Abstract
Cyclosporine is used to treat immune-mediated and allergic conditions and to prevent transplant rejection. To determine the prevalence of bacterial infections during cyclosporine therapy in dogs, 2 databases were searched and 14 articles reporting usable data were identified. In 828 dogs with atopic dermatitis receiving anti-allergic dosages of cyclosporine, the prevalence of bacterial infections was 11%; these occurred most often in the integument and urinary systems and not in multiple systems. In 95 dogs receiving cyclosporine at higher dosages for other conditions, the prevalence of bacterial infection was 17%, and these infections occurred most often in the gastrointestinal, urinary, and respiratory systems, often occurring at more than one body site. The prevalence of bacterial infections in atopic dogs treated with cyclosporine is low and occurs most often in the skin. When given for immunosuppression, the prevalence of bacterial infections is higher and can affect one or more body systems.
Résumé
Prévalence d’infections bactériennes durant la thérapie à la cyclosporine chez les chiens : un sujet évalué de manière critique. La cyclosporine est utilisée pour traiter des conditions allergiques et à médiation cellulaire et également pour prévenir le rejet de greffe. Afin de déterminer la prévalence d’infections bactériennes durant la thérapie à la cyclosporine chez le chien, deux bases de données furent recherchées et 14 articles rapportant des données utilisables furent identifiés. Chez 828 chiens avec une dermatite atopique recevant des dosages antiallergiques de cyclosporine, la prévalence d’infections bactériennes était de 11 %; celles-ci survenaient le plus souvent dans les systèmes tégumentaire et urinaire mais pas dans des systèmes multiples. Chez 95 chiens recevant de la cyclosporine à des dosages plus élevés pour d’autres conditions, la prévalence d’infections bactériennes était de 17 %, et ces infections survenaient le plus souvent dans les systèmes gastro-intestinal, urinaire et respiratoire, se rencontrant souvent dans plus d’un site corporel. La prévalence d’infections bactériennes chez des chiens atopiques recevant de la cyclosporine est faible et survient le plus souvent au niveau de la peau. Lorsqu’administrée pour immunosuppression, la prévalence d’infections bactériennes est plus élevée et elles peuvent affecter un ou plus d’un système du corps.
(Traduit par Dr Serge Messier)
Introduction
Cyclosporine is an immunomodulating drug that suppresses the expression of immunostimulating cytokines (e.g., interleukin-2 and interferon-gamma) in activated T-lymphocytes and other immune cells (1). This immunosuppressant has been used for decades in human and veterinary medicine for the treatment of immune-mediated and allergic conditions and to prevent renal allograft transplant rejection (1). In the dog, several adverse events have been associated with the use of cyclosporine, including vomiting, diarrhea, gingival hyperplasia, papillomatous skin lesions, and infections (1,2). The development of cyclosporine-induced bacterial infections can predispose patients to a higher risk of morbidity or mortality. A more precise knowledge of the prevalence of bacterial infections during cyclosporine treatment and of the factors influencing infection development could aid veterinarians in detecting and treating infections earlier; such knowledge would also be helpful to better inform pet owners during decision-making for treatment.
The veterinary literature was searched to identify the prevalence of bacterial infections in dogs treated with cyclosporine for any disease. Possible factors that could contribute to the development of these bacterial infections were also searched. For the evidence search and assessment, the methodology of critically appraised topics was used, as described recently (3), as were the headings proposed on www.bestbetsforvets.org/about-bets
Materials and methods
Clinical scenarios
The first scenario is a 5-year-old spayed female West Highland white terrier (WHWT) with atopic dermatitis (AD). There was a minimal improvement of clinical signs during treatment with previously administered therapies, and now therapy with cyclosporine at the approved dosage of 5.0 mg/kg body weight (BW) per day has been elected. The owner is concerned because she just browsed the Internet and found that cyclosporine is an immunosuppressant that could cause potentially severe bacterial infections. The owner asks how often dogs treated with this medication develop bacterial infections and what type of infections usually occur.
The second scenario is a 7-year-old castrated male Labrador retriever dog with immune-mediated polyarthritis (IMPA). The dog’s condition is in remission, and managing long-term with cyclosporine is being considered. If a cyclosporine dosage of 10.0 mg/kg BW per day is administered, would the risk of bacterial infections in this pet differ from that in the WHWT, given the higher cyclosporine dosage.
