Abstract
Introduction
Traditional Chinese medicine (TCM) has been fully committed to the treatment of coronavirus disease 2019 (COVID-19) in China. An increasing number of clinical trials have been registered to evaluate the effects of TCM for COVID-19. The aim of this study was to review the existing TCM clinical trial registrations and identify potentially promising and available TCM therapies, in order to provide a reference for the global management of COVID-19.
Methods
All clinical trials on TCM for COVID-19 registered in registry platforms worldwide were searched. The data of registration temporal trend, design, objective, interventions, and relevant information were reviewed and summarized.
Results
161 TCM trials were identified from three registries (January 26 to May 14 2020,). Of these, 94 (58.4%) were randomized controlled trials and 114 trials (70.8%) assessed therapeutic effects; while the remainder focused on prevention, rehabilitation, and the epidemiology of TCM syndromes. Eight trials (5.0%) had completed their recruitment. TCM interventions with potential for further evaluation in terms of prevention were moxibustion, Huoxiang Zhengqi pill and Jinye Baidu granules. For treatment of COVID-19, Qingfei Paidu decoction, Huashi Baidu decoction, Lianhua Qingwen capsules, Toujie Quwen granules and Xiyanping injection, and Xuebijing injection were to be tested for their therapeutic effects and symptoms relief. For rehabilitation, Tai Chi and Liuzijue were to be tested for improving patients’ lung function.
Conclusion
Some potentially promising TCM interventions have been identified and deserve further evaluation to establish their evidence base, particularly on populations outside of China.
Keywords: COVID-19 drug treatment, Medicine, Chinese medicine, Randomized controlled trial, Clinical trial protocol, Qingfei paidu decoction, Lianhua Qingwen capsules, Scoping review
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has become a major public health problem facing the world. As of October 20, 2020, 40,251,950 cases of confirmed infections had been reported from 212 countries, areas or territories around the world [1]. Since the outbreak in Hubei, China in January 2020, the Chinese government established a prevention and control policy involving both traditional Chinese medicine (TCM) and Western medicine (WM) [2]. Therefore, TCM has been fully involved in the management of the epidemic in China. Up to now, the National Health Commission of China has issued eight versions of guidelines for COVID-19, and TCM remedies have been included since the third version in January 23, 2020 [3]. For prevention of the epidemic, 23 out of 31 provinces (including autonomous regions, and municipalities) in mainland China had officially issued preventive programs by recommending Chinese herbal formulae [4]. According to official data released in 23 April, among the confirmed COVID-19 cases, 74,187 were given TCM, accounting for 91.5% of the total cases [5].
At present, China has achieved a staged success in the management of COVID-19 for more than 6 consecutive months. As of October 20, 2020, among the 85,715 confirmed cases in mainland China, 79,047 have been cured and discharged from hospitals, accounting for 93.6%, and 4634 have died, accounting for 5.5% [6]. The lower number of cases and deaths of COVID-19 reported in China compared to many countries may be due to the strict implementation of public health interventions (including isolating cases, contact tracing, quarantine of exposed persons, travel restrictions, school and workplace closures, cancelation of mass gatherings, and hand washing, among others) [7], In addition, the rapid establishment of large health-care facilities (Fangcang shelter hospitals in Wuhan) which left no patient unattended [8], and the combination of TCM and WM are believed to have played a substantial role in prevention and treatment, and this has attracted increasing attention worldwide [2]. In order to evaluate the effectiveness and safety of specific TCM therapies such as Chinese patent medicines, formulae and non-drug therapy, many clinical trials have been registered and carried out in China since the outbreak. These studies are greatly heterogeneous in terms of interventions, design, recruiting status, feasibility, and generalizability [9]. Therefore, this study was conducted to comprehensively collect and evaluate the characteristics in terms of participants, interventions and outcomes of existing TCM registered clinical trials for COVID-19, aiming to providing a reference for the global epidemic prevention and control.
2. Methods
2.1. Data source and search strategy
All the TCM clinical trial protocols on COVID-19 registered before May 14 2020, from nine trial registry platforms were retrieved. There was no limitation on study objectives, design, TCM interventions or outcomes. These platforms were: Chinese Clinical Trial Registry (ChiCTR) (http://www.chictr.org.cn), ClinicalTrials.gov (http://clinicalTrials.gov), Acupuncture-Moxibustion Clinical Trial Registry (AMCTR) (http://www.acmctr.org/index.aspx), Australian New Zealand Clinical Trials Registry (http://www.anzctr.org.au), Japan Primary Registries Network (https://jrct.niph.go.jp), the United Kingdoms’ ISRCTN registry (http://www.isrctn.com), Clinical Trials Registry-India (http://ctri.nic.in), EU Clinical Trials Register (https://www.clinicaltrialsregister.eu), and WHO International Clinical Trials Registry Platform (https://www.who.int/ictrp/en/). All the above platforms are members of the WHO Registry Network, two (ChiCTR and AMCTR) are based in China and the rest are international. The search terms included COVID-19, coronavirus disease 2019, 2019-nCoV, novel coronavirus, and SARS-CoV-2. The registered TCM trials were identified by screening the title or content of the search results. If a COVID-19 collection was available from their websites (for example ChiCTR and ClinicalTrials.gov), TCM trials were screened.
Supportive information related to these registered TCM trials were searched to identify trial publications on PubMed, Embase, Google Scholar, China National Knowledge Infrastructure (CNKI), Wanfang Data, and government websites or official media websites in China. The search terms were the same as the search on the trial registry platforms.
2.2. Data extraction and analysis
The basic characteristics of registered TCM trials were extracted, including registration identifier, date of registration, title, country and province, design, study objectives, anticipated start date, interventions and control, population, sample size, outcomes and recruiting status. The overall status of registered TCM trials on COVID-19 was reviewed and collated. The study progress or results were extracted from the documentation where possible. Trials with an appropriate design and with a potential global application were screened and data were extracted and summarized. The inclusion criteria for trials were defined as follows: (1) those with a prospective design and important outcomes including symptom improvement, virus shedding, length of hospitalization, and fatality rate; (2) those testing a intervention with a reasonable rationale for safety and effectiveness, with existing evidence of human studies on COVID-19, pneumonia, severe acute respiratory syndrome (SARS), influenza, and other viral diseases, or included in China's guideline of COVID-19 [3]; (3) interventions having the possibility of being available and applicable globally. The extraction and assessment of data characteristics were due on July 31, 2020.
