Abstract
Introduction
Lung cancer is associated with severe coronavirus disease 2019 (COVID-19) infections. Symptom overlap between COVID-19 and lung cancer may complicate diagnostic evaluation. We aimed to investigate the incidence, symptoms, differential diagnosis, and outcomes of COVID-19 in patients with lung cancer.
Methods
To determine an at-risk population for COVID-19, we retrospectively identified patients with lung cancer receiving longitudinal care within a single institution in the 12 months (April 1, 2019 to March 31, 2020) immediately preceding the COVID-19 pandemic, including an “active therapy population” treated within the last 60 days of this period. Among patients subsequently referred for COVID-19 testing, we compared symptoms, laboratory values, radiographic findings, and outcomes of positive versus negative patients.
Results
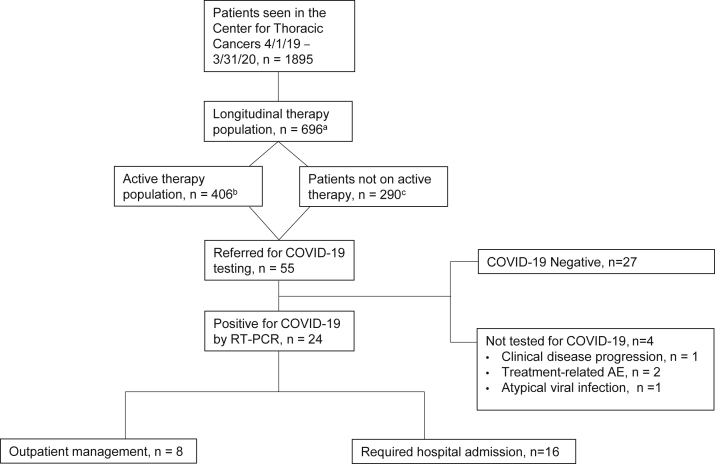
Between April 1, 2019 and March 31, 2020, a total of 696 patients received longitudinal care, including 406 (58%) in the active therapy population. Among 55 patients referred for COVID-19 testing, 24 (44%) were positive for COVID-19, representing a cumulative incidence of 3.4% (longitudinal population) and 1.5% (active therapy population). Compared with patients who were COVID-19 negative, those who were COVID-19 positive were more likely to have a supplemental oxygen requirement (11% versus 54%, p = 0.005) and to have typical COVID-19 pneumonia imaging findings (5 versus 56%, p = 0.001). Otherwise, there were no marked differences in presenting symptoms. Among patients who were COVID-19 negative, alternative etiologies included treatment-related toxicity (26%), atypical pneumonia (22%), and disease progression (22%). A total of 16 patients positive for COVID-19 (67%) required hospitalization, and seven (29%) died from COVID-related complications.
Conclusions
COVID-19 was infrequent in this lung cancer population, but these patients experienced high rates of morbidity and mortality. Oncologists should maintain a low threshold for COVID-19 testing in patients with lung cancer presenting with acute symptoms.
Keywords: COVID-19, SARS-CoV-2, NSCLC, SCLC, Lung cancer, Thoracic oncology
Introduction
Since the onset of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak in December 2019,1 evidence has accumulated indicating that patients with cancer are particularly susceptible to complications from coronavirus disease (COVID-19), the infection caused by SARS-CoV-2, with higher rates of intensive care unit (ICU) admission, need for mechanical ventilation, and death.2, 3, 4, 5, 6 Moreover, patients with a history of cancer or active cancer not on cancer therapy seem to be at higher risk for severe illness or mortality owing to COVID-19 compared with the general population.4,7
Patients with lung cancer may be particularly vulnerable to complications from COVID-19. In an initial retrospective analysis of COVID-19 outcomes among 105 patients with cancer in Wuhan, People’s Republic of China, patients with lung cancer had the second highest mortality rates owing to COVID-19, behind only those with hematologic malignancies.7 More recent studies have confirmed the high rates of hospitalization and death within thoracic oncology populations affected by COVID-19. For example, in the Thoracic Cancers International COVID-19 Collaboration (TERAVOLT) registry,8 which pooled data from 200 patients across 42 institutions and eight countries, 76% of the patients of thoracic oncology with COVID-19 required hospitalization and 33% of them died. Importantly, recent data from Luo et al.9,10 suggest that patient-specific factors, such as smoking status and chronic obstructive pulmonary disease, rather than disease-specific factors (e.g., previous surgery, systemic therapy) are the major determinants of COVID-19 infection severity among patients with lung cancer.
Although registry studies have been valuable in elucidating risk factors and clinical outcomes for severe COVID-19 infection among the patients with lung cancer, such studies have not captured the incidence of COVID-19 infection within this patient population. Furthermore, given the potential for symptoms of lung cancer or toxicities from lung cancer therapies to mimic COVID-19 infection, it is imperative to characterize the frequency of alternative diagnoses among patients with lung cancer presenting with respiratory symptoms during this pandemic. These data may guide diagnostic algorithms and clinical workflow while ensuring prompt treatment initiation for COVID-19 and minimizing transmission.
As of June 1, 2020, a total of 100,805 patients have been diagnosed with having COVID-19 in the Massachusetts, and Boston became an early epicenter in the United States.11 In this study, we retrospectively reviewed the rates of COVID-19 infection among the patients with primary lung malignancies treated at Massachusetts General Hospital (MGH), a tertiary academic medical center in Boston, during the COVID-19 pandemic. To differentiate the characteristics of COVID-19 from common complications of lung cancer, we evaluated all urgent clinic encounters from the onset through the first 3 months of the COVID-19 surge in Massachusetts.