Structured question
The aim was to answer the same question in 2 different dog populations:
What is the prevalence of bacterial infections in:
Dogs with AD treated with oral cyclosporine at the approved starting anti-allergic oral dosage of 5 mg/kg BW per day; and
Dogs with other conditions treated with oral cyclosporine at immunosuppressive dosages greater than 5 mg/kg BW per day.
Literature search
The Web of Science (Science Citation Index Expanded) and CAB abstract databases were searched for relevant articles on August 25, 2018 and November 2, 2019, using the following search string: (dog or dogs or canine) AND (cyclosporin* or cyclosporine) AND [*bacteri* AND (infect* OR pyoderma OR skin OR urinary OR nephritis OR cystitis OR kidney OR bladder OR pneumonia OR lung OR meningitis OR encephalitis OR brain OR meninge*)]. The search was limited to articles published since 2000, the time around which the oral modified cyclosporine became commercially available for use in dogs. The bibliographies of identified articles were then searched for additional relevant articles. Review articles were excluded due to the interest in original reports. Meeting abstracts were not searched because detailed information was needed.
The query identified 38 and 180 citations in the Web of Science and CAB abstracts, respectively. Fifteen articles reporting usable data were selected: 9 from the Web of Science (4–12) and 6 from the CAB Abstracts (13–18). Additionally, 12 articles were identified from screening the bibliography of previously selected articles (19–30). Finally, 1 article that was published between the 2 searches was added (31).
To avoid a publication bias, case reports detailing rare or unusual infections were not included in the final assessment; therefore, 12 articles were excluded (6–9,11,12,15,17,18,26, 28,31) and the remaining 16 articles were evaluated (4,5,10, 13,14,16,19–25,27,29,30).
Finally, upon further review, 2 articles were eliminated, as one did not specifically differentiate infections between atopic dogs and those with other conditions (10), while the other did not specify the number of dogs with infections (19).
In addition to the overall prevalence and body location of bacterial infections, some of the potential factors that could have influenced the development of infection were investigated, where available. These parameters were: the dosage and the frequency of cyclosporine administration, the disease treated with cyclosporine, the dosages and frequencies of concurrently administered medications, the type of bacteria isolated, the time to the appearance of infection, and any other factors that could potentially contribute to the development of infections.
Results
Relevant data extracted from each of the 16 selected articles are found in Appendix 1.
Appendix 1.
Summary of extracted data.
| Reference number | Author | Year | Total number of dogs | Cyclosporine dosage (frequency) | **Concurrent medications; dosage (frequency) | Length of time on cyclosporine (days) | Disease treated | Total number of dogs with infections | Type of bacterial infection | Bacteria isolated |
|---|---|---|---|---|---|---|---|---|---|---|
| A: Dogs with atopic dermatitis | ||||||||||
|
| ||||||||||
| 21 | Olivry | 2002 | 61 | 2.5 mg/kg [1] or 5.0 mg/kg [2] (daily) | Not applicable | Not specified | AD | 3 | Pyoderma or Pyotraumatic dermatitis | Not specified |
| 22 | Steffan | 2003 | 117 | 4.6 mg/kg (daily, then tapered) | Not applicable | Not specified | AD | 29 | Pyoderma, Pyotraumatic dermatitis | Not specified |
| 4 | Burton | 2004 | 41 | 4.2 to 5.0 mg/kg (daily) | Not applicable | Not specified | AD | 4 | Pyoderma | Not specified |
| 23 | Steffan | 2005 | 266 | 5.0 mg/kg (daily then every other day) | Not applicable | Not specified | AD | 25 | Enteritis [1]; Granulomatous skin lesion [1]; bacteriuria [23] | Nocardia spp. (1), Clostridum spp (1), rest not specified |
| 24 | Radowicz | 2005 | 51 | 5.0 mg/kg (daily) | Not applicable | Not specified | AD | 7 | Bacteruria [4]; bacterial skin infection [3] | Bacteruria: Staphylococcus pseudintermedius (1), beta-haemolytic Streptococcus (1), Escherichia coli (2) and Pseudomonas aeruginosa (1) |
