Abstract
Background: Abnormal extracellular matrix (ECM) remodeling plays an essential role in urothelial carcinoma (UC) invasiveness and metastasis. Focusing on the ECM structural constituent (GO: 0005201), we recognized a significant upregulation of the fibulin 2 gene (FBLN2) during UC progression in a published UC transcriptome (GSE31684). Thus, we aimed to investigate the roles of FBLN2 expression and its prognostic value in upper urinary tract UC (UTUC) and urinary bladder UC (UBUC) in our large, well-characterized cohort.
Patients and Methods: Clinicopathological data and formalin-fixed paraffin-embedded UC tissues were analyzed retrospectively. We determined FBLN2 expression using immunohistochemical staining assessed by H-scores. FBLN2 expression correlated with clinicopathological features and patient outcomes, including metastasis-free survival (MFS) and disease-specific survival (DSS). Statistical analyses were performed using Pearson's chi-square test, Kaplan–Meier estimates of DSS and MFS, and the Cox proportional hazards model. We used Ingenuity Pathway Analysis (IPA) to clarify the functional significance of dysregulated FBLN2 in UC.
Results: Data from 295 UBUC and 340 UTUC patients were available for the final evaluation. Pearson's chi-square test showed that high FBLN2 immunoexpression significantly correlated with adverse pathologic variables, such as advanced pathologic tumor stage, high histological grade, perineural invasion, vascular invasion, lymph node metastasis, and increased mitotic rate (all p < 0.05). Kaplan–Meier analysis demonstrated associations of high FBLN2 expression with worse DSS (p < 0.001) and MFS (p < 0.001). Furthermore, multivariate analysis identified high FBLN2 expression as an independent predictive risk factor for DSS [hazard ratio (HR) in UBUC, 2.306, p = 0.014; in UTUC, 2.561, p = 0.012] and MFS (HR in UBUC, 2.493, p = 0.001; in UTUC, 2.837, p = 0.001). IPA demonstrated that multiple signaling pathways were enriched, including the oxidative phosphorylation, mitochondrial dysfunction, and regulation of the epithelial–mesenchymal transition pathways.
Conclusion: High FBLN2 expression was associated with adverse pathologic features and worse oncological outcomes and may serve as a prognostic biomarker for UC.
Keywords: FBLN2, bladder cancer, upper urinary tract cancer, urothelial carcinoma, prognosis
Introduction
Urothelial carcinoma (UC) is a common urinary tract malignancy worldwide (1, 2). It may arise from the urinary bladder (UBUC) and upper urinary tract (UTUC), including the renal pelvis and ureter. Urinary bladder tumors account for 90–95% of UCs (1, 2). According to GLOBOCAN data, UBUC is the 10th most common cancer in the world, and its incidence is steadily rising (1). An estimated 550,000 new cases of UBUC were recorded worldwide in 2018 with ~200,000 deaths occurring during this same period (1). UTUCs are uncommon and account for only 5–10% of UCs in Western countries (1, 2).
For UBUC, ~75% of patients are initially diagnosed with non-muscle-invasive bladder cancer (NMIBC) while the remaining 25% are diagnosed with muscle-invasive bladder cancer (MIBC) (3, 4). Transurethral resection of the bladder tumor followed by intravesical instillation therapy constitutes the standard treatment for NMIBC (3). Different treatment modalities are suggested for MIBC, including radical cystectomy, bladder-preserving approaches, neoadjuvant or adjuvant therapy, and systemic therapy for advanced disease (4). For UTUC, approximately two-thirds of patients have invasive tumors at diagnosis. Although kidney-sparing surgery is recommended for low-risk UTUC without compromising oncological outcomes, radical nephroureterectomy with bladder cuff excision is still the standard treatment for most UTUCs (5). Despite advancements in surgical techniques and drug discovery, the prognosis for UC has not significantly improved over the past two decades (3–5).
UC tumor heterogeneity occurs at multiple levels and directly affects clinical care and treatment efficacy (6). Patients with UC typically die from tumor recurrence and metastasis (3–5). Consequently, a better understanding of the UC aggressiveness mechanism is crucial for improving patient prognosis. The hallmarks of cancer comprise six biological capabilities, including activating invasion and metastasis (7). Abnormal extracellular matrix (ECM) is a major component of a tumorigenic microenvironment as well as pre-metastatic and metastatic niches (8, 9). The process of cancer invasion and metastasis involves multifaceted molecular mechanisms in the cell and matrix interactions, which are reflected in variable up- and downregulation of multiple molecules (8, 9).
To identify transcripts that are potentially involved in UC invasiveness and metastasis, we performed data mining and processing of a public transcriptome dataset, focusing on the ECM structural constituent. We found that high transcript expression of fibulin 2 (FBLN2) was significantly associated with advanced pathologic stage and tumor metastasis in UBUC, suggesting its role in cancer progression.
The human FBLN2 gene, mapped to chromosome 3p25.1, encodes a protein that is a member of the fibulin family, which is characterized by repeated epidermal growth factor (EGF)-like domains and a unique C-terminal fibulin-type module (10). Members of this family are associated with basement membranes and elastic ECM fibers. They play a central role in matrix stabilization by forming homodimeric complexes (11–13). FBLN2 acts as a matrix organizer in the ECM to maintain tissue architecture. It is also highly expressed during organ development, including in skeletal, heart, and neuronal structures (11–13). Recently, an increasing number of studies have highlighted the involvement of FBLN2 in carcinogenesis (14). In lung and pancreatic cancers, it exhibits a pro-tumor effect (15–17). However, FBLN2 is a tumor suppressor in breast cancer and nasopharyngeal carcinoma (NPC) (18–20). Until now, the potential significance of FBLN2 in UC has not been systematically analyzed. Consequently, in the present study, we intended to evaluate FBLN2 expression and prognostic utility in our well-characterized UTUC and UBUC cohorts.
Patients and Methods
Data Mining of the Gene Expression Omnibus (GEO) Dataset
A transcriptome dataset (GSE31684), including 93 UBUC patients who received radical surgery, was collected from the NCBI GEO database. To quantify the gene expression level, we analyzed all probe sets without preselection or filtering and imported the raw files into Nexus Expression 3 software (BioDiscovery, El Segundo, CA, USA). Under supervision, comparative analyses were conducted to discover the significantly differentially expressed genes related to the ECM structural constituent (GO:0005201) by comparing tumor stage (high stage vs. low stage) and metastatic events (metastasis vs. non-metastasis). We chose differentially expressed genes (p < 0.01 and log2 ratio >0.3) for further study. From the Oncomine database, we selected two studies, Sanchez-Carbayo et al. (21) and Blaveri et al. (22), to evaluate the expression profiles of FBLN2 in UBUC.
