Abstract
Attention-deficit/hyperactivity disorder has been identified to involve the impairment of large-scale functional networks within grey matter, and recent studies have suggested that white matter, which also encodes neural activity, can manifest intrinsic functional organization similar to that of grey matter. However, the alterations in white matter functional networks in attention-deficit/hyperactivity disorder remain unknown. We recruited a total of 99 children, including 66 drug-naive patients and 33 typically developing controls aged from 6 to 14, to characterize the alterations in functional networks within white matter in drug-naive children with attention-deficit/hyperactivity disorder. Using clustering analysis, resting-state functional MRI data in the white matter were parsed into different networks. Intrinsic activity within each network and connectivity between networks and the associations between network activity strength and clinical symptoms were assessed. We identified eight distinct white matter functional networks: the default mode network, the somatomotor network, the dorsal attention network, the ventral attention network, the visual network, the deep frontoparietal network, the deep frontal network and the inferior corticospinal-posterior cerebellum network. The default mode, somatomotor, dorsal attention and ventral attention networks showed lower spontaneous neural activity in patients. In particular, the default mode network and the somatomotor network largely showed higher connectivity with other networks, which correlated with more severe hyperactive behaviour, while the dorsal and ventral attention networks mainly had lower connectivity with other networks, which correlated with poor attention performance. In conclusion, there are two distinct patterns of white matter functional networks in children with attention-deficit/hyperactivity disorder, with one being the hyperactivity-related hot networks including default mode network and somatomotor network and the other being inattention-related cold networks including dorsal attention and ventral attention network. These results extended upon our understanding of brain functional networks in attention-deficit/hyperactivity disorder from the perspective of white matter dysfunction.
Keywords: attention-deficit/hyperactivity disorder, white matter, network, resting state functional MRI
There are two distinct patterns of white matter functional networks in children with attention-deficit/hyperactivity disorder. One is the hyperactive behaviour-related hot networks including default mode network and somatomotor network, and the other is inattention-related cold networks including dorsal attention and ventral attention network.
Graphical Abstract
Graphical Abstract.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a prevalent neurodevelopmental disorder typically diagnosed in childhood and adolescence that is characterized by impaired attention and/or impulsiveness–hyperactivity (Faraone et al., 2015). Previous neuroimaging studies have led to the prominence of neural circuits or neural networks, rather than isolated brain regions, as substrates for ADHD neuropathology (Konrad and Eickhoff, 2010; Castellanos and Proal, 2012; Gallo and Posner, 2016; Samea et al., 2019). For example, many resting-state fMRI studies have consistently reported reduced functional connectivity within the default mode network (DMN), impaired anti-correlation between the DMN and the frontoparietal network (FPN) and decreased functional connectivity in attention networks (Kessler et al., 2014). A recent large (700 patients with ADHD and 580 controls) meta-analysis (Gao et al., 2019) of seed-based resting-state functional connectivity studies confirmed functional connectivity alterations in several networks, including the DMN, the FPN, the affective network, the somatosensory network and the ventral attention network (VAN), among patients with ADHD. Moreover, dysfunction of these grey matter (GM) networks has been shown to be associated with ADHD symptoms and several disrupted neurocognitive functions (de Lacy et al., 2018).
Recent studies (Ding et al., 2013, 2016; Ji et al., 2017; Huang et al., 2018) have provided robust evidence that blood-oxygen-level-dependent (BOLD) signal fluctuations in white matter (WM) are modulated by neural activity and are detectable with fMRI. WM has the vascular capacity to support hemodynamic changes detected by fMRI even with a lower density of vasculature than GM; the neural activity in WM is mainly linked to spiking, which reflects action potentials, and there is evidence provided that spiking activity may sufficient for inducing hemodynamic responses (Gawryluk et al., 2014). Therefore, these suggest that fMRI signal in WM is BOLD and is modulated by neural activity.
There is evidence supporting the functional contribution of WM. The BOLD signal in WM tracts was first detected during both event-related task and resting state and was found to be greater under stimulation than in a resting state (Wu et al., 2017; Ding et al., 2018). The detectability of neural activities was later confirmed in specific WM tracts, which strongly correlated with neural signals in cortical regions that are connected by these tracts (Li et al., 2019). The existence of an intrinsic functional network organization of WM tracts was revealed with a hierarchical structure of temporally correlated fMRI signal in WM and further demonstrated that WM functional networks interact in a similar way as GM networks (Marussich et al., 2017; Peer et al., 2017). More recently, functional connectome architecture in WM including small-world topology, high-degree hubs, and a non-random modular organization was also reported (Li et al., 2019). These results converged to provide evidence that WM networks underlying different GM networks carry synchronized activity within themselves and bear different functions. Dysfunction of WM has been detected in neuropsychiatric disorders such as epilepsy and schizophrenia (Jiang et al., 2019a, b), however no study had been carried out to explore the functionality of WM in ADHD yet.
Therefore, in this study, we explored large-scale WM functional networks for the first time by applying a clustering approach to resting-state fMRI data in children with ADHD and typically developing controls (TDC). In particular, we compared differences in spontaneous activity within each network and functional connectivity between networks between children with ADHD and TDC, we studied the relationships between WM network and clinical symptoms, and we further investigated their relationship with known GM networks. As prior studies have shown wide-spread disruptions of WM microstructure in pathways connecting prefrontal and parietooccipital areas with the striatum and the cerebellum (Silk et al., 2009; Qiu et al., 2012; Hong et al., 2014; Wu et al., 2014) and have reported altered interactions among DMN, FPN and attentional networks as aforementioned, we hypothesized that children with ADHD would present dissociated connectivity between DMN and FPN, hyperactive DMN and hypoactive attentional networks in WM and that these functional abnormalities in WM networks would be associated with clinical symptoms of ADHD.
