Abstract
Traditionally there have been differences in cancer incidence across geographic regions. When immigrants have moved from low-income to high-income countries, their incidence have changed as they have adapted to the lifestyle in the new host country. Given worldwide changes in lifestyle factors over time, we decided to examine cancer incidence in immigrant groups in Norway, a country with a recent immigration history, complete cancer registration and universal public health care. We linked immigration history for the complete population to information on cancer diagnosis from the Cancer Registry of Norway for the period 1990–2012. Age-standardized (world) overall and site-specific cancer incidence were estimated for different immigrant groups and compared to incidence among individuals born in Norway. Among 850,008 immigrants, 9,158 men and 10,334 women developed cancer, and among 5,508,429 Norwegian-born, 263,316 men and 235,020 women developed cancer. While incidence of breast and colorectal cancer were highest among individuals born in Norway and other high-income countries, other cancer types were higher in immigrants from low-income countries. Lung cancer incidence was highest in Eastern European men, and men and women from Eastern Europe had high incidence of stomach cancer. Incidence of liver cancer was substantially higher in immigrants from low-income countries than in individuals born in Norway and other high-income countries. Our results mirror known cancer challenges across the world. Although cancer incidence overall is lower in immigrants from low-income countries, certain cancers, such as lung, liver and stomach cancer, represent major challenges in specific immigrant groups.
Keywords: cancer incidence, immigration, risk factors
Cancer incidence and type distribution varies between countries and populations, with low- and middle-income countries, hereafter called low-income countries, generally having lower total cancer incidence than high-income countries.1 Infection-related cancer types, such as liver, stomach and cervical cancers, have traditionally been more common in low-income countries, while cancers predominantly caused by a high-income lifestyle, such as lung, colorectal and breast cancers, have dominated in high-income countries.2 However, in recent decades there has been a trend toward increasing occurrence of certain lifestyle-related cancers, such as lung and breast, in low-income countries.1
Early studies of immigrants in the United States found that they have higher cancer incidence than those who stay in their country of origin,3–5 a finding supported by a more recent study of Indian immigrants in Great Britain.6 Cancer incidence starts to increase the first decade after migration, coinciding with major lifestyle changes, and reaches the level of the population of the host country in 1–2 generations.7–9
Not only are lifestyle risk factors reported to be lower in immigrants than in the population of the host country at time of immigration, but the overall health of immigrants have often been better than of those who stay in the country of origin, possibly as a result of selective migration, a phenomenon named the “healthy migrant effect.”10 With time since immigration, the immigrants adapt the new culture of the host population, which negates any “healthy migrant effect,” and cancer incidence rises. Further, given the changing cancer rates globally, with rises in lifestyle-related cancers particularly in low-income countries, an important question is whether the “healthy migrant effect” remains in people who migrated recently. If so, the first few years after migration offers a window of opportunity for cancer prevention, before the immigrants assimilate the higher risk behavior of the new host populations.11
Immigration to Norway started relatively recently. Before oil was discovered in the 1970s, Norway was a poor country, and the population of immigrants was negligible. With the economy boon of the 1970s, there was a rapid rise in the immigrant population,12 which is currently 16.3%.13 Immigration to Norway has been fragmented, both in relation to country of origin and reason for immigration, resulting in a heterogeneous immigrant population.12
The Norwegian setting is well suited for studies of cancer in immigrants because of the complete cancer registration and universal public health care coverage. Thus, we took advantage of the population based registries of country background and cancer to study cancer incidence rates in Norwegian immigrants and individuals born in Norway.
Methods
Cohort and follow-up
The cohort consisted of all individuals born between 1882 and 2011 who were registered as residents of Norway for 12 or more months during the period 1990–2012. In order to define the cohort and link various registries, we used the personal identification number (PIN) assigned all Norwegian-born at birth (or 6 months after immigration). Information on country of origin, country of origin of parents and grandparents, and date of immigration was obtained from the Norwegian Population Registry—Statistics Norway. Immigrants were defined as individuals born outside of Norway with a registered date of immigration (first generation immigrants), and Norwegian-born were defined as individuals born in Norway. Included in the Norwegian-born population were individuals born in Norway of immigrant parents (second-generation immigrants; 2.3% in 2013, with 77% under the age of 1814). Immigrants were classified according to their country of origin. Countries were further collapsed into regions, broadly defined according to the WHO regional groupings,15 with some modifications based on number of immigrants from each country (Supplementary Material). We identified cancer cases in the data set during follow-up (January 1, 1990 to December 31, 2012) by linking the cohort to the Cancer Registry of Norway (CRN). Cancers are classified according to the 10th revision of the International Classification of Diseases (ICD-10), and we included codes C00-C96. The CRN has since 1952 systematically collected notifications on cancer occurrence for the Norwegian population, reporting has been mandatory by law since the start, and the registry is considered to be close to complete.16
Statistical analyses
The study population consisted of 5,508,429 Norwegian-born (95.9% of the person-years) and 850,008 immigrants (4.1% of the person-years). Individuals were followed from their birth date (Norwegian-born) or their date of immigration (immigrants) until diagnosis of cancer, death, emigration from Norway, or December 31, 2012, whichever occurred first.
We estimated age-standardized incidence rates (ASR) and 95% confidence intervals (CIs)17 by region of origin for immigrants, as well as for Norwegian-born, using the age distribution from the world standard population.18,19 The ASRs were calculated from 5-year age groups, sex and time periods (5-year bands). In comparing cancer ASRs between different immigrant groups and Norwegian-born, non-overlapping 95% CIs were considered to represent difference in cancer ASRs.20 We calculated rates for total (C00–96) excluding non-melanoma skin (C44) cancer, hereafter called total cancer, as well as for certain common cancer types with high incidence worldwide1 such as lung (C33–34), colorectal (C18–20), prostate (C61), breast (C50) and cervix (C53), as well as for stomach (C16) and liver (C22) cancers as they are known to be more prevalent in certain immigrant groups.21 As rates based on small counts tend to have poor statistical reliability,22 three regions (Rest Africa, Oceania and South/Central America) were only included in analyses of total cancer.
For immigrants, we repeated the analyses excluding the first five years of follow-up, to eliminate prevalent cancer cases. However, as this restriction did not change the results notably (ASRs overall 261.7 vs. 266.8 in men and 225.6 vs. 235.1 in women), we here present results with all available data.
To compare the immigrant groups to the rest of the population, we also calculated standardized incidence ratios (SIRs) as the ratio of observed to expected cases, using the incidence for all individuals born in Norway as the standard rate. CIs were calculated assuming a Poisson distribution.
All statistical analyses were carried out using Stata version 14.23
The study was approved by the Regional Committees for Medical and Health Research Ethics.
