Abstract
Background:
Hair loss (alopecia) is a common problem and is a major cause of psychological stress and anxiety among affected individuals. It is of utmost importance to diagnose these cases at the earliest and treat them accordingly. Trichoscopy provides a noninvasive option that can be used for early diagnosis and monitoring the progression of the hair disorders.
Aims and Objectives:
To perform trichoscopy and document the findings in patients with nonscarring alopecia's.
Materials and Methods:
A total of 100 cases satisfying the inclusion criteria were screened for general physical examination and scalp examination including hair shaft and root, and tests for hair anchorage and fragility were also done. The lesions were examined through dermoscope, photographs were taken, and findings were documented.
Results:
Among the total of 100 cases screened, 57 were female and 43 were male. The mean age of the study group was 26 ± 14.8 years. Females were affected by alopecia areata (AA) and female pattern hair loss (29.8%) equally, whereas males were most commonly affected by AA (41.8%). The common trichoscopic follicular features noted were broken hair (48%), black dots (48%), single hair follicle unit (45%), short vellus hair (44%), upright hair (41%), and yellow dots (40%). The common interfollicular features seen were honeycomb pigmentation (26%) and arborizing red lines (12%).
Conclusion:
The emergence of newer hair signs on trichoscopic studies aids in identification and has a definitive role in the diagnosis of clinically difficult cases, so it is recommended to use trichoscopy in the routine examination of nonscarring alopecia's.
Keywords: Black dots, broken hair, nonscarring alopecia, trichoscopy
INTRODUCTION
Hair is an important structure of the body that contributes to the physical appearance of an individual.[1] Hair loss (alopecia) is a common problem and is a major cause of psychological stress and anxiety among affected individuals. These alopecias can be broadly classified as scarring and nonscarring. They can also be classified as hair shaft disorders characterized by increased fragility.[2] It is of utmost importance to diagnose these cases at the earliest and treat them accordingly.[3]
The methods most commonly used to diagnose hair loss disorders include visual inspection, hair pull test, and biopsy, which is an invasive method. The need of noninvasive methods is growing in clinical practice and thereby dermatoscopy.[2]
“Dermatoscopy,” also popularly known as “dermoscopy,” is a noninvasive technology used to visualize subsurface features including epidermis, dermoepidermal junction, and superficial dermis that are not visible to the naked eye.[4]
In 2006, Lidia Rudnicka and Malgorzata Olszewska et al. coined the term “Trichoscopy” for dermoscopy of hair and the scalp.[5] Trichoscopy aids in identifying both hair shaft and hair opening disorders without the need to perform hair sampling.[6] It helps in avoiding unnecessary biopsies and also aids in choosing an ideal biopsy site.[5]
Trichoscopy helps in the differentiation of cicatricial from noncicatricial alopecia's and also aids in the diagnosis of patchy hair loss conditions such as tinea capitis, that requires immediate medication without waiting for culture reports which would otherwise take days to reveal results. Thereby trichoscopy serves as a reliable noninvasive diagnostic tool for quick evaluation and follow-up with images.[6]
This study has been undertaken to aid the diagnosis, treatment, and follow-up of nonscarring alopecias.
Aims and objectives
To perform trichoscopy and document the findings in patients with nonscarring alopecias.
MATERIALS AND METHODS
The present study was an observational study carried out in the Department of Dermatology at R.L Jalappa Hospital attached to Sri Devaraj Urs Medical College, Tamaka, Kolar, for a duration of 1½ year after obtaining ethical clearance from the Institutional Ethics Committee. All patients presenting with nonscarring alopecias were included. A total of 100 cases with hair loss were screened, and a detailed history of the patient including name, age, sex, history of presenting illness, hair grooming pattern, habits and tics, nail changes, other skin changes, systemic disease, family history of similar complaints and drug intake was taken. A written informed consent was taken from the patients and in children from parents or guardian. General physical examination, scalp examination including hair shaft and root, and tests for hair anchorage and fragility were done in all cases. The lesions were examined directly through the dermoscope Dermlite DL3N with ×10 magnification using both polarized and nonpolarized mode. Then, the dermoscopic photographs were taken with the help of an adaptor attached to iPhone 8 camera with a ×12 zoom.
A two-step procedure for dermoscopic classification of alopecia was used:
The lesions were first identified as nonscarring alopecia on the basis of the algorithm in Figure 1[7]
The patients were further evaluated in accordance with the checklist of dermoscopic features present in Table 1.[8]
Figure 1.
