The indications for early plasma transfusion, and its optimal use, remain unclear. This study suggested that plasma given before hemostasis initiation could be an important factor for improving outcomes in hemodynamically unstable patients with blunt trauma, high D‐dimer levels, or a high Injury Severity Score.
![]()
Keywords: Blood component transfusion, hemostasis, Injury Severity Score, plasma volume, Trauma Severity Index
Abstract
Aim
This study aimed to evaluate the effect of plasma transfusion before urgent hemostasis initiation on in‐hospital mortality in hemodynamically unstable patients with severe trauma.
Methods
This retrospective observational study of patients admitted to hospital between January 2011 and January 2019 grouped patients according to whether plasma transfusion was initiated before (Before group) or after (After group) hemostasis initiation. Patients with severe trauma who were unable to wait for plasma transfusion and had started hemostasis before the plasma infusion were excluded. We used multivariable logistic regression analysis to determine the effect of plasma transfusion before the initiation of urgent hemostasis on in‐hospital mortality.
Results
We included 47 and 73 patients in the Before and After groups, respectively. Blunt trauma was more common, and the D‐dimer levels and Injury Severity Score were significantly higher in the Before group than in the After group (median D‐dimer, 57.5 versus 38.1 μg/mL; P = 0.040; median Injury Severity Score, 50 versus 34; P < 0.001). Plasma given before hemostasis initiation was associated with significantly lower in‐hospital mortality (adjusted odds ratio, 0.27; 95% confidence interval, 0.078–0.900; P = 0.033) in contrast with the total plasma volume given in the first 6 or 24 h.
Conclusion
Plasma transfusion before hemostasis initiation could be an important factor for improving outcomes in hemodynamically unstable patients with blunt trauma, high D‐dimer levels, or a high Injury Severity Score.
INTRODUCTION
Uncontrolled hemorrhagic shock is an important cause of death among trauma patients. 1 Early plasma transfusion is widely used in trauma resuscitation, 2 and could lessen trauma‐induced coagulopathy and endothelial injury. 3 , 4 The Prehospital Air Medical Plasma trial reported that, compared to standard care resuscitation, prehospital plasma transfusion reduced 30‐day mortality of severely injured patients at risk of hemorrhagic shock. 5 Moreover, compared to patients with crystalloid‐only resuscitation, those who received prehospital packed red blood cells (PRBC) and plasma had the greatest reduction in mortality, followed by those who received plasma only, and PRBC only. 6 However, plasma‐first resuscitation to treat hemorrhagic shock during emergency ground transportation was not associated with reduced mortality among trial participants in urban areas. 7 The indications for early plasma transfusion, and its optimal use, remain unclear.
In this study, we aimed to evaluate the effect of plasma given before the initiation of urgent hemostasis in hemodynamically unstable patients with severe multiple injuries on in‐hospital mortality and to identify the characteristics of patients who benefited from early plasma transfusion.
METHODS
The study had a retrospective cohort design and was undertaken in a tertiary referral hospital in Isehara, Kanagawa, Japan. The hospital has a unit that specializes in trauma management and intensive care. 8 The computerized tomography scanning facility, angiography suite, and operating room adjacent to the emergency department (ED), are available at all times, and trauma surgeons are trained in emergency medicine, general surgery, cardiovascular surgery, and interventional radiology. Blood products are available at all times (including the study period), and the ED is stored with O‐PRBC and AB‐fresh frozen plasma samples (FFP). Trauma surgeons and physicians working in the ED make decisions regarding the time to initiate transfusions of PRBC and plasma in patients. In general, they consider initiating transfusion of blood products in patients with persistent profound shock after rapid infusion of 500–1,000 mL of crystalloids. Our target ratio of plasma to platelets to PRBC was established at 2:1:2.
In this study, the participants were hemodynamically unstable patients with multiple injuries admitted to the ED between January 2011 and January 2019. We included patients who had suffered severe trauma (Injury Severity Score [ISS] > 16) and could not maintain their systolic blood pressure (SBP) > 90 mmHg, regardless of primary resuscitation, which included airway management, rapid blood transfusion, and reversal of obstructive shock. Patients whose trauma was too severe to wait for plasma transfusion and had started hemostasis before the plasma infusion were excluded.