Results
Most immigrants to Norway during this period came from other European countries (Supporting Information Figure). Immigrants from the Nordic countries consisted largely of Swedes and Danes, and immigrants from other Western European countries originated mostly from Germany and Great Britain. Eastern Europe comprised the largest immigrant group, and Poland was the country of origin for most immigrants. Outside Europe, most immigrants originated from East Asia or Sub-Saharan Africa (mostly Somalia) closely followed by South Asia and the Middle East. The ratio of male to female immigrants varied considerably between countries. Among the Eastern European immigrants, 60% were men, while the number of Eastern Asian women was twice the number of Eastern Asian men. Median ages at immigration were 27 years for men and 25 years for women. Most immigrants (79% of men and 77% of women) were followed up 10 or more years before diagnosis of cancer.
A total of 498,336 cancer cases (263,316 in men and 235,020 in women) were recorded in Norwegian-born and 19,492 cancer cases (9,158 in men and 10,334 in women) in immigrants during the follow-up from 1990 to 2012. Figure 1 shows the ASRs for total cancer by region of origin. The Norwegian ASRs (298.3 in men and 248.8 in women) exceeded the ASRs for all immigrants combined (266.8 in men and 235.1 in women). Immigrants from other European countries had ASRs similar to Norwegian-born, whereas immigrants from non-European low-income countries had lower ASRs. Immigrant men had higher ASRs of lung cancer than Norwegian-born men (37.4 vs. 33.3), and especially high incidence was found among men from Eastern Europe (50.7; Fig. 2). Men from the other Nordic countries (41.6) also had higher rates than Norwegian-born men. Immigrant women had lower incidence (17.1) than Norwegian-born women (19.1), where women from high-income countries, for example, other Nordic (19.0) and other Western European countries (19.2), had similar ASRs to Norwegian-born women, and women from East Asia had a high ASR (17.9) compared with women from other low-income countries, for example, the Middle East (6.3) and South Asia (6.3).
Figure 1.
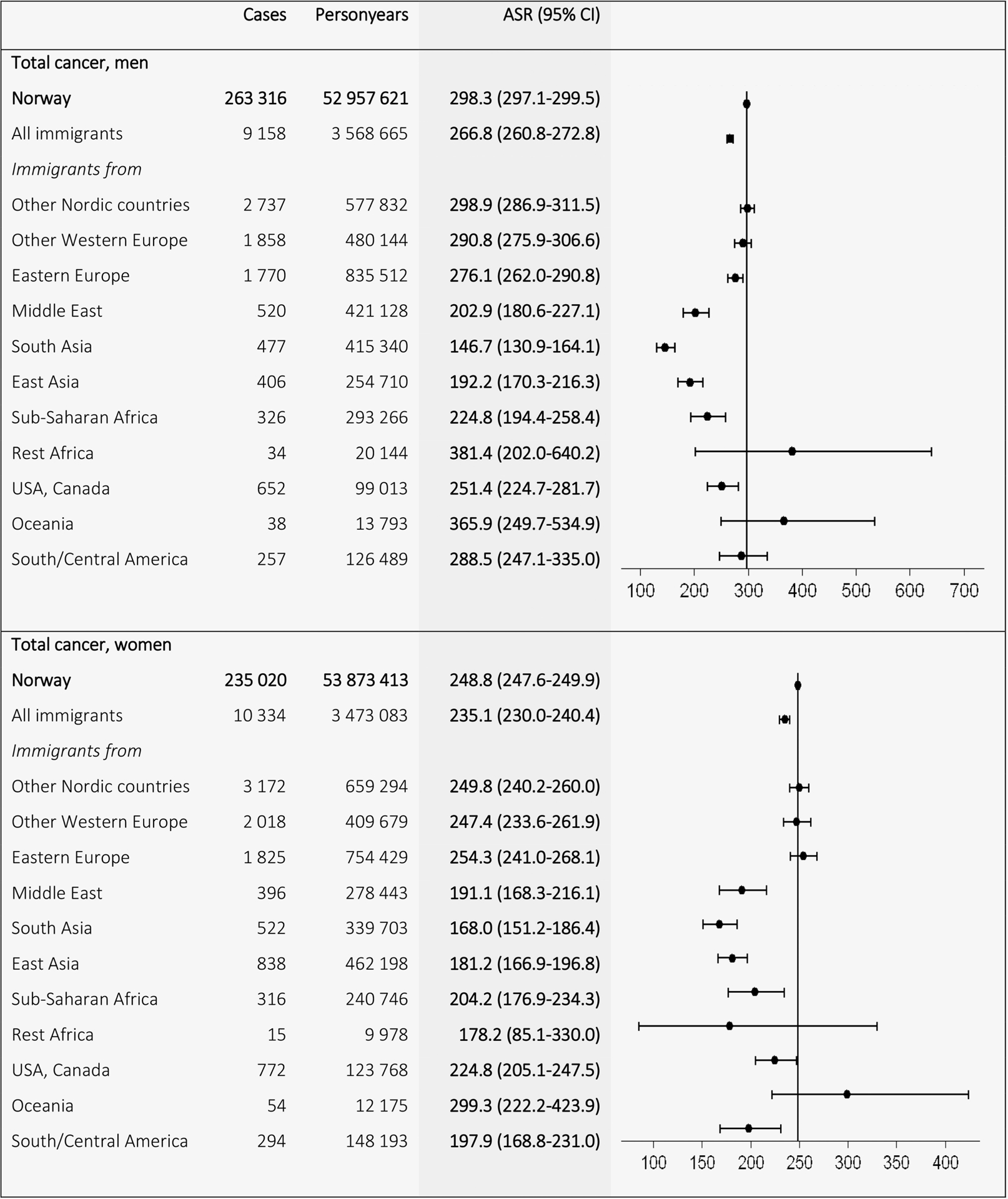
Age-standardized incidence rates (ASRs) with 95% confidence intervals (CIs) for total (C00–96) minus non-melanoma skin (C44) cancer by birth region. Standardized by the world standard population. Adjusted for calendar period (in 5-year intervals) and age (in 5-year categories).
Figure 2.