|
| ||||||||||
| A: Dogs with atopic dermatitis | ||||||||||
|
| ||||||||||
| 14 | Dip | 2013 | 48 | 5.0 mg/kg (daily) | Prednisolone, 1 mg/kg (daily, then every other day) [1] | Not specified | AD | 8 | Bacterial skin infection [2]; pyoderma [6] | Not specified |
| 29 | Little | 2015 | 112 | 3.2 to 6.6 mg/kg (daily) | Not applicable | Not specified | AD | 13 | Pyoderma | Not specified |
| 30 | Moyaert | 2017 | 132 | 5.0 mg/kg (daily) | Not applicable | Not specified | AD | 1 | Bacterial skin infection | Not specified |
|
| ||||||||||
| B: Dogs with other conditions | ||||||||||
|
| ||||||||||
| 20 | Mouatt | 2002 | 16 | 2.2 mg/kg^ (daily) | Ketoconazole, 10 mg/kg (daily) | 140 | AF | 1 | Spetic Arthritis | Not specified |
| 5 | Gregory | 2006 | 15 | 20.0 mg/kg (daily) | Azathioprine, 2 to 3 mg/kg (every other day); methylprednisolone, 10 mg/kg (single dose); prednisolone, 1 mg/kg (daily) | 10 to 270 | RT | 4 | Septic pleuritis/meningitis [1]; septic perotinitis [1]; pneumonia [1]; pyoderma and septicemia [1] | Staphylococcus spp./Mycoplasma spp. [1]; hemolytic Staphyococcus spp./Escherichia coli/gamma-Streptococcus spp. [1]; others not specified |
| 25 | Schmiedt | 2006 | 8 | 8.0 mg/kg (daily) | Capecitabine, 500 mg/m2^ (daily); ketoconazole 10 mg/kg (daily); prednisolone 0.5 mg/kg (daily) | Not specified | RT | 5 | UTI [1]; gastritis/Enteritis [4] | Beta-hemolytic, coagulase-positive Staphylococcus spp. [1]; Helicobacter pylori [4] |
| 13 | Adamo | 2007 | 10 | 6.0 to 24.0 mg/kg (daily) | Glucocorticoids, not specified [2]; ketoconazole, 8 mg/kg (daily) [1] | Not specified | MUE | 3 | UTI | Not specified |
| 27 | Hopper | 2012 | 26 | 20.0 mg/kg (daily) | Prednisolone, 1 mg/kg (daily); azathioprine, 2 to 3 mg/kg (every other day) or leflunomide, 4 to 6 mg/kg (daily) | 14; 264 | RT | 2 | Bacterial pneumonia [1]; pyoderma [1] | Not specified |
| 16 | Rhoades | 2016 | 20 | 10.0 mg/kg (daily) | Not applicable | 75 | IMPA | 1 | Bacteremia | Erysipelothrix rhusiopathiae |
Medications known to alter the metabolism of ciclosporin.
Mean dose.
[ ] — Number of dogs; AD — Atopic dermatitis; AF — Anal furunculosis/perianal fistulae; IMPA — Immune-mediated polyarthritis; MUE — Meningoencephalomyelitis of unknown etiology; RT — Renal transplantation; UTI — Urinary Tract Infection.
Dogs with atopic dermatitis
Eight articles were identified describing dogs with AD that had developed bacterial infections during treatment with cyclosporine (4,14,21–24,29,30).
Prevalence of bacterial infections
Altogether, 90/828 atopic dogs treated with cyclosporine were reported to have developed bacterial infections; a prevalence of 11% (Table 1A).
Table 1.
Prevalence of bacterial infections in A, dogs with atopic dermatitis (AD) (8 articles, 828 dogs) and B, dogs with other conditions (6 articles, 95 dogs).
| Reference number | Primary author | Year | Disease treated | Total number of dogs (T) | Number of dogs with a bacterial infection (N) | Prevalence (N/T, %) |
|---|---|---|---|---|---|---|
| A: Dogs with atopic dermatitis | ||||||
|
| ||||||
| 21 | Olivry | 2002 | AD | 61 | 3 5% | |
| 22 | Steffan | 2003 | AD | 117 | 29 | 25% |
| 4 | Burton | 2004 | AD | 41 | 4 | 10% |
| 23 | Steffan | 2005 | AD | 266 | 25 | 9% |
| 24 | Radowicz | 2005 | AD | 51 | 7 | 14% |
| 14 | Dip | 2013 | AD | 48 | 8 | 17% |
| 29 | Little | 2015 | AD | 112 | 13 | 12% |
| 30 | Moyaert | 2017 | AD | 132 | 1 | 1% |
| Total | 828 | 90 | 11% | |||
|
| ||||||
| B: Dogs with other conditions | ||||||
|
| ||||||
| 20 | Mouatt | 2002 | AF | 16 | 1 | 6% |
| 5 | Gregory | 2006 | RT | 15 | 4 | 27% |
| 25 | Schmiedt | 2006 | RT | 8 | 5 | 63% |
| 13 | Adamo | 2007 | MUE | 10 | 3 | 30% |
| 27 | Hopper | 2012 | RT | 26 | 2 | 8% |
| 16 | Rhoades | 2016 | IMPA | 20 | 1 | 5% |
| Total | 95 | 16 | 17% | |||
AF — Anal furunculosis/perianal fistulae; IMPA — Immune-mediated polyarthritis; MUE — Meningoencephalomyelitis of unknown etiology; RT — Renal transplantation.
