Abstract
Most people start experimenting with tobacco products or e-cigarettes in early adolescence and become habitual smokers in late adolescence or adulthood. These studies investigated if exposure to tobacco smoke or nicotine during early and mid-adolescence affects nicotine intake in late adolescence and early adulthood. Male and female rats were exposed to tobacco smoke from low- and high-nicotine SPECTRUM cigarettes or nicotine (0.3 mg/kg, twice a day) from postnatal day (P) 24 to 42. The self-administration sessions started at P55. The rats self-administered nicotine for 14–15 days under a fixed-ratio 1 schedule, and on the first day, the maximum number of infusions was twenty. Exposure to smoke from high, but not low, nicotine cigarettes during adolescence increased nicotine self-administration in female but not male rats. Adolescent treatment with nicotine facilitated nicotine self-administration. On the first day of nicotine self-administration, nicotine-treated rats reached the maximum number of infusions before the saline-treated control rats. Nicotine intake was also higher in the nicotine-treated female rats than in the saline-treated females. There was no sex difference in nicotine intake in controls when the data from the studies were combined. Smoke exposure led to a dose-dependent increase in plasma nicotine and cotinine levels in adolescent rats. Exposure to smoke from high-nicotine cigarettes and 0.3 mg/kg of nicotine led to plasma nicotine and cotinine levels that are similar to those in tobacco users. These findings indicate that in females, but not males, exposure to nicotine during adolescence may facilitate smoking and e-cigarette use later in life.
Keywords: Adolescent, sex differences, nicotine, tobacco smoke, self-administration, rats
1. Introduction
Nicotine addiction is a chronic disorder that is characterized by the compulsive use of tobacco products and other nicotine-containing products, withdrawal signs upon the cessation of nicotine use, and relapse after periods of abstinence (Benowitz, 2010). Worldwide there are more than 1 billion people who smoke, and cigarette use is the leading cause of lung cancer and early death (WHO, 2018). The use of tobacco products is on the decline in the US, but recently there has been a strong increase in the use of e-cigarettes. Data from the Monitoring the Future (MTF) survey indicate that e-cigarette use among high schoolers doubled from 2017 to 2019 (Miech et al., 2019). About 21 percent of 8th graders (12–13 years of age) and 40 percent of 12th graders (17–18 years of age) have used e-cigarettes (Miech et al., 2019). Daily e-cigarette use increases with age and is 2 percent for 8th graders and 12 percent for 12th graders. Considering the addictive properties of nicotine, the increase in e-cigarette use might lead to an increase in the number of young adults with a nicotine addiction. It has been well established that the vast majority of people start smoking during adolescence, and even light smoking during adolescence increases the risk for habitual smoking in adulthood (Sargent et al., 2017). Most adolescents smoke infrequently, and when they smoke, they only smoke a few cigarettes. However, in some people, this escalates to heavy smoking later in life (Sargent et al., 2017; Wellman et al., 2004). It is unknown if exposure to relatively low and rewarding doses of nicotine during early- and mid-adolescence affects the positive reinforcing properties of nicotine later in life. It is also not known if there are sex differences in the long-term effects of adolescent nicotine exposure. If exposure to nicotine during early- and mid-adolescence increases the positive reinforcing properties of nicotine, then this could contribute to the transition from infrequent smoking to daily smoking.
Numerous animal studies have shown that there are differences in the effects of nicotine between adolescent and adult rats. Experimental evidence suggests that nicotine is more rewarding and withdrawal less severe in adolescent than in adult rats (O’Dell et al., 2006; Torres et al., 2008). There are also sex differences during adolescence, with adolescent female rats being more sensitive to the rewarding effects of nicotine than adolescent male rats (Xue et al., 2018). Adolescence is a period of enhanced vulnerability for the onset of drug use (Spear, 2015). Several studies have investigated the acute and long-term effects of adolescent nicotine exposure in rodents. However, conflicting findings have been reported. One study reported that adolescent nicotine exposure decreases the rewarding properties of nicotine in adult male rats in a place conditioning procedure (Adriani et al., 2006). This is in line with a study that showed that exposure to nicotine during adolescence decreases the locomotor effects of nicotine in adult male rats (Brielmaier et al., 2007). In contrast, other studies reported that male rats exposed to nicotine during adolescence are more sensitive to the locomotor sensitization effects of nicotine in adulthood (Bracken et al., 2011; Faraday et al., 2003). Also, a study with male mice showed that exposure to nicotine during early adolescence enhances the rewarding effects of nicotine in a place conditioning procedure (Kota et al., 2009). The outcome of these studies might have been affected by the species and strain, sex, and nicotine dose.
Although several studies have explored the long-term effects of adolescent nicotine exposure in rodents, it is unknown if exposure to clinically relevant and rewarding doses of tobacco smoke and nicotine during adolescence affects the self-administration of nicotine later in life. Furthermore, it is unknown if there are sex differences in the long-term effects of adolescent nicotine and tobacco smoke exposure. Adolescent female rats are more likely to acquire nicotine self-administration than adolescent male rats and females have a higher level of nicotine intake (Lynch, 2009). In contract, other studies suggest that there are no sex differences in nicotine self-administration in adolescent rats (Chen et al., 2007; Schassburger et al., 2016). In our previous work we found that nicotine is more rewarding in adolescent female rats than in adolescent male rats (Xue et al., 2018). Nicotine also induces more pronounced behavioral sensitization in female than male rats (Harrod et al., 2004). Therefore, it is hypothesized that exposure to tobacco smoke or nicotine during adolescence leads to a greater increase in nicotine intake in female than male rats.
We investigated if exposure to relatively low and rewarding levels of tobacco smoke and nicotine affects the positive reinforcing effects of nicotine in late adolescence and early adulthood. In our nicotine study, the adolescent rats received a rewarding dose of nicotine (0.3 mg/kg)(Xue et al., 2018). The rats in the tobacco smoke group were exposed to smoke from SPECTRUM research cigarettes with a low or high level of nicotine. The high-nicotine SPECRUM cigarettes have a similar nicotine content as regular commercial cigarettes (Carmines and Gillman, 2019; Richter et al., 2016). Exposure to smoke from high-nicotine SPECTRUM cigarettes enhances reward function in the intracranial self-stimulation (ICSS) procedure in adult male and female rats (unpublished results). The same SPECTRUM research cigarettes are used in clinical studies, and similar plasma nicotine levels have been observed in smokers as in our smoke exposed adult rats (Donny et al., 2015; Hatsukami et al., 2012). The Food and Drug Administration (FDA) has approved the sale of some SPECTRUM cigarettes (FDA, 2019). Therefore, the studies described here carefully model tobacco use in humans. Tobacco smoke exposure in rats’ mimics passive smoking and can be used to investigate the pharmacokinetics of active smoking. Blood samples were collected, and plasma nicotine and cotinine levels determined to ensure that the nicotine levels in the adolescent rats are like those in tobacco users. In our studies, male and female rats were exposed to tobacco smoke and nicotine from postnatal day (P) 24 to P42, and nicotine self-administration started around P55. The developmental period during which the rats were exposed to tobacco smoke or nicotine corresponds to early- and mid-adolescence (Tirelli et al., 2003). The nicotine self-administration sessions started around P55, which is considered late adolescence or emerging adulthood (Nemati and Kolb, 2012; Spear, 2015). Therefore, these studies provide insight into the effects of early- and mid-adolescent tobacco use on the positive reinforcing properties of nicotine during late adolescence and emerging adulthood.