The following data were collected from electronic medical records on the Glasgow Coma Scale (GCS), respiratory rate, SBP, body temperature, pulse rate, blood pH, base excess, lactate level, D‐dimer level, prothrombin time – international normalized ratio (PT‐INR) on admission, and time taken to initiate hemostasis. The Revised Trauma Score, Abbreviated Injury Scale, ISS, and the probability of survival using the Trauma and Injury Severity Score (TRISS‐Ps) were used to determine the severity of patients’ injuries. Data were also collected on the total volumes of PRBC and FFP transfused in the first 6 and 24 h, before and after initiation of urgent hemostasis, the total volume of platelets transfused in the first 24 h, and whether patients had been given a massive transfusion (MT) (≥10 units of PRBC in 24 h). All patients were followed up to the time of discharge or death, whichever occurred first, and survival at 24 h after admission and at discharge were used as the outcome measures. Urgent hemostasis included neck exploration, thoracotomy, laparotomy, and interventional radiology for neck, chest, abdomen, pelvis, and extremities.
Patients were grouped according to whether plasma transfusion was initiated before or after initiating urgent hemostasis. We used multivariable logistic regression analysis to determine the plasma treatment effect before urgent hemostasis initiation on in‐hospital mortality. The logistic regression analyses were adjusted for TRISS‐Ps, D‐dimer levels, time from admission to initiation of hemostasis, PRBC given before hemostasis initiation, and the Before group (logistic regression model 1). Multivariable logistic regression analysis was also used to determine the effect of the total FFP volume transfused in the first 6 and 24 h on in‐hospital mortality. The logistic regression analyses were adjusted for TRISS‐Ps, D‐dimer levels, the time from admission to initiation of hemostasis, and PRBC and FFP transfused in the first 6 and 24 h (logistic regression models 2, 3).
Statistical analyses were carried out using spss version 25.0 for Windows (IBM, Armonk, NY, USA). Categorical variables were compared using the χ2‐test or Fisher’s exact test. Continuous variables were compared using the Mann–Whitney U‐test. Data are reported as medians and interquartile ranges. Statistical significance was defined as a P < 0.05 or assessed based on the 95% confidence intervals.
RESULTS
Patient characteristics according to the timing of plasma treatment initiation
Of 6,699 trauma patients admitted between January 2011 and January 2019, 120 met the inclusion criteria and were included in the analysis (Fig. 1). Patients with severe trauma who were unable to wait for plasma transfusion and had started hemostasis before the plasma infusion are listed in Table 1. There were five patients with severe blunt trauma. The chest was the primary bleeding control site in those patients. The median time taken to initiate primary hemostasis was 5 min. The mortality rate was 60%. The patient characteristics from the final cohort have been summarized in Table 2. There were 47 and 73 patients in the Before and After groups, respectively. The most common mechanism of injury was blunt trauma. Blunt trauma was more common in the Before group than in the After group. Compared to the participants in the After group, those in the Before group had significantly higher D‐dimer levels and more severe trauma based on their ISS and their TRISS‐Ps.
Fig. 1.
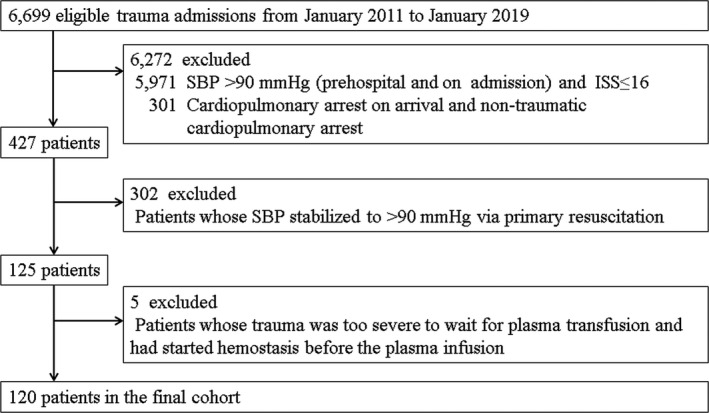
Flow diagram showing the participant selection process. ISS, Injury Severity Score; SBP, systolic blood pressure.