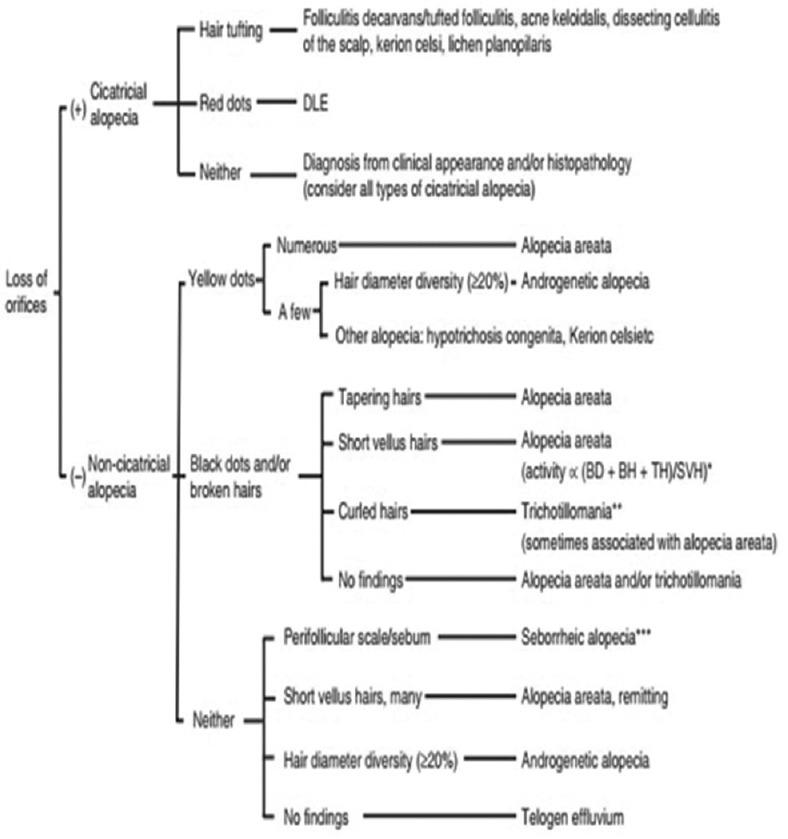
Algorithmic method for trichoscopic diagnosis of common hair loss diseases[7]
Table 1.
Checklist of dermoscopic features[8]
| Follicular features | Interfollicular features | Follicular and interfollicular features |
|---|---|---|
| YD BD (cadaverized hair) Tapering (exclamation mark) hair Broken hair Short vellus hair Coudability Comma/corkscrew hair HDD Empty follicles Peripilar sign Absence of follicular openings Tufted hair White dots Follicular hyperkeratosis Pilli torti Trichorrhexis nodosa |
Red dots Arborizing red lines Honeycomb pigment pattern Pink white appearance Dirty dots Crust formation |
Epidermal scale Pustule |
HDD – Hair diameter diversity
In all cases where diagnosis was doubtful and suspected clinically, it was further confirmed by dermoscopic examination. A routine hemogram including complete blood count with peripheral smear was done in all cases of diffuse hair loss. Special investigations such as KOH mount and fungal culture were done in suspected cases of tinea capitis for confirmation. There was one case of clinically suspected syphilitic alopecia which was further confirmed by VDRL titers.
Statistical methods used for data analysis
Data were entered into Microsoft excel data sheet and were analyzed using SPSS 22 version (IBM SPSS Statistics for Windows, version XX (IBM Corp., Armonk, N.Y., USA) software. Categorical data were represented in the form of frequencies and proportions. Chi-square was used as test of significance. Continuous data were represented as mean and standard deviation. MS Excel and MS Word were used to obtain various types of graph such as bar diagram and pie diagram. P value (probability that the result is true) <0.05 was considered statistically significant after assuming all the rules of statistical tests.
Sample size calculation
Sample size was estimated by using the proportion of yellow dots (YD) in alopecia areata (AA) (common cause) of nonscarring alopecia's detected by trichoscopy as 63.7% in patients with AA.[9]
RESULTS
A maximum of 42% cases out of total 100 patients screened were in the age group of 21–30 years, and the minimum of 2% cases were above 60 years and the mean age was 26 ± 14.8 years. The study included 100 patients with a diagnosis of alopecia (57 females and 43 males). The female patients were affected more by AA (29.8%) and female pattern hair loss (FPHL) (29.8%), whereas the male patients were most commonly affected by AA (41.8%).
Trichoscopic features
Trichoscopic features of various alopecias are summarized in Table 2.
Table 2.