Materials and Methods
Patient Population
To determine the lung cancer population at risk for COVID-19 in our center, we retrospectively identified all patients with lung cancer receiving longitudinal care in the Center for Thoracic Cancers at the MGH between April 1, 2019, and March 31, 2020, using institutional databases and manual chart review. We included in the longitudinal population all patients for whom MGH was the primary provider of lung cancer therapy (systemic therapy, radiation, and surgery) during the year before the pandemic. The electronic medical record (EMR) was manually reviewed to extract data on demographics, baseline clinical characteristics, and previous and current cancer therapies, including surgical resection, radiation therapy (RT), and systemic therapy. To ensure adequate clinical follow-up, we excluded patients who had not received cancer therapy at our institution over the study period (e.g., second opinion consultations or patients who ultimately received therapy at an outside institution), those with nonlung primary thoracic malignancies (e.g., thymoma, thymic carcinoma), patients never treated with lung cancer-directed therapy, and those who died before March 2020. As this was a retrospective review, informed consent was not obtained. All clinical, radiographic, and outcome data were compiled under an ongoing institutional review board–approved protocol at our institution with an appropriate waiver of consent.
Within the “longitudinal population” of patients who were receiving cancer therapy at MGH, the “active therapy” population was defined as those patients treated with systemic therapy (chemotherapy, immune checkpoint inhibition, combination chemotherapy plus immune checkpoint inhibition, tyrosine kinase inhibition, or any other antineoplastic therapy, including investigational agents) within the 60 days before and including March 31, 2020. Treatment modifications used to reduce the risk of COVID-19 exposure were assessed within the “active therapy” population by manual chart review of encounters between March 1, 2020, and June 1, 2020. Treatment modifications included dose delay (defined by delay in therapy administration by ≥1 wk), change in dosing schedule (e.g., transition from every second-wk infusion to every fourth-wk infusion), and cessation of therapy. Data were manually extracted from the EMR by AP, JP, MS, AD, MM, and CM.
To evaluate the proportion of patients with lung cancer examined for possible COVID-19 infection, we reviewed inpatient admissions data, outpatient respiratory infection clinic visits, and outpatient thoracic oncology clinic visits and surveyed physicians in the Center for Thoracic Cancers in the first 3 months of the COVID-19 pandemic in Massachusetts (March 1, 2020–June 1, 2020). To capture patients who may have been diagnosed with having COVID-19 outside of our institution, we manually reviewed outside facility record data using “Care Everywhere,” a program which enables electronic exchange of medical data across institutions that use Epic Systems Corporation software.
Patient demographics, baseline clinical characteristics including Eastern Cooperative Oncology Group (ECOG) performance status (PS), comorbid conditions, and previous or active cancer-directed treatments were collected on patients with suspected COVID-19 infection. COVID-19 testing location, date(s), and results were extracted. Clinical data (presenting symptoms, need for supplemental oxygen above baseline, laboratory values including complete blood count, creatinine, and liver function tests) on the day of testing were captured. Data pertaining to clinical management were also collected, including need for hospitalization and treatments administered. Among patients who ultimately tested negative for COVID-19, alternative diagnoses were based on the treating clinicians’ assessment. Such cases were also reviewed independently by CM, MM, APV, and JG to ensure consensus. All data were collected under an institutional review board–approved protocol.
SARS-CoV-2 Testing
All cases of COVID-19 infection in this series were confirmed using one of the following three assays: MGH SARS-CoV-2 assay,12 a real-time polymerase chain reaction (RT-PCR) test intended for the qualitative detection of nucleic acid from SARS-CoV-2; Xpert Xpress SARS-CoV-2 nucleic acid test (Cepheid, Sunnyvale, CA); or COBAS SARS-CoV-2 nucleic acid test (Roche Diagnostics, Indianapolis, IN). The MGH SARS-CoV-2 test was used early in the pandemic and then phased out with Food and Drug Administration clearance and implementation of the Cepheid and Roche tests in mid-March 2020. Patients referred for preprocedural COVID-19 testing were excluded from this analysis.
Radiographic Imaging
Patients with suspected COVID-19 infection underwent radiographic imaging at the discretion of the treating clinicians. Imaging findings from chest plain films or computed tomography (CT) scan were retrospectively reviewed by a dedicated thoracic radiologist (SD) who was blinded to COVID-19 testing results. Images were graded per the Radiological Society of North America guidelines, which designate findings as either negative, indeterminate, atypical, or typical for COVID-19 infection.13 The presence of lung cancer and postradiation and postsurgical changes was also noted.
Statistical Analysis
Comparisons of presenting symptoms and laboratory and radiographic findings between groups positive for and negative for COVID-19 were made using Fisher’s exact test and Wilcoxon ranked sum test. Imaging findings and oxygen supplementation were dichotomized into binary outcomes and analyzed using Fisher’s exact test. Rates of hospitalization and mortality among patients who received any versus no systemic therapy or radiation for lung cancer within 30 days of COVID-19 positivity were compared using Fisher’s exact test. All p values were based on a two-sided hypothesis and computed using Stata 12.1 (StataCorp).
Results
Study Population
Between April 1, 2019, and March 31, 2020, a total of 1895 patients were examined by medical oncologists in the Center for Thoracic Cancers (Fig. 1). Within that 12-month period, 696 patients with lung cancer received at least one form of cancer therapy (“longitudinal population”), including systemic therapy (90%), RT (30%), or surgical resection (8%) (Table 1). Among the patients in the longitudinal population ever treated with systemic therapy (90%), the most recent systemic therapy was a tyrosine kinase inhibitor (37%), chemotherapy (24%), immune checkpoint inhibitor monotherapy (19%), and chemotherapy plus immune checkpoint inhibitor (11%). In total, 58% of the patients (n = 406) in the longitudinal population received systemic therapy within 60 days of March 31, 2020 and were defined as the “active therapy” population (Table 1).
