Abstract
Background and aims:
Obesity is a pro-inflammatory risk factor for progression of CKD and cardiovascular disease. We hypothesized that implementation of caloric restriction and endurance exercise would improve adipocytokine profiles in patients with moderate to severe CKD.
Methods and Results:
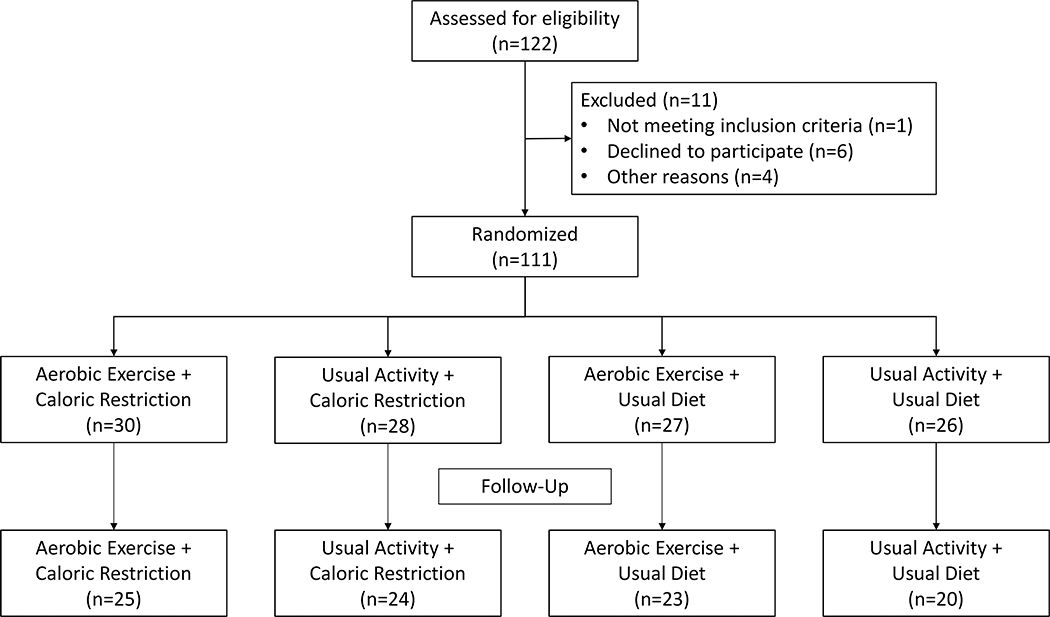
We enrolled patients with moderate to severe CKD through a multi-center pilot randomized trial of diet and exercise in a 4-arm design (dietary restriction of 10%−15% reduction in caloric intake, exercise three times/week, combined diet and exercise, and control) (NCT01150851). Adipocytokines (adiponectin and leptin) were measured at the beginning and end of the study period as secondary outcomes. Treatment effect was analyzed in a multivariable model adjusted for baseline outcome values, age, gender, site and diabetes. A total of 122 participants were consented, 111 were randomized (42% female, 25% diabetic, and 91% hypertensive), 104 started intervention and 92 completed the study (Figure 1). Plasma adiponectin levels increased significantly in response to diet by 23% (95% CI: 0.2%, 49.8%, p=0.048) among participants randomized to the caloric restriction and usual activity arm but not to exercise, whereas circulating leptin did not change by either treatment.
Conclusion:
Our data suggest that dietary caloric restriction increases plasma adiponectin levels in stage 3–4 CKD patients, with limited effect on leptin levels. These findings suggest the potential for improving the metabolic milieu of CKD with moderate calorie restriction.