Table 1.
Patients with severe trauma who were unable to wait for plasma transfusion and had started hemostasis before plasma infusion
| Case | Age, years | Gender | Mechanism of injury | BE, mmol/L | RTS | ISS | Primary bleeding control sites | Time to initiate hemostasis, min | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 | Male | Motor vehicle accident | −13.0 | 3.48 | 50 | Neck, chest | 3 | Alive |
| 2 | 26 | Male | Fall from a height | −26.4 | 0.73 | 34 | Chest | 4 | Dead |
| 3 | 50 | Female | Motor vehicle accident | −26.7 | 2.34 | 75 | Chest | 5 | Dead |
| 4 | 27 | Male | Motor vehicle accident | −18.4 | 6.82 | 34 | Abdomen | 7 | Alive |
| 5 | 79 | Female | Fall from a height | −18.8 | 1.47 | 75 | Chest | 15 | Dead |
BE, Base excess; ISS, Injury Severity Score; RTS, Revised Trauma Score.
Table 2.
Characteristics of hemodynamically unstable patients with severe trauma
| Variable |
Total (n = 120) |
Before (n = 47) |
After (n = 73) |
P‐value |
|---|---|---|---|---|
| Age (years), median (IQR, 25–75) | 54.5 (37.3–70.8) | 56.0 (37.0–71.0) | 54.0 (36.5–70.5) | 0.919 |
| Male sex (%) | 81 (67.5) | 35 (74.4) | 46 (63.0) | 0.191 |
| Mechanism of injury (%) | ||||
| Motor vehicle accident | 56 (46.7) | 26 (55.3) | 30 (41.1) | 0.099 |
| Fall from a height | 45 (37.5) | 18 (38.3) | 27 (37.0) | |
| Stabbing | 14 (11.7) | 2 (4.3) | 12 (16.4) | |
| Compression | 5 (4.1) | 1 (2.1) | 4 (5.5) | |
| Blunt mechanism (%) | 106 (88.3) | 45 (97.9) | 61 (83.6) | 0.042 |
| Vital signs on admission | ||||
| GCS total score, median (IQR 25–75) | 11.0 (6.0–14.0) | 9.0 (4.0–14.0) | 13.0 (6.0–14.5) | 0.218 |
| GCS < 9 (%) | 52 (43.3) | 23 (48.9) | 29 (39.7) | 0.320 |
| RR, breaths/min; median (IQR 25–75) | 24.0 (18.0–30.0) | 24.0 (18.0–30.0) | 27.0 (19.5–31.0) | 0.185 |
| SBP, mmHg; median (IQR 25–75) | 70.0 (54.5–88.0) | 72.0 (60.0–98.0) | 70.0 (51.0–82.0) | 0.145 |
| BT, °C; median (IQR 25–75) | 36.0 (35.5–36.7) | 36.0 (35.5–36.8) | 36.0 (35.4–36.6) | 0.969 |
| Pulse rate, b.p.m., median (IQR 25–75) | 110.0 (90.0–130.0) | 110.0 (91.0–130.0) | 110.0 (84.3–129.5) | 0.800 |
| Lowest SBP before initiation hemostasis, mmHg; median (IQR 25–75) | 55.0 (32.5–70.0) | 50.0 (0.0–70.0) | 56.0 (40.0–70.0) | 0.562 |
| Laboratory evaluations at admission, median (IQR 25–75) | ||||
| pH | 7.3 (7.1–7.4) | 7.3 (7.1–7.4) | 7.3 (7.1–7.4) | 0.809 |
| Base excess, mmol/L | −9.8 (−17.3 to −5.2) | −9.9 (−17.1 to −5.0) | −9.7 (−18.0 to −5.2) | 0.625 |
| Lactate, mg/dL | 65.0 (36.0–98.0) | 66.0 (36.0–99.0) | 58.0 (36.0–93.0) | 0.882 |
| D‐dimer, μg/mL | 43.3 (16.7–97.4) | 57.5 (25.2–140.2) | 38.1 (11.5–95.7) | 0.040 |
| PT‐INR | 1.2 (1.0–1.4) | 1.2 (1.1–1.4) | 1.2 (1.0–1.4) | 0.441 |
| Trauma score, median (IQR 25–75) | ||||
| RTS | 5.9 (3.8–6.6) | 5.6 (3.8–6.6) | 6.1 (3.8–6.7) | 0.623 |
| ISS | 43.0 (29.8–55.3) | 50.0 (41.0–59.0) | 34.0 (26.0–50.0) | <0.001 |
| TRISS‐Ps, % | 37.0 (5.3–75.2) | 25.3 (3.2–48.4) | 57.3 (7.1–84.7) | 0.008 |
After, plasma transfusion initiated after hemostasis initiation; Before, plasma transfusion initiated before hemostasis initiation; BT, body temperature; GCS, Glasgow Coma Scale; IQR, interquartile range; ISS, Injury Severity Score; PT‐INR, prothrombin time – international normalized ratio; RR, respiratory rate; RTS, Revised Trauma Score; SBP, systolic blood pressure; TRISS‐Ps, probability of survival calculated by the trauma and injury severity score.
