Abstract
The prognosis of patients with aneurysmal subarachnoid haemorrhage requiring decompressive craniectomy is usually poor. Proper selection and early performing of decompressive craniectomy might improve the patients’ outcome. We aimed at developing a risk score for prediction of decompressive craniectomy after aneurysmal subarachnoid haemorrhage. All consecutive aneurysmal subarachnoid haemorrhage cases treated at the University Hospital of Essen between January 2003 and June 2016 (test cohort) and the University Medical Center Freiburg between January 2005 and December 2012 (validation cohort) were eligible for this study. Various parameters collected within 72 h after aneurysmal subarachnoid haemorrhage were evaluated through univariate and multivariate analyses to predict separately primary (PrimDC) and secondary decompressive craniectomy (SecDC). The final analysis included 1376 patients. The constructed risk score included the following parameters: intracerebral (‘Parenchymal’) haemorrhage (1 point), ‘Rapid’ vasospasm on angiography (1 point), Early cerebral infarction (1 point), aneurysm Sac > 5 mm (1 point), clipping (‘Surgery’, 1 point), age Under 55 years (2 points), Hunt and Hess grade ≥ 4 (‘Reduced consciousness’, 1 point) and External ventricular drain (1 point). The PRESSURE score (0–9 points) showed high diagnostic accuracy for the prediction of PrimDC and SecDC in the test (area under the curve = 0.842/0.818) and validation cohorts (area under the curve = 0.903/0.823), respectively. 63.7% of the patients scoring ≥6 points required decompressive craniectomy (versus 12% for the PRESSURE < 6 points, P < 0.0001). In the subgroup of the patients with the PRESSURE ≥6 points and absence of dilated/fixed pupils, PrimDC within 24 h after aneurysmal subarachnoid haemorrhage was independently associated with lower risk of unfavourable outcome (modified Rankin Scale >3 at 6 months) than in individuals with later or no decompressive craniectomy (P < 0.0001). Our risk score was successfully validated as reliable predictor of decompressive craniectomy after aneurysmal subarachnoid haemorrhage. The PRESSURE score might present a background for a prospective randomized clinical trial addressing the utility of early prophylactic decompressive craniectomy in aneurysmal subarachnoid haemorrhage.
Keywords: subarachnoid haemorrhage, decompressive craniectomy, risk score, prediction, outcome
In this pooled analysis of 1376 patients with subarachnoid haemorrhage, we developed and successfully validated a risk score for early prediction of decompressive craniectomy, the PRESSURE score. We recommend a prospective randomized clinical trial addressing the utility of early prophylactic decompressive craniectomy after subarachnoid haemorrhage using the PRESSURE score.
Graphical Abstract
Graphical Abstract.
Introduction
Decompressive craniectomy (DC) is an effective neurosurgical treatment against intractable intracranial hypertension in different neurocritical conditions (Berge, 2007; Fung et al., 2012; Hutchinson et al., 2016; Hadjiathanasiou et al., 2018; Kolias et al., 2018; Peng et al., 2019). However, a clinical benefit from DC has been proven so far only for space-occupying cerebral infarction (Vahedi et al., 2007).
In patients with aneurysmal subarachnoid haemorrhage (SAH) presenting with severe onset or secondary ischaemic and haemorrhagic complications, DC might also be required as a lifesaving option. There is still no guideline for DC after SAH, since the present evidence is limited to mostly small mono-centric retrospective series (Fisher and Ojemann, 1994; Smith et al., 2002; D'Ambrosio et al., 2005; Buschmann et al., 2007; Park et al., 2007; Schirmer et al., 2007; Kang, 2008; Otani et al., 2008; Guresir et al., 2009a, b; Nagel et al., 2009; Dorfer et al., 2010; Otani et al., 2011; Stuart et al., 2011; Fung et al., 2012; Tuzgen et al., 2012; Hwang et al., 2014; Mori, 2014; Uozumi et al., 2014; Jussen et al., 2015; Jabbarli et al., 2017). Accordingly, indications to DC in SAH patients are still based on case-by-case decisions.
Across the previous SAH reports, DC cases can be simply dichotomized into primary (PrimDC) and secondary (SecDC) DC, depending on the time when DC was initiated (primary at admission or secondary in case of intractable intracranial hypertension). In a recent meta-analysis (Alotaibi et al., 2017), a better outcome of SAH patients after PrimDC, as compared to SecDC, was reported. In addition, a clinical benefit of an early PrimDC (performed within 24 h after SAH) over later DC cases was shown in a large retrospective series (Jabbarli et al., 2017). Similar results were also demonstrated after ischaemic stroke, where early DC within 24 h or before clinical signs of herniation improved overall mortality and functional outcomes (Dasenbrock et al., 2017; Shah et al., 2019).
In view of the above-mentioned data, early selection and timing of DC might improve the outcome of SAH patients requiring DC. An appropriate risk score, which could reliably estimate the risk of DC throughout the acute phase of SAH, might be helpful for early selection of DC candidates. Therefore, we aimed at development and validation of a novel risk score for early identification of individuals who undergo DC after SAH, using two large institutional SAH cohorts. A special attention was paid on high accuracy of the score for prediction of both PrimDC and SecDC, as separate indication subgroups.
Materials and methods
Patient population
This retrospective analysis is based on two observational institutional SAH cohorts containing consecutive cases with aneurysmal SAH treated at the university hospitals of Essen (between January 2003 and June 2016) and Freiburg (between January 2005 and December 2012). Both observational studies were approved by the local Institutional Ethics Committees (Ethik-Kommission, Albert-Ludwigs-Universität Freiburg, Registration number: 446/13 and Ethik-Kommission, Medizinische Fakultät der Universität Duisburg-Essen, Registration number: 15-6331-BO) and registered in the German clinical trial register (DRKS, Unique identifiers: DRKS00005486 and DRKS00008749).
SAH patients were included from the databases if they were admitted and treated by means of endovascular coiling or microsurgical clipping within 72 h after the bleeding event. The cases without aneurysm treatment, with DC surgery before SAH and later admission/treatment were excluded from the final analysis.
