We found no association between the number of systems of symptoms and the incidence of biphasic reactions. Currently, predicting the onset of biphasic reactions is difficult. Further prospective and nationwide studies are required to research biphasic reactions.
Keywords: Adrenaline, corticosteroid, hospitalization, incidence of biphasic reaction, systems of symptoms
Abstract
Aim
Anaphylaxis is a severe, life‐threatening, generated or systemic reaction, and biphasic reaction could occur in some cases. We investigated the clinical course of anaphylaxis in our hospital and studied the relationship between biphasic reactions and the symptoms and treatments for predicting the onset of biphasic reactions.
Methods
We retrospectively reviewed the medical records of 120 patients with anaphylaxis who were admitted to our hospital from the emergency department during April 2008–October 2015.
Results
The incidence of biphasic reactions of anaphylaxis in our hospital was 10.8% (13 patients) without significant difference when compared with that in previous reports. Regarding the development of biphasic reactions, symptoms, the number of systems of symptoms and severity of the initial reaction, and treatment with adrenaline and corticosteroid were not clearly related with biphasic reaction. Use of adrenaline in the initial treatment was approximately 60%. Of the 13 biphasic reactions, 11 (84.5%) were as equal/mild as the original symptoms.
Conclusion
This study could not show the factors predicting the onset of biphasic reactions. Further prospective and nationwide studies are required to research biphasic reactions.
INTRODUCTION
We often experience patients with anaphylaxis in the emergency department (ED). Anaphylaxis is a serious allergic reaction that develops rapidly and could be life‐threatening. There are various anaphylactic symptoms. 1 Anaphylaxis has the following systems of symptoms: skin and mucocutaneous, respiratory, cardiovascular, central nervous, and gastrointestinal.
Biphasic reactions are characterized by a uniphasic response, then an asymptomatic period, and the subsequent return of symptoms without further exposure to antigen. 2 The reported incidence of biphasic reactions differs from 0.4% to 23.3%. 3 , 4 , 5 Considering this biphasic reaction, patients with anaphylaxis are often hospitalized.
Predicting which patients will develop a biphasic reaction based on previous studies is difficult. Factors possibly causing biphasic reactions include medical history, for example, asthma, 6 heart disease, 7 prior anaphylaxis, 8 and treatment, and age. The occurrence of biphasic reaction could be associated with the severity of anaphylaxis; 5 , 9 however, its relationship with systems of symptoms is unclear. The presence of many symptoms and systems of symptoms suggests that many organs are affected and enhanced severity of the condition. The involvement of multiple organ systems, particularly gastrointestinal involvement, indicated higher hospitalization rates. 10
We hypothesized that the presence of many systems of symptoms increases the incidence of biphasic reactions. Therefore, we investigated the relationship between biphasic reactions and symptoms in patients with anaphylaxis hospitalized from the ED.
METHODS
Study design
This observational, single‐center study retrospectively reviewed the medical records of patients with anaphylaxis/anaphylactic shock to investigate the incidence and characteristics of biphasic reactions. Patients were transferred and hospitalized for emergency treatment in a secondary health‐care setting in the Juntendo University Nerima Hospital, Tokyo, Japan. The ED does not have a hospitalization facility, and we do not consider follow‐up observation in the ED as hospitalization. There was no protocol or unified treatment policy, and each emergency physician provided treatment as appropriate.
Patients with anaphylaxis were categorized into two groups according the presence/absence of biphasic response and were examined for factors related to the onset of biphasic reaction by comparing the two groups.
Definition
We defined anaphylaxis using the diagnostic criteria outlined by the 2006 Symposium on the Definition and Management of Anaphylaxis (Table 1). 11 We defined biphasic reaction as a uniphasic response, followed by an asymptomatic period of ≥1 h and the subsequent return of symptoms without further exposure to antigen. 2 We classified anaphylaxis severity according to the European Academy of Allergy and Clinical Immunology 12 (Table 2), which was the basis of the severity classification of the Japanese anaphylaxis guideline. 13
Table 1.
