Recent studies have suggested that proton pump inhibitor (PPI) use may increase the risk of contracting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and worsen the course COVID-19.1 , 2 This observation is biologically plausible, as gastric acid is a well-established line of defense against microbial pathogens, including SARS-CoV-1.3 In addition, PPI use may worsen outcomes in patients with COVID-19 through alterations of the gut microbiome and intestinal immune apparatus.4 Conversely, recent data suggest that histamine 2 receptor antagonists (H2RAs), which are less potent than PPIs in terms of acid suppression, may be beneficial in SARS-CoV-25 , 6 through direct antiviral effects.7
Considering that acid suppressive medications are among the most commonly consumed drugs in the United States, an understanding of the impact of these agents on COVID-19 outcomes is of significant importance. In particular, data informing the public on whether there is an evidence-based rationale to modify the use of these chronic medications during the pandemic are necessary. We studied whether preadmission exposure to PPIs or H2RAs was associated with worse outcomes among patients hospitalized with COVID-19.
Methods
This was a secondary analysis of a retrospective cohort study aiming to better characterize digestive manifestations in patients hospitalized with COVID-19 across 36 medical centers in North America.8 The first 50 to 100 consecutive patients with a confirmed diagnosis of COVID-19 at each participating institution were included. Clinical data from the time of symptom onset until discharge, death, or the end of the study period were manually abstracted from electronic health records by study personnel under the oversight of a primary clinician-investigator. Conventional regression and propensity score matched analyses were performed to evaluate the association between preadmission acid suppressive medication exposure and mechanical ventilation or death. Sensitivity analyses were conducted to evaluate the validity of excluding patients with unknown PPI/H2RA status and of imputing missing data. More comprehensive methods are provided in the Supplementary Material.
Results
Between April 15 and June 5, 2020, data were collected from 1992 subjects. There were 146 patients excluded from the primary analysis because PPI or H2RA use within 1 month of admission was unknown. Characteristics of the final study cohort (1846 patients with COVID-19) are shown in the Supplementary Table 1. A total of 417 patients (22.6%) had recent PPI use, 167 (9.1%) had recent H2RA use, and 29 (1.6%) had both. The baseline variables included in the final regression models are listed in Supplementary Table 2.
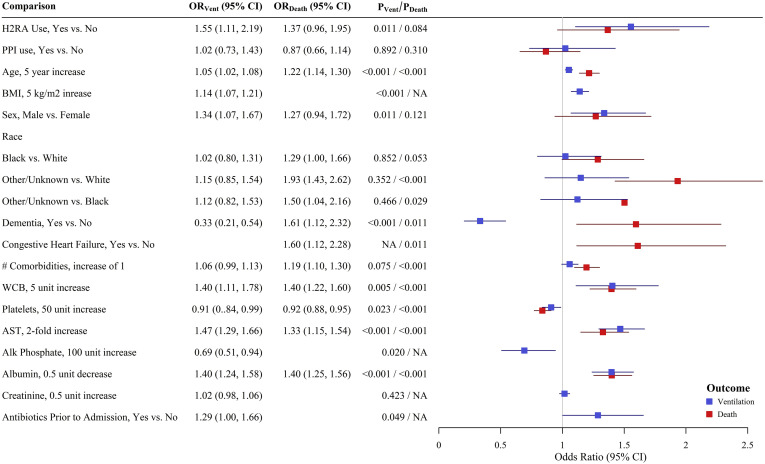
After adjusting for measured baseline confounders, PPI use was not independently associated with the need for mechanical ventilation in the primary regression analysis (odds ratio [OR] 1.02; 95% confidence interval [CI] 0.73–1.43; P = .89) (Figure 1 ). In contrast, H2RA use was associated with 1.55 times the odds of requiring mechanical ventilation relative to patients not exposed to H2RA (OR 1.55; 95% CI 1.11–2.19; P = .01) (Figure 1). Propensity score matched analysis confirmed the lack of association between PPI use and mechanical ventilation and demonstrated a significant increase in the likelihood of mechanical ventilation among patients exposed to H2RA (risk difference 9.72%; 95% CI 1.26%–18.2%; P = .02). In sensitivity analyses, removal of patients with at least 1 missing covariate value (480 observations) or inclusion of patients with unknown PPI/H2RA use (139 additional observations) did not change the findings.
Figure 1.
ORs (95% CIs) for mechanical ventilation (blue) or death (red) based on final regression models.
PPI use was not independently associated with increased death (OR 0.87; 95% CI 0.66–1.14; P = .31) (Figure 1). Similarly, H2RA exposure was not associated with increased death (OR 1.37; 95% CI 0.96–1.95; P = .08) (Figure 1). Propensity score matched analysis showed similar findings. In the sensitivity analyses, removal of patients with at least 1 missing covariate value (375 observations) did not change the findings pertaining to PPI use. However, this analysis did demonstrate a statistically significant association between H2RA exposure and in-hospital mortality (OR 1.48; 95% CI 1.04–2.12; P = .03). The sensitivity analysis that included patients with unknown PPI/H2RA status (139 additional observations) did not change the results.
Discussion
In this large-scale study of patients hospitalized with COVID-19 across 36 medical centers in North America, preadmission exposure to PPI and/or H2RA did not appear to strongly affect the likelihood of mechanical ventilation or death. Our findings do not support the putative deleterious effects of PPIs and in fact contradict the hypothesized benefits of H2RAs in SARS-CoV-2 infection. These observations suggest that modified use of these medications for the purpose of improving COVID-19 outcomes is not justified. Instead, the results reinforce the preexisting guidance of taking acid suppressive medications only when indicated and at the lowest dose that achieves the clinical objective for which they were recommended.
The observation that preadmission H2RA use increased the risk of mechanical ventilation in this cohort is noteworthy, as it opposes growing evidence of the beneficial effect of famotidine. The reasons for this finding are unclear and may not apply to famotidine specifically, as the exact H2RA consumed by patients in the cohort was not determined. However, since the estimated effect size was relatively small, there was no association between H2RA use and death, and residual confounding is always a risk in observational studies of this nature, the finding largely serves to caution against overuse of this medication and highlights the importance of additional research prior to clinical care or policy changes. It is also important to emphasize that recent studies showing the favorable effect of famotidine have evaluated active treatment rather than preadmission exposure, as we assessed in this study.
This study has several important limitations. First, PPI and H2RA exposure was not verified against pharmacy records and their consistent use could not be confirmed. However, these medications are commonly acquired over the counter, limiting the ability of prescription claims to confirm use. Furthermore, medication use in this study was collected manually by comprehensive review of electronic health records by clinicians or study coordinators under the oversight of a clinician-investigator, with likely increased accuracy compared with administrative data. Second, as noted previously, we did not specifically query the use of famotidine relative to the other H2RAs, and thus a beneficial effect of this medication may have been diluted by other drugs in this class. Third, we did not collect data on in-hospital acid suppressive medication administration, which may have been associated with preadmission exposure and could have confounded the analysis. Last, observations from this cohort reflect the earliest phase of the pandemic and may not apply to the current time.