SAH management
Initial admission and treatment of SAH patients were performed in the neurosurgical intensive care units of the centres. Ruptured aneurysms were commonly identified by digital subtraction angiography (DSA) with further treatment allocation to either coiling or clipping. In both centres, treatment of a ruptured aneurysm was performed within 24 h after admission. In SAH cases with clinical signs of brainstem herniation (fixed/dilated pupils) at admission and space-occupying intracerebral haematoma (ICH) on computed tomography (CT) scan, the diagnostic phase was limited to CT angiography with subsequent immediate surgical treatment of the patients. In these cases, diagnostic DSA was performed after the aneurysm treatment. Acute hydrocephalus was treated with an external ventricular drainage (EVD) allowing continuous monitoring of intracranial pressure. Conservative management of cerebral vasospasm included oral nimodipine and maintenance of normovolemia. Endovascular vasospasm treatment was initiated in cases of refractory cerebral vasospasm. SAH patients developing chronic hydrocephalus underwent ventriculo-peritoneal shunt placement. Along with a native CT scan at admission, SAH patients underwent additional CT scans: within 24 h after aneurysm treatment, prior to SecDC and/or shunt placement, as well as in cases of any clinical deterioration. There were no relevant changes in the management protocols for SAH over the reported years in the centres.
DC after SAH
In both centres, patients underwent PrimDC based on clinical (poor neurological condition) and radiological presentation (ICH and brain oedema on the initial CT scan) at admission, as well as intraoperative presence of brain swelling. The actual decision to perform PrimDC was up to the neurosurgeons on duty. The majority of the PrimDC cases (175/185, 94.6%) were selected for aneurysm clipping with simultaneous DC. In the remaining 10 cases, PrimDC was performed immediately after aneurysm coiling.
The patients were selected for SecDC in case of persistent intracranial pressure raise >20 mmHg despite maximal conservative treatment (continuous cerebrospinal fluid drainage via EVD, deep sedation, osmotic diuresis, maintenance of normothermia and mild hyperventilation with goal PaCO2 of 35 mmHg). In accordance to general recommendations for DC surgery (Carney et al., 2017), the size of the bone flap for DC had a minimum diameter of 12 cm.
Data management
The goal of this study was the development and validation of a novel risk score for early prediction of individuals who undergo PrimDC or SecDC after SAH. As potential score components, various demographic, clinical, radiographic and laboratory variables, which were present within 72 h after SAH, were collected (the full list of the tested parameters is shown in Table 1). Clinical condition at admission was graded according to Hunt and Hess (1968), wherein the presence of dilated/fixed pupils was additionally documented as a clinical sign of brainstem herniation (Fung et al., 2016). The cases with pupillary dysfunction related to local compression syndrome from aneurysm were not considered as brainstem herniation signs. Location and the size (maximal diameter) of the ruptured aneurysms were recorded from the DSA reports. In addition, the presence of an unequivocal narrowing of the arterial vessel lumen on the admission DSA suspicious for cerebral vasospasm was also recorded. The severity of SAH on the initial CT scans was assessed according to the original Fisher scale (Fisher et al., 1980). Further radiographic evaluation included the measurement of the severity of intraventricular haemorrhage (IVH) using the original Graeb score (Graeb et al., 1982), and the volume of aneurysmal ICH by the AxBxC/2-formula (Kothari et al., 1996). In both centres, all CT scans were reviewed by the first author (R.J.) blinded at that time for any clinical information. Occurrence of cerebral infarction within 72 h after SAH was defined as early infarction. Hypodensities resulting from ICH, EVD or surgical approach were not considered as infarcts. Medical comorbidities (arterial hypertension, smoking history, diabetes mellitus and prior anticoagulation) were documented according to the electronic health records. The functional endpoints of the study were in-hospital mortality and unfavourable outcome at 6 months after SAH defined as a modified Rankin Scale >3 (van Swieten et al., 1988). According to the standard operating procedures of both units, the follow-up evaluations of the patients were performed in the appropriate outpatient clinics on a routine basis.
Table 1.
Univariate analysis of the predictors of PrimDC and SecDC in the test cohort
| Parameter | Prim DC versus No DC |
SecDC versus NO DC |
||
|---|---|---|---|---|
| Mean (±SD) or OR (95% CI) | P-value | Mean (±SD) or OR (95% CI) | P-value | |
| Age, years | 52.9 (±12.6) versus 52.9 (±12.6) | 0.0379 | 49.7 (±10.1) versus 52.9 (±12.6) | 0.0008 |
| Sex (female) | 1.11 (0.77–1.6) | 0.5820 | 1.56 (0.94–2.6) | 0.1054 |
| Prior anticoagulation | 0.81 (0.41–1.6) | 0.6244 | 0.67 (0.23–1.98) | 0.6272 |
| Arterial hypertension | 1.2 (0.82–1.75) | 0.3841 | 1.59 (0.91–2.78) | 0.1143 |
| Smoking (history) | 0.88 (0.53–1.46) | 0.7058 | 1.0 (0.51–2.13) | 0.8565 |
| Diabetes mellitus | 0.84 (0.34–2.09) | 0.8265 | 0.34 (0.04–2.53) | 0.5018 |
| Hunt and Hess scale (IV–V) | 4.54 (3.15–6.54) | <0.0001 | 2.46 (1.48–4.08) | 0.0007 |