Trichoscopic features of various alopecia's
| Type of alopecia | Number of patients | The most common trichoscopic findings in each alopecia |
|---|---|---|
| AA | 35 | Broken hair (93.5%), BD (88.4%), YD (60%), exclamation mark hair (80%), short vellus hair (37.1%), pig tail hair (34.2%) |
| FPHL | 17 | HDD (100%), short vellus hair (94.1%), honey comb pigment pattern (64.7%), peripilar sign (58.8%), YD (41.1%), white dots (41.2%) |
| Male pattern hair loss | 14 | Short vellus hair (100%), HDD (100%), honey comb pigment pattern (85.7%), peripilar sign (57.1%), white dots (57.1%), YD (21.4%) |
| Telogen effluvium | 17 | Upright hair (100%), single hair follicle unit (100%), empty follicles (88.2%), YD (35.2%) |
| Tinea capitis | 7 | Comma hair (100%), corkscrew hair (100%), broken hair (100%), Morse code hair (42.8%), I hair (42.8%), zig zag hair (14.2%) |
| Trichotillomania | 5 | V hair (100%), trichoptilosis (100%), broken hair (100%), peripilar hemorrhage (80%), BD (80%), coiled hair (60%), flame hair (20%) |
| Anagen effluvium | 3 | YD (100%), BD (100%), empty follicles (100%) |
| Temporal triangular alopecia | 1 | White hair, diversity of diameter, vellus hair surrounded by terminal hair, empty follicles, white dots |
| Syphilitic alopecia | 1 | Focal atrichia, YD, BD |
YD – Yellow dots; AA – Alopecia areata; FPHL – Female pattern hair loss
In the present study of the total 100 patients examined, the most common trichoscopic follicular features were broken hair (BH) (48 patients, 48%), black dots (BD) (48 patients, 48%), single hair follicle units (45 patients, 45%) followed by short vellus hair (44 patients, 44%), upright hair (41 patients, 41%) and YD (40 patients, 40%) and most common interfollicular features were honeycomb pigment pattern (26 patients, 26%) followed by arborizing red lines (12 patients, 12%).
Thirty-five (35%) out of 100 cases were diagnosed to have AA, in which BH (93.5%) was most common trichoscopic finding closely followed by BD (88.4%) and YD (60%) [Figures 2 and 3].
Figure 2.
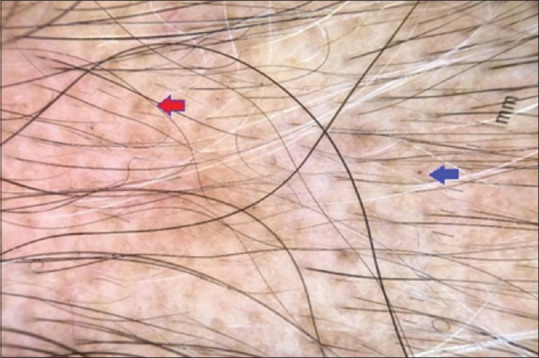
Dermoscopic picture of alopecia areata: Red arrow denotes yellow dots and blue arrow denotes black dot
Figure 3.
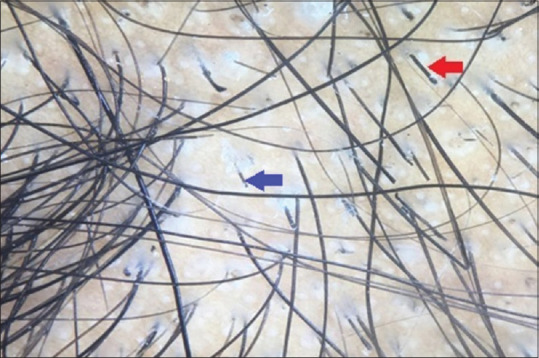
Dermoscopic picture of alopecia areata: Blue arrow denotes broken hair and red arrow denotes exclamation mark hair
Androgenetic alopecia was seen in 31% out of 100 cases, of which FPHL was seen in 17% of cases and male pattern hair loss was seen in 14% of cases. The most common trichoscopic findings of FPHL include hair diameter diversity (HDD) (100%), short vellus hair (94.1%), and honey comb pigment pattern (64.7%), whereas the most common findings of male pattern hair loss included HDD (100%), short vellus hair (100%), and honey comb pigment pattern (85.7%) [Figure 4].
Figure 4.