In conclusion, we did not observe a strong deleterious effect of preadmission PPI use or a beneficial effect of preadmission H2RA use among patients hospitalized with COVID-19. These findings caution against the modified use of these medications to improve COVID-19–related outcomes.
Acknowledgments
Teldon Alford, an author and data manager for the Alliance is now employed by Emmes, a clinical research organization that conducts research in the COVID-19 space; see the Supplementary Material for the of the North American Alliance for the Study of Digestive manifestations of COVID-19.
CRediT Authorship Contributions
Badih Joseph Elmunzer, MD (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Writing D (Conceptualization: L
Bethany Wolf, PhD (Formal analysis: Lead; Methodology: Lead; Writing ad; Formal editing: Supporting)
James Scheiman, MD (Writing alysis: Lead; Methodology: Lead; Writing ad; Formalht of Equal)
William Tierney, MD (Writing lysis: Lead; Methodology: Lead; Writing ad; Formalht of a Equal)
Jason Taylor, MD (Writing n original draft: Supporting; Writing – review & editing: Equal)
Footnotes
North American Alliance for the Study of Digestive Manifestations of COVID-19: Collaborating authors: Ambreen A. Merchant, MBBS, Division of Digestive Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA, USA. Vaishali A. Patel, MD, Division of Digestive Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA, USA. Field F. Willingham, MD, MPH Division of Digestive Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA, USA. Eric F. Howard, RN, BSN, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA. Mary K. West, RN, BSN, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA. Casey L Koza, BS, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA. Patrick S. Yachimski, MD, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA. Emad Qayed, MD, MPH, Division of Digestive Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA, USA; Division of Digestive Diseases, Department of Medicine, Grady Memorial Hospital, Atlanta, GA, USA. Rosemary Nustas, MD, Division of Digestive Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA, USA; Division of Digestive Diseases, Department of Medicine, Grady Memorial Hospital, Atlanta, GA, USA. Ali Zakaria, MD, Division of Gastroenterology, Department of Medicine, Ascension Providence Hospital/Michigan State University-College of Human Medicine, Southfield, MI, USA. Marc S. Piper, MD, MSc, Division of Gastroenterology, Department of Medicine, Ascension Providence Hospital/Michigan State University-College of Human Medicine, Southfield, MI, USA. Lujain Jaza, MD, Division of Gastroenterology and Hepatology, Department of Medicine, Saint Louis University, St. Louis, MO, USA. Nauzer Forbes, MD, MSc, Division of Gastroenterology, Department of Medicine, University of Calgary, Calgary, Alberta, Canada. Millie Chau, BSc, Division of Gastroenterology, Department of Medicine, University of Calgary, Calgary, Alberta, Canada. Luis F. Lara, MD, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, The Ohio State University Wexner Medical Center, Columbus, OH, USA. Georgios I. Papachristou, MD, PhD, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, The Ohio State University Wexner Medical Center, Columbus, OH, USA. Uchechi Okafor, BSc, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, The Ohio State University Wexner Medical Center, Columbus, OH, USA. Darwin L. Conwell, MD, MSc, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, The Ohio State University Wexner Medical Center, Columbus, OH, USA. Michael L. Volk, MD, MSc, Division of Gastroenterology, Department of Medicine, Loma Linda University, Loma Linda, CA, USA. Evan Mosier, MD, Division of Gastroenterology, Department of Medicine, Loma Linda University, Loma Linda, CA, USA. Mohamed Azab, MD, Division of Gastroenterology, Department of Medicine, Loma Linda University, Loma Linda, CA, USA. Anish Patel, MD, Division of Gastroenterology, Department of Medicine, Loma Linda University, Loma Linda, CA, USA. Liam G. Hilson, MD, Division of Gastroenterology, Department of Medicine, University of Southern California, Los Angeles, CA, USA. Selena Zhou, MD, Division of Gastroenterology, Department of Medicine, University of Southern California, Los Angeles, CA, USA. James Buxbaum, MD, Division of Gastroenterology, Department of Medicine, University of Southern California, Los Angeles, CA, USA. Vladimir M. Kushnir, MD, Division of Gastroenterology, Department of Medicine, Washington University School of Medicine, St. Louis, MO, USA. Alexandria M. Lenyo, BS, Division of Gastroenterology, Department of Medicine, Washington University School of Medicine, St. Louis, MO, USA. Ian P. Sloan, BS, Division of Gastroenterology, Department of Medicine, Washington University School of Medicine, St. Louis, MO, USA. Thomas Hollander, RN, BSN, Division of Gastroenterology, Department of Medicine, Washington University School of Medicine, St. Louis, MO, USA. Caroline G. McLeod, BS, Division of Gastroenterology and Hepatology, Department of Medicine, Medical University of South Carolina, Charleston, SC, USA. Rebecca L. Spitzer, MPH, Division of Gastroenterology and Hepatology, Department of Medicine, Medical University of South Carolina, Charleston, SC, USA. Lauren Wakefield, MHA, Division of Gastroenterology and Hepatology, Department of Medicine, Medical University of South Carolina, Charleston, SC, USA. Haley Nitchie, MHA, Division of Gastroenterology and Hepatology, Department of Medicine, Medical University of South Carolina, Charleston, SC, USA. Collins O. Ordiah MBBS, MPH, Division of Gastroenterology and Hepatology, Department of Medicine, Medical University of South Carolina, Charleston, SC, USA. Don C. Rockey, MD, Division of Gastroenterology and Hepatology, Department of Medicine, Medical University of South Carolina, Charleston, SC, USA. Teldon B. Alford, BA, Division of Gastroenterology and Hepatology, Department of Medicine, Medical University of South Carolina, Charleston, SC, USA.∗ Sunil Amin, MD, MPH, Division of Gastroenterology, Department of Medicine, University of Miami Miller School of Medicine, Miami, FL, USA. Gabriela N. Kuftinec, MD, MPH, Division of Gastroenterology, Department of Medicine, University of Miami Miller School of Medicine, Miami, FL, USA. Amar R. Deshpande, MD, Division of Gastroenterology, Department of Medicine, University of Miami Miller School of Medicine, Miami, FL, USA. Dhiraj Yadav, MD, MPH, Division of Gastroenterology and Hepatology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA. Melissa Saul, MS, Division of Gastroenterology and Hepatology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA. Melanie Mays, BS, Division of Gastroenterology and Hepatology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA. Gulsum Anderson, PhD, Division of Gastroenterology and Hepatology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA. Kelley Wood, BS, Division of Gastroenterology and Hepatology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA. Laura Mathews, BS, Division of Gastroenterology and Hepatology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA. Charlie Fox, MD, Division of Gastroenterology and Hepatology, University of Colorado Anschutz Medical Campus, Aurora, CO. Jennifer M. Kolb, MD, MS, Division of Gastroenterology and Hepatology, University of Colorado Anschutz Medical Campus, Aurora, CO. Sachin Wani, MD, Division of Gastroenterology and Hepatology, University of Colorado Anschutz Medical Campus, Aurora, CO. Swati Pawa, MD, Division of Gastroenterology, Department of Medicine, Wake Forest University School of Medicine, Winston-Salem, NC, USA. Rishi Pawa, MD, Division of Gastroenterology, Department of Medicine, Wake Forest University School of Medicine, Winston-Salem, NC, USA. Andrew Canakis, DO, Section of Gastroenterology, Department of Medicine, Boston University Medical Center, Boston, MA, USA. Christopher Huang, MD, Section of Gastroenterology, Department of Medicine, Boston University Medical Center, Boston, MA, USA. Laith H. Jamil, MD, Section of Gastroenterology and Hepatology, Department of Internal Medicine, Beaumont Health, Royal Oak, MI, USA; Oakland University William Beaumont School of Medicine, Rochester, MI, USA. Andrew M. Aneese, MD, Section of Gastroenterology and Hepatology, Department of Internal Medicine, Beaumont Health, Royal Oak, MI, USA. V. Mihajlo Gjeorgjievski, MD, Section of Gastroenterology and Hepatology, Department of Internal Medicine, Beaumont Health, Royal Oak, MI, USA. Zaid Imam, MD, Section of Gastroenterology and