Abstract
CO2 surgical lasers are widely used for procedures in veterinary and human medicine. There is evidence to suggest surgery using a CO2 laser reduces pain and swelling and improves healing time compared with surgery with a scalpel. Millions of piglets in North America are surgically castrated each year using a scalpel. Therefore, piglet welfare may be improved by making refinements to the surgical procedure. The objectives of this preliminary study were to determine the ability of a CO2 surgical laser to (1) reduce pain and (2) improve wound healing of piglets undergoing surgical castration. Two-day-old male Yorkshire × Landrace piglets were used and randomly assigned to 1 of 3 treatments (n = 10 piglets/treatment group): surgical castration with the CO2 laser, surgical castration with a scalpel, or sham (uncastrated control). Piglets were video recorded in their pens for 1 hr preprocedure and from 0 to 2, 6 to 8, and at 24 hr postprocedure for behavior scoring. Surgical site images were collected at baseline, 0, 8, 24, 48, 72, 96, 120, 144, and 168 hr postcastration for wound healing assessment. Infrared thermography images of the surgical site were also taken at baseline, 0, 0.5, 8, and 24 hr postprocedure to assess inflammation. Finally, blood was collected from each piglet at baseline and 0.5 hr postcastration to assess cortisol levels, prostaglandin E metabolite and pig-major acute phase protein concentration. Laser-castrated piglets displayed more pain behaviors across the observation period than scalpel-castrated piglets (P = 0.05). Laser-castrated piglets also displayed significantly more agonistic behavior than both scalpel-castrated piglets (P = 0.005) and sham piglets (P = 0.036); yet, laser-castrated piglets had significantly lower temperatures at the site of incision compared with scalpel-castrated piglets (P = 0.0211). There was no significant difference in wound healing or any of the blood parameters assessed between laser-castrated and scalpel-castrated piglets. There was evidence of thermal tissue damage on the scrotum of piglets that were castrated using the CO2 laser. This may have resulted in the unremarkable healing time and the increased pain behavior observed in this study. The surgical laser technique should be refined before conclusions can be made regarding the utility of a CO2 laser for piglet castration.
Keywords: animal welfare, castration, CO2 surgical laser, piglet, pain, refinement
Introduction
Male piglets in North America are routinely castrated on-farm, to prevent boar taint and minimize aggression (Sutherland, 2015). This painful procedure is done on conscious piglets, by using a scalpel to make an incision on the scrotum and removing the testicles by cutting or tearing the spermatic cord (Rault et al. 2011). Complications, such as hemorrhage, infection, excessive swelling, and intestinal herniation (resulting in preweaning mortality), may be partially attributable to the described surgical castration technique (Taylor and Weary, 2000; Morales et al., 2017). Refining the castration procedure by replacing the scalpel with a technique that decreases tissue damage and bleeding may reduce these postsurgical complications and lead to improved piglet welfare in commercial production systems.
CO2 surgical lasers are increasingly being used for procedures in veterinary and human medicine. They function by emitting a colorless, infrared light at a specific wavelength (10,600 nm) which is absorbed by intracellular water and causes tissue cells to ablate or vaporize (Mison et al., 2003). This allows for clean, precise incisions to be made on the skin and takes no more time than if a standard scalpel was used. Pain and swelling have been shown to be significantly reduced in human patients who have undergone the same surgical procedure using a CO2 laser compared with a scalpel (Tuncer et al., 2010; López-Jornet and Camacho-Alonso, 2013). The CO2 laser has also refined the canine castration procedure by nearly eliminating blood flow during the surgery and reducing the risk of scrotal hematoma, bruising, and infection compared with canine castration with a scalpel (Schultz, 2013).
The objectives of this pilot study were to determine the ability of a CO2 surgical laser to (1) reduce pain and (2) improve wound healing of piglets undergoing surgical castration. We hypothesized that surgical castration of piglets using the CO2 laser would result in decreased inflammation at the surgical site, reduced wound healing time, and less postsurgical pain compared with piglets castrated with a scalpel.
Materials and Methods
All animal use and procedures were approved by the Institutional Animal Care and Use Committee at Midwest Veterinary Services (MVS) prior to study commencement (Protocol # MCL-19065).