Materials and methods
Subjects
The Ethics Committee of The First Affiliated Hospital of Wenzhou Medical University approved this study. All participants and their parents were fully informed about the purpose and procedures of this study, and written informed consent was obtained from the parents.
A total of 99 children (66 drug-naive ADHD and 33 TDC) between the ages of 6 and 14 were recruited at the Department of Psychiatry, The First Affiliated Hospital of Wenzhou Medical University from June 2013 to March 2018. ADHD diagnosis was evaluated with the Diagnostic and Statistical Manual of Mental Disorders 5th Edition (American Psychiatric Association, 2013) and the schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL) (Kaufman et al., 2000). TDC were recruited from local primary schools and were screened by the same psychiatrists. All subjects were right-handed and were required to have a full-scale IQ above 90 on the Wechsler Intelligence Scale for Children–Chinese Revision (Gong and Cai, 1994).
Children in both groups were excluded if they were left-handed, had a history of neurological illness, had a comorbidity of other Diagnostic and Statistical Manual of Mental Disorders 5th Edition mental disorders, were receiving psychotropic medications or had significant head trauma. The first-degree relatives reported no history of psychiatric diagnosis. We excluded eight children (ADHD n = 7; TDC n = 1) with framewise displacement >0.2 mm during fMRI scanning and two children (ADHD n = 1; TDC n = 1) because their resting-state functional signal was >3 standard deviations from their own group’s mean. Thus, our final sample included 58 children with ADHD (47 males and 11 females; mean age 9.10 ± 2.22 years) and 31 TDC (21 males and 10 females; mean age 8.87 ± 1.52 years) matched with the ADHD group by age and gender. Demographic details are listed in Table1.
Table 1.
Demographic and clinical characteristics of TDC and children with ADHD
| Clinical Values | Group |
Statistics |
||
|---|---|---|---|---|
| ADHD | TDC | t/χ | P | |
| Sample size | 58 | 31 | ||
| Age (years), mean (SD) | 9.10 (2.22) | 8.87 (1.52) | 0.58 | 0.56 |
| Sex (male:female) | 47:11 | 21:10 | 1.98 | 0.16 |
| IQ scores, mean (SD) | 117.91 (12.37) | 121.45 (14.11) | −1.22 | 0.22 |
| Conners’ Parent Rating Scale, mean (SD) | ||||
| Conduct problem | 1.07 (0.49) | 0.43 (0.43) | 6.05 | <0.001 |
| Psychosomatic | 0.38 (0.41) | 0.13 (0.22) | 3.77 | <0.001 |
| Anxiety | 0.48 (0.38) | 0.34 (0.27) | 1.74 | 0.09 |
| Study problem | 1.88 (0.61) | 0.64 (0.59) | 9.34 | <0.001 |
| Hyperactivity–impulsivity | 1.50 (0.68) | 0.51 (0.48) | 8.01 | <0.001 |
| Hyperactivity index | 1.42 (0.53) | 0.49 (0.39) | 8.67 | <0.001 |
| Continuous Performance Test, mean (SD) | ||||
| Full-scale control quotient | 72.24 (23.99) | 79.61 (22.94) | −1.40 | 0.16 |
| Visual control quotient | 75.24 (25.26) | 81.74 (24.07) | −1.18 | 0.24 |
| Auditory control quotient | 74.48 (23.80) | 80.32 (23.80) | −1.10 | 0.27 |
| Full-scale attention quotient | 71.55 (22.85) | 94.39 (20.38) | −4.66 | <0.001 |
| Visual attention quotient | 73.36 (22.56) | 93.45 (18.70) | −4.24 | <0.001 |
| Auditory attention quotient | 72.43 (25.16) | 94.61 (20.45) | −4.49 | <0.001 |
The parents of all enrolled children completed the Chinese version of the revised Conners’ Parent Rating Scale (CPRS) to evaluate the behavioural problems of children with ADHD. This scale has been proven to have good reliability and validity among Chinese children with ADHD (Conners et al., 1998; Su et al., 2001). An Integrated Visual and Auditory Continuous Performance Test (IVA-CPT) (Tinius, 2003) was used to measure sustained attention and response control at the auditory and visual levels. We obtained six indexes—full-scale control/attention quotient, visual control/attention quotient and auditory control/attention quotient—for further analysis.
Image acquisition
All subjects were scanned using a GE signal HDx 3 T MR scanner with an eight-channel phase-array head coil. The data for each subject consisted of an 8-min resting-state EPI scan (31 axial slices, slice thickness = 4 mm, slice gap = 0.2 mm, repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°, matrix size = 64 × 64 and field of view = 192 mm) and a high-resolution T1-weighted structural scan acquired with a spoiled gradient-recalled echo sequence (repetition time = 7200 ms, echo time = 2.2 ms, 176 axial slices, slice thickness = 1 mm, flip angle = 7°, matrix size = 256 × 256, field of view =256 mm).
Data preprocessing
Preprocessing was performed using SPM8 (www.fil.ion.ucl.ac.uk/spm), DPABI (http://rfmri.org/dpabi) and open MATLAB scripts (http://mind.huji.ac.il/white-matter.aspx). T1 images were skull-stripped, segmented into WM, GM and cerebrospinal fluid and then normalized to the Montreal Neurological Institute template.
The preprocessing steps for functional images included the following: (1) removal of the first 10 time points for signal stabilization; (2) slice time correction; (3) realignment to the median volume and coregistration with the structural image; all registrations were visually inspected; (4) removal of linear trends to correct for signal drift; (5) nuisance regression, including 24-parameter motion correction (6 rigid-body motion parameters, their values at the previous time point and the 12 corresponding squared values) and the mean cerebrospinal fluid signals. The WM and global brain signals were not regressed out because this could have eliminated signals of interest; (6) we also used a rigorous approach to minimize the potential effects of head motion as suggested by recent publications (Power et al., 2012, 2014; Yan et al., 2013), namely temporal scrubbing using motion ‘spikes’ (framewise displacement >0.2 mm) as separate repressors to effectively censor the data at the spike without further changing the correlation values; (7) band-pass filtering (0.01–0.15 Hz) to reduce non-neuronal contributions to BOLD fluctuations; (8) spatial smoothing (4-mm FWHM) on the WM and GM separately to avoid mixing their signals; (9) normalization to the Montreal Neurological Institute template and spatially resampling to a voxel size of 3 × 3 × 3 mm3 using the DARTEL. We also examined head motion differences between the ADHD group and the TDC group using the two-sample t-test, and the group-level statistical analysis showed no difference (Supplementary Table 1).