3. Results
3.1. Numbers of registered TCM trials
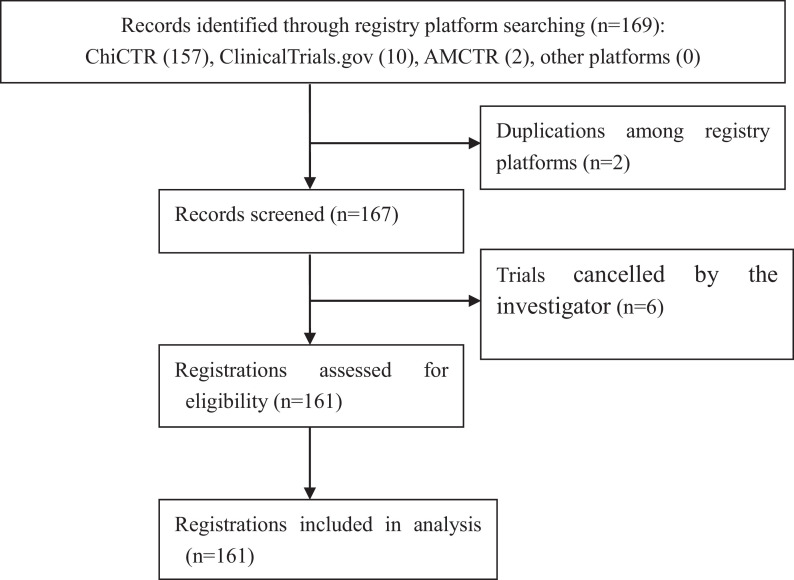
169 registered TCM clinical trials on COVID-19 were initially identified from three registries. The majority (157, 92.9%) were registered in ChiCTR, the representative registry of China on the WHO International Clinical Trial Registration Platform, accounting for 24.0% (157/653) of all trials on COVID-19 up to May 14, and the remaining 76.0% were registered trials for western medicine. Ten trials (5.9%) were registered on ClinicalTrials.gov, and 2 (1.2%) in AMCTR. No records were found from other registries. Eight trials were excluded after screening the full text of protocols, of which six (ChiCTR2000030118; ChiCTR2000030168; ChiCTR2000030478; ChiCTR2000030483; ChiCTR2000030762; ChiCTR2000030765) were withdrawn by the investigators; four (ChiCTR2000030420 & ChiCTR2000030467; ChiCTR2000030382 & AMCTR-OCN-20000332) were duplicate registrations, and the more recently registered trial was included. Therefore, 161 registered trials were finally included in this study. The flowchart of registered TCM trial registration retrieval is shown in Fig. 1 . The list of the 161 trials is provided in the Supplementary Material.
Fig. 1.
Flowchart of registered TCM trial registration searching and selection.
3.2. Characteristics of registered TCM clinical trials
All of the registered TCM trials were planned to be conducted in China, involving a total sample size of 58,501 (range: 20 to 20,000; average: 404; median: 132). In terms of the geographical distribution, 65 trials were conducted in Hubei province, the hardest hit area of the COVID-19 pandemic; 21 multi-center trials were conducted simultaneously in Hubei and other provinces of China; 72 trials were to be conducted outside of Hubei; while three trials did not report their geographic area. The first protocol of a TCM clinical study (NCT04285190) on COVID-19 was registered on January 26, 2020, and the most recent one was on May 9. The number of the registrations on February 1 was the largest, up to May 9. The number of newly confirmed cases has dropped significantly since February 17, with a total of 11,683 cases reported, accounting for 14.1% (11683/82918) of all cases; while during this period, 91 trials were registered, accounting for 56.5% (91/161) of all trials. Eight trials (ChiCTR2000029433, ChiCTR2000029434, ChiCTR2000029479, ChiCTR2000029778, ChiCTR2000030619, ChiCTR2000032717, and ChiCTR2000032767, NCT04306497) had completed participant recruitment. 76 trials were still on-going. The trends of newly registered TCM trials and confirmed cases of COVID-19 from January 26 to May 14 are presented in Supplementary Material.
All trials were divided into preventive, therapeutic, and rehabilitation studies according to the main purpose. 103 trials were single-center studies, while 58 trials were multi-center studies. The studies were divided into six categories: randomized controlled trials (RCTs), controlled clinical trials (CCTs), single-arm trials, real world clinical studies, retrospective studies, and cross-sectional studies. In those real world clinical studies, the severity of disease in participants, interventions and course of treatment were not predetermined, but were determined by doctors based on actual conditions. In the cross-sectional studies, TCM syndromes were investigated among patients with COVID-19. Participants involving in clinical trials included suspected cases in preventive trials, confirmed patients with different severity (included mild, moderate, severe and critical types) in therapeutic trials, as well as cured and discharged patients in rehabilitation trials. TCM interventions included Chinese patent medicine, herbal decoction or granules, Chinese herbal-derived injections, and non-pharmaceutical therapy. In all therapeutic trials, western convention treatment based on the guideline was used in both treatment group and control group. The details on the characteristics of registered TCM trials including study design, objectives, participants, and sample size, interventions, outcome measure, and current status are shown in Table 1 .
Table 1.
