Abstract
Background
To address the US Food and Drug Administration’s recent safety concern on robotic surgery procedures, we compared short- and long-term mortality for stage I non-small cell lung cancer (NSCLC) patients treated by robotic-assisted thoracoscopic surgical lobectomy (RATS-L) vs video-assisted thoracoscopic surgical lobectomy (VATS-L).
Methods
From the National Cancer Database, we identified 18 908 stage I NSCLC patients who underwent RATS-L or VATS-L as the primary operation from 2010 to 2014. Cox proportional hazards models were used to estimate hazard ratios (HRs) for short- and long-term mortality using unmatched and propensity score–matched analyses. All statistical tests were 2-sided.
Results
Patients treated by RATS-L had higher 90-day mortality than those with VATS-L (6.6% vs 3.8%, P = .03) if conversion to open thoracotomy occurred. After excluding first-year observation, multiple regression analyses showed RATS-L was associated with increased long-term mortality, compared with VATS-L, in cases with tumor size 20 mm or less: hazard ratio (HR) = 1.33 (95% confidence interval [CI] = 1.15 to 1.55), HR = 1.36 (95% CI = 1.17 to 1.58), and HR = 1.33 (95% CI = 1.11 to 1.61) for unmatched, N:1 matched, and 1:1 matched analyses, respectively, in the intention-to-treat analysis. Among patients without conversion to an open thoracotomy, the respective hazard ratios were 1.19 (95% CI = 1.10 to 1.29), 1.19 (95% CI = 1.10 to 1.29), and 1.17 (95% CI = 1.06 to 1.29). Similar associations were observed when follow-up time started 18 or 24 months postsurgery. No statistically significant mortality difference was found for patients with tumor size of greater than 20 mm. These associations were not related to case volume of VATS-L or RATS-L performed at treatment institutes.
Conclusions
Patients with small (≤20 mm) stage I NSCLC treated with RATS-L had statistically significantly higher long-term mortality risk than VATS-L after 1 year postsurgery.
Lung cancer is the leading cause of cancer-related mortality in the United States and worldwide (1,2). Non-small cell lung cancer (NSCLC) accounts for approximately 83% of lung cancer cases, with 16%-26% of patients diagnosed at stage I (3–5). Invasive, open surgical resection with anatomic lobectomy and mediastinal nodal dissection is the primary, evidence-based method for the treatment of early-stage NSCLC, for which a minimally invasive approach is preferred (6,7). Video-assisted thoracoscopic surgical lobectomy (VATS-L) is a minimally invasive technique for lung cancer resection and has been associated with less pain, decreased need for pain medications, less blood loss, shorter hospital stay, fewer complications, faster recovery time, and return to normal activities compared with resection by open thoracotomy (8–14). This technique is reported to be as effective as traditional thoracotomy in terms of long-term survival (15). The American College of Chest Physicians Lung Cancer Guidelines recommend that, for patients with clinical stage I NSCLC, a minimally invasive approach such as video-assisted thoracic surgery (thoracoscopy) is preferred over a thoracotomy for anatomic pulmonary resection and is indicated in experienced surgical treatment centers (6).
In the past decade, robotic-assisted thoracoscopic surgical lobectomy (RATS-L) has emerged as an alternative minimally invasive approach for lung lobectomy, offering the advantages of 3-dimensional visualization, superior visual optics, and improved maneuverability within confined spaces (16–18). A number of studies have reported that RATS-L offers a lower rate of conversion to open, more radical lymph node dissection, less bleeding, less impairment of pulmonary function, postoperative pain reduction, shorter hospital stay, and 30-day mortality rate compared with VATS-L (19–23). However, the higher cost of RATS-L compared with VATS-L has been reported (17,24,25). To date, evaluation of robotically assisted surgical treatment in oncology settings has generally focused on determining whether the complication rate at 30 days is clinically comparable with other surgical techniques (26). The relative benefits and risks of using robotically assisted surgical devices, particularly regarding long-term outcomes, have not been established, which prompted the US Food and Drug Administration’s (FDA's) recent call for more investigation on long-term safety and effectiveness of robotic devices as minimally invasive cancer surgical treatments for cancer patients (26,27). To address this gap, we used data from the National Cancer Database (NCDB) to evaluate long-term mortality for patients with stage I NSCLC treated with VATS-L or RATS-L. The NCDB is a clinical oncology database jointly sponsored by the American Cancer Society and American College of Surgeons and represents approximately 70% of newly diagnosed cancers in more than 1500 hospitals in the United States (28). Since 2010, the NCDB has been collecting information to monitor patterns and trends in the adoption and utilization of minimally invasive surgical techniques for cancer treatment, providing a unique resource to evaluate VATS-L and RATS-L for long-term outcomes in lung cancer (28).
Methods
Study Population and Patient Selection
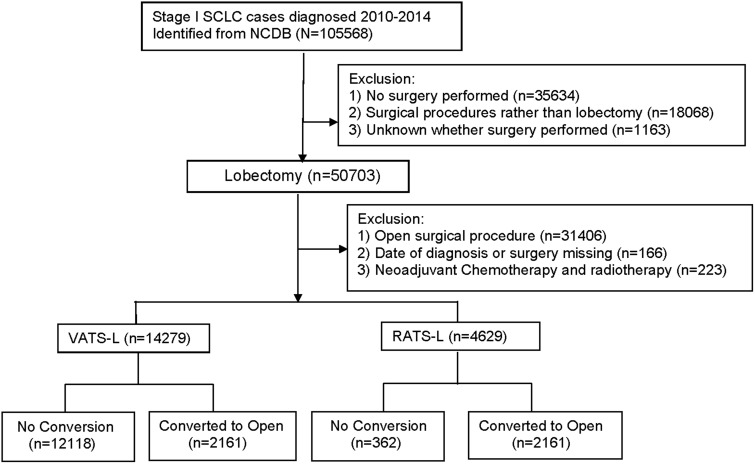
For this analysis, NSCLC included adenocarcinoma, squamous-cell carcinoma, and lung cancers other than small cell carcinoma. The 7th edition of the American Joint Committee on Cancer staging system was used to define the stage. The primary surgical approaches were coded as “open,” “endoscopic or laparoscopic” (“VATS-L” in this analysis), “endoscopic or laparoscopic converted to open” (“VATS-L converted to open”), “robotic-assisted” (“RATS-L” in this analysis), and “robotic converted to open” (“RATS-L converted to open”). As shown in Figure 1, we identified 50 703 patients with stage I NSCLC from the NCDB who underwent lobectomy as the primary treatment. We excluded patients who underwent open lobectomy, had no information on date of diagnosis or date of surgery, or who had received neoadjuvant chemotherapy and/or radiation therapy before surgery. The final analytic cohort included 18 908 patients who received VATS-L (n = 14 279) or RATS-L (n = 4629). This study was approved by the Vanderbilt University Medical Center institutional review board as a human participant exempt project.