Study Population
The study cohort consisted of 340 UTUC and 295 UBUC patients who received surgery with curative intent at a single medical center between 1998 and 2004. None of them received preoperative radiotherapy or chemotherapy. Clinical and pathologic characteristics as well as outcome data were retrospectively collected from patient medical charts. Histological grading was based on the World Health Organization criteria (23) while tumor staging was determined according to the American Joint Committee on Cancer system (24). The study was approved by the Institutional Review Board (IRB10501-005).
Immunohistochemistry and Scoring
Four-micrometer-thick sections from formalin-fixed, paraffin-embedded blocks were sectioned on pre-coated slides followed by deparaffinization, rehydration, and antigen retrieval as previously described (25, 26). Next, we blocked endogenous peroxidase using 3% hydrogen peroxide solution. The sections were then incubated with the primary antibody against FBLN2 (1:200, Catalog Number biorbyt-orb69091) for 1 h. Primary antibodies were detected using a ChemMate EnVisionTM Detection kit (DAKO, Carpinteria, CA). Sections processed without the primary anti-FBLN2 antibody were used as negative controls. As for positive controls, we included HT 1197 cell block sections known to express high FBLN2. Two independent pathologists quantified FBLN2 protein expression levels by integrating the percentage and intensity of immunostaining in the cytoplasm of UC cells to generate the H-score using the formula ΣPi(i +1), in which Pi represents the percentage of stained tumor cells (0 to 100%) and i is the intensity of immunoreactivity (0 to 3+). The immunoreactivity was divided into low and high expression levels based on the median H-score.
Functional Interpretation of The Cancer Genome Atlas (TCGA) Data and Network Analysis
The RNA sequencing and clinical data were downloaded from the TCGA BLCA (Bladder Urothelial Carcinoma) database through the c-BioPortal platform. To understand the functional significance of dysregulated FBLN2 in UC, we used Ingenuity Pathway Analysis (IPA; http://www.ingenuity.com) to analyze ingenuity canonical pathways, upstream regulators, diseases and biological functions, and gene networks. These core analyses were used to interpret the common differentially expressed genes between high FBLN2-expressing and low FBLN2-expressing UCs.
Statistical Analysis
The association between FBLN2 expression and various clinicopathological features was assessed using Pearson's chi-square test. We analyzed two end points, including metastasis-free survival (MFS) and disease-specific survival (DSS). Univariate and multivariate analyses were used to identify relevant FBLN2 expression and clinicopathological characteristics among the predictors of DSS, measured from curative surgery to the time of cancer death, and MFS, measured from curative surgery to the first metastasis. Survival curves were drawn using the Kaplan–Meier method with a log-rank test. All significant parameters for univariate analysis were included in the multivariate Cox proportional hazards model to identify the independent predictors. SPSS Statistics V.17.0 software (IBM, Armonk, NY, USA) was utilized for statistical analyses. Statistical significance was set at p < 0.05.
Results
Association Between FBLN2 Gene Expression and the ECM Structural Constituent in the UBUC Transcriptome
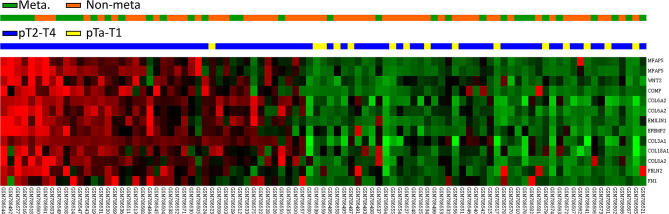
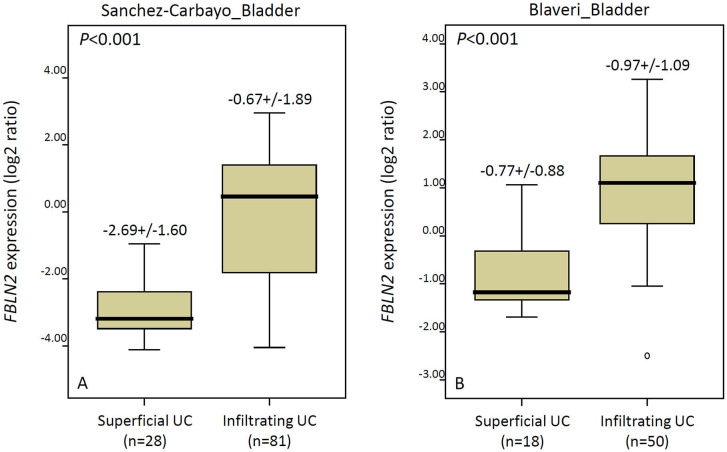
We utilized a published UBUC transcriptome dataset (GSE31684) for data mining, including 93 patients undergoing radical cystectomy. There were 78 patients with muscle-invasive disease and 28 patients with lymph node metastasis. Through transcriptomic profiling, we identified 13 probes covering 11 transcripts associated with the ECM structural constituent (GO: 0005201). These genes were significantly upregulated in the high pathologic stage and lymph node metastasis (Table 1 and Figure 1). We selected FBLN2 for further study, as the role of FBLN2 in cancer development is not straightforward; it may inhibit or promote tumorigenesis. Its function in UC is not well-elucidated. To further confirm the initial observations, we examined the Oncomine database for FBLN2 expression in bladder cancers. The Oncomine database revealed that there was a significantly higher expression of FBLN2 in infiltrating UBUC compared to superficial tumors (Figure 2, Supplementary Figures 1, 2). These findings encouraged us to elucidate the role of FBLN2 in our well-characterized UTUC and UBUC cohorts.
Table 1.