Figure 1.
Consort diagram of the study cohort. “Longitudinal therapy population” are defined as those receiving systemic therapy or radiation or undergoing surgery for thoracic cancer between April 1, 2019, and March 31, 2020. “Active therapy population” are those who received systemic therapy (chemotherapy, immune checkpoint inhibitor, tyrosine kinase inhibitor, antibody-drug conjugates, allosteric inhibitors, or combinations of these therapies) in the 60 days before March 31, 2020. Patients “not on active therapy” are those who received primary thoracic oncology care at our center in the previous 12 months but had not been treated with systemic therapy 60 days before March 31, 2020. AE, adverse event; COVID-19, coronavirus disease 2019; RT-PCR, real-time polymerase chain reaction.
Table 1.
Summary of Anticancer Therapies Received During the Study Period
| Anticancer therapy data | No. of patients |
|---|---|
| Longitudinal population, lung cancer therapy last 12 mo | 696 |
| Systemic therapy, last 12 mo; n (% of longitudinal population) | 623 (90) |
| Median time from last systemic treatment in days, (IQR) | 15 (0–104) |
| Most recent systemic therapy type, n (% of systemic therapy) | |
| Tyrosine kinase inhibitor | 229 (37) |
| Chemotherapy | 148 (24) |
| Immune checkpoint inhibitor | 120 (19) |
| Chemotherapy + immune checkpoint inhibitor | 71 (11) |
| Tyrosine kinase inhibitor + chemotherapy | 17 (3) |
| Other | 38 (6) |
| Active therapy population, last 60 d; n (% of systemic therapy) | 406 (65) |
| Tyrosine kinase inhibitor, n (%) | 192 (47) |
| Immune checkpoint inhibitor, n (%) | 67 (17) |
| Chemotherapy, n (%) | 63 (16) |
| Chemotherapy + immune checkpoint inhibitor, n (%) | 42 (10) |
| Tyrosine kinase inhibitor + chemotherapy, n (%) | 16 (4) |
| Other, n (%) | 26 (6) |
| Treatment alteration owing to COVID-19, n (% of active therapy) | 57 (14) |
| Treatment delay (≥1 wk), n (%) | 47 (82) |
| Dosing schedule alteration, n (%) | 5 (9) |
| Did not initiate an indicated therapy, n (%) | 3 (5) |
| Cessation of therapy, n (%) | 2 (4) |
| Type of therapy altered, n (% of systemic treatment delays) | |
| Immune checkpoint inhibitor | 30 (53) |
| Chemotherapy | 11 (19) |
| Chemotherapy + immune checkpoint inhibitor | 9 (16) |
| Tyrosine kinase inhibitor + chemotherapya | 7 (12) |
| Tyrosine kinase inhibitor | 0 (0) |
| RT, last 12 mo; n (% of longitudinal population) | 210 (30) |
| RT sites, n (% patients w/ radiation)b | |
| Lung | 112 (53) |
| Central nervous system | 54 (26) |
| Extrathoracic sites | 44 (21) |
| Thoracic surgery, last 12 mo; n (% of longitudinal population) | 59 (8) |
| Surgical sites, n (% patients with thoracic surgery) | |
| Lobectomy | 35 (59) |
| Wedge | 19 (32) |
| Pleurectomy, decortication, pleurodesis | 4 (7) |
| Pneumonectomy | 1 (2) |
COVID-19, coronavirus disease 2019; IQR, interquartile range; RT, radiation therapy.
Tyrosine kinase inhibitor continued in all cases, whereas the chemotherapy portion of chemotherapy plus tyrosine kinase inhibitor combination was delayed in all seven cases (100%).
First site listed if patient underwent RT to multiple sites in 12 months; 47 patients underwent RT to multiple sites.
Modifications in Systemic Therapy Owing to the COVID-19 Pandemic
A total of 57 patients (14%) in the active therapy population had a documented treatment modification as a result of the COVID-19 pandemic (Table 1). The most common treatment modification was a delay of 1 week in the administration of a patient’s ongoing systemic therapy regimen, which occurred in 47 of these patients (82%). Immune checkpoint inhibitor monotherapy was the most frequently delayed treatment type (53%). Most patients who experienced a treatment delay were receiving palliative systemic therapy (77%) and had disease control (86%) on the most recent surveillance imaging. As of the data cutoff, 33 (70%) had resumed therapy after a median duration of 38 days (interquartile range, 35) of treatment hold.
Clinical Presentations of Patients With Suspected COVID-19 Infection
A total of 55 patients with lung cancer were referred for evaluation of possible COVID-19 infection owing to concerning symptoms or imaging findings (Fig. 1). The most common symptoms within this group were cough (75%), fatigue (51%), dyspnea (45%), and fever (45%) (Table 2). Within this cohort of clinically suspected COVID-19 infection, 51 patients underwent COVID-19 testing by nasopharyngeal RT-PCR swab. COVID-19 testing was performed in the MGH Center for Thoracic Cancers outpatient clinic (79%), ambulatory COVID-19 testing centers (14%), or emergency department or hospital (7%). Four patients were referred for evaluation but did not undergo COVID-19 testing. Ultimately, these four patients were found to have symptoms attributable to progressive disease (n = 1), treatment-related adverse effects (n = 2), and an atypical pneumonia with rapid resolution of symptoms after a course of azithromycin (n = 1), although confirmatory respiratory testing was not performed.
Table 2.