Figure 1.
Flat chart for case selection process. NCDB = National Cancer Database; NSCLC = non-small cell lung cancer; RATS-L = robotic-assisted thoracoscopic surgical lobectomy; VATS-L= video-assisted thoracoscopic surgical lobectomy.
Deidentified information on patient demographics, socioeconomic status, and clinical characteristics was extracted from the NCDB NSCLC database and presented in Table 1. These included age at diagnosis (continuous variable), sex (male, female), race or ethnicity (non-Hispanic White, African American or Black, other), educational level (based on code-level estimates of the proportion of residents without a high school diploma), annual income (based on code-level estimates of median income), insurance status (private insurance, Medicare, Medicaid, other type of government insurance, or uninsured), coexisting medical conditions (0, 1, 2, or more; based on the Charlson/Deyo score, provided by the NCDB), facility type (academic research program, comprehensive cancer program, community cancer program, and integrated network cancer program), distance to treatment center (mile), tumor size (millimeters), histology (adenocarcinoma, squamous cell carcinoma, other), grade (well or moderately or poor differentiated or undifferentiated), other cancer treatment (chemotherapy and/or radiotherapy, or no further treatment), year of cancer diagnosis (since 2010-2014), case volume of VATS-L and RATS-L performed at the institutional level, and time since diagnosis to surgery (days).
Table 1.
Characteristics of patients with stage I NSCLC by surgical approaches (VATS-L and RATS-L), NCDB since 2010-2014
| VATS-L vs RATS-L |
VATS-L matched to RATS-L by propensity score |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unmatched |
VATS-L to RATS-L 1:1 matched |
VATS-L to RATS-L N:1 matched |
|||||||
| Surgical approaches | VATS-L | RATS-L | P a | VATS-L | RATS-L | P b | VATS-L | RATS-L | P b |
| No. of patients | N = 14 279 | N = 4629 | N = 4537 | N = 4537 | N = 12 056 | N = 4537 | |||
| Age at diagnosis, mean ± SD, y | 66.6 ± 10.1 | 66.9 ± 9.9 | .18 | 66.8 ± 9.6 | 66.8 ± 9.8 | .94 | 66.8 ± 9.9 | 66.8 ± 9.8 | .76 |
| Sex (%) | |||||||||
| Male | 5891 (41.3) | 2005 (43.3) | .01 | 2012 (44.4) | 1955 (43.1) | .23 | 5111 (42.4) | 1955 (43.1) | .74 |
| Female | 8388 (58.7) | 2624 (56.7) | 2525 (55.6) | 2582 (56.9) | 6945 (57.6) | 2582 (56.9) | |||
| Race or ethnicity, No. (%) | |||||||||
| Non-Hispanic White | 11 824 (82.8) | 3638 (78.6) | 3583 (79.0) | 3614 (79.6) | 9766 (81.0) | 3614 (79.6) | |||
| Black or African American | 1083 (7.6) | 394 (8.5) | <.001 | 412 (9.1) | 375 (8.3) | .10 | 1015 (8.4) | 375 (8.3) | .31 |
| Other | 845 (5.9) | 412 (8.9) | 340 (7.5) | 377 (8.3) | 794 (6.6) | 377 (8.3) | |||
| Unknown | 527 (3.7) | 185 (4.0) | 202 (4.5) | 171 (3.8) | 481 (4.0) | 171 (3.8) | |||
| Educational attainment, No. (%) | |||||||||
| <Median | 5482 (38.4) | 2054 (44.4) | 1962 (43.3) | 1971 (43.4) | 4990 (41.4) | 1971 (43.4) | |||
| >Median | 8753 (61.3) | 2566 (55.4) | <.001 | 2569 (56.6) | 2558 (56.4) | .61 | 7047 (58.4) | 2558 (56.4) | .81 |
| Data missing | 44 (0.3) | 9 (0.2) | 6 (0.1) | 8 (0.2) | 19 (0.2) | 8 (0.2) | |||
| Annual household income, No. (%) | |||||||||
| <Median | 5196 (36.4) | 1910 (41.3) | 1836 (40.5) | 1833 (40.4) | 4669 (38.7) | 1833 (40.4) | |||
| >Median | 9034 (63.3) | 2708 (58.5) | <.001 | 2690 (59.3) | 2694 (59.4) | .99 | 7362 (61.1) | 2694 (59.4) | .89 |
| Data missing | 50 (0.3) | 11 (0.2) | 10 (0.2) | 10 (0.2) | 25 (0.2) | 10 (0.2) | |||
| Insurance status, No. (%) | |||||||||
| Not insured | 263 (1.8) | 72 (1.6) | 81 (1.8) | 72 (1.6) | 199 (1.6) | 72 (1.6) | |||
| Private insurance or managed care | 4714 (33.0) | 1447 (31.3) | 1349 (31.7) | 1432 (31.5) | 3839 (31.8) | 1432 (31.5) | |||
| Medicaid | 668 (4.7) | 206 (4.5) | .001 | 184 (4.0) | 206 (4.5) | .77 | 558 (4.6) | 206 (4.5) | .77 |
| Medicare | 8386 (58.7) | 2793 (60.3) | 2731 (60.2) | 2724 (60.0) | 7217 (59.9) | 2724 (60.0) | |||
| Other government | 148 (1.1) | 64 (1.4) | 57 (1.3) | 61 (1.4) | 145 (1.2) | 61 (1.4) | |||
| Unknown | 100 (0.7) | 47 (1.0) | 45 (1.0) | 42 (1.0) | 98 (0.8) | 42 (1.0) | |||
| Facility type, No. (%) | |||||||||
| Academic or research program | 6409 (44.9) | 2199 (45.8) | 2090 (46.1) | 2071 (45.7) | 5481 (45.5) | 2071 (45.7) | |||
| Community cancer program or other | 538 (3.8) | 175 (3.8) | 157 (3.5) | 173 (3.8) | 481 (4.0) | 173 (3.8) | |||
| Comprehensive community cancer program | 5706 (40.0) | 1770 (38.2) | .03 | 1731 (38.1) | 1738 (38.3) | .10 | 4677 (38.8) | 1738 (38.3) | .80 |