Summary of differentially expressed genes associated with extracellular matrix structural constituent (GO:0005201) and showed positive associations to cancer invasiveness and metastasis in the transcriptome of urothelial carcinoma of urinary bladder (GSE31684).
| Probe | Comparing T2-4 to Ta-T1 | Comparing meta. to non-meta.# | Gene symbol | Gene title | Molecular function | ||
|---|---|---|---|---|---|---|---|
| Log ratio | p-value | log ratio | p-value | ||||
| 203886_s_at | 0.8918 | 0.0003 | 0.6266 | 0.0009 | FBLN2 | Fibulin 2 | Calcium ion binding, extracellular matrix structural constituent, protein binding |
| 204163_at | 1.1047 | <0.0001 | 0.6313 | 0.0001 | EMILIN1 | Elastin microfibril interfacer 1 | Extracellular matrix constituent conferring elasticity, extracellular matrix structural constituent, identical protein binding, protein binding |
| 205648_at | 0.6169 | 0.0014 | 0.4056 | 0.0061 | WNT2 | Wingless-type MMTV integration site family member 2 | Extracellular matrix structural constituent, signal transducer activity |
| 205713_s_at | 1.8386 | <0.0001 | 1.1823 | 0.0001 | COMP | Cartilage oligomeric matrix protein | Calcium ion binding, extracellular matrix structural constituent, protein binding |
| 209082_s_at | 0.6536 | 0.0015 | 0.4516 | 0.0042 | COL18A1 | Collagen; type XVIII; alpha 1 | Extracellular matrix structural constituent, metal ion binding, protein binding, structural molecule activity, zinc ion binding |
| 209156_s_at | 2.1304 | <0.0001 | 0.9732 | 0.0021 | COL6A2 | Collagen; type VI; alpha 2 | Extracellular matrix structural constituent, protein binding, protein binding; bridging, structural molecule activity |
| 209356_x_at | 0.5664 | 0.0027 | 0.4818 | 0.0008 | EFEMP2 | EGF-containing fibulin-like extracellular matrix protein 2 | Calcium ion binding, extracellular matrix structural constituent, protein binding, transmembrane receptor activity |
| 209758_s_at | 1.4195 | <0.0001 | 0.5778 | 0.0087 | MFAP5 | Microfibrillar associated protein 5 | Extracellular matrix structural constituent |
| 213290_at | 0.832 | 0.0002 | 0.5142 | 0.004 | COL6A2 | Collagen; type VI; alpha 2 | Extracellular matrix structural constituent, protein binding, protein binding; bridging, structural molecule activity |
| 213764_s_at | 1.8425 | <0.0001 | 0.8102 | 0.0034 | MFAP5 | Microfibrillar associated protein 5 | Extracellular matrix structural constituent |
| 214702_at | 0.8698 | <0.0001 | 0.3948 | 0.0100 | FN1 | Fibronectin 1 | Collagen binding, extracellular matrix structural constituent, heparin binding, protein binding |
| 215076_s_at | 1.4494 | <0.0001 | 0.5177 | 0.0031 | COL3A1 | Collagen; type III; alpha 1 (Ehlers-Danlos syndrome type IV; autosomal dominant) | Extracellular matrix structural constituent, structural molecule activity |
| 221900_at | 0.8558 | <0.0001 | 0.3936 | 0.0014 | COL8A2 | Collagen; type VIII; alpha 2 | Extracellular matrix structural constituent, protein binding, protein binding; bridging, structural molecule activity |
Meta., distal metastasis developed during follow-up; Non-Meta.: no metastatic event developed.
Figure 1.
Expression profiles of genes associated with the extracellular matrix structural constituent (GO:0005201) extracted from a published transcriptome of urothelial carcinoma (GSE31684) in the Gene Expression Omnibus database. FBLN2 was found to be one of the most significantly upregulated genes.
Figure 2.
The Oncomine database shows significantly upregulated FBLN2 in infiltrating urothelial carcinoma. The expression of FBLN2 is demonstrated in two datasets (Sanchez-Carbayo and Blaveri) acquired from the Oncomine database.
Clinicopathological Features of our UC Cohorts
We included 340 UTUC and 295 UBUC patients with a mean age of 65.8 years (Table 2). In the UTUC cohort, 49 patients (14.4%) had coexisting ureteral and renal pelvic tumors, and 62 patients (18.2%) had multiple tumors. There were 159 patients (46.8%) with advanced stage (pT2-4) UTUC, 284 (83.5%) with high histological grade tumors, and 28 (8.2%) with metastatic lymph nodes at diagnosis. Vascular invasion (VI) and perineural invasion (PNI) were noted in 106 (31.2%) and 19 cases (5.9%), respectively. Further, 167 lesions (49.1%) had high mitotic activity.
Table 2.
Correlations between FBLN2 expression and other important clinicopathological parameters in urothelial carcinomas.
| Parameter | Category | Upper urinary tract urothelial carcinoma | Urinary bladder urothelial carcinoma | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Case No. | FBLN2 expression | p-value | Case No. | FBLN2 expression | p-value | ||||
| Low | High | Low | High | ||||||
| Gender | Male | 158 | 77 | 81 | 0.664 | 216 | 107 | 109 | 0.868 |
| Female | 182 | 93 | 89 | 79 | 40 | 39 | |||
| Age (years) | <65 | 138 | 73 | 65 | 0.377 | 121 | 55 | 66 | 0.210 |
| ≥65 | 202 | 97 | 105 | 174 | 92 | 82 | |||
| Tumor location | Renal pelvis | 141 | 64 | 77 | 0.228 | – | – | – | – |
| Ureter | 150 | 77 | 73 | – | – | – | – | ||
| Renal pelvis and ureter | 49 | 29 | 20 | – | – | – | – | ||
| Multifocality | Single | 278 | 135 | 143 | 0.261 | – | – | – | – |
| Multifocal | 62 | 35 | 27 | – | – | – | – | ||
| Primary tumor (T) | Ta | 89 | 63 | 26 | <0.001* | 84 | 62 | 22 | <0.001* |
| T1 | 92 | 57 | 35 | 88 | 44 | 44 | |||
| T2–T4 | 159 | 50 | 109 | 123 | 41 | 82 | |||
| Nodal metastasis | Negative (N0) | 312 | 165 | 147 | <0.001* | 266 | 141 | 125 | 0.001* |
| Positive (N1–N2) | 28 | 5 | 23 | 29 | 6 | 23 | |||
| Histological grade | Low grade | 56 | 44 | 12 | 0.001* | 56 | 38 | 18 | 0.003* |
| High grade | 284 | 126 | 158 | 239 | 109 | 130 | |||
| Vascular invasion | Absent | 234 | 144 | 90 | <0.001* | 246 | 138 | 108 | <0.001* |
| Present | 106 | 26 | 80 | 49 | 9 | 40 | |||
| Perineural invasion | Absent | 321 | 168 | 153 | <0.001* | 275 | 144 | 131 | 0.001* |
| Present | 19 | 2 | 17 | 20 | 3 | 17 | |||
| Mitotic rate (per 10 high-power fields) | <10 | 173 | 104 | 69 | <0.001* | 139 | 85 | 54 | <0.001* |
| ≥10 | 167 | 66 | 101 | 156 | 62 | 94 | |||
Statistically significant.