Animals
Three Yorkshire × Landrace sows nursing 1-d-old piglets were sourced from MVS Klitz Farm (Oakland, NE) for this study. Sows were examined by a veterinarian and selected based on 4 parameters: health, body condition, past weaning history, and nonaggressive behavior. For enrollment in this study, sows had to be in good physical health, free of any complicating disease, with a body condition score of 3 ± 0.5 out of 5. They also had to have previously weaned at least 1 litter of piglets (i.e., gilts were excluded). Healthy male piglets with 2 testicles, intact tails (i.e., no tail docking prior to enrollment), and no palpable hernias were selected from these 3 sow litters, while the females and males not meeting the selection criteria were cross-fostered to other sows in the unit. Healthy, 1-d-old male piglets from other litters were then selected and cross-fostered to the study sows until each had 11 piglets (n = 33 piglets total). A record of the biological sows for each of the piglets selected was recorded and is presented in Table 1. Each piglet received 2 ear tags (1 in each ear) with an ID number and were given 1.0 mL of iron dextran (Ferrodex 100 mg/mL; Agri Laboratories, Ltd., St. Joseph, MO) intramuscular in the neck.
Table 1.
Record of male piglets and their biological sow after cross-fostering
| Piglet ID | Biological sow ID | Study sow ID | Pen no. in research facility |
|---|---|---|---|
| 1 | 17,138 | 16,176 | 1 |
| 2 | 18,063 | ||
| 3 | 18,063 | ||
| 4 | 18,063 | ||
| 5 | 16,176 | ||
| 6 | 16,176 | ||
| 7 | 16,176 | ||
| 8 | 16,176 | ||
| 9 | 16,176 | ||
| 10 | 16,176 | ||
| 11 | 16,176 | ||
| 12 | 16,177 | 18,070 | 3 |
| 13 | 16,177 | ||
| 14 | 16,177 | ||
| 15 | 16,177 | ||
| 16 | 18,070 | ||
| 17 | 18,070 | ||
| 18 | 18,070 | ||
| 19 | 18,070 | ||
| 20 | 18,070 | ||
| 21 | 18,070 | ||
| 22 | 18,070 | ||
| 23 | 16,114 | 18,159 | 2 |
| 24 | 16,114 | ||
| 25 | 16,114 | ||
| 26 | 16,114 | ||
| 27 | 16,114 | ||
| 28 | 16,114 | ||
| 29 | 18,159 | ||
| 30 | 18,159 | ||
| 31 | 18,159 | ||
| 32 | 18,159 | ||
| 33 | 18,159 |
The sows and piglets were transported using a livestock trailer to the Central States Research Center (CSRC; MVS facility, Oakland, NE) 24 hr prior to study commencement. Sows and their litters were housed in farrowing crates on raised Tenderfoot flooring (Tandem Products, Inc., Minneapolis, MN). Sows had ad libitum access to feed and water and were fed a diet that met or exceeded National Research Council (NRC, 2012) nutrient requirements for lactating sows. The facility room temperature was maintained at 24.6 ± 2.5 °C, and a heat lamp was provided to each litter of pigs. All animals were exposed to ~12 hr of light per day.
Treatments and processing procedures
On the day of the study, piglet weights were collected (mean BW = 1.8 ± 0.7 kg; 2 d old), and their ID numbers were written on their forehead and back using a black permanent marker. This was to aid in piglet identification throughout the study.
Eleven piglets were assigned to each treatment group and treatments were balanced within a litter. Piglets were randomly assigned to 1 of 3 treatments: surgical castration using a CO2 laser (VetScalpel; Aesculight, LLC, Bothell, WA), surgical castration using a scalpel or sham (uncastrated control). Treatment assignments were predetermined by randomizing piglets to treatment group in a spreadsheet (Excel; Microsoft, Redmond, WA) based on piglet and sow ID. One piglet per treatment group (n = 3 total) was deemed “extra”, to ensure appropriate study power if a piglet(s) required euthanasia due to postsurgical complications such as inguinal herniation.