| Integrated network cancer program | 1483 (10.4) | 533 (11.5) | 539 (11.9) | 523 (11.5) | 1326 (11.0) | 523 (11.5) | |||
| Data missing | 143 (1.0) | 32 (0.7) | 20 (0.4) | 32 (0.7) | 91 (0.7) | 32 (0.7) | |||
| Charlson/Deyo score, No. (%) | |||||||||
| 0 | 7397 (51.8) | 2216 (47.9) | 2225 (49.0) | 2189 (48.2) | 5992 (49.7) | 2189 (48.2) | |||
| 1 | 4994 (35.0) | 1742 (37.6) | <.001 | 1606 (35.4) | 1704 (37.6) | .71 | 4334 (36.0) | 1704 (37.6) | .95 |
| ≥2 | 1888 (13.2) | 671 (14.5) | 706 (15.6) | 644 (14.2) | 1730 (14.3) | 644 (14.2) | |||
| Tumor size, No. (%) | |||||||||
| ≤20 mm | 6749 (47.3) | 2225 (48.1) | 2206 (48.6) | 2175 (47.9) | 5720 (47.4) | 2175 (47.9) | |||
| 21-40 mm | 6416 (44.9) | 2055 (44.4) | .60 | 1986 (43.8) | 2019 (44.5) | .95 | 5410 (44.9) | 2019 (44.5) | .84 |
| Other or unknown | 1114 (7.8) | 349 (7.5) | 345 (7.6) | 343 (7.6) | 926 (7.7) | 343 (7.6) | |||
| Other cancer therapy, No. (%) | |||||||||
| None | 11 408 (79.9) | 3675 (79.4) | 3568 (78.7) | 3599 (79.3) | 9583 (79.5) | 3599 (79.3) | |||
| Radiotherapy and/or chemotherapy | 900 (6.3) | 293 (6.3) | .71 | 301 (6.6) | 285 (6.3) | .63 | 781 (6.5) | 285 (6.3) | .64 |
| Unknown | 1971 (13.8) | 661 (14.3) | 668 (14.7) | 652 (14.4) | 1692 (14.0) | 652 (14.4) | |||
| Year of diagnosis, No. (%) | |||||||||
| 2010 | 2122 (14.9) | 356 (7.7) | 442 (9.7) | 354 (7.8) | 1229 (10.2) | 354 (7.8) | |||
| 2011 | 2675 (18.7) | 673 (14.5) | 719 (15.80 | 673 (14.8) | 1967 (16.3) | 673 (14.8) | |||
| 2012 | 2793 (19.6) | 1006 (21.7) | <.001 | 856 (18.9) | 997 (21.9) | .46 | 2371 (19.7) | 997 (21.9) | .09 |
| 2013 | 3184 (22.3) | 1259 (27.2) | 1119 (24.7) | 1236 (27.3) | 3030 (25.1) | 1236 (27.3) | |||
| 2014 | 3505 (24.6) | 1335 (28.8) | 1401 (30.9) | 1277 (28.3) | 3459 (28.7) | 1277 (28.3) | |||
| Histology, No. (%) | |||||||||
| Adenocarcinoma | 8530 (59.7) | 2736 (59.1) | 2681 (59.1) | 2684 (59.2) | 7196 (59.7) | 2684 (59.2) | |||
| Squamous cell carcinoma | 2857 (20.0) | 993 (21.5) | .08 | 932 (20.5) | 972 (21.4) | .54 | 2459 (20.4) | 972 (21.4) | .93 |
| Other | 2892 (20.3) | 900 (19.4) | 924 (20.4) | 881 (19.4) | 2401 (19.9) | 881 (19.4) | |||
| Grade, No. (%) | |||||||||
| Well differentiated | 3113 (21.8) | 1060 (22.9) | 1056 (23.3) | 1034 (22.8) | 2723 (22.6) | 1034 (22.8) | |||
| Moderately differentiated | 6283 (44.0) | 2003 (43.3) | 1991 (43.9) | 1973 (43.5) | 5257 (43.6) | 1973 (43.5) | |||
| Poorly differentiated | 3693 (25.9) | 1227 (26.5) | .09 | 1159 (25.5) | 1197 (26.4) | .76 | 3153(26.2) | 1197 (26.4) | .81 |
| Undifferentiated | 142 (1.0) | 47 (1.0) | 42 (0.9) | 46 (1.0) | 122 (1.0) | 46 (1.0) | |||
| Unknown | 1048 (7.3) | 292 (6.3) | 289 (6.4) | 287 (6.3) | 801 (6.6) | 287 (6.3) | |||
| Distance to treatment center, No. (%) | |||||||||
| <25 miles | 10 262 (71.9) | 3414 (73.8) | 3345 (73.8) | 3351 (73.9) | 8835 (73.3) | 3351 (73.9) | |||
| 25-100 miles | 3327 (23.3) | 1034 (22.3) | <.001 | 1031 (22.7) | 1024 (22.5) | .61 | 2790 (23.2) | 1024 (22.5) | .56 |
| >100 miles | 642 (4.5) | 154 (3.3) | 155 (3.4) | 154 (3.4) | 414 (3.4) | 154 (3.4) | |||
| Unknown | 48 (0.3) | 27 (0.6) | 6 (0.1) | 8 (0.2) | 17 (0.1) | 8 (0.2) | |||
| Center case volume (quartile 1-4), No. (%) | |||||||||
| Quartile 1 (1-49) | 3761 (26.3) | 983 (21.2) | 1145 (25.2) | 971 (21.4) | 3075 (25.5) | 971 (21.4) | |||
| Quartile 2 (50-88) | 3403 (23.8) | 1335 (28.8) | <.001 | 1064 (23.5) | 1305 (28.8) | .79 | 2826 (23.4) | 1305 (28.8) | .80 |
| Quartile 3 (89-145) | 3558 (24.9) | 1169 (25.3) | 1146 (35.3) | 1158 (25.5) | 3080 (25.6) | 1158 (25.5) | |||
| Quartile 4 (>145) | 3557 (24.9) | 1142 (24.7) | 1182 (26.0) | 1103 (24.3) | 2075 (25.5) | 1103 (24.3) | |||
| Time since diagnosis to surgery, No. (%) | |||||||||
| 0-7 days | 4569 (32.0) | 1344 (29.0) | 1310 (28.9) | 1324 (29.2) | 3594 (29.8) | 1324 (29.2) | |||
| 8-30 days | 3572 (25.0) | 1058 (22.9) | <.001 | 1093 (24.1) | 1041 (22.9) | .96 | 2973 (24.7) | 1041 (22.9) | .06 |
| 31-90 days | 5318 (37.2 | 1928 (41.6) | 1814 (40.0) | 1881 (41.5) | 4735 (39.3) | 1881 (41.5) | |||
| >90 days | 820 (5.7) | 299 (6.5) | 320 (7.0) | 291 (6.4) | 754 (6.2) | 291 (6.4) | |||
P values derived from the χ2 test (for categorical data) and analysis of variance test (for continuous data) and used to test for unmatched cohort. All statistical tests were 2-sided. NCDB = National Cancer Database; NSCLC = non-small cell lung cancer; RATS-L = robotic-assisted thoracoscopic surgical lobectomy; VATS-L = video-assisted thoracoscopic surgical lobectomy.