The characteristics of registered TCM trials on COVID-19 (as of July 31, 2020).
| Items | Details | Number of trials | Percent (%) |
|---|---|---|---|
| Study type | Randomized controlled trials | 94 | 58.4% |
| Non-randomized controlled clinical trials | 25 | 15.5% | |
| Single-arm trials | 18 | 11.2% | |
| Real world clinical studies | 10 | 6.2% | |
| Retrospective studies | 10 | 6.2% | |
| Cross-sectional studies | 4 | 2.5% | |
| Objective | Preventive trial | 12 | 7.5% |
| Therapeutic trial | 114 | 70.8% | |
| Rehabilitation trial | 23 | 14.3% | |
| Study on TCM syndrome epidemiology | 4 | 2.5% | |
| Multi-objective | 8 | 5.0% | |
| Sample size | ≤100 | 63 | 39.1% |
| 101–300 | 65 | 40.4% | |
| 301–500 | 16 | 9.9% | |
| 501–1000 | 11 | 6.8% | |
| >1000 | 6 | 3.7% | |
| Participants (in 114 therapeutic trials) | Suspected cases*1 | 10 | 8.8% |
| Mild or moderate patients | 55 | 48.2% | |
| Severe patients | 32 | 28.1% | |
| Critical patients | 3 | 2.6% | |
| Confirmed patients (no details on severity) | 39 | 34.2% | |
| TCM Interventions | Chinese patent medicine | 40 | 24.8% |
| Decoction or granules of fixed formulae | 50 | 31.1% | |
| Decoction or granules based on TCM syndrome differentiation | 17 | 10.6% | |
| Chinese herbal-derived injections | 13 | 8.1% | |
| TCM exercises (Qigong/Daoyin/Liuzijue/ Tai Chi/Baduancao) | 12 | 7.5% | |
| Moxibustion | 5 | 3.1% | |
| Acupuncture/acupoint stimulation | 4 | 2.5% | |
| TCM psychological intervention | 1 | 0.6% | |
| TCM interventions with no details | 36 | 22.4% | |
| Comparisons (in 157 interventional studies) | TCM plus WM vs WM | 75 | 47.8% |
| Single arm | 28 | 17.8% | |
| Multiple comparisons | 14 | 8.9% | |
| TCM vs WM*2 | 10 | 6.4% | |
| TCM vs no intervention (in preventive study) | 11 | 7.0% | |
| TCM plus WM vs Placebo plus WM | 9 | 5.7% | |
| TCM vs Placebo (in rehabilitation study) | 7 | 4.5% | |
| Others | 3 | 1.9% | |
| Outcome measure (in 114 therapeutic trials) | Relief on symptoms or signs | 76 | 65.8% |
| Nucleic acid PCR test | 59 | 50.9% | |
| Case fatality / survival rate | 21 | 16.7% | |
| Length of stay | 37 | 31.6% | |
| Moderate-severe conversion rate | 44 | 37.7% | |
| Improvement in chest CT manifestations | 51 | 43.9% | |
| Lung function / lung injury score / oxygenation index | 30 | 26.3% | |
| Adverse events | 17 | 14.9% | |
| Primary sponsor | TCM hospital | 75 | 46.6% |
| TCM college | 14 | 8.7% | |
| TCM research institution | 10 | 6.2% | |
| WM hospital | 51 | 31.7% | |
| WM college | 1 | 0.6% | |
| Military hospital | 2 | 1.2% | |
| Pharmaceutical company | 6 | 3.7% | |
| Public health institution | 2 | 1.2% | |
| Recruitment status | Have not started recruiting | 74 | 46.0% |
| Have started recruiting | 76 | 47.2% | |
| Completed | 8 | 5.0% | |
| Suspended/withdrawn | 3 | 1.9% |
Note: 1. Suspected case is defined as a patient with a history of epidemiology and clinical manifestations, yet to be confirmed by etiological or serological tests according to China's guideline of COVID-19 [3].
2. TCM: traditional Chinese medicine; WM: western medicine, defined as conventional treatment according to China's guideline of COVID-19, including symptomatic treatment, antiviral drugs, antibiotics, respiratory and circulatory support, etc [3].
3.3. Registered clinical trials and interventions with potential value
3.3.1. Trials of preventive interventions
Twelve registered trials were preventive studies, including six RCTs, three CCTs, two single-arm studies, and one retrospective study. Participants included the general population in two trials and high-risk populations in close contact with confirmed cases in 10 trials. The sample sizes ranged from 80 to 20,000, with a total of 28,766, average of 2398, and median of 550. Preventive interventions included six Chinese patent medicines, moxibustion, and herbal decoction. Among 10 controlled studies, nine had no intervention in the control group and one used placebo. 58.3% (7/12) of trials reported the infection rate diagnosed by nucleic acid PCR test as the primary outcome.
Of these 12 trials, three therapies in five of the trials may have potential value given previous clinical studies and their inclusion in relevant guidelines or by expert consensus:
-
(1)
Moxibustion was recommended by the China Association of Acupuncture-Moxibustion [10] and tested in two registered trials including one RCT (Registration number: AMCTR-IPR-20000326) and one case series study (ChiCTR2000030386). However, both were assessing relief in scores of emotion and symptom scale, rather than infection rate by nucleic acid PCR test.
-
(2)
Huoxiang Zhengqi pill (HXZQ) was recommended in the guideline for COVID-19 (fourth version) issued by the National Health Commission of China for suspected cases under medical observation with symptoms of fatigue and gastrointestinal discomfort [3]. Two RCTs (ChiCTR2000029602 and ChiCTR2000029479) tested HXZQ and planned a comparison with health education and no intervention in high-risk populations or healthy people in the community. Both used the incidence of COVID-19 based on nucleic acid PCR test as the primary outcome, and the sample sizes were 600 and 20,000 respectively. The study of ChiCTR2000029479 was published in China Journal of Chinese Materia Medica on April 30, 2020, showing that the incidence rate of cold in TCM group combined use of HXZQ and another Chinese patent medicine (10,627 cases) was significantly lower than that in non-intervention group (10,972 cases) (0.02% versus 0.23%, P<0.001), no confirmed COVID-19 case occurred in either group [11].
-
(3)
Jinye Baidu granules (JYBD) were officially recommended for preventing COVID-19 in some provinces of China [4]. JYBD was developed by Tongji hospital, Huazhong University of Science and Technology, and had been used for preventing SARS in Tongji Hospital in 2003 [12]. Some experimental and clinical studies found that JYBD had an antiviral effect [13], [14], [15]. The details of the above interventions and trials are shown in Table 2 .
