the most common cause of monogenic obesity, with a prevalence ranging from 2% to 6% in juvenile-onset obesity populations.1, 2, 3, 4, 5 We have previously shown that individuals with obesity caused by heterozygous pathogenic MC4R mutations can be treated efficiently with liraglutide, a glucagon-like peptide-1 receptor agonist (GLP-1 RA).5
GLP-1 is a gut hormone secreted from endocrine cells in the gastrointestinal tract.6 Upon food intake, GLP-1 stimulates insulin secretion and regulates appetite by affecting central appetite-regulating areas of the brain.7, 8, 9 The central appetite-regulating pathways associated with GLP-1 RA-mediated anorexia are incompletely known, but GLP-1 receptors (GLP-1Rs) have been identified in numerous areas of the brain associated with regulation of food intake, including the hypothalamus. In the hypothalamus, it has been suggested that the GLP-1 RA liraglutide may act through GLP-1Rs present on anorexigenic proopiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) neurons, which, upon stimulation, release α-melanocyte-stimulating hormone (MSH), interacting with MC4 receptors and producing satiety.10 In this scenario, functional MC4Rs would be a prerequisite for conveying liraglutide-mediated anorexia. However, it was also suggested that liraglutide may act through a local gamma-aminobutyric acid (GABA) neuron, inhibiting the orexigenic agouti-related peptide (AgRP)/neuropeptide Y (NPY) neurons.10
The importance of the MC4R in appetite and thus body weight regulation is evident by the obese phenotype of the MC4R−/− knockout mice, whereas mice with heterozygous mutations, MC4R−/+, display an intermediary phenotype between that of homozygous and wild-type mice.11 In humans, homozygous carriers of pathogenic MC4R mutations also display more severe obesity than heterozygous carriers, probably reflecting the fact that heterozygous carriers have retained some signaling capacity of their MC4Rs.12
We have previously shown that heterozygous individuals with pathogenic MC4R mutations and matched control participants lost exactly the same amount of body weight, suggesting that the appetite-inhibiting effect of GLP-1 is independent of the MC4R.5 In order to examine the complete independence of the MC4R in the appetite-inhibiting effect of GLP-1, we assessed the effect of the GLP-1 RA liraglutide on body weight in a homozygous carrier with a pathogenic MC4R mutation with no MC4Rs.
Results
Patient Characteristics
The case, a 51-year-old woman, suffers from a wide range of co-morbidities including type 2 diabetes (T2D) (diagnosed in 1999), hypertension (diagnosed in 2018), hypercholesterolemia, rheumatic disease (treated with prednisolone 10 mg once daily), as well as depression and borderline personality disorder, all for which she is being treated pharmaceutically. For a complete drug list, see Table 1. The patient had a normal birth weight (3,500 g) but describes that, from early childhood, she experienced extreme hunger and, at the age of 20, weighed 140 kg with a height of 164 cm. Her highest weight was 186 kg. Obesity runs in the family, both on the paternal and maternal side. No other known inherited diseases run in the family, including T2D.
Table 1.
Data from a Woman with Homozygous MC4R Mutation from before to after 4 Months of Daily Liraglutide Injections
| Variables | Week 0 | Week 16 | Difference |
|---|---|---|---|
| Weight (kg) | 152.2 | 142.5 | −9.7 |
| Height (m) | 1.64 | 1.64 | N/A |
| BMI (kg/m2) | 56.6 | 53.0 | −3.6 |
| Systolic blood pressure (mmHg) | 122 | 112 | −10 |
| Diastolic blood pressure (mmHg) | 78 | 76 | −2 |
| Pulse (beats/min) | 83 | 67 | −16 |
| Fasting plasma glucose (mmol/L) | 6.3 | 5.0 | −1.3 |
| Fasting HbA1C (mmol/mol) | 54 | 49 | −5 |
| Fasting serum insulin (pmol/L) | 66 | 48 | −18 |
| Fasting C-peptide (pmol/L) | 1,152 | 1,099 | −53 |
| Triglycerides (mmol/L) | 2.41 | 1.78 | −0.63 |
| Total fat mass (kg) | 75.5 | 71.2 | −4.3 |
| Total lean mass (kg) | 76.9 | 71.3 | −5.6 |
| Total fat (%) | 49.6 | 49.9 | 0.3 |
In 2006, the patient underwent a gastric bypass operation and lost approximately 40 kg. However, the patient returned to her preoperative weight within a few years to her current weight status, which was her weight when entering this project.