P values derived from the Cochran-Mantel-Haenszel test and analysis of covariance test and used to test for propensity score–matched groups. All statistical tests were 2-sided.
Main Outcomes
The primary outcomes of this study were long-term total mortality for patients who survived at least 12 months post VATS-L or RATS-L in the intention-to-treat or end treatment analyses. The intention-to-treat group included all cases in the study, and the end treatment group included cases without conversion to open lobectomy during operation of VATS-L or RATS-L. Because 1 major limitation of NCDB data is the lack of information on cause of death, we used landmark analyses (follow-up time starting from 12, 18, or 24 months postsurgery) to access the possible influence of death-related surgical complications, with an assumption that death occurring close to diagnosis was more likely to be associated with surgical complications. Other outcomes of interest included short-term (≤90 days) mortality risk, rates of conversion from VATS-L or RATS-L to open surgery, and 30-day rehospitalization rates after VATS-L or RATS-L procedures.
Statistical Analyses
Descriptive statistics (means and proportions) were used to describe the distributions of each variable. To examine the differences in patient characteristics between VATS-L and RATS-L, χ2 (for categorical data) and analysis of variance (for continuous data) tests were applied for participants included in the unmatched analyses, and Cochran–Mantel–Haenszel and analysis of covariance tests were used to assess characteristics of participants included in the propensity score–matched analyses. Rates of conversion to open surgery and 30-day unplanned hospitalizations between VATS-L and RATS-L were compared using a χ2 test. The Kaplan–Meier method was used to derive 30-day and 90-day mortality rates as well as to generate overall survival curves. Mortality differences between VATS-L and RATS-L were examined by the log-rank test.
To control for potential confounders, we carried out 3 sets of analyses: unmatched multivariable analysis and propensity score 1:1 or N:1 matched analyses. We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of surgical approaches of interest (VATS-L and RATS-L) associated with all-cause mortality. For the unmatched analysis, potential confounding factors adjusted included age at diagnosis, sex, race or ethnicity, median census-tract education and income levels, Charlson/Deyo score, facility type, distance to treatment center, tumor size, histology, grade, other cancer treatment, year of cancer diagnosis, center volume of surgery (VATS-L and RATS-L) performed, and time since diagnosis to surgery. These factors were further evaluated for their potential modifications on the association of surgical approaches (VATS-L or RATS-L) with survival (Supplementary Table 1, available online). We identified a statistically significant interaction between tumor size and surgical approaches (VATS-L or RATS-L) and thus present the results stratified by tumor size.
Propensity scores were derived to reflect the probability of receiving VATS-L vs RATS-L treatment, conditional on age at diagnosis, sex, race or ethnicity, education, income, Charlson/Deyo score, facility type, distance to treatment center, tumor size, histology, grade, other cancer treatment, year of cancer diagnosis, case volume of surgery (VATS-L and RATS-L) performed at the institutional level, and time since diagnosis to surgery. For propensity score–matched analyses, patients who received VATS-L were matched to those treated by RATS-L based on their propensity score, with a caliper size of 0.0001 (1:1 matching) or 0.05-0.00001 (N:1 matching). After matching, 4537 pairs of VATS-L to RATS-L cases were included in the 1:1 matched analysis; 12 056 VATS-L cases and 4537 RATS-L cases were included in N:1 matched (2, 3, or 4 VATS-L to 1 RATS-L) analyses. Love plots and mirror histograms showed all covariates were balanced after propensity score matching (Supplementary Figure 1, available online; Figure 2). Cox regression model was applied for the analyses. All analyses were conducted using SAS version 9.1 (SAS Institute, Cary, NC). All statistical tests were based on 2-sided probability. A P value of less than .05 was considered statistically significant.
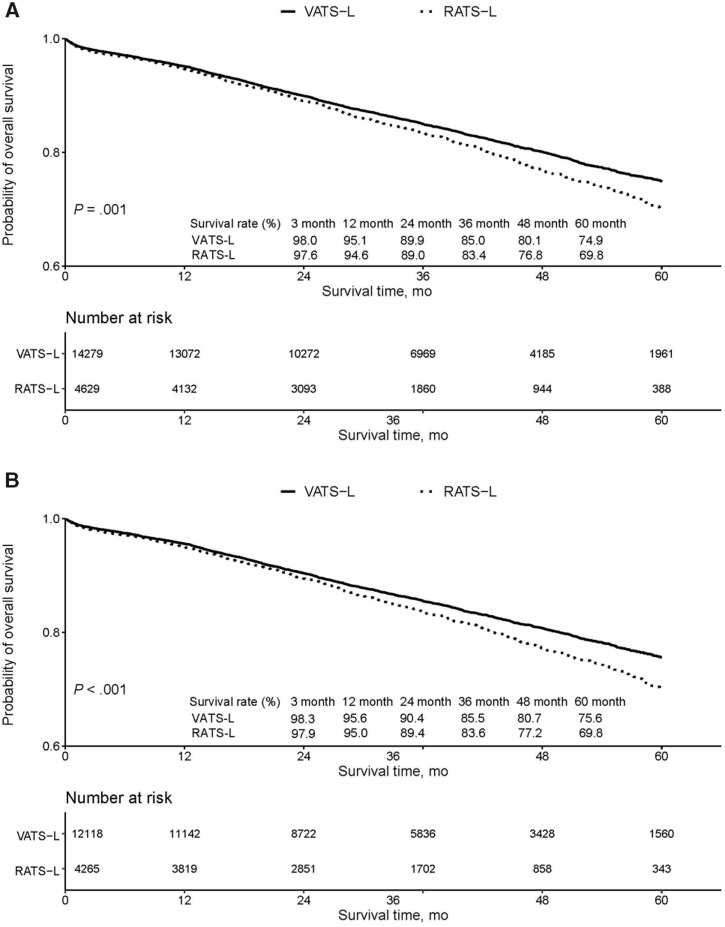
Figure 2.
Survival curves among patients with stage I non-small cell lung cancer according to surgical approaches. Survival curves were calculated and plotted using the Kaplan–Meier method. A) Survival rate table shows 3-, 12-, 24-, 36-, 48-, and 60-month survival rates since surgery in intention-to-treat cases (all video-assisted thoracoscopic surgical lobectomy [VATS-L] and robotic-assisted thoracoscopic surgical lobectomy [RATS-L] cases, with or without conversion to open lobectomy). B) Survival rate table shows 3-, 12-, 24-, 36-, 48-, and 60-month survival rates since surgery in end treatment cases (VATS-L and RATS-L cases without conversion to open lobectomy). Two-sided log-rank test was used to calculate the P values.
