Abstract
Current mortality due to the Covid-19 pandemic (approximately 1.2 million by November 2020) demonstrates the lack of an effective treatment. As replication of many viruses - including MERS-CoV - is supported by enhanced aerobic glycolysis, we hypothesized that SARS-CoV-2 replication in host cells (especially airway cells) is reliant upon altered glucose metabolism. This metabolism is similar to the Warburg effect well studied in cancer. Counteracting two main pathways (PI3K/AKT and MAPK/ERK signaling) sustaining aerobic glycolysis inhibits MERS-CoV replication and thus, very likely that of SARS-CoV-2, which shares many similarities with MERS-CoV. The Warburg effect appears to be involved in several steps of COVID-19 infection. Once induced by hypoxia, the Warburg effect becomes active in lung endothelial cells, particularly in the presence of atherosclerosis, thereby promoting vasoconstriction and micro thrombosis. Aerobic glycolysis also supports activation of pro-inflammatory cells such as neutrophils and M1 macrophages. As the anti-inflammatory response and reparative process is performed by M2 macrophages reliant on oxidative metabolism, we speculated that the switch to oxidative metabolism in M2 macrophages would not occur at the appropriate time due to an uncontrolled pro-inflammatory cascade. Aging, mitochondrial senescence and enzyme dysfunction, AMPK downregulation and p53 inactivation could all play a role in this key biochemical event. Understanding the role of the Warburg effect in COVID-19 can be essential to developing molecules reducing infectivity, arresting endothelial cells activation and the pro-inflammatory cascade.
Keywords: SARS-CoV-2, Warburg effect, Macrophage, AMPK, Atherosclerosis
Highlights
-
•
Enhanced aerobic glycolysis supports replication of many viruses including MERS-CoV.
-
•
PI3K/AKT and MAPK/ERK inhibitors arrest MERS-CoV replication.
-
•
This metabolism likely sustains SARS-CoV-2 replication in host cells, in particular airway cells .
-
•
The Warburg effect also supports activation of endothelial cells and pro-inflammatory cells .
List of abbreviations
- 2-DG
2-deoxy-d-glucose
- ACC
acetyl-CoA carboxylase
- ACE2
angiotensin-converting enzyme 2
- Acetyl-Coa
acetyl coenzyme A
- ACLY
ATP citrate lyase
- ACO2
mitochondrial aconitase
- Acyl-CoA
acyl coenzyme A
- AKG
α-ketoglutarate
- AKT
Protein kinase B
- AMPK
AMP-activated protein kinase
- Ang
angiotensin
- ARDS
acute respiratory distress syndrome
- ARG1
arginase 1
- ATF3
activating transcription factor
- CAD
cis-Aconitate decarboxylase
- CD36
cluster of differentiation 36
- COVID-19
Coronavirus disease 2019
- DCs
dendritic cells
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- F1,6BP
fructose 1,6-bisphosphate
- F6P
fructose 6-phosphate
- FAO
fatty acid oxidation
- FAS
fatty acid synthesis
- GLS1
Glutaminase 1
- Glu
glutamate
- GLUT
glucose transporter
- HCMV
human cytomegalovirus
- HIF-1α
hypoxia-inducible factor-1 alpha
- IDH
isocitrate dehydrogenase
- Ils
interleukins
- iNOS
inducible NO synthase
- LDHA
lactate dehydrogenase A
- Malonyl-CoA
malonyl-coenzyme A
- MAPK
mitogen-activated protein kinase
- MCT
monocarboxylate transporter
- MERS-CoV
Middle East Respiratory Syndrome coronavirus
- MOF
multi-organ failure
- mTOR
mammalian target of rapamycin
- MYC
Myelocytomatosis Viral oncogene
- NADPH,H+
nicotinamide adenine dinucleotide phosphate
- NK
natural killer
- NO
nitric oxide
- NRF2
nuclear factor erythroid 2 p45-related factor 2
- OAA
oxaloacetate
- OXPHOS
oxidative phosphorylation
- PAH
pulmonary arterial hypertension
- PC
pyruvate carboxylase
- PDGF
platelet-derived growth factor
- PDH
|pyruvate dehydrogenase
- PDK1
pyruvate dehydrogenase Kinase 1
- PFK1
phosphofructokinase1
- PI3K
Phosphoinositide 3-kinase
- PK
pyruvate kinase
- PKM2
pyruvate kinase muscle isozyme 2
- PPP
pentose phosphate pathway
- R5P
ribose 5-phosphate
- RAS
rat sarcoma viral oncogene homolog
- ROS
reactive oxygen species
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SDH
succinate dehydrogenase
- SGLT1
sodium glucose cotransporter 1
- SOD2
superoxide dismutase 2
- TCA
tricarboxylic acid cycle
- TGEV
transmissible gastroenteritis virus
- TNF-α
tumor necrosis factor-α
- VSMC
vascular smooth muscle cells
- ZIKV
Zika virus
1. Introduction
Coronavirus SARS-CoV-2 is responsible for the Coronavirus Disease 2019 (COVID-19), a viral pandemic which has resulted to approximately 1.2 million deaths worldwide as of early November 2020. Mortality rates are higher among elderly patients, especially those suffering from hypertension, obesity, diabetes, metabolic syndrome, cardiac or renal failure [1]. Available anti-viral drugs may target all stages of virus replication, from viral cell entry to the release of new viruses, but none of them have appeared to be effective. Therefore, finding more efficient strategies is urgently needed.
Considering that many viruses induce metabolic reprogramming in host cells in a similar way to the Warburg effect in cancer cells (i.e. enhancement of glycolysis with lactate production) [[2], [3], [4], [5], [6]], we examined the hypothesis that glycolytic metabolism also supports SARS-CoV-2 replication in airway cells, namely type I and type II pneumocytes which represent more than 95% of the alveolar surface [7].
Furthermore, we provide arguments supporting a role for the Warburg effect’s involvement in several steps of COVID-19 infection, as hypoxia promotes aerobic glycolysis, in particular in the endothelial cells, especially in the presence of atherosclerosis. Moreover, we will discuss how the Warburg effect supports activation of pro-inflammatory macrophages and cytotoxic immune cells against pathogens [8]. Finally, we will speculate that the switch to oxidative metabolism in macrophages implied in the anti-inflammatory response is altered and/or does not occur at the appropriate time in severe COVID-19 disease. As we will see, this switch can be altered by many factors such as chronic hypoxia, mitochondrial senescence, enzymatic dysfunctions or deregulations, AMPK and p53 inactivation. Understanding the Warburg effect in this broader perspective can be essential in developing new therapeutics for reducing infectivity and mortality.
2. The Warburg effect sustains the metabolism and replication of numerous cell types
In order to replicate, numerous cell types (cancerous or not) increase their nutrient consumption (in particular glucose and glutamine). At the beginning of the 20th century, Otto Warburg first observed that cultured cancer cells have a high rate of glycolysis and secrete lactate, even in the presence of oxygen (O2) [9]. This so-called aerobic glycolysis or “Warburg effect” has been extensively studied in cancer cells over the past years, and thus a brief presentation will facilitate further comprehension of its possible role in COVID-19.