Hepatology, Department of Internal Medicine, Beaumont Health, Royal Oak, MI, USA. Fadi Odish, MD, Section of Gastroenterology and Hepatology, Department of Internal Medicine, Beaumont Health, Royal Oak, MI, USA. Ahmed I. Edhi, MD, Section of Gastroenterology and Hepatology, Department of Internal Medicine, Beaumont Health, Royal Oak, MI, USA. Molly Orosey, DO, Section of Gastroenterology and Hepatology, Department of Internal Medicine, Beaumont Health, Royal Oak, MI, USA. Abhinav Tiwari, MD, Section of Gastroenterology and Hepatology, Department of Internal Medicine, Beaumont Health, Royal Oak, MI, USA. Soumil Patwardhan, MBBS, Section of Gastroenterology and Hepatology, Department of Internal Medicine, Beaumont Health, Royal Oak, MI, USA. Benita K. Glamour, BA, Division of Gastroenterology, Department of Medicine, University Hospitals of Cleveland Medical Center, Cleveland, OH, USA. Zachary L Smith, DO, Division of Gastroenterology, Department of Medicine, University Hospitals of Cleveland Medical Center, Cleveland, OH, USA. Amy E. Hosmer, MD, Division of Gastroenterology, Department of Medicine, University Hospitals of Cleveland Medical Center, Cleveland, OH, USA. Nancy Furey, RN, BSN, MBA, Division of Gastroenterology, Department of Medicine, University Hospitals of Cleveland Medical Center, Cleveland, OH, USA. Amitabh Chak, MD, Division of Gastroenterology, Department of Medicine, University Hospitals of Cleveland Medical Center, Cleveland, OH, USA. Katherine A. Hanley, MMS, PAC, Division of Gastroenterology, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA. Jordan Wood, BS, Division of Gastroenterology, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA. Rajesh N. Keswani, MD, MS, Division of Gastroenterology, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA. Harsh K. Patel, MBBS, Department of Gastroenterology, Ochsner Health, New Orleans, LA, USA. Janak N. Shah, MD, Department of Gastroenterology, Ochsner Health, New Orleans, LA, USA. Emil Agarunov, BS, Division of Gastroenterology, Department of Medicine, Columbia University Medical Center, New York, NY, USA. Nicholas G. Brown, MD, Division of Gastroenterology, Department of Medicine, Columbia University Medical Center, New York, NY, USA. Anish A. Patel, MD, Division of Gastroenterology, Department of Medicine, Columbia University Medical Center, New York, NY, USA. Amrita Sethi, MD, Division of Gastroenterology, Department of Medicine, Columbia University Medical Center, New York, NY, USA. Evan L. Fogel, MD, MSc, Division of Gastroenterology and Hepatology, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA. Gail McNulty, RN, BSN, Division of Gastroenterology and Hepatology, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA. Abdul Haseeb, MD, Division of Gastroenterology and Nutrition, Department of Medicine Loyola University Medical Center, Chicago, IL, USA. Judy A. Trieu, MD, Division of Gastroenterology and Nutrition, Department of Medicine Loyola University Medical Center, Chicago, IL, USA. Rebekah E. Dixon, BS, The Dr. Henry D. Janowitz Division of Gastroenterology, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA. Jeong Yun Yang, MD, The Dr. Henry D. Janowitz Division of Gastroenterology, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA. Christopher J. DiMaio, MD, The Dr. Henry D. Janowitz Division of Gastroenterology, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA. Robin B. Mendelsohn, MD, Gastroenterology, Hepatology and Nutrition service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA. Delia Calo, MD, Gastroenterology, Hepatology and Nutrition service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA. Olga C. Aroniadis, MD, MSc, Division of Gastroenterology, Department of Medicine, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, USA. Joseph F. LaComb, BS, Division of Gastroenterology, Department of Medicine, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, USA. Lilian Cruz, BS, Division of Gastroenterology, Department of Medicine, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, USA. Olga Reykhart, BS, Division of Gastroenterology, Department of Medicine, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, USA. Bryan G. Sauer, MD, Division of Gastroenterology, Department of Medicine, University of Virginia Medical School, Charlottesville, VA, USA. Galina Diakova, MS, Division of Gastroenterology, Department of Medicine, University of Virginia Medical School, Charlottesville, VA, USA. Duyen T. Dang, MD, Division of Gastroenterology, Department of Medicine, Henry Ford Health System, Detroit, MI, USA. Cyrus R. Piraka, MD, Division of Gastroenterology, Department of Medicine, Henry Ford Health System, Detroit, MI, USA. Eric D. Shah, MD, MBA, Section of Gastroenterology and Hepatology, Department of Medicine, Dartmouth-Hitchcock Health, Lebanon, NH, USA. Molly Caisse, BS, Natalia H. Zbib, MD, Section of Gastroenterology and Hepatology, Department of Medicine, Dartmouth-Hitchcock Health, Lebanon, NH, USA. John A. Damianos, MD, Section of Gastroenterology and Hepatology, Department of Medicine, Dartmouth-Hitchcock Health, Lebanon, NH, USA. Heiko Pohl, MD, Section of Gastroenterology and Hepatology, Department of Medicine, Dartmouth-Hitchcock Health, Lebanon, NH, USA. Stephanie Mitchell, RN, Section of Digestive Diseases and Nutrition, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA. Michael S. Bronze MD, Section of Digestive Diseases and Nutrition, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA. Ashwinee Condon, MD, Division of Gastroenterology, Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, California, USA. Adrienne Lenhart, MD, Division of Gastroenterology, Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, California, USA. Raman Muthusamy, MD, MAS, Division of Gastroenterology, Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, California, USA. Kulwinder S. Dua, MD, Division of Gastroenterology, Department of Medicine, Medical College of Wisconsin, Milwaukee, Wisconsin, USA. Vikram S. Kanagala, MD, Division of Gastroenterology, Department of Medicine, Medical College of Wisconsin, Milwaukee, Wisconsin, USA. James Esteban, MD, Division of Gastroenterology, Department of Medicine, Medical College of Wisconsin, Milwaukee, Wisconsin, USA. Ayesha Kamal, MD, Division of Gastroenterology, Department of Medicine, Johns Hopkins Medical Institutions, Baltimore, MD, USA. Marcia I. Canto, MD, Division of Gastroenterology, Department of Medicine, Johns Hopkins Medical Institutions, Baltimore, MD, USA. Vikesh K. Singh, MD, MS, Division of Gastroenterology, Department of Medicine, Johns Hopkins Medical Institutions, Baltimore, MD, USA. Maria Ines Pinto-Sanchez, MD, MSc, Division of Gastroenterology, Department of Medicine, McMaster University Hamilton Health Sciences, Hamilton, ON, Canada. Joy M. Hutchinson, RD, MSc, Division of Gastroenterology, Department of Medicine, McMaster University Hamilton Health Sciences, Hamilton, ON, Canada. Richard S. Kwon, MD, Division of Gastroenterology and Hepatology, Department of Medicine, Michigan Medicine, Ann Arbor, MI, USA. Sheryl J. Korsnes, MA, Division of Gastroenterology and Hepatology, Department of Medicine, Michigan Medicine, Ann Arbor, MI, USA. Akbar K. Waljee, MD, MS, Division of Gastroenterology and Hepatology, Department of Medicine, Michigan Medicine, Ann Arbor, MI, USA. Weijing Tang, MA, Division of Gastroenterology and Hepatology, Department of Medicine, Michigan Medicine, Ann Arbor, MI, USA. Yueyang Zhang, BS, Division of Gastroenterology and Hepatology, Department of Medicine, Michigan Medicine, Ann Arbor, MI, USA. Ji Zhu, PhD Division of Gastroenterology and Hepatology, Department of Medicine, Michigan Medicine, Ann Arbor, MI, USA. Harminder Singh, MD, MPH, Division of Gastroenterology and Hepatology, Department of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada. Zahra Solati, MSc, Division of Gastroenterology and Hepatology, Department of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada. Nick Hajidiacos, MD, Division of Gastroenterology and Hepatology, Department of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada.
CRediT Authorship Contributions
Rebecca Spitzer, MPH (Data curation: Equal; Writing – review & editing: Supporting),
Rebecca Dixon, BS (Data curation: Supporting; Writing – review & editing: Supporting)
Ambreen Merchant, MBBS (Data curation: Supporting; Writing – review & editing: Supporting)
Eric Howard, BSN (Data curation: Supporting; Writing – review & editing: Supporting)
Vaishali Patel, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Mary West, BSN (Data curation: Supporting; Writing – review & editing: Supporting)