| Dilated/fixed pupils | 6.08 (3.67–10.06) | <0.0001 | 1.46 (0.55–3.89) | 0.3995 |
| Fisher scale (III–IV) | 30.77 (4.24–223.0) | <0.0001 | 2.17 (0.85–5.58) | 0.1322 |
| Aneurysm (anterior circulation) | 5.74 (3.29–10.02) | <0.0001 | 2.83 (1.45–5.51) | 0.0016 |
| Aneurysm size, mm | 8.7 (±4.8) versus 6.7 (±4.2) | <0.0001 | 8.1 (±5.1) versus 6.7 (±4.2) | 0.0118 |
| Treatment modality (clipping) | 68.14 (32.66 - 142.2) | <0.0001 | 2.05 (1.21–3.46) | 0.0110 |
| Acute hydrocephalus | 0.88 (0.61–1.27) | 0.5096 | 3.67 (1.64–8.18) | 0.0005 |
| Presence of IVH | 1.17 (0.83–1.64) | 0.3865 | 1.48 (0.89–2.46) | 0.1553 |
| Severity of IVH (Graeb score) | 2.89 (±3.8) versus 2.17 (±3.1) | 0.1207 | 2.58 (±3.3) versus 2.17 (±3.1) | 0.1771 |
| Presence of ICH | 20.07 (13.01–30.95) | <0.0001 | 3.88 (2.29–6.56) | <0.0001 |
| ICH volume, ml | 38.86 (±35.2) versus 13.9 (±16.8) | <0.0001 | 13.4 (±16) versus 13.9 (±16.8) | 0.6165 |
| Rebleeding | 3.88 (1.91–7.85) | 0.0004 | 2.79 (0.99–7.87) | 0.0595 |
| Early angiographic vasospasm | 2.76 (1.62–4.69) | 0.0004 | 3.51 (1.91–6.45) | 0.0001 |
| Early Infarction | 2.97 (2.07–4.25) | <0.0001 | 4.22 (2.5–7.11) | <0.0001 |
| WBC, × 109/l | 14.02 (±4.5) versus 13 (±4.7) | 0.0068 | 14.7 (±4.7) versus 13 (±4.7) | 0.0052 |
| Haemoglobin, × 101 g/l | 12.4 (±2) versus 12.7 (±1.9) | 0.1099 | 12.9 (±1.9) versus 12.7 (±1.9) | 0.6074 |
| CRP, × 10−3 g/l | 0.7 (±1.6) versus 1.0 (±1.9) | 0.1504 | 1.0 (±2.1) versus 1.0 (±1.9) | 0.5202 |
| CSF Interleukin-6, × 10−6 g/l | 4.16 (±6.14) versus 3.4 (±6.51) | 0.7950 | 4.39 (±7.3) versus 3.4 (±6.51) | 0.9597 |
| CSF cells, × 106/l | 2705 (±7204) versus 1229 (±2963) | 0.5562 | 1380 (±1853) versus 1229 (±2963) | 0.3275 |
| Troponin-I, 10−9 g/l | 0.6 (±2) versus 2.5 (±31.7) | 0.2435 | 0.5 (±1) versus 2.5 (±31.7) | 0.9254 |
| Maximal systolic BP, mmHg | 161.6 (±22.6) versus 165.4 (±25.8) | 0.0513 | 168.2 (±24.7) versus 165.4 (±25.8) | 0.2476 |
| Minimal systolic BP, mmHg | 125.5 (±88.6) versus 124.1 (±57.4) | 0.2574 | 122.9 (±24.2) versus 124.1 (±57.4) | 0.9612 |
| Mean MAP, mmHg | 89.3 (±14.8) versus 90.5 (±14.6) | 0.1131 | 93.1 (±15.3) versus 90.5 (±14.6) | 0.2552 |
| Maximal body temperature, °C | 37.2 (±0.9) versus 37 (±1.9) | 0.5385 | 37.2 (±0.9) versus 37 (±1.9) | 0.3009 |
For laboratory parameters, the first value at admission was considered. P-values set in boldface indicate statistical significance.
SD = standard deviation; OR = odds ratio; CI = confidence interval; WBC = white blood cells; CRP = C-reactive protein; CSF = cerebrospinal fluid; BP = blood pressure; MAP = mean arterial pressure.
Statistical analysis
Data of the patients from the university Hospital of Essen were used for the creation of the risk score (‘test cohort’), whereas the external validation of the score was performed on the SAH cohort from Freiburg (‘validation cohort’). After the creation and validation of the score, further analyses regarding the associations between DC and SAH outcome were performed in the pooled data of both cohorts.
Score creation
Prior to eventual inclusion to the risk score, all recorded ‘admission’ variables were subsequently tested through univariate and multivariate analysis for the associations with PrimDC and SecDC separately. Univariate assessments were performed using the Student’s t- test for normally distributed and the Mann–Whitney U test for non-normally distributed continuous data, as well as the Fisher exact or chi-square tests for categorical variables. Significant parameters were considered for further multivariate analysis, where all included variables were converted to dichotomous variables to make them eligible as score components. Initial clinical condition (Hunt and Hess) was as assessed as good (I–III) and poor (IV–V) grades, and the Fisher scale as low (I–II) and high (III–IV) grades. The remaining continuous variables (age, aneurysm size, ICH volume, white blood cells count) were dichotomized according to the cut-off estimation in the receiver operating characteristic curve and the appropriate area under the curve (AUC) values.
Thereafter, independent predictors of PrimDC and SecDC in the test cohort were identified using multivariate binary logistic regression analysis. The significant parameters from both analyses were then included as final score components. The appropriate adjusted odds ratios (aOR) were divided by the smallest coefficient and then rounded to the nearest whole number.
Score validation
Based upon the presence of the components of the new score, a total point value was calculated for each SAH patient from the final analysis. For both cohorts separately, the association between the risk score and PrimDC/SecDC was tested using the receiver operating characteristic curves, bivariate and multivariate analyses. In addition, the Hosmer–Lemeshow test for goodness of fit for logistic regression models and the bootstrapping method were also applied during the score validation.
Association of DC with SAH outcome
Using univariate and multivariate analyses, the relationship between DC and outcome endpoints was evaluated in the pooled data from both cohorts. Therefore, the whole cohort was analysed in different subgroups depending on: (i) indication to DC (PrimDC, SecDC or no DC); (ii) timing of DC measured in days since the bleeding event; (iii) risk score values (low- and high-risk groups); and (iv) clinical manifestation (presence of brainstem herniation at admission).