The patient was screened for MC4R mutations while she was attending a diabetes clinic and volunteered to enter a biobank project for which plasma and DNA samples were collected. The patient has had many former weight loss attempts without success. Based on our previous study, which showed successful treatment with the GLP-1 RA liraglutide for heterozygous carriers with pathogenic MC4R mutations,5 we contacted the patient, who volunteered to participate in the present study with liraglutide treatment.
Genetic Characteristics
The patient is a homozygous mutation carrier of the T allele of the rs747681609 variant. The C-to-T allele mutation changes the 165th amino acid of the MC4R protein from arginine to glutamine (p.Arg165Gln). The variant is in silico predicted to be pathogenic and/or deleterious (e.g., by SIFT and PolyPhen), and it has a PHRED-scaled combined annotation dependent depletion (CADD) score of 33, predicting its deleteriousness to be above the 1,000th quartile of SNP variants in the genome. ClinVar categorizes the variant as likely pathogenic for obesity. Several in vitro validations document the pathogenic function of the variant.13,14 Thus, the pathogenic MC4 Arg165Gln receptor is not present on the cell surface and does not bind its ligand α-MSH.14 The mutation is very rare and reported in only 7 of 123,034 individuals in the Genome Aggregation Database (gnomAD), where 6 of these are of European origin and the last carrier is East Asian.15 The variant has previously been reported in relation to extreme and early onset obesity, mostly in Europeans,2,4,12,16 but also in Pima Indians.17
Anthropometrics and Blood Analyses Before and After Liraglutide Treatment
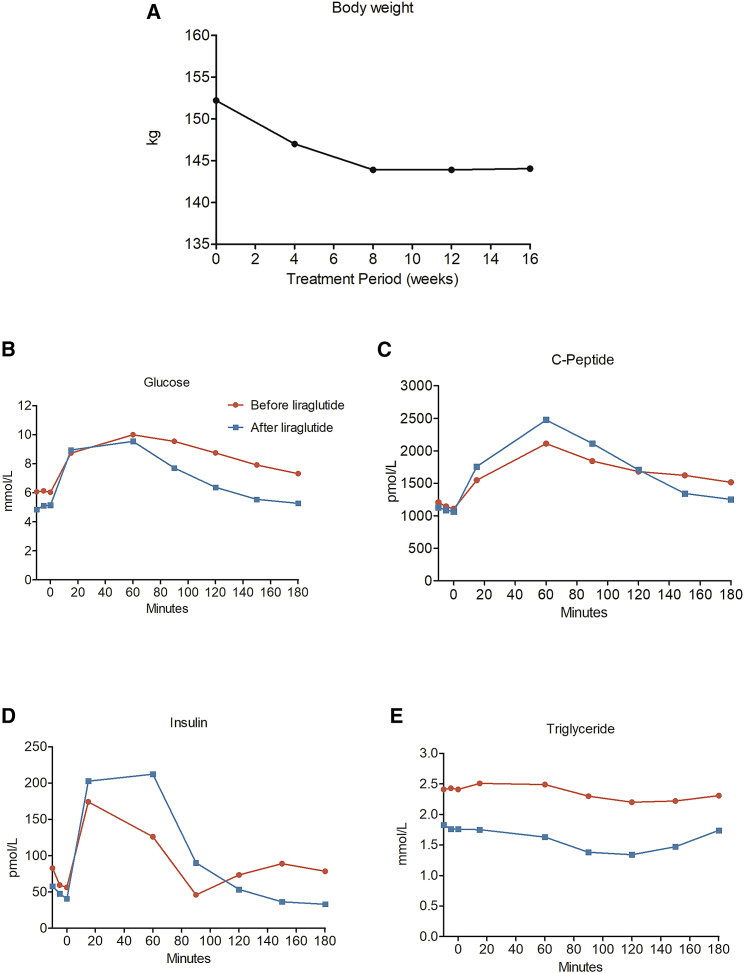
During 16 weeks of liraglutide treatment, the patient lost a total of 9.7 kg (from 152.2 kg to 142.5 kg). BMI was reduced by 3.6 units (from 56.6 kg/m2 to 53.0 kg/m2). The weight loss was achieved within the initial 8 weeks of treatment, with no additional weight loss between week 8 to week 16. Fasting plasma glucose was reduced by 1.3 mmol/L (from 6.3 to 5.0 mmol/L), and fasting insulin levels decreased by 18 pmol/L (from 66 to 48 pmol/L). Hemoglobin A1c (HbA1c) decreased by 5 mmol/mol (from 54 to 49 mmol/mol). Triglyceride levels decreased by 0.6 mmol/L (from 2.4 to 1.8 mmol/L).