Interventions and outcomes according to the timing of plasma initiation
Table 3 compares the interventions and outcomes between the groups. There was no significant difference between the groups in the time taken to initiate urgent hemostasis, total volume of PRBC and FFP in the first 6 h, total volume of PRBC and platelets transfused in the first 24 h, or the proportion of patients who underwent MT in the first 24 h. Patients in the Before group were significantly more likely to have PRBC transfused before the initiation of urgent hemostasis, and had a significantly higher FFP : PRBC ratio in the first 6 h. Moreover, they had significantly higher total FFP volume transfused and FFP : PRBC ratio in the first 24 h. Although the PRBC volume transfused in the first 24 h after the initiation of hemostasis (post‐PRBC) did not differ significantly between the groups, the post‐PRBC : total PRBC ratio was significantly lower in the Before group. There were no significant differences between the groups in terms of complication, 24‐h survival, or survival to discharge rates.
Table 3.
Interventions and outcomes among hemodynamically unstable patients with severe trauma
| Variable |
Total (n = 120) |
Before (n = 47) |
After (n = 73) |
P‐value |
|---|---|---|---|---|
| Time from admission to initiate hemostasis, min; median (IQR 25–75) | 56.0 (37.0–88.5) | 50.0 (31.5–107.5) | 57.0 (40.0–75.0) | 0.847 |
| Transfusions given before hemostasis initiation, units; median (IQR 25–75) | ||||
| PRBC | 4.0 (2.0–8.0) | 6.0 (4.0–8.0) | 4.0 (0.0–4.0) | <0.001 |
| FFP | 0.0 (0.0–2.0) | 4.0 (2.0–6.0) | 0.0 (0.0–0.0) | <0.001 |
| Ratio of FFP : PRBC before initiation hemostasis | 0.0 (0.0–0.5) | 0.5 (0.3–0.8) | 0.0 (0.0–0.0) | <0.001 |
| Total amount of blood transfusions in the first 6 h, units, median (IQR 25–75) | ||||
| PRBC | 12.0 (6.0–20.0) | 12.0 (8.0–20.0) | 12.0 (6.0–20.0) | 0.633 |
| FFP | 8.0 (4.0–14.0) | 10.0 (4.0–14.0) | 8.0 (2.0–14.0) | 0.156 |
| Ratio of FFP: PRBC in the first 6 h | 0.7 (0.4–0.9) | 0.8 (0.6–1.0) | 0.6 (0.4–0.8) | 0.005 |
| Total amount of blood transfusion in the first 24 h, units; median (IQR 25–75) | ||||
| PRBC | 16.0 (8.0–24.0) | 18.0 (10.0–26.0) | 14.0 (6.0–22.0) | 0.142 |
| FFP | 10.0 (4.0–19.5) | 12.0 (8.0–22.0) | 8.0 (4.0–18.0) | 0.028 |
| Ratio of FFP : PRBC in the first 24 h | 0.7 (0.4–1.0) | 0.8 (0.6–1.0) | 0.6 (0.4–0.8) | <0.001 |
| Platelets | 10.0 (0.0–20.0) | 10.0 (0.0–30.0) | 0.0 (0.0–20.0) | 0.375 |
| Massive transfusion (≥10 units of RBC during 24 h) (%) | 82 (68.3) | 37 (78.7) | 45 (61.6) | 0.050 |
| PRBC given in the first 24 h following hemostasis initiation, units; median (IQR 25–75) | 8.0 (4.0–18.0) | 8.0 (2.0–18.0) | 8.0 (4.0–20.0) | 0.196 |