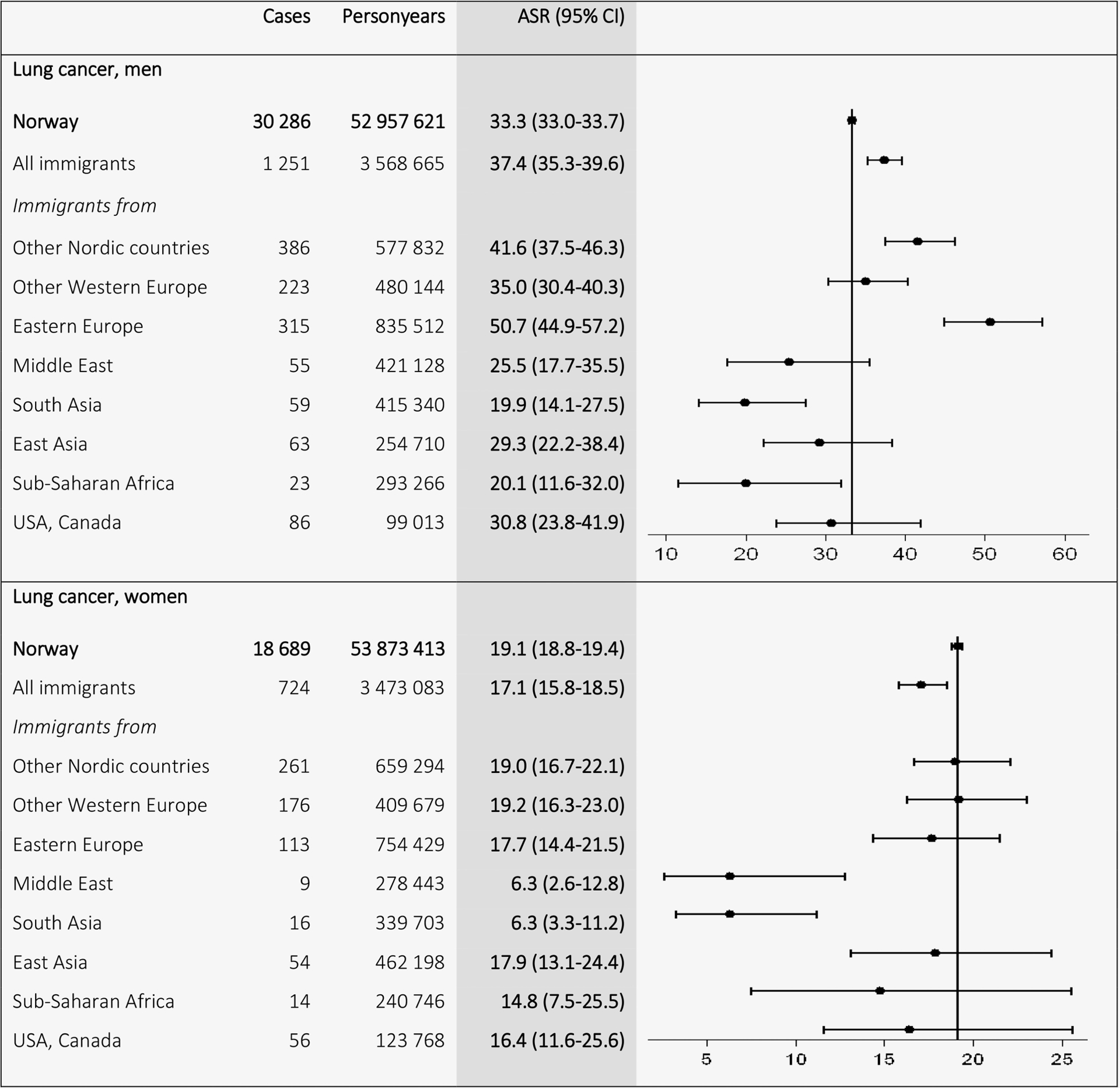
Age-standardized incidence rates (ASRs) with 95% confidence intervals (CIs) for lung cancer (C33–34) by birth region. Standardized by the world standard population. Adjusted for calendar period (in 5-year intervals) and age (in 5-year categories).
For colorectal cancer, Norwegian ASRs (38.8 in men and 30.7 in women) were higher than the ASRs among the different immigrant groups, especially among immigrants from low-income countries, where the lowest ASRs were found in immigrants from South Asia (7.2 in men and 9.2 in women) (Fig. 3). For prostate cancer, the Norwegian ASRs (78.5) exceeded ASRs for all immigrant groups however closely followed by men of African descent (71.1). Immigrants from other low-income countries, for example, East (26.8) and South (28.4) Asia, showed the lowest ASRs for prostate cancer (Fig. 4).
Figure 3.
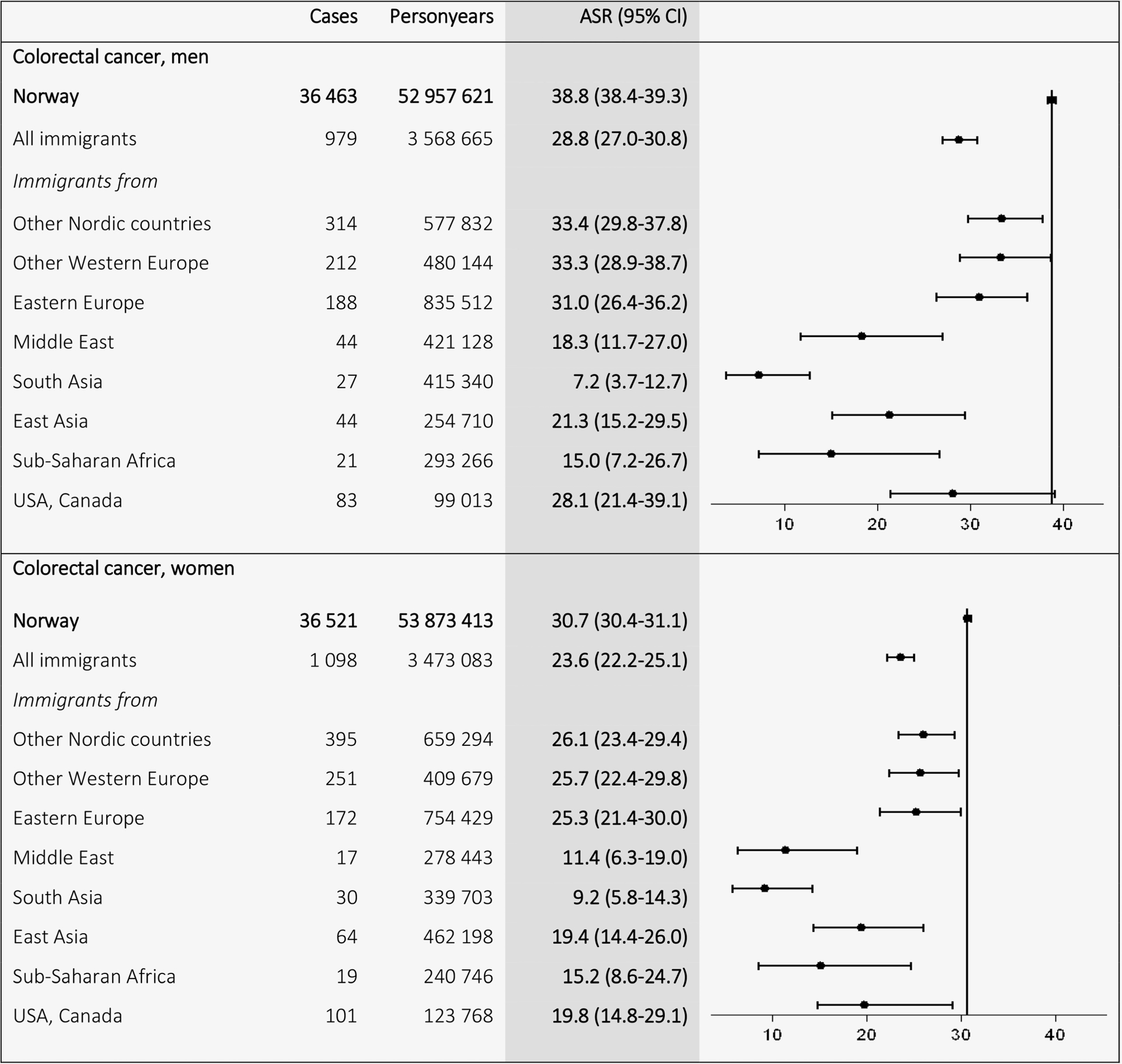
Age-standardized incidence rates (ASRs) with 95% confidence intervals (CIs) for colorectal cancer (C18–20) by birth region. Standardized by the world standard population. Adjusted for calendar period (in 5-year intervals) and age (in 5-year categories).