Construction of WM functional networks
WM network clustering was performed using an analysis pipeline that was described in a previous study (Peer et al., 2017). In brief, the steps were as follows: WM and GM masks were created to select voxels for clustering across all the children: We used the T1 segmentation results of each subject to create WM and GM masks, which are used in following clustering analysis. We identified each voxel as WM, GM or cerebrospinal fluid based on its maximum probability in the segmentation images, which resulted in WM, GM and cerebrospinal fluid mask for each subject. Then, we averaged these masks across subjects and for each voxel we calculated the percentage of subjects that was classified as WM or GM. For WM, voxels identified in >60% of subjects were used for group-level mask, whereas for GM, threshold of >20% was used. Meanwhile, we only keep voxels identified as WM or GM and containing functional data in >80% of the subjects. Finally, we used the Harvard–Oxford atlas (Desikan et al., 2006) to remove subcortical structures including thalamus, caudate nucleus, putamen, globus pallidus and nucleus accumbens from WM mask. Pearson's correlation coefficients between each WM voxel and other WM voxels were computed for each subject and then averaged across all 89 subjects to obtain a group-level correlation matrix. K-means clustering was performed on the average group-level correlation matrix to identify the clusters of WM voxels with similar connectivity patterns to the rest of the voxels. The number of clusters ranged from 2 to 22, and the stability of clustering was measured for each number of clusters by Dice's coefficient.
Functional activity within WM networks
Signal amplitudes were extracted from each WM network using Fourier transform (MATLAB's FFT function). The resulting frequency graphs were averaged separately across subjects in the ADHD and TDC groups to produce an average frequency–power graph for each network. For each WM network, the averaged amplitudes were obtained for each subject, which were then used to compare the differences between the ADHD and TDC groups.
Functional connectivity between WM networks
We extracted the average time courses from each WM network by averaging across all voxels belonging to the network for each subject. The Pearson's correlation between the average time courses of any two WM networks was computed for each subject and then transformed to the Fisher z-score for the statistical analysis.
Furthermore, we assessed the functional relationships between WM and GM networks to explore whether the WM networks act in concert with GM networks. We first identified a number of functional networks within the GM by the same clustering procedure. Then, average time courses from each GM network were extracted. The temporal correlations were calculated between each GM network and each WM network.
Correlations between WM functional networks and clinical measures
We further investigated the relationships between clinical measures (CPRS scores and IVA-CPT scores) and functional connectivity and signal amplitudes by Pearson correlation analysis with age as a covariant.
Statistical analysis
For demographic data, we performed two-sample t-test or χ2 test to investigated group differences. For the functional activity within WM networks and functional connectivity between WM networks, we used the analysis of covariance with age and gender as covariates to detect the differences between ADHD and TDC.
We analysed the relationships between clinical measures (CPRS scores and IVA-CPT scores) and functional connectivity and signal amplitudes by Pearson correlation analysis with age as a covariant.
For all analyses, P < 0.05 was used to determine significance. To address concerns that may arise from multiple comparisons across the eight networks, we used Bonferroni correction with the threshold of P < 0.05.
Data availability
Data from this study are available for researches upon reasonable request.
Results
WM networks
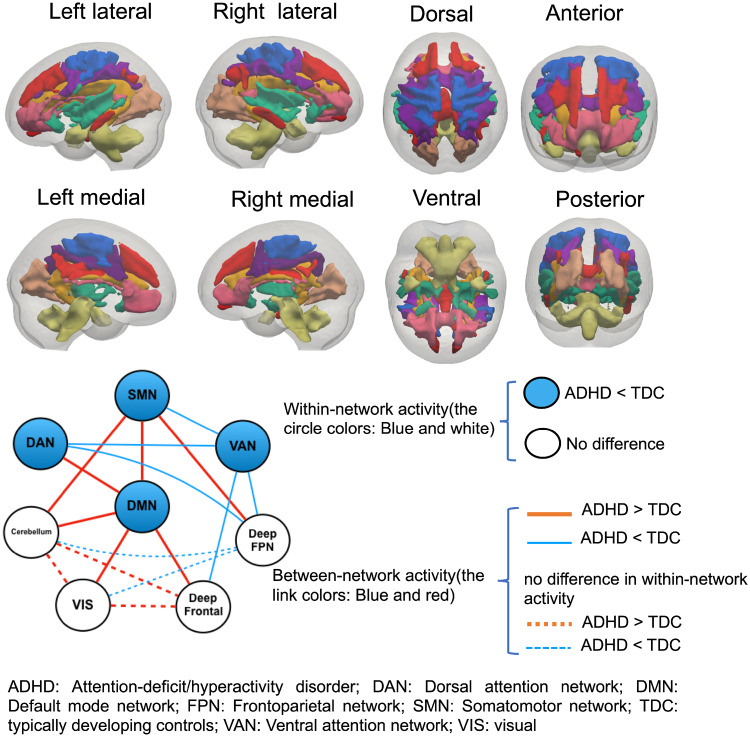
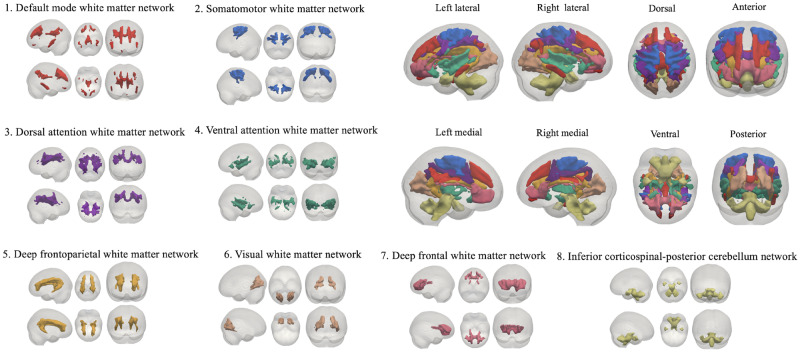
Using a clustering approach on the resting-state voxelwise correlation matrices of WM, we identified the eight most stable and proper WM functional segregations among our subjects according to Dice's coefficient (Supplementary Fig. 1). Therefore, we conducted analyses based on the resulting eight networks and defined them according to the correspondence between our WM networks and the known resting-state GM networks (Yeo et al., 2011). The identified networks were the DMN, the somatomotor network (SMN), the dorsal attention network (DAN), the VAN, the visual network, the deep FPN, the deep frontal network and the inferior corticospinal-posterior cerebellum network. The presentation of the eight networks is presented in Fig. 1.