Emad Qayed, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Rosemary Nustas, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Ali Zakaria, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Mark Piper, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Lujain Jaza, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Nauzer Forbes, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Millie Chae, BS (Data curation: Supporting; Writing – review & editing: Supporting)
Luis Lara, MD (Conceptualization: Supporting; Writing – review & editing: Supporting)
Georgios Parachristou, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Michael Volk, MD (Conceptualization: Supporting; Writing – review & editing: Supporting)
Liam Hilson, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Selena Zhou, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Vladimir Kushnir, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Alexandia Lenyo, BS (Data curation: Supporting; Writing – review & editing: Supporting)
Caroline McLeod, BS (Data curation: Supporting; Writing – review & editing: Supporting)
Sunil Amin, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Gabriela Kuftinec, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Dhiraj Yadav, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Charlie Fox, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Jennifer Kolb, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Swati Pawa, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Rishi Pawa, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Andrew Canakis, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Christopher Huang, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Laith Jamil, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Andrew Aneese, MD (Formal analysis: Supporting; Writing – review & editing: Supporting)
Benita Glamour, BA (Data curation: Supporting; Writing – review & editing: Supporting)
Zachary Smith, DO (Data curation: Supporting; Writing – review & editing: Supporting)
Katherine Hanley, PA (Data curation: Supporting; Writing – review & editing: Supporting)
Jordan Wood, BS (Data curation: Supporting; Writing – review & editing: Supporting)
Harsh Patel, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Janak Shah, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Emil Agarunov, BS (Data curation: Supporting; Writing – review & editing: Supporting)
Amritha Sethi, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Evan Fogel, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Gail McNulty, BSN (Data curation: Supporting; Writing – review & editing: Supporting)
Abdul Haseeb, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Judy Trieu, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Jeong Yang, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Robert Mendelsohn, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Delia Calo, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Olga Aroniadis, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Joseph LaComb, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Bryan Sauer, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Duyen Dang, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Cyrus Piraka, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Eric Shah, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Heiko Pohl, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Stephanie Mitchell, BSN (Data curation: Supporting; Writing – review & editing: Supporting)
Ashwinee Condon, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Adrienne Lenhart, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Kulwinder Dua, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Vikram Kanagala, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Ayesha Kamal, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Vikesh Singh, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Maria Ines Pinto-Sanchez, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Joy Hutchinson, MSc (Data curation: Supporting; Writing – review & editing: Supporting)
Richard Kwon, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Sheryl Korsnes, MA (Data curation: Supporting; Writing – review & editing: Supporting)
Harminder Singh, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Zahra Solati, MSc (Data curation: Supporting; Writing – review & editing: Supporting)
Amar Deshpande, MD (Data curation: Supporting; Writing – review & editing:
Supporting)
Don Rockey, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Teldon Alford, BA (Data curation: Supporting; Writing – review & editing: Supporting)
Field Willingham, MD (Data curation: Supporting; Writing – review & editing:
Supporting)
Patrick Yachimski, MD (Data curation: Supporting; Writing – review & editing:
Supporting)
Darwin Conwell, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Evan Mosier, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Mohamed Azab, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Anish Patel, MD (Data curation: Supporting; Writing – review & editing: Supporting)
James Buxbaum, MD (Data curation: Supporting; Writing – review & editing:
Supporting)
Sachin Wani, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Amitabh Chak, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Amy Hosmer, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Rajesh Keswani, MD (Data curation: Supporting; Writing – review & editing:
Supporting)
Christopher DiMaio, MD (Data curation: Supporting; Writing – review & editing:
Supporting)
Michael Bronze, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Raman Muthusamy, MD (Data curation: Supporting; Writing – review & editing:
Supporting)
Marcia Canto, MD (Data curation: Supporting; Writing – review & editing: Supporting)
V. Mihajlo Gjeorgjievski, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Ziad Imam, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Fadi Odish, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Ahmad Edhi, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Molly Orosey, DO (Data curation: Supporting; Writing – review & editing: Supporting)
Abhivav Tiwari, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Soumil Patwardhan, MD (Data curation: Supporting; Writing – review & editing:
Supporting)
Nicholas Brown, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Anish Patel, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Collins Ordiah, MBBS (Data curation: Supporting; Writing – review & editing:
Supporting)
Ian Sloan, BS (Data curation: Supporting; Writing – review & editing: Supporting)
Lilian Cruz, BS (Data curation: Supporting; Writing – review & editing: Supporting)
Casey Koza, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Uchechi Okafor, BSc (Data curation: Supporting; Writing – review & editing: Supporting)
Thomas Hollander, BSN (Data curation: Supporting; Writing – review & editing: Supporting)
Nancy Furey, BSN (Data curation: Supporting; Writing – review & editing: Supporting)
Olga Reykhart, BS (Data curation: Supporting; Writing – review & editing: Supporting)
Natalia Zbib, MD (Data curation: Supporting; Writing – review & editing: Supporting)
John Damianos, MD (Data curation: Supporting; Writing – review & editing: Supporting)
James Esteban, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Nick Hajidiacos, MD (Data curation: Supporting; Writing – review & editing: Supporting). Melissa Saul, MS (Data curation: Supporting; Writing – review & editing: Supporting)
Melanie Mays, BS (Data curation: Supporting; Writing – review & editing: Supporting)
Gulsum Anderson, PhD (Data curation: Supporting; Writing – review & editing: Supporting)
Kelley Wood, BS (Data curation: Supporting; Writing – review & editing: Supporting)
Laura Mathews, BS (Data curation: Supporting; Writing – review & editing: Supporting)
Galina Diakova, MS (Data curation: Supporting; Writing – review & editing: Supporting)
Molly Caisse, BS (Data curation: Supporting; Writing – review & editing: Supporting)
Lauren Wakefield, MHA (Data curation: Supporting; Writing – review & editing: Supporting)
Haley Nitchie, MHA (Data curation: Supporting; Writing – review & editing: Supporting)
Akbar Waljee, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Weijing Tang, MA (Data curation: Supporting; Writing – review & editing: Supporting)
Yueyang Zhang, BS (Data curation: Supporting; Writing – review & editing: Supporting)
Ji Zhu, PhD (Conceptualization: Supporting; Writing – review & editing: Supporting)
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.11.007.