Figure 4.
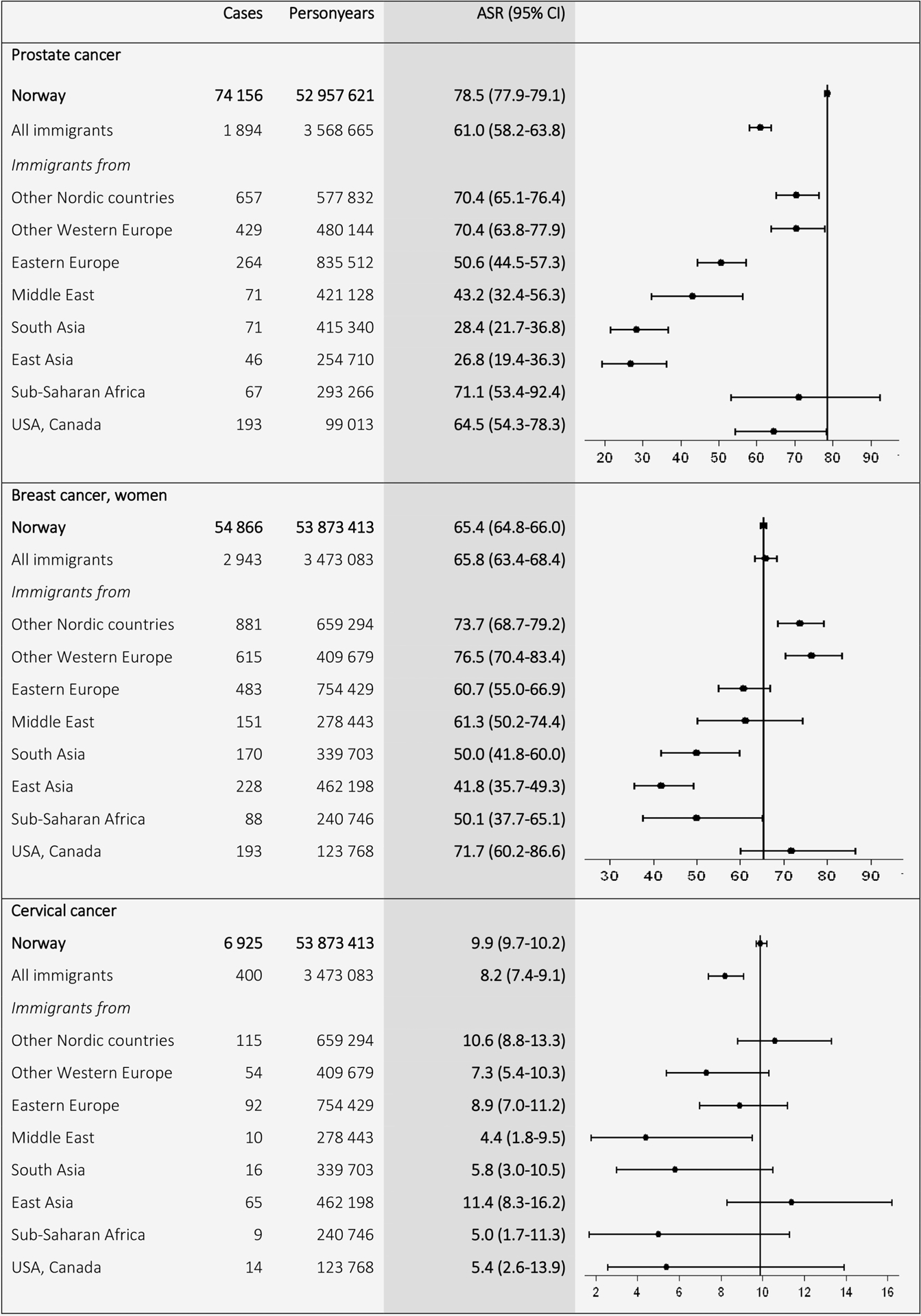
Age-standardized incidence rates (ASRs) with 95% confidence intervals (CIs) for prostate (C61), breast (C50), and cervical cancer (C53) by birth region. Standardized by the world standard population. Adjusted for calendar period (in 5-year intervals) and age (in 5-year categories).
For breast cancer, Nordic (73.7) and other Western European (76.5) women had higher ASRs than Norwegian-born women (65.4), who had similar ASRs to women from the Middle East (61.3) and Eastern Europe (60.7). The lowest ASRs were found in women from low-income countries, for example, East (41.8) and South (50.0) Asia and Sub-Saharan Africa (50.1; Fig. 4). Cervical cancer ASR for Norwegian-born (9.9) was higher than for all immigrants combined (8.2; Fig. 4).
The highest ASRs for stomach cancer (Fig. 5) were found among immigrants from Eastern Europe (13.4 in men and 7.5 in women), as compared to ASRs in Norwegian-born men (8.5) and women (4.2). For liver cancer (Fig. 6), the ASRs for immigrants from low-income countries exceeded the ASRs for Norwegian-born men and women. The highest ASRs were found in East Asian (19.7) and Sub-Saharan (15.3) men and in Asian (6.3) and Sub-Saharan (6.1) women.
Figure 5.