Keywords: chronic kidney disease, adiponectin, leptin, exercise, diet
Introduction
Obesity is an established risk factor for development and progression of chronic kidney disease (CKD).1–3 Adipose tissue is an active endocrine organ that releases a number of bioactive proteins termed adipokines, which play key roles in modulating insulin resistance, inflammation, and endothelial dysfunction.4 Some adipokines such as leptin, resistin, tumor necrosis factor alpha (TNF-α), plasminogen activator inhibitor (PAI)-1, have been implicated in the pathogenesis of diabetes and obesity-related kidney diseases and CKD progression.3,5,6 To the contrary, other adipokines such as adiponectin may exert anti-inflammatory and kidney protective effects.7
CKD patients experience dysregulated adipokine metabolism, due to impaired renal clearance and the effects of co-existing systemic inflammation or protein-energy wasting.8 Previous studies support a direct role of dietary restriction and exercise training in improving systemic dysmetabolism and endothelial dysfunction among obese individuals and those with type II diabetes.9,10 On the other hand, a number of studies have failed to show improvements in markers of inflammation and endothelial dysfunction following rigorous aerobic exercise among sedentary individuals with type II diabetes and overweight children.11–13 Whether caloric restriction and/or exercise can benefit the adipokinome among patients with pre-existing oxidative and inflammatory burden, such as CKD, is not studied in detail.
We recently reported the results of a pilot randomized clinical trial examining the efficacy, feasibility and safety of supervised endurance exercise and dietary calorie restriction in patients with Stage 3–4 CKD (NCT01150851).14 In this post-hoc analysis, we aimed to investigate the effects of endurance exercise and dietary calorie restriction individually or in combination on adipocytokine levels among individuals with Stage 3–4 CKD.
Methods
Study Design
We performed a post-hoc analysis of a pilot randomized 2×2 factorial design trial of dietary caloric restriction and endurance exercise among individuals with stage 3–4 CKD (NCT01150851).14 Participants were randomized to one of 4 interventions, for a duration of 4 months: dietary restriction: 10–15% daily caloric restriction, supervised low-impact endurance exercise regimen: three times/week, combined dietary restriction and supervised exercise regimen, or control (usual exercise and diet). Details of the exercise intervention have been previously described.14 In brief, participants randomized to the low-impact endurance exercise intervention were scheduled to perform supervised physical activity for a maximum of 30–45 minutes three times per week for 4 months in duration. Participants alternated aerobic exercise with the use of a treadmill, an elliptical cross-trainer, a Nu-Step cross-trainer, and a recumbent stationary bicycle. The exercise prescription was customized to reflect each subject’s individual baseline fitness status, with the goal for all participants being to achieve an exercise intensity that represented 60%–80% VO2 peak. Participants randomized to the dietary intervention were counseled to reduce their daily caloric intake by 10–15%. Dietary recalls before the initiation of the dietary regimen were used to assess the number of kilocalories to reduce by and to determine how to achieve such reductions in energy.
Eligible trial participants were 18–75 years of age, with an estimated glomerular filtration rate (eGFR) 15–60 mL/min/1.73m2, body mass index (BMI) ≥ 25 kg/m2, life expectancy ≥ one year, and the ability to understand and provide informed consent. Exclusion criteria included any acute inflammatory condition, pregnancy, high-dose anti-oxidant use, chronic use of anti-inflammatory medication, significant cardiac or vascular disease, significant occlusive atherosclerotic disease or ischemic disease, significant physical immobility or disabilities, type 1 diabetes mellitus, or type 2 diabetes mellitus requiring insulin therapy, and history of poor adherence to medical regimen8.
The study was approved by the Institutional Review Boards at participating sites (Vanderbilt University Medical Center (VUMC), the Veterans Affairs Tennessee Valley Healthcare System Nashville (VATVHS), University of Washington (UW), Providence Health Care Providence Medical Research Center (PMRC), and Springfield College (SC)). The study began in October 2010 and was completed in February 2014. The safety profile of the initial study was overseen by a Data Safety Monitoring Board.
A total of 122 participants were consented, 111 were randomized, 104 started intervention and 92 completed the original study (completed all baseline and 1, 2, and 4-month visits).