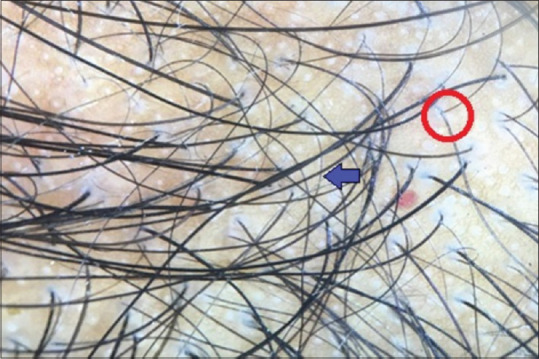
Dermoscopic picture of androgenetic alopecia: Blue arrow denotes short vellus hair, red circle denotes hair diameter diversity
Seventeen (17%) cases of telogen effluvium was presented in this study of which trichoscopic findings includes upright hair and single hair follicle unit in all cases followed by empty follicles in 88.2% cases. Tinea capitis was seen in seven (7%) cases out of total hundred cases, the findings of which included comma hair, corkscrew hair and BH in 100% cases followed by Morse code hair and I hair in 42.8% cases [Figure 5]. Five cases of total hundred cases were diagnosed as trichotillomania, which typically showed the presence of v hair (100%), trichoptilosis (100%), BH (100%) followed by peripilar hemorrhage (80%) and BD (80%) [Figure 6]. Three patients presented with anagen effluvium. The most common dermoscopic findings included YD (100%), BD (100%) and empty follicles (100%). There was one case of temporal triangular alopecia which showed the presence of white hair, diversity of diameter, vellus hair surrounded by terminal hair, empty follicles and white dots. One case of AA had VDRL positive titers (1:32) suspecting syphilitic alopecia which on trichoscopy showed the presence of focal atrichia, YD and BD [Figure 7].
Figure 5.
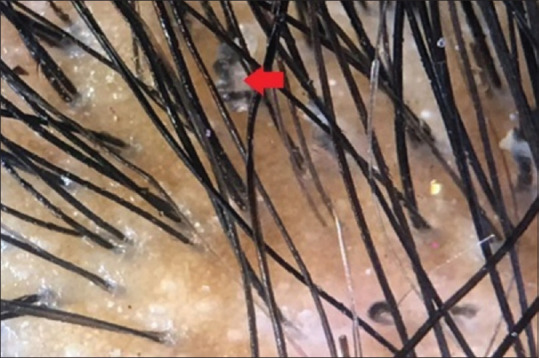
Dermoscopic picture of tinea captis: Red arrow denotes corkscrew hair
Figure 6.
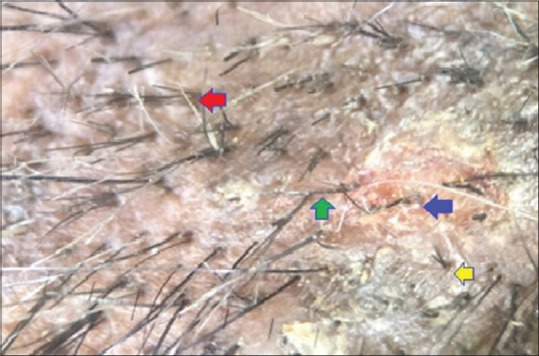
Dermoscopic picture trichotillomania: Red arrow denotes trichoptillosis, green arrow denotes kinking of hair, blue arrow denotes peripilar hemorrhage, yellow arrow denotes v hair
Figure 7.
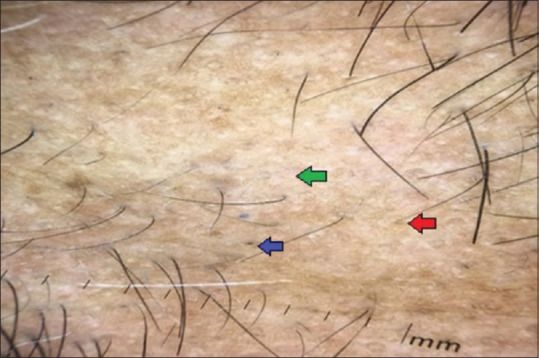
Dermoscopic findings of AA with positive VDRL titers suspecting syphilitic alopecia: Green arrow denotes focal atrichia, blue arrow denotes black dot, red arrow denotes yellow dot
DISCUSSION
Nonscarring alopecia's is a common condition that affect male and female patients which may in turn lead to social withdrawal, depression, anxiety and stress.[9] Trichoscopy can help in early diagnosis and prompt treatment of the affected individuals. The trichoscopic patterns observed consistently with certain alopecia's can be used for their diagnosis, monitoring the disease activity and thereby overcomes the need to take unnecessary biopsy.[9]
The age of the patient has an important role in the interpretation of alopecia's. There are certain alopecia's which are known to occur in particular age groups.[8] The mean age was 26 years in this study which is consistent with mean age of 24.9 years in other study.[10] In this study of 100 patients, 57% were females and 43% were males similar to other study wherein 56% were females and 44% were males.[8]
Trichoscopic features of nonscarring alopecia's
Trichoscopy of AA: YD findings in AA patients were consistent with other studies.[8,11,12] YD are considered to be the most sensitive dermoscopic feature of AA. These are marked by distinctive yellow to yellow-pink, round or polycyclic dots that vary in size and are uniform in color.[12] The trichoscopic findings of YD in AA vary depending on type of dermoscope equipment used and also as an account of different shampooing habits between European, Asian and Latin American populations. The highest prevalence of YD was seen in dark-skinned patients as per Fitzpatrick's skin Type V ranging from 6 to 100% with a mean value of 72% with a handheld dermoscope. In white-skinned individuals of Fitzpatrick's phototype I–III, YD were seen in 81% with hand held dermoscope. These YD in individuals with yellowish skin and those belonging to Fitzpatrick's phototype IV ranged from 40 to 65% with a mean value of 60% with a dermoscope.[13]
The BD in this study are higher than the other studies.[8,11,12] This is because BD are due to changes in hair color and cuticle resistance. The cuticle of Asian's fail under extension stress as large pieces while maintaining their original shape. In contrast to that, Caucasian hair cuticles tend to collapse into small fragments, these factors result in higher percentage of BD in Asians of 40%–78% with a mean value of 57% versus 29%–57% BD in Caucasians with a mean value of 49%. The highest BD proportion was found in dark skinned patients such as Egyptian, Turkish and Indians of range between 0% and 63% with a mean value of 61%, these findings are in accordance to our study in which the proportion of BD is 88.4%.[13]