Contributor Information
North American Alliance for the Study of Digestive Manifestations of COVID-19:
Ambreen A. Merchant, Vaishali A. Patel, Field F. Willingham, Eric F. Howard, Mary K. West, Casey L. Koza, Patrick S. Yachimski, Emad Qayed, Rosemary Nustas, Ali Zakaria, Marc S. Piper, Lujain Jaza, Nauzer Forbes, Millie Chau, Luis F. Lara, Georgios I. Papachristou, Uchechi Okafor, Darwin L. Conwell, Michael L. Volk, Evan Mosier, Mohamed Azab, Anish Patel, Liam G. Hilson, Selena Zhou, James Buxbaum, Vladimir M. Kushnir, Alexandria M. Lenyo, Ian P. Sloan, Thomas Hollander, Caroline G. McLeod, Rebecca L. Spitzer, Lauren Wakefield, Haley Nitchie, Collins O. Ordiah, Don C. Rockey, Teldon B. Alford, Sunil Amin, Gabriela N. Kuftinec, Amar R. Deshpande, Dhiraj Yadav, Melissa Saul, Melanie Mays, Gulsum Anderson, Kelley Wood, Laura Mathews, Charlie Fox, Jennifer M. Kolb, Sachin Wani, Swati Pawa, Rishi Pawa, Andrew Canakis, Christopher Huang, Laith H. Jamil, Andrew M. Aneese, V. Mihajlo Gjeorgjievski, Zaid Imam, Fadi Odish, Ahmed I. Edhi, Molly Orosey, Abhinav Tiwari, Soumil Patwardhan, Benita K. Glamour, Zachary L. Smith, Amy E. Hosmer, Nancy Furey, Amitabh Chak, Katherine A. Hanley, Jordan Wood, Rajesh N. Keswani, Harsh K. Patel, Janak N. Shah, Emil Agarunov, Nicholas G. Brown, Anish A. Patel, Amrita Sethi, Evan L. Fogel, Gail McNulty, Abdul Haseeb, Judy A. Trieu, Rebekah E. Dixon, Jeong Yun Yang, Christopher J. DiMaio, Robin B. Mendelsohn, Delia Calo, Olga C. Aroniadis, Joseph F. LaComb, Lilian Cruz, Olga Reykhart, Bryan G. Sauer, Galina Diakova, Duyen T. Dang, Cyrus R. Piraka, Eric D. Shah, Molly Caisse, Natalia H. Zbib, John A. Damianos, Heiko Pohl, Stephanie Mitchell, Michael S. Bronze, Ashwinee Condon, Adrienne Lenhart, Raman Muthusamy, Kulwinder S. Dua, Vikram S. Kanagala, James Esteban, Ayesha Kamal, Marcia I. Canto, Vikesh K. Singh, Maria Ines Pinto-Sanchez, Joy M. Hutchinson, Richard S. Kwon, Sheryl J. Korsnes, Akbar K. Waljee, Weijing Tang, Yueyang Zhang, Ji Zhu, Harminder Singh, Zahra Solati, and Nick Hajidiacos
Supplementary Material
Methods
This was a secondary analysis of a large-scale retrospective cohort study aiming to better understand COVID-19 and the digestive system. The study was conducted through an alliance of 36 medical centers in the United States and Canada. Institutional review board approval was obtained at each site before patient identification and data collection.
Adult patients who were hospitalized with a confirmed diagnosis of COVID-19 were considered eligible. To limit sampling bias, we aimed to include the first 50 to 100 consecutive patients meeting eligibility criteria at each participating institution. Potentially eligible patients were identified by site investigators using multiple methods, including data warehouse queries, electronic research subject identification tools, and COVID-19 patient lists provided by relevant hospital entities.
Clinical data from the time of symptom onset until discharge, death, or the end of the study period, including the use of PPI or H2RA within 1 month of admission, were manually abstracted through review of electronic health records. Data abstraction was performed by clinical research coordinators, medical students, internal medicine and gastroenterology trainees, as well as faculty gastroenterologists, depending on site. Every site had a designated clinician investigator who oversaw and vouched for the integrity of data collection. Data quality was ensured to the greatest extent possible using a multifaceted strategy that has been described previously and included manual review of all incoming data by a dedicated data management specialist.1 The data collection form is provided later in this Supplementary Material.
The association between acid suppressive medication use and mechanical ventilation or death was evaluated using 2 separate multivariable generalized linear regression models. PPI and H2RA use, the independent variables of interest, as well as age, race, and sex were included in both models. Lasso regression, an agnostic, data-driven approach to variable selection, was used to select the other covariates. Only laboratory values at admission were included as covariates in the models because in-hospital laboratory values (highest/lowest) could have occurred after mechanical ventilation (a primary outcome) and could have been confounded by in-hospital treatments. To account for clustering by center, generalized estimating equation models with a random center effect were used.