Data collection and laboratory measurements
Demographic, anthropometric, lifestyle, medication, physical examination and laboratory data were collected at baseline (2 weeks prior to initiation of intervention phase). Follow-up visits were conducted at 2 months and 4 months. eGFR was calculated by the CKD-EPI 2012 cystatin C-based equation9. Bioelectrical impedance analysis (Quantum III machine; RJL Systems, Clinton Township, MI) for assessment of percentage body fat and dual energy x-ray absorptiometry (Lunar Prodigy iDEXA machine, v.11.40.004, software versions 2003–2011; General Electric, Madison, WI) for body composition measurements were performed at all study visits. VO2 peak (MedGraphics Ultima; Medical Graphics Corp., St. Paul, MN) was measured at baseline and 4 months to monitor changes in cardiorespiratory fitness. All participants were issued an ActiGraph GT3X accelerometer (Actigraph, Fort Walton Beach, Florida), which was worn for 1 week after the initial baseline visit and for 1 week either before or after both the month 2 and month 4 study visits.10,11 The triaxial accelerometer estimates the duration and intensity of physical activity by capturing the magnitude of acceleration (intensity) in three dimensions and then summing the magnitudes as counts per minute (higher counts per minute indicate more physical activity)10.
Nutritional, metabolic, oxidative stress, and inflammatory biomarkers were measured from blood and urine samples. All adipokine measures were collected at month 1, month 2 and month 4. Adiponectin and leptin were measured using Bio-Rad Luminex flow cytometry (Millepore, Billerica, MA). Analytical coefficients of variation across several control samples for these analytes ranged from 6.55% to 13.0%.
Statistical analyses
Primary analyses were performed on an intent-to-treat basis, including all randomized participants, regardless of adherence or compliance status. Baseline descriptive statistics on demographics, medical history, and clinical, laboratory, and lifestyle characteristics were tabulated according to intervention arm.
We examined the effect of each intervention on 4-month change in circulating adiponectin and leptin. For all analyses, leptin and adiponectin were log-transformed. We used generalized estimating equations, accounting for within-participant clustering across time, to determine whether the relative change in adiponectin or leptin differed according to the intervention arms (over the usual diet, usual activity arm). The model included a fixed effect term for each active intervention arm, site, diabetes status, age, race and gender, and an indicator variable for month, indicator variables for each active intervention crossed with the month 4 indicator variable, and subject-specific random intercepts. We constructed 95% confidence intervals of the estimated effects with standard, linear mixed-model methods. Because the parent trial reported a significant effect of caloric restriction on change in body fat percentage15, we secondarily additionally adjusted the GEE models for changes in body fat (by DEXA), to assess whether accounting for weight loss would attenuate the associations of the interventions on adipokines.
We additionally examined effects of each intervention separately, by comparing outcomes among 1) participants randomized to caloric restriction (with or without endurance exercise) to those randomized to endurance exercise alone or usual diet and exercise and 2) participants randomized to endurance exercise (with or without caloric restriction) to those randomized to caloric restriction alone or usual diet and exercise.
To examine the impact of adherence, a post-hoc subgroup analysis excluded participants if they were randomized to the endurance exercise intervention but did not attend a minimum of 75% of their supervised sessions, of if they were randomized to the caloric restriction intervention but did not achieve their calorie goal at least two of three follow-up time points. The same model fitting and hypothesis testing procedures implemented for the intent-to-treat population were implemented among the compliant sub-population.
Statistical analyses were conducted with Stata v16.0 and R version 3.3.0 (R Foundation for Statistical Computing). The nominal level of significance was defined as P<0.05 (2-sided).
Results
A total of 111 participants were randomized into the 4 intervention arms of the study, with a mean age of 55 ± 11 years. Of these participants, 47 were female (42%), 28 had diabetes (25%), and 100 (91%) were hypertensive (Table 1). Mean baseline eGFR was 41±18.6 mL/min/1.73m2. Of the 111 participants who were randomized, 104 started intervention and 92 completed the study.
Table 1.