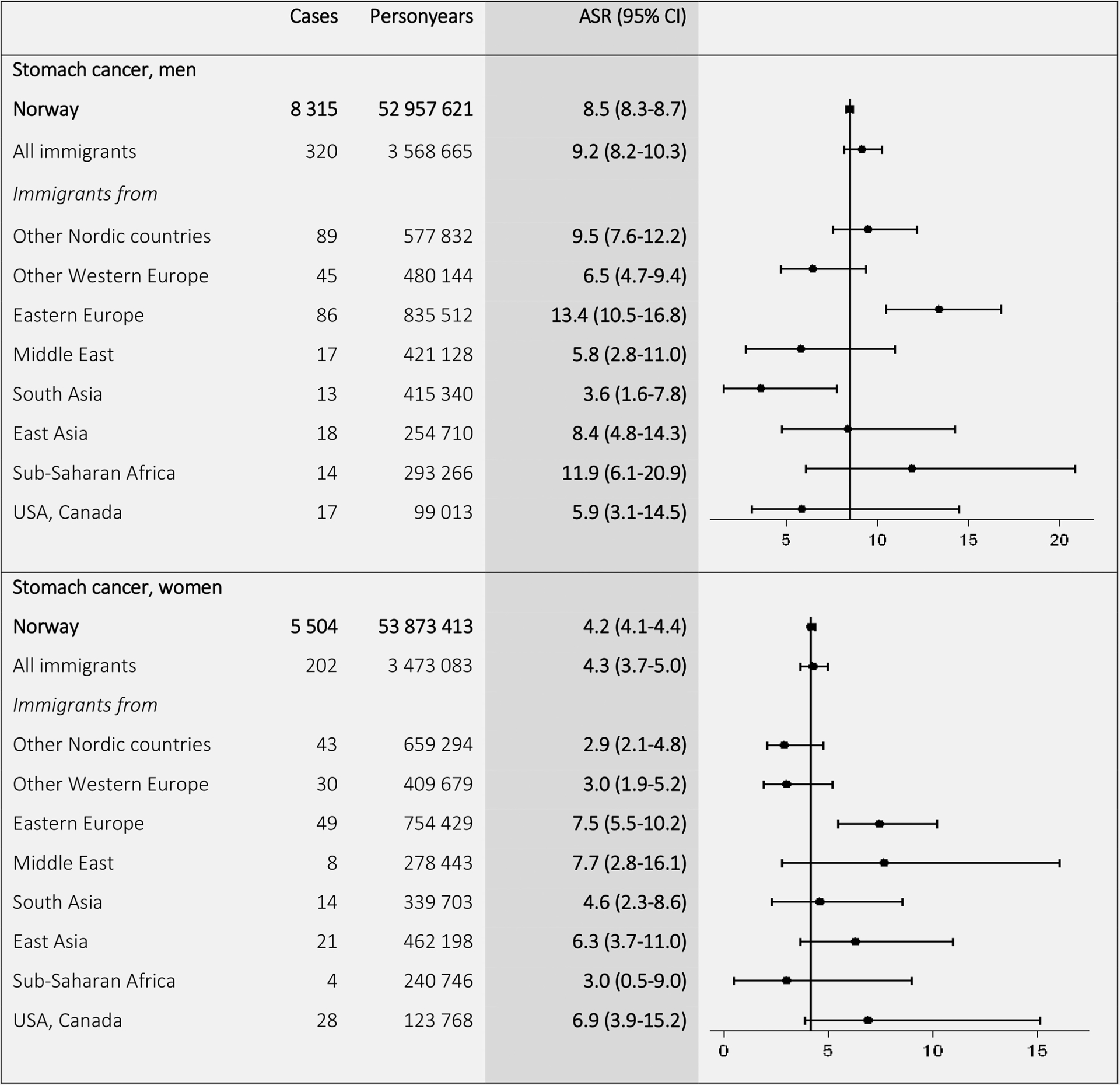
Age-standardized incidence rates (ASRs) with 95% confidence intervals (CIs) for stomach cancer (C16) by birth region. Standardized by the world standard population. Adjusted for calendar period (in 5-year intervals) and age (in 5-year categories).
Figure 6.
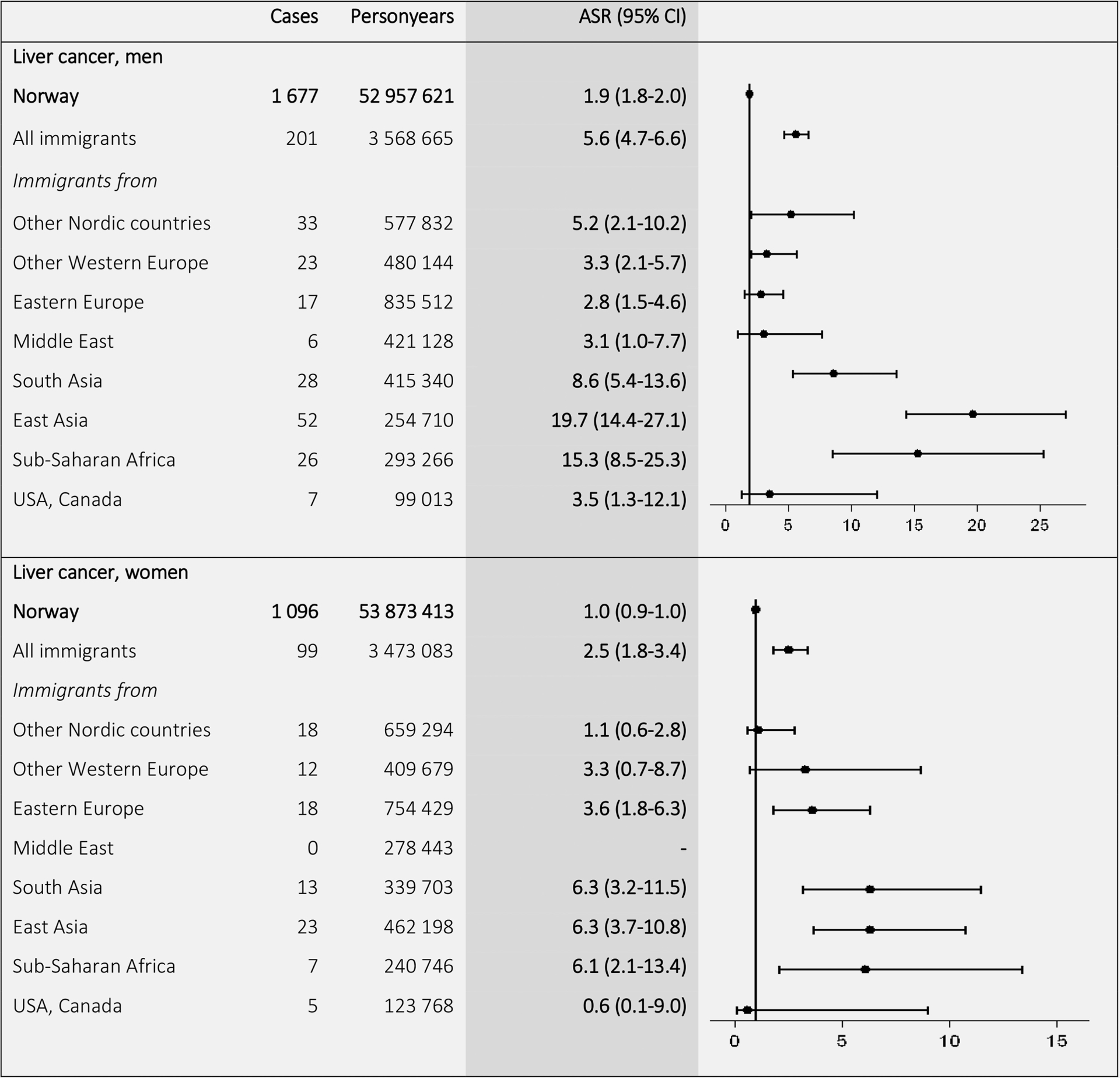
Age-standardized incidence rates (ASRs) with 95% confidence intervals (CIs) for liver cancer (C22) by birth region. Standardized by the world standard population. Adjusted for calendar period (in 5-year intervals) and age (in 5-year categories).