To conduct the castration procedure, piglets were removed from their pen, placed on a table in the supine position and restrained by 2 individuals. The surgical site was then disinfected using gauze soaked in isopropyl alcohol 70% (Vet One; MWI, Boise, ID). Piglets were castrated by making 1 horizontal incision on the scrotum with the CO2 laser (set to 15 W, continuous) or scalpel, based on their treatment group. Testicles were removed by ablating (CO2 laser) or cutting (scalpel) the spermatic cord. The CO2 laser was calibrated after each litter of pigs to ensure proper functionality (all calibrations yielded 79.7 ± 1.4%; therefore, the actual power of the CO2 laser at the level of the piglet was 12 W. This is a normal deviation for the surgical laser unit). Piglets in the sham treatment group were restrained in the same manner, the handle of the scalpel was used to simulate the incision, and the scrotum was manipulated to resemble a surgical castration. Piglets were then returned to their pen. All procedures occurred between 09:30 and 10:30 hr and were conducted by the same individual with extensive experience in surgically castrating piglets. The length of time piglets were restrained was similar across all treatment groups (~20 s). Two piglets (1 CO2 laser-castrated and 1 scalpel-castrated) herniated postcastration and were euthanized using a pentobarbital sodium injection (Fatal-Plus 390 mg/mL; Vortech Pharmaceuticals, Ltd., Dearborn, MI); all other castrated piglets (n = 20) recovered without incident.
Behavior recording and scoring
Piglets were video recorded for 1 hr preprocedure using a high-definition video camera (Sony Handycam HDR-CX405, Sony USA Inc., New York, NY) mounted on a tripod and placed outside of each farrowing pen. After processing, piglets were video recorded for two 3 hr periods: 0 to 2 hr post- and 6 to 8 hr postcastration. Finally, 24 hr postprocedure, piglets were recorded for 1 hr (i.e., 8 hr of video data were collected in total for each litter of pigs). The videos were randomized across time point and pen ID using a random number generator (random.org). The behavior of each piglet was scored continuously by 1 experienced observer for the first 15 min of every hour of data collected using BORIS software (Behavioral Observation Research Interactive Software v 7.7.3, Torino, Italy) and a detailed ethogram (Table 2). The observer was masked to treatment and time point; however, they could observe which piglets had been castrated and which had not. A total of 3,600 min (60 hr) of behavior recordings were scored and analyzed for this study.
Table 2.
Ethogram used to score piglet behavior, grouped into feeding, locomotion, nonspecific behaviors, castration-related pain behaviors, posture, and social cohesion (adapted from Hay et al., 2003)
| Behavior | Description |
|---|---|
| Suckling | Teat in mouth and suckling movements |
| Nosing udder | Nose in contact with udder, up and down head movements |
| Playing | Springing, bouncy movements with or without littermates |
| Agonistic | Biting or fighting other littermates |
| Walking | Moving forward at a normal pace |
| Running | Trot or gallop |
| Awake inactive | No special activity, but awake |
| Sleeping | Lying down, eyes closed |
| Nosing | Snout in contact with a substrate |
| Chewing | Nibbling at littermates or substrates |
| Trembling | Shivering, as with cold |
| Spasms | Quick and involuntary contractions of the muscles |
| Scratching | Rubbing the rump against the floor, pen walls, or littermates |
| Tail wagging | Tail’s movement from side to side (or up and down) |
| Stiffness | Lying with extended and tensed legs |
| Lying | Body weight supported by side or belly |
| Sitting | Body weight supported by hindquarters and front legs |
| Standing | Body weight supported by 4 legs |
| Kneeling | Body weight supported by front carpal joints and hind legs |
| Isolated | Alone, or with 1 littermate, distance of 40 cm separates the animal(s) from the closest group of littermates |
| Desynchronized | Activity different from that of most littermates (at least 75%) |
Piglet behaviors were analyzed individually and then grouped into categories to assess the activity level of piglets across the observation period and the total proportion of pain behaviors displayed. Pain behaviors included tail wagging, trembling, scratching, and stiffness (Hay et al., 2003). The active behavior category included walking, running, playing, suckling, nosing, and chewing (Viscardi et al., 2018). Inactive behaviors included sleeping and awake inactive.