The Family Smoking Prevention and Tobacco Control Act provides the Food and Drug Administration (FDA) with the authority to regulate the manufacturing of cigarettes (Congress, 2009). The Tobacco Control Act allows the FDA to mandate lower nicotine levels in tobacco products. The FDA is considering reducing the amount of nicotine in cigarettes so that they become minimally addictive or even non-addictive (FDA, 2018). Therefore, the present work with low- and high-nicotine cigarettes can inform the FDA about the addictive properties of low- versus high-nicotine cigarettes.
2. Materials and Methods
2.1. Animals
Early adolescent male and female Wistar rats (P21, 45–55 g, Charles River, Raleigh, NC) were used for this study. The rats were socially housed (2 per cage) with a rat of the same sex in a climate-controlled vivarium on a reversed 12 h light-dark cycle (light off at 7 AM). Food and water were available ad libitum in the home cage. The rats were gently handled for 2–3 min per day for several days before the onset of the experiments. The rats were also handled for about 1 min before the nicotine injections and the smoke exposure sessions. The experimental protocols were approved by the University of Florida Institutional Animal Care and Use Committee.
2.2. Drugs
Nicotine hydrogen tartrate was purchased from Sigma (Sigma-Aldrich, St. Louis, MO, USA) and dissolved in sterile saline (0.9 % sodium chloride). The nicotine solution was administered subcutaneously (SC) in a volume of 1 ml/kg body weight or the rats self-administered 0.1 ml intravenously (IV). Nicotine doses are expressed as base. SPECTRUM research cigarettes with a low (NRC 200) or high level of nicotine (NRC600) were used for experiments 1 and 3 (NIDA Drug Supply Program). The SPECTRUM research cigarettes had a nicotine yield of 0.07 mg/cig (NRC200) and 0.84 mg/cig (NRC600). The yield refers to the amount of nicotine in tobacco smoke that is produced by one cigarette under standard laboratory smoking conditions. These cigarettes contain 600–700 mg of tobacco, and the nicotine concentration in the tobacco is 0.93 mg/g (NRC200) and 15.7 mg/g (NRC600)(Richter et al., 2016).
2.3. Experimental design
2.3.1. Experiment 1. Adolescent tobacco smoke exposure and nicotine self-administration
The rats arrived in the vivarium at P21 and were exposed to tobacco smoke for one hour per day from P24 to P42. One group of rats (males and females) was exposed to smoke from low-nicotine cigarettes, and when this was completed, another group of rats was exposed to smoke from high-nicotine cigarettes. Rats that were part of the air-control group were placed adjacent to the smoke exposure chamber and were therefore exposed to the same sounds as the smoke-exposed animals. For this study, we used 3 groups of adolescent male rats (male air n=9, male low-nicotine smoke n=8, male high-nicotine smoke n=10) and 3 groups of adolescent female rats (female air n=9, female low-nicotine smoke n=10, female high-nicotine smoke n=11). The rats were exposed to tobacco smoke for five days per week (no smoke exposure on weekends). During the first smoke exposure session, one cigarette was burnt at a time (1 cig/10 min round, total of 6 cig/1h session) during the second session two cigarettes were burnt at a time (12 cigs/1h session), and during the following sessions, three cigarettes were burnt at a time (18 cigs/1h session). Food training started at P26, and catheter implantations started at P50. Drug self-administration sessions started between P56 and P59. The rats self-administered nicotine for 14 days, and the self-administration sessions were conducted five days per week. During the first session the maximum number of infusions was set to 20 to prevent the rats from overdosing on nicotine. Nicotine self-administration in rats exposed to smoke with low or high levels of nicotine was compared to that of the air-control rats.
2.3.2. Experiment 2. Adolescent nicotine exposure and nicotine self-administration
The rats arrived at P21, and the nicotine injections started at P24. The adolescent male (saline n=10, nicotine n=9) and female (saline n=9, nicotine n=9) rats received two nicotine injections (0.3 mg/kg, SC) per day on weekdays. The rats received one injection in the morning (10 AM) and one injection in the afternoon (4 PM). Food training started at P26, and the catheter implantations started at P45. Nicotine self-administration sessions started at P55. The rats self-administered nicotine for 15 days, and the self-administration sessions were conducted five days per week. During the first session, the maximum number of infusions was set to 20, and the time required to obtain the maximum number of infusions was recorded.
2.3.3. Experiment 3. Adolescent smoke and nicotine exposure and plasma nicotine and cotinine levels
The rats arrived at P21, and catheters for blood collection were implanted at P29 and P30. The rats were exposed to smoke from low-nicotine cigarettes on P31 and to smoke from high-nicotine cigarettes on P33 (n=5–10/group). Blood samples were collected immediately after the 1-h smoke exposure sessions (3 cig/10 min round, total of 18 cig/1h session). The rats received 0.1, 0.3, and 0.6 mg/kg of nicotine (SC) on P36, P38, and P40, respectively. Blood samples were collected 15 min after the nicotine injections. The plasma half-life of nicotine in adolescent rats is 1 h and the half-life of cotinine is 7 h (Abobo et al., 2012; Craig et al., 2014). Cotinine is not detected in plasma 24 h after nicotine injections or smoke exposure (Abobo et al., 2012; Craig et al., 2014). However, to ensure that all cotinine was cleared from the systems, in this experiment there was a 48-h interval between nicotine injections and smoke exposure sessions.
2.4. Blood collection
Blood was collected from a catheter that was implanted in the right jugular vein. The blood samples (400–500 μl) were collected in heparinized blood collection tubes (BD Microtainer Tubes) and kept on ice during the blood collection procedure. The samples were centrifuged for 10 min at 2000 g at 4°C, and then plasma was collected. The samples were stored in a −80°C freezer until later use.
2.5. Plasma nicotine and cotinine levels
2.5.1. Chemicals and reagents
To determine plasma nicotine and cotinine levels, liquid chromatography-mass spectrometry (LC-MS) grade acetonitrile, formic acid, isopropanol, acetonitrile, water, methanol, and ammonium acetate were used (Fisher Scientific, Fair Lawn, NJ, USA). Blank rat plasma treated with heparin was purchased from VWR International (Suwanee, GA, USA).