The patient also exhibited improvements in postprandial glucose, insulin, and C-peptide values. Accordingly, incremental area under the curve (iAUC) for glucose decreased from 439 mmol/L × min to 428 mmol/L × min, iAUC for insulin increased from 6,217 pmol/L × min to 12,027 pmol/L × min, and iAUC for C-peptide increased from 103,623 mmol/mol × min to 130,277 mmol/mol × min.
Before treatment, the patient had impaired glucose tolerance (defined as a plasma glucose between 8.7 to 11.1 mmol/L after a 2-h oral glucose tolerance test [OGTT]), with a postprandial glucose concentration after 2 h of 8.7 mmol/L, which was reduced to 6.4 mmol/L after treatment and thus normalized to glucose tolerant.
Finally, systolic blood pressure was reduced by 10 mmHg (from 122 to 112 mmHg), and diastolic blood pressure was reduced from 78 to 75 mmHg. Pulse also decreased by 16 bpm (from 83 to 67 bpm). Fat mass was reduced by 4.3 kg, and lean body mass decreased by 5.6 kg, but there was no net change in fat percentage (49.6% before and 49.9% after liraglutide treatment).
The patient experienced mild gastrointestinal side effects in the form of nausea and stomach pain during the first 4 weeks of treatment but did not experience any side effects during the remaining 12 weeks. The patient reported that she fell less hungry and more satiated during the first 8 weeks of treatment, but this effect was reduced, although still present to some extent, during the last 8 weeks of treatment. For all data, please see Table 1 and Figure 1.
Figure 1.
Weight and Metabolism Data from a Woman Homozygous for Pathogenic MC4R Mutation
(A–E) Body weight (A), fasting and postprandial levels of glucose (B), C-peptide (C), insulin (D), and triglycerides (E) before and after 16 weeks of treatment with a GLP-1 RA (liraglutide).
Discussion
In this case story, we report weight loss during 16 weeks of treatment with liraglutide in a woman with a previous gastric bypass opaeration and morbid obesity due to a complete loss of function of homozygous MC4R mutation. The weight loss (9.7 kg) was equivalent to 3.6 BMI units and stabilized during the last 2 months of treatment. The weight loss response is comparable to the effect of liraglutide in common obesity.5,18, 19, 20 In common obesity, both lean and fat mass is lost during weight loss.21 In the present study, the losses in lean body mass and fat mass were almost equal (4.3 kg fat mass and 5.6 kg lean body mass). This is in contrast to our previously published article, where we observed a decrease of ~1 kg in lean body mass and ~5 kg in fat mass in heterozygous MC4R mutation carriers and control participants alike.5
The treatment led to reductions in fasting glucose, fasting insulin, systolic blood pressure, and triglyceride levels, although the patient was within normal limits when the study began. Furthermore, postprandial glucose was reduced, and insulin and C-peptide responses increased immediately in response to glucose but were reduced after 150 min compared to before treatment. Thus, the insulin response improved with liraglutide treatment and the patient no longer exhibited impaired glucose tolerance.
