Abstract
Aim
The coronavirus disease 2019 (COVID-19) pandemic presents significant challenges to healthcare systems globally. Orthopaedic surgeons are at risk of contracting COVID-19 due to their close contact with patients in both outpatient and theatre environments. The aim of this review was to perform a literature review, including articles of other coronaviruses, to formulate guidelines for orthopaedic healthcare staff.
Methods
A search of Medline, EMBASE, the Cochrane Library, World Health Organization (WHO), and Centers for Disease Control and Prevention (CDC) databases was performed encompassing a variety of terms including ‘coronavirus’, ‘covid-19’, ‘orthopaedic’, ‘personal protective environment’ and ‘PPE’. Online database searches identified 354 articles. Articles were included if they studied any of the other coronaviruses or if the basic science could potentially applied to COVID-19 (i.e. use of an inactivated virus with a similar diameter to COVID-19). Two reviewers independently identified and screened articles based on the titles and abstracts. 274 were subsequently excluded, with 80 full-text articles retrieved and assessed for eligibility. Of these, 66 were excluded as they compared personal protection equipment to no personal protection equipment or referred to prevention measures in the context of bacterial infections.
Results
There is a paucity of high quality evidence surrounding COVID-19. This review collates evidence from previous coronavirus outbreaks to put forward recommendations for orthopaedic surgeons during the COVID-19 pandemic. The key findings have been summarized and interpreted for application to the orthopaedic operative setting.
Conclusion
For COVID-19 positive patients, minimum suggested PPE includes N95 respirator, goggles, face shield, gown, double gloves, and surgical balaclava.
Space suits not advised.
Be trained in the correct technique of donning and doffing PPE.
Use negative pressure theatres if available.
Minimize aerosolization and its effects (smoke evacuation and no pulse lavage).
Minimize further unnecessary patient-staff contact (dissolvable sutures, clear dressings, split casts).
Keywords: COVID-19, coronavirus, SARS-CoV-2, personal protective equipment, facemasks, orthopaedics, theatre
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has created unprecedented challenges to healthcare systems globally.1,2 The coronavirus primarily targets the human respiratory system; symptoms are wide-ranging from symptoms of the common cold to severe respiratory distress requiring escalation of care and ventilation. COVID-19 is the third known zoonotic coronavirus disease after severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS).3
Personal protective equipment (PPE) remains a major concern for healthcare personnel with the percentage of cases in healthcare workers being as high as 20%.4 Orthopaedic teams are at risk of contracting COVID-19 from a variety of sources in both inpatient and outpatient settings. Orthopaedic staff have close patient contact during the management of both nonoperative orthopaedic cases (casting and manipulation) and operative environments (power tools), with the latter generating aerosols which may contain viable virus.5
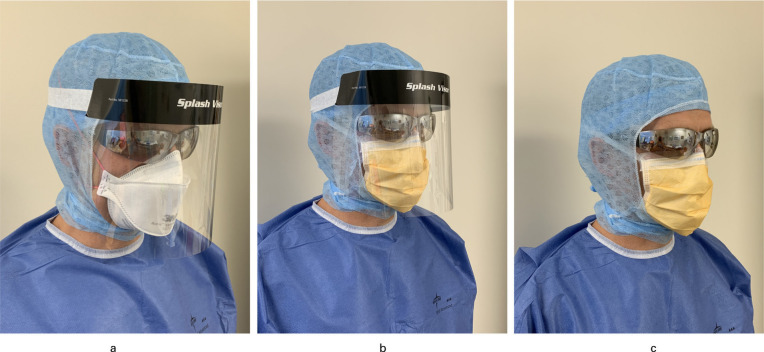
A variety of respiratory protection devices are on the market with the most common ones encountered in healthcare being the surgical mask, the N95 respirator (3MM1860) and the Powered Air Purifying Respirator (PAPR). Routine surgical masks are considered disposable coverings which protect the mouth and nose, and are not fit-tested. This is in contrast to respirators which are fit-tested.6 PAPRs have a higher assigned protective factor than N95 Respirators and are reusable7 (APF = 25 for loose-fitting, 50 for half mask and 100 for full facepiece vs N95 APF = 10). AFP is a number assigned by the Occupational Safety and Health Administration, USA, and refers to the level of protection each respirator is expected to provide. An APF of 50 will reduce the exposure of the user to 1/50th the concentration of the contaminant in the air.8 Figure 1 shows both the surgical mask and N95 respirator in use.