Table 2.
Characteristics of TCM preventive interventions and trials worthy of attention.
| Intervention | Category | Trials | Rationality | Ingredients |
|---|---|---|---|---|
| Moxibustion | TCM external treatment | AMCTR-IPR-20,000,326 | Included in guideline | Artemisiae Argyi Folium (Aiye) |
| Huoxiang Zhengqi pill | Chinese patent medicine | ChiCTR2000029602 ChiCTR2000029479 |
Included in guideline | Pericarpium Arecae (Dafupi), Radix Angelicae Dahuricae (Baizhi), Caulis Perillae (Zisu), Poria (Fuling), Rhizoma Pinelliae Preparata (Banxiaqu), Atractylodis Macrocephalae Rhizoma (Baizhu), Pericarpium Citri Reticulatae (Chenpi), Cortex Magnoliae Officinalis (Houpo), Radix Platycodi (Jiegeng), Pogostemonis Herba (Huoxiang), Glycyrrhizae Radix Et Rhizoma Praeparata Cum Melle (Zhigancao), Rhizoma Zingiberis Recens (Shengjiang), Fructus Jujubae (Dazao) |
| Jinye Baidu granules | Chinese patent medicine | ChiCTR2000029728 | Used for preventing SARS | Lonicerae Japonicae Flos (Jinyinhua), Isatidis Folium (Daqingye), Taraxaci Herba (Pugongying), Houttuyniae Herba (Yuxingcao) |
3.3.2. Trials of therapeutic interventions
114 registered trials were therapeutic trials, including 67 RCTs, 18 CCTs, 12 single-arm studies, 10 real world clinical studies, and 7 retrospective studies. Participants in the majority of trials (91.2%, 104/114) were confirmed COVID-19 cases, while 5.3% (6/114) recruited both confirmed and suspected cases, and 3.5% (4/114) recruited only suspected cases. Suspected cases were defined as people with an exposure history and clinical manifestations of COVID-19, yet to be confirmed by etiological or serological tests according to China's guideline on COVID-19 [3]. The sample sizes ranged from 20 to 3000, with the total of 28,109, average of 247 and median of 120, and the majority (94/114, 82.5%) of trials had a sample size below 300. Therapeutic interventions included herbal decoction/granules (in 52 trials), Chinese patent medicines (22), herbal-derived injections (12), acupuncture/ acupoint stimulation (4), TCM exercise (Daoyin, Qigong, Liuzijue, Baduanjin) (5), and TCM psychological intervention (1). The remaining 18 trials did not report details of the interventions. Eight trials used TCM interventions alone in the treatment group, while the majority used a combination of TCM and Western medicine, compared with WM. Outcome measures used the therapeutic trials are shown in Table 1.
Amongst the 114 trials, six Chinese patent medicines, formulae and injections in 20 trials might be of potential value due to the comprehensive consideration of their availability, previous basis, current clinical application and study progress, and inclusion in the national guidelines. The background and protocol information are as follows:
-
(1)
Qingfei Paidu decoction (QFPD): It was recommended by China's guideline for treating COVID-19 at mild, moderate, severe, and critical types since February 7, 2020 [16]. Six registered trials evaluated the effect of QFPD: One (ChiCTR2000030810) was a registered RCT comparing QFPD versus WM with sample size of 100; one (ChiCTR2000029778) was a controlled clinical trial comparing QFPD plus WM versus WM with sample size of 600; Three (ChiCTR2000030864, ChiCTR2000030883, and ChiCTR2000032767) were single-arm studies; one (ChiCTR2000030806) was a retrospective study evaluating the effectiveness of QFPD plus ulinastatin, a human urinary trypsin inhibitor. The trial on ChiCTR2000029778 was published in Biomedicine & Pharmacotherapy on July 2020, showing that the combination of QFPD with WM demonstrated effects of anti-inflammatory and mitigating the extent of multi-organ impairment compared with those of WM alone in patients with mild and moderate COVID-19 [17].
-
(2)
Huashi Baidu decoction/granules (HSBD): It was formulated by the national medical team of TCM in Hubei and recommended by the national guideline (7th version) for treating severe COVID-19 [3]. One RCT (ChiCTR2000030988) with sample size of 204 evaluated the effectiveness of HSBD granules comparing with WM. Besides, three studies on HSBD had been carried out in Jinyintan Hospital (75 severe cases), Dongxihu Fangcang Hospital (124 moderate cases), and Jiangjunlu Street Health Center (894 mild and moderate cases), respectively. The results showed significant improvement in symptoms and CT images of lungs, shortening hospital stay and reducing rate of viral clearance by PCR, and no adverse events or liver and kidney damage were found [18].
-
(3)
Lianhua Qingwen capsules (LHQW) is a Chinese patent medicine developed in 2003 for treating SARS, and also showed a similar therapeutic effectiveness reduction of the duration of illness and of viral shedding compared with oseltamivir in the treatment of influenza A (H1N1) virus infection [19], and was safer and cheaper than oseltamivir [20]. A new study showed that it might exert anti-viral and anti-inflammatory activity against SARS-CoV-2 [21]. LHQW was recommended by the national guideline (4th version) for treating COVID-19 [3]. Two multicenter RCTs (ChiCTR2000029433 and ChiCTR2000029434) evaluated the effectiveness of LHQW plus WM versus WM alone on 240 suspected cases and 240 confirmed cases, respectively. The study of ChiCTR2000029434 was published in Phytomedicine on 16 May 2020, showing that LHQW group was superior to control group in terms of recovery rate, median time to symptom relief, time to normal of fever, fatigue, and coughing, and the rate of improvement in chest computed tomographic (CT) manifestations scan, and no serious adverse events were reported [22].