2.5.2. Instrumentation and analytical conditions
A Waters Acquity Class I ultra-performance liquid chromatography (UPLC) coupled with a Xevo TQ-S micro triple quadrupole mass spectrometer (Milford, MA, USA) was used for the quantitative analysis of nicotine and cotinine. The chromatographic separation of nicotine and cotinine was achieved on a Waters Acquity UPLC BEH C18 column (1.7 μm, 2.1 × 50 mm) with a gradient using a mobile phase consisting of 1 mM aqueous ammonium acetate (A) and acetonitrile (B). The flow rate of the mobile phase was maintained at 0.3 ml/min with a linear gradient as follows: 5% B until 0.5 min, 5% to 95% B until 1.5 min, maintained at 95% B until 2 min, decreased to 5% B from 2 to 2.2 min and maintained at 5% B until 3 min. The column oven and autosampler temperatures were maintained at 40 and 10°C, respectively. The detection of analytes was achieved using the multiple reaction monitoring (MRM) method with electrospray ionization (ESI) in the positive mode. The compound parameters (mass transitions, cone voltage, and collision energy) of the analytes and the internal standards (IS) are shown in Table S1. The source parameters such as capillary voltage, source temperature, desolvation temperature, desolvation gas, and cone gas were held at 0.5 kV, 150°C, 450°C, 950 l/h, and 60 l/h, respectively. Data acquisition and analysis were performed by MassLynx software version 4.1.
2.5.3. Sample preparation
Rat plasma samples were thawed at room temperature and vortex-mixed using a BenchMixer (San Francisco, CA, USA) for 5 minutes before analysis. A sample aliquot of 25 μl was transferred into a 96 well plate and quenched with 200 μl of acetonitrile containing 50 ng/ml each IS (nicotine D4 and cotinine D3). The samples were vortexed for 5 minutes at 750 rpm and subsequently filtered through a 96 well 0.45 μ membrane filtration plate under centrifugation at 2000 g for 5 min at 4°C. One microliter of the filtrate was subjected to UPLC-MS/MS analysis. The linearity samples were prepared by spiking 2 μl of 12.5X of respective mixed stock solution of nicotine and cotinine in 23 μl of blank rat plasma samples to obtain linearity range from 5 to 400 ng/ml. Additionally six sets of four quality control (QC) samples (lower limit of quantification, low, medium, and high quality controls) of 5, 10, 250 and 320 ng/ml were prepared by spiking 2 μl of 12.5X of respective nicotine and cotinine mixed stock solution each in 23 μl of blank plasma samples. These samples were then vortex mixed for 5 min at 750 rpm, both linearity and QC samples were processed like the test samples.
2.6. Tobacco smoke exposure
The rats were exposed to tobacco smoke in standard polycarbonate rodent cages (38 × 28 × 20 cm; L x W x H) with corncob bedding and a wire top (Small et al., 2010; Yamada et al., 2010). The rats were not restrained (whole-body exposure) during the smoke exposure sessions, and water and food were available. The rats were moved to the exposure cages immediately before the smoke exposure sessions and returned to their home cages after the exposure sessions. Tobacco smoke was generated using a microprocessor-controlled cigarette smoking-machine (model TE-10, Teague Enterprises, Davis, CA)(Teague et al., 1994). Tobacco smoke was generated by burning SPECTRUM research cigarettes using a standardized smoking procedure (35 cm3 puff volume, one puff per minute, 2 seconds per puff). Mainstream smoke and sidestream smoke were mixed and diluted in a special chamber. The smoke machine produces a mixture of 10% mainstream smoke and 90% sidestream smoke, based on total suspended particle matter (TSP). The smoke was aged for 2 – 4 min and diluted with air before being introduced into the exposure chambers. The exposure conditions were monitored for carbon monoxide (CO) and the TSP level. CO levels were assessed using a continuous CO analyzer that measures CO levels between 0 – 2000 parts per million (Monoxor II, Bacharach, New Kensington, PA USA). The TSP count in the exposure chamber was measured by pumping smoke out the chamber into a chemical hood through a pre-weighed filter (Pallflex Emfab Filter, Pall Corporation, Port Washington, NY USA) for 5 min. The TSP count was calculated by dividing the weight increase of the filter by the volume (m3) of the smoke. The TSP level depends on the number of cigarettes that are burnt during one 10-min round (i.e., burnt at one time). In our previous work, we showed that burning four research cigarettes (3R4F) during one round leads to a TSP levels of 147±4 mg/m3 and CO levels of 493±14 ppm (Small et al., 2010). Decreasing the number of cigarettes that are burnt per round leads to lower TSP and CO levels. In the present studies, 1 to 3 cigarettes were burnt per round, which led to TSP levels of 20–90 mg/m3 and CO levels of 150–451 ppm (Table S2). Also, the TSP and CO levels of 4 cigarettes per round was estimated, which result in the TSP levels of 151 mg/m3 and CO levels of 721 ppm (Table S2).
2.7. Catheter implantation and operant responding for nicotine
The rats were anesthetized and prepared with a catheter in the right jugular vein (See supplemental materials for details). The nicotine and smoke exposed rats that were used in the self-administration studies were prepared with catheters around P45. A separate group of drug naïve adolescent rats were prepared with catheters at P29 and P30 for blood collection. The surgery was conducted as described before (Chellian et al., 2019; Qi et al., 2015; Yamada and Bruijnzeel, 2011). The rats were singly housed after the implantation of the catheters. Food training and nicotine self-administration sessions were conducted as described before (Bruijnzeel et al., 2009; Yamada and Bruijnzeel, 2011; Zislis et al., 2007). First, the rats were trained to respond for food pellets (45 mg, F0021, Bio-Serv, Frenchtown, NJ). The rats were trained to respond on the right lever (RL, active lever) to receive food pellets. Responding on the left lever (LL, inactive lever) was recorded but did not have scheduled consequences. Instrumental training started under an FR1-TO1s reinforcement schedule, and after several sessions, the time-out period was increased to 10 s (FR1-TO10s). For the first 5 days of food training, the rats were fed 70 percent of their baseline food intake in the home cage. After 2–3 days of food training, the rats’ food intake was back at baseline levels (70% home cage and 30% or more in operant chamber). After 5 days, the rats were fed 90 percent of their ad lib home cage intake and they obtained the remainder in the operant chambers. Because the food pellets are highly palatable most rats’ daily food intake was 30–40 percent above baseline levels. During the nicotine self-administration studies, the rats received about 95% of their normal ad lib food intake in the home cage. The rats were fed immediately after the operant sessions. A mild level of food restriction facilitates food training and nicotine self-administration in rats (Donny et al., 1998). The rats self-administered nicotine (1 h session/day) at the 0.03 mg/kg/infusion dose for 14–15 days. During the first day, the maximum number of nicotine infusions was set to 20. In the second experiment, but not the first experiment, the time required to self-administer 20 infusions was recorded. Active lever pressing resulted in the delivery of a nicotine infusion (0.1 ml infused over a 5.6-s period). The initiation of the delivery of an infusion was paired with a cue light, which remained illuminated throughout the time-out period. Inactive lever responding was recorded during the nicotine self-administration sessions but did not have scheduled consequences. Both levers were retracted during the 10 s time-out period. The self-administration sessions were conducted five days per week.
2.8. Statistics
The effect of tobacco smoke exposure on nicotine intake was analyzed using a three-way ANOVA with smoke exposure condition and sex as between-subjects factors and time (self-administration sessions) as a within-subjects factor. The effect of adolescent nicotine treatment on nicotine intake was analyzed using a three-way ANOVA with nicotine treatment and sex as between-subjects factors and time (session) as a within-subjects factor. An area under the curve (AUC) analysis was conducted to compare nicotine intake between the females treated with nicotine and the female control rats. The first day of nicotine self-administration is depicted in the figures but was not included in the data analysis because there was a cap on the number of nicotine infusions. Plasma nicotine and cotinine levels were analyzed using a two-way ANOVA with the treatment condition and sex as between-subjects factors. For all statistical analyses, significant interaction effects in the ANOVAs were followed by Bonferroni’s post hoc tests. P values of less or equal to 0.05 were considered significant. Data were analyzed with SPSS version 25 and GraphPad Prism version 7.