More BH are seen in this study when compared to other studies.[8,11,12] BH are found to be more in patients with acute AA with active hair loss. The proportion of BH on an average is around 0%–71% with a mean value of 49% in patients with AA which is in accordance to our study where BH was seen in 93.5% cases, suggesting more patients had increased activity of hair loss.[13] Though findings of short vellus hair in present study are in contrast to other studies,[8,11,12] however in dark skinned individuals it is seen in 34%–94% with a mean value of 59%, which is in accordance to our study which reported short vellus hair in 37.1% cases.[13] Short vellus hair is common in remitting phase and long-standing cases of AA. The proportion of short vellus hair in AA is found to be 34%–100% with a mean value of 61% in patients in a study.[13] This percentage difference in the proportion of short vellus hair is due to difference in skin phototypes. The short vellus hair vary according to the skin phototype and are more prevalent in white-or yellowish-skinned phototype of I–IV as compared to dark skin phototype V of Fitzpatrick's scale populations.[13]
Exclamation mark hair in this study are higher than the other studies.[8,11,12] The number of exclamation mark hair are usually high in progressive, nonprogressive and acute form of AA which accounts for their higher proportion. The proportion of exclamation mark hair was found to be 80% in our study indicative of both acute form of disease and long standing nonprogressive disease.[13]
The findings of pig tail hair in this study is consistent with other study.[11] Pig tail hair are short and seen in first few weeks. These are regrowing, regularly coiled hair with tapered ends. They indicate hair regrowth and not a frequent finding with a mean value of just 21% in few earlier studies.[13]
The largest trichoscopic study on AA concluded that BD, YD correlated with the severity of disease whereas tapering hair (TH), BH correlated with disease activity. They noted that YD and short vellus hair were the most sensitive diagnostic markers whereas BD, TH and BH were the most specific markers[9,14] [Table 3].
Table 3.
Comparative findings of alopecia areata with other studies
| Percentage findings in AA (in this study), n=35 | Percentage findings in AA (in other studies)[11], n=50 | Percentage findings in AA (in other studies)[8], n=49 | Percentage findings in AA (in other studies)[12], n=126 | |
|---|---|---|---|---|
| YD | 60 | 88 | 83.7 | 84.1 |
| BD | 88.4 | 58 | 63.3 | 48.4 |
| Broken hair | 93.5 | 56 | 57.1 | 9.5 |
| Short vellus hair | 37.1 | 66 | 46.9 | 62 |
| Exclamation mark hair | 80 | 26 | 42.9 | 30.9 |
| Pig tail hair | 34.2 | 14 |
AA – Alopecia areata; YD – Yellow dots
Trichoscopy of androgenetic alopecia: The findings of YD in androgenetic alopecia in this study are in contrast to the other studies.[10,15,16] YD in androgenetic alopecia are formed by absence of terminal hair and intact sebaceous glands which produce sebum, which are seen commonly in patients with advanced alopecia. The percentage of YD is variable but is lesser than in AA which is in accordance to our study (<60%).[16] The HDD is seen in 100% of cases in this study which is in accordance to other studies.[10,15,16] A variation of more than 20% of HDD in males and more than 10% of HDD in females is considered significant.[16] The frequency of white dots was more when compared to other studies,[15,16] due to dark skin colour which makes them more prominent.[15] Peripilar sign is seen in all patients of alopecia with fair skin whereas these ratios are lower in Asian patients.[15] The findings of peripilar sign in this study is consistent with other studies[15,16] The proportion of honey comb pigment pattern in this study is higher than the other studies.[10,15,16] This pattern is seen in cases with long standing sun exposure and dark skin tone, which accounts for the higher proportion of honey comb pigment pattern in our study[15] [Table 4].