To further compare the incidence rates, we calculated SIRs (Supporting Information Tables 1 and 2). The results showed lower total cancer incidence among immigrants from low-income countries (SIRs from 0.48 in men and 0.64 in women from South Asia to 0.69 in men and 0.78 in women from Sub-Saharan Africa), compared with the Norwegian-born population. Men from Eastern Europe had high risk of lung cancer (SIR 1.54), and Eastern European men (SIR 1.74) and women (SIR 1.86) had high risk of stomach cancer. Immigrants from low-income countries had higher risk of liver cancer than Norwegian-born, especially individuals born in East Asia (SIR 12.35 in men and 6.85 in women).
Discussion
Our findings show that during the period 1990–2012, all immigrants to Norway combined had lower total cancer incidence than Norwegian-born, with differences between immigrant groups and cancer types. While incidence of breast and colorectal cancer were highest among individuals born in Norway and other high-income countries, other cancer types were higher in immigrants from low-income countries. We found an elevated incidence of lung cancer among Eastern European men, followed by men from the Nordic countries. We also found a high incidence of stomach cancer in immigrants from Eastern Europe. Immigrants from low-income countries (Asia and Sub-Saharan Africa) had high incidence of liver cancer.
The elevated incidence of lung cancer in Eastern European men is likely entirely determined by smoking habits in the immigrant groups, which partly reflect smoking prevalence in their countries of origin.24 The high lung cancer incidence among Nordic immigrant men in Norway could reflect a selection of working class immigrants with high smoking prevalence.25 The high incidence of stomach cancer in immigrants from Eastern Europe may be caused by an Helicobacter pylori infection contracted in their country of origin26 in combination with smoking.27 The very high liver cancer incidence found in immigrants from low-income countries is possibly caused by a hepatitis B or C virus infection.28 Immigrants from these countries tend to have high incidence of cancers related to exposure to infectious agents,2, and increased incidence of liver cancer has been found in Asian immigrants to Australia29 and North America,21 and Sub-Saharan immigrants to France.30
On the other hand, incidence of lifestyle-related cancers, such as breast and colorectal cancer, were highest in individuals born in Norway and other high-income countries, probably linked to a high-income lifestyle (i.e., diet, obesity and physical inactivity)31 and reproductive histories.32 The lower rates of breast cancer in women from low-income countries could also to some extent reflect poor utilization of Norwegian health care services, that is, low attendance to mammographic screening.33–35 A surprising finding was the high incidence of cervical cancer in women born in Norway and the other Nordic countries, compared to most immigrant groups. The incidence of cervical cancer decreases following screening if pre-cancerous cell changes are found and treated before the disease is developed, and results from a recent study of attendance to screening in Norway have shown that the rates are generally lower for immigrants.36 Additionally, many Muslim countries have low rates of cervical cancer.1 The number of cervical cancer cases are relatively small in many immigrant groups, and results should be interpreted with caution.
In general, our findings confirm findings of other recent studies of immigrants from low-income to high-income countries. The differences in cancer rates overall and in specific cancer types are likely due to a combination of different factors, such as differences in lifestyle and socioeconomic factors, reason for immigration, and the “healthy migrant effect.”
Certain lifestyle factors associated with cancer risk may differ between immigrants and host populations. In particular, diet may be healthier and the prevalence of obesity and alcohol consumption lower in immigrants than in some high-income host populations before and immediately after arrival.37–39 On the other hand, self-reported physical inactivity tend to be higher in immigrants compared to the host population in Norway,40 and smoking prevalence is very high in some immigrant groups.41
After moving to the host country, immigrants go through various degrees of acculturation. Many factors, for example, socioeconomic status and occupation, can influence the acculturation process,42 and affect cancer incidence. Immigrants from low- income countries are likely to have lower socioeconomic status than Norwegian-born,43 and they are over-represented in occupations where they could be exposed to factors associated with cancer.25,44 In addition, widespread high-income diets and increasing obesity prevalence worldwide could influence acculturation in recent immigrants.11
Variation in cancer incidence could also be linked to reason for immigration, which may vary across immigrant groups.12 In 2014, reasons for immigration to Norway were reported as due to labour (43%), family reunion (33%), refugees (14%) and students (10%). Labour immigrants mostly originate from Europe, while immigrants from Asia and Africa are typically refugees or family immigrants.45 The reason for immigration vary across gender. There is a male overrepresentation among refugees and labour immigrants and a female overrepresentation among family immigrants.46
The “healthy migrant effect,” in which immigrants are assumed to be healthier than the average population in the country of origin,10 does not appear to be supported by the results, as cancer incidence in immigrants from high-income countries is very similar to that of the Norwegian-born population. On the contrary, smoking rates may be higher in immigrants from some countries than in the population that remains, on the basis that they are a selected group, that is, labour immigrants with high smoking prevalence.
Strengths and limitations
A strength of the study was the large study population, consisting of the entire Norwegian population between the years 1990 and 2012. The design enabled us to identify accurately all immigrants in Norway, from many regions around the world, and to follow them prospectively. Another strength was the complete cancer registration.
A limitation of the study was the size of the different immigrant groups, comprising a relatively small proportion of the entire Norwegian population and producing at times uncertain effect estimates. We included individuals born in Norway of immigrant parents in the Norwegian-born population, but if they have rates in between immigrants and Norwegian-born with Norwegian-born parents, then the differences may have been underestimated. However, this was a small group, consisting of young individuals not yet reached the age where cancer normally occur. The immigrant groups identified in this study are often heterogeneous and diverse, and could mask variations by country of origin, however investigating group differences still have empirical value. Additionally, immigration patterns to Norway have shifted over time, and older and younger immigrants, with different reason for immigration, originate from different countries. To disentangle the effect of age, calendar period and region of origin, we calculated age- and period-specific ASRs, and adjusted for age in 5-year categories and time period in 5-year intervals.