3. Results
3.1. Experiment 1. Adolescent tobacco smoke exposure and nicotine self-administration
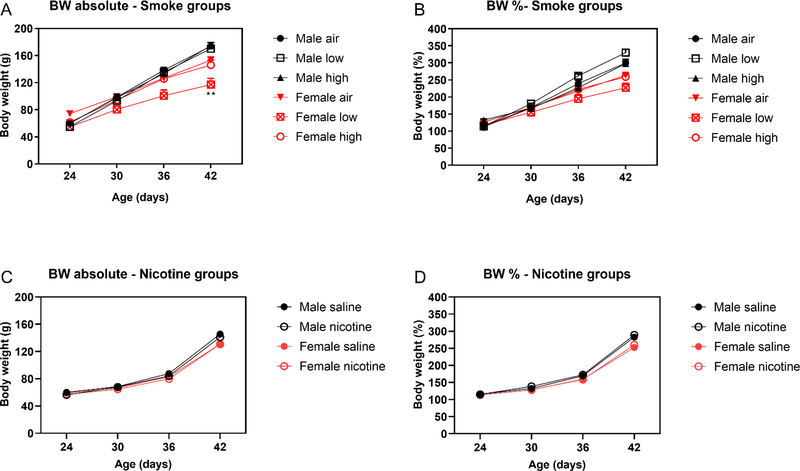
Before the onset of the smoke exposure sessions, there were no differences in body weights between the groups (Table S3). The rats were exposed to tobacco smoke or air from P24–42. During this period, the males gained more weight than the females (Time F3,153=1449.466, P<0.001; Sex F1,51=16.148, P<0.001; Time x Sex F3,153=53.87 P<0.001, Fig. 1A). Smoke exposure affected weight gain, and the effect on weight gain was dependent on the sex of the rats (Treatment F2,51=5.607, P<0.01; Time x Sex x Treatment F6,153=8.533, P<0.001). The posthoc test showed that female rats exposed to tobacco smoke with a low level of nicotine gained less weight than the air-control females. When the body weights of the rats were expressed as a percentage of baseline values, a similar pattern of results was observed. The males gained more weight than the females and smoke exposure affected weight gain (Time F3,153=1308.356, P<0.001; Sex F1,51=26.283, P<0.001; Time x Sex F3,153=46.554, p<0.001; Treatment x Sex F6,153=5.333, p<0.01; Time x Sex x Treatment F6,153=10.428, P<0.001, Fig. 1B). The posthoc test did not reveal significant differences between the groups. On the first day and the last day of nicotine self-administration, the females weighed less than the males. There was no effect of prior smoke exposure on the body weights on the first or last day of nicotine self-administration (Table S3).
Figure 1. Body weights of adolescent male and female rats exposed to tobacco smoke or nicotine.
The figures depict the absolute body weights of the rats exposed to tobacco smoke (A) and nicotine (C) and the body weights expressed as a percentage of the baseline values (B, D). The adolescent male and female rats were exposed to tobacco smoke or nicotine from P24–42. Weight gain was slightly reduced in the female rats exposed to smoke with a low level of nicotine, but this effect was not observed when the body weights were expressed as a percentage of the baseline values. Asterisks indicate lower body weight compared to the females exposed to air. N = 8–11/group. Data are expressed as means ± SEM. ** P < .01. Abbreviations: BW, body weight; low, smoke with low level of nicotine; high, smoke with high level of nicotine.
Exposure to tobacco smoke with a low level of nicotine during early- and mid-adolescence did not affect the self-administration of nicotine. Nicotine intake initially decreased and then stabilized (Session F12,3846.369, P<0.0001; Session x Sex F12,384=2.567, P<0.01, Fig. 2A). A similar pattern was observed in active lever responding (RL)(Session F12,420=6.015, P<0.0001; Session x Sex F12,420=2.949, P<0.01, Fig. S1A). The females started out with a relatively high level of inactive lever (LL) responding (Sex F1,32=5.00, P<0.05; Sex x Session F12,384=1.932, P<0.05, Fig. 2B). One group in particular, female low-nicotine smoke exposure, had a very high level of inactive lever responding (Sex F1,32=5.00, P<0.05; Sex x Session F12,384=1.932, P<0.05; Sex x Treatment F1,32=4.791).
Figure 2. Adolescent exposure to tobacco smoke with a high level of nicotine increases nicotine self-administration in female rats.
The rats were exposed to tobacco smoke with low (A, B) or high levels (C, D) of nicotine during early and mid-adolescence and nicotine self-administration started during late adolescence. E) Depicts difference in nicotine intake between the male and female rats exposed to smoke with a high level of nicotine during adolescence. Asterisks indicate significantly different from the male HNS rats and plus signs from the female air rats. The rats had access to nicotine 5 days per week and total nicotine intake and responding on the inactive lever were recorded. Abbreviations: LNS, low nicotine smoke; HNS, high nicotine smoke; LL, left lever. N = 8–11/group. Bonferroni post-hoc test: +,* P < .05, ** P < .01. Data are expressed as means ± SEM.
Exposure to tobacco smoke with a high level of nicotine increased nicotine self-administration in the females but not in the males (Sex F1,35=8.537, P<0.01; Session F12,420=5.644, P<0.0001; Session x Sex x Treatment F12,420=39.959, P<0.05, Fig. 2C). The posthoc analysis showed that the females exposed to smoke with a high level of nicotine had a higher level of nicotine intake than females exposed to air and also compared to males exposed to smoke with a high level of nicotine (Fig. 2E). A similar pattern of results was obtained when active lever responses were analyzed (Session F12,420=5.003, P<0.0001; Sex F1,38=6.754, P<0.05; Session x Sex x Treatment F12,420=2.04, P<0.05, Fig. S1B). Responding on the inactive lever initially increased and then decreased (Session F12,420=2.445, P<0.01, Fig. 2D).
3.2. Experiment 2. Adolescent nicotine exposure and nicotine self-administration
There were no differences in the body weights of the groups before the onset of the nicotine injections (Table S4). During the period that the rats received the nicotine injections, all groups gained weight, and the males gained more weight than the females (Time F3,99=3617.663, P<0.0001; Time x Sex F3,99=20.609, P<0.0001, Fig. 1C). There was no effect of nicotine-treatment on weight gain. When the body weights were expressed as a percentage of the baseline values, the males gained more weight than the females and there was no effect of the nicotine treatment (Time F3,99=2222.91, P<0.0001; Sex F1,33=18.83, P<0.0001; Time x Sex F3,99=16.694, P<0.0001, Fig. 1D). At the onset of the nicotine self-administration sessions and on the last day of nicotine self-administration, the females weighed less than the males and there was no effect of prior nicotine treatment (P55, Sex F1,33=126.455, P<0.0001; P69, Sex F1,33=329.773, P<0.0001, Table S4).