Figure 1.
Eight WM functional networks were identified by clustering analysis: (1) the DMN, (2) the SMN, (3) the DAN, (4) the VAN, (5) the visual network, (6) the deep FPN, (7) the deep frontal network and (8) the inferior corticospinal-posterior cerebellum network.
Functional activity within WM networks
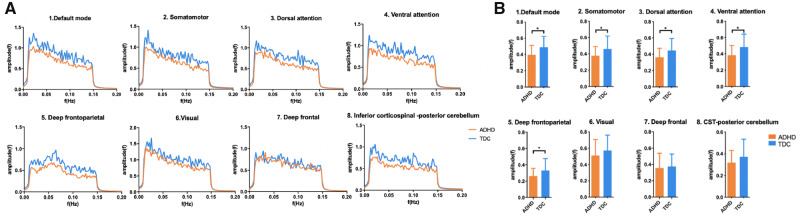
For all WM networks, the signal amplitudes were derived by Fourier transform, and the values corresponding to the frequency are shown in Fig. 2A. A decreasing trend in amplitude with increased frequency was found in each network, suggesting greater activity in WM at lower frequencies. All the networks except for the deep FPN exhibited maximal activity at the lowest frequency of 0.01 Hz, whereas the deep FPN showed a maximal amplitude at ∼0.07 Hz. Compared with TDC, children with ADHD showed lower amplitudes in all the networks, with the DMN, the SMN, the deep FPN, the DAN and the VAN exhibiting significant differences after correction (Fig. 2B).
Figure 2.
Analysis of signal amplitude for each WM functional network. (A) Power–frequency graphs showing spontaneous neural activity in each WM network. (B) Differences in the average amplitude of each network between children with ADHD and TDC. Asterisks represent significant differences with age and gender as covariates (*P < 0.05, Bonferroni corrected).
Functional connectivity between WM networks
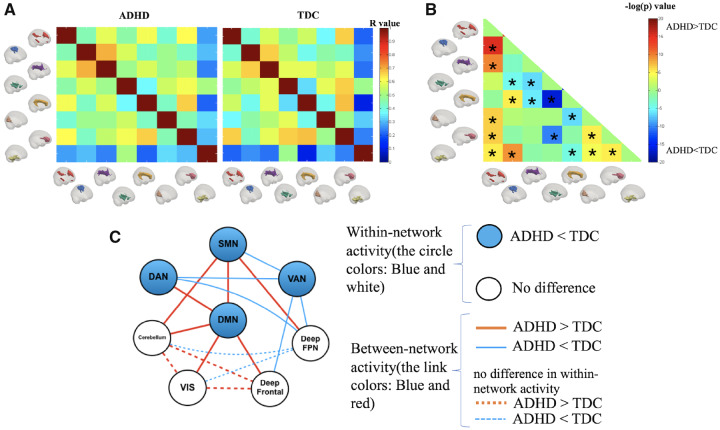
For each group, an 8 × 8 matrix of pairwise network correlation coefficients was calculated (Fig. 3A). These matrices were then compared between the two groups to reveal differences in functional connectivity, as noted in Fig. 3B. We identified that the between-network connectivity of the following 10 network pairs was significantly higher in children with ADHD: DMN–SMN, DMN–DAN, DMN–cerebellum, DMN–Visual, DMN–deep frontal, SMN–deep FPN, SMN–cerebellum, Visual–cerebellum, Visual–deep frontal and cerebellum–deep frontal. In contrast, children with ADHD showed lower connectivity between the following seven network pairs: VAN–SMN, VAN–DAN, VAN–deep FPN, VAN–deep frontal, deep FPN–DAN, deep FPN–Visual and deep FPN–cerebellum. Detailed illustrations of between-group differences in all networks are provided in Supplementary Fig. 2.
Figure 3.
Connectivity between the WM network. (A) Matrices of the Pearson correlation coefficient showing the between-network connectivity for each group. (B) Differences in between-network connectivity. Hot colour means ADHD > TDC, and cool colour means ADHD < TDC. Asterisks represent significant differences with age, gender as covariates (P < 0.05, Bonferroni corrected). (C) Summary of WM network activities, including within-network activity reflected by signal amplitude and between-network activity revealed by the Pearson correlation. The node represents the WM network (blue: lower amplitude in the ADHD group, white: no difference in the amplitude between groups), and the link represents the connectivity between networks (red: increased connectivity in ADHD, blue: decreased connectivity in ADHD).