| Post‐PRBC : total PRBC; median (IQR 25–75) | 0.7 (0.5–0.9) | 0.6 (0.1–0.8) | 0.8 (0.5–1.0) | <0.001 |
| Complications (%) | ||||
| MOF (Denver score >3) | 6 (5.0) | 3 (6.4) | 3 (4.1) | 0.678 |
| ALI | 22 (18.3) | 10 (21.3) | 12 (16.4) | 0.504 |
| Allergic reaction or transfusion‐related reaction | 3 (2.5) | 1 (2.1) | 2 (2.7) | — |
| 24‐h survival (%) | 87 (72.5) | 37 (78.7) | 50 (68.5) | 0.221 |
| Survival to discharge (%) | 61 (50.8) | 24 (51.1) | 37 (50.7) | 0.968 |
After, plasma transfusion initiated after hemostasis initiation; ALI, acute lung injury; Before, plasma transfusion initiated before hemostasis initiation; FFP, fresh frozen plasma; IQR, interquartile range; MOF, multiple organ failure; PRBC, packed red blood cells; Post‐PRBC, PRBC volume transfused in first 24 h after initiation of hemostasis; total PRBC, total amount of PRBC in the first 24 h; RBC, red blood cells.
Factors associated with in‐hospital mortality
The risk factors for in‐hospital mortality are shown in Tables 4, 5, and 6. Although the total volume of plasma given in the first 6 or 24 h did not have a significant association with in‐hospital mortality, plasma given before initiation of urgent hemostasis was associated with lower in‐hospital mortality. In contrast, a greater volume of PRBC transfused in the first 6 or 24 h and a longer time taken to initiate hemostasis were associated with higher in‐hospital mortality.
Table 4.
Independent risk factors for in‐hospital mortality among hemodynamically unstable patients with severe trauma, logistic regression model 1
| Variable | Adjusted odds ratio (95% CI) | P‐value |
|---|---|---|
| TRISS‐Ps | 0.96 (0.94–0.98) | <0.001 |
| D‐dimer levels | 1.00 (1.00–1.01) | 0.438 |
| Time from admission to initiate hemostasis | 1.03 (1.01–1.04) | 0.001 |
| PRBC given before hemostasis initiation | 1.12 (0.98–1.28) | 0.144 |
| Before group | 0.26 (0.08–0.90) | 0.033 |
CI, confidence interval; Before group, plasma transfusion initiated before hemostasis initiation; PRBC, packed red blood cells; TRISS‐Ps, probability of survival calculated by the Trauma and Injury Severity Score.
Table 5.
Independent risk factors for in‐hospital mortality among hemodynamically unstable patients with severe trauma, logistic regression model 2
| Variable | Adjusted odds ratio (95% CI) | P‐value |
|---|---|---|
| TRISS‐Ps | 0.96 (0.94–0.98) | <0.001 |
| D‐dimer levels | 1.00 (1.00–1.01) | 0.207 |
| Time from admission to initiate hemostasis | 1.03 (1.01–1.04) | 0.001 |
| Total amount of PRBC in the first 6 h | 1.10 (1.03–1.18) | 0.007 |
| Total amount of FFP in the first 6 h | 0.95 (0.88–1.03) | 0.229 |
CI, confidence interval; FFP, fresh frozen plasma; PRBC, packed red blood cells; TRISS‐Ps, probability of survival calculated by the trauma and injury severity score.
Table 6.
