Abstract
Human histone H1.5, in mice called H1b, belongs to the family of linker histones (H1), which are key players in chromatin organization. These proteins sit on top of nucleosomes, in part to stabilize them, and recruit core histone modifying enzymes. Through subtype-specific deposition patterns and numerous post-translational modifications, they fine-tune gene expression and chromatin architecture, and help to control cell fate and homeostasis. However, even though it is increasingly implicated in mammalian development, H1.5 has not received as much research attention as its relatives. Recent studies have focused on its prognostic value in cancer patients and its contribution to tumorigenesis through specific molecular mechanisms. However, many functions of H1.5 are still poorly understood. In this review, we will summarize what is currently known about H1.5 and its function in cell differentiation and carcinogenesis. We will suggest key experiments that are required to understand the molecular network, in which H1.5 is embedded. These experiments will advance our understanding of the epigenetic reprogramming occurring in developmental and carcinogenic processes.
Keywords: histones, histone isoforms, H1.5, development, cancer
Introduction
Linker Histone Basics
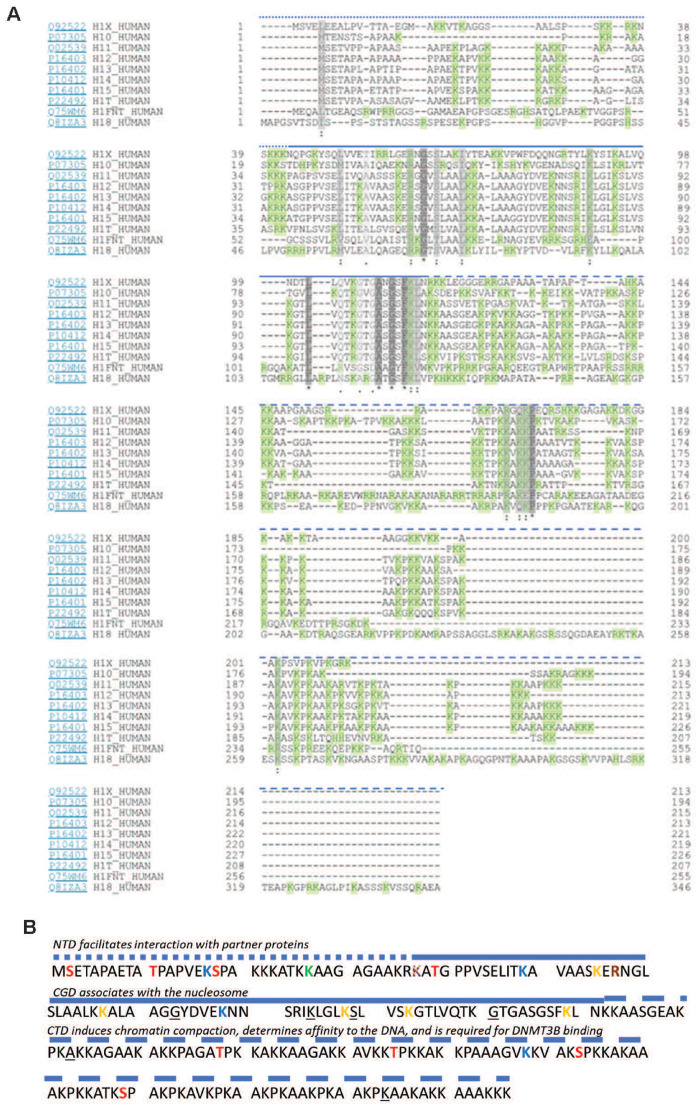
The protein of interest is called H1.5 in humans, and denoted H1b in mice. Hence, we will use both terms dependent on the experimental system in question. H1.5 is one of eleven mammalian linker histones (Table 1 and Fig. 1), of which five—H1.1–5—are ubiquitously expressed. It is encoded on the major histone cluster 1 (HIST1). This gene locus for H1.5, H1–5, is located on chromosome 6 in humans (chromosome 13 in mice) and encodes most of the linker and core histone genes [1]. There is astonishingly little known about the modifications of the HIST1 locus and the (epigenetic) control of histone expression, and no information on the regulation of H1.5 in specific. However, it is known that H1.5 is present as a single gene copy in the human genome. The somatic linker histone transcripts including H1.5 mRNA lack introns and a poly A tail—instead they are capped by an RNA hairpin/stem–loop structure [2, 3].
Table 1:
Nomenclature of the eleven mammalian H1 subtypes
| Class | Human | Mouse | Expression—cell type | Expression—timing |
|---|---|---|---|---|
| Mitotic linker histone | H1X | H1X | Somatic cells [6] | Replication-independent [1] |
| Replacement linker histone | H1.0 | H1f0/H10 | Terminally differentiated cells [1] | Replication-independent [1] |
| Somatic linker histones | H1.1 | H1a | Somatic cells [1] | Replication-dependent, during S-phase [1, 2] |
| H1.2 | H1c | |||
| H1.3 | H1d | |||
| H1.4 | H1e | |||
| H1.5 | H1b | |||
| Germ cell linker histones | H1t/H1.6 | H1t | Spermatids/spermatocytes (testes-specific) [1, 46] | |
| H1T2/H1.7 | H1t2 | |||
| TISP64/H1.9/HILS1 | TISP64 | |||
| H1oo/H1.8 | H1oo | Oocytes [1] |
Figure 1:
Comparison of human histone 1 isoforms with a flashlight on H1.5. (A) Alignment of the 11 linker histone subtypes. Grey—similarity (the darker, the greater the extend of similarity between subtypes). Green—positively charged amino acid residues. Heavily dashed blue line, N-terminus; straight blue line, globular domain; lightly dashed blue line, C-terminus [based on Uniprot and NCBI protein-tools as well as (1)]. (B) H1.5 protein sequence. Phosphorylated residues (marked red): T10, S17, T39, T138, T155, S173, S189. Methylated residues (marked green): K27. Acetylated residues (marked blue): K17, K49, K78, 168. β-Hydroxybutyrylated residues (marked yellow): K37, K55, K67, K88, K93, K109. Succinylated residues (marked purple): K37. Citrullinated residues (marked orange): R57. Sites mutated in lymphoma patients are underlined: 73G, 84K, 89S, 101G, 123A, 214K. NTD, N-terminal domain; CGD, central globular domain; CTD, C-terminal domain. N-terminus, straight blue line; globular domain, lightly dashed blue line; C-terminus [based on Uniprot and NCBI protein-tools as well as (1, 29)]
As the name suggests, linker histones are involved in DNA-packaging and binds to the linker-DNA in between nucleosomes [4]. This interaction is believed to be sequence-independent and based on electrostatic bonds between the negatively charged DNA phosphate backbone and the basic C- and N-terminal tails of H1 [1]. H1 acts as ‘liquid-like glue’ to promote the formation of tetra-nucleosomes [5] and higher order chromatin structures [1]. Consistently, H1 is generally depleted from the transcription start sites of active genes to allow access of the transcriptional machinery, and therefore has historically been viewed as an epigenetic repressor [1]. Today we know that the effect of H1-binding on chromatin organization and gene regulation is subtype-specific and depends on post-translational modifications (PTMs) [1]. PTMs alter the charge of specific amino acid residues, which influence the H1-variants’ residency time on the nucleosome, its interaction with other proteins (DNA methylases, histone modifying enzymes, transcription factors) and therefore the effect it has on chromatin structure and gene expression [6]. Notably, the expression, binding pattern along the chromatin, and PTMs of H1-variants change throughout differentiation [6].