Symptoms and Laboratory and Radiographic Findings of Those Referred for COVID-19 Testing
| Clinical findings | Underwent C19 Testing |
C19 Positive |
C19 Negative |
Two-Sided p Value |
|---|---|---|---|---|
| n = 51 | n = 24 | n = 27 | ||
| Symptoms reported, n (%) | ||||
| Fever | 23 (45) | 14 (58) | 9 (33) | 0.095 |
| Fatigue | 26 (51) | 12 (50) | 14 (52) | 1.000 |
| Cough | 38 (75) | 17 (71) | 21 (78) | 0.749 |
| Dyspnea | 23 (45) | 10 (42) | 13 (48) | 0.780 |
| Altered taste or smell | 4 (8) | 1 (4) | 3 (11) | 0.612 |
| Diarrhea or abdominal pain | 8 (16) | 4 (17) | 4 (15) | 1.000 |
| Asymptomatic | 2 (4) | 0 (0) | 2 (7) | 0.492 |
| Laboratory findings, n (%) | 31 (61) | 14 (58) | 17 (63) | |
| Acute kidney injurya | 7 (23) | 3 (21) | 4 (24) | 1.000 |
| White blood cell count, median (min-max) | 7.65 (3.07–16) | 5.91 (3.07–16.00) | 8.08 (4.37–12.42) | 0.284 |
| Absolute neutrophil count, median (min-max) | 5.74 (1.87–14.00) | 4.19 (2.36–14.00) | 6.07 (1.87–10.49) | 0.508 |
| Absolute lymphocyte count, median (min-max) | 0.63 (0.28–2.34) | 0.63 (0.36–1.40) | 0.87 (0.28–2.34) | 0.292 |
| Liver function test elevationb | 8 (26) | 5 (36) | 3 (18) | 0.412 |
| Imaging obtained, n (%) | 38 (75) | 16 (67) | 22 (81) | |
| Imaging modality | 0.324 | |||
| Chest radiograph | 17 (45) | 9 (56) | 8 (36) | |
| CT chest | 21 (55) | 7 (44) | 14 (64) | |
| Imaging findings | ||||
| COVID-19 pneumonia imaging classificationc | 0.001 | |||
| Negative | 17 (45) | 4 (25) | 13 (60) | |
| Indeterminate | 8 (21) | 2 (12) | 6 (27) | |
| Atypical | 3 (8) | 1 (6) | 2 (9) | |
| Typical | 10 (26) | 9 (56) | 1 (5) | |
| Lung cancer-related finding | 26 (68) | 10 (63) | 16 (73) | 0.725 |
| Treatment-related changesd | 25 (66) | 9 (56) | 16 (73) | 0.323 |
| New pulmonary embolism | 1 (3) | 0 | 1 (5) | 0.421 |
| Supportive oxygen therapy, n (%) | ||||
| Baseline O2 required by nasal cannula | 4 (8) | 2 (8) | 2 (7) | 1.000 |
| Max O2 required above baselinee | 0.005 | |||
| No supplemental O2 required | 35 (69) | 11 (46) | 24 (89) | |
| Nasal cannula, 1–2 liter | 5 (10) | 3 (13) | 2 (7) | |
| Nasal cannula, 3–6 liter | 6 (12) | 5 (21) | 1 (4) | |
| Nonrebreather | 3 (6) | 3 (13) | 0 | |
| Bilevel positive airway pressure | 1 (2) | 1 (4) | 0 | |
| Mechanical ventilation | 1 (2) | 1 (4) | 0 |
ALT, alanine transaminase; AST, aspartate transaminase; COVID-19, coronavirus disease 2019; Cr, creatinine; CT, computed tomography; TB, tuberculosis.
Acute kidney injury defined by Kidney Disease | Improving Global Outcomes 2012 Guidelines17 (Cr increased ≥0.3 mg/dL from baseline or ≥1.5 times baseline).
Liver function test elevation defined by AST, ALT, and TB greater than the upper limit of normal at presentation.
According to the Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19.13
Changes related to previous surgery, radiation, pleurodesis, or PleurX catheter
No patients received high-flow nasal cannula as their maximal level of supplemental oxygen therapy.
Among the patients tested for COVID-19, a total of 24 (47%) were positive by SARS-CoV-2 RT-PCR. Across the longitudinal and active therapy populations, this represents an estimated cumulative incidence of 3.4% and 1.5%, respectively. A total of 18 (75%) were diagnosed with having COVID-19 by their initial RT-PCR test, whereas six (25%) initially tested negative and were diagnosed in subsequent testing (median tests for entire cohort, 1; range, 1–4 tests). In those who were initially tested negative, the median time from first negative RT-PCR to positive finding was 10 days (range, 1–63 d). In two of the six cases, a clear alternative diagnosis was made at the time of initial COVID-19 negative test (liver abscess and postobstructive pneumonia), which was performed more than 30 days before eventual COVID-19 positivity in both cases. By contrast, among the other four patients who were negative for COVID-19 on initial testing, subsequent testing was performed and found to be positive within 1 to 11 days. For these six cases, symptoms and additional workup at the time of presentation are presented in Supplementary Table 1.