Results
Among the 18 908 patients included in the study, 75.5% received VATS-L and 24.5% received RATS-L as the primary treatment. The range of postoperative follow-up time was 0-84.2 months. Overall, compared with those who received VATS-L, patients who underwent RATS-L were less likely to be White or women and to have private insurance and more likely to have lower educational levels and/or annual incomes (less than the median), have existing medical conditions, be diagnosed with cancer in recent years, and have longer time since diagnosis to surgery; however, age at cancer diagnosis, tumor size, histology, grade, and other cancer treatments were similar (Table 1). After propensity score matching, there were no statistically significant differences observed in the distributions of all variables between these 2 groups of patients.
Overall, 30-day unplanned readmission rates and 30- and 90-day mortality rates were similar between the 2 groups (Table 2). Also, no statistically significant differences were observed between VATS-L and RATS-L in 30-day unplanned readmission rates or 30- or 90-day mortality rates if no conversion to an open thoracotomy occurred during the procedure. VATS-L had a higher rate of conversion to open thoracotomy than RATS-L (15.2% vs 7.8%; P < .01). Compared with VATS-L converted to open thoracotomy, on the other hand, RATS-L converted to open had a higher 30-day unplanned readmission rate (7.1% vs 4.6%; P = .07) and a higher 90-day mortality rate (6.6% vs 3.8%; P = .03). Characteristics related to surgical type conversion are shown in Supplementary Table 2 (available online).
Table 2.
Select short-term outcomes of patients with stage I NSCLC by VATS-L and RATS-L
| Surgical approaches | VATS-L | RATS-L | P a |
|---|---|---|---|
| Converted to open thoracotomy, No. (%) | |||
| No | 12 118 (84.9) | 4265 (92.1) | <.001 |
| Yes | 2161 (15.1) | 364 (7.9) | |
| 30-day unplanned readmission by surgical approaches (%)b | |||
| Procedure with or without conversion | 608 (4.3) | 201 (4.3) | .66 |
| Procedure without conversion | 508 (4.2) | 175 (4.1) | .32 |
| Procedure converted to open | 100 (4.6) | 26 (7.1) | .07 |
| 30-day mortality after operation by surgical approaches (%) | |||
| Procedure with or without conversion | 157 (1.1) | 60 (1.3) | .28 |
| Procedure without conversion | 106 (0.9) | 47 (1.1) | .18 |
| Procedure converted to open | 51 (2.4) | 13 (3.6) | .17 |
| 90-day mortality after operation surgical approaches (%) | |||
| Procedure with or without conversion | 288 (2.0) | 110 (2.4) | .14 |
| Procedure without conversion | 206 (1.7) | 87 (2.0) | .15 |
| Procedure converted to open | 82 (3.8) | 23 (6.3) | .03 |
Differences between VATS-L and RATS-L in rate of conversion to open surgery and 30-day unplanned hospitalization rate were examined by using χ2 test (2-sided); 30- and 90-day mortality rates were examined by using lifetable method, and χ2 is calculated by using log-rank test (2-sided). NSCLC = non-small cell lung cancer; RATS-L = robotic-assisted thoracoscopic surgical lobectomy; VATS-L = video-assisted thoracoscopic surgical lobectomy.
The 30-day unplanned readmission includes patients who had an unplanned readmission only and those who had both a planned and an unplanned readmission.
Survival curves of all VATS-L and RATS-L patients showed no difference in mortality up to 12 months postsurgery, after which a gradual increasing mortality difference was observed in RATS-L compared with VATS-L (Figure 2A). A similar trend was also observed for VATS-L and RATS-L without conversion (Figure 2B).
Hazard ratios for all-cause mortality associated with VATS-L and RATS-L with adjustment for or matched on multiple variables are presented in Table 3. Because there was a statistically significant interaction between surgical approaches (VATS-L or RATS-L) and tumor size on long-term survival (Pinteraction = .007 to .02; Table 3), analyses for long-term survival or mortality risk were stratified by tumor size. We found that, compared with VATS-L, in both intention-to-treat cases or cases with nonconversion, RATS-L was associated with an increased long-term all-cause mortality risk across all 3 analytic models when tumor size was 20 mm or smaller. For example, among cases with tumor size 20 mm or less, when follow-up started from 12 months postsurgery, hazard ratios for all-cause mortality were higher in RATS-L than VATS-L for all participants (HR = 1.33, 95% CI = 1.15 to 1.55; HR = 1.36, 95% CI = 1.17 to 1.58; and HR = 1.33, 95% CI = 1.11 to 1.61 for unmatched, N:1 matched, and 1:1 matched analyses, respectively) and in the nonconversion subgroup (HR = 1.19, 95% CI = 1.10 to 1.29; HR = 1.19, 95% CI = 1.10 to 1.29; and HR = 1.17, 95% CI = 1.06 to 1.29 for unmatched, N:1 matched, and 1:1 matched analyses, respectively). We did not find a statistically significant difference in mortality for patients with larger (>20 mm) stage I NSCLC. Similar association patterns were also observed when follow-up started from 18 or 24 months postsurgery and were seen across all 3 types of analytic models (Table 3). We did not find that patients who received RATS-L or VATS-L had a statistically significant difference in 3-month mortality (Table 3), nor was the association modified by tumor size.
Table 3.