Statistical analyses were performed with the help of PRISM (version 5.0, GraphPad Software Inc., San Diego, CA, USA) and SPSS (version 21, SPSS Inc., IBM, Chicago, IL, USA). Variables were expressed as mean ± standard deviation (SD) or percentage of patients, as appropriate. Differences with a P < 0.05 were regarded as statistically significant. Missing data were replaced using multiple imputation.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
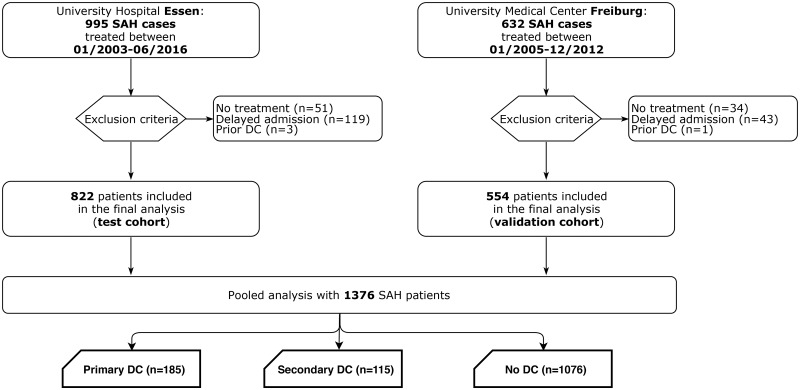
Data of 1627 SAH patients were available in the observational cohorts of both centres. Of them, 1376 individuals (822 and 554 in the test and validation cohorts, respectively) were eligible for this study (Fig. 1). There was no difference with regards to basic demographic, radiographic and clinical characteristics of the analysed cohorts (see Supplementary Table 1). According to the inclusion criteria, all SAH patients in the final analysis were treated within 72 h after the bleeding event. There was no association between the timing of aneurysm treatment and the rates of early CT infarctions (P = 0.633/P = 0.354) and DC (P = 0.941/P = 0.293 in the test and validation cohorts, respectively). However, the test cohort was characterized by a significantly higher proportion of patients undergoing PrimDC after SAH (21% versus 2.2% in the validation cohort, P < 0.0001). In turn, there was no difference in the proportion of patients with SecDC between the cohorts (8.3% versus 8.5%, P = 0.9210).
Figure 1.
Flow-chart demonstrating the selection process of SAH patients eligible for this study in both cohorts.
Risk score construction
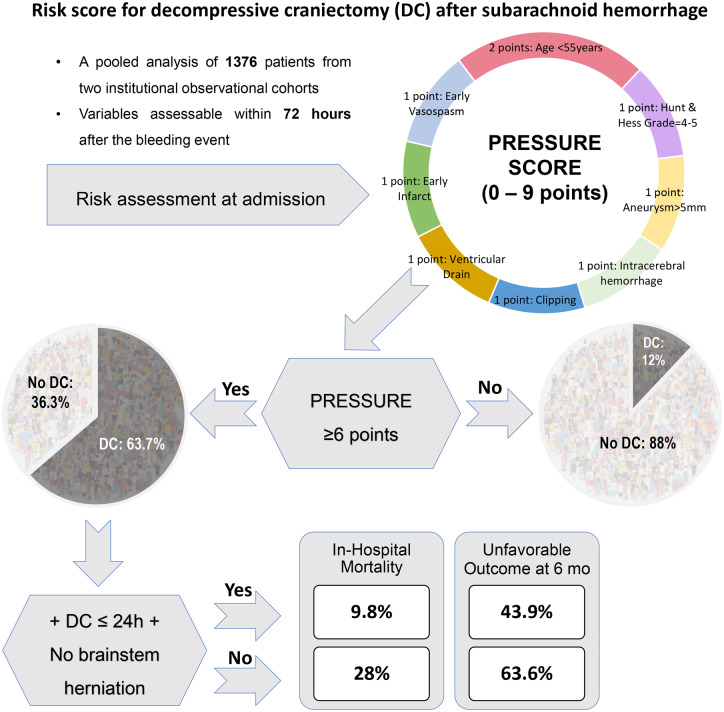
In the test cohort, 30 parameters at admission were correlated with the PrimDC and SecDC (Table 1). Accordingly, 13 and 10 significant variables were included in the multivariate analysis of PrimDC and SecDC predictors, respectively (see Supplementary Table 2). Based on both multivariate analyses, the following parameters were included to the new risk score for DC after SAH: presence of ICH, early vasospasm on DSA, early CT infarction, aneurysm sac size ≥ 5 mm, microsurgical clipping, and younger age (≤55 years old), poor initial clinical condition (Hunt and Hess grades IV–V) and the need for EVD. According to the aOR values, all score components except age (2 points) were weighted with 1 point. Based on the included risk factors, the new score was named PRESSURE (Table 2).
Table 2.
Components, weights and acronym explanation of the PRESSURE score
| Component | Weights | Acronym explanation |
|---|---|---|
| Presence of ICH | 1 | Parenchymal bleeding |
| Early DSA Vasospasm | 1 | Rapid Vasospasm |
| Early CT infarction | 1 | Early Infarct |
| Aneurysm size >5 mm | 1 | Size of aneurysm > 5 mm |
| Clipping | 1 | Surgery of aneurysm |
| Age <55 years | 2 | Under 55 years |
| Hunt and Hess IV – V | 1 | Reduced consciousness |
| Need for EVD | 1 | External ventricular drain |
Risk score validation
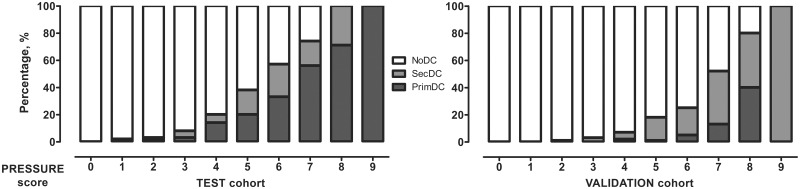
In the receiver operating characteristic curves, the new risk score showed high diagnostic accuracy for the prediction of PrimDC and SecDC in the test (AUC = 0.842/0.818) and validation (AUC = 0.903/0.823) cohorts, respectively (hereinafter). There was a strong association between the PRESSURE score and DC in both cohorts (P < 0.0001/P < 0.0001, Fig. 2). Multivariate analysis confirmed independent association between the risk score and the PrimDC [aOR = 3.15 per point increase (95% confidence interval [CI]: 2.43–4.08), P < 0.0001/aOR = 2.07 per point increase (95% CI: 1.12–3.81), P = 0.02] and SecDC [aOR = 2.56 per point increase (95% CI: 1.93–3.39), P < 0.0001/aOR = 1.9 per point increase (95% CI: 1.42–2.55), P < 0.0001] in the analysed cohorts. According to the Hosmer–Lemeshow test (P = 0.972/P = 0.531), there was no evidence for poor calibration of the PRESSURE score. The bootstrapping method confirmed the robustness of the score against possible variability in both cohorts.