2.1. The Warburg effect in cancer cells
Aerobic glycolysis promotes cancer cells invasiveness, aggressiveness, and drug resistance [10,11]. The shift from oxidative metabolism to increased glycolysis with lactate production is promoted by hypoxia and the hypoxia-inducible factor-1 alpha (HIF-1α) [12]. This switch is related to pyruvate dehydrogenase (PDH) inhibition by pyruvate kinase dehydrogenase 1 (PDK1), a process stimulated by HIF-1α, phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PI3K/AKT) and the mitogen-activated protein kinases/extracellular signal-regulated kinase (MAPK/ERK) signaling pathways [[13], [14], [15]]. These key signaling pathways are activated by loss of suppressors such as P53, and activation of oncogenes such as Rat Sarcoma Viral oncogene homolog (RAS) and Myelocytomatosis Viral oncogene (MYC) [16]. Importantly, it is noteworthy that together with promotion of the Warburg effect [17], PI3K/AKT pathway and phosphorylated sugars activate ATP citrate lyase (ACLY), thus linking enhancement of aerobic glycolysis and acetyl-CoA production [for references, see Ref. [18]. Acetyl-CoA molecules sustain de novo fatty acid synthesis (FAS) required for membrane formation. Lactate derived from pyruvate is expulsed in the microenvironment (ME) promoting tissue acidity and immune cells exhaustion [19].
Glycolysis and its branched metabolic pathways (such as the pentose phosphate pathway (PPP) and the serine pathway) sustain the production of molecules composing nucleotides (such as ribose 5-phosphate (R5P), glycine and methyl groups). PPP furnishes also nicotinamide adenine dinucleotide phosphate (NADPH,H+), a reduced cofactor required for FAS and redox system. As mitochondrial tricarboxylic acid (TCA) cycle functioning is downregulated by the Warburg effect, cancer cells often increase glutamine metabolism to reload the TCA cycle into α-ketoglutarate (AKG), thus furnishing molecules for biosynthesis. Glutamine metabolism also provides nitrogen groups for nucleotide synthesis and can sustain the ACLY reaction, in particular via a reversed isocitrate dehydrogenase (IDH) route [10,20]. As the Warburg effect and glutamine metabolism concomitantly sustain FAS and nucleotides synthesis, they appear as key targets for cancer cells inhibition [21].
2.2. The Warburg effect likely sustains the replication of SARS-CoV-2 in airway cells
Many viruses alter the host cell metabolism in a similar way to the Warburg effect, enhancing glycolysis and therefore producing rapid energy and “bricks” for nucleotide replication and specific protein synthesis [2,6]. Enhanced aerobic glycolysis has been shown in Zika virus (ZIKV) with up-regulation of glycolytic genes, including membrane glucose transporter 1 (GLUT1), several enzymes of glycolysis, and MCT4, the transporter expulsing lactate outside cells [6]. During infection, human cytomegalovirus (HCMV) increases the expression of glucose transporter 4 (GLUT4) which has a higher glucose transport capacity than GLUT1 [22]. In intestinal cells, the coronavirus transmissible gastroenteritis virus (TGEV) increases glucose absorption via the apical transporters Na+-dependent glucose transporter 1 (SGLT1), and the basal glucose transporter 2 (GLUT2), an uptake stimulated by the epidermal growth factor receptor (EGFR) [23].
Host cells metabolism and activation of signaling pathways is reprogrammed by viral proteins as showed in adenovirus-E4-ORF1 [24]. The two key signaling pathways - PI3/AKT/mTOR and MAPK/ERK - promote the replication of Middle East Respiratory Syndrome coronavirus (MERS-CoV) [5], enterovirus 71 (EV71) [25] and ZIKV [26]. In MERS-CoV, PI3/AKT/mTOR and MAPK/ERK inhibitors (including rapamycin) inhibit the virus replication in vitro, regardless if the inhibitors are introduced prior or after viral infection [5].
Thus, considering that SARS-CoV-2 and MERS-CoV are betacoronavirus which share numerous similarities [27], we hypothesize that SARS-CoV-2 replication is supported in airway cells by the Warburg effect promoted by PI3/AKT/mTOR and MAPK/ERK pathways (Fig. 1 ). Interestingly, during the reviewing process of this manuscript, a study reported that SARS-CoV-2 replication is supported in colon cancer cells by increasing carbon metabolism, and this replication is inhibited by 2-deoxy-d-glucose (2-DG), a glycolysis inhibitor [28]. However, the scientific demonstration of our hypothesis remains to be tested in airway cells, in which the SARS-CoV-2 virus develops. Such studies need to give priority to the following considerations: 1) that the metabolism may be cell type-dependent; 2) the Warburg effect may be activated independent of the infection itself (as an example in response to hypoxia); 3) and the virus could promote alternative pathways (in particular glutaminolysis) to adapt nutrient conditions to biosynthetic or bioenergetic demands. Thus, inhibition solely of glycolysis would be inefficient as a means to inactivate the viral replication.
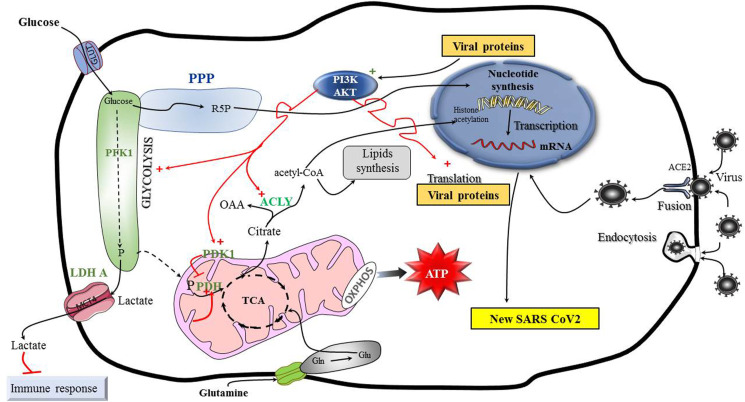
Fig. 1.
The Warburg effect likely supports replication of SARS CoV2 in airway cells.
Enhanced aerobic glycolysis sustains the replication of many viruses in a similar way to the Warburg effect. Glycolysis sustains the production of molecules required for nucleotide synthesis, such as R5P, glycine and methyl groups (not represented). This metabolism is stimulated in particular by the PI3K/AKT signaling pathway which promotes PDK1 activity. PDK1 inhibits PDH, thus blocking the entry of pyruvate into mitochondria. A part of the glucose is transformed into pyruvate, which is further transformed by LDH into lactate. This molecule is expulsed outside the cell in the microenvironment by monocarboxylate transporter 4 (MCT4) where it participates to immune response inhibition. AKT also stimulates ACLY reaction sustaining production of acetyl-CoA molecules for histone acetylation (required for transcription) and de novo FAS (required for membrane replication). ACLY also produces OAA which sustains aspartate formation required for nucleotide synthesis (not figured). Glutamine metabolism participates in supporting nucleotide synthesis and ATP production. Glutamine can also support citrate synthesis by sustaining glutamate production, and TCA cycle functioning. Of note, AKG carboxylation followed by IDH1 reverse reaction can also sustain citrate synthesis (not figured).
ACE2: viral receptor angiotensin converting enzyme 2; ACLY: ATP citrate lyase, AKT or Protein Kinase B, AKG: α-ketoglutarate, Glu: glutamate, Gln: glutamine, GLUT1: membrane glucose transporter 1, IDH1: isocitrate dehydrogenase 1, LDH: lactate dehydrogenase, MCT4: monocarboxylate transporter 4, OAA: oxaloacetate, OXPHOS: oxidative phosphorylation, P: pyruvate, PDH: pyruvate dehydrogenase, PDK1: pyruvate dehydrogenase kinase1, PI3K/AKT: phosphatidylinositol 3-kinase/protein kinase B; PFK1: phosphofructokinase1, PPP: pentose phosphate pathway, R5P: ribose 5-phosphate, TCA: tricarboxylic acid cycle.