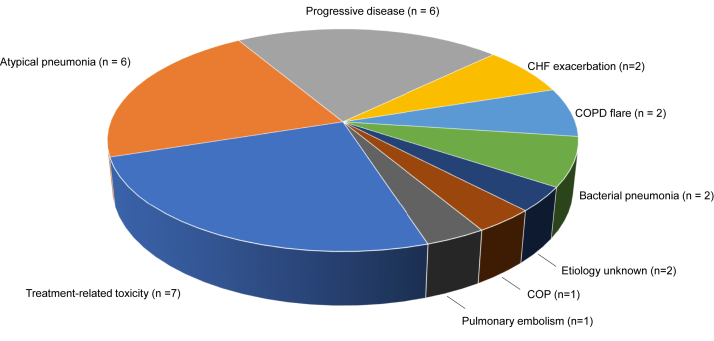
Of the 51 patients tested for COVID-19, a total of 27 (53%) were negative by RT-PCR testing. In this COVID-negative cohort, 15 patients (56%) had a single COVID-19 test, seven (26%) had two tests, and five (18%) underwent testing three times, all of which were negative. Additional workup for the group negative for COVID-19 included CT or plain film imaging of the chest (81%) and laboratory evaluation (63%). After medical evaluation, 15 patients (56%) were found to have a definite alternate cause for their symptoms and 10 (37%) had a likely alternative cause (Fig. 2). The most common alternative diagnoses included treatment-related toxicity (n = 7), atypical pneumonia (n = 6), and disease progression (n = 6).
Figure 2.
Alternative diagnoses in patients negative for COVID-19 infection. Of the 27 patients negative for COVID-19, alternative diagnoses were identified in 25. In two cases, the clinical and radiographic symptoms were attributed to the following two possible causes: case 1: grade I immune checkpoint inhibition-induced pneumonitis and an atypical pneumonia; case 2: grade II immune checkpoint inhibition-induced pneumonitis and an atypical pneumonia. COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; COP, cryptogenic organizing pneumonia; COVID-19, coronavirus disease 2019.
Among the six patients diagnosed with having an atypical pneumonia, two underwent more than one COVID-19 test, two underwent additional viral respiratory testing for common pathogens (influenza and respiratory syncytial virus), and two were empirically treated with azithromycin for potential bronchitis. Notably, the limited testing for respiratory viruses other than SARS-CoV-2 was the result of institutional restrictions placed on the use of swabs or reagents to ensure adequate SARS-CoV-2 testing materials. Among the entire cohort negative for COVID-19, two cases remained without an identifiable diagnosis despite evaluation. In the first, a patient with stage IV NSCLC and a history of deep venous thrombosis on therapeutic anticoagulation presented with low-grade fevers. Evaluation included SARS-COV-2 RT-PCR (negative on two tests), blood and urine cultures (negative), and a CT chest (no evidence of progressive disease or acute abnormalities). The symptoms later resolved after 29 days without intervention. In the second case, testing was prompted by new, multifocal bilateral ground-glass opacities on routine imaging in a patient with extensive-stage SCLC on active surveillance. As the patient was asymptomatic, no further workup was performed after COVID-19 testing returned negative.
Symptoms at the time of presentation between patients positive for versus negative for COVID-19 are summarized in Table 2. With the exception of a greater need for supplemental oxygen initiation among those with COVID-19 infection (54% versus 11%, p = 0.005), there were no significant differences in presenting symptoms or laboratory abnormalities between patients positive for and negative for COVID-19.
Radiographic Imaging Findings Among Patients Referred for COVID-19 Testing
Radiographic imaging was obtained in 38 patients (75%), with 45% evaluated by plain film and 55% by CT imaging. Among patients with RT-PCR–confirmed COVID-19 infection (Table 2), imaging findings were “typical” for COVID-19 infection13 in nine (56%), “negative” in four (25%), “indeterminate” in two (12%), and “atypical” in one (6%). RT-PCR–confirmed COVID-19 cases were significantly more likely than RT-PCR–negative cases to have findings typical for COVID-19 pneumonia on the basis of imaging classification (56% versus 5%; p = 0.001).
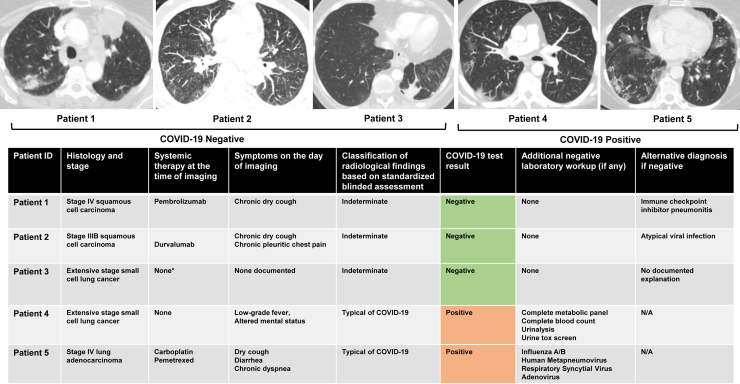
Five patients were referred for COVID-19 testing based primarily on radiographic findings on surveillance imaging, and two were found to be positive for COVID-19. Of these positive cases, retrospective chart review revealed that one patient had low-grade fever and altered mental status at the time of surveillance imaging and one reported chronic dyspnea and new dry cough and diarrhea. In the three patients who tested negative, alternative etiologies for radiographic findings were treatment-related pneumonitis, atypical pneumonia, and cause unknown (Fig. 3).
Figure 3.
Clinical summary of patients referred for COVID-19 testing on the basis of results of routine imaging studies. Five patients with advanced lung cancer were referred for COVID-19 testing on the basis of routine restaging imaging studies. Top panel: representative CT chest images for each patient. Bottom panel: table summarizing cancer histopathology, systemic anticancer therapy, symptoms on day of imaging, imaging findings, COVID-19 RT-PCR results, additional workup, and alternative diagnoses. ∗At the time of imaging, patient was on active treatment with ATRA and arsenic trioxide for concurrent diagnosis of APML. No active lung cancer-directed systemic therapy. COVID-19, coronavirus disease 2019; CT, computed tomography; N/A, not applicable; RT-PCR, real-time polymerase chain reaction.