Baseline Characteristics Among Randomized Study Participants
| Baseline Characteristics | Endurance Exercise/Caloric Restriction | Usual Activity/Caloric Restriction | Endurance Exercise/Usual Diet | Usual Activity/Usual Diet |
|---|---|---|---|---|
| N | 30 | 28 | 27 | 26 |
| Age, years | 52.9 (8.6) | 55.6 (12.6) | 54.5 (11.7) | 57.3 (9.7) |
| Race | ||||
| White | 22(73.3) | 19(67.9) | 17(63) | 16(61.5) |
| Black | 7(23.3) | 9(32.1) | 7(25.9) | 8(30.8) |
| Other | 1(3.3) | 0(0) | 3(11.1) | 2(7.7) |
| Male Gender | 17(56.7) | 15(53.6) | 15(55.6) | 17(65.4) |
| BMI, kg/m2 | 34 (6.7) | 33.5 (7.3) | 32.5 (5.8) | 38.8 (11.9) |
| Waist circumference, in | 43.9 (5.9) | 43.3 (6) | 42 (5.1) | 46.5 (7.6) |
| Hip circumference, in | 46.3 (6) | 45.9 (5.3) | 45.1 (4.5) | 49 (7.9) |
| Systolic BP, mmHg | 124.7 (17.7) | 132 (22.8) | 129.9 (18.6) | 131.3 (16.9) |
| Weekday energy intake, kcal | 1769 (691) | 1619 (549) | 1669 (577) | 1738 (657) |
| Weekend energy intake, kcal | 1787 (649) | 1561 (466) | 1765 (583) | 1859 (763) |
| DEXA body fat, % | 40.6 (7.4) | 40.4 (8.5) | 40.2 (7.6) | 40 (8.1) |
| BIA body fat % | 35.6 (10.5) | 35 (10.7) | 34.3 (8.7) | 36 (10.2) |
| Physical Activity, counts per minute | 868 (408, 1765) | 616 (290, 1271) | 850 (529, 3065) | 888 (349, 1723) |
| Percent time spent | ||||
| in sedentary time | 72.2 (16.1) | 69.5 (14) | 70 (13.8) | 77 (13.8) |
| in light activity | 22 (11.8) | 25.5 (10.4) | 24.3 (11.8) | 19.1 (12) |
| in moderate activity | 3 (2.4) | 2.9 (4.1) | 3.7 (4.6) | 3 (3.8) |
| in vigorous activity | .1 (.3) | .2 (.7) | .2 (.8) | 0 (.1) |
| VO2 Max, ml/kg/min | 20.4 (5.4) | 19.2 (4.3) | 20.5 (5.1) | 19 (4.4) |
| Tobacco Use | ||||
| Never | 18(60) | 11(39.3) | 7(25.9) | 14(56) |
| Current | 3(10) | 2(7.1) | 3(11.1) | 2(8) |
| Former | 9(30) | 15(53.6) | 17(63) | 9(36) |
| Prevalent Disease | ||||
| Heart Failure | 1(3.3) | 2(7.1) | 2(7.4) | 0(0) |
| Coronary artery disease | 2(6.7) | 2(7.1) | 2(7.4) | 0(0) |
| Diabetes mellitus | 7(23.3) | 6(21.4) | 7(25.9) | 8(30.8) |
| Hypertension | 27(90) | 26(92.9) | 25(92.6) | 22(88) |
Continuous variables are presented as mean (±SD) or median (25th percentile, 75th percentile) and categorical variables as n (%).
Adipocytokine levels
There was no correlation among participants between adiponectin and leptin levels (ρ=0.015, p=0.74). Baseline adiponectin concentrations were similar across intervention arms. (Table S1) Participants in the endurance exercise plus usual diet group had slightly lower baseline leptin concentrations.
In intent-to-treat analyses, there was a significant effect of caloric restriction on the median plasma levels of adiponectin or leptin (Table 2). However, changes in plasma adiponectin levels were significantly higher by 23% (95% CI: 0.2%, 49.8%, p=0.048) among participants randomized to the caloric restriction and usual activity arm, and by 15.7% (95% CI 0.3%, 33.5%, p=0.045) among those randomized to any intervention including caloric restriction (Table 3). Additional adjustment for change in body fat percentage attenuated the results (percent change in adiponectin with caloric restriction: 13.1 % (95%CI: −2.2%, 30.7%, p=0.098), Supplemental Table S2). In analyses restricted to participants with the highest level of compliance to the interventions, the effect of caloric restriction was stronger, with plasma adiponectin levels increased by 27% (95% CI: 2.5%, 56.9%, p=0.029) among participants randomized to the caloric restriction and usual activity arm, and by 21.3% (95% CI 3.6%, 42.1%, p=0.016) among those randomized to any intervention including caloric restriction (Supplemental Table 3).
Table 2.