Types of bacterial infections
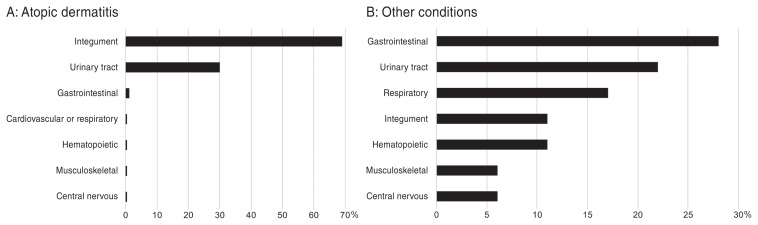
The localization of the identified bacterial infections in atopic dogs is depicted in Figure 1A. None of the dogs developed an infection in more than 1 body system, but at least 1 dog in each study developed a skin infection. The most affected system was the integument (61/90 dogs, 68%). Among these 61 dogs with skin infections, there were 60 dogs with pyoderma, bacterial folliculitis, or pyotraumatic dermatitis (98%; 67% of all infections) and 1 dog with granulomatous skin lesions associated with Nocardia spp. (2%; 1% of all infections). The second most commonly affected system was the urinary tract (27/90 dogs; 30%) with all 27 dogs diagnosed with bacteriuria and only 1 exhibiting clinical signs of urinary tract infection (23). One article reported 9 dogs with evidence of bacteriuria both before and after cyclosporine therapy; these dogs were excluded from the final count of dogs with bacteriuria (23). The only other body system affected in atopic dogs was the gastrointestinal tract, with 1 dog diagnosed with bacterial enteritis (23).
Figure 1.
Frequency of infection of body systems in A — dogs with atopic dermatitis (AD), and B — dogs with other conditions.
Isolated bacteria
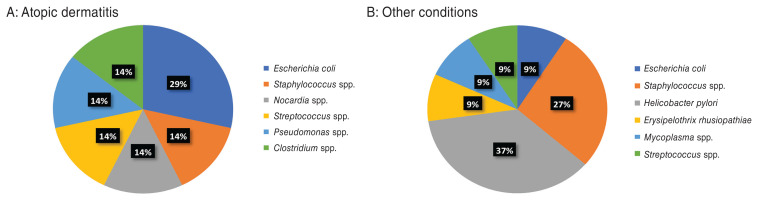
Seven bacterial isolates were identified in 2 of 8 articles (Figure 2A). In 2 dogs (29%), Escherichia coli was cultured, and the remaining 5 species (Staphylococcus spp., Nocardia spp., Streptococcus spp., Pseudomonas spp., and Clostridium spp.) were identified in 1 dog each (14%). Five of seven isolates (71%; Escherichia coli, Staphylococcus spp., Nocardia spp., Streptococcus spp., Pseudomonas spp.) were found in the urine, 1 (14%; a Clostridium spp.) was cultured from the gastrointestinal tract, and 1 (a Nocardia spp.) was grown from a deep cutaneous granuloma. The relatively low reporting of staphylococcal bacteria isolated is likely due to that species not being cultured or identified explicitly in 83/90 skin infections (92%).
Figure 2.
Frequency of bacterial genera isolated from dogs treated with ciclosporin. A — dogs with atopic dermatitis (AD); B — dogs with other conditions.
Time to development of infection
None of the studies describing the treatment of dogs with AD reported the time to development of bacterial infection in the affected patients.