Importantly, AMP-activated protein kinase (AMPK) is the key sensor of energy in eukaryotic cells which switches off ATP-consuming processes, and promotes oxidative functioning, in particular fatty acid oxidation (FAO) [30]. AMPK is a well-known inhibitor of the Warburg effect and of PI3K/AKT/mTOR [31]. In ZIKV, AMPK attenuates virus replication, inhibits inflammatory mediators such as tumor necrosis factor-α (TNF-α) and up-regulates genes demonstrating antiviral properties [6]. Thus, it is likely that in MERS-CoV, PI3K/AKT/mTOR and MAPK/ERK supporting viral replication, are not efficiently counteracted by AMPK. Thus, AMPK activators could be tested in laboratory studies to prevent MERS-CoV and in the current context, SARS-CoV-2 replication.
3. The Warburg effect may sustain many other aspects of COVID-19 disease
3.1. The Warburg effect is promoted in endothelial cells by hypoxia
Hypoventilation induces local pulmonary arterial vasoconstriction, redirecting the blood flow to better ventilated areas, thus maintaining oxygenation [32]. Pneumonia induces local hypoxia in pulmonary alveoli, a condition which induces the Warburg effect in endothelial cells in close contact with pneumocytes, while local acidity favors interstitial edema and stress of these cells [32]. Furthermore, hypoxia promotes vasoconstriction and thrombosis in micro-vessels [33]. Importantly, aerobic glycolysis is stimulated by PDK1 (the PDH inhibitor) and ERK in platelets, resulting in thromboxane activation and micro thrombosis [34]. In turn, platelet-derived growth factor (PDGF) activates the Warburg effect via PI3K signaling pathway and HIF-1α [34]. Extensive thrombosis can lead to pulmonary arterial hypertension (PAH) with HIF-1α activation in a feedback loop [35]. Finally, extension of infected lung areas relying on the Warburg effect can lead to severe hypoxia, PAH and acute respiratory distress syndrome (ARDS) [36,37]. It is noteworthy that AMPK likely attenuates vasoconstriction by counteracting PI3K pathway activated by PDGF, while it may counteract platelet activation by inhibiting acetyl-CoA carboxylase (ACC), the first enzyme of FAS sustaining arachidonic acid synthesis, a pathway crucial for thromboxane generation [38]. Furthermore, AMPK possibly increases the production in endothelium of angiotensin 1-7 (Ang1-7) [39], a vasodilator derived from Ang1-9 secreted by angiotensin-converting enzyme 2 (ACE2) [40]. ACE2 is the membrane receptor of SARS-CoV-2 [41], and cleavage of this receptor by viral proteins increases infectivity but functionally alters ACE2 [42]. Thus, Ang1-9 and Ang1-7 production are impaired, and Ang1-7 does not counteract the vasoconstrictor effect of angiotensin II (Ang II) on lung vessels [43,44]. As AMPK phosphorylates and stabilizes ACE2 [39], it may promote the production of Ang1-7, thus attenuating the vasoconstriction effect of Ang II.
In summary, the Warburg effect is promoted in endothelial cells of lung vessels by hypoxia and this activation sustains vasoconstriction and platelet micro thrombosis. PI3K signaling and HIF-1α are activated in a feedback loop. If this cascade is not arrested, in particular by AMPK activation, it can result in extensive lung damage.
3.2. The Warburg effect is promoted by atherosclerosis
Atherosclerosis is a vascular pathology caused by atheroma deposits in the wall of the vessels, narrowing the lumen and restricting the blood flow. This pathological injury, which increases with aging, sustains a chronic inflammatory state of the cardiovascular system [45]. It favors the Warburg effect which sustains proliferation of vascular smooth muscle cells (VSMC) [46]. Atherosclerosis appears as a common comorbidity factor in severe COVID-19 disease because it is frequently observed in patients with metabolic syndrome, diabetes, obesity, arterial hypertension, cardiovascular disorders and chronic nephropathy [1,47]. All these pathologies promote the occurrence of a low-grade inflammation state, a condition increased with aging (“inflammaging”). This chronic state of inflammation impairs the endothelial function, immune response and antioxidant defense, while it increases development of cancers [48,49].
Aging increases the Warburg effect in cells, in particular via oxidative stress and production of reactive oxygen species (ROS) promoting HIF-1α activation; mitochondrial senescence, decreased oxidative phosphorylation (OXPHOS), dysfunction of AMPK and p53 pathways conspire to promote aerobic glycolysis in elderly patients [47,50].
3.3. The Warburg effect supports the pro-inflammatory innate and adaptive immune response
The Warburg effect also sustains defense against bacterial and virus infection. Indeed, aerobic glycolysis supports activation and proliferation of neutrophils and activation of M1 macrophages, all cells involved in the innate defense [8,51]. These cells rely on high consumption of glucose to sustain a rapid and efficient response against microbes with secretion of high amounts of nitric oxide (NO), pro-inflammatory lipid molecules (as eicosanoids), and cytokines, in particular of interleukins (Ils) (as IL-1 and IL-6) and TNF-α [52,53]. Furthermore, aerobic glycolysis sustains the secondary proliferation of cytotoxic and B lymphocytes which have been activated by antigens presented by macrophages and dendritic cells [54]. On the contrary, the activation of the anti-inflammatory response with secretion of IL-10 is mainly supported by M2 macrophages (M2) whose metabolism principally relies on FAO activation [52,53,55]. This efficient way of ATP production sustains the cleaning and recycling processes ensured by M2, in particular autophagy [53] (Fig. 2 ). Importantly, M1 versus M2 activation is regulated by two enzymatic pathways competing for arginine, namely the inducible NO synthase (iNOS) and the arginase pathways. These pathways inhibit each other, since they are regulated by two opposite signaling: i) the PI3K/AKT/mTOR sustains the iNOS axis in M1 which produces high levels of NO and pro-inflammatory molecules [52,53,56]; - ii) the AMPK signaling activates in M2 the Arginase 1 (ARG1) reaction which hydrolyzes l-arginine into urea and ornithine; this latter molecule is a precursor of proline and polyamines, which are involved in tissue repair and wound healing [52,53]. In addition to these opposing functions, there would also be regulation of the TCA cycle exercised at the level of the mitochondrial aconitase (ACO2) [57]: NO inhibits this enzyme, which results in a “truncated” TCA cycle [58], a process reinforced by isocitrate dehydrogenase 2 (IDH2) inactivation [59]. Decarboxylation of aconitate by CAD (cis-Aconitate decarboxylase) (also name as immunoresponsive gene 1 (IRG1), or ACOD1) increases itaconate concentration in mitochondria, resulting in succinate dehydrogenase (SDH) inhibition and HIF-1α activation [60]. ACO2 and/or IDH2 inhibition promotes citrate mitochondrial export in M1. Acetyl-CoA sustains histone acetylation (promoting transcription of genes sustaining inflammatory response) and also the production of pro-inflammatory lipids.
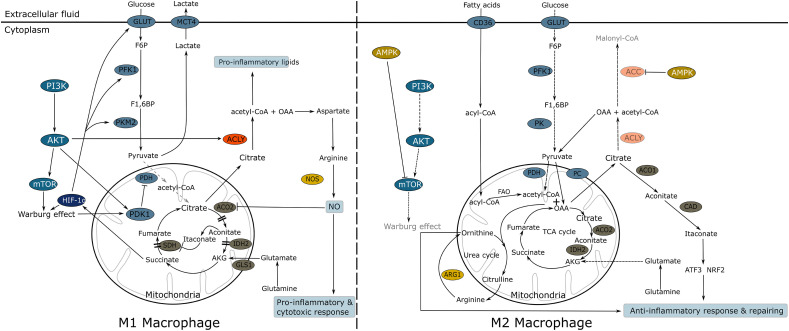
Fig. 2.
Schematic view of M1 and M2 macrophage metabolism.