Fig. 1.
Advised PPE use in different scenarios. a) COVID-19 positive case and suspected cases: N95 respirator, goggles, surgical balaclava, face shield, gown and double gloves. b) Non-COVID-19 case with high risk of aerosolization: taped surgical mask, goggles, surgical hood, face shield, gown and double gloves. c) Non COVID-19 case with low risk of aerosolization: taped surgical mask, goggles, surgical hood, gown and double gloves.
The SARS coronavirus 2 (SARS-CoV-2) demonstrates similar transmission to that of other coronaviruses between carriers through respiratory droplets and contact routes9-11 and the viral shedding pattern in symptomatic patients has been described as resembling that of influenza.12 Coronavirus transmission can occur in two ways: via direct and indirect contact. Direct contact occurs via droplet transmission from close contact (within one metre) with someone who has respiratory symptoms (i.e. cough or sneeze). These infective respiratory droplets have risk of contacting mucosal surfaces (mouth and nose) or conjunctiva (eyes). Indirect contact occurs via fomites (objects/surfaces) in the immediate environment around the infected individual.13 COVID-19 viral RNA has been detected in the blood of a small number of patients,14 however, there have been no documented cases of blood borne transmission.
The World Health Organization (WHO) recommends airborne precautions for settings in which aerosol generating procedures and treatments are performed, based on risk assessment.13 However, there is no current clear guideline for prevention measures that can be taken by orthopaedic surgeons in the operating theatre to reduce the risk of COVID-19 transmission. There is a pressing need to establish the evidence base around Orthopaedic procedures.
The aim of this review is to inform ‘best practice’ preventative measures to minimize staff and patient exposure to COVID-19.
Methods
Medline, EMBASE, the Cochrane Library, WHO, and Centers for Disease Control and Prevention (CDC) database searches were performed by two independent reviewers (TB, SB). The search included articles and guidelines published between 2001 and following inclusion terms: ‘COVID-19’, ‘coronavirus’, ‘influenza’, ‘SARS’, ‘severe acute respiratory distress syndrome’, ‘MER’ and ‘middle eastern respiratory distress syndrome’. Secondary filters included ‘orthopaedics’, ‘surgery’, ‘personal protective equipment’, ‘PPE’, ‘positive pressure’, ‘negative pressure’, ‘theatre’, ‘space suit’, ‘aerosols’, ‘aerosolization’, ‘smoke’, ‘laminar flow’, ‘training’. All English full-text articles were included.
Articles were included if they studied any of the other coronaviruses or if the basic science could potentially applied to COVID-19 (i.e. use of an inactivated virus with a similar diameter to COVID-19).
Two reviewers independently identified and screened 354 articles based on the titles and abstracts. 274 were subsequently excluded, with 80 full-text articles retrieved and assessed for eligibility. Of these, 66 were excluded as they compared personal protection equipment to no personal protection equipment or referred to prevention measures in the context of bacterial infections.
Results
Personal protective equipment (PPE)
While a Cochrane review15 and numerous studies were identified regarding the use of PPE, including use in the SARS epidemic16-19, most articles demonstrated the importance of different facets of the PPE (gown-/-surgical mask-/hand washing/gloves) but mostly contrasted with subjects not wearing PPE. This is of limited applicability in the orthopaedic theatre setting as hand washing, gown, surgical mask, and gloves are standardized practice for all theatre cases.