The extent to which missing or incorrect emigration data may have influenced our results could not be examined. Some immigrants may emigrate back to their country of origin and fail to notify the Norwegian population registry, and thus their vital statistics remain “cancer free and immortal”. In some cases, an emigration is registered in the system several years after the person actually left the country.47 70% of the registered emigrations (22,883 individuals) were attributed to immigrants in 2011, mostly from the Nordic or other Western European countries.48 The “salmon bias” hypothesis suggests that as immigrants age or fall ill they migrate back to their country of origin to die.49 However, health services and treatment are most likely better in Norway, and many immigrants have already been joined by their families.
The adult immigrant population in Norway is relatively young, especially immigrants from low-income countries, and have not yet reached the age groups characterized with high lung, colorectal and prostate cancer rates. This is especially the case for prostate cancer, where 85% of cases are diagnosed after the age of 65 years, after which ASR increase with age.50 However, the ASRs presented in our study are standardized, that is, adjusted for the effect of differences in age-distribution across populations. The rates are weighted averages of age-specific rates, using the age distribution of the world standard population.18,19
Conclusions
This study showed that total cancer incidence was lower in immigrants than in Norwegian-born, with differences in incidence patterns depending on country of origin and the cancer type in question. The extent of the differences varied across gender and immigrant groups. Immigrants from high-income countries had a relatively similar cancer burden compared to Norwegian-born, with high incidence of lifestyle-related cancers. The high incidence of lung cancer among Eastern European men, and to some extent among men from other Nordic countries, is a main concern, and current tobacco control should attempt to reach these populations. Immigrants from Eastern Europe also have high rates of stomach cancer. Additional attention should be given to the high rates of liver cancer among immigrants from low-income countries. It is important that health care personnel are attentive of symptoms of these cancers that, although rare, are present in some immigrant groups. Since immigrants constitute a significant and increasing proportion of the population in many high-income countries, monitoring their cancer rates and health needs can indicate the extent of risk reduction achievable by public health programs.
Supplementary Material
What’s new?
Despite the growing incidence of certain lifestyle-related cancers in low-income countries, differences remain in cancer incidence across geographic regions. Some of those differences are significant, as highlighted in the present comparison of cancer incidence between immigrant groups in Norway and Norwegian-born individuals. In particular, breast and colorectal cancer incidence were higher among Norwegian-born individuals, while other cancers, including those of the liver, lung and stomach, were more common among immigrants. Overall, immigrants to Norway had lower total cancer incidence than Norwegian-born individuals, though cancer burden was similar between immigrants from high-income countries and individuals born in Norway.
Grant sponsor:
Norwegian Cancer Society; Grant number: 161326
Abbreviations:
- ASR
age-standardized incidence rate
- CI
confidence interval
- CRN
Cancer Registry of Norway
- ICD-10
10th revision of the International Classification of Diseases
- PIN
personal identification number
- SIR
Standardized Incidence Ratio
- WHO
World Health Organization
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to declare.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 2.Hemminki K, Forsti A, Khyatti M, et al. Cancer in immigrants as a pointer to the causes of cancer. Eur J Public Health 2014;24 Suppl 1:64–71. [DOI] [PubMed] [Google Scholar]
- 3.Dunn JE. Cancer epidemiology in populations of the United States–with emphasis on Hawaii and California–and Japan. Cancer Res 1975;35:3240–5. [PubMed] [Google Scholar]
- 4.Tominaga S Cancer incidence in Japanese in Japan, Hawaii, and western United States. Natl Cancer Inst Monogr 1985;69:83–92. [PubMed] [Google Scholar]
- 5.Petrakis NL. Some preliminary observations on the influence of genetic admixture on cancer incidence in american negroes. Int J Cancer 1971; 7:256–8. [DOI] [PubMed] [Google Scholar]
- 6.Ali R, Barnes I, Kan SW, Beral V. Cancer incidence in British Indians and British whites in Leicester, 2001–2006. Br J Cancer 2010;103:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas DB, Karagas MR. Cancer in first and second generation Americans. Cancer Res 1987; 47:5771–6. [PubMed] [Google Scholar]
- 8.Ziegler RG, Hoover RN, Pike MC, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst 1993; 85:1819–27. [DOI] [PubMed] [Google Scholar]
- 9.Kolonel LN, Hinds MW, Hankin JH. Cancer patterns among migrant and native-born Japanese in Hawaii in relation to smoking, drinking, and dietary habits In: Gelboin HV, MacMahon B, Matsushima T, Sugimura T, Takayama S, Takebe H, eds. Genetic and environmental factors in experimental and human cancer. Tokyo: Japan Scientific Press, 1980. [Google Scholar]
- 10.Kennedy S, Kidd MP, McDonald JT, et al. The Healthy Immigrant Effect: Patterns and Evidence from Four Countries. J Int Migr Integr 2015;16:317–32. [Google Scholar]
- 11.Parkin DM, Khlat M. Studies of cancer in migrants: rationale and methodology. Eur J Cancer 1996;32A:761–71. [DOI] [PubMed] [Google Scholar]
- 12.Syse A, Strand BH, Naess O, et al. Differences in all-cause mortality: a comparison between immigrants and the host population in Norway 1990–2012. Demogr Res 2016;34:615–56. [Google Scholar]
- 13.Statistics Norway; 2016. Key figures for immigration and immigrants. Available at: http://www.ssb.no/en/innvandring-og-innvandrere/nokkeltall/immigration-and-immigrants. (Accessed on August 17, 2016).
- 14.Dzamarija MT. Categories of immigration background in the Norwegian population (Reports 16/2014, English summary). Oslo: Statistics Norway, 2014. Available at: https://www.ssb.no/befolkning/artikler-og-publikasjoner/_attachment/175380?_ts=14a61e5cd90. Accessed date 09 Aug 2016. [Google Scholar]
- 15.World Health Organization; 2015. Regional and income groupings. Available at: http://www.who.int/gho/publications/world_health_statistics/EN_WHS2015_Annex.pdf?ua=1. (Accessed on October 3, 2016).