Cyclosporine dosage and concurrent medications
The dosages of cyclosporine were extractable from all 8 selected articles, and are summarized in Appendix 1. These dosages ranged from 2.5 to 6.6 mg/kg BW per day with most articles (5/8, 63%) reporting dogs receiving an average dosage of 5 mg/kg BW per day; 1 trial allowed tapering the dosing to every other day (23). The dogs that developed an infection at locations other than the skin had been prescribed the same dosage (5 mg/kg BW per day) as that given to most dogs with AD. Thus, it seems that the dosage of cyclosporine did not influence the location of bacterial infections in atopic dogs.
The concurrent use of another medication was reported in only 1 trial (14) in which 1 dog treated with cyclosporine and prednisolone at 1 mg/kg BW per day developed a bacterial skin infection. In that article, another dog treated solely with cyclosporine was also reported as having a bacterial skin infection while 6 others had an unspecified pyoderma (14).
Dogs with other conditions
Six articles describing infections in dogs treated with cyclosporine as an immunosuppressant were reviewed (5,13,16, 20,25,27). In these articles, this drug was used to manage immune-mediated polyarthritis (16), anal furunculosis (20), meningoencephalomyelitis of unknown etiology (13), or immunosuppression after renal transplantation (5,25,27).
Prevalence of bacterial infections
Altogether, 16/95 dogs (a prevalence of 17%) developed bacterial infections while undergoing cyclosporine therapy for immune-mediated conditions or after renal transplantation (Table 1B).
Types of bacterial infections
The localization of the bacterial infections reported in dogs with immune-mediated conditions or after renal transplantation is depicted in Figure 1B. As 2/16 dogs (13%) had developed an infection at more than 1 body site (5), there was a total of 18 infections. Among these dogs with multiple infections, one had a bacterial septicemia suspected to be secondary to a chronic pyoderma and the other had a septic pleuritis and meningitis. Altogether, the affected body systems were, in descending order of frequency, the gastrointestinal tract (5/18, 28%), the urinary system (4/18, 22%), respiratory system (3/18, 17%), hematopoietic and integumentary systems (2/18 each, 11%), and musculoskeletal and nervous systems (1/18 each, 6%).
Isolated bacteria
Eleven bacterial isolates were identified in 8 dogs in 3 articles (Figure 2B) (5,16,25). In 1 dog, 2 bacterial species were isolated and in one, 3 species were grown. The most identified bacterial species was Helicobacter pylori (4 isolates, 37%), all cultured from the gastrointestinal tract. The second most common bacterial genus was Staphylococcus spp. (3 isolates, 27%), and these bacteria were isolated from the urinary tract, the integument, and the pleura/meninges in 1 dog each. The remaining 4 isolates were identified in 1 dog each (9%), and they were found in the integument (E. coli and Streptococcus spp.), the pleura/meninges (Mycoplasma spp.), and the blood (Erysipelothrix rhusiopathiae).
Time to development of infection
The time to development of bacterial infection following the initiation of cyclosporine therapy was reported in 4 articles (7 dogs; Appendix 1) (5,16,20,27). These articles described a time to infection varying between 8 and 264 d, with a median time to infection of 18 d.
Cyclosporine dosage and concurrent medications
The dosages of cyclosporine were extractable from all 6 selected studies, and they are summarized in Appendix 1. The dosages used for the management of immune-mediated diseases or the prevention of renal transplant rejection ranged from 2.2 to 24.0 mg/kg BW per day with 4 articles reporting at least 1 dog treated with a dosage of cyclosporine of 20 mg/kg BW per day or greater. The dogs that developed a bacterial infection in more than one body site were receiving 20 mg/kg BW per day of cyclosporine (5).
Only 1 article reported the use of cyclosporine monotherapy for the management of IMPA (16). The remaining 5 articles all described the use of concurrent medications. A potentiation of the pharmacokinetics of cyclosporine with ketoconazole was stated in 3 articles for 7 dogs (13,20,25) including 1 that reported a cyclosporine dosage less than 5 mg/kg BW per day (20). The other added immunosuppressive medications were glucocorticoids (13 dogs) (5,13,25,27), azathioprine (6 dogs) (5,27), capecitabine (5 dogs) (25), and leflunomide (2 dogs) (27).