Table 4.
Comparative findings of androgenetic alopecia with other studies
| Percentage findings in AGA (in this study), n=31 | Percentage findings in other study)[15], n=206 | Percentage findings in other study)[16], n=950 | Percentage findings in other study)[10], n=31 | |||||
|---|---|---|---|---|---|---|---|---|
| MPHL | FPHL | MPHL | FPHL | MPHL | FPHL | MPHL | FPHL | |
| YD | 21.4 | 41.1 | 28.4 | 18.7 | 20.1 | 24.1 | 100 | 44.5 |
| HDD | 100 | 100 | 100 | 100 | 100 | 100 | 95.1 | 88.9 |
| White dots | 57.1 | 41.2 | 28.6 | 8.2 | 27.3 | 24 | - | - |
| Peripilar sign | 57.1 | 58.8 | 46 | 64.9 | 44 | 44.5 | 9 | 11.1 |
| Honey comb pigment | 85.7 | 64.7 | 25.4 | 13.4 | 33.2 | 30.5 | 40.9 | 11.1 |
MPHL – Male pattern hair loss; FPHL – Female pattern hair loss; AGA – Androgenetic alopecia; YD – Yellow dots; HDD – Hair diameter diversity
Trichoscopy of tinea capitis: The most specific feature of tinea capitis is comma hair, which is a common finding in all earlier studies.[10,17,18] The findings of corkscrew hair which is higher in this study is suggestive of endothrix variant of tinea capitis.[19] The presence of zigzag hair which is consistent with other studies is usually seen due to bend at the narrow paler parts of the infected hair shafts due to underlying structural weakness.[17,18,20] BH is considered an additional dermoscopic feature of tinea capitis but not the specific feature and is a common finding in the present study which is in contrast to other studies.[10,17,18] Morse code hair are irregularly interrupted hair with normally pigmented and paler narrowed intervals on dermoscope.[17] The findings of morse code hair in this study were consistent with other study.[17] Further studies have to be done to prove the association of morse code hair with tinea capitis.[17] I-hair are short BH with an accentuated distal end. This study is one among the few studies to describe I-hair[17] [Table 5].
Table 5.
Comparative findings of tinea capitis with other studies
| Percentage findings in tinea capitis (in this study), n=7 | Percentage findings in tinea capitis (in other studies)[10], n=7 | Percentage findings in tinea capitis (in other studies)[17], n=40 | Percentage findings in tinea capitis (in other studies)[18], n=43 | |
|---|---|---|---|---|
| Comma hair | 100 | 85.7 | 60 | 70 |
| Corkscrew hair | 100 | 14.3 | 20 | 30 |
| Zig zag hair | 14.2 | - | 30 | 20 |
| Broken hair | 100 | 14.3 | 17.5 | 25.6 |
| Morse code hair | 42.8 | - | 27.5 | - |
| I hair | 42.8 | - | 5 | - |
Trichoscopy of trichotillomania: BD in trichotillomania in this study are similar to few other studies showing above 80% involvement whereas it is just 30% in another study.[10,21,22] These are not the specific features of trichotillomania, as they are also seen in conditions such as AA thereby accounting for the difference in the findings.[10] The characteristic features of Trichotillomania such as flame hair, v-hair, tulip hair, and hair powder, ‘partially coiled’ hair (hook hair) occurs due to manual pulling of hair. The flame hair leads to residue which is semi-transparent, wavy and cone shaped. The findings of flame hair in this study are similar to other studies.[10,21,22] BH and trichoptilosis are diagnostic of Trichotillomania. BH in trichotillomania are seen due to mechanical force used to pull the hair which results in transverse fracture with cuticle broken at different length with longitudinal splitting or fraying.[21] The traumatic hair pull results in characteristic peripilar hemorrhage. Peripilar hemorrhage is considered diagnostic for Trichotillomania which were seen in 80% cases in our study which was consistent with other study[10] [Table 6].
Table 6.