Figure 2.
Proportion of the patients with PrimDC, SecDC or NoDC in different values of the PRESSURE score.
In the pooled data analysis, 63.7% of the patients scoring ≥6 points required DC, as compared with 12% among the cases with the PRESSURE value <6 points, OR = 12.87 (95% CI: 9.3–17.80), P < 0.0001. The difference in the DC rates at the PRESSURE cut-off of 6 points remained significant, when comparing in the test [OR = 15.14 (95% CI: 9.93–23.08), P < 0.0001] and validation [OR = 11.16 (95% CI: 6.13–20.3), P < 0.0001] cohorts separately. Accordingly, SAH patients scoring PRESSURE ≥6 points were regarded as individuals at ‘high risk for DC’.
Finally, the PRESSURE score showed independent association with the functional study endpoints in both cohorts: aOR = 1.55 (95% CI: 1.27–1.89), P < 0.0001/aOR = 1.47 (95% CI: 1.17–1.85), P = 0.001 for in-hospital mortality and aOR = 1.7 (95% CI: 1.44–2.0), P < 0.0001/aOR = 1.57 (95% CI: 1.3–1.9), P < 0.0001 for unfavourable outcome.
DC and SAH outcome
In the whole cohort, the individuals selected for DC showed poorer outcome, than SAH patients without DC: (i) 26.5% (PrimDC) versus 42.1% (SecDC) versus 10.7% (NoDC) for in-hospital mortality (P < 0.0001 for PrimDC versus no DC/P < 0.0001 for SecDC versus no DC); (ii) 64.9% versus 78.3% versus 33.3% for unfavourable outcome (P < 0.0001/P < 0.0001, respectively). In turn, SAH patients with PrimDC were at lower risk for in-hospital mortality (P = 0.0074) and unfavourable outcome (P = 0.0142), than the counterparts with SecDC. The multivariate analysis showed independent association between the timing of DC and functional outcome (P = 0.003).
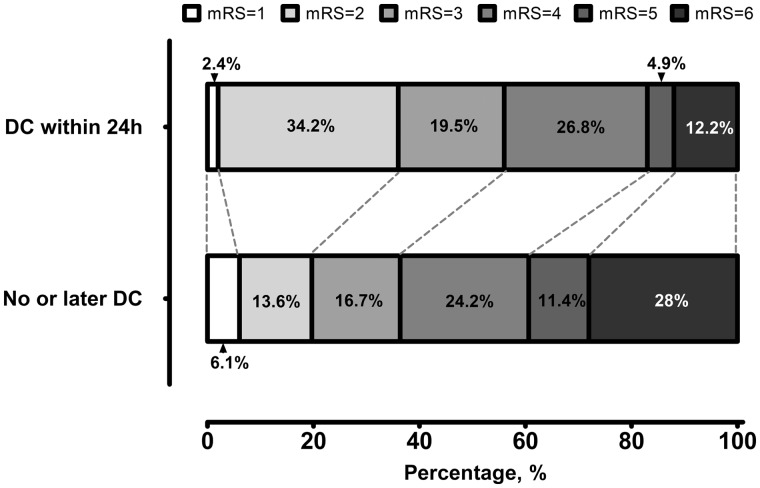
After restricting the analysis to the SAH patients at ‘high risk for DC’ (PRESSURE ≥ 6 points), but without dilated/fixed pupils at admission (n = 173), we compared the outcome of SAH patients who underwent early PrimDC (performed within 24 h after SAH) with the remaining patients (i.e. with later or no DC). Herein, patients with early PrimDC showed lower risk of unfavourable outcome [OR = 0.45 (95% CI: 0.22–0.91), P = 0.0297] and in-hospital mortality [OR = 0.28 (95% CI: 0.09–0.83), P = 0.0197, see Fig. 3 with full modified Rankin Scale values at 6 months post-SAH of the patients in this subgroup]. Independent association between early PrimDC and the study endpoints could be confirmed in the multivariate analysis adjusted for demographic and clinical characteristics of the patients, treating centre and the PRESSURE score value (P < 0.0001/P = 0.018 for unfavourable outcome and in-hospital mortality, respectively, see Supplementary Table 3).
Figure 3.
Outcome of SAH individuals with PRESSURE ≥ 6 points. Functional outcome at 6 months after SAH in the subgroup of patients with ≥6 points on the PRESSURE score (‘high risk for DC’) and without fixed/dilated pupils at admission. The analysis is based on the pooled data from both cohorts.
Discussion
In this pooled analysis of 1376 patients from two institutional observational SAH cohorts, we developed a new risk score for the prediction of DC after SAH. Despite significantly different rates of DC in the analysed cohorts, the PRESSURE score successfully underwent internal and external validation, showed a high diagnostic accuracy and robustness in prediction of both DC events: PrimDC and SecDC. The presented score might become a useful tool for early selection of individuals who are highly likely to require DC during SAH.