To assess the validity of findings from the primary regression analysis, we performed a propensity score matched analysis of the association between PPI or H2RA use with mechanical ventilation or death. Propensity scores were estimated for PPI or H2RA use based on the predicted probability of exposure, estimated from generalized linear mixed models with either PPI/H2RA use as the outcome and all other patient factors (excluding mechanical ventilation and death) as predictors. A random center effect was used in this model as well. Four-to-one control-to-treatment caliper matching with caliper = 0.2 was applied to the propensity scores to obtain matched patients by H2RA or PPI use.
Missing data were assumed to be missing at random and were imputed before model development. In the primary analysis, we excluded patients whose PPI and/or H2RA use within 1 month of admission was unknown. Sensitivity analyses were subsequently conducted to evaluate the validity of excluding patients with unknown PPI/H2RA status and of imputation.
Supplementary Table 1.
Patient Characteristics
| Patient Characteristic | Overall (N = 1846) |
|---|---|
| Demographics | |
| Age, y, mean (SD) | 59.9 (16.4) |
| Sex, male, n (%) | 1044 (56.6) |
| Race, n (%) | |
| White | 680 (36.8) |
| Black | 774 (41.9) |
| Other/Unknown | 392 (21.2) |
| Body mass index, mean (SD) | 31.5 (8.14) |
| PPI use, Yes, n (%) | 417 (22.6) |
| H2 blocker use, Yes, n (%) | 167 (9.1) |
| Patient health characteristics | |
| Comorbidities, Yes, n (%) | |
| Hypertension | 1146 (62.1) |
| Coronary artery disease/myocardial infarction | 284 (15.4) |
| Congestive heart failure | 194 (10.5) |
| COPD | 171 (9.26) |
| Asthma | 240 (13.0) |
| Obstructive sleep apnea | 197 (10.7) |
| Peripheral vascular disease | 91 (4.93) |
| Cerebrovascular accident or TIA | 170 (9.21) |
| Dementia | 118 (6.39) |
| Diabetes Mellitus | 658 (35.6) |
| ESRD | 175 (9.48) |
| Current malignancy | 117 (6.34) |
| Prior malignancy | 171 (9.26) |
| Digestive disease | 183 (9.91) |
| Other comorbidities | 829 (44.9) |
| No comorbidities | 203 (11.0) |
| No. Comorbidities, median (min-max; IQR) | 2 (0-10; 3) |
| Chemotherapy or Immunosuppression, n (%) | |
| Yes | 219 (11.9) |
| No | 1621 (87.8) |
| Unknown | 6 (0.33) |
| Current or recent ACE or ARB use, Yes, n (%) | 556 (30.1) |
| Current or recent NSAID use, n (%) | |
| Yes | 506 (27.4) |
| No | 1100 (59.6) |
| Unknown | 240 (13.0) |
| Current or recent antibiotic use, n (%) | |
| Yes | 560 (30.3) |
| No | 1251 (67.8) |
| Unknown | 35 (1.90) |
| Admission clinical lab measures | |
| White blood cell count, mean (SD) | 7.18 (4.00) |
| Hemoglobin, mean (SD) | 12.9 (2.20) |
| Platelets. mean (SD) | 207.5 (90.3) |
| Aspartate, mean (SD) | 50.7 (54.1) |
| Alanine aminotransferase, mean (SD) | 37.5 (34.5) |
| Alkaline phosphate, mean (SD) | 82.3 (48.8) |
| Bilirubin, mean (SD) | 0.64 (0.63) |
| Albumin, mean (SD) | 3.63 (0.52) |
| Creatinine, mean (SD) | 1.65 (2.76) |
| Outcomes | |
| Mechanical ventilation required, Yes, n (%) | 584 (31.6) |
| ICU admission, Yes, n (%) | 795 (43.1) |
| In-hospital death, Yes, n (%) | 327 (17.7) |
| HLOS, days, median (min-max, IQR) | 8 (0.4-113; 13) |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; HLOS, hospital length of stay; ICU, intensive care unit; IQR, interquartile range; NSAID, nonsteroidal anti-inflammatory drug; TIA, transient ischemic attack.
Supplementary Table 2.
Variables Included in the Final Regression Models for Mechanical Ventilation or Death
| Mechanical ventilation | Death |
|---|---|
| H2RA use | H2RA use |
| PPI use | PPI use |
| Age | Age |
| Body mass index | |
| Sex | Sex |
| Race | Race |
| Dementia | Dementia |
| Congestive heart failure | |
| Number of comorbidities | Number of comorbidities |
| White blood cell count at admission | White blood cell count at admission |
| Platelets at admission | Platelets at admission |
| Aspartate aminotransferase at admission | Aspartate aminotransferase at admission |
| Alkaline phosphatase at admission | |
| Albumin at admission | Albumin at admission |
| Creatinine at admission | |
| Antibiotics before admission |
-
1)Please complete the below data collection form (DCF) in REDCap at the time of discharge or death. Data will appear in the DMC19 database once entry and verification are complete.
-
2)We aim to capture inpatients with a confirmed COVID-19 diagnosis, regardless of whether they have digestive manifestations. After prevalence is defined in hospitalized patients, and as the numbers grow, we may focus on patients who are known to have GI manifestations and/or include outpatients.
-
3)Please make all efforts to collect data on the first 50 to 100 consecutive patients at your hospital or health system.
-
4)Eligible patients can and should be identified by any means necessary, which may include, but is not limited to, institutional laboratory records, data warehouse queries, electronic health record research subject identification tools/dashboards, and discussions with the infectious disease or critical care services, etc. You may elect to use the emergency ICD-10 code of U07.1 – 2019-nCov acute respiratory disease – to help identify eligible patients.
-
5)Please triple-check data for accuracy before submission. Although we are performing central data monitoring, we cannot verify incoming data against source documents, nor are we performing on-site monitoring visits. Therefore, the overall quality of the data is assured primarily at the site level.
-
6)Along the lines of #5, coordinators should confer with a clinician during data collection to ensure that clinical context is accounted for as much as possible in the interpretation of questions that involve an element of subjectivity.
-
7)All data fields should have affirmative, negative, and unknown options. Therefore, missing data will be assumed to be inadvertent and this will generate a query.
-
8)Please maintain a secure key at your site that allows patient identification on the basis of subject ID#. This may be used in the future for to collect data pertaining to long-term outcomes.
Authors for this North American Alliance for the Study of Digestive Manifestations of COVID-19 study
Primary authors: B. Joseph Elmunzer, MD, and Bethany J. Wolf, PhD.
Writing committee: B. Joseph Elmunzer, MD, Bethany J. Wolf, PhD, Jason R. Taylor, MD, William Tierney, MD, James Scheiman, MD.
Steering Committee: B. Joseph Elmunzer, MD (Chair), Rebecca L. Spitzer, MPH, Rebekah E. Dixon, BS, Collins O. Ordiah, MBBS, Jennifer M. Kolb, MD, MS, Sachin Wani, MD, Olga C. Aroniadis, MD, Robin B. Mendelsohn, MD, Christopher J. DiMaio, MD, Don C. Rockey, MD, Amit G. Singal, MD, MS, Amar R. Deshpande, MD, Swati Pawa, MD, Darwin L. Conwell, MD, MSc, Raman Muthusamy, MD, MAS, William M. Tierney, MD, Dhiraj Yadav, MD, MPH.