-
(4)
Xuebijing injection: Its efficacy in treating patients with severe community-acquired pneumonia was demonstrated by a multicenter randomized placebo control trial published in 2019 [23]. It was recommended for treating severe COVID-19 by the national guideline (4th version) [3]. Two trials evaluated the effectiveness of Xuebijing in the treatment of severe patients with COVID-19. A multicenter controlled clinical trial (ChiCTR2000029381) compared Xuebijing with WM in 400 patients; the RCT (ChiCTR2000030388) compared Xuebijing plus WM with WM in 60 patients.
-
(5)
Xiyanping injection was recommended by the national guideline (5th version) for treating COVID-19 [3]. Four RCTs (ChiCTR2000029756, ChiCTR2000030117, ChiCTR2000030218/NCT04275388, and ChiCTR2000032412) evaluated the effectiveness of xiyanping plus WM (lopinavir / ritonavir, alpha-interferon) versus WM on mild or moderate cases, and their sample sizes were 238, 80, 348, and 426 respectively. It should be noted that ChiCTR2000030218 and NCT04275388 were duplicates and registered by the same applicant, study period, design, sample size, and interventions.
-
(6)
Toujie Quwen granules (TJQW), formulated by Guangzhou Eight People's Hospital, had been used for treating COVID-19 in several hospitals of Guangdong province. Two multicenter controlled studies (CCT: ChiCTR2000031089; RCT: ChiCTR2000031888) evaluated the effectiveness of TJQW granules plus basic treatment versus basic treatment with or without antiviral therapy for moderate or mild patients. A previous case series study involving 121 non-severe patients found that the proportion of patients with improved symptoms and/or chest CT manifestations ranged from 71%~84% after taking TJQW for six days [24].
The details of the above interventions and trials are shown in Table 3 .
Table 3.
Characteristics of TCM treatment interventions and trials worthy of attention.
| Intervention | Category | Trials | Rationality | Ingredients |
|---|---|---|---|---|
| Qingfei Paidu decoction | New formula for COVID-19 | ChiCTR2000030810, ChiCTR2000029778; ChiCTR2000030864; ChiCTR2000030883; ChiCTR2000030806; ChiCTR2000032767 |
Included in guideline | Ephedrae Herba (Mahuang) Glycyrrhizae Radix Et Rhizoma Praeparata Cum Melle (Zhigancao), Armeniacae Semen (Xingren), Gypsum Fibrosum (Shengshigao), Cinnamomi Ramulus (Guizhi), Alismatis Rhizoma (Zexie), Polyporus (Zhuling), Atractylodis Macrocephalae Rhizoma (Baizhu), Poria (FulLing), Bupleuri Radix (Chaihu), Scutellariae Radix (Huangqin), Pinellinae Rhizoma Praeparatum (Jiangbanxia), Zingiberis Rhizoma recens (Shengjiang), Asteris Radix (Ziwan), Farfarae Flos (Kuandonghua), Belamcandae Rhizoma (Shegan), Asari Radix et Rhizoma (Xixin), Dioscoreae Rhizoma (Shanyao), Aurantii Fructus immaturus (Zhishi), Citri reticulatae Pericarpium (Chenpi), Pogostemonis Herba (Huoxiang). |
| Huashi Baidu decoction | New formula for COVID-19 | ChiCTR2000030988 | Included in guideline | Ephedrae Herba (Shengmahuang), Armeniacae Semen (Xingren), Gypsum fibrosum (Shengshigao), Glycyrrhizae Radix Et Rhizoma (Gancao), Pogostemonis Herba (Huoxiang), Magnoliae officinalis Cortex (Houpo), Atractylodis Rhizoma (Cangzhu), Tsaoko Fructus (Caoguo), Pinellinae Rhizoma Praeparatum (Fabanxia), Poria (Fuling), RheiRadix et Rhizoma (Shengdahuang), Astragali Radix (Shenghuangqi), Lepidii/Descurainiae Semen (Tinglizi), Paeoniae Radix rubra (Chishao). |
| Lianhua Qingwen capsules | Chinese patent medicine | ChiCTR2000029433; ChiCTR2000029434 | Included in guideline; used for treating H1N1 influenza | Forsythiae Fructus (Lianqiao), Lonicerae Japonicae Flos (Jinyinhua), Ephedrae Herba (Mahuang), Armeniacae Semen Amarum (Kuxingren), Gypsum Fibrosum (Shigao), Isatidis Radix (Banlangen), Dryopteridis Crassirhizomatis Rhizome (Mianmaguanzhong), Houttuyniae Herba (Yuxingcao), Pogostemonis Herba (Guanghuoxiang), Rhei Radix Et Rhizome (Dahuang), Rhodiolae Crenulatae Radix Et Rhizome (Hongjingtian), l-Menthol (Bohenao), Glycyrrhizae Radix Et Rhizoma (Gancao) |
| Xuebijing injection | Chinese herbal-derived injection | ChiCTR2000029381; ChiCTR2000030388 | Included in guideline; used for treating severe community-acquired pneumonia | Radix Paeoniae Rubra (Chishao), Radix Angelica Sinensis (Danggui), Chuanxiong Rhizoma (Chuanxiong), Carthami Flos (Honghua), Radix Salviae Miltiorrhizae (Danshen) |
| Xiyanping injection | Chinese herbal-derived injection | ChiCTR2000029756, ChiCTR2000030117, ChiCTR2000030218/NCT04275388 | Included in guideline | Sulfonate Andrographolide (Chuanxinlian) |
| Toujie Quwen granules | New formula for COVID-19 | ChiCTR2000031089; ChiCTR2000031888 | Evidence from case series study for treating COVID-19 | Forsythiae Fructus (Lianqiao), Cremastrae Pseudobulbus (Shancigu), Lonicerae Japonicae Flos (Jinyinhua), Scutellariae Radix (Huangqin), Bupleuri Radix (Chaihu), Artemisiae Annuae Herba (Qinghao), Cicadae Periostracum (Chantui), Peucedani Radix (Qianhu), Fritillariae Cirrhosae Bulbus (Chuanbeimu), Mume Fructus (Wumei), Scrophulariae Radix (Xuanshen), Eupolyphaga Steleophaga (Tubiechong), Atractylodis Rhizoma (Cangzhu) Astragali Radix (Huangqi), Pseudostellariae Radix (Taizishen), Poria (Fuling) |
3.3.3. Trials of rehabilitation interventions
Twenty-three registered trials were rehabilitation trials, including 18 RCTs, two CCTs, and three single-arm studies. Participants were cured and discharged patients with negative nucleic acid PCR tests. The total sample size was 5080, with ranging from 28 to 1500, average of 221, and median of 120. TCM rehabilitation interventions included Chinese patent medicine, herbal decoction, moxibustion, and TCM exercise (Tai Chi, Liuzijue, Qigong, Baduancao). Outcome indexes included aerobic exercise capacity measured by the six-minute walk test, quality of life, returning positive rate of PCR test, and chest CT. Among the interventions evaluated in the 23 trials, moxibustion, Tai Chi, and Liuzijue were recommended by China's rehabilitation guideline for cured COVID-19 patients [25]. Three TCM therapies were potential value due to their availability and study design.