DC after SAH: the current evidence
There are several retrospective institutional series reporting on indications to and outcome after DC in SAH patients (Fisher and Ojemann, 1994; Smith et al., 2002; D'Ambrosio et al., 2005; Buschmann et al., 2007; Park et al., 2007; Schirmer et al., 2007; Kang, 2008; Otani et al., 2008; Guresir et al., 2009a, b; Nagel et al., 2009; Dorfer et al., 2010; Otani et al., 2011; Stuart et al., 2011; Fung et al., 2012; Tuzgen et al., 2012; Hwang et al., 2014; Mori, 2014; Uozumi et al., 2014; Jussen et al., 2015; Jabbarli et al., 2017). Due to negative selection bias, individuals necessitating DC after SAH are characterized with higher morbidity and mortality rates, than SAH patients without DC. In fact, DC remains mostly an ultima ratio against intractable intracranial hypertension, which is basically the consequence of severe early brain injury and secondary ischaemic complications after SAH. At the same time, these causal processes evoking DC are also acknowledged as strong outcome predictors of SAH (Jabbarli et al., 2015a, b). As noted in a recent meta-analysis on DC in SAH (Alotaibi et al., 2017), due to the lack of robust control groups, the effect of DC on functional outcomes versus that of other interventions for refractory intracranial hypertension is still unknown. As to the proper DC management in SAH patients, several studies highlighted the essential role of early DC for favourable outcome (Smith et al., 2002; Schirmer et al., 2007; Otani et al., 2008; Jussen et al., 2015; Jabbarli et al., 2017). This conclusion is in line with a general consensus from stroke and traumatic brain injury trials, which pointed to the utmost importance of DC timing (Dasenbrock et al., 2017; Shackelford et al., 2018; Shah et al., 2019).
Clinical value of DC after SAH: a scope for prophylactic use with the PRESSURE score?
So far, indication for and timing of DC after SAH are based on individual decisions of treating neurosurgeons. In this study, we developed and validated the novel risk score for prediction of necessity for DC after aneurysm rupture. Based on the risk factors assessable at admission, the PRESSURE score facilitates reliable and early selection of DC candidates after SAH. Since the individuals with SecDC showed the lowest chances for favourable outcome, early decompression of these patients at admission might improve the treatment results.
And even more, early DC might be a reasonable option not only for SAH individuals requiring SecDC. In a subgroup analysis of SAH patients who were at ‘high risk for DC’ (≥6 points on the PRESSURE score), a positive effect of early DC on the functional outcome after SAH was shown. In particular, the significant difference in favour of early PrimDC (as against later DC or no DC at all) was observed only after the restriction of the analysis to the cases without brainstem herniation and without prolonged utilization of conservative intracranial pressure treatment (i.e. for DCs performed within 24 h after SAH). This finding is of eminent importance, since the clinical utility of DC is currently limited to the cases with medically refractory intracranial hypertension and/or clinical deterioration (Nirula et al., 2014). Because of rather disappointing functional outcome of SAH individuals undergoing DC, many authors expressed criticism regarding the clinical value of DC after SAH (D'Ambrosio et al., 2005; Buschmann et al., 2007; Kang, 2008; Dorfer et al., 2010). As noted above, this is due to negative selection bias of SAH individuals undergoing DC. Therefore, a PRESSURE score-based rethinking of indications to DC after SAH towards a more preventive (‘prophylactic’) use might bring new insight to the clinical value of DC after SAH. At the same time, certain risks of DC-related complications (like rebleeding or infection) as well as the need for reoperation for cranioplasty should be accounted when considering the initiation of DC. Nevertheless, our findings might provide a basis for a prospective randomized clinical trial evaluating the value of a prophylactic early DC in SAH individuals.
Study limitations
The major limitation of the study is related to the retrospective nature of the presented analysis. The absence of strictly defined study protocols for DC after SAH in both institutions might limit the generalizability of the study results. Particularly, indications to PrimDC strongly depended on individual decisions of neurosurgeons on duty. Overall, the constructed score, at least partially, incorporates the selection bias accompanying DC indications in everyday practice, since there are no guidelines for DC after SAH based on certain measurable criteria. This circumstance may especially refer to the association between the treatment modality (clipping) and DC. On the other hand, objective intraoperative findings (like the presence of brain swelling or intraoperative complications) might also contribute to the decision to perform DC. In summary, the selection of the score components was based on strict statistical evaluation, and the parameters included to the PRESSURE are in line with common predictors of DC reported previously for SAH patients (Fisher and Ojemann, 1994; Smith et al., 2002; D'Ambrosio et al., 2005; Buschmann et al., 2007; Park et al., 2007; Schirmer et al., 2007; Kang, 2008; Otani et al., 2008; Guresir et al., 2009a, b; Nagel et al., 2009; Dorfer et al., 2010; Otani et al., 2011; Stuart et al., 2011; Fung et al., 2012; Tuzgen et al., 2012; Hwang et al., 2014; Mori, 2014; Uozumi et al., 2014; Jussen et al., 2015; Jabbarli et al., 2017).
Although the inclusion of eight parameters makes the score somewhat complex, all components of the PRESSURE score are easily assessable. The included Hunt and Hess scale was reported to be inferior to the World Federation of Neurological Surgeons (WFNS) scale (Teasdale et al., 1988) with regards to outcome prediction. However, the Hunt and Hess scale is still widely accepted and a recent publication did not show any advantage of the WFNS over the Hunt and Hess scale (Dengler et al., 2018). Finally, due to a limited number of levels, the modified Rankin Scale used for outcome assessment may be less responsive to changes in neurological and functional condition of the patients than some other stroke scales (Broderick et al., 2017).
Conclusion
Timing of DC seems to be of paramount importance for functional outcome of SAH patients requiring decompressive surgery. The presented score has been successfully validated as a reliable predictor of DC in two separate SAH cohorts and allows early selection of patients at admission. The PRESSURE score might present a background for prospective randomized clinical trial addressing the utility of the early prophylactic DC for SAH patients.
Supplementary material
Supplementary material is available at Brain Communications online.
Funding
We acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.
Competing interests
The authors report no competing interests.
Supplementary Material
Glossary
- aOR =
adjusted odds ratio
- AUC =
area under the curve
- CT =
computed tomography
- DC =
decompressive craniectomy
- DSA =
digital subtraction angiography
- EVD =
external ventricular drainage
- ICH =
intracerebral haematoma
- IVH =
intraventricular haemorrhage
- PrimDC =
primary decompressive craniectomy
- SAH =
(aneurysmal) subarachnoid haemorrhage
- SD =
standard deviation
- SecDC =
secondary decompressive craniectomy
Contributor Information
Ramazan Jabbarli, Department of Neurosurgery, University Hospital of Essen, D-45147 Essen, Germany; Department of Neurosurgery, Medical Center, University of Freiburg, D-79106 Freiburg, Germany.