Circulating adipokine concentrations in each randomization group at all time points
| Endurance Exercise/Caloric Restriction | Endurance Exercise/Usual Diet | Usual Activity/Caloric Restriction | Usual Activity / Usual Diet | |
|---|---|---|---|---|
| Adiponectin, μg/ml | ||||
| Baseline | 11.0 (6.7, 15.0) | 13.5 (7.3, 22.0) | 11.0 (5.5, 20.0) | 13.0 (5.7, 21.0) |
| Month 1 | 10.5 (6.7, 14.5) | 12.0 (8.0, 17.0) | 11.0 (5.5, 21.0) | 14.0 (5.5, 21.0) |
| Month 2 | 10.4 (6.7, 14.5) | 11.5 (7.3, 17.0) | 13.0 (6.0, 19.0) | 11.0 (5.3, 20.0) |
| Month 4 | 12.0 (6.8, 14.5) | 13.0 (9.7, 15.0) | 10.5 (5.7, 20.0) | 12.0 (6.0, 17.0) |
| Leptin, ng/ml | ||||
| Baseline | 24.9 (10.1, 31.0) | 14.9 (11.0, 21.9) | 17.8 (10.3, 26.8) | 20.1 (13.5, 35.1) |
| Month 1 | 18.0 (8.0, 32.5) | 13.7 (8.5, 19.0) | 14.2 (6.5, 27.8) | 18.0 (10.9, 26.3) |
| Month 2 | 18.9 (10.2, 26.7) | 12.0 (9.8, 21.4) | 15.6 (7.4, 29.3) | 22.9 (10.2, 36.1) |
| Month 4 | 17.2 (9.5, 28.9) | 14.5 (10.3, 22.8) | 15.7 (7.4, 25.6) | 19.1 (13.6, 39.1) |
Descriptive statistics are presented as median (interquartile range).
Table 3.
Effect of interventions on percent change (95% CIs) in circulating adipokine concentrations over 4 months
| Adiponectin | Leptin | |||
|---|---|---|---|---|
| Percent change (95% CI)* | P | Percent change (95% CI)* | P | |
| Usual Activity/Diet | 0.0 (ref) | - | 0.0 (ref) | - |
| Exercise/Caloric Restriction | 17.2 (−4.1, 43.2) | 0.126 | −8.9 (−30.3, 18.9) | 0.491 |
| Usual Activity/Caloric Restriction | 22.5 (0.2, 49.8) | 0.048 | 6.2 (−18.7, 38.8) | 0.659 |
| Exercise/Usual Diet | 7.1 (−12.9, 31.7) | 0.515 | 6.1 (−19.4, 39.7) | 0.673 |
| 2-arm trial analysis | ||||
| Usual Activity | reference | - | reference | - |
| Exercise | 0.7 (−12.8, 16.2) | 0.927 | −5.2 (−21.6, 14.7) | 0.583 |
| Usual Diet | reference | - | reference | - |
| Caloric Restriction | 15.7 (0.3, 33.4) | 0.045 | −4.7 (−21.2, 15.3) | 0.624 |
Adjusted for age, gender, race, study site and baseline diabetes status.
We found no significant change in circulating leptin concentrations in any of the intervention arms (Table 3). Additionally, no significant alteration in leptin levels was observed in analyses restricted to the most compliant participants (Supplemental Table 3).
Discussion
In this secondary analysis of a pilot randomized clinical trial, a 10–15% restriction of caloric intake increased plasma adiponectin levels but did not affect leptin concentrations, among patients with stage 3–4 CKD.