The patient underwent a roux-en-y gastric bypass (RYGB) in 2006, which led to a transient weight loss of ~40 kg. Because increases in endogenous GLP-1 levels are considered a key component in RYGB-induced weight loss,22 one could ask whether exogenous GLP-1 in the form of liraglutide would work in this patient, when the assumed increase in endogenous GLP-1 after RYGB only worked transiently. However, after RYGB, meal ingestion elicits a rapid and short-lasting rise in endogenous GLP-1 response, spanning only about 90 min.23,24 In contrast, with liraglutide treatment, there is a 24-h maintained exposure, which will lower the drive to ingest foods, including small meals and snacks, which might not produce marked endogenous GLP-1 responses even after RYGB. It is also possible that the acylated liraglutide molecule may access regions of the brain that are not normally reached by endogenous (and intact) GLP-1.10 Thus, it is probably important to distinguish between the actions of endogenous GLP-1 and exogenously administered GLP-1 in the form of a GLP-1 RA such as liraglutide.
Conclusions
We conclude that 16 weeks of treatment with 3.0 mg of GLP-1 RA liraglutide resulted in a weight loss of 9.7 kg in a woman with severe morbid obesity and T2D due to a homozygous mutation in the MC4R as well as reductions in fasting and postprandial glucose levels, leading to normal glucose tolerance. This finding supports the notion that MC4Rs are not required for the appetite-inhibiting effects of liraglutide. Furthermore, our findings support the use of GLP-1 RAs in the treatment of obesity and T2D caused by MC4R mutations.
Strengths and Limitations
The results from this case report are limited by the fact that we were only able to obtain data from one patient. A larger group of homozygous carriers would have been preferable. However, these patients are extremely rare. Accordingly, with our previous article on heterozygous carriers with pathogenic MC4R mutation,5 together with this case report, there is good support for a conclusion that GLP-1 RAs act independently of the MC4R in individuals with severe obesity due to both heterozygous and homozygous pathogenic mutations in the MC4R.
STAR★Methods
Key Resources Table
| Reagent and Resource | Source | Identifier |
|---|---|---|
| Biological samples | ||
| Plasma and serum | Homozygous muation carrier | H-1-2013-093/ NCT02082496 |
| Genomic DNA | Homozygous muation carrier | H-1-2013-093/ NCT02082496 |
| Critical Commercial Assays | ||
| QIAamp DNA Blood mini kits | QIAGEN, Germany | RRID:SCR_008539 |
| Dynal Myone Streptavidin C1 magnetic beads | Invitrogen, USA | RRID:SCR_008410 |
| Software and Algorithms | ||
| Prism 5.0 | GraphPad, USA | RRID:SCR_002798 |
| Covaris sonicator, | SonoLab Software, USA | RRID:SCR_016302 |
| Other | ||
| 2100 Bioanalyzer | Agilent, USA | RRID:SCR_018043 |
Lead Contact and Materials Availability
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Signe S. Torekov, torekov@sund.ku.dk. Materials availability statement: this study did not generate new unique reagents.
Experimental Model and Subject Details
Subject details
The case story involves a 51 years old woman homozygous for pathogenic MC4R mutation.
Ethical issues
The project and associated biobank were approved by the ethical committee in Copenhagen (reference number: H-1-2013-093) and the study was performed in accordance with the Helsinki Declaration II. Participation was voluntary and the participant could at any time retract her consent to participate. ClinicalTrials.gov: NCT02082496.