The Relationship between Differentiation and Cancer
Cell differentiation involves a cell-type specific reorganization of chromatin structure, which allows for temporally and spatially distinct gene expression patterns [7]. The higher order chromatin structures establish a genomic/cellular status quo, which constitutes a barrier to reprogramming events [8]. Aberrant levels, modifications and DNA-binding patterns of H1 promote abnormal chromatin architecture [8]. This results in altered levels of gene expression, and ultimately an anomalous phenotype—different from the proper terminally differentiated phenotype of the cell [8]. The new variety of cells may favour the selection of the most proliferative and invasive phenotype, which promotes tumour growth and metastasis [7]. Reprogramming events in carcinogenesis often involve de-differentiation of cells to a more pluripotency-like state, as these cells are highly proliferative [7]. Therefore, understanding the role H1-variants play in differentiation can help us find specific mechanisms, by which they are involved in carcinogenesis.
Linker Histones as Guardians of the Genome
Besides cellular development, the linker histones play a role in the preservation of genome integrity. First, H1-induced chromatin compaction protects against DNA damage and silences transposable elements in Drosophila [1]. Second, linker histones help to orchestrate the DNA damage repair [1]. Thus, H1-variants may prevent somatic mutations that could otherwise give rise to tumour cells. However, the subtype-specific mechanisms, by which linker histones contribute to differentiation and tumorigenesis in vivo remain unclear/equivocal. This may be due the fact that many studies are conducted in model organisms like yeast and Drosophila that have a single H1 protein, whereas mammals express a total of 11 H1-variants [1]. Each of these variants is suspected to have its own entourage of writers, erasers, and readers/interaction partners [6]. The emerging complex interaction systems of the individual linker histones are not well understood. H1.5 is of particular interest because of its role in cell differentiation, a growing number of specific interaction partners, unique effects on nucleosome spacing and mRNA splicing, as well as its implication in various cancer types. In the following we will emphasize that H1.5 is an active player in the cellular reprogramming events taking place in differentiation and cancer. We will explore a variety of research endeavours with the aim to connect their findings and to point to the limitations of the presented data.
Linker Histone Knockout Studies in Mice show That Less Isn’t Always Less
The main function of linker histones is chromatin organization, and this is believed to be conserved among the different subtypes [9]. The consensus arose from knockout (KO) studies in various organisms [9]. Important KO studies were conducted in the Skoultchi lab. Depletion of the replacement linker histone H1.0, which accounts for up to 80% of the linker histone protein content in terminally differentiated cells [10], did not cause an abnormal phenotype in mice [11]. Instead, it was found that the H1.0 KO induced an up-regulation of the replication-dependent H1c, H1d, and H1e (human H1.2, H1.3, and H1.4), which suggests that mammalian H1-subtypes share a degree of functional redundancy [11]. This conclusion was supported by further experiments, in which double KO mice for H1.0 and one of the replication-associated variants, that were up-regulated in response to H1.0 depletion, did not exhibit any signs of aberrant development either [11]. A subsequent study with mice lacking a single H1 variant—H1.0, H1a, H1c, H1d, or H1e—that additionally carried a human transgene, revealed that the different homo- and heterozygous KO mice had varying abilities to silence the transgene [12]. Therefore, Alami et al. [12] suggested that each H1-subtype has slightly different effects on higher order chromatin architecture. Furthermore, they were first to hypothesize that linker histones are not merely inducers of chromatin compaction and gene repression but can fine-tune gene expression as well [12].
This notion was supported by further experiments, in which the three replication-dependent variants that were up-regulated in response to H1.0 KO, were knocked out simultaneously to create triple KO mice [13]. These mice lacking H1c, H1d, H1e (human H1.2, H1.3, and H1.4) contained only ∼50% of the wildtype H1 protein content [13]. The triple KO mice died at E11.5 (mid-gestation), at which they exhibited various developmental defects [13]. Molecular analysis of the embryonic stem cells from triple KO mice found a global decrease in nucleosome repeat length (NRL) and oligonucleosome compaction as well as aberrant levels of core histone modifications. Astonishingly, these were not associated with global dysregulation of gene expression [13]. Thus, linker histones are likely to have specific and necessary effects on gene expression. Indeed in the triple KO mice, only 29 genes showed abnormal mRNA levels, of which 4 (14%) belonged to the group of imprinted genes, whose expression is controlled by DNA methylation of their imprinting control regions [13]. Bisulphite sequencing of the imprinting control regions proved that the DNA methylation of these genes was significantly lower and could account for their altered expression [13]. However, non-imprinted genes, known to be regulated by promoter methylation, did not show aberrant levels of DNA methylation, which originally implicated linker histones in the activity/recruitment of DNA methyltransferases (DNMTs) at specific loci [13]. It appears, that the H1-subtypes have similar modes of action—interaction with the nucleosome, recruitment of DNMTs and histone modifiers—but differ in subtle ways that allow them to have unique effects on chromatin architecture and gene expression. Hence, their functions are only partially redundant and single isoforms should be explored in more detail.