On the first day of nicotine self-administration, the nicotine-treated rats obtained the maximum number of infusions before the saline-treated control rats (Treatment F1,34=16.622, P<0.0001, Fig. 3A). There was a significant effect of nicotine treatment, and the post-hoc analysis found a statistically significant effect in females only. Over the 15-day self-administration period, nicotine intake slightly increased and then decreased and stabilized (Session F13,429=24.425, p<0.0001, Fig. 3B). Exposure to nicotine during adolescence differently affected nicotine intake in the males and the females (Treatment x Sex x Session F13,429=2.097, p<0.05, Fig. 3B). The posthoc analysis showed that nicotine intake was higher in the nicotine-treated female rats than in the saline-treated female rats on the third day of nicotine self-administration. An area under the curve (AUC) analysis indicated that the female nicotine-treated rats self-administered more nicotine than the female control rats on Days 2–4 (female-saline 1.093 ± 1.517, female-nicotine 0.1493 ± 0.1531, t=2.066, df=52, P<0.05). The number of responses on the active lever decreased over time and during the first several sessions the female rats treated with nicotine responded more on the active lever than the female rats treated with saline (Session F13,429=17.57, p<0.0001; Treatment x Session F13,429=1.885, p<0.05; Treatment x Sex x Session F13,429=2.435, p<0.01, Fig. S2). Responding on the inactive lever increased and then decreased (Session F13,429=2.15, p<0.0001). The increase in inactive lever responding was more pronounced in the nicotine-treated rats than in the saline-treated rats (Treatment x Session F13,429=1.948, p<0.05, Fig. 3C).
Figure 3. Adolescent nicotine-treatment increases nicotine self-administration in female rats.
Rats treated with nicotine during early and mid-adolescence reached the maximum number of infusion (20) before rats treated with saline. The effect of nicotine pre-treatment on the speed of nicotine intake was most pronounced in the females (A). The rats had access to nicotine 5 days per week and total nicotine intake (B) and responding on the inactive lever (C) were recorded. Asterisks indicate a significant difference compared to the female-saline rats. Bonferroni post-hoc test: ** P < .01. N = 9–10/group. Abbreviations: LL, left lever. Data are expressed as means ± SEM.
An additional analysis was conducted to determine if there were sex differences in nicotine intake in the drug naïve control groups. To conduct this analysis, the data of the control animals of experiment 1 and 2 were combined (male-air, male-saline, female-air, female-saline. There was no sex difference in nicotine intake when the data from these two studies were combined (Fig. 4).
Figure 4. Similar levels of nicotine intake in male and female rats.
The self-administration data of the saline and air-control groups of the first 2 experiments were combined and it was determined if there was a sex difference in nicotine intake in late adolescent rats. The males and females self-administer the same amount of nicotine when they have access to a standard dose of nicotine, 0.03 mg/kg/inf under an FR1 schedule of reinforcement. N=18–19/group. Data are expressed as means ± SEM.
3.3. Experiment 3. Smoke exposure and nicotine and cotinine levels
Adolescent rats were exposed to smoke from SPECTRUM research cigarettes or nicotine, and blood samples were collected to determine plasma nicotine and cotinine levels. There was an effect of the treatment condition on plasma nicotine (F4,69=144.1, P<0.0001, Fig. 5A) and cotinine levels (F4,70=46.11, P<0.0001, Fig. 5B). The posthoc comparison showed that exposure to smoke from high-nicotine cigarettes led to higher plasma nicotine levels than exposure to smoke from low-nicotine cigarettes. The administration of nicotine led to a dose-dependent increase in plasma nicotine levels. Adolescent rats treated with 0.3 mg/kg of nicotine had higher plasma nicotine levels than rats treated with 0.1 mg/kg of nicotine, and rats treated with 0.6 mg/kg of nicotine had higher plasma nicotine levels than rats treated with 0.3 mg/kg of nicotine. Treatment with nicotine had a similar effect on plasma cotinine levels, with the exception that plasma cotinine levels were similar after treatment with 0.3 and 0.6 mg/kg of nicotine. There were no sex differences in the effect of tobacco smoke exposure or nicotine treatment on plasma nicotine and cotinine levels in the adolescent rats.
Figure 5. Nicotine and cotinine levels in rats after exposure to tobacco smoke or nicotine.
Adolescent male and female rats were exposed to tobacco smoke from low- or high-nicotine cigarettes for 1 h and samples were collected immediately after smoke exposure. Adolescent rats were also exposed to a low (0.1 mg/kg), intermediate (0.3 mg/kg), and high dose of nicotine (0.6 mg/kg) and blood samples were collected 15 min later. Asterisks indicate a higher level of nicotine or cotinine compared to rats exposed to tobacco smoke with a low level of nicotine. Dollar signs indicate a higher level of nicotine or cotinine compare to rats of the same sex treated with 0.1 mg/kg of nicotine. Plus signs indicate higher levels of nicotine compared to rats of the same sex treated with 0.3 mg/kg of nicotine. Bonferroni post-hoc test: * P < 0.05, **, $ $ P < 0.01, ***, +++, $ $ $ P < 0.001.. N=5–10/group. Abbreviations: NIC, nicotine. Data are expressed as means ± SEM.
4. Discussion
The goal of the present study was to investigate the effects of early- and mid-adolescent tobacco smoke exposure and nicotine treatment on the positive reinforcing properties of nicotine in late adolescence and early adulthood. The rats were treated with tobacco smoke or nicotine from P24–42, and the nicotine self-administration sessions started around P55. Exposure to tobacco smoke with a low level of nicotine during early- and mid-adolescence did not affect nicotine self-administration. Exposure to tobacco smoke with a high level of nicotine during the same developmental period increased nicotine intake in the females. The females that had been exposed to smoke with a high level of nicotine had a higher level of nicotine intake than the air-control females and the males exposed to smoke with a high level of nicotine. Exposure to nicotine during early- and mid-adolescence also facilitated nicotine intake. The nicotine-treated rats reached the maximum number of nicotine infusions before the saline-treated control rats, and this effect was more pronounced in the females. Furthermore, the nicotine-treated female rats self-administered more nicotine than the saline-treated females. Plasma nicotine and cotinine levels in the adolescent rats after exposure to tobacco smoke and nicotine were similar to those in smokers. These findings suggest that exposure to clinically relevant and rewarding doses of tobacco smoke and nicotine during early and mid-adolescence may facilitate tobacco or e-cigarette use during early adulthood. The present finding suggests that females are more sensitive to the long-term effects of adolescent nicotine exposure than males. Our animal work is in line with clinical research that suggests that females are also more sensitive to the effects of nicotine than males (O’Dell and Torres, 2014). Adolescent females who experiment with cigarettes are more likely to become regular smokers and nicotine dependent than adolescent males who experiment with cigarettes (Sylvestre et al., 2018).