Masks and respirators
Articles on PPEs are related to previous pandemics. Noti et al demonstrated increased efficacy of a tightly sealed N95 respirator (3MM1860) compared to a tightly sealed mask (Kimberly Clark 47625) in a laboratory simulation for influenza virus (virus elimination 99.8% vs 94.5%).20 A poorly fitting N95 respirator had the worst performance (64.5%). Park et al reported the use of respirators in their standard PPE equipment during the MERS outbreak.21 However, a recent systematic review and meta-analysis suggested N95 respirators did not perform any better than surgical masks (RR = 0.37; 95% CI 0.33 to 1.14; p > 0.05).22
Wong et al described the use of PAPRs when members of staff failed their N95 fit testing.23 However, to the best of the authors' knowledge, there have been no clinical studies that demonstrate superiority with the use of PAPR compared to N95 respirators in preventing viral infections.
Space suits
Derrick & Gomersall showed that surgical helmets commonly used with space suits (Stryker T4 and Stackhouse FreedomAire surgical helmets) were inadequate at filtering sub-micrometre-sized particles in performance testing (N100 mask reduces particle count by a minimum of a factor of 100 vs surgical helmet-hood of 4.8).24
Donning and soffing PPE
Hannum et al compared three groups of hospital employees who underwent a variety of respirator training (A: One-to-one training, B: Classroom instruction, C: No formal training). Both Groups A and B had higher pass rates on qualitative fit testing opposed to C (94% to 91% vs 79%, p = 0.036).25
Phan et al observed healthcare workers, varying from nurses, doctors, and students, in day-to-day practice. They showed that, upon observing staff removing their PPE, 90% performed this incorrectly.26 Common mistakes included removal of the gown incorrectly, removing the face shield of the mask, and touching potentially contaminated surfaces.
Zamora et al examined the difference in self contamination rates with participants wearing enhanced respiratory and contact precautions (respirator, goggles, face shield, gown, neck towel and gloves) versus a full-bodied PAPR in a prospective, randomized, controlled crossover study. Participants wearing the former were more likely to experience skin/base clothing contamination and had larger total areas of contamination (p < 0.0001). However, participants donning PPAR committed more procedure violations (p = 0.0034) as well as the procedure of donning & doffing taking considerably longer (p < 0.0001).27
Theatre airflow
Two articles discuss the use of negative pressure theatres, and their conversion from positive pressure theatres, during the MERS28 and SARS21 epidemics, with the theoretical advantage of preventing the spread of the virus outside of the theatre. No articles confirm clinical benefit nor that positive pressure theatres lead to viral infections.
Hsing-Wang et al studied the removal and retention of viral aerosols by comparing an alumina nanofiber filter with commercially available high efficiency particulate arrestor (HEPA) filters.29 HEPA filters are commonly used in laminar flow rooms as part of the ventilation system.30 Coronavirus has a diameter of 60-120nm1 and they demonstrated that three HEPA filters had an efficiency of > 95% with particles of 60 nm in diameter, with one HEPA filter having an efficiency of close to 100%.29
Orthopaedic devices
Jewett et al demonstrated that orthopaedic devices, such as the Stryker (Michigan, USA) bone saw and Hall drill, produced blood containing aerosols of less than 5 μm in diameter.5 A review article by Liu et al highlighted the presence of viruses in surgical smoke from diathermy, including human immunodeficiency virus (HIV) and human papillomaviruses (HPV).31
Discussion
There is a paucity of high quality evidence surrounding COVID-19. This review collates evidence from previous coronavirus outbreaks to put forward recommendations for orthopaedic surgeons during the COVID-19 pandemic. The former is possible as both routes of transmission9-11 and viral shedding pattern mimic that of other coronaviruses.12 These are summarized in Table I.
Table I.