H1.5 on Its Own
H1.5 Chromatin Binding
FRAP-based assays using GFP-H1.5 fusion proteins showed that H1.5 is a high affinity H1-subtype that typically exhibits a residency time of several minutes [14]. A first ‘eccentricity’ of H1.5 with regards to DNA-binding was found, when human H1-variants were separately overexpressed in Xenopus (frog) oocytes. All of the somatic linker histones except H1.5 did increase NRL upon overexpression [15]. One must be cautious to transfer these in vitro findings gained in an engineered system to the in vivo situation [6]. However, the observation that the other somatic H1-subtypes increase NRL is in line with observations in the triple KO mouse embryonic stem cells (mESCs), where H1 deficiency was accompanied by a decrease in NRL [13]. Therefore, H1.5 might indeed lack the ability to alter nucleosome spacing. Other variant-specific effects, which H1.5 might have on higher order chromatin structures, have yet not been investigated.
H1.5 as Determinant of Alternative Splicing
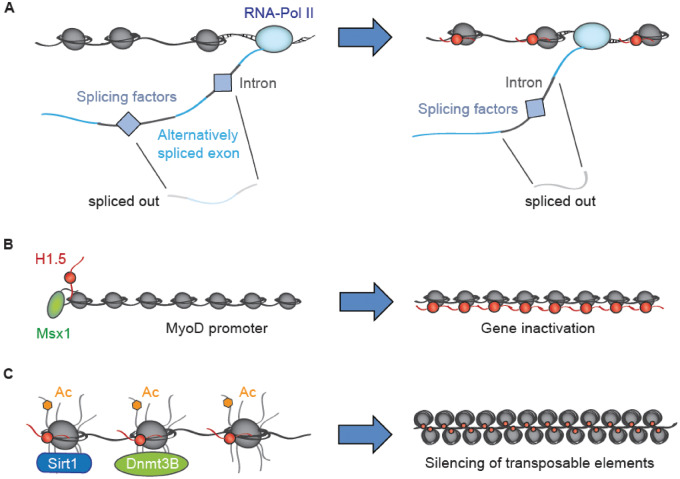
H1.5 is the only linker histone that has been implicated in the control of mRNA splicing [16] (Fig. 2A). In human lung fibroblasts, Glaich et al. recently discovered that H1.5 is enriched around splicing sites, especially on genes that are highly alternatively spliced. This enrichment correlated with the inclusion of the alternatively spliced exon into the mature mRNA [16]. The authors also observed that H1.5 occupancy causes RNA polymerase II pausing, perhaps by blocking the enzyme’s access to the DNA through stabilizing the nucleosome [16]. The slower pace of transcription decreases competition between the splice sites and thus increases the chance of the alternatively spliced exon to be recognized and incorporated [16]. Accordingly, H1.5 knockdown (KD) in the human lung fibroblasts significantly reduced the inclusion of alternatively spliced exons for all genes analysed without affecting their expression levels, i.e. the same number of transcription-events occurred, but the alternatively spliced exon was included less frequently [16]. Therefore, H1.5 does not just regulate gene expression through chromatin modulation and transcription factor binding, but also helps to establish appropriate levels of different splicing variants. The in vivo functions of splicing variants for most proteins are not understood, but it is well established that differentiation as well as cell transformation (epithelial to mesenchymal transition) require specific splicing networks [17]. Therefore, aberrant H1.5 enrichment or depletion might be associated with altered splicing isoforms. In conclusion, H1.5’s role in the calibration of gene expression/regulation of alternative splicing underscores its relevance for the establishment and maintenance of the cellular equilibrium.
Figure 2:
Overview over selected H1.5 functions. (A) H1.5 regulates splicing. H1.5 binding is enriched around exon borders. This is believed to slow down transcription, which decreases competition between splicing sites and hence promotes the incorporation of alternatively spliced exons into the mature mRNA. (B) Msx1 binds to the MyoD promoter and recruits H1.5, which results in chromatin condensation by an unknown mechanism and ultimately in suppression of MyoD expression. (C) Proposed mechanism of H1.5-mediated chromatin compaction. H1.5 has been shown to interact with SIRT1 and DNMT3B, which induce histone deacetylation and DNA methylation, respectively, ultimately leading to chromatin compaction. This results in the inactivation of specific genes and presumably helps silencing transposable elements
A Promiscuous Protein—H1.5’s Many Interaction Partners
H1.5 in Cell Differentiation
The triple KO mESCs were utilized for numerous subsequent studies investigating specific aspects of linker histone function, such as their roles in topology-associated domains [18] or in the repression of pluripotency genes [19]. This caused the other murine replication-dependent somatic linker histones—H1b and H1a (human H1.5 and H1.1, respectively)—to be studied to a lesser extent. Nevertheless, other data suggest a relevance of H1.5 in differentiation, even though more research is necessary to gain an overall understanding. In human embryonic stem cells (hESCs), pluripotency factors occupy the promoter of H1.5 (to a lower extent the H1.3 promoter and sporadically the H1.1 promoter), which suggests a pivotal role of these subtypes—especially H1.5—in differentiation and development [10]. H1.0 becomes the predominant H1 variant in differentiated cells, which is accompanied by a decrease in the expression of H1.1–5 [10]. Terme et al. [10], showed that de-differentiation of keratinocytes to induced pluripotent stem cells (iPSs) involves the up-regulation of H1-subtypes, most notably H1.3 and H1.5. Furthermore, the H1.5 DNA-binding pattern has been shown to depend on the developmental stage of the cell and changes with differentiation in a tissue-specific manner [20]. A study by Li et al. investigated hESCs, lung fibroblasts, neural cells, keratinocytes, and hepatocytes. The authors found that H1.5 enrichment occurred in ‘blocks’ within genic and intergenic regions, and that H1.5 exhibited a statistically significant preference for binding genes encoding membrane/membrane-related proteins [20]. Furthermore, H1.5 binding correlated with chromatin compaction and gene silencing [20]. The authors propose that the observed chromatin condensation is conveyed by PTMs on core histones due to H1.5 binding to the nucleosome. This claim was substantiated by chromatin immunoprecipitation experiments, which demonstrated that H1.5 binds the general protein deacetylase sirtuin 1 (SIRT1) and that H1.5 DNA occupancy is associated with H3K9 methylation [20]. H1.5 KD experiments in human fibroblasts resulted in dysregulation of 10% of genes as well as a disturbed H3K9me2 deposition pattern, which was reproduced in SIRT1 KD cells [20]. It is to note that SIRT1 KD did not result in an altered H1.5 deposition pattern, i.e. H1.5 influences SIRT1 binding to DNA but H1.5’s genomic distribution is independent of SIRT1 [20]. Li et al. [20] conclude that H1.5 recruits SIRT1, causing hypoacetylation of H3K9, which allows for the methylation of the latter. The large proportion of genes affected by H1.5 deficiency and the observation that H1.5 KD fibroblasts grow less efficiently [20] leads to the hypothesis that H1b KO mice (human H1.5) would carry severe developmental defects. However, an H1b knockout model is still missing. One of the reasons, why H1.5’s relevance in development may have been underestimated, is that its expression pattern during development remains controversial. Glaich et al. [16] conducted next-generation RNA-sequencing, which showed that H1.5 mRNA levels substantially increased over the course of differentiation from hESCs over primitive endoderm and lung precursors to lung fibroblasts. In contrast, western blot analysis by Li et al. [20] demonstrated that hESCs and lung fibroblasts contain similar amounts of H1.5 protein. The latter is in line with the real-time PCR results obtained by Terme et al. [10], who found that the mRNA expression remains constant during differentiation of hESCs but that the relative contribution of H1.5 to the linker histone content decreases. Similar experiments showed that in NT2-cells, total H1.5 expression decreases significantly as they differentiate into neural cells [10]. Please refer to Table 2 for an overview of altered H1.5 levels.