Comparison of hazard ratios between VATS-L and RATS-L associated with all-cause death stratified by tumor size
| All (unmatched)a |
N:1 matched by PSb |
1:1 matched by PSb |
||||
|---|---|---|---|---|---|---|
| Analytic models | Death, all | Adj. HR (95% CI) | Death, all | Adj. HR (95% CI) | Death, all | Adj. HR (95% CI) |
| Follow-up within 3 mo postsurgery | ||||||
| VATS-L (ref.), RATS-L (all cases) | 398, 18 908 | 1.20 (0.96 to 1.50) | 350, 16 593 | 1.18 (0.94 to 1.49) | 196, 9074 | 1.21 (0.91 to 1.60) |
| Tumor size: 1-20 mm | 135, 8975 | 1.27 (0.87 to 1.86) | 122, 7895 | 1.20 (0.82 to 1.77) | 74, 4381 | 1.08 (0.69 to 1.71) |
| Tumor size: 21-40 mm | 214, 8471 | 1.34 (0.99 to 1.80) | 186, 7429 | 1.35 (1.00 to 1.83) | 101, 4005 | 1.58 (1.06 to 2.36) |
| Tumor size: other or unknown | 49, 1463 | 0.59 (0.27 to 1.28) | 42, 1269 | 0.55 (0.24 to 1.23) | 21, 688 | 0.51 (0.21 to 1.26) |
| Pinteraction | .22 | .12 | .07 | |||
| VATS-L (ref.), RATS-L (no conversion) | 293, 16 383 | 1.11 (0.97 to 1.26) | 256, 14 487 | 1.11 (0.97 to 1.26) | 143, 8069 | 1.16 (0.98 to 1.37) |
| Tumor size: 1-20 mm | 96, 7741 | 1.17 (0.94 to 1.46) | 87, 6875 | 1.14 (0.92 to 1.42) | 49, 3885 | 1.23 (0.92 to 1.63) |
| Tumor size: 21-40 mm | 164, 7393 | 1.16 (0.98 to 1.38) | 142, 6526 | 1.17 (0.99 to 1.39) | 81, 3581 | 1.22 (0.98 to 1.53) |
| Tumor size: other or unknown | 33, 1243 | 0.67 (0.41 to 1.09) | 27, 1086 | 0.66 (0.39 to 1.12) | 13, 601 | 0.65 (0.36 to 1.17) |
| Pinteraction | .23 | .14 | .13 | |||
| Follow-up starting from 12 mo postsurgery | ||||||
| VATS-L (ref.), RATS-L (all cases) | ||||||
| Tumor size: 1-20 mm | 915, 8298 | 1.33 (1.15 to 1.55) | 766, 7279 | 1.36 (1.17 to 1.58) | 447, 4014 | 1.33 (1.11 to 1.61) |
| Tumor size: 21-40 mm | 1189, 7634 | 1.03 (0.90 to 1.19) | 983, 6681 | 1.02 (0.89 to 1.18) | 539, 3597 | 0.95 (0.80 to 1.13) |
| Tumor size: other or unknown | 274, 1271 | 0.95 (0.70 to 1.29) | 220, 1098 | 0.97 (0.71 to 1.31) | 110, 595 | 1.12 (0.77 to 1.63) |
| Pinteraction | .02 | .01 | .03 | |||
| VATS-L (ref.), RATS-L (no conversion) | ||||||
| Tumor size: 1-20 mm | 765, 7170 | 1.19 (1.10 to 1.29) | 652, 6348 | 1.19 (1.10 to 1.29) | 395, 3566 | 1.17 (1.06 to 1.29) |
| Tumor size: 21-40 mm | 1022, 6700 | 1.01 (0.94 to 1.08) | 847, 5906 | 1.01 (0.93 to 1.09) | 474, 3234 | 0.97 (0.89 to 1.06) |
| Tumor size: other or unknown | 226, 1090 | 1.01 (0.86 to 1.19) | 180, 948 | 1.02 (0.87 to 1.20) | 94, 529 | 1.13 (0.92 to 1.39) |
| Pinteraction | .007 | .008 | .02 | |||
| Follow-up starting from 18 mo postsurgery | ||||||
| VATS-L (ref), RATS-L (all cases) | ||||||
| Tumor size: 1-20 mm | 575, 7606 | 1.36 (1.16 to 1.61) | 625, 6625 | 1.40 (1.18 to 1.65) | 362, 3623 | 1.40 (1.14 to 1.72) |
| Tumor size: 21-40 mm | 960, 6920 | 1.03 (0.88 to 1.20) | 785, 6022 | 1.01 (0.86 to 1.18) | 424, 3233 | 0.94 (0.78 to 1.14) |
| Tumor size: other or unknown | 197, 1126 | 0.91 (0.63 to 1.31 | 158, 970 | 0.95 (0.66 to 1.36) | 80, 524 | 1.06 (0.68 to 1.64) |
| Pinteraction | .02 | .008 | .02 | |||
| VATS-L (ref.), RATS-L (no conversion) | ||||||
| Tumor size: 1-20 mm | 632, 6571 | 1.20 (1.10 to 1.31) | 532, 5780 | 1.21 (1.10 to 1.32) | 323, 3219 | 1.18 (1.06 to 1.32) |
| Tumor size: 21-40 mm | 822, 6073 | 1.01 (0.93 to 1.10) | 676, 5329 | 1.00 (0.92 to 1.09) | 374, 2909 | 0.97 (0.88 to 1.08) |
| Tumor size: other or unknown | 159, 959 | 1.00 (0.82 to 1.21 | 127, 833 | 1.04 (0.86 to 1.26) | 68, 456 | 1.13 (0.89 to 1.43) |
| Pinteraction | .01 | .01 | .04 | |||
| Follow-up starting at 24 mo postsurgery | ||||||
| VATS-L, RATS-L (all cases) | ||||||
| Tumor size: 1-20 mm | 588, 6562 | 1.39 (1.15 to 1.67) | 470, 5625 | 1.47 (1.21 to 1.78) | 277, 3055 | 1.44 (1.13 to 1.82) |
| Tumor size: 21-40 mm | 755, 5865 | 1.05 (0.88 to 1.26) | 605, 5015 | 1.03 (0.86 to 1.24) | 325, 2684 | 0.97 (0.78 to 1.21) |
| Tumor size: other or unknown | 147, 938 | 0.81 (0.52 to 1.26) | 114, 794 | 0.88 (0.57 to 1.36) | 61, 426 | 0.85 (0.51 to 1.41) |
| Pinteraction | .03 | .009 | .03 | |||
| VATS-L (ref.), RATS-L (no conversion) | ||||||
| Tumor size: 1-20 mm | 483, 5646 | 1.21 (1.09 to 1.34) | 394, 4892 | 1.24 (1.12 to 1.38) | 246, 2706 | 1.18 (1.04 to 1.34) |
| Tumor size: 21-40 mm | 644, 5134 | 1.03 (0.94 to 1.13) | 519, 4430 | 1.02 (0.93 to 1.13) | 289, 2420 | 0.99 (0.88 to 1.11) |
| Tumor size: other or unknown | 118, 793 | 0.91 (0.72 to 1.15) | 90, 677 | 0.98 (0.78 to 1.24) | 51, 378 | 0.99 (0.75 to 1.30) |
| Pinteraction | .02 | .01 | .11 | |||
In unmatched model: adjusting for age at diagnosis, sex, race or ethnicity, educational level, annual income, insurance status, coexisting medical conditions, distance to treatment center, facility type, histology, grade, tumor size, other cancer treatment, year of cancer diagnosis, time since diagnosis to surgery, and center case volume of surgery (VATS-L and RATS-L) performed. CI = confidence interval; HR = hazard ratio; PS = propensity score; RATS-L = robotic-assisted thoracoscopic surgical lobectomy; VATS-L = video-assisted thoracoscopic surgical lobectomy.
In 1:1 and N:1 PS-matched models: adjusting for PS-matched set.
We also evaluated the potential role of center case volume of VATS-L or RATS-L, which reflects the institutional experience of minimally invasive operations performed, on survival. We found that, although all-cause mortality risk was inversely associated with case volume (Supplementary Table 3, available online), the latter did not explain the interactive association of surgical type and tumor size on long-term mortality (Supplementary Table 4, available online).