Summary of main recommendations
| Summary of main recommendations |
|---|
| For COVID+ ve patients, minimum suggested PPE includes: N95 respirator, goggles, face shield, gown, double gloves, surgical balaclava5,20 |
| Do not use a space suit1,24 |
| Be trained in the correct technique of donning and doffing PPE25,26 |
| Use negative pressure theatres if available21,28 |
| Minimize aerosolization and its effects (smoke evacuation and no pulse lavage)14,31 |
| Minimize further unnecessary patient-staff contact (dissolvable sutures, clear dressings, split casts) |
It is commonly believed that respirators (i.e. N95) would routinely perform better than surgical masks across all settings. However, laboratory and clinical trials findings are contradictory, with evidence centred around influenza cases. In a laboratory-based study, the authors evaluated the effectiveness of surgical masks and N95 respirators in preventing viral infection using manikins. The findings from this study showed that sealed masks were not as effective as sealed N95 respirators at blocking influenza virus (94.5% compared with 99.8% blocked).20 To the contrary, a recent meta-analysis of randomized clinical trials showed no statistical significance between surgical masks and N95 respirators.22 However, it is important to note that these trials were not conducted in the theatre environment. Healthcare professionals are exposed to aerosols in the theatre through the use of orthopaedic devices.5 It is on this basis that the authors recommend healthcare professionals wear N95 masks in confirmed and suspected cases of COVID 19. In non-COVID 19 cases, the use of surgical masks, rather than the N95 masks, is recommended.
PAPRs theoretically offer increased protection compared with N95 masks7 and have been considered an alternative for individuals who fail N95 fit testing.23 The PAPR respirator coupled with the full body suit has been shown to have reduced skin/base layer contamination rates than the respirator/gown/goggles/face shield combination.27 However, no clinical evidence was found that the respirator itself had superiority over N95 respirators. Other important considerations are that PAPRs are more expensive32 and reusable. They require desterilization after each case and are more cumbersome to put on and remove, potentially leading to contamination if performed incorrectly.27 Considering this, the authors recommend the use of N95 respirators with showering and changing of base layer straight after the procedure due to the theoretical risk of base-layer contamination.
Considering eyes and contact transmission routes the authors advocate the use of goggles and face shields. It is important to note that this is the authors’ preference, with Public Health England advocating either the use of goggles or face shield.33 Both are recommended in the non-COVID 19 case and in high aerosolization procedures, but it is at the surgeon’s discretion if they decide not to use the face shield in procedures without high-energy devices. For all cases, it is recommended that a surgical balaclava, gown and double gloves are used as standard practice to minimize potential skin contact with the virus.
A demonstration of full PPE is shown in Figure 1.
Numerous articles have described the importance of training in PPE in both donning and doffing.25,26 It is particularly important in the case of N95 respirators, with an ill-fitting mask providing sub-optimal protection.20 It is recommended that formalized training, whether that be one-to-one or group sessions, is provided.
Surgical ‘space suits’ with an open fan have been reviewed as a potential alternative to PPE. Coronavirus has a diameter which varies between 60 to 140 nm1 and space suits poorly filter sub micrometre particles in the laboratory setting,24 and as such are considered to not confer adequate PPE. The authors do not recommend that space suits are used with the fan system deactivated. Theatre staff who commonly use space suits will have experienced ‘battery drop-out’ where the space suit environment becomes hot and humid, causing impaired vision on the face shields. Finally, removing the space suit can be cumbersome and potentially increases the risk of aerosolization of the virus. Therefore, the authors do not recommend the use of space suits with or without the fan system.
The use of negative pressure theatres is well documented, in both the SARS28 and MERS21 epidemic theatres. Theatres were converted from positive to negative environments. The level of evidence is low, but the theoretical advantage is the protection of staff working in adjacent areas. With regards to positive pressure theatres, there is the theoretical possibility of the viruses being transmitted to adjacent areas. However, no evidence was identified to support or refute this. HEPA filters are incorporated in orthopaedic theatres and have been shown to demonstrate an efficiency of > 95% in filtering 60 nm particles, with one filter close to 100% in the laboratory setting.29 It is unclear if this is transferable to the clinical setting. The authors therefore interpret the available information on positive pressure theatres with caution. The recommendation of the use of negative pressure theatres is on the basis of theoretical advantage and prior use in other epidemics. The authors cannot support or dispute the use of positive pressure theatres due to the paucity of evidence in this area.