Adiponectin plays a significant role in the regulation of insulin sensitivity, and low levels have been associated with type 2 diabetes, metabolic syndrome, and cardiovascular disease risk. Adiponectin has been postulated to have anti-atherosclerotic and anti-inflammatory properties,16–18 mediated by inhibition of endothelial cell activation, monocyte adhesion to endothelium, secretion of TNF alpha from macrophages, phagocytic activity of macrophages, and proliferation of vascular smooth muscle cells. Paradoxically, although hypoadiponectinemia is a risk factor for cardiovascular complications, adiponectin levels are higher in CKD patients and have been associated with CKD progression.19 This association may be confounded by the fact that adiponectin is cleared by the kidneys or that higher adiponectin is an adaptive response for kidney protection. Nevertheless, it has been postulated that hypoadiponectinemia may be a novel putative CV risk factor in patients with mild and moderate CKD.20,21 Other studies have reported that high adiponectin levels are associated with CV outcomes and all-cause mortality in moderate CKD and hemodialysis patients.22–24
In our study, we found that adiponectin levels were significantly increased among individuals randomized to the dietary intervention alone, but not to the combined diet-plus-exercise intervention. Notably, in the exercise/caloric restriction group, the diet compliance was 62.1%, whereas in the usual activity/caloric restriction group, the diet compliance was 71.4%. It is possible that individuals who exercised had more difficulty adhering to the caloric restriction intervention, and resultantly did not experience improvements in adiponectin.
It is unclear whether associations of high adiponectin and CKD represent a causal process, an adaptive response for kidney protection, or confounding by low eGFR and possibly the metabolic milieu of CKD. While no other randomized trial has tested the effects of diet and exercise on circulating adipokines in CKD, a small study in which six obese individuals with advanced diabetic nephropathy underwent a 12-week very low-calorie ketogenic weight reduction diet with encouragement of exercise reported an improvement in metabolic profile.25 Similarly, a randomized controlled study of sleeve gastrectomy versus best medical care in obese patients with stages 3–4 CKD found that the intervention increased serum adiponectin at 12 months.26 Among a small cohort of 25 kidney transplant recipients and 15 age-matched non dialysis patients with CKD stage 3 who participated in a 12-week supervised exercise program with short cell phone text reminds, serum adiponectin increased substantially in the transplant group but not among those with CKD.27 In a systematic review of randomized control trials examining effects of exercise on adiponectin levels in the general population, fewer than half demonstrated that adiponectin levels increased with exercise.28 Interestingly, a negative correlation between adiponectin levels and visceral adipose tissue storage and a positive correlation of this adipokine with subcutaneous adipose tissue storage are described in the literature. In this sense, it is possible that visceral fat loss can increase adiponectin levels in overweight subjects, while subcutaneous fat loss can result in a reduction in adiponectin levels in normal-weight subjects. Our study was not designed to examine associations of changes in adiponectin with visceral and subcutaneous adipose tissue, although this could represent a future direction of investigation.
Leptin, known as the satiety hormone, regulates appetite and energy balance as well as stimulates the sympathetic nervous system, modulates inflammation, and immune system 29 In obesity, leptin levels are paradoxically high, but because of leptin resistance in these patients, there is an inability to detect satiety although high energy reserve and increased leptin levels. In patients with CKD, leptin levels are higher with lower eGFR.30 Furthermore, it has been suggested that uremic plasma may cause excessive leptin production by adipocytes, and some mechanisms such as fat metabolism, hyperinsulinemia and inflammation may also contribute an increase in leptin levels.31 Leptin is also associated with oxidative stress in endothelial cells and the inflammatory process. It has been shown that leptin stimulates macrophage activation, produce of TNF alpha and free oxygen radicals.32 Leptin is related to the proliferation and migration of vascular smooth muscle and endothelial cells.33 These findings suggest that obesity-related hyperleptinemia may have detrimental effects on the cardiovascular system. Our study found no effect of either exercise or caloric restriction on circulating levels of leptin. This could be related to a lack of direct effect of the study interventions. It is also possible that the study duration was too short to impact leptin levels, especially since the fat distribution was not significantly changed in the study, as previously reported.
Strengths of this study include a dietary intervention designed to decrease overall calories rather than have a specific nutrient intake, potentially improving patient tolerance and adherence. The exercise intervention was supervised by clinical exercise physiologists and personalized to the specific patient’s physiologic capability. This is one of the largest randomized trials examining healthy lifestyle interventions in patients with CKD. There are also limitations to be acknowledged. This was a post-hoc analysis of a pilot study intended to test feasibility and justify a more comprehensive, and potentially pragmatic study, and not designed with the biomarker outcomes of this analysis in mind. Additionally, our study duration was relatively short, at 4 months, limiting our ability to evaluate longer-term adherence and biologic effects. Furthermore, other markers which may be correlated with adiponectin and leptin were not considered in the analyses, but may have affected the metabolic milieu in these CKD patients.