To better illustrate the ADHD pattern of the within- and between-network activities compared with TDC, the results are summarized in Fig. 3C, showing abnormalities in both within-network and between-network activities. These abnormalities existed in one intrinsic network (DMN), two sensory networks (SMN and VIS), three task-control networks (DAN, VAN and deep FPN) and two other networks (cerebellum and deep frontal). In the ADHD group, the DMN and the SMN mainly showed higher connectivity with other networks, whereas the DAN and the VAN generally exhibited lower connectivity, which suggests that abnormal WM networks exhibit two distinct patterns, with DMN and SMN as ‘hot’ networks and the DAN and the VAN as ‘cold’ networks.
We also examined the relationship between WM and GM networks by calculating the temporal correlations between each WM network and each GM network. We found that most WM networks showed high (r > 0.6) functional connectivity with their corresponding GM networks, which may suggest good activity coupling between WM and GM networks and further validates the existence of WM functional networks (Supplementary Fig. 3).
Correlation between WM network functional connectivity and clinical measures
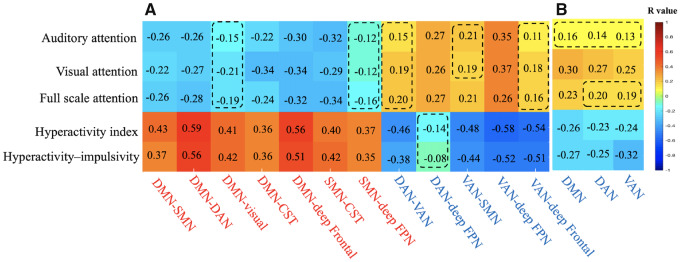
We next explored the relationship of significantly increased or decreased functional connectivity with CPRS scores and IVA-CPT scores for all subjects. We found that increased connectivity of the DMN and the SMN was positively correlated with the hyperactivity–impulsivity score and the hyperactivity index in the CPRS and negatively correlated with the attention quotient in the IVA-CPT. In contrast, decreased connectivity of the DAN and the VAN had a positive correlation with the attention quotient in the IVA-CPT but a negative correlation with the hyperactivity–impulsivity score and the hyperactivity index in the CPRS (Fig. 4A, Supplementary Table 2 and Supplementary Fig. 4).
Figure 4.
Correlation between (A) functional connectivity, (B) within-network activity and scores of hyperactivity behaviour and attention. The rows indicate functional connectivity/within-network activity, with red indicating higher activity in ADHD and blue indicating lower activity in ADHD, while the columns indicate clinical measures in terms of hyperactivity behaviour and attention performance. The number in each cell is the correlation coefficient (r) with red cells indicating a positive correlation and blue cells indicating a negative correlation. The dashed box indicates a nonsignificant correlation (P > 0.05).
We also found that the within-network activity of the DMN, the DAN and the VAN, which showed lower amplitudes in children with ADHD, was negatively correlated with hyperactivity behaviour and positively correlated with poor attention performance (Fig. 4B, Supplementary Table 2 and Supplementary Fig. 4).
Discussion
In this study, we uncovered for the first time the intrinsic functional organization of WM in children with ADHD using clustering analysis with rs-fMRI data in WM. We revealed that children with ADHD illustrated decreased intrinsic neural activity in four of the eight distinct WM networks, i.e. the DMN, the SMN, the DAN and the VAN, which are defined according to the correspondence with the known GM networks. The DMN and the SMN seem to be ‘hot’ networks that showed higher connectivity with other networks and were correlated with more severe hyperactive behaviour, while the DAN and the VAN were considered ‘cold’ networks that showed lower connectivity with other networks and were correlated with poor attention performance. Taken together, our findings extend upon the existing understanding of brain functional networks in ADHD from the perspective of WM functional networks and suggest two patterns of WM network alterations that might underlie ADHD psychopathology of hyperactivity and attention deficit.
Previous studies have provided microstructural and functional evidence of abnormalities within circuits or networks in patients with ADHD. Diffusion MRI studies found altered fractional anisotropy in several distributed WM tracts, including frontostriatal fibre tracts (Wu et al., 2014), the corticospinal tract (Bu et al., 2019) and other WM bundles (Hong et al., 2014), which indicates the existence of disrupted WM microstructure. Regarding functional aspects, reduced functional connectivity within regions of the DMN (Posner et al., 2014; Gao et al., 2019), the DAN (Zhu et al., 2008; Cortese et al., 2012; McCarthy et al., 2013), the VAN and the FPN (Hart et al., 2013; Yerys et al., 2019) has been reported. The reduced signal amplitude within the WM functional network in our study supported and extended upon these findings of abnormalities in ADHD from the perspective of WM dysfunction, suggesting reduced neural activities in WM underlying disrupted WM microstructural and hypoactivity of the GM network. In contrast, for the motor network of GM, strong connectivity (O'Halloran et al., 2018) within the motor cortex, as well as atypical strength and patterns (McLeod et al., 2016) of functional connections within and between hemispheres, has been reported. It is plausible that decreased neural activity in the SMN of WM, as observed in our study, could affect information transmission between cortical regions and thus be associated with alterations in how the motor network is connected within and between hemispheres.
In addition to different within-network activity from TDC, we identified abnormal between-network connectivity in children with ADHD. Notably, increased connectivity was mainly observed in the DMN and the sensory networks (i.e., the SMN and visual network), while decreased connectivity was observed for the task-control networks (i.e., the DAN, VAN and deep FPN). Previous fMRI studies of ADHD examining the interaction between the DMN and other networks have revealed deficient regulation of DMN activity from task-control networks (Sonuga-Barke and Castellanos, 2007; Fair et al., 2010; McCarthy et al., 2013; Posner et al., 2014; Sidlauskaite et al., 2016), and similar findings for DMN were also presented in individuals with familial risk for depression and patients with OCD (Posner et al., 2016, 2017). Normally, the DMN can be deactivated during external goal-directed tasks and activated during internally focused cognition. Persistent DMN hyperactivity in ADHD interferes with sustained attention, which manifests as mind-wandering and lapses or errors in goal-directed behaviour (Gallo and Posner, 2016). In our study, we found that the DMN of WM had increased connectivity with all other WM networks and absent connections with the FPN of WM, and the increased between-network connectivity for DMN was correlated with increased inattention. Our results suggest that the DMN interference model in ADHD is further supported and extended by WM system since diminished connectivity between DMN and FPN (cognitive control network) also exits in BOLD signal from WM. As previous fMRI study of GM network of ADHD has indicated that mind-wandering dominated by interference of DMN may impair the normal attention function(Posner et al., 2014), here, we postulated that DMN in WM plays a similar important role in attention regulation as it does in GM.