Marvin Darkwah Oppong, Department of Neurosurgery, University Hospital of Essen, D-45147 Essen, Germany.
Roland Roelz, Department of Neurosurgery, Medical Center, University of Freiburg, D-79106 Freiburg, Germany.
Daniela Pierscianek, Department of Neurosurgery, University Hospital of Essen, D-45147 Essen, Germany.
Mukesch Shah, Department of Neurosurgery, Medical Center, University of Freiburg, D-79106 Freiburg, Germany.
Philipp Dammann, Department of Neurosurgery, University Hospital of Essen, D-45147 Essen, Germany.
Christian Scheiwe, Department of Neurosurgery, Medical Center, University of Freiburg, D-79106 Freiburg, Germany.
Klaus Kaier, Institute of Medical Biometry and Statistics, Faculty of Medicine and Medical Center, University of Freiburg Institute for Medical Biometry and Medical Informatics, University Medical Center Freiburg, D-79106 Freiburg, Germany.
Karsten H Wrede, Department of Neurosurgery, University Hospital of Essen, D-45147 Essen, Germany.
Jürgen Beck, Department of Neurosurgery, Medical Center, University of Freiburg, D-79106 Freiburg, Germany.
Ulrich Sure, Department of Neurosurgery, University Hospital of Essen, D-45147 Essen, Germany.
References
- Alotaibi NM, Elkarim GA, Samuel N, Ayling OGS, Guha D, Fallah A, et al. Effects of decompressive craniectomy on functional outcomes and death in poor-grade aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Neurosurg 2017; 127: 1315–25. [DOI] [PubMed] [Google Scholar]
- Berge E. Decompressive surgery for cerebral oedema after stroke: evidence at last. Lancet Neurol 2007; 6: 200–1. [DOI] [PubMed] [Google Scholar]
- Broderick JP, Adeoye O, Elm J. Evolution of the modified Rankin scale and its use in future stroke trials. Stroke 2017; 48: 2007–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann U, Yonekawa Y, Fortunati M, Cesnulis E, Keller E. Decompressive hemicraniectomy in patients with subarachnoid hemorrhage and intractable intracranial hypertension. Acta Neurochir (Wien) 2007; 149: 59–65. [DOI] [PubMed] [Google Scholar]
- Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery 2017; 80: 6–15. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio AL, Sughrue ME, Yorgason JG, Mocco JD, Kreiter KT, Mayer SA, et al. Decompressive hemicraniectomy for poor-grade aneurysmal subarachnoid hemorrhage patients with associated intracerebral hemorrhage: clinical outcome and quality of life assessment. Neurosurgery 2005; 56: 12–9; dicussion 19–20. [DOI] [PubMed] [Google Scholar]
- Dasenbrock HH, Robertson FC, Vaitkevicius H, Aziz-Sultan MA, Guttieres D, Dunn IF, et al. Timing of decompressive hemicraniectomy for stroke: a nationwide inpatient sample analysis. Stroke 2017; 48: 704–11. [DOI] [PubMed] [Google Scholar]
- Dengler NF, Sommerfeld J, Diesing D, Vajkoczy P, Wolf S. Prediction of cerebral infarction and patient outcome in aneurysmal subarachnoid hemorrhage: comparison of new and established radiographic, clinical and combined scores. Eur J Neurol 2018; 25: 111–9. [DOI] [PubMed] [Google Scholar]
- Dorfer C, Frick A, Knosp E, Gruber A. Decompressive hemicraniectomy after aneurysmal subarachnoid hemorrhage. World Neurosurg 2010; 74: 465–71. [DOI] [PubMed] [Google Scholar]
- Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 1980; 6: 1–9. [DOI] [PubMed] [Google Scholar]
- Fisher CM, Ojemann RG. Bilateral decompressive craniectomy for worsening coma in acute subarachnoid hemorrhage. Observations in support of the procedure. Surg Neurol 1994; 41: 65–74. [DOI] [PubMed] [Google Scholar]
- Fung C, Inglin F, Murek M, Balmer M, Abu-Isa J, Z'Graggen WJ, et al. Reconsidering the logic of World Federation of Neurosurgical Societies grading in patients with severe subarachnoid hemorrhage. J Neurosurg 2016; 124: 299–304. [DOI] [PubMed] [Google Scholar]
- Fung C, Murek M, Z’Graggen WJ, Krähenbühl AK, Gautschi OP, Schucht P, et al. Decompressive hemicraniectomy in patients with supratentorial intracerebral hemorrhage. Stroke 2012; 43: 3207–11. [DOI] [PubMed] [Google Scholar]
- Graeb DA, Robertson WD, Lapointe JS, Nugent RA, Harrison PB. Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology 1982; 143: 91–6. [DOI] [PubMed] [Google Scholar]
- Guresir E, Raabe A, Setzer M, Vatter H, Gerlach R, Seifert V, et al. Decompressive hemicraniectomy in subarachnoid haemorrhage: the influence of infarction, haemorrhage and brain swelling. J Neurol Neurosurg Psychiatry 2009. a; 80: 799–801. [DOI] [PubMed] [Google Scholar]
- Guresir E, Schuss P, Vatter H, Raabe A, Seifert V, Beck J. Decompressive craniectomy in subarachnoid hemorrhage. Neurosurg Focus 2009. b; 26: E4. [DOI] [PubMed] [Google Scholar]
- Hadjiathanasiou A, Schuss P, Ilic I, Borger V, Vatter H, Guresir E. Decompressive craniectomy for intracerebral haematoma: the influence of additional haematoma evacuation. Neurosurg Rev 2018; 41: 649–54. [DOI] [PubMed] [Google Scholar]
- Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968; 28: 14–20. [DOI] [PubMed] [Google Scholar]
- Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med 2016; 375: 1119–30. [DOI] [PubMed] [Google Scholar]
- Hwang US, Shin HS, Lee SH, Koh JS. Decompressive surgery in patients with poor-grade aneurysmal subarachnoid hemorrhage: clipping with simultaneous decompression versus coil embolization followed by decompression. J Cerebrovasc Endovasc Neurosurg 2014; 16: 254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbarli R, Oppong MD, Dammann P, Wrede KH, El Hindy N, Özkan N, et al. Time is brain! Analysis of 245 cases with decompressive craniectomy due to subarachnoid hemorrhage. World Neurosurg 2017; 98: 689–94.e2. [DOI] [PubMed] [Google Scholar]