In the UBUC cohort, 123 patients (41.7%) had MIBC (pT2-4), and most patients (81%) had high histological grade tumors at initial diagnosis. Concerning lymph node status, 266 patients (92.2%) were N0 and 29 (7.8%) were N1–2. There were 156 lesions (52.9%) with high mitosis. Moreover, VI and PNI were observed in 20 (6.8%) and 49 tumors (16.6%), respectively.
FBLN2 Immunoexpression and Patient Characteristic Correlations in UC
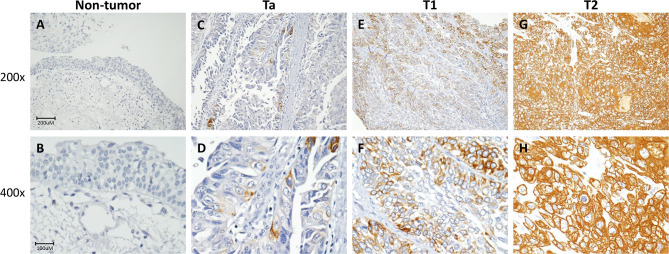
Immunohistochemical staining showed stepwise increments in FBLN2 immunoreactivity from low stage UC to high stage UC (Figure 3). Table 2 shows the association of FBLN2 immunoexpression with clinicopathological characteristics. In UTUC patients, high FBLN2 expression was significantly associated with advanced pathologic tumor stage, high histological grade, lymph node metastasis, VI, PNI, and high mitotic activity (all p < 0.001). Similarly, in UBUC patients, we found significant associations between high FBLN2 expression and advanced pT status (p < 0.001), high histological tumor grade (p = 0.003), lymph node metastasis (p = 0.001), VI (p < 0.001), PNI (p = 0.001), and high mitotic rate (p < 0.001).
Figure 3.
FBLN2 immunohistochemistry. Representative sections of non-tumor (A,B), non-invasive (Ta) (C,D), superficial (T1) (E,F), and muscle-invasive urothelial carcinoma (T2) (G,H) demonstrate stepwise increments in FBLN2 expression (original magnification: 200× and 400×).
Prognostic Significance of FBLN2 Expression
There were 113 patients, including 61 UTUCs and 52 UBUCs, who died of UC. In addition, 146 patients, including 70 UTUCs and 76 UBUCs, had subsequent metastatic tumor development. Univariate and multivariate analyses were performed to assess the prognostic impacts of FBLN2 staining on cancer metastasis and patient death in UC.
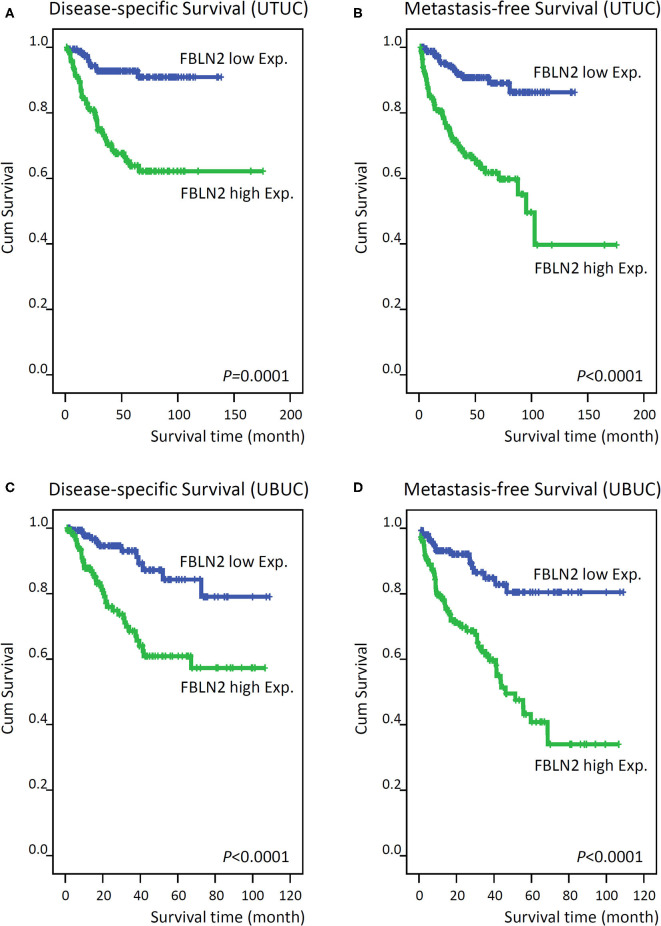
In UTUC (Table 3), 29.4% of patients with high FBLN2-expressing tumors died of the disease, whereas only 6.5% of patients with low FBLN2-expressing tumors did. Furthermore, 32.9% of high FBLN2-expressing tumors developed metastasis, whereas only 8.2% of the tumors with low FBLN2-expressing tumors did. Kaplan–Meier analysis showed a significant association of high FBLN2 expression with worse DSS (Figure 4A; p = 0.0001) and worse MFS (Figure 4B; p < 0.0001). Apart from FBLN2 immunoexpression, we found that tumor location, multifocal tumors pathologic stage, lymph node metastasis, histological grade, PNI, and VI were significant prognosticators of MFS and DSS in the univariate analysis. Multivariate Cox regression analysis showed that FBLN2 expression was independently predictive for DSS [hazard ratio (HR), 2.561; 95% confidence interval (CI), 1.219–4.968; p = 0.012] and MFS (HR, 2.837; 95% CI, 1.504–5.349; p = 0.001).
Table 3.