In the present study, exposure to tobacco smoke with a high level of nicotine or nicotine during early- and mid-adolescence increased nicotine self-administration in the female rats. Exposure to nicotine during adolescence leads to an up-regulation of α4- and β2-nicotinic acetylcholine receptors (nAChRs) in the cerebral cortex of both male and female rats at P42 (Hoegberg et al., 2015). However, the nicotine-induced increase in α4- and β2-nAChR levels is greater in females than males at P42 (Hoegberg et al., 2015). This sex difference in nAChR upregulation could potentially be one of the mechanisms by which adolescent nicotine or smoke exposure leads to a higher level of nicotine self-administration in females than males. Adolescent (P31-P45) nicotine injections (0.35 mg/kg, SC; once daily for 15 days) increase locomotor activity to a similar degree in male and female rats (Quick et al., 2014). Therefore, it is unlikely that a long-term effect of nicotine or smoke exposure on locomotor activity led to the sex differences in nicotine intake. Only a few other studies have investigated the long-term effects of adolescent nicotine exposure on the rewarding effects of nicotine, and these studies reported conflicting findings. In one study, adolescent male rats were exposed to nicotine from P34–43, and the rewarding effects of nicotine were investigated with a place conditioning procedure in adulthood. Exposure to nicotine during adolescence decreased the rewarding effects of nicotine in adulthood (Adriani et al., 2006). In contrast, a place condition study with male mice suggests that exposure to nicotine during adolescence increases the rewarding effects of nicotine in adulthood (Kota et al., 2009). In a recent study, Kallupi et al. investigated the effects of adolescent nicotine vapor exposure on the self-administration of nicotine in adulthood (Kallupi et al., 2019). Adolescent male rats were exposed to vapor with two levels of nicotine (0.4 and 7 mg/m3). After the exposure period, both groups of rats displayed somatic withdrawal signs, which indicates that they had become dependent. Exposure to the low dose of nicotine did not affect nicotine intake, but exposure to the high dose led to a higher level of nicotine intake. The observation that the low dose of nicotine does not have a long-term effect on nicotine intake in male rats is in line with our work presented here. The work by Kallupi et al. suggests that exposure to vapor with a high level of nicotine during adolescence will increase nicotine intake later in life. In our study, plasma nicotine levels after tobacco smoke and nicotine exposure were like those in tobacco users (40–80 ng/ml). In the vapor study, in contrast, the adolescent rats were exposed to much higher levels of nicotine. The rats were exposed to vapor with a nicotine level of 7 mg/m3 (14 h/day), and a prior study by the same group showed that exposure to 1 mg/m3 (8 h session) leads to a plasma nicotine level of 140 ng/m3 (George et al., 2010). This suggests that in the recent vapor study, the plasma nicotine levels may have above 900 ng/ml. Therefore, the nicotine level in the rats exposed to the vapor with a high level of nicotine might have been ten times higher than in human smokers. Furthermore, signs of nicotine poisoning can be observed at about 200 ng/ml (Benowitz et al., 1987). Therefore, in the vapor study, rats might have exposed to nicotine levels that are toxic and aversive. Exposure to the vapor with a high concentration of nicotine (7 mg/m3) also led to a rapid 40% decrease in body weight (Kallupi et al., 2019). This is a substantial decrease in body weight especially considering the fact 3.2 mg/kg/day of nicotine, which leads to a plasma nicotine level of 65 ng/ml, rapidly leads to dependence in rats but does not cause weight loss (Grunberg et al., 1984; O’Dell et al., 2006; Paterson et al., 2007). Nonetheless, it is an interesting observation that exposure to vapor with a high level of nicotine leads to a higher level of nicotine intake later in life. It remains to be determined if exposing adolescent male rats to nicotine levels that are somewhat higher than those in our study, but lower than those in the study by Kallupi et al. would also increase nicotine intake later in life.
In the present study, we investigated the effects of tobacco smoke exposure and nicotine treatment on plasma nicotine and cotinine levels. The adolescent rats were exposed to tobacco smoke from low (NRC200) or high (NRC600) nicotine SPECTRUM cigarettes, or they were treated with a low (0.1 mg/kg), intermediate (0.3 mg/kg), or high (0.6 mg/kg) dose of nicotine. Exposure to tobacco smoke from low-nicotine cigarettes led to a plasma nicotine level of about 6 ng/ml, and exposure to smoke from high-nicotine cigarettes led to a plasma nicotine level of about 40 ng/ml. The plasma nicotine levels after exposure to smoke from the high-nicotine cigarettes (NRC 600) were similar to those in humans after smoking these high-nicotine cigarettes (Kamens et al., 2019). Similar nicotine levels were also observed in a heavy smoker after smoking their regular cigarettes (Russell et al., 1976). The adolescent rats were also exposed to nicotine in order to be able to compare plasma nicotine levels after smoke exposure with those after treatment with widely used doses of nicotine (Harrison et al., 2002; Le Foll and Goldberg, 2005). In our previous work, we showed that 0.1 and 0.3 mg/kg of nicotine stimulates the brain reward system in adolescent rats as measured with the ICSS procedure (Xue et al., 2018). The 0.6 mg/kg dose elevates the brain reward thresholds in adult rats in the ICSS procedure, which indicates that this dose is aversive (Igari et al., 2013). This dose might, however, been rewarding in adolescent rats (Torres et al., 2008). In the present study, we found that plasma nicotine levels were higher after the administration of 0.6 mg/kg of nicotine than after the administration of 0.3 mg/kg of nicotine. In contrast, there was no significant difference in cotinine levels after the administration of 0.3 and 0.6 mg/kg of nicotine. Plasma nicotine levels peak 10 min after SC nicotine administration in early adolescent rats (P25) and cotinine levels peak 2 h after SC nicotine administration (Craig et al., 2014). Therefore, it might be possible that significant differences in cotinine levels between the 0.3 and 0.6 mg/kg of nicotine groups could have been detected at later time point. Overall, these findings indicate that exposure to tobacco smoke with a high level of nicotine and 0.3 mg/kg of nicotine induce similar plasma nicotine levels in rats as in tobacco users.
When the nicotine intake data from the control groups of the two self-administration studies were combined, there was no difference in nicotine intake between the males and the females. In our study, the rats were tested under an FR1 schedule and received a standard dose (0.03 mg/kg/inf) of nicotine. This is in line with our previous studies in which we found no sex difference in nicotine intake (FR1, 0.03 mg/kg/inf), and no sex difference in the rewarding effects of nicotine in adult rats (Chellian et al., 2019; Xue et al., 2018). Several other studies did not observe a sex difference in nicotine intake when standard nicotine doses (0.03–0.06 mg/kg/infusion) were used and the rats were tested under fixed-ratio schedules (Chaudhri et al., 2005; Donny et al., 2000; Feltenstein et al., 2012; Grebenstein et al., 2013). Experimental evidence suggests that females have higher levels of nicotine intake when the rats are tested under a progressive ratio (PR) schedule and during the acquisition phase of nicotine self-administration (Chen et al., 2007; Donny et al., 2000; Park et al., 2007). Flores et al. recently conducted a meta-analysis to determine if there are sex differences in the self-administration of nicotine (Flores et al., 2017). The meta-analysis included operant schedules with different response requirements, adolescent and adult rats, and rats with short access and long access (23 h) to nicotine. Results of the random effect analysis showed that when all studies were combined, the females self-administered more nicotine than the males. The sex differences were mainly observed with higher response requirements (>FR1) and when the rats had long access to nicotine (23 h). The meta-analysis did not identify a significant difference in nicotine intake between the males and the females when the rats were tested under an FR1 schedule. Our finding is in line with previous studies that there are no sex differences in nicotine intake when the rats are tested under an FR1 schedule of reinforcement and have access to an intermediate dose (0.03 mg/kg/inf) of nicotine.