Comparative findings of trichotillomania with other studies
| Percentage findings in TTM (in this study), n=5 | Percentage findings in TTM (in other studies)[21], n=44 | Percentage findings in TTM (in other studies)[10], n=10 | Percentage findings in TTM (in other studies)[22], n=10 | |
|---|---|---|---|---|
| BD | 80 | 100 | 90 | 30 |
| Flame hair | 20 | 25 | - | 30 |
| V hair | 100 | - | 80 | 30 |
| Trichoptilosis | 100 | 34 | 80 | 80 |
| Coiled hair | 60 | 39 | - | 80 |
| Broken hair | 100 | - | 100 | 30 |
| Peripilar hemorrhage | 80 | - | 60 | 30 |
TTM – Trichotillomania; BD – Black dots
In this study, the findings of anagen effluvium due to chemotherapy induced alopecia on trichoscopy included YD, BD and empty follicles in all cases. The additional feature included pohl pinkus constriction hair which was seen in one case in this study, these are similar to the findings reported earlier.[23] Though trichoscopy has little role in diagnosis of telogen effluvium the findings of YD is consistent with other studies.[8,10]
The findings of temporal triangular alopecia in this study included diversity of diameter of hair, vellus hair and empty follicles which were in accordance to other studies.[24,25]
This study included one case of AA which had VDRL positive titers (1:32 dilutions) thereby suspecting syphilitic alopecia which on trichoscopy showed the presence of focal atrichia, YD and BD. No characteristic findings of syphilitic alopecia have been demonstrated on trichoscopy till date.[26] In few earlier case reports, findings described were in accordance to this study such as YD in the centre of the alopecia patch with few BD at the periphery, focal atrichia and hypopigmentation of the hair shaft.[27] There are few case reports that have shown the presence of empty hair follicles, vellus hair, red-brown background and irregularly dilated capillaries with red blood cells extravasation.[28]
CONCLUSION
Trichoscopy serves as a valuable diagnostic tool in early diagnosis by avoiding biopsy and also helps in assessing the various stages and severity of nonscarring alopecias. In this study, trichoscopy helped us to reach a conclusive diagnosis of patients in whom the diagnosis was not clear, it was also supplemented by relevant investigations such as KOH and other blood investigations whenever indicated.
With new hair signs being found on trichoscopic studies, it aids in identification and has a definitive role in the diagnosis of clinically difficult cases, so it is recommended to use trichoscopy in the routine examination of nonscarring alopecia's.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Vinay K, Sawatkar GU, Dogra S. Hair manifestations of endocrine diseases: A brief review. Indian J Dermatol Venereol Leprol. 2018;84:528–38. doi: 10.4103/ijdvl.IJDVL_671_17. [DOI] [PubMed] [Google Scholar]
- 2.Mubki T, Rudinicka L, Olszewska M, Shapiro J. Evaluation and diagnosis of the hair loss patient. J Am Acad Dermatol. 2014;71:415. doi: 10.1016/j.jaad.2014.04.070. [DOI] [PubMed] [Google Scholar]
- 3.Tosti A, Duque BE. Dermoscopy in hair disorders. J Egypt Women Dermatol Soc. 2010;7:1–4. [Google Scholar]
- 4.Nirmal B. Dermatoscopy: Physics and principles. Indian J Dermatopathol Diagn Dermatol. 2017;4:27–30. [Google Scholar]
- 5.Jain N, Doshi B, Khopkar U. Trichoscopy in alopecias: Diagnosis simplified. Int J Trichology. 2013;5:170–8. doi: 10.4103/0974-7753.130385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elghblawi E. Frontier in hair loss and trichoscopy: A review. J Surg Dermatol. 2016;1:80–96. [Google Scholar]
- 7.Rudnicka L, Rakowska A, Olszewska M. Trichoscopy: How it may help the clinician. Dermatol Clin. 2013;31:29–41. doi: 10.1016/j.det.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Karadaǧ Köse Ö, Güleç AT. Clinical evaluation of alopecias using a handheld dermatoscope. J Am Acad Dermatol. 2012;67:206–14. doi: 10.1016/j.jaad.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Inui S, Nakajima T, Nakagawa K, Itami S. Clinical significance of dermoscopy in alopecia areata: Analysis of 300 cases. Int J Dermatol. 2008;47:688–93. doi: 10.1111/j.1365-4632.2008.03692.x. [DOI] [PubMed] [Google Scholar]