Univariate log-rank and multivariate analyses for disease-specific and metastasis-free survivals in upper urinary tract urothelial carcinoma.
| Parameter | Category | Case No. | Disease-specific survival | Metastasis-free survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| No. of event | p-value | RR | 95% CI | p-value | No. of event | p-value | RR | 95% CI | p-value | |||
| Gender | Male | 158 | 28 | 0.8286 | – | – | – | 32 | 0.7904 | – | – | – |
| Female | 182 | 33 | – | – | – | 38 | – | – | – | |||
| Age (years) | <65 | 138 | 26 | 0.9943 | – | – | – | 30 | 0.8470 | – | – | – |
| ≥65 | 202 | 35 | – | – | – | 40 | – | – | – | |||
| Tumor side | Right | 177 | 34 | 0.7366 | – | – | – | 38 | 0.3074 | – | – | – |
| Left | 154 | 26 | – | – | – | 32 | – | – | – | |||
| Bilateral | 9 | 1 | – | – | – | 0 | – | – | – | |||
| Tumor location | Renal pelvis | 141 | 24 | 0.0079* | 1 | – | 0.476 | 31 | 0.0659 | – | – | – |
| Ureter | 150 | 22 | 0.763 | 0.411–1.417 | 25 | – | – | – | ||||
| Renal pelvis and ureter | 49 | 15 | 1.675 | 0.462–6.067 | 14 | – | – | – | ||||
| Multifocality | Single | 273 | 48 | 0.0026* | 1 | – | 0.340 | 52 | 0.0127* | 1 | – | 0.001* |
| Multifocal | 62 | 18 | 1.833 | 0.528–6.372 | 18 | 2.678 | 1.525–4.703 | |||||
| Primary tumor (T) | Ta | 89 | 2 | <0.0001* | 1 | – | 0.177 | 4 | <0.0001* | 1 | – | 0.118 |
| T1 | 92 | 9 | 3.575 | 0.763–16.756 | 15 | 3.178 | 1.034–9.772 | |||||
| T2–T4 | 159 | 50 | 4.226 | 0.926–19.284 | 51 | 2.251 | 0.704–7.192 | |||||
| Nodal metastasis | Negative (N0) | 312 | 42 | <0.0001* | 1 | – | <0.001* | 55 | <0.0001* | 1 | – | 0.001* |
| Positive (N1–N2) | 28 | 19 | 5.421 | 2.932–10.022 | 15 | 2.893 | 1.555–5.385 | |||||
| Histological grade | Low grade | 56 | 4 | 0.0215* | 1 | – | 0.045* | 3 | 0.0027* | 1 | – | 0.158 |
| High grade | 284 | 57 | 2.765 | 1.021–7.488 | 67 | 1.781 | 0.799–3.967 | |||||
| Vascular invasion | Absent | 234 | 24 | <0.0001* | 1 | – | 0.204 | 26 | <0.0001* | 1 | – | 0.002* |
| Present | 106 | 37 | 1.478 | 0.809–2.700 | 44 | 2.539 | 1.403–4.595 | |||||
| Perineural invasion | Absent | 321 | 50 | <0.0001* | 1 | – | <0.001* | 61 | <0.0001* | 1 | – | 0.027* |
| Present | 19 | 11 | 3.970 | 1.907–8.265 | 9 | 2.310 | 1.102–4.842 | |||||
| Mitotic rate (per 10 high-power fields) | <10 | 173 | 27 | 0.167 | – | – | 30 | 0.0823 | – | - | ||
| FBLN2 expression | ≥10 | 167 | 34 | – | – | 40 | – | – | ||||
| Low | 170 | 11 | <0.0001* | 1 | – | 0.012* | 14 | <0.0001* | 1 | – | 0.001* | |
| High | 170 | 50 | 2.561 | 1.219–4.968 | 56 | 2.837 | 1.504–5.349 | |||||
Statistically significant.
Figure 4.
Kaplan–Meier plots show that FBLN2 overexpression conferred significant prognostic effects on the disease-specific survival and metastasis-free survival of upper tract urothelial carcinoma (A,B, respectively) and urinary bladder urothelial carcinoma (C,D, respectively) patients.
In UBUC (Table 4) patients with high FBLN2-expressing tumors, 39.9% had tumor metastasis and 27.0% died of the disease, whereas only 11.6% of patients with low FBLN2-expressing tumors had subsequent metastatic tumors and 8.2% experienced cancer deaths. Following univariate analysis, we observed that advanced pT status, lymph node metastasis, high histological grade, VI, PNI, high mitotic activity, and high FBLN2 expression (Figures 4C,D; all p < 0.0001) were associated with worse DSS and MFS. In the multivariate analysis, FBLN2 expression status (HR, 2.306; 95% CI, 1.185–4.489; p = 0.014), pathologic stage, and mitotic activity were markedly associated with DSS. In addition, high FBLN2 expression (HR, 2.493; 95% CI, 1.427–4.353; p = 0.001), high pathologic stage, and high mitotic activity significantly correlated with worse MFS.
Table 4.
Univariate log-rank and multivariate analyses for disease-specific and metastasis-free survivals in urinary bladder urothelial carcinoma.