Before the onset of the experiments, it was hypothesized that exposure to tobacco smoke or nicotine during adolescence would lead to a greater increase in nicotine self-administration in females than males. We observed an effect of nicotine and smoke exposure in the females, but there were no long-term effects of nicotine and smoke exposure in males. It cannot be ruled out that an effect of adolescent tobacco smoke or nicotine exposure in male rats could have been detected under different test conditions. Previous work has shown that noncontingent drug administration may increase the acquisition of drug self-administration (Lacy et al., 2018). In the present study, the rats were trained to respond for food pellets before the onset of nicotine self-administration, which leads to a relatively high level of nicotine intake during the first self-administration sessions. Without food training, nicotine intake gradually increases over time, and it might be possible that adolescent tobacco smoke or nicotine exposure affects the acquisition of nicotine intake in male rats. Furthermore, in the present study, the rats were tested under an FR schedule, and the rats had a high level of nicotine intake. Therefore, the lack of effect of adolescent tobacco smoke and nicotine exposure might have been due to a ceiling effect. Future studies may investigate if differences between nicotine exposed and control animals can be detected with progressive ratio schedules or with behavioral economics procedures (Hursh and Roma, 2013; Stafford et al., 1998). It is unlikely that the lack of long-term effects of early and mid-adolescent tobacco smoke and nicotine exposure in the males was due to low nicotine levels. Plasma nicotine levels in the adolescent rats were similar to those in smokers, and previous work has shown that these nicotine levels are rewarding in adolescent male and female rats (Kamens et al., 2019; Xue et al., 2018).
In conclusion, the present study suggests that exposure to tobacco smoke and nicotine during early- and mid-adolescence increases nicotine self-administration in females, but not in the males. Consequently, exposure to nicotine during early and mid-adolescence might increase the risk for tobacco and e-cigarette use in females later in life. These studies also showed that in contrast to high-nicotine cigarettes, low nicotine cigarettes do not have a long-term effect on nicotine intake in female rats. Therefore, the low-nicotine cigarettes may be less likely to cause nicotine addiction than the high-nicotine cigarettes.
Supplementary Material
Acknowledgments:
We thank the NIDA Drug Supply Program for providing the Nicotine Research Cigarettes.
Funding: This work was supported by a NIDA/NIH and FDA Center for Tobacco Products (CTP) grant (DA042530) and NIDA grant (DA046411) to AB. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Footnotes
Declarations of interest: none
References
- Abobo CV, Ma J, Liang D, 2012. Effect of menthol on nicotine pharmacokinetics in rats after cigarette smoke inhalation. Nicotine Tob Res 14, 801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriani W, Deroche-Gamonet V, Le MM, Laviola G, Piazza PV, 2006. Preexposure during or following adolescence differently affects nicotine-rewarding properties in adult rats. Psychopharmacology (Berl) 184, 382–390. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, 2010. Nicotine addiction. N Engl J Med 362, 2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Lake T, Keller KH, Lee BL, 1987. Prolonged absorption with development of tolerance to toxic effects after cutaneous exposure to nicotine. Clinical Pharmacology & Therapeutics 42, 119–120. [DOI] [PubMed] [Google Scholar]
- Bracken AL, Chambers RA, Berg SA, Rodd ZA, McBride WJ, 2011. Nicotine exposure during adolescence enhances behavioral sensitivity to nicotine during adulthood in Wistar rats. Pharmacology Biochemistry and Behavior 99, 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brielmaier JM, McDonald CG, Smith RF, 2007. Immediate and long-term behavioral effects of a single nicotine injection in adolescent and adult rats. Neurotoxicol Teratol 29, 74–80. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Prado M, Isaac S, 2009. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol.Psychiatry 66, 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmines E, Gillman IG, 2019. Comparison of the Yield of Very Low Nicotine Content Cigarettes to the Top 100 United States Brand Styles. Beiträge zur Tabakforschung International/Contributions to Tobacco Research 28, 253–266. [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA, 2005. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology 180, 258–266. [DOI] [PubMed] [Google Scholar]
- Chellian R, Wilson R, Polmann M, Knight P, Behnood-Rod A, Bruijnzeel AW, 2019. Evaluation of sex differences in the elasticity of demand for nicotine and food in rats. Nicotine & Tobacco Research 22, 925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Matta SG, Sharp BM, 2007. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology 32, 700–709. [DOI] [PubMed] [Google Scholar]
- Congress U, 2009. Family smoking prevention and tobacco control federal reform act. Pub. Law, 2009. [Google Scholar]
- Craig EL, Zhao B, Cui JZ, Novalen M, Miksys S, Tyndale RF, 2014. Nicotine pharmacokinetics in rats is altered as a function of age, impacting the interpretation of animal model data. Drug Metab Dispos 42, 1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF, 1998. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology 136, 83–90. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S, 2000. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 151, 392–405. [DOI] [PubMed] [Google Scholar]
- Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, al’Absi M, Carmella SG, Cinciripini PM, Dermody SS, Drobes DJ, Hecht SS, Jensen J, Lane T, Le CT, McClernon FJ, Montoya ID, Murphy SE, Robinson JD, Stitzer ML, Strasser AA, Tindle H, Hatsukami DK, 2015. Randomized Trial of Reduced-Nicotine Standards for Cigarettes. N Engl J Med 373, 1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraday MM, Elliott BM, Phillips JM, Grunberg NE, 2003. Adolescent and adult male rats differ in sensitivity to nicotine’s activity effects. Pharmacology Biochemistry and Behavior 74, 917–931. [DOI] [PubMed] [Google Scholar]
- FDA, 2018. Tobacco product standard for nicotine level of combusted cigarettes. Fed Regist 83, 11818–11843. [Google Scholar]
- FDA, 2019. FDA permits sale of two new reduced nicotine cigarettes through premarket tobacco product application pathway.