Discussion
Limitations of our analysis was the relative lack of detail about the type of infection identified in dogs treated with cyclosporine. Numerous articles that could have been added herein merely stated the word “infection” as an adverse event. One could thus not assume that the infection was bacterial; it was suspected that some dogs with bacterial infections were not accounted for. Another limitation was that over half of the articles did not identify the specific bacteria isolated, which may have falsely under- or over-represented some bacterial species. When reporting the diagnosis of “pyoderma,” the depth of infection was typically not stated. As dogs with AD appear predisposed to develop bacterial skin infections (32), the role played by cyclosporine in the spread of these pyodermas is unclear, especially as the specific prevalence of pyoderma development in dogs with AD has not been established. Furthermore, most articles did not differentiate true infections from mere bacterial contamination or colonization. Although bacteria were isolated in each case, one cannot assume that the bacteria were inducing the disease. A good example of this is that only 1 dog of the 27 with bacteriuria exhibited clinical signs of UTI. Additionally, a positive culture for Helicobacter pylori was obtained in 4 dogs, but another article showed no correlation between high numbers of Helicobacter spp. and true gastritis in dogs (33). In future studies, and to avoid confusion, efforts should be made to better differentiate infections from contaminations or colonizations, for example by reporting the presence of phagocytized bacteria and describing clinical signs of infection in greater details. Finally, several studies described the use of a combination of drugs to immunosuppress dogs, for example, for the prevention of renal transplant rejection. As a result, it is unclear if the infections observed were caused by the cyclosporine, the added immunosuppressants, or the combination thereof.
In summary, the prevalence of bacterial infections in atopic dogs treated with cyclosporine is approximately 11% while that in dogs treated for other immune-mediated conditions or after renal transplantation is approximately 17%.
In dogs with AD, bacterial infections most often occurred in the integument and urinary systems, which should prompt the monitoring of these sites for clinical signs of infection during treatment. Confounding issues are that AD, by itself, can predispose dogs to secondary bacterial skin infections and that bacteriuria can be present in the absence of dysuria, which might have arisen before cyclosporine therapy was initiated. The treatment of dogs with cyclosporine at dosages of ≤ 5 mg/kg BW per day appears not to predispose the dogs to developing infections at multiple body sites. In contrast, in dogs receiving cyclosporine for the treatment of immune-mediated diseases or after renal transplantation, infections can occur in various body systems, and they might be present at more than one body site. The most affected systems are the gastrointestinal, urinary, and respiratory systems. The median time to development of infection was 18 d. It could not be determined if the higher cyclosporine dosage contributed to bacterial infection development because of the concurrent use of additional immunosuppressive drugs in most dogs.
Additional studies are needed to establish more accurately the prevalence of bacterial infections in dogs treated with cyclosporine, and to identify specific factors influencing the development of such infections. Patients enrolled in such studies should be screened for pre-existing infections and excluded whenever one is identified. Detailed information about the bacterial infections should be recorded, including the location of the infection, the identification of bacterial species, and the time at which the infection occurred in relation to the initiation of cyclosporine (or other) therapy. Where possible, concurrent immunosuppressive medications should be excluded in order to more precisely identify the role of cyclosporine in the development of these infections.