Method Details
Genotyping
The variant was genotyped through deep sequencing of the MC4R on a custom target region capture sequencing platform.25 The methods for DNA extraction, target region capture, and NGS have previously been extensively described.25 The final captured DNA libraries were sequenced using the Illumina HiSeq2000 Analyzer as paired-end 90 bp reads (following the manufacturer’s standard cluster generation and sequencing protocols). The depth at the variant position of the rs747681609 variant was 221 and all but two reads mapped to call the non-reference T-allele. The PRED-scaled genotype quality for the site was the maximal supported by the VCF format. The genotype was called using the GATK pipeline.26
Study drug
Liraglutide was administered as FlexPen devices (Saxenda®, Novo Nordisk A/S, Bagsvaerd, Denmark) by subcutaneous injection in the abdomen or thigh. Dosing was initiated at 0.6 mg daily, increasing to 3.0 mg daily over a 5 week period (0.6 mg, 1.2 mg, 1.8 mg, 2.4 mg and 3.0 mg per week) continuing until 16 weeks of treatment. The liraglutide injection period was not accompanied by any lifestyle counselling, diet or exercise program.
Visits and measurements
Before and after 16 weeks of liraglutide treatment the patient met in the morning after an overnight fast in an outpatient clinic. Morning weight was measured in light indoor clothes (weight model: Tanita WB-110MA, Tokyo, Japan), height, waist-and hip circumference (measured with non-elastic tape), blood pressure and pulse (Omron M6, Omron Healthcare Co. Ltd., Kyoto, Japan) was measured and BMI calculated as the weight in kilos over the height in meters squared (kg/m2).
Oral glucose tolerance test (OGTT)
A cannula was inserted in an antecubital vein and fasting blood samples were drawn at time point −10, −5 and 0 (for measurement of plasma glucose, plasma HbA1C, serum insulin, plasma C-peptide, and plasma triglycerides) prior to ingestion of 75 g glucose dissolved in 125 mL water. Subsequently, blood samples were drawn 15, 30, 60, 90, 120, 150 and 180 minutes after glucose ingestion for measurements of postprandial plasma glucose, plasma C-peptide, serum insulin and plasma triglycerides.
Dual energy X-ray absorptiometry (DEXA)
Total fat mass and total lean body mass (fat free mass) were assessed by DEXA (Hologic Discovery A, Massachusetts, USA), and performed by trained personnel.
Plasma Analyses
Plasma glucose was measured with the glucose oxidase technique (YSI model 2300 STAT Plus; Yellow Springs Instruments, Yellow Springs, OH). HbA1C was measured with a high performance liquid chromatography (HPLC) technique (Tosoh Bioscience GmbH, Griesheim, Germany).
Quantification and Statistical Analyses
Total and incremental areas under the curve (AUC) were calculated in GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego California USA, using the trapezoidal method. All data (N = 1) can be found in Figure 1 and Table 1.
Data and Code Availability
The published article includes all datasets generated or analyzed during this study.
Acknowledgments
We would like to thank the study participant for volunteering for this project. This work would not have been possible without professional help from Alis Sloth Andersen and Sussi Polman (Department of Endocrinology, Hvidovre University Hospital). The project was supported by funding from The Danish Diabetes Academy supported by the Novo Nordisk Foundation, The Lundbeck Foundation, the Novo Nordisk Foundation, the Augustinus Foundation, the Region Zealand Health Scientific Research Foundation, Aase and Ejnar Danielsens Foundation, the Michaelsen Foundation and supported by the Tripartite Immunometabolism Consortium (TrIC)-Novo Nordisk Foundation (grant number NNF15CC0018486). This study is part of the research activities in TARGET (The Impact of our Genomes on Individual Treatment Response in Obese Children, https://cbmr.ku.dk/research/human-genomics-and-metagenomics-in-metabolism/target/). The funding sponsors were not involved in study design, collection of data or data analysis, approval of the manuscript, or decision to submit for publication.
Author Contributions
Conceptualization and Methodology: S.S.T., J.J.H., S.M., T.H., E.W.I., and J.C.H. Formal Analysis: E.W.I. and C.T.H. Investigation: E.W.I. and S.V. Resources: I.B., C.T.H., T.H., N.G., O.P., and J.C.H. Writing – Original Draft: E.W.I. with help from S.S.T. Writing – Review & Editing: J.J.H., S.M., S.S.T., T.H., J.C.H., C.T.H., S.V., I.B., N.G., and O.P. Supervision: S.S.T. and J.J.H. Project Administration: E.W.I. The corresponding authors E.W.I. and S.S.T. confirm full access to data and final responsibility for the decision to submit for publication.