Diagnostic criteria for anaphylaxis
| Anaphylaxis is highly likely when any one of the following three criteria is fulfilled: |
|---|
| Criterion 1 |
| Acute onset of an illness (minutes to several hours) with involvement of the skin and mucosal tissue |
| A. Respiratory compromise (e.g., dyspnea, wheeze/bronchospasm, stridor, reduced peak expiratory flow, hypoxemia) |
| B. Reduced BP or associated symptoms and signs of end‐organ dysfunction (e.g., hypotonia, collapse, syncope, incontinence) |
| Criterion 2 |
| Two or more of the following that occur rapidly after exposure to a likely allergen for the patient |
| A. Involvement of the skin and mucosal tissue (e.g., generalized hives, itchy/flushed, swollen lips/tongue/uvula) |
| B. Respiratory compromise (e.g., dyspnea, wheeze/bronchospasm, stridor, reduced peak expiratory flow, hypoxemia) |
| C. Reduced BP or associated symptoms and signs (e.g. hypotonia, syncope, incontinence) |
| D. Persistent gastrointestinal symptoms and signs (e.g., crampy abdominal pain, vomiting) |
| Criterion 3 |
| A. Systolic BP of <90 mmHg or >30% decrease from that person’s baseline (in adults) |
| B. Age‐specific low systolic BP (in infants and children) |
| <70 mmHg in infants aged from 1 month up to 1 year |
| Less than (70 mmHg + [2 × age]) in children aged 1–10 years |
| <90 mmHg in children aged 11–17 years |
BP, blood pressure.
Table 2.
Grading the severity of anaphylaxis
| Grade | Skin | GI tract | Respiratory | Cardiovascular | Neurological |
|---|---|---|---|---|---|
| 1 Mild | Sudden itching of eyes and nose, generalized pruritus, flushing, urticaria, angioedema | Oral pruritus, oral “tingling”, mild lip swelling, nausea or emesis, mild abdominal pain | Nasal congestion and/or sneezing, rhinorrhea, throat pruritus, throat tightness, mild wheezing | Tachycardia (increase >15 b.p.m.) | Change in activity level plus anxiety |
| 2 Moderate | Any of the above | Any of the above, crampy abdominal pain, diarrhea, recurrent vomiting | Any of the above, hoarseness, “barky” cough, difficulty swallowing, stridor, dyspnea, moderate wheezing | As above | Light headedness, feeling of “impending doom” |
| 3 Severe | Any of the above | Any of the above, loss of bowel control | Any of the above, cyanosis or saturation <92%, respiratory arrest | Hypotension † and/or collapse, dysrhythmia, severe bradycardia and/or cardiac arrest | Confusion, loss of consciousness |
The severity score should be based on the organ system most affected.
Hypotension defined as systolic blood pressure <90 mmHg (adult).
The five systems of symptoms are skin and mucocutaneous, respiratory, cardiovascular, gastrointestinal, and central nervous. The systems are the same as the items in the assessment of severity.
Outcome measures
We compared the following factors between the uniphasic and biphasic groups: sex, age, medical history, causative substance, severity, the systems of symptoms, the number of systems of symptoms involved, and treatment with adrenaline, corticosteroid, and H1/H2 blocker. Regarding severity, we examined whether more biphasic reactions occurred in severe cases (grade 3) in cases satisfying the anaphylaxis definition. 11 Grade 1 cases are not considered anaphylaxis in the Japanese guideline and do not require hospitalization, so grade 1 cases were not included. Patients with symptoms of two to three systems and those with symptoms of four to five systems were grouped into the small and large number of systems groups. These two groups were compared to determine whether patients with symptoms in more systems had more biphasic reactions.
Participants
We evaluated patients with anaphylaxis/anaphylactic shock who were hospitalized and needed follow‐up from April 2008 to October 2015.
Exclusion criteria
We excluded patients aged <15 years because emergency physicians do not treat pediatric cases in our hospital. We also excluded patients with duplicate registration, or who were not hospitalized (including transfers), who did not satisfy the definition of anaphylaxis, who were not treated by emergency physicians because of non‐standardized medical record documentation, and who had incomplete data.
Statistical analysis
The Mann–Whitney U‐test was used for analyzing continuous data and Fisher’s exact tests for analyzing ordinal data. P‐values of <0.05 were considered statistically significant. We used EZR, a free software for statistical analyses. 14
No multivariate analyses were carried out because of the small sample size. Instead, we calculated and evaluated Cramer’s coefficient of association along with the significance of differences. This coefficient is an index for the strength of association, and a result of >0.1 was considered relevant.
RESULTS
We included 120 patients in this study (39 men; median age, 47 years). Table 3 summarizes patient characteristics. Seventy‐eight (65.0%), 25 (20.8%), and four patients (3.3%) had a history of allergies, asthma, and heart diseases. The sample included 107 (89.2%) and 13 (10.8%) patients in the uniphasic and biphasic groups.
Table 3.