Table 2:
Summary of the H1.5 levels and their effects
| Cases of H1.5 down-regulation | Cases of H1.5 up-regulation |
|---|---|
|
|
| H1.5 KO/KD experiments | H1.5 overexpression experiments |
|
|
Further experiments would be needed to clarify whether H1.5 levels increase in lung differentiation (ensure that the western blot analysis was conducted with antibodies that are insensitive to PTMs, use physiological cell samples rather than a cell line). But the divergent levels of H1.5 in the differentiation process of two cell-types are further evidence for the notion that H1-variants have a cell and tissue-specific function. To prove this, it would be necessary to not only acquire conclusive data on H1.5’s mRNA and protein expression levels but also determine the regulatory mechanisms underlying the cell-type specific expression patterns. That would require a detailed molecular analysis of the chromatin alterations/epigenetic modifications occurring at the H1.5 promoter. The epigenetic changes and protein interactions occurring on the HIST1 locus in general, are usually omitted in studies investigating H1.5 expression [10, 16, 20, 21]. Therefore, the connections of H1.5 expression to cellular processes—differentiation and disease—remain unknown. Nevertheless, the mechanisms of gene regulation could provide insights into the distinct functions of the H1-variants and hold curative potential.
H1.5’s Intermezzo with Transcription Factors
Among H1.5’s interaction partners, transcription factors are important readers of PTMs and mediators of physiological effects. The first evidence that H1.5 is involved in transcriptional processes was found in murine satellite cells, where H1b forms a heterodimer with the transcriptional regulator Msh homeobox 1 (Msx1) that binds to the core enhancer of myoblast determination protein 1 (MyoD) and induces H3 hypoacetylation and H3K9 methylation, which promotes a repressive chromatin state (Fig. 2B) [22]. As MyoD is a key transcription factor indicative of myogenic commitment, this further underlines the role of H1b in differentiation. A similar mechanism of action was unveiled by Mackey-Cushman et al. [23], who found that in human T-regulatory cells the forkhead box transcription factor (FoxP3) physically interacts with H1.5 to influence its binding to DNA. FoxP3 enhances H1.5 binding to the interleukin-2 (IL-2) promoter, which results in histone deacetylation, decreased levels of H3K4me3 (activating mark) and overall lower expression levels of the inflammation marker IL-2 [23]. However, FoxP3 significantly depleted H1.5 from the promoter of cytotoxic T-lymphocyte-associated Protein 4 (CTLA4), accompanied by an increase in H3 acetylation [23]. The study used Jurkat T-cells that were transfected with viral vectors inducing overexpression of FoxP3 and H1.5 [23], presenting the potential that the H1.5 interaction with a single transcription factor can evoke both, gene activation and silencing. It is not known what determines whether the FoxP3-H1.5 interaction results in H1.5 eviction or enrichment. One possibility is that it is the result of differential local epigenetic landscapes. To dis-/prove this, it would be necessary to redo the experiments and investigate the epigenetic modifications on the IL2 and CTL4 promoters. The work by Lee et al. and Mackey-Cushman et al. highlights H1.5’s role in the formation of repressive chromatin. To date it is not known whether H1.5-induced chromatin compaction at the MyoD and IL-2 loci depends on SIRT1. It is possible that H1.5 recruits multiple different histone deacetylases depending on protein concentrations, cell type, or interaction with additional factors. These complex interaction systems and their unique roles in gene regulation, remain largely unexplored.
What Makes H1.5 So Attractive?
The exact mechanisms, by which H1.5 associates with its partners, remain unknown. Gene analysis of the H1-family shows that the globular domain is more strictly conserved between subtypes compared to the C- and N-terminal tails—the latter being the most divergent linker histone region (Fig. 1) [24]. This could suggest that the N-terminus domain conveys the subtype-specific functions of the linker histones, as has been shown for the binding of H1.5 to Msx1 [22]. H1.5’s association with FOXP3 and Peptidyl-prolyl cis–trans isomerase NIMA-interacting1 (PIN1) depends on specific protein domains of the H1.5 partner—the leucine zipper domain of FOXP3, the WW domain of PIN1. This suggests that these proteins bind H1.5 like their other substrates based on complementary protein structure and amino acid sequence [22, 23, 25]. The direct association opposes the notion that H1.5 requires chaperones to interact with the aforementioned protein partners. However, FOXP3’s ability to induce both, H1.5 binding to and dissociation from DNA, implies that there is a local factor involved in determining H1.5’s ability to bind DNA. As aforementioned, it is conceivable that this is due to differential epigenetic marks on the respective promoters. However, the epigenetic ground state of the latter were not analysed by Mackey-Cushman et al. Therefore, the effect of other previously established histone/DNA modifications on H1.5 binding remains elusive.
H1.5—How to Approach the Molecular Interaction Network
The lack of high-grade PTM- and subtype-specific antibodies prevents the use of chromatin immunoprecipitation or ‘Cut n’ Tag’ assays to determine H1.5’s DNA-binding pattern and its protein partners. Therefore, it might be easier to produce H1.5 KO cells (e.g. by CRISPR-Cas), compare their gene expression pattern and then investigate the loci of aberrantly expressed genes with the proteomics of chromatin assay, where modified nucleic acid derivatives complementary to a target sequence are used to pulldown DNA-binding-proteins for isolation [26]. This method is not currently suitable for genome-wide screening but would help to decipher H1.5 binding at a small scale and may aid in finding interaction suspects. These could be used as bait in subsequent chromatin immunoprecipitation assays, followed by the verification of H1.5 binding. This identification step would best be carried out by mass spectrometry, as this technique can indisputably distinguish between H1-variants and reliably detects the presence of PTMs [20]. Indeed, Harshman et al.’s [27] work suggests that until the immunoreagents have achieved a higher grade, mass spectrometry might be the superior method to distinguish between H1-variants and their numerous PTMs.