- 16.Larsen IK, Smastuen M, Johannesen TB, et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer 2009;45: 1218–31. [DOI] [PubMed] [Google Scholar]
- 17.Consonni D, Coviello E, Buzzoni C, et al. A command to calculate age-standardized rates with efficient interval estimation. Stata J 2012;12:688–701. [Google Scholar]
- 18.Doll R, Payne P, Waterhouse JAH. Cancer Incidence in Five Continents. Geneva: Union Internationale Contre le Cancer, 1966. [Google Scholar]
- 19.Segi M Cancer Mortality for Selected Sites in 24 Countries (1950–57). Sendai, Japan: Department of Public Health, Tohoku University of Medicine, 1960. [Google Scholar]
- 20.Schenker N, Gentleman JF. On Judging the Significance of Differences by Examining the Overlap Between Confidence Intervals. Am Stat 2001; 55:182–6. [Google Scholar]
- 21.Singh GK, Hiatt RA, Trends and disparities in socioeconomic and behavioural characteristics, life expectancy, and cause-specific mortality of native-born and foreign-born populations in the United States, 1979–2003. Int J Epidemiol 2006; 35:903–19. [DOI] [PubMed] [Google Scholar]
- 22.Fay MP, Feuer EJ, Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med 1997;16:791–801. [DOI] [PubMed] [Google Scholar]
- 23.StataCorp. Stata Statistical Software: Release 14. College Station, TX: Statacorp LP2014. [Google Scholar]
- 24.Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA 2014;311:183–92. [DOI] [PubMed] [Google Scholar]
- 25.Bang KM, Kim JH. Prevalence of cigarette smoking by occupation and industry in the United States*. Am J Ind Med 2001;40:233–9. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y, Park J, Nam BH, et al. Stomach cancer incidence rates among Americans, Asian Americans and Native Asians from 1988 to 2011. Epidemiol Health 2015;37:e2015006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenner H, Arndt V, Bode G, et al. Risk of gastric cancer among smokers infected with Helicobacter pylori. Int J Cancer 2002;98:446–9. [DOI] [PubMed] [Google Scholar]
- 28.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245–55. [DOI] [PubMed] [Google Scholar]
- 29.McCredie M, Williams S, Coates M. Cancer mortality in East and Southeast Asian migrants to New South Wales, Australia, 1975–1995. Br J Cancer 1999;79:1277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouchardy C, Wanner P, Parkin DM. Cancer mortality among sub-Saharan African migrants in France. Cancer Causes Control 1995;6:539–44. [DOI] [PubMed] [Google Scholar]
- 31.Mousavi SM, Fallah M, Sundquist K, et al. Age- and time-dependent changes in cancer incidence among immigrants to Sweden: colorectal, lung, breast and prostate cancers. Int J Cancer 2012;131:E122–8. [DOI] [PubMed] [Google Scholar]
- 32.Andreeva VA, Unger JB, Pentz MA. Breast cancer among immigrants: a systematic review and new research directions. J Immigr Minor Health 2007;9:307–22. [DOI] [PubMed] [Google Scholar]
- 33.Latif F, Helgeland J, Bukholm G, et al. Ethnicity differences in breast cancer stage at the time of diagnosis in Norway. Scand J Surg 2015; 104:248–53. [DOI] [PubMed] [Google Scholar]
- 34.Kristiansen M, Thorsted BL, Krasnik A, et al. Participation in mammography screening among migrants and non-migrants in Denmark. Acta Oncol 2012;51:28–36. [DOI] [PubMed] [Google Scholar]
- 35.Lagerlund M, Maxwell AE, Bastani R, et al. Sociodemographic predictors of non-attendance at invitational mammography screening–a population-based register study (Sweden). Cancer Causes Control 2002;13:73–82. [DOI] [PubMed] [Google Scholar]
- 36.Moen KA, Kumar B, Qureshi S, et al. Differences in cervical cancer screening between immigrants and nonimmigrants in Norway: a primary healthcare register-based study. Eur J Cancer Prev, 2016. October 4 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarich PE, Ding D, Sitas F, et al. Co-occurrence of chronic disease lifestyle risk factors in middle-aged and older immigrants: a cross-sectional analysis of 264,102 Australians. Prev Med 2015;81:209–15. [DOI] [PubMed] [Google Scholar]
- 38.Satia-Abouta J, Patterson RE, Neuhouser ML, et al. Dietary acculturation: applications to nutrition research and dietetics. J Am Diet Assoc 2002; 102:1105–18. [DOI] [PubMed] [Google Scholar]
- 39.Goel MS, McCarthy EP, Phillips RS, et al. Obesity among US immigrant subgroups by duration of residence. JAMA 2004;292:2860–7. [DOI] [PubMed] [Google Scholar]
- 40.Kumar BN, Grøtvedt L, Meyer HE, et al. The Oslo Immigrant Health Profile (Report 7/2008). Oslo: The Norwegian Institute of Public Health, 2008. Available at: https://brage.bibsys.no/xmlui/bitstream/handle/11250/220525/Kumar_2008_The.pdf?sequence=3&isAllowed=y. Accessed date 09 Sept 2016. [Google Scholar]
- 41.Vedoy TF. The role of education for current, former and never-smoking among non-western immigrants in Norway. Does the pattern fit the model of the cigarette epidemic? Ethn Health 2013;18:190–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abraido-Lanza AF, Armbrister AN, Florez KR, et al. Toward a theory-driven model of acculturation in public health research. Am J Public Health 2006;96:1342–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blom S, Henriksen K. Living conditions among immigrants in Norway 2005/2006 (Reports 2/2009). Oslo: 2008. Available at: https://www.ssb.no/a/english/publikasjoner/pdf/rapp_200902_en/rapp_200902_en.pdf. Accessed date 10 Aug 2016. [Google Scholar]
- 44.Statistics Norway; Immigration and immigrants: Labour market and earnings. Available at: http://www.ssb.no/en/innvandring-oginnvandrere?de=Labour+market+and+earnings. (Accessed on April 27, 2016). [Google Scholar]
- 45.Statistics Norway; Immigrants by reason for immigration, 1 January 2015. Available at: http://www.ssb.no/en/innvgrunn/.(Accessed on May 21, 2016). [Google Scholar]
- 46.Daugstad G, Sandnes T. Gender and Migration. Similarities and disparities among women and men in the immigrant population (Reports 10/2008). Oslo: Statistics Norway, 2008. Available at: https://www.ssb.no/a/english/publikasjoner/pdf/rapp_200810_en/rapp_200810_en.pdf. Accessed date 18 Aug 2016. [Google Scholar]
- 47.Skjerpen S, Stambøl LS, Tønnesen M. Emigration among immigrants in Norway (Reports 17/2015, English summary). Oslo: Statistics Norway, 2015. Available at: http://www.ssb.no/en/befolkning/artikler-og-publikasjoner/utvandring-blant-innvandrere-i-norge. Accessed date 10 Aug 2016. [Google Scholar]
- 48.Pettersen SV. Emigration from Norway 1971–2011 (Reports 30/2013, English summary). Oslo: Statistics Norway, 2013. Available at: https://www.ssb.no/befolkning/artikler-og-publikasjoner/_attachment/133741?_ts=140952ed0c0. Accessed date 10 Aug 2016. [Google Scholar]
- 49.Lu Y, Qin L. Healthy migrant and salmon bias hypotheses: a study of health and internal migration in China. Soc Sci Med 2014;102:41–8. [DOI] [PubMed] [Google Scholar]
- 50.Gronberg H Prostate cancer epidemiology. Lancet 2003;361:859–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