It is advised that surgeons minimize the risk of aerosolization during all procedures. While COVID-19 viral RNA has been shown to be present in a number of patients there have been no documented cases of blood borne transmission.14 Unnecessary aerosolization may lead to the theoretical spread of viral RNA. Therefore, the authors recommend avoiding pulse lavage and promote the use of smoke evacuation during electrocautery. Despite the other potential health issues associated with surgical smoke, viruses have been shown to be present in smoke aerosols.31 Smoke evacuation devices are cheap and remove air, smoke, and sub-micron particles from the surgical field.
Other practical considerations are to use absorbable sutures whenever possible to limit the patient’s return to clinic and/or prevent prolonged close contact for nursing staff when removing staples/sutures. Likewise, it is recommended to use clear dressings and consider the use of surgical glue, rather than steri-strips, so wounds can be reviewed easily. Unnecessary dressing changes should be avoided and removable casts are also advised.
In conclusion, there is an appreciative lack-of high level evidence at present. The recommendations are based on previous studies on other coronaviruses as well as considering the practicality of the suggestions put forward.
Footnotes
Author contributions: T. E. Baldock: Independently performed the initial search and reviewed articles, Subsequently reviewed relevant articles and wrote the original draft, Reviewed and edited the manuscript.
S. M. Bolam: Independently performed the initial search and reviewed articles, Subsequently reviewed relevant articles and wrote the original draft, Reviewed and edited the manuscript.
R. Gao: Reviewed relevant articles, Reviewed and edited the manuscript.
M. F. Zhu: Reviewed relevant articles, Reviewed and edited the manuscript.
M. P. J Rosenfeldt: Reviewed relevant articles, Reviewed and edited the manuscript.
S. W. Young: Reviewed relevant articles, Reviewed and edited the manuscript.
J. T. Munro: Curated the initial idea, Reviewed relevant articles and reviewed and edited the manuscript, Supervised the project.
A. P. Monk: Curated the initial idea, Reviewed relevant articles and reviewed and edited the manuscript, Supervised the project.
Funding statement: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest: All authors declare no conflict of interest.
References
- 1.Zhu N, Zhang D, Wang W, et al. . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID-19 based on current evidence: John Wiley and Sons Inc., 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Lancet The Lancet COVID-19: protecting health-care workers. Lancet. 2020;395(10228):922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jewett DL, Heinsohn P, Bennett C, Rosen A, Neuilly C. Blood-Containing aerosols generated by surgical techniques: a possible infectious hazard. Am Ind Hyg Assoc J. 1992;53(4):228–231. [DOI] [PubMed] [Google Scholar]
- 6.Health C for D and R N95 Respirators and Surgical Masks (Face Masks). FDA [Internet]. 2020. http://www.fda.gov/medical-devices/personal-protective-equipment-infection-control/n95-respirators-and-surgical-masks-face-masks (date last accessed 2 Apr 2020).
- 7.Occupational Safety and Health Administration Assigned Protection Factors for the Revised Respiratory Protection Standard [Internet]. Occupational Safety and Health Administration; [cited p. 5. Report No.: OSHA 3352-02 2009. 2009. https://www.osha.gov/Publications/3352-APF-respirators.pdf (date last accessed 1 Apr 2020).
- 8.Respirator Types Occupational Safety and Health Administration [Internet]. 2020. https://www.osha.gov/video/respiratory_protection/resptypes_transcript.html (date last accessed 14 Apr 2020).