Motor and sensory circuits have been implicated in the pathophysiology of ADHD (Valera et al., 2010; Proal et al., 2011; O'Halloran et al., 2018). Previous studies using intrinsic functional connectivity analysis have reported high network centrality in the somatomotor cortex (Di Martino et al., 2013) and the visual cortex (Wang et al., 2009) in patients with ADHD. Thus, it is plausible that the functional connectivity in the motor and visual networks of WM could be abnormal. Carmona et al. (2015) revealed an increased degree of functional connectivity in the somatomotor cortex and the visual cortex and a decreased degree of connectivity in executive control systems in children with ADHD. In the current study, we found that the motor-sensory system of WM, which contains the SMN, the visual network and the cerebellum, showed increased connectivity with most networks but decreased connectivity for two network pairs—visual–FPN and SMN–VAN. Therefore, the current study not only provides evidence for the predominance of sensorimotor processing and less interaction with attentional and executive control regions in ADHD but also suggests that WM activity may play a corresponding role in cortical activity.
In addition to increased connectivity, we observed several decreased between-network connectivity, predominantly in the task-control network. Compared with TDC, we found significantly decreased between-network connectivity in children with ADHD in both the DAN and the VAN. The DAN functions in goal-directed top-down attention processing, while the VAN supports the identification of salient or novel environmental factors. Normally, activation of the DAN and the VAN increases together when reorientation of attention is required. Hypofunction of these two networks has been proposed in ADHD (Castellanos and Proal, 2012; Cortese et al., 2012; McCarthy et al., 2013). Clinical symptoms of ADHD- and ADHD-related deficits in adaptive switching to external salient stimuli have been linked to deficient VAN engagement and disrupted information exchange between the VAN and the DAN (Helenius et al., 2011; Cubillo et al., 2012). Our finding regarding decreased connectivity of the WM of the attention network is in line with that of GM. We speculate that the decreased connectivity between WM networks underpins deficits in the function of the GM network. Thus, we provide further evidence of deficiency of the DAN and the VAN in attention processing. Moreover, the decreased connectivity between the deep FPN and other networks, especially between the deep FPN and attention networks, identified in our study might suggest less engagement of the FPN of WM in ADHD. Since sustained attention requires both engagement of the FPN, the DAN and the VAN and suppression of the DMN, our results suggest a similar disrupted functional connectivity pattern within the WM network, which could play a role in distractibility in ADHD.
Our findings must be considered in light of some limitations. First, the limited number of TDC in the current study may bias the clustering results. It is necessary to include a larger and more balanced sample in further research to examine the stability of the current results. Second, as fibre tracts cross each other, mixed signals from different functional systems may be detected in the same WM locations. Therefore, it is difficult to clarify the precise source of the fMRI signals in the WM. We would like to compare the WM network with WM tracts identified by diffusion tensor imaging to further confirm the overlap between functional networks and fibre tracts in future studies. Third, the conclusion should be taken with caution since we recruited ADHD children without comorbidities and treatment to reduce clinical confounding factors, which make our sample differ from typical ADHD population. Fourth, though there may be profound differences between ADHD presentations and importance of subtyping analysis, we were not able to perform subtyping analysis due to small sample size and imbalance number of subjects in each group. Further studies are needed to examine alterations in functional WM networks for ADHD subtypes.
Conclusion
In summary, we for the first time characterized the intrinsic functional organization of WM networks in children with ADHD. Our findings demonstrated two distinct WM network patterns—the DMN and the SMN as ‘hot’ networks and the DAN and the VAN as ‘cold’ networks—suggesting that abnormal WM network interactions may be implicated in hyperactivity and attention deficit. These results offer a new perspective for studying WM in ADHD and help further understand the network basis of ADHD.
Supplementary material
Supplementary material is available at Brain Communications online.
Funding
This study was supported by the National Natural Science Foundation (Grant No. 81671669) and a Science and Technology Project of Sichuan Province (Grant No. 2017JQ0001).
Competing interests
The authors report no competing interests.
Supplementary Material
Glossary
- ADHD =
attention-deficit/hyperactivity disorder
- BOLD =
blood-oxygen-level-dependent
- CPRS =
Conners’ Parent Rating Scale
- DAN =
dorsal attention network
- DMN =
default mode network
- FPN =
frontoparietal network
- GM =
grey matter
- IVA-CPT =
Integrated Visual and Auditory Continuous Performance Test
- SMN =
somatomotor network
- TDC =
typically developing controls
- VAN =
ventral attention network
- WM =
white matter
Contributor Information
Xuan Bu, Huaxi MR Research Center (HMRRC), Functional and Molecular Imaging Key Laboratory of Sichuan Province, Department of Radiology, West China Hospital of Sichuan University, Chengdu, Sichuan 610041, China; Psychoradiology Research Unit of the Chinese Academy of Medical Sciences (2018RU011), West China Hospital of Sichuan University, Chengdu, Sichuan 610041, China.
Kaili Liang, Huaxi MR Research Center (HMRRC), Functional and Molecular Imaging Key Laboratory of Sichuan Province, Department of Radiology, West China Hospital of Sichuan University, Chengdu, Sichuan 610041, China; Psychoradiology Research Unit of the Chinese Academy of Medical Sciences (2018RU011), West China Hospital of Sichuan University, Chengdu, Sichuan 610041, China.