Declaration of Interests
T.H. hold stock in Novo Nordisk A/S. J.J.H. has acted as a consultant and received speaker honoraria for Novo Nordisk. S.S.T. has received a research grant from Novo Nordisk. S.M. has served as a consultant or adviser to Novartis Pharmaceuticals, Novo Nordisk, Merck, Sharp and Dome, Pfizer A/S, Abbott Laboratories, Sanofi Aventis, Astra Zeneca, Johnson & Johnson, Rosche, Mankind, BMS, Antarcia, Boehringer Ingelheim, Eli Lilly, and Amgen. S.M. has received a fee for speaking from Novo Nordisk, Merck, Sharp and Dome, Astra Zeneca, Johnson and Johnson, Abbott Laboratories, Pfizer A/S, Roche, Schering-Plough, Sanofi-Aventis, Eli Lilly, Novartis Pharmaceuticals, BMS, and Boehringer Ingelheim. The remaining authors have nothing to disclose.
Published: April 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.xcrm.2020.100006.
Contributor Information
Eva W. Iepsen, Email: epwi@sund.ku.dk.
Signe S. Torekov, Email: torekov@sund.ku.dk.
Supplemental Information
References
- 1.Vaisse C., Clement K., Durand E., Hercberg S., Guy-Grand B., Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J. Clin. Invest. 2000;106:253–262. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farooqi I.S., Yeo G.S., Keogh J.M., Aminian S., Jebb S.A., Butler G., Cheetham T., O’Rahilly S. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J. Clin. Invest. 2000;106:271–279. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hainerová I., Larsen L.H., Holst B., Finková M., Hainer V., Lebl J., Hansen T., Pedersen O. Melanocortin 4 receptor mutations in obese Czech children: studies of prevalence, phenotype development, weight reduction response, and functional analysis. J. Clin. Endocrinol. Metab. 2007;92:3689–3696. doi: 10.1210/jc.2007-0352. [DOI] [PubMed] [Google Scholar]
- 4.Larsen L.H., Echwald S.M., Sørensen T.I., Andersen T., Wulff B.S., Pedersen O. Prevalence of mutations and functional analyses of melanocortin 4 receptor variants identified among 750 men with juvenile-onset obesity. J. Clin. Endocrinol. Metab. 2005;90:219–224. doi: 10.1210/jc.2004-0497. [DOI] [PubMed] [Google Scholar]
- 5.Iepsen E.W., Zhang J., Thomsen H.S., Hansen E.L., Hollensted M., Madsbad S., Hansen T., Holst J.J., Holm J.-C., Torekov S.S. Patients with Obesity Caused by Melanocortin-4 Receptor Mutations Can Be Treated with a Glucagon-like Peptide-1 Receptor Agonist. Cell Metab. 2018;28:23–32.e3. doi: 10.1016/j.cmet.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Orci L., Pictet R., Forssmann W.G., Renold A.E., Rouiller C. Structural evidence for glucagon producing cells in the intestinal mucosa of the rat. Diabetologia. 1968;4:56–67. doi: 10.1007/BF01241034. [DOI] [PubMed] [Google Scholar]
- 7.Flint A., Raben A., Astrup A., Holst J.J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Invest. 1998;101:515–520. [Google Scholar]
- 8.Nauck M.A., Kleine N., Orskov C., Holst J.J., Willms B., Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–744. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- 9.Tang-Christensen M., Larsen P.J., Göke R., Fink-Jensen A., Jessop D.S., Møller M., Sheikh S.P. Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. Am. J. Physiol. 1996;271:R848–R856. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- 10.Secher A., Jelsing J., Baquero A.F., Hecksher-Sørensen J., Cowley M.A., Dalbøge L.S., Hansen G., Grove K.L., Pyke C., Raun K. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Invest. 2014;124:4473–4488. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huszar D., Lynch C.A., Fairchild-Huntress V., Dunmore J.H., Fang Q., Berkemeier L.R., Gu W., Kesterson R.A., Boston B.A., Cone R.D. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 12.Farooqi I.S., Keogh J.M., Yeo G.S., Lank E.J., Cheetham T., O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 13.Yeo G.S., Lank E.J., Farooqi I.S., Keogh J., Challis B.G., O’Rahilly S. Mutations in the human melanocortin-4 receptor gene associated with severe familial obesity disrupts receptor function through multiple molecular mechanisms. Hum. Mol. Genet. 2003;12:561–574. doi: 10.1093/hmg/ddg057. [DOI] [PubMed] [Google Scholar]