Independent risk factors for in‐hospital mortality among hemodynamically unstable patients with severe trauma, logistic regression model 3
| Variable | Adjusted odds ratio (95% CI) | P‐value |
|---|---|---|
| TRISS‐Ps | 0.96 (0.94–0.98) | <0.001 |
| D‐dimer levels | 1.00 (1.00–1.01) | 0.276 |
| Time from admission to initiate hemostasis | 1.02 (1.01–1.04) | 0.001 |
| Total amount of PRBC in the first 24 h | 1.06 (1.01–1.11) | 0.018 |
| Total amount of FFP in the first 24 h | 0.97 (0.93–1.01) | 0.138 |
CI, confidence interval; FFP, fresh frozen plasma; PRBC, packed red blood cells; TRISS‐Ps, probability of survival calculated by the trauma and injury severity score.
DISCUSSION
In this study, plasma transfusion before the initiation of urgent hemostasis was associated with a reduction in in‐hospital mortality in hemodynamically unstable patients with severe multiple injuries who did not respond to primary resuscitation, whereas the total volume of plasma given in the first 6 or 24 h did not have a significant association with in‐hospital mortality. Blunt trauma was more common, and the D‐dimer levels and ISS were significantly higher in the Before group than in the After group.
It is important to consider how patient selection might have influenced the study results. In a large previous study, 7 the researchers concluded that early initiation of plasma transfusion before hospital admission was not associated with decreased mortality. The characteristics of patients in the previous study differed from those in our study, as most of them had a partial or complete response to the initial resuscitation and were less likely to have experienced blunt trauma and had a MT. They also had higher GCS scores, lower ISS, lower INRs before initiation of plasma transfusion, lower base deficits, and lower lactic acid concentrations on admission. Taken together, these results suggested that early plasma transfusion could have a beneficial effect in some patients who have experienced severe trauma or who are likely to develop the characteristics before achieving bleeding control. 7 The logistic challenges of early plasma transfusion in all trauma patients with hemorrhagic shock might outweigh any benefits. It is important to avoid unnecessary plasma transfusions, even though it is relatively safe. In this study, the participants in the Before group had more blunt trauma and higher D‐dimer levels and ISS than those in the After group. Therefore, it might be beneficial to target patients with more severe coagulopathy, who are likely to take longer to stop bleeding, for plasma transfusion before hemostasis initiation. In contrast, it could be possible to delay giving plasma even in hemodynamically unstable patients when the bleeding stops for a moment (e.g., a patient with an amputated limb, enabling the use of a tourniquet or clamp on exposed vessels, or a penetrating major vascular injury enabling the use of a clamp within a few minutes). However, we were unable to identify specific patient characteristics that could be useful to decide whether to initiate early plasma transfusion before hemostasis initiation. Further studies are needed to identify the characteristics of patients who are more likely to benefit from early plasma initiation.
Hyperfibrinolysis is the most lethal phenotype of trauma‐induced coagulopathy. 9 Shock‐induced systemic hyperfibrinolysis is attenuated by plasma‐first resuscitation. 10 For this reason, we believe that plasma transfusion before initiation of urgent hemostasis could be beneficial to facilitate urgent hemostasis and treat coagulopathy. Recovering from coagulopathy before the initiation of hemostasis could be a determinant of a favorable outcome. In this study, plasma transfusion before the initiation of hemostasis might have led to reduced mortality because the majority of patients had severe coagulopathy and acidosis following admission. However, in the critical setting, it is unclear whether trauma‐induced coagulopathy can be ameliorated by only plasma supplementation with a few units of FFP in continuous bleeding. It is difficult to determine the optimal volume of plasma before or after the initiation of hemostasis. Additionally, giving high volumes of plasma in relation to PRBC can have harmful effects, such as acute lung injury‐induced acute respiratory distress syndrome. 11 , 12 We used laboratory evaluations, such as hemoglobin, fibrinogen, and PT‐INR, to decide whether to continue transfusion of PRBC and plasma when the patients became hemodynamically stable. Furthermore, we suggested transfusion of FFP and PRBC in a ratio of approximately 0.8 in the acute phase. However, as there are differing opinions regarding the optimal ratio of FFP : PRBC, 13 , 14 , 15 , 16 further studies are needed.