Alternatively, the DNA adenine methyltransferase identification technique could be used to establish the genome-wide H1.5 DNA-binding pattern. However, this approach requires the fusion of a 30 kDa enzyme to a 23 kDa linker histone, whose DNA affinity is already altered by a single phosphate/citrulline group [6].
Differentiation Gone Wrong—H1.5’s Role in Cancer
H1.5 Mutations Occur in Cancer
In simple terms, cancer can be described as abnormal cell growth. A plethora of genetic and epigenetic disturbances can induce a cell to divide more frequently and become invasive. Today we know that linker histones help to silence transgenes, protect against DNA damage and contribute to the DNA repair response. Therefore, they maintain genome stability, which decreases the mutation rate, and thus constitute a line in the defence against cancer. Linker histones also recruit core histone and DNA modifying enzymes, determine nucleosome spacing and play specific roles in gene expression. These processes are needed to maintain the cell’s/chromatin’s status quo, and their disruption enables cell transformation and tumour formation.
H1.5 was first implicated in cancer by Sjöblom et al. [28], who analysed samples from colorectal tumours for mutations. They found that the human H1.5 gene is more frequently mutated in colorectal cancer than would be expected based on the background mutation rate [28]. A similar trend was seen in follicular lymphomas. Twenty-seven per cent of tumours had mutations in linker histone subtypes H1.2–5, of which 20% were located in the H1.5 gene [29]. These mutations abolished the ability of the H1-variants to bind DNA-methyltransferase 3B (DNMT3B)—a de novo DNA-methyltransferase—substantiating the importance of linker histones for the establishment of epigenetic marks [29]. Interestingly, the mutations that interfered with DNMT3B binding were located in the more conserved C-terminal domain of the H1-variants, which raises the possibility that the somatic linker histones are redundant with respect to DNMT3B recruitment [29].
A crude comparison of cancer cell lines and ‘normal’ cell lines based on the NextBio data bank showed that H1.5 mRNA is significantly down-regulated in cancer cells [20]. However, the data’s variance and multitude of outliers support the notion that H1.5 expression diverges between cell types and hints at the possibility that reduction and overexpression of H1.5 could both contribute to cell transformation. Subsequent studies of H1 expression in cancer and cell growth identified H1.5 as a putative marker in prostate cancer [30].
H1.5 and Prostate Cancer—a Dysfunctional Relationship
Two independent immunohistological studies confirmed H1.5’s suitability as a marker for prostate cancer progression through the investigation of prostatic adenocarcinoma biopsy samples [31, 32]. Both found that H1.5 was generally absent from nuclei of benign prostatic glands, only 9% [31] and 11% [32] respectively expressed H1.5. At a first glance this fits with the observation by Li et al. [20] that H1.5 deficiency decreases cell growth, but there are no prospective studies published that identify H1.5 overexpression as a cause of increased cell proliferation/tumorigenesis in prostate tissue.
Furthermore, the studies on prostate cancer found that high-grade prostatic intraepithelial neoplasia, the predecessor of prostatic adenocarcinoma, only stained weakly for H1.5 [31, 32]. However, for prostatic adenocarcinomas an increase in nuclear reactivity to H1.5 correlated with a higher Gleason score [31, 32]. The Gleason score is used in the prognosis of prostate cancer. The higher its number, the more de-differentiated cancer cells are in the prostatic lesion and the faster the tumour grows and metastasizes, which is accompanied by higher patient mortality [33]. Therefore, antibody-based histological evaluation of nuclear reactivity to H1.5 is a putative tool in the prognosis of prostatic lesions, given the specificity of the antibodies is ensured [31, 32]. Also, as a higher degree of dedifferentiation is accompanied by an increase in H1.5 expression, it could be hypothesized that the overexpression of H1.5 contributes to the repression of prostatic genes. This encourages probing into the expression of such tissue-specific genes in patients’ biopsy samples to determine whether they correlate with H1.5 overexpression. Another possible mechanism, by which H1.5 might contribute to prostate cancer aggressiveness, is alternative splicing. One of the main drivers of prostate cancer is the androgen receptor, which has several splicing variants [34]. Some of them lack the ligand-binding domain or have a ‘disrupted’ ligand-binding domain, which causes them to be constitutively active and promote cell growth [34]. It is known that these isoforms tend to be overexpressed in prostate cancer [34] and can accelerate the transition to castration-resistant prostate cancer [35]. This raises the question whether aberrant H1.5 deposition around the alternatively spliced exons could contribute to the expression of cancer-related androgen receptor variants. The answer remains elusive until the androgen receptor gene is investigated with regards to H1.5 binding.
H1.5 and the Progression of Cancer in the Female Genital Tract
The same trend in nuclear reactivity for H1.5 in cancer progression was observed in a study of uterine smooth muscle cell tumours. The malignant leiomyosarcomas exhibited a 5.6 times higher nuclear reactivity for H1.5 staining compared to the benign leiomyoma samples [36]. The association of H1.5 overexpression with more progressed tumours in two different types of cancer indicates that H1.5-dependent mechanisms could promote cell transformation and an invasive phenotype. A similar study investigating ovarian granulosa cell tumours, rare neoplasms arising from sex cord and stromal cells, presents conflicting results [37]. Granulosa cell tumours were devoid of H1.5 staining, whereas healthy ovarian tissue showed high nuclear reactivity to anti-H1.5 antibodies [37]. Taken together, these immunohistological studies suggest that the degree of H1.5 expression is tissue-dependent and indicate that both loss and overexpression of H1.5 can contribute to cancer development. This is in line with the observation that H1.5 has specific interaction partners and distinct effects on gene expression, which are only active in particular cell types. However, to indisputable confirm this it would be necessary to not only conduct large-scale multi-centre studies [37], but also analyse the nuclei in the cancerous lesions for H1.5 mutagenesis as mutated epitopes could avoid antibody binding. The apparent involvement of H1.5 in prostate cancer and tumours of the female genital tract suggest that it might have sexually dimorphic functions. This raises the possibility that it interacts with sex hormones. However, to our knowledge there are no studies investigating the interplay of H1.5 with sex hormones. The only established connection between linker histones and hormones is a decrease in the phosphorylation of H1.3, H1.4, and H1.5 in response to prolonged glucocorticoid exposure [38]. Therefore, mechanisms, by which sex hormones influence H1.5 PTMs or its’ binding to chromatin seem plausible and should be investigated further.