-
(1)
Tai Chi: it is a kind of traditional Chinese shadow boxing. Patients with moderate COVID-19 were trained by TCM Tai Chi doctors in some mobile cabin hospitals in Wuhan. A RCT (ChiCTR2000029460) involving 100 cured cases with viral clearance on PCR evaluated the effect of Tai Chi plus conventional rehabilitation therapy versus conventional rehabilitation therapy for lung function and quality of life.
-
(2)
Liuzijue is a traditional breathing exercise of Qigong in TCM. A previous study found that Liuzijue promoted function of lung and quality of life in older people with chronic obstructive pulmonary disease (COPD) at 6 months and was a good alternative home exercise program for the elderly in the rehabilitation of COPD [26]. A RCT (ChiCTR2000030933) evaluated the effectiveness of Liuzijue compared with respiratory muscle training for respiratory function in 108 COVID-19 patients who had been cured and discharged. Maximal inspiratory pressure, 6-minute walking test, daily living activity and lung function were measured. Another RCT (ChiCTR2000032367) evaluated the efficacy of Liuzijue on improving respiratory symptoms and physical and mental health of mild COVID-19 patients during hospitalization and after discharge [27].
4. Discussion
From the number of registered clinical trials, both TCM and western medicine responded quickly to the COVID-19 pandemic during the early months in China. From the outbreak in January to basic control in March, China encouraged the application of TCM in the prevention and treatment of epidemics, based on the historical and contemporary experience from SARS and H1N1 influenza [4,28,29]. In response to the new communicable disease like COVID-19, doctors and TCM practitioners used the existing Chinese patent medicines or Chinese herbal-derived injections which were previously designed for other virus infections such as SARS and H1N1 influenza, which have been tested for effectiveness and safety [30], and also developed and applied new herbal formulae in clinical practice (Table 3). Therefore, a series of clinical studies were registered and conducted to evaluate the efficacy and safety of these interventions [9,31]. This study systematically reviewed all of the available TCM clinical trial registrations on COVID-19. Since the number of new cases and the number of newly registered clinical trials in China had decreased significantly since April, most of the registered trials failed to complete. The number of TCM trials registered in ChiCTR accounted for 23.8% of all trials, and the research scope covered prevention, treatment, rehabilitation, and TCM syndrome prevalence, indicating that TCM has been investigated to support their use in the management of COVID-19 in China [2].
Based on the analysis of the contents of the existing registered trials and the current epidemic situation in China, we believed that among the 161 trials, only a few of the trials may be able to complete and their TCM interventions but may be possibly promising and have the potential to be applied abroad. Most of trials might not be able to continue, or may have little significance to the application of TCM in the global management of COVID-19, which might be explained by the following three aspects: firstly, due to the rapid control of the epidemic, the number of new cases in China reduced greatly and a large number of existing patients had been cured, making it difficult for clinical trials to recruit a sufficient sample size. The increase of registered trials lagged behind the growth of new confirmed cases. In fact, both RCTs of Remdesivir in China, one (NCT04257656) [32] in severe patients and the other (NCT04252664) in mild and moderate patients were terminated or suspended because no eligible patients could be enrolled. Secondly, the generally low methodological quality and planned small sample size made many trials unlikely to generate strong evidence about the effectiveness of both TCM and WM in China [9,31,33,34]. Lastly, interventions in these trials lacked sufficient evidence in previous clinical practice, or were not available out of China, for example the individual treatment based on TCM syndrome differentiation.
For the application of TCM experience in the management of the COVID-19 pandemic worldwide, there are some suggestions. Firstly, collaboration between TCM and WM in China's epidemic prevention and control, as well as the effect of TCM in treating COVID-19: the majority (91.5%) of patients received integrated Chinese and western medicine treatment in China. At present, a series of clinical observations, studies and systematic reviews have initially found that the combination of TCM and WM was superior to WM alone in improving symptoms, shortening the course of disease and length of hospital stay, or reducing moderate-severe conversion rate [17,22,[35], [36], [37], [38]]. Secondly, based on the principle of compassionate use of a drug, as well as the national conditions of and relevant medical and pharmacy laws and regulations, countries with severe epidemics could consider the feasibility of introducing potential promising TCM therapies including herbal formulae, patent medicines, injections, or non-drug therapies for prevention, treatment and rehabilitation. Thirdly, it is worth paying attention to the study progress of the promising TCM therapies in relevant registered trials and applying the available TCM interventions that have widely used evidence in China.
Regarding the specific TCM therapies for the prevention, treatment and rehabilitation of COVID-19, the following could be recommended for further evaluation and application worldwide. For prevention on high-risk population or suspected cases, moxibustion, Huoxiang Zhengqi oral liquid, and Jinye Baidu granules could be considered (Table 2). For treatment, Qingfei Paidu decoction in mild, moderate and severe cases, Huashi Baidu decoction, Lianhua Qingwen capsules, Toujie Quwen granules and Xiyanping injection in mild and moderate cases, and Xuebijing injection in severe cases could be considered (Table 3). For rehabilitation of cured patients, TCM exercises including Tai Chi and Liuzijue might be beneficial to the patients’ lung function and quality of life. We suggested that interim analyses be conducted if no eligible patients could be enrolled in these on-going trials in China. If the trials have some preliminary promising evidence suggesting safety and effectiveness on COVID-19, their interventions can continue to be recruit participants for clinical study and be used in clinical practice outside China, in compliance with local laws.