Finally, the prevalence, clinical type, and bacteria involved in pyoderma should be established in dogs with AD in the absence of anti-allergic therapy, for example, in clinical trials with a placebo arm. Such data will help determine if any of the immuno-modulating drugs used for AD have an explicit role in predisposing the dogs to develop skin and other infections. CVJ
Footnotes
Conflict of interest: Dr. Olivry declared having been a consultant, received lecturing honoraria and research funding from Novartis Animal Health or Elanco.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Guaguère E, Steffan J, Olivry T. Cyclosporin A: A new drug in the field of canine dermatology. Vet Dermatol. 2004;15:61–74. doi: 10.1111/j.1365-3164.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- 2.Nuttall T, Reece D, Roberts E. Life-long diseases need life-long treatment: Long-term safety of ciclosporin in canine atopic dermatitis. Vet Rec. 2014;174:3–12. doi: 10.1136/vr.102471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callander J, Anstey AV, Ingram JR, Limpens J, Flohr C, Spuls PI. How to write a critically appraised topic: Evidence to underpin routine clinical practice. Br J Dermatol. 2017;177:1007–1013. doi: 10.1111/bjd.15873. [DOI] [PubMed] [Google Scholar]
- 4.Burton G, Burrows A, Walker R, et al. Efficacy of cyclosporin in the treatment of atopic dermatitis in dogs — Combined results from two veterinary dermatology referral centres. Aust Vet J. 2004;82:681–685. doi: 10.1111/j.1751-0813.2004.tb12153.x. [DOI] [PubMed] [Google Scholar]
- 5.Gregory CR, Kyles AE, Bernsteen L, Mehl M. Results of clinical renal transplantation in 15 dogs using triple drug immunosuppressive therapy. Vet Surg. 2006;35:105–112. doi: 10.1111/j.1532-950X.2006.00121.x. [DOI] [PubMed] [Google Scholar]
- 6.Smith PM, Haughland SP, Jeffery ND. Brain abscess in a dog immunosuppressed using cyclosporin. Vet J. 2007;173:675–678. doi: 10.1016/j.tvjl.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Baxter CG, Vogelnest LJ. Multifocal papular deep bacterial pyoderma in a boxer dog caused by pseudomonas aeruginosa. Aust Vet J. 2008;86:435–439. doi: 10.1111/j.1751-0813.2008.00351.x. [DOI] [PubMed] [Google Scholar]
- 8.Paul AE, Mansfield CS, Thompson M. Presumptive Nocardia spp. infection in a dog treated with cyclosporin and ketoconazole. N Z Vet J. 2010;58:265–268. doi: 10.1080/00480169.2010.69301. [DOI] [PubMed] [Google Scholar]
- 9.MacNeill AL, Steeil JC, Dossin O, Hoien-Dalen PS, Maddox CW. Disseminated nocardiosis caused by nocardia abscessus in a dog. Vet Clin Pathol. 2010;39:381–385. doi: 10.1111/j.1939-165X.2010.00247.x. [DOI] [PubMed] [Google Scholar]
- 10.Peterson AL, Torres SM, Rendahl A, Koch SN. Frequency of urinary tract infection in dogs with inflammatory skin disorders treated with ciclosporin alone or in combination with glucocorticoid therapy: A retrospective study. Vet Dermatol. 2012;23:201–e43. doi: 10.1111/j.1365-3164.2012.01044.x. [DOI] [PubMed] [Google Scholar]
- 11.Park KM, Nam HS, Woo HM. Successful management of multidrug-resistant pseudomonas aeruginosa pneumonia after kidney transplantation in a dog. J Vet Med Sci. 2013;75:1529–1533. doi: 10.1292/jvms.13-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banovic F, Koch S, Robson D, Jacob M, Olivry T. Deep pyoderma caused by Burkholderia cepacia complex associated with ciclosporin administration in dogs: A case series. Vet Dermatol. 2015;26:287–e64. doi: 10.1111/vde.12210. [DOI] [PubMed] [Google Scholar]
- 13.Adamo PF, Rylander H, Adams WM. Ciclosporin use in multi-drug therapy for meningoencephalomyelitis of unknown aetiology in dogs. J Small Anim Pract. 2007;48:486–496. doi: 10.1111/j.1748-5827.2006.00303.x. [DOI] [PubMed] [Google Scholar]
- 14.Dip R, Carmichael J, Letellier I, et al. Concurrent short-term use of prednisolone with cyclosporine A accelerates pruritus reduction and improvement in clinical scoring in dogs with atopic dermatitis. BMC Vet Res. 2013;9:173. doi: 10.1186/1746-6148-9-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siak MK, Burrows AK. Cutaneous nocardiosis in two dogs receiving ciclosporin therapy for the management of canine atopic dermatitis. Vet Dermatol. 2013;24:453–456. e102–103. doi: 10.1111/vde.12046. [DOI] [PubMed] [Google Scholar]