Left side, M1 macrophage triggers a pro-inflammatory response. The Warburg effect is activated by PI3K/AKT/mTor signaling pathway, which concomittantly activates FAS sustaining proinflammatory molecules synthesis (leucotriens and arachidonic acid). OAA sustains arginosuccinate (not figured) and arginine production, leading to NO formation by iNOS. The expression of PK in its embryonic form promotes the functioning of branched pathways on glycolysis upstream, as PPP furnishing NADPH,H+ for iNOS functioning (not figured). ACO2 inhibition by NO, results in a “truncated” TCA cycle: citrate is exported in the cytosol, further sustaining ACLY functioning and the production of pro-inflammatory lipids. In mitochondria, decarboxylation of aconitate in itaconate, results in succinate dehydrogenase (SDH) inhibition, a process favoring HIF-1α activation promoting glycolysis.
Right side, M2 macrophage triggers an anti-inflammatory response. AMPK inhibits PI3K/AKT: glycolysis and FAS are thus downregulated or blocked. AMPK also inhibits ACC, the first enzyme of FAS. FAO is promoted and feeds the TCA cycle, providing great amount of acetyl-coA, ATP and NADH,H+. PDH is blocked by NADH,H+ and acetyl-CoA, while pyruvate carboxylase (PC) is activated, regenerating OAA for TCA functioning. Cytosolic pyruvate (derived from glycolysis or OAA transformation) sustains PC activity. In mitochondria, OAA condensates with acetyl-coA to form citrate. High amount of citrate is produced and exported in the cytosol. Citrate sustains the production of itaconate, an anti-inflammatory molecule promoting activation of NRF2 and ATF3. Concomitantly, the break exercised by NO on ACO2 is released, allowing complete TCA functioning. The urea cycle – coupled with TCA –sustains the production of arginine, transformed by ARG1 into ornithine required for repairing (proline and polyamine synthesis).
ACLY: ATP citrate lyase, ACC: acetyl-CoA carboxylase, ACO2: aconitase 2, ARG1: arginase 1, AKT or Protein Kinase B, AKG: α-ketoglutarate, AMPK: AMP-activated protein kinase, FAO: Fatty acid β-oxidation, CAD: cis-Aconitate decarboxylase, also known as ACOD1 or Irg1, FAS: Fatty acid synthesis, F6P: fructose 6-phosphate, F1,6P: fructose-1,6-bisphosphate, GLUT: membrane glucose transporter, IDH2: isocitrate dehydrogenase 2, HIF-1α: Hypoxia-inducible factor-1α, MCT4: monocarboxylate transporter 4, NADPH,H+: nicotinamide adenine dinucleotide phosphate, NRF2: nuclear factor erythroid 2-related factor 2, iNOS: inducible nitric oxide synthase, NOS: nitric oxide synthase, OAA: oxaloacetate, PC: pyruvate carboxylase, PDH: pyruvate dehydrogenase, PDK1: pyruvate dehydrogenase kinase1, PFK1: phosphofructokinase1, PI3K/AKT: phosphatidylinositol 3-kinase, PKM2: pyruvate kinase embryonic form, PK; pyruvate kinase, SDH: succinate dehydrogenase, TCA: tricarboxylic acid cycle.
In contrast, in M2 macrophages, AMPK inhibits FAS (in particular by inactivation of ACC), and citrate feeds the cytosolic production of itaconitate. This molecule promotes activation of ATF3 (a negative regulator of cytokines production as IL-6) and of nuclear factor erythroid 2-related factor 2 (NRF2) inducing resolution of inflammation and repair [61,62] (Fig. 2).
Thus schematically, a truncated TCA cycle (in relation to NO production) sustains metabolism of pro-inflammatory M1 whereas oxidative metabolism (supported by FAO) sustains anti-inflammatory M2 activation. It is tempting to speculate that the deregulation or alteration of the metabolic shift from M1 to M2 is a major event in development of the uncontrolled pro-inflammatory response observed in severe COVID-19 patients. Aging, diabetes, oxidative damage, AMPK deregulation, loss of p53 (frequently observed in cancer) can impact or disrupt this shift [63,64]. The complex mechanisms regulating ACO1 and ACO2, INOS and ARG1, itaconitate production, ornithine and NO synthesis, remain to be studied [58,65,66].
4. Discussion and therapeutic perspectives
As we have seen, SARS-CoV2 replication is likely supported by the Warburg effect in cells expressing ACE2, particularily in nasal epithelial and pneumocytic cells which represent the body’s airway entry site for the virus. This hypothesis has been recently reinforced by studies showing that increasing glycolysis supports the virus replication in colon cancer cells and in blood monocytes [28,67]. In monocytes, SARS-CoV2 replication is arrested when pyruvate is only given, this substrate sustaining TCA cycle and oxidative metabolism [67]. This observation provides arguments to consider that hypoxia - the major inducer of the Warburg effect - is an essential condition for promoting SARS-CoV-2 replication. Of note, pneumonia is usually first seen in the basal part of lungs with the lowest ventilation/perfusion ratio [[1], [32]].
In most patients, COVID-19 infection is quickly controlled and recovery is fairly rapid, as the virus is effectively fought by neutrophils and mobile monocytes/macrophages which migrate to the sites of inflammation. This pro-inflammatory innate response (representing the first line of defense of the immune system) with high secretion levels of NO, pro-inflammatory lipids, IL-1, Il-6 and TNF-α, is further followed by dendritic cell (DC) activation. Macrophages and DCs present antigens, a process leading to T cytotoxic lymphocytes and B lymphocytes activation with development of a memory response. Of note, all these activations are supported by the Warburg effect [68]. Thereafter, the anti-inflammatory and restorative healing response occurs, promoted by M2 macrophages and IL-10. As we have seen, AMPK signaling likely plays a key role in the coordination and regulation of the shift towards the anti-inflammatory and repairing response. Indeed, AMPK inhibits PI3K/AKT, and therefore down regulates aerobic glycolysis, FAS and NO synthesis. P53 deregulation (especially promoted by cancer and aging), may also impact the shift towards the anti-inflammatory response. In consequence, the pro-inflammatory response is triggered and the cytokine storm develops with abnormal IL-1, IL-6 and TNF-α secretion, promoting in turn the Warburg effect [69]. The uncontrolled cytokine cascade and extensive micro-vessel thrombosis (also supported by the Warburg effect) conspires to promote the occurrence of ARDS, lung destruction and multi-organ failure (MOF). Further studies should clarify the mechanisms altering the shift from M1 to M2 phenotypes. Of note, Zinc is required for ACE2, ACO2 and mitochondrial superoxide dismutase 2 (SOD2) functioning. This latter enzyme reduces oxidative damage created by reactive oxygen ion superoxide, in particular on enzymes such as ACO2, an iron sulfur cluster containing enzyme sensitive to oxidation and linked to diabetes [66]. Thus, correction of Zinc deficiency could be important especially in diabetic and elderly patients [64,70].
In clinical research, the metabolic phenotype of lung and blood macrophages could be studied by using markers reflecting either glycolytic function (such as expression of MCT4) or oxidative function (such as expression of MCT1) [71].
Recent in vitro studies performed on blood monocytes argue that the cytokine cascade is the result of the virus replication inside monocytes and macrophages, a replication favored by increasing glucose concentration (and thus by diabetes), and promoting the secretion of inflammatory cytokines by these cells [67]. Blood monocytes could be the cell mediator of the cytokine cascade and/or multi-organ dissemination of the virus. Since only 1% of patients had detectable levels of SARS-CoV-2 in the blood, this suggests that viremia does not underlie the dissemination process [72,73]. Further in vivo studies should specify the sequence of contamination, as the virus enters the body mainly through respiratory tract cells and ileal absorptive enterocytes [29].