Notably, this study revealed the following important outcomes. First, we found that a high volume of PRBC transfused in the first 24 h was associated with significantly increased mortality. Plasma transfusion before initiation of urgent hemostasis might enable reduction in the total volume of PRBC transfused, resulting in reduced medical care costs. Second, when evaluating the effectiveness of early plasma transfusion, it is important to consider the risk of transfusion‐related complications, such as multiple organ failure, acute lung injury‐induced acute respiratory distress syndrome, allergic reactions, and transfusion‐related reactions. 17 , 18 , 19 However, recent studies have shown that transfusion‐related complications are rare. 3 , 5 In this study, the incidence rate of transfusion‐related complications was very low in both groups and did not differ between them. In some reports, 20 platelet transfusions seemed to improve hemostasis and survival, although this was not our observation.
Our multivariable logistic analyses considered some important factors, as follows. First, early plasma treatment might improve mortality and increase the risk of complications. 5 , 6 , 11 , 12 Therefore, we used in‐hospital mortality as an objective variable to analyze the independent risk factors. Second, we did not include age, mechanism of injury, or Revised Trauma Score in the multivariable logistic regression analysis because variables did not differ significantly between the two groups. Instead, we included TRISS‐Ps as a confounder for severity. Third, the time from hospital arrival to initiation of hemostasis is critical in saving the lives of patients with multiple traumatic injuries, 21 and a delay in laparotomy in patients with intra‐abdominal hemorrhage after trauma is associated with increased mortality. 22 Therefore, it is important to consider the time taken to initiate hemostasis, as a potential confounder when evaluating factors associated with mortality. Although the time taken to initiate hemostasis did not differ significantly between the groups, we included it as a potential confounder in the multivariable logistic regression analyses. Finally, we used D‐dimer levels as an evaluation factor for coagulopathy because many cases in early periods had missing values of fibrin/fibrinogen degradation products or fibrinogen at admission.
The limitations of the study included the small sample size, retrospective study design, and the long study period. Moreover, the study was undertaken at a single center. Statistically, this study had limited power due to the small sample size, and therefore, some clinically important associations might not have been statistically significant. A prospective, multicenter study is needed to confirm our study findings. In addition, the patients in our study had severe trauma that was challenging to manage, requiring specialist care, and the study was carried out at a hospital that specializes in trauma. This could limit the generalizability of the results. The decision to initiate transfusions was made by the primary physician, which might have led to selection bias. Although we excluded patients whose trauma was too severe to wait for plasma transfusion and who had started hemostasis before the plasma infusion, survival biases could remain. In addition, the study was carried out as part of routine clinical practice, and there was no set resuscitation protocol. We were unable to estimate the cost‐effectiveness ratio of early plasma transfusion. Furthermore, medical techniques, such as the use of freeze‐dried plasma, are constantly advancing and are becoming more widely used. 23
CONCLUSIONS
Plasma transfusion before hemostasis initiation could be an important factor for improving outcomes in hemodynamically unstable patients with blunt trauma, high D‐dimer levels, or a high ISS.
Thus, patient selection and the use of plasma transfusion are important to obtain optimal benefits. To address unanswered questions regarding early plasma transfusion, further studies are needed after considering the targeted patients, cost‐effectiveness ratio, and other prevention and treatment techniques for managing coagulopathy.
DISCLOSURE
Approval of the research protocol: The research protocol was approved by the Tokai University Institutional Review Board for Clinical Research (Approval number: 18R‐326), and conformed to the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013). All methods were carried out in accordance with relevant guidelines and regulations.
Informed consent: The institutional review board waived the need for patient consent.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interest: None.
ACKNOWLEDGMENTS
We would like to thank Editage for English language editing.
Funding information
No funding information provided.