Discussion
In response to the recent US FDA’s call for studies on the effects of robotic devices in minimally invasive cancer surgeries, especially on long-term oncologic endpoints (26,27), we evaluated all-cause mortality, both short-term and long-term, associated with VATS-L and RATS-L in stage I NSCLC patients using data from the NCDB. We found that, compared with VATS-L, RATS-L was associated with about up to 40% higher long-term all-cause mortality among patients with small cancer (≤20 mm). VATS-L had a higher rate than RATS-L to convert to open thoracotomy. However, once conversion occurred, RATS-L had a higher 90-day mortality, although the difference became statistically insignificant after multivariate adjustment. We did not find these 2 surgery types had a different mortality outcome when tumor size was larger than 20 mm. To our knowledge, this is the first large study to date to evaluate both short- and long-term survival outcomes of VATS-L vs RATS-L for the treatment of stage I NSCLC.
Studies have documented that intraoperative conversion rates from VATS-L to open thoracotomy range up to 23% in lung cancer patients (29–35). An earlier analysis using NCDB data from 2010 to 2012 (36) reported a statistically significantly higher conversion rate associated with VATS-L (17.5%) compared with RATS-L (10.3%). Similarly, a study using the Premier Healthcare Database reported that, compared with RATS-L, VATS-L was associated with a statistically significantly higher conversion rate (13.1% vs 6.3%), although the rates of intraoperative complications, bleeding, transfusion, and iatrogenic complications were similar between these 2 minimally invasive cohorts (19). In line with the previous studies, we confirmed in our study a higher conversion rate in VATS-L (15.2%) vs RATS-L (7.8%), although patients did not differ statistically significantly in tumor size, histology, and grade. Interestingly, we observed that once a conversion occurred, patients who underwent RATS-L had a higher 30-day unplanned readmission rate and elevated 90-day mortality than those who received VATS-L, although no statistically significant difference in 90-day mortality was observed in multivariate analyses.
Few studies have investigated long-term survival outcomes of RATS-L vs VATS-L in treating early-stage lung cancer patients (37–39). Yang et al. (37) compared 470 stage I NSCLC patients (172 RATS-L, 141 VATS-L, and 157 open lobectomy) and reported the 5-year overall survival rates for the RATS-L, VATS-L, and open lobectomy matched groups were 77.6%, 73.5%, and 77.9%, respectively, without a statistically significant difference. Similarly, Park et al. (38,39) evaluated 325 consecutive patients who underwent robotic lobectomy for early-stage NSCLC to assess long-term oncologic efficacy and reported that long-term stage-specific survival did not differ statistically significantly from that of VATS-L or thoracotomy. However, the previous studies did not evaluate the potential modifying effect of tumor size on the association of surgical approach (VATS-L or RATS-L) with mortality. In this large-scale study that included 18 908 stage I NSCLC patients who underwent VATS-L (n = 14 279) or RATS-L (n = 4629), we found that, compared with VATS-L, RATS-L was associated with a statistically significantly higher long-term all-cause mortality (from 1 year postsurgery up to 7 years of observation) in treating patients with small tumors (≤20 mm). The results were consistently seen in the intention-to-treat analyses or analyses including only patients without conversion to open thoracotomy during operation. Furthermore, we provided evidence that this tumor size–specific association is independent of center case volume of VATS-L or RATS-L performed. These findings are consistently seen in our unmatched, N:1, or 1:1 propensity score–matched analyses. Further investigations are needed to confirm our findings and reveal the underlying reasons for such tumor size–specific associations for long-term mortality between these 2 minimally invasive surgical approaches in treating early-stage NSCLC.
We acknowledge that the present analysis has several noticeable limitations inherited from the NCDB data, especially for evaluation of long-term survival. First, the NCDB provides data only on all-cause death, not on disease-specific death; therefore, associations of VATS-L and RATS-L with cancer-specific mortality could not be examined and compared. Second, despite the detailed information on clinical characteristics and first-line treatment, we did not have information on many factors that may be associated with overall and lung cancer–specific survival, such as lifestyle factors, particularly posttreatment cigarette smoking habits, comorbidity, performance status, physical activity, weight, health-related quality of life, and genetic factors (37–40). Thus, we could not determine if the higher all-cause mortality of RATS-L derived from the surgical procedure or from the differences between RATS-L– and VATS-L–treated patients on other mortality risk factors. In addition, data from the NCDB were collected for patients diagnosed and/or treated at Commission on Cancer–accredited facilities, which are more likely to be located in larger, more urban areas compared with facilities not accredited by the Commission on Cancer. Therefore, our results may not be generalizable to all cancer patients treated in the United States (40).
In summary, this is the first large study to our knowledge to report that patients with small (≤20 mm) stage I NSCLC treated with RATS-L, compared with VATS-L, had a higher long-term all-cause mortality and that such an association was not related to center case volume of VATS-L or RATS-L performed. Our study supports the United States Food and Drug Administration’s call for additional research to evaluate the long-term safety and effectiveness of robotic devices in minimally invasive cancer surgeries. Future studies should include assessments of disease recurrence or specific mortality and account for other mortality-associated factors in order to draw a definitive conclusion.
Funding
None.
Notes
Conflicts of interest: The authors have declared no conflicts of interest.
Role of the author: YC, EG, SD and XS contributed to conception and design of the study; YC, HC and FW conducted data analysis; CB and XS obtained the data; YC and XS drafted paper; all authors contributed interpretation of results and manuscript review and approved the final version of the manuscript.
Acknowledgments: The authors acknowledge the National Cancer Database (jointly sponsored by the American Cancer Society and American College of Surgeons) for collecting, assembling and providing data and Dr Mary Shannon Byers for technical support in preparing the manuscript. Dr Fei Wang is supported by the program of China Scholarships Council.
Supplementary Material
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 3. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. [DOI] [PubMed] [Google Scholar]
- 4. Monsalve AF, Hoag JR, Resio BJ, et al. Variable impact of prior cancer history on the survival of lung cancer patients. Lung Cancer. 2019;127:130–137. [DOI] [PubMed] [Google Scholar]
- 5. American Cancer Society. Non-small cell lung cancer survival rates. https://www.cancer.org/cancer/non-small-cell-lung-cancer/./survival-rates.html. Accessed November 7, 2019.