Background of 120 patients in this study with anaphylaxis
| Total cases | 120 |
| Male sex | 39 (32.5) |
| Age, median (IQR) | 47 (30.0, 62.5) |
| History: allergy | 78 (65.0) |
| History: asthma | 25 (20.8) |
| History: heart disease | 4 (3.3) |
| Biphasic reaction | 13 (10.8) |
Data are shown as n (%) unless otherwise specified.
IQR, interquartile range.
Causes are listed in Figure 1. Food was most common (63 cases, 52.5%), followed by drugs (34 cases, 28.3%), insect bites (three cases, 2.5%), and animals (two cases, 1.6%). Causes were unidentified in 18 cases (15.0%).
Fig. 1.
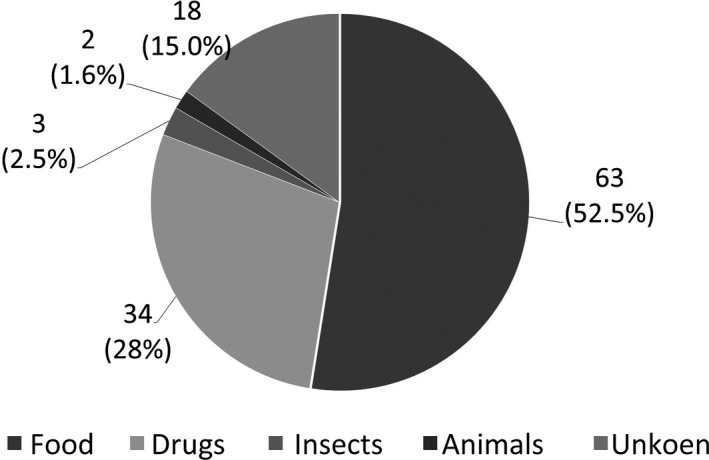
Causative substances of anaphylaxis in this study.
Table 4 shows comparisons between the uniphasic and biphasic groups. Men were less common in the biphasic group, possibly with a weak association (P = 0.218, Cramer’s V = 0.099). The median age was higher in the biphasic group than in the uniphasic group (47.0 versus 55.0), although the difference was not statistically significant. There were no significant differences between the two groups regarding histories of allergy, asthma, and heart disease, with small coefficients of association (Cramer’s V < 0.1). There was no significant difference regarding food, drugs, insects, animals, or unknown causes between the two groups (Cramer’s V < 0.1).
Table 4.
Comparison between uniphasic and biphasic anaphylaxis in this study
| Uniphasic, n = 107 | Biphasic, n = 13 | P‐value † | Cramer’s V ‡ | |
|---|---|---|---|---|
| Male sex | 37 (34.6) | 2 (15.3) | 0.218 | 0.099 |
| Age, years (IQR) | 47 (30.5, 62.0) | 55 (30.0, 66.0) | 0.615 § | − |
| History of allergy | 69 (64.5) | 9 (69.2) | 1.000 | 0.003 |
| History of asthma | 22 (20.6) | 3 (23.0) | 0.733 | 0.014 |
| History of heart disease | 4 (3.7) | 0 (0.0) | 1.000 | 0.010 |
| Food | 56 (52.3) | 7 (53.8) | 1.000 | 0.017 |
| Drug | 31 (29.0) | 3 (23.0) | 0.757 | 0.011 |
| Insect | 3 (2.8) | 0 (0.0) | 1.000 | 0.030 |
| Others | 2 (1.9) | 0 (0.0) | 1.000 | 0.059 |
| Unknown | 15 (14.0) | 3 (23.0) | 0.411 | 0.041 |
| Skin and mucosa | 104 (97.2) | 13 (100.0) | 1.000 | 0.03 |
| Gastrointestinal | 68 (63.4) | 10 (76.9) | 0.539 | 0.059 |
| Respiratory | 80 (74.8) | 12 (92.3) | 0.295 | 0.097 |
| Cardiovascular | 51 (47.7) | 6 (46.1) | 1.000 | 0.017 |
| Shock | 38 (35.5) | 3 (23.0) | 0.539 | 0.081 |
| Neurological | 25 (23.4) | 3 (23.0) | 1.000 | 0.030 |
| Severity (grade 3) | 57 (53.2) | 5 (38.4) | 0.385 | 0.065 |
| Many (4–5) systems | 32 (29.9) | 4 (30.8) | 1.000 | 0.023 |
| Adrenaline | 70 (65.4) | 8 (61.5) | 0.767 | 0.003 |
| Corticosteroid | 98 (91.6) | 13 (100.0) | 0.595 | 0.048 |
| H1 blocker | 104 (97.2) | 13 (100.0) | 1.000 | 0.030 |
| H2 blocker | 105 (98.1) | 12 (92.3) | 0.293 | 0.030 |
Data are shown as n (%) unless otherwise specified.