Clinical Outcomes Among COVID-19 Cohort
Patients positive for COVID-19 (n = 24) were predominantly current or former smokers (87%), white (84%), and older (median age 75 y, range, 57–87) (Table 3). At the time of last medical oncology assessment before COVID-19 infection, 16 patients (67%) had a documented ECOG PS of 0 to 2. A total of 11 patients (46%) had metastatic disease, and six (25%) were receiving active treatment at the time of COVID-19 infection. One-half (three of six) of the patients on active therapy were receiving a tyrosine kinase inhibitor. A total of 15 patients (63%) had received previous thoracic RT, of whom four patients (17%) received thoracic RT within 3 months of infection.
Table 3.
Demographics of Thoracic Oncology Patients Positive for COVID-19
| Patient characteristics | COVID-19 Positive, n = 24 |
|---|---|
| Age, median y | 75 (57 -87) |
| Sex, n (%) | |
| Female | 11 (46) |
| Male | 13 (54) |
| Smoking status, n (%) | |
| Never | 3 (13) |
| Former | 19 (79) |
| Current | 2 (8) |
| ECOG PS, n (%) | |
| 0 - 2 | 16 (67) |
| 3 - 4 | 8 (33) |
| Lung cancer histology, n (%) | |
| Adenocarcinoma | 12 (50) |
| Squamous cell lung cancer | 7 (29) |
| SCLC | 2 (8) |
| Other | 3 (13) |
| Stage of disease, n (%) | |
| I | 5 (21) |
| II | 3 (13) |
| III | 6 (25) |
| IV | 11 (46) |
| Ever treated with systemic therapy | 23 (96) |
| Days since last systemic treatment, median (IQR) | 190 (407) |
| Treatment within 30 d | 6 (25) |
| Most recent systemic therapy, n (%) | |
| Chemotherapy | 5 (21) |
| Immune checkpoint inhibitor monotherapy | 5 (21) |
| Tyrosine kinase inhibitor | 4 (17) |
| Radiation therapy, n (%) | |
| History of thoracic radiation | 15 (63) |
| Median time since thoracic radiation, d (IQR) | 396 (597) |
| Radiation within 3 mo | 4 (17) |
| Comorbid conditions, n (%) | |
| Chronic obstructive pulmonary disease | 6 (25) |
| Hypertension | 17 (71) |
| Diabetes | 6 (25) |
COVID-19, coronavirus disease 2019; ECOG Eastern Cooperative Oncology Group; IQR, interquartile range; PS, performance status.
A total of 16 patients (67%) required hospital admission, and two patients (8%) received ICU-level care (Table 4). During COVID-19 course, supplemental oxygen was required in 54% of the cases (Table 2) with maximal level of support being nasal cannula (34%), nonrebreather (13%), bilevel positive airway pressure (4%), or mechanical ventilation (4%). No patient received high-flow nasal cannula as their maximal level of supplemental oxygen therapy. Furthermore, more than half of the patients (58%) received antibiotic therapy (Supplementary Table 2). One patient received remdesivir and tocilizumab. Hydroxychloroquine and corticosteroids were used in 21% and 4%, respectively. No patient received renal replacement therapy or extracorporeal membrane oxygenation.
Table 4.
COVID-19 Hospitalization and Related Outcomes
| COVID-19 related outcomes | COVID-19 positive, n = 24 (%) |
|---|---|
| Level of care required, n % | |
| Required hospital admission | 16 (67) |
| Required ICU-level care | 2 (8) |
| Required intubation | 1 (4) |
| Discharged at time of data extractiona | 8/16 (50) |
| Median length of hospitalization, days (IQR) | 7 (2) |
| COVID-19 outcomes, n % | |
| Fully recovered | 12 (50) |
| Recovered with residual complications | 5 (20) |
| Died from COVID-19 (mortality rate) | 7 (29) |
COVID-19, coronavirus disease 2019; ICU, intensive care unit; IQR, interquartile range.
One patient remained in hospital at the time of data extraction.
At the time of hospital admission, six of 16 patients (38%) had a documented code status of do not resuscitate (DNR) or do not intubate (DNI). Of the 10 patients (62%) who were “full code” on admission, three transitioned to DNR or DNI and four to comfort measures only (CMO). Both patients who received ICU-level care were “full code” on admission, with one patient transitioning to CMO.
Eight patients positive for COVID-19 (33%) died during the study period with seven deaths (29%) attributed to COVID-19 complications, including acute respiratory distress syndrome (n = 5) (Table 4). One patient positive for COVID-19 died from disease progression in the central nervous system. Of the seven patients who died from COVID-19 infection, the median age was 75 years (range, 63–87 y). Six patients (85%) had a documented ECOG PS of 3 at last medical oncology assessment before COVID-19 infection, and 85% were active or former smokers. Three of seven patients had documented progression of disease on the most recent oncologic staging assessment. Hospitalization rates and mortality were slightly higher among the patients positive for COVID-19 who were on active therapy within the preceding 30 days, but this was not statistically significant (Supplementary Table 3). All seven patients who died from COVID-19 (or their appointed health care proxies) elected de-escalation of care during hospitalization, transitioning code status to DNR or DNI or CMO.
Discussion
The COVID-19 pandemic has introduced marked new challenges in the care of patients with thoracic malignancies. Clinicians are now faced with recognizing and treating a new disease entity, counseling patients on risk reduction strategies, and making decisions on whether to modify oncologic care in the middle of a global pandemic—all despite limited data on the effects of COVID-19 infection in patients with lung cancer. Beyond the apparent increased morbidity of COVID-19 infection within a lung cancer population,7,8 the marked overlap of COVID-19–related syndromes with the symptoms of lung cancer progression or toxicities from lung cancer therapies makes a COVID-19 diagnosis exceptionally challenging.