In conclusion, caloric restriction was associated with increases in adiponectin levels, but with no change in leptin. While suggesting an overall metabolic improvement, our results are hypothesis generating, and the effect of weight loss and/or dietary restriction on glomerular and tubular damage by changes in adiponectin in CKD patients warrants further study.
Supplementary Material
Figure 1.
Consort flow diagram.
Acknowledgements
This study was in part supported by National Institutes of Health grants R01HL070938 from National Heart, Lung, and Blood Institute; K24DK62849, P30DK020593, and P30DK035816 from National Institute of Diabetes and Digestive and Kidney Diseases; P30ES000267 from the National Institute of Environmental Health Sciences; and Clinical Translational Science Awards UL1-TR000445 and UL1TR000423 from the National Center for Advancing Translational Sciences.
The funding agencies had no involvement with the design, implementation, analysis, and interpretation of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Obermayr RP, Temml C, Knechtelsdorfer M, et al. Predictors of new-onset decline in kidney function in a general middle-european population. Nephrol Dial Transplant 2008;23(4):1265–1273. [DOI] [PubMed] [Google Scholar]
- 2.Wickman C, Kramer H. Obesity and kidney disease: potential mechanisms. Semin Nephrol 2013;33(1):14–22. [DOI] [PubMed] [Google Scholar]
- 3.Briffa JF, McAinch AJ, Poronnik P, Hryciw DH. Adipokines as a link between obesity and chronic kidney disease. Am J Physiol Renal Physiol 2013;305(12):F1629–1636. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112(17):2735–2752. [DOI] [PubMed] [Google Scholar]
- 5.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004;92(3):347–355. [DOI] [PubMed] [Google Scholar]
- 6.Teta D Adipokines as uremic toxins. J Ren Nutr 2012;22(1):81–85. [DOI] [PubMed] [Google Scholar]
- 7.Sweiss N, Sharma K. Adiponectin effects on the kidney. Best Pract Res Clin Endocrinol Metab 2014;28(1):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nanayakkara PW, Le Poole CY, Fouque D, et al. Plasma adiponectin concentration has an inverse and a non linear association with estimated glomerular filtration rate in patients with K/DOQI 3 – 5 chronic kidney disease. Clin Nephrol 2009;72(1):21–30. [DOI] [PubMed] [Google Scholar]
- 9.Roberts CK, Won D, Pruthi S, et al. Effect of a short-term diet and exercise intervention on oxidative stress, inflammation, MMP-9, and monocyte chemotactic activity in men with metabolic syndrome factors. J Appl Physiol (1985). 2006;100(5):1657–1665. [DOI] [PubMed] [Google Scholar]
- 10.Anderssen SA, Carroll S, Urdal P, Holme I. Combined diet and exercise intervention reverses the metabolic syndrome in middle-aged males: results from the Oslo Diet and Exercise Study. Scand J Med Sci Sports. 2007;17(6):687–695. [DOI] [PubMed] [Google Scholar]
- 11.Mori TA, Dunstan DW, Burke V, et al. Effect of dietary fish and exercise training on urinary F2-isoprostane excretion in non-insulin-dependent diabetic patients. Metabolism: clinical and experimental 1999;48(11):1402–1408. [DOI] [PubMed] [Google Scholar]
- 12.Van Craenenbroeck AH, Van Craenenbroeck EM, Van Ackeren K, et al. Effect of Moderate Aerobic Exercise Training on Endothelial Function and Arterial Stiffness in CKD Stages 3–4: A Randomized Controlled Trial. Am J Kidney Dis 2015;66(2):285–296. [DOI] [PubMed] [Google Scholar]