–, not applicable.
Fisher’s exact test.
Cramer’s coefficient of association.
Mann–Whitney U‐test was used in analysis of age.
Regarding symptoms, no significant differences between the two groups were noted in any of skin and mucocutaneous, gastrointestinal, respiratory, cardiovascular, shock, and central nervous system categories; however, there was a weak association with the respiratory category (Cramer’s V = 0.097).
Regarding severity, the incidence tended to be slightly higher for grade 2; however, no significant differences were found between grade 2 and 3 with small coefficient of association (Cramer’s V < 0.1). Regarding the number of systems of symptoms, there were no significant differences between the small and large number of systems groups, and the coefficient of association was small (Cramer’s V < 0.1).
Seventy of 107 patients (65.4%) in the uniphasic group and eight of 13 patients (61.5%) in the biphasic group used intramuscular adrenaline injections. Corticosteroids were given to 98 (91.6%) and 13 patients (100%) in the uniphasic and biphasic groups, respectively. Corticosteroids used were hydrocortisone/methylprednisolone. H1‐blockers were used in 104 (97.2%) and 13 patients (100%) in the uniphasic and biphasic groups. H2‐blockers were used in 105 (98.1%) and 12 patients (92.3%) in the uniphasic and biphasic groups. No significant differences between the two groups were noted for adrenaline, corticosteroids, or H1/H2 blocker use, and the coefficients of association were also small.
No deaths occurred in this study. Of the 13 biphasic reactions, 11 (84.5%) were as equal or mild as the original symptoms.
DISCUSSION
Biphasic anaphylactic reaction was first reported in 1984 by Popa and Lerner, 15 and many cases have since been reported. Although small‐scale, single‐center studies have investigated biphasic reactions in Japan, and the occurrence is unclear.
The reported incidence of biphasic reaction in recent publications is generally <10%. 16 In this study, the incidence was 10.8% and did not significantly differ from that in recent reports.
This study showed a tendency of higher incidence in women; however, no previous reports have indicated sex difference in the incidence of biphasic reactions.
The cause was food in the majority of anaphylactic cases. Causative foods were diverse and were not associated with biphasic reactions. No associations were noted between biphasic reactions and medical history. Although allergy history should be considered, predicting the onset of biphasic reaction based on the medical history appears to be difficult.
Severe cases mean that allergic reactions are intense, possibly with an association between the severity and biphasic reactions. However, we found no association between the anaphylaxis severity and occurrence of biphasic reaction. Because anaphylaxis can rapidly progress, diagnosis and treatment need to be promptly carried out in parallel. Clinical symptoms are pieces of information that can be directly obtained from patients through clinical examinations. Thus, we speculated that making decisions about hospitalization is useful if the occurrence of biphasic reaction can be predicted from the characteristics of symptoms and number of systems of symptoms. However, the results of the present study did not support the hypothesis that the number of systems of symptoms involved is associated with the occurrence of biphasic reaction. We supposed that the hypothesis was not proven because of complex mechanisms underlying anaphylactic symptom development and because subjective symptoms vary among patients. It would be ideal to be able to predict the occurrence of a biphasic reaction from the background and clinical manifestations of the initial response in each patient. However, this currently seems difficult.
Treatment with adrenaline was not associated with the onset of biphasic reactions. Adrenaline is the most effective treatment of anaphylaxis with different guidelines emphasizing it as the first‐line treatment. 17 , 18 As administrations of adrenaline were all intramuscularly injected and single in this study, we were unable to examine whether the incidence of biphasic reaction changed depending on the method or amount. Delay in administering adrenaline, inadequate adrenaline dose given for the initial response, or large adrenaline dose requirement could increase the incidence of a biphasic reaction. 3 In this study, records about time from the onset/initial visit to adrenaline injection were insufficient, making it impossible to analyze this information. Adrenaline is evidently useful, and the result in this study should not be interpreted to controvert its use. As adrenaline is not commonly given in practice, 18 ensuring that adrenaline is used in cases for which it is indicated is necessary. Adrenaline potentially decreases the risk of biphasic reactions. 3 The need for adrenaline injection was recognized in a patient who was exposed to a likely allergen who experienced only a single system of symptoms (e.g., cutaneous). 17 The use of adrenaline in more cases might have reduced the incidence of biphasic reactions in this study.