It is noteworthy that the Warburg effect may down regulate the adaptive immune response, if we consider that the increase acidity in the extracellular compartment favors inhibition of the cytotoxic immune response in cancer studies [for references, see 10]. In favor, an increased serum lactate dehydrogenase (LDH) level reflects the severity of COVID-19 [74], as well as lymphopenia which means a decreased number of NK cells and cytotoxic T lymphocytes [75,76]. Impairment of immune defense can increase sensitivity to bacterial co-infection, and in this setting overcoming the Warburg Effect has been considered as a key factor to improve tolerance to septic shock, often resulting in MOF and death [77].
As we have seen, atherosclerosis is a pathologic feature frequently observed in elderly patients, a process promoting the Warburg effect in endothelial and smooth muscular cells of the vessels. This pathologic process is favored by many factors (diabetes, obesity, metabolic syndrome, lipids abnormalities) inducing a state of low-grade inflammation, a condition increasing with aging. In parallel, elderly patients have a global reduction in the capacity to cope with infection, which is exemplified by the mortality rate: from 1 to 3% in patients between 50 and 59 years, it increases to 9.8% from 70 to 79 years and it is approximately 14% for around 85 years old [1].
From a therapeutic point of view, counteracting the metabolism sustaining SARS-CoV-2 replication and/or macrophages activation can be essential. AMPK activators such as metformin [78,79], lipoic acid [80], resveratrol [81], and ivermectin [82] should be tested in vitro and in vivo in a preventive or curative intent. Interestingly, lipoic acid also inhibits furin, a convertase involved in increasing SARS-CoV-2 infectivity and virulence [80,83], while ivermectin inhibits the virus replication in vitro [84].
Obviously, glucose transporter (GLUT) inhibitors should be tested, as well as phlorizin, a molecule which inhibits glucose absorption by the apical transporters Na+-dependent glucose transporter 1 (SGLT1) of intestinal cells, as showed with the coronavirus transmissible gastroenteritis virus (TGEV) [23]. This molecule also inhibits glucose uptake by the alveolar-airway barrier [85]. Consideration should be given to inhibitors of aerobic glycolysis and its branched pathways ;as well as FAS-sustained membrane replication [86]. The effect of glutamine metabolism inhibitors should be studied in particular with respect to the metabolic vulnerabilities of SARS-CoV-2 replication. In the past, l-asparaginase greatly increased the efficiency of anti-nucleotide agents in acute leukemia. However, targeting one specific pathway may result in modest viral replication inhibition, as the virus can up regulate or become dependent on alternative pathway(s) to meet bioenergetic or biosynthetic needs. As an example, human fibroblasts infected with HCMV failed to produce virions when starved for glutamine 24 h after infection [87]. Such resistance can be favored by the competition for nutrients occurring at the site of inflammation, a process well-observed in the microenvironment of cancer cells [88]. A (non-exhaustive) list of candidate inhibitors (well-studied in cancer experiments) is presented in Table 1 . Animal models like ferret or hamster model of Covid-19 can be useful for testing molecules because in vitro studies are not always confirmed by in vivo studies.
Table 1.
Non-exhaustive list of glycolysis, glutaminolysis, and fatty acid synthesis inhibitors.
| Pathway targeted | Inhibitors |
|---|---|
| Glycolysis | |
| GLUTs SGLTs |
Fasentin, Phloretin (GLUT2 inhibitor), Ritonavir (GLUT4 inhibitor), Silybin/Silibinin, STF-31 (GLUT1 inhibitor) Phloridzin (SGLT1 inhibitor), Dapagliflozin (SGLT2 inhibitor) |
| HK2 | Astragalin, Benserazide, 2-deoxyglucose, Genistein-27, Lonidamine, Resveratrol |
| PFK1 PFK2/PFKFB3 GAPDH PKM2 LDH-A |
Citrate sodium, Sulforaphane 3PO, PFK15 3-bromopyruvate (inhibits also HK2, PGK1, IDH) Resveratrol, Apigenin FX11, Oxamate |
| Inhibiting the Warburg effect by reconnecting TCA cycle | |
| PDH activation | Lipoïc acid |
| PDK inhibition | Dichloroacetate |
| AMPK activator | Metformine, Lipoïc acid, Resveratrol, Ivermectin |
| Lactate exchanges | |
| MCTs | AZD-3965, Oxamate |
| glutaminolyis | |
|
ASCT2 (SLC1A5) |
Benzylserine, GPNA,V-9302 |
| GLS1 | Azaserine, Acivicin, BPTES, CB-839, DON, Zaprinast |
| IDH | IDH305, Olutasidenib, AG-120 (IDH1 inhibitor), AG-221 |
| Lipid synthesis | |
| ACLY | Bempedoic acid, Cucurbitacin B, Hydroxycitrate |
| FAS inhibition | TVB-2640, Cerulenin, Epigallocatechin Gallate, Orlistat |
| Mevalonate and cholesterol synthesis | Statins |
ACLY: ATP citrate lyase; AMPK: AMP-activated protein kinase; ASCT2: glutamine transporter 2, BPTES: Bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide; DON: 6-Diazo-5-oxo-l-norleucine; FAS: fatty acid synthesis; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GLUTs: glucose transporters; HK2: hexokinase 2; IDH: isocitrate dehydrogenase; LDH-A: lactate dehydrogenase A; PDH: pyruvate dehydrogenase, PDK: pyruvate kinase dehydrogenase; PFK: phosphofructokinase; PFK15: 1-(4-pyridinyl)-3-(2-quinolinyl)-2-propen-1-one; PGK1: phosphoglycerate kinase1; PKM2: pyruvate kinase M 2; 3PO: 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one, SGLT1: Sodium dependent glucose transporter 1.
Finally, in the context of the current pandemic and in the perspective of new ones, the exploration of all aspects of the Warburg effect in COVID-19 is certainly fundamental to the discovery of new treatments.
Authors’ contribution
Philippe Icard: conception, writing, revision; Hubert Lincet: figure design and revision; Zherui Wu: figure and table design; Antoine Coquerel: literature search and revision; Patricia Forgez: literature search and revision; Marco Alifano: revision and supervision; Ludovic Fournel: editing and revision.
Declaration of competing interest
The authors have no conflict of interest to declare.
Acknowledgments
The authors want to thank Diana Berzan, Antonin Ginguay, Yen-Lan Nguyen and Mauro Loi for their contribution in references search and English language editing.