- 13.Kirkman DL, Ramick MG, Muth BJ, et al. Effects of aerobic exercise on vascular function in nondialysis chronic kidney disease: a randomized controlled trial. Am J Physiol Renal Physiol 2019;316(5):F898–F905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikizler TA, Robinson-Cohen C, Ellis C, et al. Metabolic Effects of Diet and Exercise in Patients with Moderate to Severe CKD: A Randomized Clinical Trial. J Am Soc Nephrol 2018;29(1):250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikizler TA, Robinson-Cohen C, Ellis C, et al. Metabolic Effects of Diet and Exercise in Patients with Moderate to Severe CKD: A Randomized Clinical Trial. Journal of the American Society of Nephrology: JASN 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bluher M, Mantzoros CS. From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century. Metabolism 2015;64(1):131–145. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi M, Shibata R, Takahashi H, et al. Association of adiponectin with carotid arteriosclerosis in predialysis chronic kidney disease. Am J Nephrol 2011;34(3):249–255. [DOI] [PubMed] [Google Scholar]
- 18.Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 2000;20(6):1595–1599. [DOI] [PubMed] [Google Scholar]
- 19.Kim HY, Bae EH, Ma SK, et al. Association of serum adiponectin level with albuminuria in chronic kidney disease patients. Clin Exp Nephrol 2016;20(3):443–449. [DOI] [PubMed] [Google Scholar]
- 20.Guebre-Egziabher F, Bernhard J, Funahashi T, Hadj-Aissa A, Fouque D. Adiponectin in chronic kidney disease is related more to metabolic disturbances than to decline in renal function. Nephrol Dial Transplant 2005;20(1):129–134. [DOI] [PubMed] [Google Scholar]
- 21.Becker B, Kronenberg F, Kielstein JT, et al. Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: the mild and moderate kidney disease study. J Am Soc Nephrol 2005;16(4):1091–1098. [DOI] [PubMed] [Google Scholar]
- 22.Rhee CM, Nguyen DV, Moradi H, et al. Association of Adiponectin With Body Composition and Mortality in Hemodialysis Patients. Am J Kidney Dis 2015;66(2):313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menon V, Li L, Wang X, et al. Adiponectin and mortality in patients with chronic kidney disease. J Am Soc Nephrol 2006;17(9):2599–2606. [DOI] [PubMed] [Google Scholar]
- 24.Machiba Y, Inaba M, Mori K, et al. Paradoxical positive association of serum adiponectin with all-cause mortality based on body composition in Japanese haemodialysis patients. Sci Rep 2018;8(1):14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman AN, Chambers M, Kamendulis LM, Temmerman J. Short-term changes after a weight reduction intervention in advanced diabetic nephropathy. Clin J Am Soc Nephrol 2013;8(11):1892–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacLaughlin HL, Hall WL, Patel AG, et al. Weight loss, adipokines, and quality of life after sleeve gastrectomy in obese patients with stages 3–4 CKD: a randomized controlled pilot study. Am J Kidney Dis 2014;64(4):660–663. [DOI] [PubMed] [Google Scholar]
- 27.Muras-Szwedziak K, Masajtis-Zagajewska A, Pawłowicz E, Nowicki M. Effects of a Structured Physical Activity Program on Serum Adipokines and Markers of Inflammation and Volume Overload in Kidney Transplant Recipients. Ann Transplant 2019;24:569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simpson KA, Singh MA. Effects of exercise on adiponectin: a systematic review. Obesity (Silver Spring). 2008;16(2):241–256. [DOI] [PubMed] [Google Scholar]
- 29.La Cava A Leptin in inflammation and autoimmunity. Cytokine 2017;98:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Briley LP, Szczech LA. Leptin and renal disease. Semin Dial 2006;19(1):54–59. [DOI] [PubMed] [Google Scholar]
- 31.Alix PM, Guebre-Egziabher F, Soulage CO. Leptin as an uremic toxin: Deleterious role of leptin in chronic kidney disease. Biochimie 2014;105:12–21. [DOI] [PubMed] [Google Scholar]
- 32.Singhal A, Farooqi IS, Cole TJ, et al. Influence of leptin on arterial distensibility: a novel link between obesity and cardiovascular disease? Circulation 2002;106(15):1919–1924. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Mamputu JC, Wiernsperger N, Renier G. Signaling pathways involved in human vascular smooth muscle cell proliferation and matrix metalloproteinase-2 expression induced by leptin: inhibitory effect of metformin. Diabetes 2005;54(7):2227–2234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.