Corticosteroids are used in many cases for treating anaphylaxis. In this study, corticosteroids were used in 111 of 120 patients with anaphylaxis (92.5%); this proportion was greater than that for adrenaline, which should be given a higher priority. Corticosteroids are theoretically predicted to be effective in preventing biphasic reactions because corticosteroids suppress immune responses. Corticosteroid use was reported to be effective in preventing biphasic reactions, 19 and studies in Japan also have reported that it is likely to be effective, 20 , 21 although no significant differences were determined. A 2015 practice parameter update stated that corticosteroid use has no role in the acute management of anaphylaxis. 17 Additionally, there are reports on corticosteroid‐induced anaphylaxis. 22 Montoro et al. 23 reported that the incidence of immediate reaction in patients receiving oral/parenteral corticosteroid treatment was 0.3%. Thus, discontinuing routine corticosteroid use could be better if appropriately timed adrenaline injection is ensured.
Cases of biphasic anaphylactic reaction characterized by increased symptom severity in the second phase than in the initial phase and even fatal cases have been reported, albeit rare. 3 However, symptom intensity in the second phase is generally comparable to/weaker than that in the initial phase. 6 , 19 One study reported that the frequency of clinically important events was rare and that there were no fatalities. 4 Most secondary responses occur within 8 h after resolving the first event. 3 , 5 , 6 , 19 Considering the onset of biphasic reaction, 8‐h follow‐up observation is recommended. 3 , 21 However, once symptoms improve, keeping the patient in ED for a prolonged period for follow‐up observation is difficult because of limited bed and manpower availability. Therefore, patients with anaphylaxis need to be hospitalized. However, some patients do not wish to be hospitalized, and some cannot be hospitalized for various reasons. Hospitalization could negatively affect patients’ social life. It is a “clinical dilemma” to decide whether to hospitalize patients with anaphylaxis. If we can predict the onset of biphasic reactions, we might be able to reduce unnecessary hospitalization. The clinical impact of this study might be small because of the negative results and limited study setting.
A prospective, multicenter study on biphasic anaphylactic reactions investigating the incidence and risk factors is necessary in Japan.
Limitation
This was a single‐center, retrospective observational study; the sample could have been skewed toward relatively mild anaphylaxis cases. The sample size was small; only adult patients were included. Non‐anaphylaxis cases with anaphylaxis‐like symptoms might have been included; thus, we cannot rule out the contribution of such cases to the results. Such clinical diagnosis can be erroneous. As the clinical practice guidelines for anaphylaxis in Japan were published in 2014 and there was no clear protocol for treating anaphylaxis before that, adrenaline might not have sufficiently become common use. We did not follow‐up patients after discharge, suggesting that biphasic reactions occurring after discharge were overlooked.
CONCLUSION
We found no association between the number of systems of symptoms and the incidence of biphasic reactions. Currently, predicting the onset of biphasic reactions is difficult. Further prospective and nationwide studies are required to research biphasic reactions.
DISCLOSURE
Approval of the research protocol: This study was approved by the ethics committee of Juntendo University Nerima Hospital.
Informed consent: Opt‐out done.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interest: None.
Funding informationNo funding information provided.