Our work has two limitations. Firstly, due to the criteria for deciding potential value were subjective, and the inadequate reporting of key details in some registered trials and lack of timely updates on research status in the registries, there may be some bias in the judgment of research methodological quality and potential value. Secondly, this study included registration protocols of TCM studies for COVID-19, not published clinical trials. Without a systematic review of existing completed or published clinical research results, we cannot yet recommend specific TCM interventions for global epidemic management. Therefore, the purpose of this study is to screen the potential promising trials and available TCM interventions, in order to provide reference for interested readers.
5. Conclusion
Of the 161 registered TCM trials for COVID-19, only a few will be able to complete due to lack of resources. The interventions involved in these possibly promising studies include some specific formulae, Chinese patent medicines, herbal-derived injections and non-drug therapies which may have the potential feasibility of being evaluated internationally so that the evidence base for their use can be established for the management of COVID-19 pandemic.
Author contribution
Hui Luo: Conceptualization, Investigation, Formal analysis, Writing - original draft, Writing - review & editing. Ming Yang: Investigation, Data curation, Methodology. Qiao-Ling Tang: Conceptualization, Formal analysis. Xiao-Yang Hu: Methodology, Writing - review & editing. Merlin L. Willcox: Methodology, Writing - review & editing. Jian-Ping Liu: Conceptualization, Methodology, Supervision, Writing - review & editing.
Financial support
Jian-Ping Liu is funded by the Key project of the National Natural Science Foundation of China (No. 81830115) “Key techniques and outcome research for therapeutic effect of traditional Chinese medicine as complex intervention based on holistic system and pattern differentiation & prescription”. Qiao-Ling Tang is funded by a project from School of TCM, Beijing University of Chinese Medicine (2020-yjgg-10). Merlin Willcox's salary is funded by a National Institute of Health Research (NIHR) Academic Clinical Lectureship, under grant CL-2016-26–005. Xiao-yang Hu's salary is funded by the NIHR School for Primary Care Research fellowship, and the views expressed are those of the author and not necessarily those of the NIHR or the Department of Health and Social Care.
Declaration of Competing Interests
None.
Acknowledgments
The authors would like to thank Ziyu Tian, Yao Zhang, Yingying Zhang, Qiubai Jin, and Yaxi Shang from Beijing University of Chinese Medicine for their assistance on data extraction.
Data availability
Data and any supplementary material related to this article can be obtained from the corresponding author on request.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eujim.2020.101251.
Appendix. Supplementary materials
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int. (Accessed 21 October 2020).
- 2.Sun C. Carry out the spirit of General Secretary Xi Jinping's important instructions, strengthen the frontline work of epidemic prevention and control. Qiushi Journal. 2020;(7) http://www.qstheory.cn/dukan/qs/2020-04/01/c_1125791378.htm [Google Scholar]
- 3.National Health Commission of the People's Republic of China. Diagnosis and Treatment Protocol for COVID-19 (Trial Version 8). http://en.nhc.gov.cn/2020-09/07/c_81565.htm. (Accessed 21 October 2020).
- 4.Luo H., Tang Q., Shang Y. Can Chinese medicine be used for prevention of Corona Virus Disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin. J. Integr. Med. 2020;26(4):243–250. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The State Council Information Office of the People's Republic of China. News Conference of the Information Office of the State Council: yu Yanhong Briefing the Important Role of TCM in COVID-19 Containment and Treatment. http://ghs.satcm.gov.cn/gongzuodongtai/2020-03-27/14281.html. (Accessed 5 August 2020).
- 6.National Health Commission of the People's Republic of China. Oct 21: daily briefing on novel coronavirus cases in China. http://en.nhc.gov.cn/2020-10/21/c_81920.htm. (Accessed 21 October 2020).
- 7.Lai S., Ruktanonchai N.W., Zhou L. Effect of non-pharmaceutical interventions to contain COVID-19 in China. Nature. 2020 doi: 10.1038/s41586-020-2293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S.M., Zhang Z.J., Yang J.T. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395(():1305–1314. doi: 10.1016/S0140-6736(20)30744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei X.X., Zhao M.Z., Zhao C. The global registry of COVID-19 clinical trials: indicating the design of traditional Chinese medicine clinical trials. TMR Mod. Herb. Med. 2020;3(3):1–7. doi: 10.12032/TMRmhm202003068. [DOI] [Google Scholar]
- 10.China Association of Acupuncture-Moxibustion. Guiding Opinions on Acupuncture-moxibustion Intervention for COVID-19 (version 2). http://www.caam.cn/article/2193-. (Accessed 5 August 2020).
- 11.Yan B.H., Jiang Z.W., Zeng J.P. Large-scale prospective clinical study on prophylactic intervention of COVID-19 intervention in community population using Huoxiang Zhengqi Oral Liquid and Jinhao Jiere Granules. Zhongguo Zhong Yao Za Zhi. 2020;45(13):2993–3000. doi: 10.19540/j.cnki.cjcmm.20200430.501. [DOI] [PubMed] [Google Scholar]
- 12.Pharmacy Department of Tongji hospital. Drugs for prevention and treatment of COVID-19 (3): jinye Baidu granule. https://mp.weixin.qq.com/s/PkKAYmAkNVeSRcToCyIeaA. (Accessed 5 August 2020).