H1.5’s Partners in Crime
The molecular H1.5 interaction network has only recently been investigated with regards to cancer. Glioblastomas commonly overexpress nucleophosmin (NPM1), which is a histone chaperone that binds H1.5 [39]. The investigation of several glioma cell lines found that siRNA-induced reduction in H1.5 protein content causes reactivation of apoptotic pathways that are deactivated in glioblastomas [39]. This was more pronounced when NPM1 was also knocked down, however NPM1 KD alone could not trigger apoptosis [39]. Interestingly, NPM1 overexpression could reverse the pro-apoptotic effect mediated by H1.5 KD [39]. However, so far no patient samples were investigated to compare H1.5 expression between the healthy and cancerous tissue [39]. Therefore, it cannot be concluded that H1.5 overexpression abates apoptosis. The authors suggest that H1.5 depletion induces apoptosis in glioma cells, which is regulated by NPM1 availability [37]. The mechanism by which H1.5 deficiency promotes cell death in gliomas remains to be elucidated, but it underscores the crucial and opposing roles H1.5 can play within different cell types. Furthermore, it commends us to specifically investigate the gene regulation of the individual linker histones, as a disturbance in their expression possibly contributes to abnormal cell growth.
The research linking H1.5 to cancer is promising, yet limited. Without knowing the exact H1.5 deposition pattern and the tissue-specific interacting proteins, as well as the binding mode and requirements, it will be impossible to pinpoint the exact mechanisms, by which H1.5 is involved in cancer. Furthermore, the studies employed antibodies against H1.5 in general, without testing whether PTMs could interfere with the binding. That the latter is necessary to experiments involving linker histones has been exemplified by Sang et al. [21], who investigated the role of site-specific phosphorylation of H1.5 in Ras-mediated glioma progression. As it is currently disputed, whether this paper is lacking scientific integrity, we present the results below but advise the reader to take them as a starting point for thought [40]. The glioblastoma cells expressing a cancerogenic Ras mutant (RasG12V/Y40C), had lower levels of H1.5 phosphorylation at threonine 10 (H1.5T10ph) compared to wildtype-cells [21]. Furthermore, the mutant cells, exhibited increased cell growth and migration, as well as altered expression levels of Ras target-genes, known to be involved in cancer [21]. The changes in gene expression, cell growth, and ability to migrate were reversed by the overexpression of an H1.5T10ph mimetic [21]. This demonstrates the influence of single site-specific PTMs of linker histones on gene expression/cellular phenotype/health. The contribution also established that H1.5T10 is phosphorylated by glycogen synthase kinase 3 (GSK3) [21]. GSK3 is a multifunctional protein kinase that has been known to phosphorylate H1.5 at threonine 10 during M-phase for the past decade [41]. The recent paper by Sang et al. was first to show that this modification occurs independently of the cell-cycle in glioma cells, and could therefore play a role in carcinogenesis.
GSK3 has been implicated in numerous disease conditions such as inflammation, Alzheimer’s, diabetes, and cancer [42]. Therefore, GSK3 could provide a link between these diseases and H1.5 phosphorylation/modification. Interestingly, GSK3 inhibition helps stem cells to maintain pluripotency. This could be in part due to its effect on H1-mediated chromatin remodelling [43]. However, the precise underlying mechanisms are still unclear.
Kinase inhibitors compromise an important field of anticancer medication, as they mitigate cell growth and survival, as well as therapy resistance [44]. Kinase inhibitor activity is commonly assessed by their ability to decrease total H1 phosphorylation [45], even though the latter has not been the deliberate aim of any cancer treatment. In the light of the results presented by Sang et al., the question arises whether meddling with linker histone phosphorylation could indirectly undermine the effectiveness of small molecule drugs that obstruct the activity of cyclin-dependent kinases and other protein kinases.
Conclusions
H1 proteins regulate chromatin marks and -architecture to preserve genome integrity, thereby establishing a barrier to cellular reprogramming [8]. Overexpression of H1 conveys protection against DNA double-strand breaks by minimizing the access of damaging factors to the DNA [6]. Ironically, H1 depletion transmits hyper-resistance to DNA damage, supposedly by granting access of the repair machinery to the DNA [6]. Thus, H1 levels need to be controlled in a localized manner to direct protection and maintenance of the genome. Aberrant H1 expression/distribution might therefore be a mechanism, by which all variants are implicated in carcinogenesis [8].
H1.5 specifically induces chromatin compaction, e.g. through the establishment of repressive histone tags, and DNA methylation, which depends on its interaction with SIRT1 and DNMT3B, respectively, as well as other potential interaction partners (Fig. 2C). However, we can expect future research to discover an array of additional interaction partners.
Due to its ability to establish chromatin condensation, H1.5 is suspected to play a role in the silencing of transposable elements, further promoting genome stability [6]. Furthermore, this raises the possibility that H1.5 is involved in carcinogenesis in a manner similar to promoter methylation. H1.5 binding could induce repressive chromatin around tumour suppressor genes, whereas aberrant H1.5 eviction from the loci of genes associated with pluripotency/cell growth could increase their expression. Definite proof of this hypothesis can only be established through the comparative analysis of the H1.5 deposition pattern on DNA between tumour and healthy cells.
H1.5 is frequently mutated in tumours, and it appears that these mutations induce a loss of function/decrease H1.5’s ability to recruit certain proteins [28]. This is in line with the H1.5 deficiency in granulosa cell tumours [37] and a general tendency for down-regulation in cancer cell lines [20]. Contrary, H1.5 depletion can help to reactivate apoptosis in glioblastoma cells [39], and has been found to be overexpressed in malignant carcinomas of the prostate and uterine smooth muscle, where the H1.5 overexpression correlates with tumour growth and metastasis [31, 32, 36]. H1.5’s role in cancer likely depends on the tissue and requires the interaction with cell-type specific transcription factors, H1.5 modifying enzymes and associated PTMs, the interplay with distinct oncogenic signalling pathways, and aberrant incorporation of alternatively spliced exons. Therefore, it will be necessary to determine the proteins that bind to H1.5 in each cell-type individually, as well as the PTMs that allow H1.5 to adapt certain tissue-specific functions. The interaction and modification profile of H1.5 is suspected to change during cell differentiation and carcinogenesis. The relevance of H1.5 expression patterns during developmental and tumorigenic processes remains debated and can only be established/clarified through the simultaneous investigation of H1.5 mRNA and protein levels, and the mechanisms by which the H1.5 gene promoter is modified to control transcription.