- 10.Chiramel MJ, Sharma VK, Khandpur S, Sreenivas V. Relevance of trichoscopy in the differential diagnosis of alopecia: A cross-sectional study from North India. Indian J Dermatol Venereol Leprol. 2016;82:651–8. doi: 10.4103/0378-6323.183636. [DOI] [PubMed] [Google Scholar]
- 11.Guttikonda AS, Aruna C, Ramamurthy DV, Sridevi K, Alagappan SK. Evaluation of clinical significance of dermoscopy in alopecia areata. Indian J Dermatol. 2016;61:628–33. doi: 10.4103/0019-5154.193668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmoudi H, Salehi M, Moghadas S, Ghandi N, Teimourpour A, Daneshpazhooh M. Dermoscopic findings in 126 patients with alopecia areata: A cross-sectional study. Int J Trichol. 2018;10:118–23. doi: 10.4103/ijt.ijt_102_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waśkiel A, Rakowska A, Sikora M, Olszewska M, Rudnicka L. Trichoscopy of alopecia areata: An update. J Dermatol. 2018;45:692–700. doi: 10.1111/1346-8138.14283. [DOI] [PubMed] [Google Scholar]
- 14.Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006;55:799–806. doi: 10.1016/j.jaad.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 15.Kibar M, Aktan S, Bilgin M. Scalp dermatoscopic findings in androgenetic alopecia and their relations with disease severity. Ann Dermatol. 2014;26:478–84. doi: 10.5021/ad.2014.26.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu R, Xu F, Han Y, Sheng Y, Qi S, Miao Y, et al. Trichoscopic findings of androgenetic alopecia and their association with disease severity. J Dermatol. 2015;42:602–7. doi: 10.1111/1346-8138.12857. [DOI] [PubMed] [Google Scholar]
- 17.Amer M, Helmy A, Amer A. Trichoscopy as a useful method to differentiate tinea capitis from alopecia areata in children at Zagazig University Hospitals. Int J Dermatol. 2017;56:116–20. doi: 10.1111/ijd.13217. [DOI] [PubMed] [Google Scholar]
- 18.Isa RI, Amaya B Y, Pimentel MI, Arenas R, Tosti A, Cruz AC, et al. Dermoscopy in tinea capitis: A prospective study on 43 patients. Med Cutan Iber Lat Am. 2014;42:18–22. [Google Scholar]
- 19.Lin YT, Lin YC. The dermoscopic comma, zigzag, and bar code-like hairs: Markers of fungal infection of the hair follicles. Dermatol Sin. 2014:160–3. [Google Scholar]
- 20.Elghblawi E. Tricoscopy findings in tinea capitis. A rapid method of diagnosis. Eur J Pediat Dermatol. 2016;26:71–4. [Google Scholar]
- 21.Rakowska A, Slowinska M, Olszewska M, Rudinicka L. New trichoscopy findings in trichotillomania: Flame hairs, V-sign, hook hairs, hair powder, tulip hairs. Acta Derm Venereol. 2014;94:303–6. [Google Scholar]
- 22.Ankad BS, Naidu MV, Beergouder SL, Sujana L. Trichoscopy in trichotillomania: A useful diagnostic tool. Int J Trichology. 2014;6:160–3. doi: 10.4103/0974-7753.142856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudnicka A, Olszewsk M, Rudinicka L. Anagen effluvium. In: Rudnicka L, Olszweska M, Rakowska A, editors. Atlas of Trichoscopy. 1st ed. UK: Springer London Ltd; 2012. pp. 245–8. [Google Scholar]
- 24.Fernández-Crehuet P, Vaño-Galván S, Martorell-Calatayud A, Arias-Santiago S, Grimalt R, Camacho-Martínez FM. Clinical and trichoscopic characteristics of temporal triangular alopecia: A multicenter study. J Am Acad Dermatol. 2016;75:634–7. doi: 10.1016/j.jaad.2016.04.053. [DOI] [PubMed] [Google Scholar]
- 25.Karadaǧ Köse Ö, Güleç AT. Temporal triangular alopecia: Significance of trichoscopy in differential diagnosis. J Eur Acad Dermatol Venereol. 2015;29:1621–5. doi: 10.1111/jdv.12656. [DOI] [PubMed] [Google Scholar]
- 26.Ye Y, Zhang X, Zhao Y, Gong Y, Yang J, Li H, et al. The clinical and trichoscopic features of syphilitic alopecia. J Dermatol Case Rep. 2014;8:78–80. doi: 10.3315/jdcr.2014.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doche I, Hordinsky MK, Valente NYS, Romiti R, Tosti A. Syphilitic alopecia: Case reports and trichoscopic findings. Skin Appendage Disord. 2017;3:222–4. doi: 10.1159/000477415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piraccini BM, Broccoli A, Starace M, Gasapari V, Antunno DA, Patrizi A, et al. Hair and scalp manifestations in secondary syphilis: Epidemiology, clinical features and trichoscopy. Dermatology. 2015;231:171 6. doi: 10.1159/000431314. [DOI] [PubMed] [Google Scholar]