References
- 1.Siordia J.A. Epidemiology and clinical features of COVID-19: a review of current literature. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2020;127:104357. doi: 10.1016/j.jcv.2020.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchez E.L., Lagunoff M. Viral activation of cellular metabolism. Virology. 2015;479–480:609–618. doi: 10.1016/j.virol.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilz N.C., Jahn K., Lorenz M., Lüdtke A., Hübschen J.M., Geyer H., Mankertz A., Hübner D., Liebert U.G., Claus C. Rubella viruses shift cellular bioenergetics to a more oxidative and glycolytic phenotype with a strain-specific requirement for glutamine. J. Virol. 2018;92 doi: 10.1128/JVI.00934-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramière C., Rodriguez J., Enache L.S., Lotteau V., André P., Diaz O. Activity of hexokinase is increased by its interaction with hepatitis C virus protein NS5A. J. Virol. 2014;88:3246–3254. doi: 10.1128/JVI.02862-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kindrachuk J., Ork B., Hart B.J., Mazur S., Holbrook M.R., Frieman M.B., Traynor D., Johnson R.F., Dyall J., Kuhn J.H., Olinger G.G., Hensley L.E., Jahrling P.B. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob. Agents Chemother. 2015;59:1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh S., Singh P.K., Suhail H., Arumugaswami V., Pellett P.E., Giri S., Kumar A. AMP-activated protein kinase restricts Zika virus replication in endothelial cells by potentiating innate antiviral responses and inhibiting glycolysis. J. Immunol. Baltim. Md. 2020;1950(204):1810–1824. doi: 10.4049/jimmunol.1901310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elsevier; 2020. Anatomy and histology of the laboratory rat in toxicology and biomedical research - agricultural and biological sciences textbooks.https://textbooks.elsevier.com/web/product_details.aspx?isbn=9780128118375 (n.d.) [Google Scholar]
- 8.Nonnenmacher Y., Hiller K. Biochemistry of proinflammatory macrophage activation. Cell. Mol. Life Sci. CMLS. 2018;75:2093–2109. doi: 10.1007/s00018-018-2784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 10.Icard P., Lincet H. A global view of the biochemical pathways involved in the regulation of the metabolism of cancer cells. Biochim. Biophys. Acta. 2012;1826:423–433. doi: 10.1016/j.bbcan.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Heiden M.G.V., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu H., Forbes R.A., Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J. Biol. Chem. 2002;277:23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 13.Kim J., Tchernyshyov I., Semenza G.L., Dang C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metabol. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Semenza G.L. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov. Today. 2007;12:853–859. doi: 10.1016/j.drudis.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Chae Y.C., Vaira V., Caino M.C., Tang H.-Y., Seo J.H., Kossenkov A.V., Ottobrini L., Martelli C., Lucignani G., Bertolini I., Locatelli M., Bryant K.G., Ghosh J.C., Lisanti S., Ku B., Bosari S., Languino L.R., Speicher D.W., Altieri D.C. Mitochondrial akt regulation of hypoxic tumor reprogramming. Canc. Cell. 2016;30:257–272. doi: 10.1016/j.ccell.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stine Z.E., Walton Z.E., Altman B.J., Hsieh A.L., Dang C.V. MYC, metabolism, and cancer. Canc. Discov. 2015;5:1024–1039. doi: 10.1158/2159-8290.CD-15-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courtnay R., Ngo D.C., Malik N., Ververis K., Tortorella S.M., Karagiannis T.C. Cancer metabolism and the Warburg effect: the role of HIF-1 and PI3K. Mol. Biol. Rep. 2015;42:841–851. doi: 10.1007/s11033-015-3858-x. [DOI] [PubMed] [Google Scholar]
- 18.Icard P., Wu Z., Fournel L., Coquerel A., Lincet H., Alifano M. ATP citrate lyase: a central metabolic enzyme in cancer. Canc. Lett. 2020;471:125–134. doi: 10.1016/j.canlet.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Husain Z., Huang Y., Seth P., Sukhatme V.P. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J. Immunol. Baltim. Md. 2013;1950(191):1486–1495. doi: 10.4049/jimmunol.1202702. [DOI] [PubMed] [Google Scholar]
- 20.DeBerardinis R.J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C.B. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. U.S.A. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akins N.S., Nielson T.C., Le H.V. Inhibition of glycolysis and glutaminolysis: an emerging drug discovery approach to combat cancer. Curr. Top. Med. Chem. 2018;18:494–504. doi: 10.2174/1568026618666180523111351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu Y., Maguire T.G., Alwine J.C. Human cytomegalovirus activates glucose transporter 4 expression to increase glucose uptake during infection. J. Virol. 2011;85:1573–1580. doi: 10.1128/JVI.01967-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai L., Hu W.W., Xia L., Xia M., Yang Q. Transmissible gastroenteritis virus infection enhances SGLT1 and GLUT2 expression to increase glucose uptake. PloS One. 2016;11 doi: 10.1371/journal.pone.0165585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frese K.K., Lee S.S., Thomas D.L., Latorre I.J., Weiss R.S., Glaunsinger B.A., Javier R.T. Selective PDZ protein-dependent stimulation of phosphatidylinositol 3-kinase by the adenovirus E4-ORF1 oncoprotein. Oncogene. 2003;22:710–721. doi: 10.1038/sj.onc.1206151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong W.-R., Chen Y.-Y., Yang S.-M., Chen Y.-L., Horng J.-T. Phosphorylation of PI3K/Akt and MAPK/ERK in an early entry step of enterovirus 71. Life Sci. 2005;78:82–90. doi: 10.1016/j.lfs.2005.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scaturro P., Stukalov A., Haas D.A., Cortese M., Draganova K., Płaszczyca A., Bartenschlager R., Götz M., Pichlmair A. An orthogonal proteomic survey uncovers novel Zika virus host factors. Nature. 2018;561:253–257. doi: 10.1038/s41586-018-0484-5. [DOI] [PubMed] [Google Scholar]
- 27.Rabaan A.A., Al-Ahmed S.H., Haque S., Sah R., Tiwari R., Malik Y.S., Dhama K., Yatoo M.I., Bonilla-Aldana D.K., Rodriguez-Morales A.J. SARS-CoV-2, SARS-CoV, and MERS-COV: a comparative overview. Inf. Med. 2020;28:174–184. [PubMed] [Google Scholar]
- 28.Bojkova D., Klann K., Koch B., Widera M., Krause D., Ciesek S., Cinatl J., Münch C. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020;583:469–472. doi: 10.1038/s41586-020-2332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., Feldman J., Muus C., Wadsworth M.H., Kazer S.W., Hughes T.K., Doran B., Gatter G.J., Vukovic M., Taliaferro F., Mead B.E., Guo Z., Wang J.P., Gras D., Plaisant M., Ansari M., Angelidis I., Adler H., Sucre J.M.S., Taylor C.J., Lin B., Waghray A., Mitsialis V., Dwyer D.F., Buchheit K.M., Boyce J.A., Barrett N.A., Laidlaw T.M., Carroll S.L., Colonna L., Tkachev V., Peterson C.W., Yu A., Zheng H.B., Gideon H.P., Winchell C.G., Lin P.L., Bingle C.D., Snapper S.B., Kropski J.A., Theis F.J., Schiller H.B., Zaragosi L.-E., Barbry P., Leslie A., Kiem H.-P., Flynn J.L., Fortune S.M., Berger B., Finberg R.W., Kean L.S., Garber M., Schmidt A.G., Lingwood D., Shalek A.K., Ordovas-Montanes J. HCA lung biological network. Electronic address: lung-network@humancellatlas.org, HCA lung biological network, SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035. doi: 10.1016/j.cell.2020.04.035. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardie D.G., Schaffer B.E., Brunet A. AMPK: an energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 2016;26:190–201. doi: 10.1016/j.tcb.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faubert B., Boily G., Izreig S., Griss T., Samborska B., Dong Z., Dupuy F., Chambers C., Fuerth B.J., Viollet B., Mamer O.A., Avizonis D., DeBerardinis R.J., Siegel P.M., Jones R.G. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metabol. 2013;17:113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liljestrand G. Chemical control of the distribution of the pulmonary blood flow. Acta Physiol. Scand. 1958;44:216–240. doi: 10.1111/j.1748-1716.1958.tb01623.x. [DOI] [PubMed] [Google Scholar]