- 6. Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5):e278S–e313S. [DOI] [PubMed] [Google Scholar]
- 7. Whitson BA, Groth SS, Andrade RS, et al. Survival after lobectomy versus segmentectomy for stage I non-small cell lung cancer: a population-based analysis. Ann Thorac Surg. 2011;92(6):1943–1950. [DOI] [PubMed] [Google Scholar]
- 8. Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc. 1992;2(3):244–247. [PubMed] [Google Scholar]
- 9. Nwogu CE, D’Cunha J, Pang H, et al. VAT lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg. 2015;99(2):399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139(2):366–378. [DOI] [PubMed] [Google Scholar]
- 11. Cattaneo SM, Park BJ, Wilton AS, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg. 2008;85(1):231–235. [DOI] [PubMed] [Google Scholar]
- 12. Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2007;83(6):1965–1970. [DOI] [PubMed] [Google Scholar]
- 13. Yang CJ, Kumar A, Klapper JA, et al. A national analysis of long-term survival following thoracoscopic versus open lobectomy for stage I non-small-cell lung cancer. Ann Surg. 2019;269:163–171. [DOI] [PubMed] [Google Scholar]
- 14. Yang CJ, Kumar A, Deng JZ, et al. A national analysis of short-term outcomes and long-term survival following thoracoscopic versus open lobectomy for clinical stage II non-small-cell lung cancer. Ann Surg. 2019; doi: 10.1097/SLA.0000000000003231. [DOI] [PubMed] [Google Scholar]
- 15. Boffa DJ, Kosinski AS, Furnary AP, et al. Minimally invasive lung cancer surgery performed by thoracic surgeons as effective as thoracotomy. J Clin Oncol. 2018;36(23):2378–2385. [DOI] [PubMed] [Google Scholar]
- 16. Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg. 2002;21(5):864–868. [DOI] [PubMed] [Google Scholar]
- 17. Veronesi G, Novellis P, Voulaz E, et al. Robot-assisted surgery for lung cancer: state of the art and perspectives. Lung Cancer. 2016;101:28–34. [DOI] [PubMed] [Google Scholar]
- 18. Paul S, Jalbert J, Isaacs AJ, et al. Comparative effectiveness of robotic-assisted vs thoracoscopic lobectomy. Chest. 2014;146(6):1505–1512. [DOI] [PubMed] [Google Scholar]
- 19. Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-assisted, video-assisted thoracoscopic and open lobectomy: propensity-matched analysis of recent premier data. Ann Thorac Surg. 2017;104(5):1733–1740. [DOI] [PubMed] [Google Scholar]
- 20. Louie BE, Farivar AS, Aye RW, et al. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg. 2012;93(5):1598–1604. [DOI] [PubMed] [Google Scholar]
- 21. Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg. 2014;97(6):1901–1906. [DOI] [PubMed] [Google Scholar]
- 22. Nelson DB, Mehran RJ, Mitchell KG, et al. Robotic assisted lobectomy for non-small cell lung cancer: a comprehensive Institutional experience. Ann Thorac Surg. 2019;108(2):370–376. [DOI] [PubMed] [Google Scholar]
- 23. Farivar AS, Cerfolio RJ, Knight AW, et al. Comparing robotic lung resection with thoracotomy and video-assisted thoracoscopic surgery cases entered into the Society of Thoracic Surgeons database. Innovations (Phila). 2014;9(1):10–15. [DOI] [PubMed] [Google Scholar]
- 24. Swanson SJ, Miller DL, McKenna RJ, Jr, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier). J Thorac Cardiovasc Surg. 2014;147(3):929–937. [DOI] [PubMed] [Google Scholar]
- 25. Wei S, Chen M, Chen N, Liu L. Feasibility and safety of robot-assisted thoracic surgery for lung lobectomy in patients with non-small cell lung cancer: a systematic review and meta-analysis. World J Surg Oncol. 2017;15(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. US Food and Drug Administration. Caution when using robotically-assisted surgical devices in women's health including mastectomy and other cancer-related surgeries: FDA safety communication. https://www.fda.gov/medical-devices/safety-communications/caution-when-using-robotically-assisted-surgical-devices-womens-health-including-mastectomy-and./. Accessed July 7, 2019.
- 27. Ong M. FDA tightens regulation of robotic devices in minimally invasive surgery for cancer. https://cancerletter.com/articles/20190301_2/. Accessed July 7, 2019. [Google Scholar]
- 28. Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3(12):1722–1728. [DOI] [PubMed] [Google Scholar]
- 29. Gharagozloo F, Tempesta B, Margolis M, et al. Video-assisted thoracic surgery lobectomy for stage I lung cancer. Ann Thorac Surg. 2003;76(4):1009–1015. [DOI] [PubMed] [Google Scholar]
- 30. McKenna RJ, Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg. 2006;81(2):421–426. [DOI] [PubMed] [Google Scholar]
- 31. Roviaro G, Varoli F, Vergani C, et al. Video-assisted thoracoscopic major pulmonary resection: technical aspects, personal series of 259 patients and review of the literature. Surg Endosc. 2004;18(11):1551–1558. [DOI] [PubMed] [Google Scholar]
- 32. Solaini L, Prusciano F, Bagioni P, et al. Video-assisted thoracic surgery (VAT) of the lung: analysis of intraoperative and postoperative complications over 15 years and review of the literature. Surg Endosc. 2008;22(2):298–310. [DOI] [PubMed] [Google Scholar]
- 33. Puri V, Patel A, Majumder K, et al. Intraoperative conversion from video-assisted thoracoscopic surgery lobectomy to open thoracotomy: a study of causes and implications. J Thorac Cardiovasc Surg. 2015;149(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Byun CS, Lee S, Kim DJ, et al. Analysis of unexpected conversion to thoracotomy during thoracoscopic lobectomy in lung cancer. Ann Thorac Surg. 2015;100(3):968–973. [DOI] [PubMed] [Google Scholar]
- 35. Amore D, Di Natale D, Scaramuzzi R, et al. Reasons for conversion during VAT lobectomy: what happens with increased experience. J Vis Surg. 2018;4:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang CF, Sun Z, Speicher PJ, et al. Use and outcomes of minimally invasive lobectomy for stage I non-small cell lung cancer in the national database. Ann Thorac Surg. 2016;101(3):1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang HX, Woo KM, Sima CS, et al. Long-term survival based on the surgical approach to lobectomy for clinical stage I nonsmall cell lung cancer: comparison of robotic, video-assisted thoracic surgery, and thoracotomy lobectomy. Ann Surg. 2017;265(2):431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park BJ. Robotic lobectomy for non-small cell lung cancer: long-term oncologic results. Thorac Surg Clin. 2014;24(2):157–162. [DOI] [PubMed] [Google Scholar]
- 39. Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg. 2012;143(2):383–389. [DOI] [PubMed] [Google Scholar]
- 40. Carte GC, Barrett AM, Kaye JA, et al. A comprehensive review of nongenetic prognostic and predictive factors influencing the heterogeneity of outcomes in advanced non-small-cell lung cancer. Cancer Manag Res. 2014;6:437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.