- 13.Jiang J.J., Xie Y.M., Wang Y.Y. Study of Jinye Baidu granule in treatment of wind-warmth lung heat disease (heat in lung-wei pattern): a randomized, double-blind, parallel-controlled trial. China Journal of Chinese Materia Medica. 2017;42(8):1467–1473. doi: 10.19540/j.cnki.cjcmm.2017.0043. [DOI] [PubMed] [Google Scholar]
- 14.Jiang H., Chen S.H., Wen L.Z. Effects of Jinye Baidu Granule on fetal growth and development with maternal active human cytomegalovirus infection. Chin. J. Integ. Med. 2016;12(4):250–254. doi: 10.1007/s11655-006-0250-3. [DOI] [PubMed] [Google Scholar]
- 15.Chen S.H., Xiong J.W., Xing W. A study on the traditional Chinese medicine Jinyebaidu for prevention and treatment of intrauterine infection with guinea pigs cytomegalovirus. J. Huazhong Univ. Sci Technolog. Med. Sci. 2005;25(6):721–723. doi: 10.1007/bf02896182. [DOI] [PubMed] [Google Scholar]
- 16.National Administration of Traditional Chinese Medicine of China. Notice on recommending the use of Qingfei Paidu Decoction in the treatment of coronavirus disease 2019. http://yzs.satcm.gov.cn/zhengcewenjian/2020-02-07/12876.html. (Accessed 5 August 2020).
- 17.Xin X., Cheng X., Zhu B. Clinical retrospective study on the efficacy of Qingfei Paidu decoction combined with Western medicine for COVID-19 treatment. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The State Council Information Office of China. Press conference on the important role of TCM in preventing and treating COVID-19 and effective drugs. http://www.scio.gov.cn/xwfbh/xwbfbh/wqfbh/42311/42768/index.htm. (Accessed 5 August 2020).
- 19.Duan Z.P., Jia Z.H., Zhang J. Natural herbal medicine Lianhuaqingwen capsule anti-influenza A (H1N1) trial: a randomized, double blind, positive controlled clinical trial. Chin. Med. J. (Engl) 2011;124(18):2925–2933. doi: 10.3760/cma.j.issn.0366-6999.2011.18.024. [DOI] [PubMed] [Google Scholar]
- 20.Niu Q.Q., Chen Y., Liu Y. Efficacy and safety of Lianhua Qingwen capsule for influenza: a systematic review. Zhongguo Zhong Yao Za Zhi. 2017;42(8):1474–1481. doi: 10.19540/j.cnki.cjcmm.2017.0044. [DOI] [PubMed] [Google Scholar]
- 21.Li R.F., Hou Y.L., Huang J.C. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020;156(() doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu K., Guan W., Bi Y. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z., Zhou Q., Wang Y. XueBiJing injection versus placebo for critically III patients with severe community-acquired pneumonia: a randomized controlled trial. Crit. Care Med. 2019;47(9):e735–e743. doi: 10.1097/ccm.0000000000003842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xinhuanet. Pheumonia I formula was used for treating COVID-19 in several hospitals of Guangdong province. http://www.xinhuanet.com/health/2020-02/18/c_1125591065.htm. (Accessed 5 August 2020).
- 25.National Health Commission of China, National Administration of TCM of China. The rehabilitation guidance of traditional Chinese medicine for the recovery of COVID-19 (for trial implementation). http://www.gov.cn/zhengce/zhengceku/2020-02/24/content_5482544.htm. (Accessed 5 August 2020).
- 26.Xiao C.M., Zhuang Y.C. Efficacy of Liuzijue Qigong in individuals with chronic obstructive pulmonary disease in remission. J. Am. Geriatr. Soc. 2015;63(7):1420–1425. doi: 10.1111/jgs.13478. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S.P., Lv Z.Z., Zhu Q.G. Efficacy of Liu-zi-jue in Patients with 2019 Novel Coronavirus Pneumonia (COVID-19): structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21(1):416. doi: 10.1186/s13063-020-04383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen W., Lim C.E.D., Kang H.J. Chinese herbal medicines for the treatment of type A H1N1 influenza: a systematic review of randomized controlled trials. PLoS ONE. 2011;6(12):e28093. doi: 10.1371/journal.pone.0028093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization . WHO Library Cataloguing-in-Publication Data; Geneva, Switzerland: 2004. SARS: Clinical Trials On Treatment Using a Combination of Traditional Chinese medicine and Western medicine.https://apps.who.int/iris/handle/10665/43029 [Google Scholar]
- 30.Tu Y.F., Chien C.S., Yarmishyn A.A. A review of SARS-CoV-2 and the ongoing clinical trials. Int J Mol Sci. 2020;21(7):2657. doi: 10.3390/ijms21072657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q., Wang Y.K., Qi C.S. Clinical trial analysis of 2019‐nCoV therapy registered in China. J. Med. Virol. 2020;92(():540–545. doi: 10.1002/jmv.25733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y., Zhang D., Du G. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu R.F., Gao Y.L., Robert S.H. Systematic review of the registered clinical trials of Coronavirus Disease 2019 (COVID-19) medRxiv. 2020 doi: 10.1101/2020.03.01.20029611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao C., Zhao W.L. Lessons from COVID-19 clinical trials and the concept of national clinical trials network. Chin. J. Evid. Based Med. 2020;20(5):497–503. doi: 10.7507/1672-2531.202003069. [DOI] [Google Scholar]
- 35.Fan A.Y., Gu S., Alemi S.F. Chinese herbal medicine for COVID-19: current evidence with systematic review and meta-analysis. J Integr Med. 2020 doi: 10.1016/j.joim.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong W.Z., Wang G., Du J. Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19:a pilot randomized clinical trial. Integr. Med. Res. 2020 doi: 10.1016/j.imr.2020.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J.B., Wang Z.X., Jing J. Exploring an integrative therapy for treating COVID-19: a randomized controlled trial. Chin. J. Integr. Med. 2020 doi: 10.1007/s11655-020-3426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z.W., Chen X.R., Lu Y.F. Clinical characteristics and therapeutic procedure for four cases with 2019 Novel Coronavirus Pneumonia receiving combined Chinese and Western medicine treatment. Biosci. Trends. 2020;14(1):64–68. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.