| Parameter | Category | Case No. | Disease-specific survival | Metastasis-free survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| No. of event | p-value | RR | 95% CI | p-value | No. of event | p-value | RR | 95% CI | p-value | |||
| Gender | Male | 216 | 41 | 0.4446 | – | – | – | 60 | 0.2720 | – | – | – |
| Female | 79 | 11 | – | – | – | 16 | – | – | – | |||
| Age (years) | <65 | 121 | 17 | 0.1136 | – | – | – | 31 | 0.6875 | – | – | – |
| ≥65 | 174 | 35 | – | – | – | 45 | – | – | – | |||
| Primary tumor (T) | Ta | 84 | 1 | <0.0001* | 1 | – | <0.001* | 4 | <0.0001* | 1 | – | 0.006* |
| T1 | 88 | 9 | 4.908 | 0.532–45.247 | 23 | 3.922 | 1.132–13.597 | |||||
| T2–T4 | 123 | 42 | 22.382 | 2.587–193.647 | 49 | 6.639 | 1.943–22.693 | |||||
| Nodal metastasis | Negative (N0) | 266 | 41 | 0.0002* | 1 | – | 0.489 | 61 | <0.0001* | 1 | – | 0.060 |
| Positive (N1–N2) | 29 | 11 | 1.283 | 0.634–2.598 | 15 | 1.816 | 0.975–3.381 | |||||
| Histological grade | Low grade | 56 | 2 | 0.0013* | 1 | – | 0.911 | 5 | 0.0007* | 1 | – | 0.907 |
| High grade | 239 | 50 | 1.094 | 0.226–5.282 | 71 | 0.937 | 0.316–2.782 | |||||
| Vascular invasion | Absent | 246 | 37 | 0.0024* | 1 | – | 0.121 | 54 | 0.0001* | 1 | – | 0.845 |
| Present | 49 | 15 | 0.581 | 0.293–1.155 | 22 | 0.941 | 0.513–1.727 | |||||
| Perineural invasion | Absent | 275 | 44 | 0.0001* | 1 | – | 0.075 | 66 | 0.0007* | 1 | – | 0.252 |
| Present | 20 | 8 | 2.122 | 0.927–4.854 | 10 | 1.531 | 0.739–3.174 | |||||
| Mitotic rate (per 10 high-power fields) | <10 | 139 | 12 | <0.0001* | 1 | – | 0.023* | 23 | <0.0001* | 1 | – | 0.050* |
| FBLN2 expression | ≥ 10 | 156 | 40 | 2.190 | 1.115–4.305 | 53 | 1.677 | 0.999–2.815 | ||||
| Low | 147 | 12 | 0.0001* | 1 | – | 0.014* | 17 | <0.0001* | 1 | – | 0.001* | |
| High | 148 | 40 | 2.306 | 1.185–4.489 | 59 | 2.493 | 1.427–4.353 | |||||
Statistically significant.
Pathway, Functional Enrichment, and Interaction Network Analyses of FBLN2
The selection criteria of the differentially expressed genes included the top 200 most significant positively and negatively correlated genes to FBLN2 expression in TCGA BLCA. Detailed information on the 400 differentially expressed genes is provided in Supplementary Tables 1,2. To further examine the associated gene signatures and functions of FBLN2 in UC, these genes were analyzed by IPA. The data demonstrate the enrichment conditions of differentially expressed genes in canonical signaling pathways. Multiple signaling pathways were enriched, including oxidative phosphorylation, mitochondrial dysfunction, sirtuin signaling pathway, and regulation of the epithelial–mesenchymal transition pathway. The IPA results identified CCR2, TGFB1, and TWIST1 among the top upstream regulators.
Additionally, in the disease and functional enrichment analyses, we identified that FBLN2 may induce the alteration of numerous biological functions and diseases, such as cancer, organismal injury and abnormalities, connective tissue disorders, tissue development and cellular assembly and organization, and cellular function and maintenance. Subsequently, the differentially expressed genes were categorized into 28 networks. The top 3 networks had higher scores and more than 25 focused molecules. Genes encoding the collagen family proteins, including COL10A1, COL14A1, COL16A1, COL1A2, COL3A1, COL5A1, COL5A2, COL6A1, COL6A2, COL6A3, COL8A1, COL8A2, OL1A2, and COL3A1, along with 13 other dysregulated genes were involved in diseases and functions related to cancer, connective tissue disorders, and organismal injury and abnormalities, whereas 25 dysregulated genes, including ADAM12, ADAMTS10, and ADAMTS2, were involved in diseases and functions related to connective tissue disorders, hereditary disorders, and organismal injury and abnormalities. In addition, 9 nuclear genes encoding mammalian mitochondrial ribosomal proteins and 20 other dysregulated genes were involved in diseases and functions associated with metabolic diseases, organismal injury and abnormalities, and protein synthesis.
Discussion
UC is a common and heterogeneous malignancy in the urinary system. Until recently, treatment for UC had seen little progress, and survival rates did not improve significantly over the last three decades. Therefore, the discovery of novel prognostic biomarkers remains an urgent concern in order to improve the clinical outcomes of UC patients. With advances in high-throughput technologies, many scholars have investigated the gene expression levels of UC samples and uploaded these data to public databases, such as GEO and TCGA (27, 28). These data offer chances for biomarker discovery, validation, and clinical application.
Using a public domain dataset (GSE31684), we demonstrated that FBLN2 was a significantly upregulated gene related to the ECM structural constituent and associated with advanced tumor stage and metastatic disease in UBUC. The Oncomine database also revealed that infiltrating UBUC expressed significantly higher FBLN2 than superficial tumors. Furthermore, the prognostic impact of FBLN2 immunoexpression was validated in our well-characterized UC cohort. We demonstrated that FBLN2 expression status was independently predictive for both MFS and DSS in UTUC and UBUC. In addition, high FBLN2 expression was significantly associated with adverse pathologic tumor characteristics, such as advanced pathologic tumor stage, high histological tumor grade, lymph node metastasis, VI, PNI, and high mitotic rate. These results indicate that FBLN2 plays a key role in UC development and progression. Thus, incorporation of FBLN2 immunoexpression with standard pathologic and prognostic parameters may improve UC risk stratification.
Radical operation is the only curative therapy for UC. However, cancer metastasis and progression frequently occur over time, resulting in patient mortality (3–5). Deregulated ECM dynamics play important roles in cancer invasiveness and progression (29, 30). The ECM consists of a complex network of highly modified macromolecules, including fibrous proteins, proteoglycans, glycosaminoglycans, and glycoproteins, with different physical and biochemical properties (29, 30). It not only supports tissue architecture and integrity but also modulates and regulates cell functions such as adhesion, migration, proliferation, and differentiation (29, 30). Abnormal ECM dynamics result in cellular dysfunctions that promote cellular transformation and metastasis (29, 30). Fibulins, a family of glycoproteins, are related to elastic ECM fibers and basement membranes. They severed as a scaffold for the ECM (11–13). FBLN2 can form homodimeric complexes and bind to many ECM components, such as integrin, fibronectin, and laminin, to stabilize the organization of ECM structures (10–13). Therefore, we selected FBLN2 for further study.