Statisticians: Bethany J. Wolf, PhD, Weijing Tang, MA, Yueyang Zhang, BS, Ji Zhu, PhD.
Data Coordinating Center: Teldon B. Alford, BA, Lauren Wakefield, MHA, Haley Nitchie, MHA, Collins O. Ordiah, MBBS, Rebecca L. Spitzer, MPH.
Clinical sites in order of number of patients contributed
Emory University: Ambreen A. Merchant MBBS, Vaishali A. Patel MD, Field F. Willingham MD, MPH.
Vanderbilt University: Eric F. Howard RN, BSN, Mary K. West RN, BSN, Casey L Koza BS, Patrick S. Yachimski MD.
Grady Memorial Hospital: Emad Qayed MD, MPH, Rosemary Nustas MD.
Ascension Providence Hospital/Michigan State University: Ali Zakaria MD, Marc S. Piper MD, MSc.
Saint Louis University: Jason R. Taylor MD, Lujain Jaza MD.
University of Calgary: Nauzer Forbes MD, MSc, Millie Chau BSc.
The Ohio State University Wexner Medical Center: Luis F. Lara MD, Georgios I. Papachristou MD, PhD, Uchechi Okafor BSc, Darwin L. Conwell MD, MSc.
Loma Linda University: Michael L. Volk MD, MSc, Evan Mosier MD, Mohamed Azab MD, Anish Patel MD.
University of Southern California: Liam G. Hilson MD, Selena Zhou MD, James Buxbaum MD.
Washington University School of Medicine: Vladimir M. Kushnir MD, Alexandria M. Lenyo BS, Ian P. Sloan BS, Thomas Hollander RN, BSN.
Medical University of South Carolina: Caroline G. McLeod BS, Rebecca L. Spitzer MPH, Lauren Wakefield MHA, Haley Nitchie MHA, Collins O. Ordiah MBBS, MPH, Don C. Rockey MD, Bethany J. Wolf PhD, B. Joseph Elmunzer MD.
University of Miami Miller School of Medicine: Sunil Amin MD, MPH, Gabriela N. Kuftinec MD, MPH, Amar R. Deshpande MD.
University of Pittsburgh: Dhiraj Yadav MD, MPH, Melissa Saul MS, Melanie Mays BS, Gulsum Anderson PhD, Kelley Wood BS, Laura Mathews BS.
University of Colorado: Charlie Fox MD, Jennifer M. Kolb MD, MS, Sachin Wani MD.
Wake Forest University School of Medicine: Swati Pawa MD, Rishi Pawa MD.
Boston University: Andrew Canakis DO, Christopher Huang MD.
Beaumont Health: Laith H. Jamil MD, Andrew M. Aneese MD, V. Mihajlo Gjeorgjievski, MD, Zaid Imam MD, Fadi Odish MD, Ahmed I. Edhi MD, Molly Orosey DO, Abhinav Tiwari MD, Soumil Patwardhan MBBS.
University Hospitals of Cleveland Medical Center: Benita K. Glamour BA, Zachary L Smith DO, Amy E. Hosmer MD, Nancy Furey RN, BSN, MBA, Amitabh Chak MD.
Northwestern University: Feinberg School of Medicine: Katherine A. Hanley MMS, PAC, Jordan Wood BS, Rajesh N. Keswani MD, MS.
Ochsner Health: Harsh K. Patel MBBS, Janak N. Shah MD.
Columbia University Medical Center: Emil Agarunov BS, Nicholas G. Brown MD, Anish A. Patel MD, Amrita Sethi MD.
Indiana University School of Medicine: Evan L. Fogel MD, MSc, Gail McNulty RN, BSN.
Loyola University Medical Center: Abdul Haseeb MD, Judy A. Trieu MD.
Icahn School of Medicine at Mount Sinai: Rebekah E. Dixon BS, Jeong Yun Yang MD, Christopher J. DiMaio MD.
Memorial Sloan Kettering Cancer Center: Robin B. Mendelsohn MD, Delia Calo MD.
Renaissance School of Medicine at Stony Brook University: Olga C. Aroniadis MD, MSc, Joseph F. LaComb BS, Lilian Cruz BS, Olga Reykhart BS.
University of Virginia Medical School: James M. Scheiman MD, Bryan G. Sauer MD, Galina Diakova MS.
Henry Ford Health System: Duyen T. Dang MD, Cyrus R. Piraka MD.
Dartmouth-Hitchcock Health and VA White River Junction: Eric D. Shah MD, MBA, Molly Caisse BS, Natalia H. Zbib MD, John A. Damianos MD, Heiko Pohl MD.
University of Oklahoma Health Sciences Center: William M. Tierney MD, Stephanie Mitchell RN, Michael S. Bronze MD.
David Geffen School of Medicine at UCLA: Ashwinee Condon MD, Adrienne Lenhart MD, Raman Muthusamy MD, MAS.
Medical College of Wisconsin: Kulwinder S. Dua MD, Vikram S. Kanagala MD, James Esteban MD.
Johns Hopkins Medical Institutions: Ayesha Kamal MD, Marcia I. Canto MD, Vikesh K. Singh MD, MS.
McMaster University Hamilton Health Sciences: Maria Ines Pinto-Sanchez MD, MSc (37), Joy M. Hutchinson RD, MSc.
Michigan Medicine: Richard S. Kwon MD, Sheryl J. Korsnes MA, Akbar K. Waljee, MD, MS, Weijing Tang MA, Yueyang Zhang BS, Ji Zhu PhD.
University of Manitoba: Harminder Singh MD, MPH, Zahra Solati MSc, Nick Hajidiacos MD.