In this study, we retrospectively estimated the incidence of COVID-19 among a thoracic oncology population within a tertiary academic medical center in one of the early epicenters in the United States. We found that roughly 3.4% of the patients with lung cancer overall and 1.5% receiving active therapy tested positive for COVID-19 within the first 3 months of the pandemic. Our cumulative incidence of COVID-19 infection is comparable with other larger series that have reported a prevalence of 1% to 3% in patients with active or previous malignancy.2,14 Notably, the 1.5% incidence in our active therapy population is similar to the 1.46% prevalence rate in the state of Massachusetts as of June 1, 2020.
At our center, on the basis of earlier reports of increased COVID-19 complications among oncology populations in People’s Republic of China2,3 and Italy,15 we proactively pursued strategies to minimize COVID-19 infection risk, including restrictions on all visitors, implementation of a universal face mask policy for providers and patients alike, transition of all nonessential visits to a virtual format, and, in some cases, modifications in systemic therapy. Reassuringly, in our active therapy population, 86% of the patients were able to continue active therapy. Furthermore, for those who experienced a delay in systemic therapy, most had successfully resumed treatment by the time of data cutoff. As the pandemic continues, guidelines on optimal methods to safely delivery cancer treatment will be crucial.16 Future studies are also needed to understand the impact of these modifications on cancer-specific outcomes.
In contrast to other recent COVID-19 registry series, our data set was uniquely poised to highlight the diagnostic challenges faced by thoracic oncologists because we collected data on all patients referred for COVID-19 testing. Overall, we found that 44% of those referred for COVID-19 testing were positive for COVID-19. Importantly, 25% of COVID-19 RT-PCR–positive cases required two or more tests to confirm infection, underscoring the continued diagnostic constraints of RT-PCR testing. Furthermore, our study highlights the need for thoracic oncologists to maintain a broad differential diagnosis for patients presenting with acute symptoms, as nearly 50% of the patients referred for COVID-19 testing in our cohort were found to have an alternative cause. These alternative diagnoses included treatment-related complications, progressive disease, atypical pneumonia, pulmonary embolism, congestive heart failure, and chronic obstructive pulmonary disease flare. The presenting signs and symptoms of COVID-19 infection among patients with lung cancer were difficult to distinguish from these other etiologies.
Although clinical symptoms were largely poor predictors of COVID-19 status in this series, radiographic imaging of the chest provided diagnostic clarity in a subset of cases. Radiographic changes consistent with lung cancer and previous treatment (radiation and surgery) were common in both positive and negative cohorts of COVID-19, but acute imaging findings “typical” for COVID-19 in the COVID-19 pneumonia classification system were marked more common in patients with RT-PCR–confirmed COVID-19 compared with patients with negative results (56% versus 5%, respectively; p = 0.001). These findings underscore the diagnostic challenges faced by thoracic oncologists and suggest that clinicians should have a low threshold for COVID-19 testing and radiographic imaging in patients presenting with new respiratory symptoms.
Similar to previously reported data,3,6,8 we found that more than half of our patients with lung cancer infected with COVID-19 required hospitalization and nearly one-third died. Patients who died were often older than 70, had ECOG PS 3, and had disease progression on recent scans. Findings from larger cohorts, such as the CCC19 consortium, have also indicated age and poor PS as risk factors for mortality or poor outcomes in oncology patients positive for COVID-19.5 Of note, no specific systemic therapies have to date been linked with increased risk of severe COVID-19 infection in NSCLC.8,9 In our study, hospitalization and mortality rates were slightly higher among patients on active therapy within 30 days before COVID-19 positivity, but such comparisons are limited by a small sample size (Supplementary Table 3).
In our hospitalized cohort, 62% of the patients had a documented code status of “full code” on admission. All seven patients who died from COVID-19 (or their appointed health care proxies) elected to de-escalate care during hospitalization, transitioning code status to CMO or DNR or DNI. Whether ICU-level care would have improved clinical outcomes in our cohort remains unknown, but the incidence of elective de-escalation of care indicates that metrics of ICU utilization and rates of mechanical ventilation alone may underestimate the severity of COVID-19 infection in an oncology patient population. These findings emphasize the ongoing importance of critical illness conversations in all patients with advanced malignancies.
Our study has several limitations. First, this was a single institution, retrospective study with a small sample size and limited duration of follow-up. Second, we may have underestimated the true incidence of COVID-19 in our study population owing to false-negative RT-PCR testing, an inability to capture patients who were diagnosed outside of our hospital system, and excluding patients who underwent testing for COVID-19 as a preprocedural protocol. It should be noted, however, that our study period was relatively early in the pandemic, and preprocedural asymptomatic testing had not been implemented at the start of our study period. To minimize the number of patients who may have been diagnosed at other institutions, we restricted our study population to those receiving longitudinal care at MGH and manually reviewed all external hospital records available to us through our EMR system. In defining the “at-risk” population receiving care at our institution, we did not include patients with active disease who had not received any lung cancer therapy within the preceding year (e.g., patients receiving best supportive care). The impact of COVID-19 on this population remains an important area for future investigation. Another limitation of this analysis is that 22% of the patients in the group negative for COVID-19 were clinically diagnosed as having atypical pneumonia, but we were unable to perform comprehensive viral testing to find a definitive organism owing to restrictions on viral respiratory testing (except for COVID-19) at our institution to conserve resources during the surge. Finally, our study was conducted before the approval of the Food and Drug Administration to remdesivir and corticosteroids as therapy for COVID-19. Although the efficacy of these therapies has not yet been proven in patients with lung cancer, it is possible that clinical outcomes would have been improved by guideline-directed administration in hospitalized patients.