Depending on the tissue context, FBLN2 may act as either a tumor promoter or a tumor suppressor in different cancer models (15–20). For example, in lung cancer, Baird et al. demonstrated that FBLN2 can drive malignant progression and promote tumor cell adherence to collagen and collagen cross-linking (15). They found that FBLN2 was abundant in the ECM of human lung cancer. Knockdown of the FBLN2 gene in lung cancer cells inhibited tumor growth and lymph node metastatic properties in a xenograft and orthotopic 129/SV mice model. However, in NPCs, Law et al. found that FBLN2 was a tumor suppressor and had antiangiogenic effects (20). FBLN2 was downregulated in NPCs but highly expressed in adjacent normal tissues. Exogenous expression of the FBLN2 gene in NPCs suppressed cell proliferation, migration, invasion, and angiogenesis in vitro. FBLN2 re-expression also inhibited tumor growth and angiogenesis in vivo (20). However, the roles of FBLN2 in UC remain unclear.
In the present study, we demonstrated that high FBLN2 immunoexpression was significantly associated with aggressive UTUC and UBUC characteristics, such as high tumor stage and grade, PNI, VI, lymph node metastasis, and high mitotic rate, suggesting important roles of FBLN2 in UC progression and metastasis. These findings have some clinical applications. In NMIBC, it is critical to recognize the high-risk patients who will progress to MIBC (3, 4). FBLN2 immunoexpression status may serve as a useful marker to classify these patients because high FBLN2 expression is significantly related to muscle invasiveness and high-grade UBUC. For low-risk UTUC, kidney-sparing management may be a primary treatment without compromising oncological outcomes (5). However, in ureteroscopic biopsy tumors with high FBLN2 expression, radical nephroureterectomy with bladder cuff excision should be suggested. High FBLN2 immunoactivity was observed in high-grade, advanced-stage, and lymph node metastatic UTUC. For advanced or metastatic UC, cisplatin-based combination chemotherapy or checkpoint inhibitor immunotherapy has survival benefits (4, 5), suggesting the need for early identification of patients with progressive and metastatic potential. Here, we showed that UC patients with high FBLN2 expression and aggressive pathologic features were more likely to develop distant metastasis. These findings may accelerate early recognition and aggressive UC management, such as early radical surgery, systemic chemotherapy, or immunotherapy.
Although the exact mechanism underlying the role of FBLN2 in UC progression has not been well-studied, its expression may indicate a distinctive subtype in UC with aggressive characteristics and worse oncological prognosis. Several metabolic altered pathways are involved in UC tumorigenesis and progression, representing potential therapeutic targets (31). Using the IPA database, we identified pivotal canonical pathways according to the differential gene expressions. Among the predicted pathways, oxidative phosphorylation and mitochondrial dysfunction were the top pathways with a significant correlation, suggesting critical roles of FBLN2 in metabolic changes and UC progression. Moreover, FBLN2 was also significantly associated with the sirtuin signaling pathway, which is involved in epithelial to mesenchymal transition promotion and UC progression (32). Therefore, further studies are necessary to elucidate the exact molecular mechanisms underlying FBLN2 effects on these pathways in UC.
There are some limitations in our research. First, this is a single-institution retrospective study. Second, the precise molecular mechanism resulting in disease progression and adverse outcomes in FBLN2-overexpressing UC has not been well-clarified. Third, no standardized immunostaining and scoring scheme for FBLN2 expression is currently available. In this study, we followed the standard protocol of immunostaining and estimated both the intensity and percentage of positively stained UC cells to yield an H-score on account of its excellent correlation to immunoblots (33). In addition, two independent pathologists evaluated all slides to reduce discrepancy and come to a consensus for every case. Finally, prospective multicenter studies are necessary to verify our findings.
In conclusion, we demonstrated that high FBLN2 immunoexpression is associated with aggressive tumor characteristics and independent prognostication of worse oncological outcomes in a large, well-characterized UC cohort. Thus, FBLN2 may serve as a potential prognostic biomarker to identify high-risk UC patients for individualized therapy.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB10501-005) of Chi Mei Medical Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
W-ML, Y-LS, and C-FL: study concept and design. T-CC, SH, P-IL, and Y-CW: acquisition of data. H-LK and C-FL: analysis and interpretation of data. W-ML, Y-LS, and C-FL: drafting of the manuscript. Y-LS and C-FL: critical revision of the manuscript for important intellectual content. W-ML and C-FL: statistical analysis. All authors agreed to be accountable for the content of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by Kaohsiung Medical University Hospital, Taiwan (KMUH105-5R48, KMUH106- 6R56), Kaohsiung Medical University Research Center Grant, Taiwan (KMU-TC108A02, KMU-TC108B07), Kaohsiung Medical University, Taiwan (KMU-KI109002), NSYSU-KMU Joint Research Project, Taiwan (NSYSUKMU109-I008-3), and Ministry of Science and Technology, Taiwan (MOST109-2314-B-037-110-MY3).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.570340/full#supplementary-material
Oncomine database illustration of FBLN2 expression in Sanchez-Carbayo Bladder 2.
Oncomine database illustration of FBLN2 expression in Blaveri Bladder 2.
IPA. Top 200 most significant positively correlated genes to FBLN2 expression in bladder cancer.