| Subject ID # | |
| Institution | |
| Email address (of individual entering data) |
| Patient characteristics | |
|---|---|
| Age (years) | |
| Sex |
|
| Race (check all that apply) |
|
| Ethnicity |
|
| Body mass index at presentation (kg/m2): available? |
|
| Body mass index at presentation (kg/m2) | |
| Is the patient a health care worker? |
|
| Cigarette smoking status |
|
| Vaping status |
|
| Alcoholism |
|
| Cannabis use |
|
| Illicit drug use |
|
| Comorbidities (select all that apply) |
|
| If other, specify | |
| Recent (within 6 months) or current (at admission) immunosuppression or chemotherapy |
|
| If yes, specify | (medication, dose, route, duration) |
| Recent (within 1 month of admission) or current (at admission) angiotensin-converting enzyme inhibitor use |
|
| Recent (within 1 month of admission) or current (at admission) angiotensin receptor blocker (ARB) use |
|
| Recent (within 1 month of admission) or current (at admission) nonsteroidal anti-inflammatory drug use |
|
| Recent (within 1 month of admission) or current (at admission) antibiotic use |
|
| Recent (within 1 month of admission) or current (at admission) PPI use |
|
| Recent (within 1 month of admission) or current (at admission) H2 blocker use |
|
| COVID-19 parameters | |
|---|---|
| History of known contact with COVID-19 positive individual(s) |
|
| Highest level of care |
|
| Duration of symptoms prior to first seeking medical attention (days) | |
| Duration of symptoms prior to hospitalization (days) | |
| Duration of hospitalization (days) | |
| If admitted to ICU, duration of ICU stay (days) | |
| Required mechanical ventilation |
|
| Required extracorporeal membrane oxygenation (ECMO) |
|
| Required vasopressor support |
|
| Final discharge disposition |
|
| COVID-specific treatments (select all that apply) |
|
| If other, specify |
|
| Symptomatology | |
|---|---|
| Respiratory or systemic symptoms (select all that apply) |
|
| If other, specify | |
| Gastrointestinal symptoms or signs (select all that apply) |
|
| If other, specify | |
| Timing of gastrointestinal symptoms (if any) relative to respiratory/systemic symptoms (if any) |
|
| Duration of gastrointestinal symptoms |
|
| Did gastrointestinal symptoms remain after resolution of other COVID-19 symptoms? |
|
| If yes, how long (days) | |
| Prominence of gastrointestinal symptoms |
|
| Were digestive manifestations (gastrointestinal or hepatic) specifically addressed in the Assessment/Plan section of any progress notes for 3 or more days during the hospitalization? |
|
| Did the gastroenterology or hepatology service consult on the patient during the hospitalization, as evidence by consult notes? |
|
| Were stool studies other than FOBT (fecal occult blood test) obtained during the hospitalization? |
|
| Gastrointestinal diagnoses established shortly before, during, or shortly after COVID-19 illness (select all that apply) |
|
| If other, specify | |
| Was COVID-specific treatment prescribed specifically for digestive manifestations? |
|
| Imaging and endoscopy | |
|---|---|
| Was abdominal computed tomography (CT) performed and abnormal shortly before, during, or shortly after COVID-19 illness? |
|
| If yes, list abnormal findings in the impression section of the most concerning CT report | |
| Was abdominal magnetic resonance imaging (MRI) performed and abnormal shortly before, during, or shortly after COVID-19 illness? |
|
| If yes, list abnormal findings in the impression section of the most concerning MRI report | |
| Was abdominal ultrasound (US) performed and abnormal shortly before, during, or shortly after COVID-19 illness? |
|
| If yes, list abnormal findings in the impression section of the most concerning US report | |
| Was endoscopy performed during COVID-19 illness? (select all that apply) |
|
| If performed, how many total endoscopic sessions did the patient undergo? (EGD+colonoscopy or EUS+ERCP at the same time is considered 1 session) | |
| If performed, did the patient require respiratory support BEFORE their first procedure? (select all the apply) |
|
| If performed, what kind of anesthesia did the patient receive DURING their first procedure? (select all that apply) |
|
| If performed, did the patient experience an adverse cardiopulmonary event related to any of the endoscopic procedures? (select all that apply) |
|
| Endoscopic findings (please list abnormal findings in impression section of endoscopy report(s)) | |
| Histologic findings (please list abnormal findings in impression section of pathology report(s)) | |
| Laboratory data and other hepatologic considerations | |
|---|---|
|
Prior to COVID-19, ideally when patient was healthy: White blood cells (WBC) Hemoglobin Platelets Aspartate aminotransferase (AST) Alanine aminotransferase (ALT) Alkaline phosphatase (ALK-phos) Total bilirubin International normalized ratio (INR) Albumin Factor V level Lipase Creatinine |
|
|
At time of hospital admission: White blood cells (WBC) Hemoglobin Platelets Aspartate aminotransferase (AST) Alanine aminotransferase (ALT) Alkaline phosphatase (ALK-phos) Gamma-Glutamyl Transferase (highest) U/L Total bilirubin Direct bilirubin International normalized ratio (INR) Albumin Factor V level Lipase
|
|
|
Highest or lowest level during illness: WBC (highest and lowest) Hemoglobin (lowest) Platelets (lowest) AST (highest) ALT (highest) ALK-phos (highest) Gamma-Glutamyl Transferase (highest) U/L Total Bilirubin (highest) Direct Bilirubin (highest) INR (highest) Albumin (lowest) Factor 5 (lowest) Lipase (highest)
Absolute lymphocyte count (lowest) C-Reactive Protein (CRP) (highest) Procalcitonin (highest) Troponin (highest) Ferritin (highest) ng/mL or ug/L Interleukin-6 (highest) pg/mL |
|
| Duration between first onset of symptoms and highest AST (days) | |
| Duration between first onset of symptoms and highest ALT (days) | |
| Duration between first onset of symptoms and highest total bilirubin (days) | |
| Duration between first hospital day and highest AST (days) | |
| Duration between first hospital day and highest ALT (days) | |
| Duration between first hospital day and highest total bilirubin (days) | |
| Abnormal LFTs were |
|
| Were the increased LFTs suspected to be due to a drug reaction (based on review of progress/consult notes)? |
|
| If yes, which medication(s) were suspected | (medication, dose, route, duration) |
| If yes, was this a COVID-specific treatment that was believed to increase LFTs (based on review of progress/consult notes)? |
|
| Anti-HAV IgM |
|
| Anti-HCV |
|
| HCV RNA |
|
| HBsAg |
|
| Anti-HBc |
|
| Anti-HBc IgM |
|
| Anti-HBs |
|
| Epstein-Barr Virus antibody IgM (Anti-EBV IgM) |
|
| Cytomegalovirus antibody IgM (Anti-CMV IgM) |
|
| Anti-nuclear antibody (ANA) |
|
| Anti-smooth muscle antibody (ASMA) |
|
| Anti-mitochondrial antibody (AMA) |
|
| Did the patient develop evidence of decompensated liver disease during or shortly after COVID-19 illness? (select all that apply) |
|
| Was a liver biopsy performed during the COVID-19 hospitalization? |
|
| If yes, histologic findings (please list abnormal findings in impression section of pathology report(s)). | |
References
- 1.Almario C.V. Am J Gastroenterol. 2020;115:1707–1715. doi: 10.14309/ajg.0000000000000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee S.W. Gut. 2021;70:76–84. doi: 10.1136/gutjnl-2020-322248. [DOI] [PubMed] [Google Scholar]
- 3.Darnell M.E. J Virol Methods. 2004;121:85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedberg D.E. Gastroenterology. 2015;149:883–885. doi: 10.1053/j.gastro.2015.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedberg D.E. Gastroenterology. 2020;159:1129–1131. doi: 10.1053/j.gastro.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mather J.F. Am J Gastroenterol. 2020;115:1617–1623. doi: 10.14309/ajg.0000000000000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C. Acta Pharm Sin B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elmunzer B.J. Clin Gastroenterol Hepatol. 2020 Sep 30 PMID: 33010411. [Google Scholar]
Reference
- 1.Elmunzer B.J. Clin Gastroenterol Hepatol. 2020 Sep 30 PMID: 33010411. [Google Scholar]