Qingxia Lin, Department of Psychiatry, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang 325003, China.
Yingxue Gao, Huaxi MR Research Center (HMRRC), Functional and Molecular Imaging Key Laboratory of Sichuan Province, Department of Radiology, West China Hospital of Sichuan University, Chengdu, Sichuan 610041, China; Psychoradiology Research Unit of the Chinese Academy of Medical Sciences (2018RU011), West China Hospital of Sichuan University, Chengdu, Sichuan 610041, China.
Andan Qian, Department of Radiology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang 325003, China.
Hong Chen, Department of Psychiatry, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang 325003, China.
Wanying Chen, Department of Psychiatry, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang 325003, China.
Meihao Wang, Department of Radiology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang 325003, China.
Chuang Yang, Department of Psychiatry, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang 325003, China.
Xiaoqi Huang, Huaxi MR Research Center (HMRRC), Functional and Molecular Imaging Key Laboratory of Sichuan Province, Department of Radiology, West China Hospital of Sichuan University, Chengdu, Sichuan 610041, China; Psychoradiology Research Unit of the Chinese Academy of Medical Sciences (2018RU011), West China Hospital of Sichuan University, Chengdu, Sichuan 610041, China.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th edn Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- Bu X, Yang C, Liang K, Lin Q, Lu L, Zhang L, et al. Quantitative tractography reveals changes in the corticospinal tract in drug-naive children with attention-deficit/hyperactivity disorder. J Psychiatry Neurosci 2019; 45: 190024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona S, Hoekzema E, Castellanos FX, Garcia-Garcia D, Lage-Castellanos A, Van Dijk KR, et al. Sensation-to-cognition cortical streams in attention-deficit/hyperactivity disorder. Hum Brain Mapp 2015; 36: 2544–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci 2012; 16: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners' Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 1998; 26: 257–68. [DOI] [PubMed] [Google Scholar]
- Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry 2012; 169: 1038–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Smith A, Taylor E, Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with attention deficit hyperactivity disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex 2012; 48: 194–215. [DOI] [PubMed] [Google Scholar]
- de Lacy N, Kodish I, Rachakonda S, Calhoun VD. Novel in silico multivariate mapping of intrinsic and anticorrelated connectivity to neurocognitive functional maps supports the maturational hypothesis of ADHD. Hum Brain Mapp 2018; 39: 3449–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31: 968–80. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Zuo XN, Kelly C, Grzadzinski R, Mennes M, Schvarcz A, et al. Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biol Psychiatry 2013; 74: 623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Huang Y, Bailey SK, Gao Y, Cutting LE, Rogers BP, et al. Detection of synchronous brain activity in white matter tracts at rest and under functional loading. Proc Natl Acad Sci USA 2018; 115: 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Newton AT, Xu R, Anderson AW, Morgan VL, Gore JC. Spatio-temporal correlation tensors reveal functional structure in human brain. PLoS One 2013; 8: e82107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Xu R, Bailey SK, Wu TL, Morgan VL, Cutting LE, et al. Visualizing functional pathways in the human brain using correlation tensors and magnetic resonance imaging. Magn Reson Imaging 2016; 34: 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Posner J, Nagel BJ, Bathula D, Dias TG, Mills KL, et al. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol Psychiatry 2010; 68: 1084–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers 2015; 1: 15020. [DOI] [PubMed] [Google Scholar]
- Gallo EF, Posner J. Moving towards causality in attention-deficit hyperactivity disorder: overview of neural and genetic mechanisms. Lancet Psychiatry 2016; 3: 555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Shuai D, Bu X, Hu X, Tang S, Zhang L, et al. Impairments of large-scale functional networks in attention-deficit/hyperactivity disorder: a meta-analysis of resting-state functional connectivity. Psychol Med 2019; 1–11. [DOI] [PubMed] [Google Scholar]
- Gawryluk JR, Mazerolle EL, D'Arcy RC. Does functional MRI detect activation in white matter? A review of emerging evidence, issues, and future directions. Front Neurosci 2014; 8: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong YX, Cai TS. The Wechsler Intelligence Scale for Children Revised in China(C-WISC). Chin J Clin Psychol 1994; 1: 1–6. [Google Scholar]
- Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry 2013; 70: 185–98. [DOI] [PubMed] [Google Scholar]
- Helenius P, Laasonen M, Hokkanen L, Paetau R, Niemivirta M. Impaired engagement of the ventral attentional pathway in ADHD. Neuropsychologia 2011; 49: 1889–96. [DOI] [PubMed] [Google Scholar]
- Hong SB, Zalesky A, Fornito A, Park S, Yang YH, Park MH, et al. Connectomic disturbances in attention-deficit/hyperactivity disorder: a whole-brain tractography analysis. Biol Psychiatry 2014; 76: 656–63. [DOI] [PubMed] [Google Scholar]
- Huang Y, Bailey SK, Wang P, Cutting LE, Gore JC, Ding Z. Voxel-wise detection of functional networks in white matter. Neuroimage 2018; 183: 544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Luo C, Li X, Li Y, Yang H, Li J, et al. White-matter functional networks changes in patients with schizophrenia. Neuroimage 2019. a; 190: 172–81. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Song L, Li X, Zhang Y, Chen Y, Jiang S, et al. Dysfunctional white-matter networks in medicated and unmedicated benign epilepsy with centrotemporal spikes. Hum Brain Mapp 2019. b; 40: 3113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G-J, Liao W, Chen F-F, Zhang L, Wang K. Low-frequency blood oxygen level-dependent fluctuations in the brain white matter: more than just noise. Sci Bull 2017; 62: 656–7. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao UK-S-P. K-SADS-PL. J Am Acad Child Adolesc Psychiatry 2000; 39: 1208. [DOI] [PubMed] [Google Scholar]