References
- 1. Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J. Trauma 2006; 60: S3–11. [DOI] [PubMed] [Google Scholar]
- 2. Radwan ZA, Bai Y, Matijevic N et al An emergency department thawed plasma protocol for severely injured patients. JAMA Surg. 2013; 148: 170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holcomb JB, Tilley BC, Baraniuk S et al Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015; 313: 471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pati S, Potter DR, Baimukanova G, Farrel DH, Holcomb JB, Schreiber MA. Modulating the endotheliopathy of trauma: factor concentrate versus fresh frozen plasma. J. Trauma Acute Care Surg. 2016; 80: 576–85. [DOI] [PubMed] [Google Scholar]
- 5. Sperry JL, Guyette FX, Brown JB et al Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N. Engl. J. Med. 2018; 379: 315–26. [DOI] [PubMed] [Google Scholar]
- 6. Guyette FX, Sperry JL, Peitzman AB et al Prehospital blood product and crystalloid resuscitation in the severely injured patient: a secondary analysis of the prehospital air medical plasma trial. Ann. Surg. 2019. 10.1097/SLA.0000000000003324. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 7. Moore HB, Moore EE, Chapman MP et al Plasma‐first resuscitation to treat haemorrhagic shock during emergency ground transportation in an urban area: a randomised trial. Lancet 2018; 392: 283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Otsuka H, Sato T, Sakurai K et al Impact of emergency physicians competent in severe trauma management, surgical techniques, and interventional radiology on trauma management. Acute Med. Surg. 2018; 5: 342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moore HB, Moore EE, Gonzalez E et al Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J. Trauma Acute Care Surg. 2014; 77: 811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moore HB, Moore EE, Morton AP et al Shock‐induced systemic hyperfibrinolysis is attenuated by plasma‐first resuscitation. J Trauma Acute Care Surg 2015; 79: 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inaba K, Branco BC, Rhee P et al Impact of plasma transfusion in trauma patients who do not require massive transfusion. J. Am. Coll. Surg. 2010; 210: 957–65. [DOI] [PubMed] [Google Scholar]
- 12. Endo A, Shiraishi A, Fushimi K, Murata K, Otomo Y. Outcomes of patients receiving a massive transfusion for major trauma. Br. J. Surg. 2018; 105: 1426–34. [DOI] [PubMed] [Google Scholar]
- 13. Scalea TM, Bochicchio KM, Lumpkins K et al Early aggressive use of fresh frozen plasma does not improve outcome in critically injured trauma patients. Ann. Surg. 2008; 248: 578–84. [DOI] [PubMed] [Google Scholar]
- 14. Holcomb JB, del Junco DJ, Fox EE et al The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time‐varying treatment with competing risks. JAMA Surg. 2013; 148: 127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hagiwara A, Kushimoto S, Kato H et al Can early aggressive administration of fresh frozen plasma improve outcomes in patients with severe blunt trauma?—a report by the Japanese Association for the Surgery of Trauma. Shock 2016; 45: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mesar T, Larentzakis A, Dzik W, Chang Y, Velmahos G, Yeh DD. Association between ratio of fresh frozen plasma to red blood cells during massive transfusion and survival among patients without traumatic injury. JAMA Surg. 2017; 152: 574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Minei JP, Cuschieri J, Sperry J et al Inflammation and the host response to injury collaborative research program. The changing pattern and implications of multiple organ failure after blunt injury with hemorrhagic shock. Crit. Care Med. 2012; 40: 1129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Watson GA, Sperry JL, Rosengart MR et al Inflammation and host response to injury investigators. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J. Trauma 2009; 67: 221–30. [DOI] [PubMed] [Google Scholar]
- 19. Sauaia A, Moore EE, Johnson JL et al Temporal trends of postinjury multiple‐organ failure: Still resource intensive, morbid, and lethal. J. Trauma Acute Care Surg. 2014; 76: 582–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cardenas JC, Zhang X, Fox EE et al Platelet transfusions improve hemostasis and survival in a substudy of the prospective, randomized PROPPR trial. Blood Adv. 2018; 2: 1696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gruen RL, Brohi K, Schreiber M et al Haemorrhage control in severely injured patients. Lancet 2012; 380: 1099–108. [DOI] [PubMed] [Google Scholar]
- 22. Clarke JR, Trooskin SZ, Doshi PJ, Greenwald L, Mode CJ. Time to laparotomy for intra‐abdominal bleeding from trauma does affect survival for delays up to 90 minutes. J. Trauma 2002; 52: 420–5. [DOI] [PubMed] [Google Scholar]
- 23. Shlaifer A, Siman‐Tov M, Radomislensky I et al The impact of prehospital administration of freeze‐dried plasma on casualty outcome. J. Trauma Acute Care Surg. 2019; 86: 108–15. [DOI] [PubMed] [Google Scholar]