- 14.Nijenhuis W.A., Kruijtzer J.A., Wanders N., Vrinten D.H., Garner K.M., Schaaper W.M., Meloen R.H., Gispen W.H., Liskamp R.M., Adan R.A. Discovery and in vivo evaluation of new melanocortin-4 receptor-selective peptides. Peptides. 2003;24:271–280. doi: 10.1016/s0196-9781(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 15.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nordang G.B.N., Busk O.L., Tveten K., Hanevik H.I., Fell A.K.M., Hjelmesæth J., Holla Ø.L., Hertel J.K. Next-generation sequencing of the monogenic obesity genes LEP, LEPR, MC4R, PCSK1 and POMC in a Norwegian cohort of patients with morbid obesity and normal weight controls. Mol. Genet. Metab. 2017;121:51–56. doi: 10.1016/j.ymgme.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Ma L., Tataranni P.A., Bogardus C., Baier L.J. Melanocortin 4 receptor gene variation is associated with severe obesity in Pima Indians. Diabetes. 2004;53:2696–2699. doi: 10.2337/diabetes.53.10.2696. [DOI] [PubMed] [Google Scholar]
- 18.Wadden T.A., Hollander P., Klein S., Niswender K., Woo V., Hale P.M., Aronne L., NN8022-1923 Investigators Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int. J. Obes. 2013;37:1443–1451. doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- 19.Pi-Sunyer X., Astrup A., Fujioka K., Greenway F., Halpern A., Krempf M., Lau D.C., le Roux C.W., Violante Ortiz R., Jensen C.B., Wilding J.P., SCALE Obesity and Prediabetes NN8022-1839 Study Group A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015;373:11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 20.Astrup A., Rössner S., Van Gaal L., Rissanen A., Niskanen L., Al Hakim M., Madsen J., Rasmussen M.F., Lean M.E., NN8022-1807 Study Group Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 21.Sargeant J.A., Henson J., King J.A., Yates T., Khunti K., Davies M.J. A Review of the Effects of Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors on Lean Body Mass in Humans. Endocrinol. Metab. (Seoul) 2019;34:247–262. doi: 10.3803/EnM.2019.34.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.le Roux C.W., Welbourn R., Werling M., Osborne A., Kokkinos A., Laurenius A., Lönroth H., Fändriks L., Ghatei M.A., Bloom S.R., Olbers T. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann. Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 23.Holst J.J. Postprandial insulin secretion after gastric bypass surgery: the role of glucagon-like peptide 1. Diabetes. 2011;60:2203–2205. doi: 10.2337/db11-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobsen S.H., Olesen S.C., Dirksen C., Jørgensen N.B., Bojsen-Møller K.N., Kielgast U., Worm D., Almdal T., Naver L.S., Hvolris L.E. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes. Surg. 2012;22:1084–1096. doi: 10.1007/s11695-012-0621-4. [DOI] [PubMed] [Google Scholar]
- 25.Gao R., Liu Y., Gjesing A.P., Hollensted M., Wan X., He S., Pedersen O., Yi X., Wang J., Hansen T. Evaluation of a target region capture sequencing platform using monogenic diabetes as a study-model. BMC Genet. 2014;15:13. doi: 10.1186/1471-2156-15-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DePristo M.A., Banks E., Poplin R., Garimella K.V., Maguire J.R., Hartl C., Philippakis A.A., del Angel G., Rivas M.A., Hanna M. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes all datasets generated or analyzed during this study.