- 9.Liu J, Liao X, Qian S, et al. . Community Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, Shenzhen, China, 2020. Emerg Infect Dis [Internet]. 2020. Jun;26(6). Available from:. http://www.ncbi.nlm.nih.gov/pubmed/32125269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan JF-W, Yuan S, Kok K-H, et al. . A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou L, Ruan F, Huang M, et al. . SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations [Internet]. 2020. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (date last accessed 31 Mar 2020).
- 14.Chen W, Lan Y, Yuan X, et al. . Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect. 2020;9(1):469–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verbeek JH, Rajamaki B, Ijaz S, et al. . Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst Rev. 2019;2(12). Jul 1 [cited 2020 Apr 2]; Available from:[Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teleman MD, Boudville IC, Heng BH, Zhu D, Leo YS. Factors associated with transmission of severe acute respiratory syndrome among health-care workers in Singapore. Epidemiol Infect. 2004;132(5):797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho AS, Sung JJY, Chan-Yeung M. An outbreak of severe acute respiratory syndrome among hospital workers in a community hospital in Hong Kong. Ann Intern Med. 2003;139(7):564–567. Oct 7. [DOI] [PubMed] [Google Scholar]
- 18.Lau JTF, Fung KS, Wong TW, et al. . Sars transmission among hospital workers in Hong Kong. Emerg Infect Dis. 2004;10(2):280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seto WH, Tsang D, Yung RWH, et al. . Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). The Lancet. 2003;361(9368):1519–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noti JD, Lindsley WG, Blachere FM, et al. . Detection of infectious influenza virus in cough aerosols generated in a simulated patient examination room. Clinical Infectious Diseases. 2012;54(11):1569–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park J, Yoo SY, Ko J-H, et al. . Infection prevention measures for surgical procedures during a middle East respiratory syndrome outbreak in a tertiary care hospital in South Korea. Sci Rep. 2020;10(1):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long Y, Hu T, Liu L, et al. . Effectiveness of N95 respirators versus surgical masks against influenza: a systematic review and meta-analysis. J Evid Based Med. 2020. Mar 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong J, Goh QY, Tan Z, et al. . Preparing for a COVID-19 pandemic: a review of operating room outbreak response measures in a large tertiary hospital in Singapore. Can J Anesth/J Can Anesth. 2020;395 Mar 11 [cited 2020 Apr 1]; Available from:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derrick JL, Gomersall CD. Surgical helmets and SARS infection. Emerg Infect Dis. 2004;10(2):277–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannum D, Cycan K, Jones L, et al. . The effect of respirator training on the ability of healthcare workers to pass a qualitative fit test. Infect Control Hosp Epidemiol. 1996;17(10):636–640. [DOI] [PubMed] [Google Scholar]
- 26.Phan LT, Maita D, Mortiz DC, et al. . Personal protective equipment doffing practices of healthcare workers. J Occup Environ Hyg. 2019;16(8):575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zamora JE, Murdoch J, Simchison B, Day AG. Contamination: a comparison of 2 personal protective systems. Can Med Assoc J. 2006;175(3):249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow TT, Kwan A, Lin Z, Bai W. Conversion of operating theatre from positive to negative pressure environment. J Hosp Infect. 2006;64(4):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H-W, Wu C-Y, Tepper F, Lee J-H, Lee CN. Removal and retention of viral aerosols by a novel alumina nanofiber filter. J Aerosol Sci. 2009;40(1):65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51(2):79–84. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Song Y, Hu X, Yan L, Zhu X. Awareness of surgical smoke hazards and enhancement of surgical smoke prevention among the gynecologists. J Cancer. 2019;10(12):2788–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Board on Health Sciences Policy; Institute of Medicine The Use and Effectiveness of Powered Air Purifying Respirators in Health Care: Workshop Summary In Washington (DC): National Academies Press (US), 2015. [PubMed] [Google Scholar]
- 33.COVID-19: personal protective equipment use for aerosol generating procedures [Internet] GOV.UK. 2020. https://www.gov.uk/government/publications/covid-19-personal-protective-equipment-use-for-aerosol-generating-procedures (date last accessed 14 Apr 2020).