- 16.Rhoades AC, Vernau W, Kass PH, Herrera MA, Sykes JE. Comparison of the efficacy of prednisone and cyclosporine for treatment of dogs with primary immune-mediated polyarthritis. J Am Vet Med Assoc. 2016;248:395–404. doi: 10.2460/javma.248.4.395. [DOI] [PubMed] [Google Scholar]
- 17.Ho K, Kennis RA, Sandey M, White A. Successful medical management of cutaneous nocardia species infection in a dog receiving ciclosporin (atopica) Vet Rec Case Rep. 2017;5:e000493. [Google Scholar]
- 18.Yaemsiri S, Sykes JE. Successful treatment of disseminated nocardiosis caused by Nocardia veterana in a dog. J Vet Intern Med. 2018;32:418–422. doi: 10.1111/jvim.14855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathews KA, Holmberg DL, Miller CW. Kidney transplantation in dogs with naturally occurring end-stage renal disease. J Am Anim Hosp Assoc. 2000;36:294–301. doi: 10.5326/15473317-36-4-294. [DOI] [PubMed] [Google Scholar]
- 20.Mouatt JG. Cyclosporin and ketoconazole interaction for treatment of perianal fistulas in the dog. Aust Vet J. 2002;80:207–211. doi: 10.1111/j.1751-0813.2002.tb10814.x. [DOI] [PubMed] [Google Scholar]
- 21.Olivry T, Steffan J, Fisch RD, et al. Randomized controlled trial of the efficacy of cyclosporine in the treatment of atopic dermatitis in dogs. J Am Vet Med Assoc. 2002;221:370–377. doi: 10.2460/javma.2002.221.370. [DOI] [PubMed] [Google Scholar]
- 22.Steffan J, Alexander D, Brovedani F, Fisch RD. Comparison of cyclosporine A with methylprednisolone for treatment of canine atopic dermatitis: A parallel blinded randomized controlled trial. Vet Dermatol. 2003;14:11–22. doi: 10.1046/j.1365-3164.2003.00318.x. [DOI] [PubMed] [Google Scholar]
- 23.Steffan J, Parks C, Seewald W Group NAVDCS. Clinical trial evaluating the efficacy and safety of cyclosporine in dogs with atopic dermatitis. J Am Vet Med Assoc. 2005;226:1855–1863. doi: 10.2460/javma.2005.226.1855. [DOI] [PubMed] [Google Scholar]
- 24.Radowicz SN, Power HT. Long-term use of cyclosporine in the treatment of canine atopic dermatitis. Vet Dermatol. 2005;16:81–86. doi: 10.1111/j.1365-3164.2005.00435.x. [DOI] [PubMed] [Google Scholar]
- 25.Schmiedt C, Penzo C, Schwab M, Dubielzig R, McAnulty J. Use of capecitabine after renal allograft transplantation in dog erythrocyte antigen-matched dogs. Vet Surg. 2006;35:113–124. doi: 10.1111/j.1532-950X.2006.00122.x. [DOI] [PubMed] [Google Scholar]
- 26.Mohri T, Takashima K, Yamane T, Sato H, Yamane Y. Purulent pericarditis in a dog administered immune-suppressing drugs. J Vet Med Sci. 2009;71:669–672. doi: 10.1292/jvms.71.669. [DOI] [PubMed] [Google Scholar]
- 27.Hopper K, Mehl ML, Kass PH, Kyles A, Gregory CR. Outcome after renal transplantation in 26 dogs. Vet Surg. 2012;41:316–327. doi: 10.1111/j.1532-950X.2011.00924.x. [DOI] [PubMed] [Google Scholar]
- 28.Hilligas J, Van Wie E, Barr J, et al. Vertebral osteomyelitis and multiple cutaneous lesions in a dog caused by Nocardia pseudobrasiliensis. J Vet Intern Med. 2014;28:1621–1625. doi: 10.1111/jvim.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Little PR, King VL, Davis KR, Cosgrove SB, Stegemann MR. A blinded, randomized clinical trial comparing the efficacy and safety of oclacitinib and ciclosporin for the control of atopic dermatitis in client-owned dogs. Vet Dermatol. 2015;26:23–30. e7–8. doi: 10.1111/vde.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moyaert H, Van Brussel L, Borowski S, et al. A blinded, randomized clinical trial evaluating the efficacy and safety of lokivetmab compared to ciclosporin in client-owned dogs with atopic dermatitis. Vet Dermatol. 2017;28:593–e145. doi: 10.1111/vde.12478. [DOI] [PubMed] [Google Scholar]
- 31.Cain CL, Cole SD, Bradley CW, II, Canfield MS, Mauldin EA. Clinical and histopathological features of Burkholderia cepacia complex dermatitis in dogs: A series of four cases. Vet Dermatol. 2018;29:457–e156. doi: 10.1111/vde.12677. [DOI] [PubMed] [Google Scholar]
- 32.Seckerdieck F, Mueller RS. Recurrent pyoderma and its underlying primary diseases: A retrospective evaluation of 157 dogs. Vet Rec. 2018;182:434. doi: 10.1136/vr.104420. [DOI] [PubMed] [Google Scholar]
- 33.Suárez-Esquivel M, Alfaro-Alarcón A, Guzmán-Verri C, Barquero-Calvo E. Analysis of the association between density of Helicobacter spp. and gastric lesions in dogs. Am J Vet Res. 2017;78:1414–1420. doi: 10.2460/ajvr.78.12.1414. [DOI] [PubMed] [Google Scholar]