Most of the specifics of H1.5’s functions are currently unknown (Table 3). Exploring them further promises fascinating insights into the interplay between higher order chromatin structures and specific regulation of gene expression as H1.5 (and the other linker histones) are pertinent to both. These future studies will be brought forward by advancements in research tools, especially high-grade subtype and modification specific anti-H1 antibodies, and the development of new techniques.
Table 3:
Outstanding questions
| - By which mechanism does H1.5T10 phosphorylation counteract Ras-mediated carcinogenesis? |
| - How is H1.5 expression regulated? How is it regulated by sex-hormones? |
| - What are physiological-binding patterns of H1.5 in various tissues? How do they change during cell differentiation and transformation? |
| - What is the role of H1.5 in silencing transposable elements in mammals? |
| - What role does the local chromatin landscape play in H1.5 binding? |
| - Which chromatin modifiers can be recruited by H1.5? |
| - Do aberrant H1.5 levels affect alternative splicing events that are relevant for tumorigenesis? |
Acknowledgements
We thank Prof. A. Baniahmad (Institute for Human Genetics, University Clinic Jena, Germany) for his useful comments and C.A. Hübner (Institute for Human Genetics, University Clinic Jena, Germany) for financial support.
Funding
Advanced Medical Scientist Program from the Interdisciplinary Center for Clinical Research (IZKF) at the Jena University Hospital, Germany (973685 to O.E.).
Conflict of interest statement. None declared.
References
- 1. Hergeth SP, Schneider R. The H1 linker histones: multifunctional proteins beyond the nucleosomal core particle. EMBO Rep 2015;16:1439–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rattray AMJ, Müller B. The control of histone gene expression. Biochem Soc Trans 2012;40:880–5. [DOI] [PubMed] [Google Scholar]
- 3. Medrzycki M, Zhang Y, Cao K. et al. Expression analysis of mammalian linker-histone subtypes. J Vis Exp 2012;61:3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maze I, Noh K-M, Soshnev AA, Allis CD. Every amino acid matters: essential contributions of histone variants to mammalian development and disease. Nat Rev Genet 2014;15:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gibbs EB, Kriwacki RW. Linker histones as liquid-like glue for chromatin. Proc Natl Acad Sci USA 2018;115:11868–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fyodorov DV, Zhou B-R, Skoultchi AI, Bai Y. Emerging roles of linker histones in regulating chromatin structure and function. Nat Rev Mol Cell Biol 2018;19:192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sivakumar A, de Las Heras JI, Schirmer EC. Spatial genome organization: from development to disease. Front Cell Dev Biol 2019;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang T, Chuffart F, Bourova-Flin E, Wang J, Mi J, Rousseaux S, Khochbin S. Histone variants: critical determinants in tumour heterogeneity. Front Med 2019;13:289–97. [DOI] [PubMed] [Google Scholar]
- 9. Izzo A, Kamieniarz K, Schneider R. The histone H1 family: specific members, specific functions? Biol Chem 2008;389:333–43. [DOI] [PubMed] [Google Scholar]
- 10. Terme J-M, Sesé B, Millán-Ariño L, Mayor R, Belmonte JCI, Barrero MJ, Jordan A. Histone H1 variants are differentially expressed and incorporated into chromatin during differentiation and reprogramming to pluripotency. J Biol Chem 2011;286:35347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fan Y, Sirotkin A, Russell RG, Ayala J, Skoultchi AI. Individual somatic H1 subtypes are dispensable for mouse development even in mice lacking the H1(0) replacement subtype. Mol Cell Biol 2001;21:7933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alami R, Fan Y, Pack S, Sonbuchner TM, Besse A, Lin Q, Greally JM, Skoultchi AI, Bouhassira EE. Mammalian linker-histone subtypes differentially affect gene expression in vivo. Proc Natl Acad Sci 2003;100:5920–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fan Y, Nikitina T, Zhao J, Fleury TJ, Bhattacharyya R, Bouhassira EE, Stein A, Woodcock CL, Skoultchi AI. Histone H1 depletion in mammals alters global chromatin structure but causes specific changes in gene regulation. Cell 2005;123:1199–212. [DOI] [PubMed] [Google Scholar]
- 14. Flanagan TW, Files JK, Casano KR, George EM, Brown DT. Photobleaching studies reveal that a single amino acid polymorphism is responsible for the differential binding affinities of linker histone subtypes H1.1 and H1.5. Biol Open 2016;5:372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Öberg C, Izzo A, Schneider R, Wrange Ö, Belikov S. Linker histone subtypes differ in their effect on nucleosomal spacing in vivo. J Mol Biol 2012;419:183–97. [DOI] [PubMed] [Google Scholar]
- 16. Glaich O, Leader Y, Lev Maor G, Ast G. Histone H1.5 binds over splice sites in chromatin and regulates alternative splicing. Nucleic Acids Res 2019;47:6145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baralle FE, Giudice J. Alternative splicing as a regulator of development and tissue identity. Nat Rev Mol Cell Biol 2017;18:437–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geeven G, Zhu Y, Kim BJ, Bartholdy BA, Yang S-M, Macfarlan TS, Gifford WD, Pfaff SL, Verstegen MJAM, Pinto H, Vermunt MW, Creyghton MP, Wijchers PJ, Stamatoyannopoulos JA, Skoultchi AI, de Laat W. Local compartment changes and regulatory landscape alterations in histone H1-depleted cells. Genome Biol 2015;16:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Cooke M, Panjwani S, Cao K, Krauth B, Ho P-Y, Medrzycki M, Berhe DT, Pan C, McDevitt TC, Fan Y. Histone H1 depletion impairs embryonic stem cell differentiation. PLoS Genet 2012;8:e1002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li J-Y, Patterson M, Mikkola HKA, Lowry WE, Kurdistani SK. Dynamic distribution of linker histone H1.5 in cellular differentiation. PLoS Genet 2012;8:e1002879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sang B, Sun J, Yang D, Xu Z, Wei Y. Ras-AKT signaling represses the phosphorylation of histone H1.5 at threonine 10 via GSK3 to promote the progression of glioma. Artif Cells Nanomed Biotechnol 2019;47:2882–90. [DOI] [PubMed] [Google Scholar]