- Jabbarli R, Reinhard M, Niesen WD, Roelz R, Shah M, Kaier K, et al. Predictors and impact of early cerebral infarction after aneurysmal subarachnoid hemorrhage. Eur J Neurol 2015. a; 22: 941–7. [DOI] [PubMed] [Google Scholar]
- Jabbarli R, Reinhard M, Roelz R, Shah M, Niesen WD, Kaier K, et al. Early identification of individuals at high risk for cerebral infarction after aneurysmal subarachnoid hemorrhage: the BEHAVIOR score. J Cereb Blood Flow Metab 2015. b; 35: 1587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jussen D, Marticorena S, Sandow N, Vajkoczy P, Horn P. Ultra-early decompressive hemicraniectomy in aneurysmal intracerebral hemorrhage: a retrospective observational study. Minerva Anestesiol 2015; 81: 398–404. [PubMed] [Google Scholar]
- Kang SD. Emergent clipping without prophylactic decompressive craniectomy in patients with a large aneurysmal intracerebral hematoma. J Korean Neurosurg Soc 2008; 44: 353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolias AG, Viaroli E, Rubiano AM, Adams H, Khan T, Gupta D, et al. The current status of decompressive craniectomy in traumatic brain injury. Curr Trauma Rep 2018; 4: 326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996; 27: 1304–5. [DOI] [PubMed] [Google Scholar]
- Mori N. Decompressive craniectomy for poor-grade aneurysmal subarachnoid hemorrhage. Austin J Neurosurg 2014; 1: 1006. [Google Scholar]
- Nagel A, Graetz D, Vajkoczy P, Sarrafzadeh AS. Decompressive craniectomy in aneurysmal subarachnoid hemorrhage: relation to cerebral perfusion pressure and metabolism. Neurocrit Care 2009; 11: 384–94. [DOI] [PubMed] [Google Scholar]
- Nirula R, Millar D, Greene T, McFadden M, Shah L, Scalea TM, et al. Decompressive craniectomy or medical management for refractory intracranial hypertension: an AAST-MIT propensity score analysis. J Trauma Acute Care Surg 2014; 76: 944–52; discussion 952–5. [DOI] [PubMed] [Google Scholar]
- Otani N, Takasato Y, Masaoka H, Hayakawa T, Yoshino Y, Yatsushige H, et al. Surgical outcome following decompressive craniectomy for poor-grade aneurysmal subarachnoid hemorrhage in patients with associated massive intracerebral or Sylvian hematomas. Cerebrovasc Dis 2008; 26: 612–7. [DOI] [PubMed] [Google Scholar]
- Otani N, Takasato Y, Masaoka H, Hayakawa T, Yoshino Y, Yatsushige H, et al. Clinical characteristics and surgical outcomes of patients with aneurysmal subarachnoid hemorrhage and acute subdural hematoma undergoing decompressive craniectomy. World Neurosurg 2011; 75: 73–7. [DOI] [PubMed] [Google Scholar]
- Park CK, Min KS, Lee MS, Kim YG, Kim DH. Use of prophylactic decompressive craniectomy in middle cerebral artery aneurysmal subarachnoid hemorrhage patients presenting with associated large hematoma. Korean J Cerebrovasc Surg 2007; 9: 94–100. [Google Scholar]
- Peng G, Huang C, Chen W, Xu C, Liu M, Xu H, et al. Risk factors for decompressive craniectomy after endovascular treatment in acute ischemic stroke. Neurosurg Rev 2019. doi: 10.1007/s10143-019-01167-4. [DOI] [PubMed] [Google Scholar]
- Schirmer CM, Hoit DA, Malek AM. Decompressive hemicraniectomy for the treatment of intractable intracranial hypertension after aneurysmal subarachnoid hemorrhage. Stroke 2007; 38: 987–92. [DOI] [PubMed] [Google Scholar]
- Shackelford SA, Del Junco DJ, Reade MC, Bell R, Becker T, Gurney J, et al. Association of time to craniectomy with survival in patients with severe combat-related brain injury. Neurosurg Focus 2018; 45: E2. [DOI] [PubMed] [Google Scholar]
- Shah A, Almenawer S, Hawryluk G. Timing of decompressive craniectomy for ischemic stroke and traumatic brain injury: a review. Front Neurol 2019; 10: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EC, Carter BS, Ogilvy CS. Proposed use of prophylactic decompressive craniectomy in poor-grade aneurysmal subarachnoid hemorrhage patients presenting with associated large Sylvian hematomas. Neurosurgery 2002; 51(1): 117–24; discussion 124. doi: 10.1097/00006123-200207000-00018. [DOI] [PubMed] [Google Scholar]
- Stuart RM, Claassen J, Schmidt M, Helbok R, Kurtz P, Fernandez L, et al. Multimodality neuromonitoring and decompressive hemicraniectomy after subarachnoid hemorrhage. Neurocrit Care 2011; 15: 146–50. [DOI] [PubMed] [Google Scholar]
- Teasdale GM, Drake CG, Hunt W, Kassell N, Sano K, Pertuiset B, et al. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry 1988; 51: 1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuzgen S, Kucukyuruk B, Aydin S, Ozlen F, Kizilkilic O, Abuzayed B. Decompressive craniectomy in patients with cerebral infarction due to malignant vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosci Rural Pract 2012; 3: 251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozumi Y, Sakowitz O, Orakcioglu B, Santos E, Kentar M, Haux D, et al. Decompressive craniectomy in patients with aneurysmal subarachnoid hemorrhage: a single-center matched-pair analysis. Cerebrovasc Dis 2014; 37: 109–15. [DOI] [PubMed] [Google Scholar]
- Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard JP, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial). Stroke 2007; 38: 2506–17. [DOI] [PubMed] [Google Scholar]
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.