- Kessler D, Angstadt M, Welsh RC, Sripada C. Modality-spanning deficits in attention-deficit/hyperactivity disorder in functional networks, gray matter, and white matter. J Neurosci 2014; 34: 16555–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp 2010; 31: 904–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Biswal BB, Wang P, Duan X, Cui Q, Chen H, et al. Exploring the functional connectome in white matter. Hum Brain Mapp 2019; 40: 4331–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Newton AT, Anderson AW, Ding Z, Gore JC. Characterization of the hemodynamic response function in white matter tracts for event-related fMRI. Nat Commun 2019; 10: 1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marussich L, Lu KH, Wen H, Liu Z. Mapping white-matter functional organization at rest and during naturalistic visual perception. Neuroimage 2017; 146: 1128–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy H, Skokauskas N, Mulligan A, Donohoe G, Mullins D, Kelly J, et al. Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention-deficit/hyperactivity disorder in childhood. JAMA Psychiatry 2013; 70: 1329–37. [DOI] [PubMed] [Google Scholar]
- McLeod KR, Langevin LM, Dewey D, Goodyear BG. Atypical within- and between-hemisphere motor network functional connections in children with developmental coordination disorder and attention-deficit/hyperactivity disorder. Neuroimage Clin 2016; 12: 157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Halloran L, Cao Z, Ruddy K, Jollans L, Albaugh MD, Aleni A, et al. Neural circuitry underlying sustained attention in healthy adolescents and in ADHD symptomatology. Neuroimage 2018; 169: 395–406. [DOI] [PubMed] [Google Scholar]
- Peer M, Nitzan M, Bick AS, Levin N, Arzy S. Evidence for functional networks within the human brain's white matter. J Neurosci 2017; 37: 6394–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Cha J, Wang Z, Talati A, Warner V, Gerber A, et al. Increased default mode network connectivity in individuals at high familial risk for depression. Neuropsychopharmacology 2016; 41: 1759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Park C, Wang Z. Connecting the dots: a review of resting connectivity MRI studies in attention-deficit/hyperactivity disorder. Neuropsychol Rev 2014; 24: 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Song I, Lee S, Rodriguez CI, Moore H, Marsh R, et al. Increased functional connectivity between the default mode and salience networks in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp 2017; 38: 678–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD Barnes KA Snyder AZ Schlaggar BL Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 2012; 59: 2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD Mitra A Laumann TO Snyder AZ Schlaggar BL Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage 2014; 84: 320–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proal E, Reiss PT, Klein RG, Mannuzza S, Gotimer K, Ramos-Olazagasti MA, et al. Brain gray matter deficits at 33-year follow-up in adults with attention-deficit/hyperactivity disorder established in childhood. Arch Gen Psychiatry 2011; 68: 1122–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Rifkin-Graboi A, Tuan TA, Zhong J, Meaney MJ. Inattention and hyperactivity predict alterations in specific neural circuits among 6-year-old boys. J Am Acad Child Adolesc Psychiatry 2012; 51: 632–41. [DOI] [PubMed] [Google Scholar]
- Samea F, Soluki S, Nejati V, Zarei M, Cortese S, Eickhoff SB, et al. Brain alterations in children/adolescents with ADHD revisited: a neuroimaging meta-analysis of 96 structural and functional studies. Neurosci Biobehav Rev 2019; 100: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidlauskaite J, Sonuga-Barke E, Roeyers H, Wiersema JR. Default mode network abnormalities during state switching in attention deficit hyperactivity disorder. Psychol Med 2016; 46: 519–28. [DOI] [PubMed] [Google Scholar]
- Silk TJ, Vance A, Rinehart N, Bradshaw JL, Cunnington R. White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Hum Brain Mapp 2009; 30: 2757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev 2007; 31: 977–86. [DOI] [PubMed] [Google Scholar]
- Su LY, Li XR, Huang CX, Luo XR, Zhang JS. Norms of the Conners parent symptom questionnaire in Chinese urban children. Chin J Clin Psychol 2001; 9: 241–3. [Google Scholar]
- Tinius TP. The integrated visual and auditory continuous performance test as a neuropsychological measure. Arch Clin Neuropsychol 2003; 18: 439–54. [PubMed] [Google Scholar]
- Valera EM, Spencer RM, Zeffiro TA, Makris N, Spencer TJ, Faraone SV, et al. Neural substrates of impaired sensorimotor timing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 2010; 68: 359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhu C, He Y, Zang Y, Cao Q, Zhang H, et al. Altered small-world brain functional networks in children with attention-deficit/hyperactivity disorder. Hum Brain Mapp 2009; 30: 638–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Yang Z, Bailey SK, Zhou J, Cutting LE, Gore JC, et al. Functional connectivity and activity of white matter in somatosensory pathways under tactile stimulations. Neuroimage 2017; 152: 371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YH, Gau SS, Lo YC, Tseng WY. White matter tract integrity of frontostriatal circuit in attention deficit hyperactivity disorder: association with attention performance and symptoms. Hum Brain Mapp 2014; 35: 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C-G Cheung B Kelly C Colcombe S Craddock RC Di Martino A, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage 2013; 76: 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 2011; 106: 1125–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Tunc B, Satterthwaite TD, Antezana L, Mosner MG, Bertollo JR, et al. Functional connectivity of frontoparietal and salience/ventral attention networks have independent associations with co-occurring attention-deficit/hyperactivity disorder symptoms in children with autism. Biol Psychiatry Cogn Neurosci Neuroimaging 2019; 4: 343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CZ, Zang YF, Cao QJ, Yan CG, He Y, Jiang TZ, et al. Fisher discriminative analysis of resting-state brain function for attention-deficit/hyperactivity disorder. Neuroimage 2008; 40: 110–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study are available for researches upon reasonable request.