REFERENCES
- 1. Sampson HA. Anaphylaxis and emergency treatment. Pediatrics 2003; 111(6 Pt 3): 1601–8. [PubMed] [Google Scholar]
- 2. Lieberman P. Biphasic anaphylactic reactions. Ann. Allergy Asthma Immunol. 2005; 95: 217–26. [DOI] [PubMed] [Google Scholar]
- 3. Tole JW, Lieberman P. Biphasic anaphylaxis: review of incidence, clinical predictors, and observation recommendations. Immunol. Allergy Clin. North Am. 2007; 27: 309–26. [DOI] [PubMed] [Google Scholar]
- 4. Grunau BE, Li J, Yi TW et al Incidence of clinically important biphasic reactions in emergency department patients with allergic reactions or anaphylaxis. Ann. Emerg. Med. 2014; 63: 736–44.e2. [DOI] [PubMed] [Google Scholar]
- 5. Scranton SE, Gonzalez EG, Waibel KH. Incidence and characteristics of biphasic reactions after allergen immunotherapy. J. Allergy Clin. Immunol. 2009; 123: 493–8. [DOI] [PubMed] [Google Scholar]
- 6. Confino‐Cohen R, Goldberg A. Allergen immunotherapy‐induced biphasic systemic reactions: Incidence, characteristics, and outcome: A prospective study. Ann. Allergy Asthma Immunol. 2010; 104: 73–8. [DOI] [PubMed] [Google Scholar]
- 7. Brady WJ, Luber S, Joyce TP. Multiphasic anaphylaxis: Report of a case with prehospital and emergency department considerations. J. Emerg. Med. 1997; 15: 477–81. [DOI] [PubMed] [Google Scholar]
- 8. Lee S, Bellolio MF, Hess EP, Campbell RL. Predictors of biphasic reactions in the emergency department for patients with anaphylaxis. J. Allergy Clin. Immunol. Pract. 2014; 2: 281–7. [DOI] [PubMed] [Google Scholar]
- 9. Brown SGA, Stone SF, Fatovich DM et al Anaphylaxis: clinical patterns, mediator release, and severity. J. Allergy Clin. Immunol. 2013; 132: 1141–9.e5. [DOI] [PubMed] [Google Scholar]
- 10. Steele R, Camacho‐Halili M, Rosenthal B, Davis‐Lorton M, Aquino M, Fonacier L. Anaphylaxis in the community setting: determining risk factors for admission. Ann. Allergy Asthma Immunol. 2012; 109: 133–6. [DOI] [PubMed] [Google Scholar]
- 11. Sampson HA, Muñoz‐Furlong A, Campbell RL et al Second symposium on the definition and management of anaphylaxis: summary report – Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium. Ann. Emerg. Med. 2006; 47: 373–80. [DOI] [PubMed] [Google Scholar]
- 12. Muraro A, Roberts G, Clark A et al The management of anaphylaxis in childhood: position paper of the European academy of allergology and clinical immunology. Allergy Eur. J. Allergy Clin. Immunol. 2007; 62: 857–71. [DOI] [PubMed] [Google Scholar]
- 13. Japanese Society of Allergology . Anaphylaxis guideline [Internet]. 2014. [cited 8 Nov 2020]. Available from: https://anaphylaxis-guideline.jp/pdf/anaphylaxis_guideline.PDF.
- 14. Kanda Y. Investigation of the freely available easy‐to‐use software “EZR” for medical statistics. Bone Marrow Transplant. 2013; 48: 452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Popa VT, Lerner SA. Biphasic systemic anaphylactic reaction: three illustrative cases. Ann. Allergy 1984; 53: 151–5. [PubMed] [Google Scholar]
- 16. Lee S, Bellolio MF, Hess EP, Erwin P, Murad MH, Campbell RL. Time of onset and predictors of biphasic anaphylactic reactions: a systematic review and meta‐analysis. J. Allergy Clin. Immunol. Pract. 2015; 3: 408–16.e1. [DOI] [PubMed] [Google Scholar]
- 17. Lieberman P, Nicklas RA, Randolph C et al Anaphylaxis—a practice parameter update 2015. Ann. Allergy Asthma Immunol. 2015; 115: 341–84. [DOI] [PubMed] [Google Scholar]
- 18. Simons FER, Ebisawa M, Sanchez‐Borges M et al World allergy organization anaphylaxis guidelines: 2015 update of the evidence base. World Allergy Organ. J. 2015; 8: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ellis AK, Day JH. Incidence and characteristics of biphasic anaphylaxis: a prospective evaluation of 103 patients. Ann. Allergy Asthma Immunol. 2007; 98: 64–9. [DOI] [PubMed] [Google Scholar]
- 20. Nakanishi K, Nagai Y, Akimoto T et al Clinical analysis of anaphylaxis patients in an Emergency Medical Center. JJAAM 2006; 17: 137–41. [Google Scholar]
- 21. Oya S, Nakamori T, Kinoshita H. Incidence and characteristics of biphasic and protracted anaphylaxis: evaluation of 114 inpatients. Acute Med Surg. 2014; 1: 228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moreno Escobosa MC, Cruz Granados S, Moya Quesada MC, Amat López J. Anaphylaxis due to methylprednisolone. J. Investig. Allergol. Clin. Immunol. 2008; 18: 407–8. [PubMed] [Google Scholar]
- 23. Montoro J, Valero A, Serra‐Baldrich E, Amat P, Lluch M, Malet A. Anaphylaxis to paramethasone with tolerance to other corticosteroids. Allegy 2000; 55: 197–8. [DOI] [PubMed] [Google Scholar]