- 22. Lee H, Habas R, Abate-Shen C. Msx1 cooperates with histone H1b for inhibition of transcription and myogenesis. Science (80 )2004;304:1675–8. [DOI] [PubMed] [Google Scholar]
- 23. Mackey-Cushman SL, Gao J, Holmes DA, Nunoya J-I, Wang R, Unutmaz D, Su L. FoxP3 interacts with linker histone H1.5 to modulate gene expression and program Treg cell activity. Genes Immun 2011;12:559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ponte I, Romero D, Yero D, Suau P, Roque A. Complex evolutionary history of the mammalian histone H1.1-H1.5 gene family. Mol Biol Evol 2017;34:545–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raghuram N, Strickfaden H, McDonald D, Williams K, Fang H, Mizzen C, Hayes JJ, Th’ng J, Hendzel MJ. Pin1 promotes histone H1 dephosphorylation and stabilizes its binding to chromatin. J Cell Biol 2013;203:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Déjardin J, Kingston RE. Purification of proteins associated with specific genomic loci. Cell 2009;136:175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harshman SW, Chen MM, Branson OE, Jacob NK, Johnson AJ, Byrd JC, Freitas MA. Isolation and analysis of linker histones across cellular compartments. J Proteomics 2013;91:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sjöblom T, Jones S, Wood LD. et al. The consensus coding sequences of human breast and colorectal cancers. Science (80-) 2006;314:268–74. [DOI] [PubMed] [Google Scholar]
- 29. Li H, Kaminski MS, Li Y, Yildiz M, Ouillette P, Jones S, Fox H, Jacobi K, Saiya-Cork K, Bixby D, Lebovic D, Roulston D, Shedden K, Sabel M, Marentette L, Cimmino V, Chang AE, Malek SN. Mutations in linker histone genes HIST1H1 B, C, D, and E; OCT2 (POU2F2); IRF8; and ARID1A underlying the pathogenesis of follicular lymphoma. Blood 2014;123:1487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sato S, Takahashi S, Asamoto M, Nakanishi M, Wakita T, Ogura Y, Yatabe Y, Shirai T. Histone H1 expression in human prostate cancer tissues and cell lines. Pathol Int 2012;62:84–92. [DOI] [PubMed] [Google Scholar]
- 31. Khachaturov V, Xiao G-Q, Kinoshita Y, Unger PD, Burstein DE. Histone H1.5, a novel prostatic cancer marker: an immunohistochemical study. Hum Pathol 2014;45:2115–9. [DOI] [PubMed] [Google Scholar]
- 32. El-Rashidy MA, Bedeer AE, Kabel AM. Histone H1.5 expression in prostatic carcinoma: an immunohistochemical study. J Cancer Res Treat 2016;4:21–5. [Google Scholar]
- 33. Munjal A, Leslie SW. Gleason Score. Treasure Island (FL): StatPearls, 2020. [Google Scholar]
- 34. Haile S, Sadar MD. Androgen receptor and its splice variants in prostate cancer. Cell Mol Life Sci 2011;68:3971–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Testa U, Castelli G, Pelosi E. Cellular and molecular mechanisms underlying prostate cancer development: therapeutic implications. Medicines (Basel, Switzerland )2019;6:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Momeni M, Kalir T, Farag S, Kinoshita Y, Roman TY, Chuang L, Fishman DA, Burstein DE. Immunohistochemical detection of promyelocytic leukemia zinc finger and histone 1.5 in uterine leiomyosarcoma and leiomyoma. Reprod Sci 2014;21:1171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Momeni M, Kalir T, Farag S, Chuang L, Fishman D, Burstein DE. Expression of H1.5 and PLZF in granulosa cell tumors and normal ovarian tissues: a short report. Cell Oncol 2014;37:229–34. [DOI] [PubMed] [Google Scholar]
- 38. Banks GC, Deterding LJ, Tomer KB, Archer TK. Hormone-mediated dephosphorylation of specific histone H1 isoforms. J Biol Chem 2001;276:36467–73. [DOI] [PubMed] [Google Scholar]
- 39. Holmberg Olausson K, Elsir T, Moazemi Goudarzi K, Nistér M, Lindström MS. NPM1 histone chaperone is upregulated in glioblastoma to promote cell survival and maintain nucleolar shape. Sci Rep 2015;5:16495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Expression of Concern: Ras-AKT signaling represses the phosphorylation of histone H1.5 at threonine 10 via GSK3 to promote the progression of glioma. Artif Cells Nanomed Biotechnol 2020;48:713. [DOI] [PubMed] [Google Scholar]
- 41. Happel N, Stoldt S, Schmidt B, Doenecke D. M phase-specific phosphorylation of histone H1.5 at threonine 10 by GSK-3. J Mol Biol 2009;386:339–50. [DOI] [PubMed] [Google Scholar]
- 42. Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther 2015;148:114–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tee W-W, Reinberg D. Chromatin features and the epigenetic regulation of pluripotency states in ESCs. Development 2014;141:2376–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kanev GK, de Graaf C, de Esch IJP, Leurs R, Würdinger T, Westerman BA, Kooistra AJ. The landscape of atypical and eukaryotic protein kinases. Trends Pharmacol Sci 2019;40:818–32. [DOI] [PubMed] [Google Scholar]
- 45. Wang L, Harshman SW, Liu S, Ren C, Xu H, Sallans L, Grever M, Byrd JC, Marcucci G, Freitas MA. Assaying pharmacodynamic endpoints with targeted therapy: Flavopiridol and 17AAG induced dephosphorylation of histone H1.5 in acute myeloid leukemia. Proteomics 2010;10:4281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Talbert PB, Ahmad K, Almouzni G, Ausió J, Berger F, Bhalla PL, Bonner WM, Cande W, Chadwick BP, Chan SWL, Cross GAM, Cui L, Dimitrov SI, Doenecke D, Eirin-López JM, Gorovsky MA, Hake SB, Hamkalo BA, Holec S, Jacobsen SE, Kamieniarz K, Khochbin S, Ladurner AG, Landsman D, Latham JA, Loppin B, Malik HS, Marzluff WF, Pehrson JR, Postberg J, Schneider R, Singh MB, Smith M, Thompson E, Torres-Padilla M-E, Tremethick D, Turner BM, Waterborg J, Wollmann H, Yelagandula R, Zhu B, Henikoff S. A unified phylogeny-based nomenclature for histone variants. Epigenet Chromatin 2012;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]