In summary, COVID-19 infections were identified in a relatively small proportion of patients with lung cancer during the initial wave of the pandemic in our institution, but these patients experienced high rates of morbidity and mortality. There is a critical need for ongoing investigation into optimal patient triage strategies, indications for testing, test modalities, and ways to mitigate exposure of patients while still ensuring the safe administration of cancer-directed therapies during the COVID-19 pandemic.
Acknowledgments
This research was supported by a Stand Up To Cancer (SU2C)—American Cancer Society Lung Cancer Dream Team Translational Research Grant (grant number: SU2C-AACR-DT17-15). The SU2C is a program of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, a scientific partner of SU2C. This research was also supported by the Mark Foundation and an ALK Positive or LUNGevity award.
Footnotes
Drs. Piper-Vallillo and Mooradian contributed equally to this work.
Disclosure: Dr. Mooradian has served as a compensated consultant or received honoraria from AstraZeneca, Immunia, and Nektar Pharmaceuticals. Dr. Zubiri has served as a compensated consultant for Merck. Dr. Piotrowska has received consulting honoraria from AstraZeneca, Eli Lilly, InCyte, Medtronic, C4 Therapeutics, and Blueprint Medicines and receives institutional research support from Novartis, Takeda, Spectrum, AstraZeneca, Tesaro, and Cullinan Oncology. Dr. Lin has served as a compensated consultant or received honoraria from Chugai Pharma, Boehringer Ingelheim, Pfizer, C4 Therapeutics, Nuvalent, Turning Point Therapeutics, and Genentech; received institutional research funds from Hengrui Therapeutics, Turning Point Therapeutics, Neon Therapeutics, Relay Therapeutics, and Novartis; received CME funding from OncLive, MedStar Health, and Northwell Health; and received travel support from Pfizer. Dr. Dagogo-Jack has served as a compensated consultant or received honoraria from Boehringer Ingelheim, AstraZeneca, Foundation Medicine, and American Lung Association; travel support from Pfizer and Array; and research support from Array, Pfizer, Genentech, and Guardant Health. Dr. Farago has served as a compensated consultant or received honoraria from Bayer, Loxo Oncology, Genentech, Roche, Bristol-Myers Squibb, AstraZeneca, AbbVie, PharmaMar, Boehringer Ingelheim, Merck, H3 Biomedicine, Pfizer, and Syros; institutional research support from Bayer, Loxo Oncology Inc., Genentech, Roche, Bristol-Myers Squibb, AstraZeneca, AbbVie, PharmaMar, Merck, Ignyta, Amgen, and Novartis. Dr. Sequist has served as a compensated consultant or received honoraria from AstraZeneca, Janssen, and Genentech; institutional research support from Norvartis, AstraZeneca, Boehringer Ingelheim, Genentech, Blueprint, and Loxo Oncology. Dr. Heist has received institutional research support from Novartis, Abbvie, Agios, Daichii Sankyo, Corvus, GenentechRoche, Mirati, Exelixis, Debiopharm, Incyte, Takeda, and Eli Lilly and has received honoraria or served as a compensated consultant for Gainor has served as a compensated consultant or received honoraria from Bristol-Myers Squibb, Genentech, Ariad/Takeda, Loxo/Eli Lilly, Blueprint, Oncorus, Regeneron, Gilead, AstraZeneca, Pfizer, Incyte, Novartis, Merck, Agios, Amgen, and Array; research support from Novartis, Genentech/Roche, and Ariad / Takeda; institutional research support from Bristol-Myers Squibb, Tesaro, Moderna, Blueprint, Jounce, Array BioPharma, Merck, Adaptimmune, Novartis, and Alexo; and has an immediate family member who is an employee of Ironwood Pharmaceuticals. The remaining authors declare no conflict of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2020.100124.
Supplementary Data
References
- 1.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6:1108–1110. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desai A., Sachdeva S., Parekh T., Desai R. COVID-19 and cancer: lessons from a pooled meta-analysis. JCO Glob Oncol. 2020:557–559. doi: 10.1200/GO.20.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuderer N.M., Choueiri T.K., Shah D.P. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta V., Goel S., Kabarriti R. Case fatality rate of cancer patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020;10:935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai M., Liu D., Liu M. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garassino M.C., Whisenant J.G., Huang L.-C. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo J., Rizvi H., Egger J.V., Preeshagul I.R., Wolchok J.D., Hellmann M.D. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020;10:1121–1128. doi: 10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo J., Rizvi H., Preeshagul I.R. COVID-19 in patients with lung cancer. Ann Oncol. 2020;31:1386–1396. doi: 10.1016/j.annonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massachusetts Department of Public Health COVID Dashboard. https://www.mass.gov/doc/covid-19-dashboard-may-15-2020/download
- 12.Massachusetts General Hospital SARS-CoV-2 test EUA summary. https://www.fda.gov/media/136699/download
- 13.Simpson S., Kay F.U., Abbara S. Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Rad Cardiothorac Imaging. 2020;2 doi: 10.1148/ryct.2020200152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L., Zhu F., Xie L. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trapani D., Marra A., Curigliano G. The experience on coronavirus disease 2019 and cancer from an oncology hub institution in Milan, Lombardy Region. Eur J Cancer. 2020;132:199–206. doi: 10.1016/j.ejca.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institute for Health and Care Excellence COVID-19 rapid guideline: delivery of Systemic Anticancer Treatments. https://www.nice.org.uk/guidance/ng161 [PubMed]
- 17.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.