IPA. Top 200 most significant negatively correlated genes to FBLN2 expression in bladder cancer.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M, Burger M, Comperat EM, Gontero P, Mostafid AH, Palou J, et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and Carcinoma In Situ) - 2019 update. Eur Urol. (2019) 76:639–57. 10.1016/j.eururo.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 4.Alfred Witjes J, Lebret T, Comperat EM, Cowan NC, De Santis M, Bruins HM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. (2017) 71:462–75. 10.1016/j.eururo.2016.06.020 [DOI] [PubMed] [Google Scholar]
- 5.Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, Burger M, et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol. (2018) 73:111–22. 10.1016/j.eururo.2017.07.036 [DOI] [PubMed] [Google Scholar]
- 6.Kamoun A, de Reynies A, Allory Y, Sjödahl G, Robertson AG, Seiler R, et al. A consensus molecular classification of muscle-invasive bladder cancer. Eur Urol. (2020) 77:420–33. 10.1016/j.eururo.2019.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 8.Buchheit CL, Weigel KJ, Schafer ZT. Cancer cell survival during detachment from the ECM: multiple barriers to tumour progression. Nat Rev Cancer. (2014) 14:632–41. 10.1038/nrc3789 [DOI] [PubMed] [Google Scholar]
- 9.Buchheit CL, Rayavarapu RR, Schafer ZT. The regulation of cancer cell death and metabolism by extracellular matrix attachment. Semin Cell Dev Biol. (2012) 23:402–11. 10.1016/j.semcdb.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 10.Pan TC, Sasaki T, Zhang RZ, Fässler R, Timpl R, Chu ML. Structure and expression of fibulin-2, a novel extracellular matrix protein with multiple EGF-like repeats and consensus motifs for calcium binding. J Cell Biol. (1993) 123:1269–77. 10.1083/jcb.123.5.1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Vega S, Iwamoto T, Yamada Y. Fibulins: multiple roles in matrix structures and tissue functions. Cell Mol Life Sci. (2009) 66:1890–902. 10.1007/s00018-009-8632-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Argraves WS, Greene LM, Cooley MA, Gallagher WM. Fibulins: physiological and disease perspectives. EMBO Rep. (2003) 4:1127–31. 10.1038/sj.embor.7400033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timpl R, Sasaki T, Kostka G, Chu ML. Fibulins: a versatile family of extracellular matrix proteins. Nat Rev Mol Cell Biol. (2003) 4:479–89. 10.1038/nrm1130 [DOI] [PubMed] [Google Scholar]
- 14.Obaya AJ, Rua S, Moncada-Pazos A, Cal S. The dual role of fibulins in tumorigenesis. Cancer Lett. (2012) 325:132–8. 10.1016/j.canlet.2012.06.019 [DOI] [PubMed] [Google Scholar]
- 15.Baird BN, Schliekelman MJ, Ahn YH, Chen Y, Roybal JD, Gill BJ, et al. Fibulin-2 is a driver of malignant progression in lung adenocarcinoma. PLoS One. (2013) 8:e67054. 10.1371/journal.pone.0067054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Senapati S, Gnanapragassam VS, Moniaux N, Momi N, Batra SK. Role of MUC4-NIDO domain in the MUC4-mediated metastasis of pancreatic cancer cells. Oncogene. (2012) 31:3346–56. 10.1038/onc.2011.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaturvedi P, Singh AP, Moniaux N, Senapati S, Chakraborty S, Meza JL, et al. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res. (2007) 5:309–20. 10.1158/1541-7786.MCR-06-0353 [DOI] [PubMed] [Google Scholar]
- 18.Tan H, Zhang J, Fu D, Zhu Y. Loss of fibulin-2 expression is involved in the inhibition of breast cancer invasion and forms a new barrier in addition to the basement membrane. Oncol Lett. (2017) 14:2663–68. 10.3892/ol.2017.6539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi CH, Smith DJ, West WW, Hollingsworth MA. Loss of fibulin-2 expression is associated with breast cancer progression. Am J Pathol. (2007) 170:1535–45. 10.2353/ajpath.2007.060478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Law EW, Cheung AK, Kashuba VI, Pavlova TV, Zabarovsky ER, Lung HL, et al. Anti-angiogenic and tumor-suppressive roles of candidate tumor-suppressor gene, Fibulin-2, in nasopharyngeal carcinoma. Oncogene. (2012) 31:728–38. 10.1038/onc.2011.272 [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Carbayo M, Socci ND, Lozano J, Saint F, Cordon-Cardo C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J Clin Oncol. (2006) 24:778–89. 10.1200/JCO.2005.03.2375 [DOI] [PubMed] [Google Scholar]
- 22.Blaveri E, Simko JP, Korkola JE, Brewer JL, Baehner F, Mehta K, et al. Bladder cancer outcome and subtype classification by gene expression. Clin Cancer Res. (2005) 11:4044–55. 10.1158/1078-0432.CCR-04-2409 [DOI] [PubMed] [Google Scholar]
- 23.Eble JN, Sauter G, Epstein JI. editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press: (2004). [Google Scholar]
- 24.Edge SB, Byrd DR, Compton CC. editors. AJCC Cancer Staging Manual. 7th ed American Joint Committee on Cancer. Chicago, IL: Springer-Verlag: (2010). [Google Scholar]
- 25.Li WM, Huang CN, Ke HL, Li CC, Wei YC, Yeh HC, et al. MCM10 overexpression implicates adverse prognosis in urothelial carcinoma. Oncotarget. (2016) 7:77777–92. 10.18632/oncotarget.12795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HY, Li CC, Huang CN, Li WM, Yeh HC, Ke HL, et al. INHBA overexpression indicates poor prognosis in urothelial carcinoma of urinary bladder and upper tract. J Surg Oncol. (2015) 111:414–22. 10.1002/jso.23836 [DOI] [PubMed] [Google Scholar]
- 27.Netto GJ. Molecular biomarkers in urothelial carcinoma of the bladder: are we there yet? Nat Rev Urol. (2011) 9:41–51. 10.1038/nrurol.2011.193 [DOI] [PubMed] [Google Scholar]
- 28.Choi W, Ochoa A, McConkey DJ, Aine M, Höglund M, Kim WY, et al. Genetic alterations in the molecular subtypes of bladder cancer: illustration in the cancer genome atlas dataset. Eur Urol. (2017) 72:354–65. 10.1016/j.eururo.2017.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. (2012) 196:395–406. 10.1083/jcb.201102147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. (2014) 15:786–801. 10.1038/nrm3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woolbright BL, Ayres M, Taylor JA, III. Metabolic changes in bladder cancer. Urol Oncol. (2018) 36:327–37. 10.1016/j.urolonc.2018.04.010 [DOI] [PubMed] [Google Scholar]
- 32.Monteiro-Reis S, Lameirinhas A, Miranda-Goncalves V, Felizardo D, Dias PC, Oliveira J, et al. Sirtuins' deregulation in bladder cancer: SIRT7 is implicated in tumor progression through epithelial to mesenchymal transition promotion. Cancers. (2020) 12:E1066. 10.3390/cancers12051066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan CH, Li CF, Yang WL, Gao Y, Lee SW, Feng Z, et al. The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis. Cell. (2012) 149:1098–111. 10.1016/j.cell.2012.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Oncomine database illustration of FBLN2 expression in Sanchez-Carbayo Bladder 2.
Oncomine database illustration of FBLN2 expression in Blaveri Bladder 2.
IPA. Top 200 most significant positively correlated genes to FBLN2 expression in bladder cancer.
IPA. Top 200 most significant negatively correlated genes to FBLN2 expression in bladder cancer.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.