- Feltenstein MW, Ghee SM, See RE, 2012. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug & Alcohol Dependence 121, 240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores RJ, Uribe KP, Swalve N, O’Dell LE, 2017. Sex differences in nicotine intravenous self-administration: A meta-analytic review. Physiology & behavior 203, 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Grieder TE, Cole M, Koob GF, 2010. Exposure to chronic intermittent nicotine vapor induces nicotine dependence. Pharmacology Biochemistry and Behavior 96, 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebenstein P, Burroughs D, Zhang Y, LeSage MG, 2013. Sex differences in nicotine self-administration in rats during progressive unit dose reduction: implications for nicotine regulation policy. Pharmacol Biochem Behav 114–115, 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg NE, Bowen DJ, Morse DE, 1984. Effects of nicotine on body weight and food consumption in rats. Psychopharmacology (Berl) 83, 93–98. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A, 2002. Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 160, 56–66. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Mactutus CF, Bennett K, Hasselrot U, Wu G, Welch M, Booze RM, 2004. Sex differences and repeated intravenous nicotine: behavioral sensitization and dopamine receptors. Pharmacology Biochemistry and Behavior 78, 581–592. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Heishman SJ, Vogel RI, Denlinger RL, Roper-Batker AN, Mackowick KM, Jensen J, Murphy SE, Thomas BF, Donny E, 2012. Dose–response effects of spectrum research cigarettes. Nicotine & Tobacco Research 15, 1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegberg BG, Lomazzo E, Lee NH, Perry DC, 2015. Regulation of alpha4beta2alpha5 nicotinic acetylcholinergic receptors in rat cerebral cortex in early and late adolescence: Sex differences in response to chronic nicotine. Neuropharmacology 99, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Roma PG, 2013. Behavioral economics and empirical public policy. Journal of the experimental analysis of behavior 99, 98–124. [DOI] [PubMed] [Google Scholar]
- Igari M, Alexander JC, Ji Y, Qi X, Papke RL, Bruijnzeel AW, 2013. Varenicline and cytisine diminish the dysphoric-like state associated with spontaneous nicotine withdrawal in rats. Neuropsychopharmacology 39, 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, de Guglielmo G, Larrosa E, George O, 2019. Exposure to passive nicotine vapor in male adolescent rats produces a withdrawal-like state and facilitates nicotine self-administration during adulthood. European Neuropsychopharmacology 29, 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Silva CP, Nye RT, Miller CN, Singh N, Sipko J, Trushin N, Sun D, Branstetter SA, Muscat JE, 2019. Pharmacokinetic profile of Spectrum reduced nicotine cigarettes. Nicotine & Tobacco Research 22, 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Robinson SE, Damaj MI, 2009. Enhanced nicotine reward in adulthood after exposure to nicotine during early adolescence in mice. Biochem Pharmacol 78, 873–879. [DOI] [PubMed] [Google Scholar]
- Lacy RT, Schorsch HK, Austin BP, 2018. Adolescent d-amphetamine exposure enhances the acquisition of cocaine self-administration in male and female rats. Exp Clin Psychopharmacol 26, 18. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR, 2005. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl) 178, 481–492. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, 2009. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacology Biochemistry and Behavior 94, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech R, Johnston L, O’Malley PM, Bachman JG, Patrick ME, 2019. Trends in Adolescent Vaping, 2017–2019. New England Journal of Medicine 381, 1490–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemati F, Kolb B, 2012. Recovery from medial prefrontal cortex injury during adolescence: implications for age-dependent plasticity. Behav Brain Res 229, 168–175. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A, 2006. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology (Berl) 186, 612–619. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV, 2014. A mechanistic hypothesis of the factors that enhance vulnerability to nicotine use in females. Neuropharmacology 76 Pt B, 566–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Belluzzi JD, Han S-H, Cao J, Leslie FM, 2007. Age, sex and early environment contribute to individual differences in nicotine/acetaldehyde-induced behavioral and endocrine responses in rats. Pharmacology Biochemistry and Behavior 86, 297–305. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A, 2007. Chronic bupropion attenuated the anhedonic component of nicotine withdrawal in rats via inhibition of dopamine reuptake in the nucleus accumbens shell. European Journal of Neuroscience 25, 3099–3108. [DOI] [PubMed] [Google Scholar]
- Qi X, Yamada H, Corrie LW, Ji Y, Bauzo RM, Alexander JC, Bruijnzeel AW, 2015. A critical role for the melanocortin 4 receptor in stress-induced relapse to nicotine seeking in rats. Addict Biol 20, 324–335. [DOI] [PubMed] [Google Scholar]
- Quick SL, Olausson P, Addy NA, Taylor JR, 2014. Repeated nicotine exposure during adolescence alters reward-related learning in male and female rats. Behav Brain Res 261, 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter P, Pappas RS, Bravo R, Lisko JG, Damian M, Gonzales‐Jimenez N, Gray N, Keong LM, Kimbrell JB, Kuklenyik P, 2016. Characterization of SPECTRUM variable nicotine research cigarettes. Tobacco regulatory science 2, 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MA, Feyerabend C, Cole PV, 1976. Plasma nicotine levels after cigarette smoking and chewing nicotine gum. Br Med J 1, 1043–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent JD, Gabrielli J, Budney A, Soneji S, Wills TA, 2017. Adolescent smoking experimentation as a predictor of daily cigarette smoking. Drug and Alcohol Dependence 175, 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schassburger RL, Pitzer EM, Smith TT, Rupprecht LE, Thiels E, Donny EC, Sved AF, 2016. Adolescent rats self-administer less nicotine than adults at low doses. Nicotine & Tobacco Research 18, 1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small E, Shah HP, Davenport JJ, Geier JE, Yavarovich KR, Yamada H, Sabarinath SN, Derendorf H, Pauly JR, Gold MS, Bruijnzeel AW, 2010. Tobacco smoke exposure induces nicotine dependence in rats. Psychopharmacology (Berl) 208, 143–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, 2015. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol Behav 148, 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR, 1998. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology (Berl) 139, 169–184. [DOI] [PubMed] [Google Scholar]
- Sylvestre M-P, Chagnon M, Wellman RJ, Dugas EN, O’Loughlin J, 2018. Sex differences in attaining cigarette smoking and nicotine dependence milestones among novice smokers. American journal of epidemiology 187, 1670–1677. [DOI] [PubMed] [Google Scholar]
- Teague SV, Pinkerton KE, Goldsmith M, Gebremichael A, Chang S, Jenkins RA, Moneyhun JH, 1994. A sidestream cigarette smoke generation and exposure system for environmental tobacco smoke studies. Inhalation Toxicology 6, 79–93. [Google Scholar]
- Tirelli E, Laviola G, Adriani W, 2003. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neuroscience & Biobehavioral Reviews 27, 163–178. [DOI] [PubMed] [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O’Dell LE, 2008. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav 90, 658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman RJ, DiFranza JR, Savageau JA, Dussault G, 2004. Short term patterns of early smoking acquisition. Tob Control 13, 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2018. WHO global report on trends in prevalence of tobacco smoking 2000–2025, Second edition., Geneva. [Google Scholar]
- Xue S, Behnood-Rod A, Wilson R, Wilks I, Tan S, Bruijnzeel AW, 2018. Rewarding Effects of Nicotine in Adolescent and Adult Male and Female Rats as Measured Using Intracranial Self-stimulation. Nicotine & Tobacco Research 22, 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Bishnoi M, Keijzers KF, van Tuijl IA, Small E, Shah HP, Bauzo RM, Kobeissy FH, Sabarinath SN, Derendorf H, Bruijnzeel AW, 2010. Preadolescent tobacco smoke exposure leads to acute nicotine dependence but does not affect the rewarding effects of nicotine or nicotine withdrawal in adulthood in rats. Pharmacol.Biochem.Behav. 95, 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Bruijnzeel AW, 2011. Stimulation of alpha2-adrenergic receptors in the central nucleus of the amygdala attenuates stress-induced reinstatement of nicotine seeking in rats. Neuropharmacology 60, 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW, 2007. Effects of the CRF receptor antagonist D-Phe CRF(12–41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology 58, 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.