- 33.Gupta N., Zhao Y.-Y., Evans C.E. The stimulation of thrombosis by hypoxia. Thromb. Res. 2019;181:77–83. doi: 10.1016/j.thromres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Manne B.K., Münzer P., Badolia R., Walker-Allgaier B., Campbell R.A., Middleton E., Weyrich A.S., Kunapuli S.P., Borst O., Rondina M.T. PDK1 governs thromboxane generation and thrombosis in platelets by regulating activation of Raf 1 in the MAPK pathway. J. Thromb. Haemost. JTH. 2018;16:1211–1225. doi: 10.1111/jth.14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dabral S., Muecke C., Valasarajan C., Schmoranzer M., Wietelmann A., Semenza G.L., Meister M., Muley T., Seeger-Nukpezah T., Samakovlis C., Weissmann N., Grimminger F., Seeger W., Savai R., Pullamsetti S.S. A RASSF1A-HIF1α loop drives Warburg effect in cancer and pulmonary hypertension. Nat. Commun. 2019;10:2130. doi: 10.1038/s41467-019-10044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng H., Xiao Y., Deng X., Luo J., Hong C., Qin X. The Warburg effect: a new story in pulmonary arterial hypertension. Clin. Chim. Acta Int. J. Clin. Chem. 2016;461:53–58. doi: 10.1016/j.cca.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lepropre S., Kautbally S., Octave M., Ginion A., Onselaer M.-B., Steinberg G.R., Kemp B.E., Hego A., Wéra O., Brouns S., Swieringa F., Giera M., Darley-Usmar V.M., Ambroise J., Guigas B., Heemskerk J., Bertrand L., Oury C., Beauloye C., Horman S. AMPK-ACC signaling modulates platelet phospholipids and potentiates thrombus formation. Blood. 2018;132:1180–1192. doi: 10.1182/blood-2018-02-831503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J., Dong J., Martin M., He M., Gongol B., Marin T.L., Chen L., Shi X., Yin Y., Shang F., Wu Y., Huang H.-Y., Zhang J., Zhang Y., Kang J., Moya E.A., Huang H.-D., Powell F.L., Chen Z., Thistlethwaite P.A., Yuan Z.-Y., Shyy J.Y.-J. AMP-activated protein kinase phosphorylation of angiotensin-converting enzyme 2 in endothelium mitigates pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2018;198:509–520. doi: 10.1164/rccm.201712-2570OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 41.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2014;88:1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shenoy V., Qi Y., Katovich M.J., Raizada M.K. ACE2, a promising therapeutic target for pulmonary hypertension. Curr. Opin. Pharmacol. 2011;11:150–155. doi: 10.1016/j.coph.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alifano M., Alifano P., Forgez P., Iannelli A. Renin-angiotensin system at the heart of COVID-19 pandemic. Biochimie. 2020;174:30–33. doi: 10.1016/j.biochi.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Z., Liu M., Li L., Chen L. Involvement of the Warburg effect in non-tumor diseases processes. J. Cell. Physiol. 2018;233:2839–2849. doi: 10.1002/jcp.25998. [DOI] [PubMed] [Google Scholar]
- 46.Werle M., Kreuzer J., Höfele J., Elsässer A., Ackermann C., Katus H.A., Vogt A.M. Metabolic control analysis of the Warburg-effect in proliferating vascular smooth muscle cells. J. Biomed. Sci. 2005;12:827–834. doi: 10.1007/s11373-005-9010-5. [DOI] [PubMed] [Google Scholar]
- 47.Katsuumi G., Shimizu I., Yoshida Y., Minamino T. The pathological role of vascular aging in cardio-metabolic disorder. Inflamm. Regen. 2016;36:16. doi: 10.1186/s41232-016-0021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baylis D., Bartlett D.B., Patel H.P., Roberts H.C. Understanding how we age: insights into inflammaging. Longev. Heal. 2013;2:8. doi: 10.1186/2046-2395-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varricchi G., Bencivenga L., Poto R., Pecoraro A., Shamji M.H., Rengo G. The emerging role of T follicular helper (TFH) cells in aging: influence on the immune frailty. Ageing Res. Rev. 2020:101071. doi: 10.1016/j.arr.2020.101071. [DOI] [PubMed] [Google Scholar]
- 50.Lee H.-C., Wei Y.-H. Mitochondria and aging. Adv. Exp. Med. Biol. 2012;942:311–327. doi: 10.1007/978-94-007-2869-1_14. [DOI] [PubMed] [Google Scholar]
- 51.Awasthi D., Nagarkoti S., Sadaf S., Chandra T., Kumar S., Dikshit M. Glycolysis dependent lactate formation in neutrophils: a metabolic link between NOX-dependent and independent NETosis. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2019;1865:165542. doi: 10.1016/j.bbadis.2019.165542. [DOI] [PubMed] [Google Scholar]
- 52.Mills C.D. M1 and M2 macrophages: oracles of health and disease. Crit. Rev. Immunol. 2012;32:463–488. doi: 10.1615/critrevimmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 53.O’Neill L.A.J., Pearce E.J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016;213:15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun H., Li X. Metabolic reprogramming in resting and activated immune cells. Metabolomics Open Access. 2017;7 doi: 10.4172/2153-0769.1000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu P.-S., Wang H., Li X., Chao T., Teav T., Christen S., Di Conza G., Cheng W.-C., Chou C.-H., Vavakova M., Muret C., Debackere K., Mazzone M., Huang H.-D., Fendt S.-M., Ivanisevic J., Ho P.-C. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 2017;18:985–994. doi: 10.1038/ni.3796. [DOI] [PubMed] [Google Scholar]
- 56.Rath M., Müller I., Kropf P., Closs E.I., Munder M. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front. Immunol. 2014;5:532. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tong W.-H., Rouault T.A. Metabolic regulation of citrate and iron by aconitases: role of iron-sulfur cluster biogenesis. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2007;20:549–564. doi: 10.1007/s10534-006-9047-6. [DOI] [PubMed] [Google Scholar]
- 58.Palmieri E.M., Gonzalez-Cotto M., Baseler W.A., Davies L.C., Ghesquière B., Maio N., Rice C.M., Rouault T.A., Cassel T., Higashi R.M., Lane A.N., Fan T.W.-M., Wink D.A., McVicar D.W. Nitric oxide orchestrates metabolic rewiring in M1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat. Commun. 2020;11:698. doi: 10.1038/s41467-020-14433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jha A.K., Huang S.C.-C., Sergushichev A., Lampropoulou V., Ivanova Y., Loginicheva E., Chmielewski K., Stewart K.M., Ashall J., Everts B., Pearce E.J., Driggers E.M., Artyomov M.N. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 60.Lampropoulou V., Sergushichev A., Bambouskova M., Nair S., Vincent E.E., Loginicheva E., Cervantes-Barragan L., Ma X., Huang S.C.-C., Griss T., Weinheimer C.J., Khader S., Randolph G.J., Pearce E.J., Jones R.G., Diwan A., Diamond M.S., Artyomov M.N. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metabol. 2016;24:158–166. doi: 10.1016/j.cmet.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luan H.H., Medzhitov R. Food fight: role of itaconate and other metabolites in antimicrobial defense. Cell Metabol. 2016;24:379–387. doi: 10.1016/j.cmet.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mills E.L., Ryan D.G., Prag H.A., Dikovskaya D., Menon D., Zaslona Z., Jedrychowski M.P., Costa A.S.H., Higgins M., Hams E., Szpyt J., Runtsch M.C., King M.S., McGouran J.F., Fischer R., Kessler B.M., McGettrick A.F., Hughes M.M., Carroll R.G., Booty L.M., Knatko E.V., Meakin P.J., Ashford M.L.J., Modis L.K., Brunori G., Sévin D.C., Fallon P.G., Caldwell S.T., Kunji E.R.S., Chouchani E.T., Frezza C., Dinkova-Kostova A.T., Hartley R.C., Murphy M.P., O’Neill L.A. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556:113–117. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan L.J., Levine R.L., Sohal R.S. Oxidative damage during aging targets mitochondrial aconitase. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11168–11172. doi: 10.1073/pnas.94.21.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin G., Brownsey R.W., MacLeod K.M. Regulation of mitochondrial aconitase by phosphorylation in diabetic rat heart. Cell. Mol. Life Sci. CMLS. 2009;66:919–932. doi: 10.1007/s00018-009-8696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bailey J.D., Diotallevi M., Nicol T., McNeill E., Shaw A., Chuaiphichai S., Hale A., Starr A., Nandi M., Stylianou E., McShane H., Davis S., Fischer R., Kessler B.M., McCullagh J., Channon K.M., Crabtree M.J. Nitric oxide modulates metabolic remodeling in inflammatory macrophages through TCA cycle regulation and itaconate accumulation. Cell Rep. 2019;28:218–230. doi: 10.1016/j.celrep.2019.06.018. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lushchak O.V., Piroddi M., Galli F., Lushchak V.I. Aconitase post-translational modification as a key in linkage between Krebs cycle, iron homeostasis, redox signaling, and metabolism of reactive oxygen species. Redox Rep. Commun. Free Radic. Res. 2014;19:8–15. doi: 10.1179/1351000213Y.0000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Codo A.C., Davanzo G.G., Monteiro L.de B., de Souza G.F., Muraro S.P., Virgilio-da-Silva J.V., Prodonoff J.S., Carregari V.C., de Biagi Junior C.A.O., Crunfli F., Jimenez Restrepo J.L., Vendramini P.H., Reis-de-Oliveira G., Bispo Dos Santos K., Toledo-Teixeira D.A., Parise P.L., Martini M.C., Marques R.E., Carmo H.R., Borin A., Coimbra L.D., Boldrini V.O., Brunetti N.S., Vieira A.S., Mansour E., Ulaf R.G., Bernardes A.F., Nunes T.A., Ribeiro L.C., Palma A.C., Agrela M.V., Moretti M.L., Sposito A.C., Pereira F.B., Velloso L.A., Vinolo M.A.R., Damasio A., Proença-Módena J.L., Carvalho R.F., Mori M.A., Martins-de-Souza D., Nakaya H.I., Farias A.S., Moraes-Vieira P.M. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metabol. 2020;32:437–446. doi: 10.1016/j.cmet.2020.07.007. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palsson-McDermott E.M., O’Neill L.A.J. The Warburg effect then and now: from cancer to inflammatory diseases. BioEssays News Rev. Mol. Cell. Dev. Biol. 2013;35:965–973. doi: 10.1002/bies.201300084. [DOI] [PubMed] [Google Scholar]
- 69.Tarasenko T.N., Jestin M., Matsumoto S., Saito K., Hwang S., Gavrilova O., Trivedi N., Zerfas P.M., Barca E., DiMauro S., Senac J., Venditti C.P., Cherukuri M., McGuire P.J. Macrophage derived TNFα promotes hepatic reprogramming to Warburg-like metabolism. J. Mol. Med. Berl. Ger. 2019;97:1231–1243. doi: 10.1007/s00109-019-01786-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wessels I., Rolles B., Rink L. The potential impact of Zinc supplementation on COVID-19 pathogenesis. Front. Immunol. 2020;11:1712. doi: 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mikkilineni L., Whitaker-Menezes D., Domingo-Vidal M., Sprandio J., Avena P., Cotzia P., Dulau-Florea A., Gong J., Uppal G., Zhan T., Leiby B., Lin Z., Pro B., Sotgia F., Lisanti M.P., Martinez-Outschoorn U. Hodgkin lymphoma: a complex metabolic ecosystem with glycolytic reprogramming of the tumor microenvironment. Semin. Oncol. 2017;44:218–225. doi: 10.1053/j.seminoncol.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. J. Am. Med. Assoc. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 74.Sun C., Zhang X.B., Dai Y., Xu X.Z., Zhao J. [Clinical analysis of 150 cases of 2019 novel coronavirus infection in nanyang city, henan province], zhonghua jie He He hu xi za zhi zhonghua jiehe He huxi zazhi chin. J. Tuberc. Respir. Dis. 2020;43:E042. doi: 10.3760/cma.j.cn112147-20200224-00168. [DOI] [PubMed] [Google Scholar]
- 75.Zheng Y., Zhang Y., Chi H., Chen S., Peng M., Luo L., Chen L., Li J., Shen B., Wang D. The hemocyte counts as a potential biomarker for predicting disease progression in COVID-19: a retrospective study. Clin. Chem. Lab. Med. 2020;58:1106–1115. doi: 10.1515/cclm-2020-0377. [DOI] [PubMed] [Google Scholar]
- 76.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bar-Or D., Carrick M., Tanner A., Lieser M.J., Rael L.T., Brody E. Overcoming the Warburg effect: is it the key to survival in sepsis? J. Crit. Care. 2018;43:197–201. doi: 10.1016/j.jcrc.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 78.Guimarães T.A., Farias L.C., Santos E.S., de Carvalho Fraga C.A., Orsini L.A., de Freitas Teles L., Feltenberger J.D., de Jesus S.F., de Souza M.G., Santos S.H.S., de Paula A.M.B., Gomez R.S., Guimarães A.L.S. Metformin increases PDH and suppresses HIF-1α under hypoxic conditions and induces cell death in oral squamous cell carcinoma. Oncotarget. 2016;7:55057–55068. doi: 10.18632/oncotarget.10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee J., Hong E.M., Kim J.H., Jung J.H., Park S.W., Koh D.H., Choi M.H., Jang H.J., Kae S.H. Metformin induces apoptosis and inhibits proliferation through the AMP-activated protein kinase and insulin-like growth factor 1 receptor pathways in the bile duct cancer cells. J. Canc. 2019;10:1734–1744. doi: 10.7150/jca.26380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Farhat D., Léon S., Ghayad S.E., Gadot N., Icard P., Le Romancer M., Hussein N., Lincet H. Lipoic acid decreases breast cancer cell proliferation by inhibiting IGF-1R via furin downregulation. Br. J. Canc. 2020;122:885–894. doi: 10.1038/s41416-020-0729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Price N.L., Gomes A.P., Ling A.J.Y., Duarte F.V., Martin-Montalvo A., North B.J., Agarwal B., Ye L., Ramadori G., Teodoro J.S., Hubbard B.P., Varela A.T., Davis J.G., Varamini B., Hafner A., Moaddel R., Rolo A.P., Coppari R., Palmeira C.M., de Cabo R., Baur J.A., Sinclair D.A. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metabol. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu J., Liang H., Chen C., Wang X., Qu F., Wang H., Yang K., Wang Q., Zhao N., Meng J., Gao A. Ivermectin induces autophagy-mediated cell death through the AKT/mTOR signaling pathway in glioma cells. Biosci. Rep. 2019;39 doi: 10.1042/BSR20192489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ji H.-L., Zhao R., Matalon S., Matthay M.A. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol. Rev. 2020;100:1065–1075. doi: 10.1152/physrev.00013.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Icard P., Saumon G. Alveolar sodium and liquid transport in mice. Am. J. Physiol. 1999;277:L1232–L1238. doi: 10.1152/ajplung.1999.277.6.L1232. [DOI] [PubMed] [Google Scholar]
- 86.Abu-Farha M., Thanaraj T.A., Qaddoumi M.G., Hashem A., Abubaker J., Al-Mulla F. The role of lipid metabolism in COVID-19 virus infection and as a drug target. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21103544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chambers J.W., Maguire T.G., Alwine J.C. Glutamine metabolism is essential for human cytomegalovirus infection. J. Virol. 2010;84:1867–1873. doi: 10.1128/JVI.02123-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sukumar M., Roychoudhuri R., Restifo N.P. Nutrient competition: a new Axis of tumor immunosuppression. Cell. 2015;162:1206–1208. doi: 10.1016/j.cell.2015.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]