Wound healing
Still-images of each piglet’s scrotum were collected using a point-and-shoot camera (Olympus Stylus Tough TG-4; Olympus Corporation, Tokyo, Japan), with 1 individual briefly handling the piglets to facilitate capturing a clear picture of the surgical site. Pictures were taken preprocedure (baseline) and at 0, 8, 24, 48, 72, 96, 120, 144, and 168 hr postcastration. Images were randomized using a random number generator (random.org) and scored using a 6-point scale developed by Sutherland et al., (2010a), by 1 individual blinded to piglet treatment and time point. Wounds with a score of 1 were fully healed (no scab) and wounds with a score of 6 had signs of fresh blood.
Infrared thermography (IRT) imaging
IRT images of the surgical castration site were collected from each piglet preprocedure (baseline) and at 0, 0.5, 8, and 24 hr postprocedure using a research-grade infrared camera (FLUKE TiX580; FLUKE Corporation, Everett, WA). The camera was calibrated to the ambient temperature and relative humidity of the room prior to taking images. One individual briefly handled the piglets to facilitate image capture while another held the IRT camera in-line with the surgical site at a distance of ~0.5 m. In castrated piglets, the incision and surrounding tissues of the scrotum were captured in a single image; in sham piglets, an image of the scrotum was collected. The majority (80%) of IRT data collection coincided with still-image capture of the castration site for wound healing assessment, so piglets were only handled once to minimize stress.
Infrared images were analyzed using research-grade software (SmartView 4.3; FLUKE Corporation, Everett, WA). For each image collected, the temperature of the incision (in castrated piglets) and the average temperature of the surrounding tissues of the scrotum were recorded and analyzed. The difference between the temperatures taken at the 2 sites on the scrotum was also calculated, by subtracting the temperature of the surrounding tissues by the temperature of the incision. These data were used to assess the degree of inflammation associated with the surgical castration procedure.
Blood sample collection and processing
A blood sample (4.0 mL) from each piglet was collected from the jugular vein using a 20-gauge needle (TycoHealth Care, Mansfield, MA) at baseline and 30 min postcastration. Piglets were briefly removed from their pen and restrained in the supine position during sample collection. Blood was immediately transferred into serum separator tubes (BD Vacutainer, Franklin Lakes, NJ) and placed on ice. Once all of the samples at each time point were collected, blood was centrifuged at 3,000 × g for 10 min. The serum was pipetted from the tube, placed into cryovials, and stored at −80 °C until analysis.
Serum samples were submitted to the Iowa State University-Pharmacology Analytical Support Team at the Iowa State University Veterinary Diagnostic Laboratory for cortisol determination. Samples were also analyzed by a laboratory technician at Kansas State University to determine prostaglandin E metabolite (PGEM) and pig-major acute phase protein concentration. All laboratory personnel were blinded to piglet treatment and time point.
Cortisol determination
Baseline (preprocedure) and 0.5 hr postprocedure serum samples were analyzed for cortisol using the commercially available Cortisol Coated Tube RIA kit (MP Biomedicals catalog no. 07-221105R; Irvine, CA). Samples were run in duplicate. There was a quality control (QC) high and a QC low-concentration run to assess drift from beginning to end of the gamma counter analysis and across runs. The average concentration of the QC low was 48.8 ng/mL and the QC high was 263.7 ng/mL. The intra- and inter-assay coefficient of variation was 10.3% and 10.6%, respectively.
PGEM determination
PGEM was determined from serum samples collected at baseline (preprocedure) and 0.5 hr postcastration. A commercially available PGEM ELISA kit was used (Cayman Chemical catalog no. 514531; Ann Arbor, MI) with minor modifications. Briefly, samples were purified by adding 1.5 mL ice cold acetone to 375 µL serum. Samples were then incubated at −20 °C for 30 min followed by centrifugation at 3,000 × g for 5 min. The supernatant was transferred to 13 × 100 mm glass tubes and evaporated using a CentriVap Concentrator (Labconco catalog no. 7810014; Kansas City, MO) and reconstituted with 375 µL of appropriate kit buffer. A 300-µL aliquot of the reconstituted sample was derivatized with proportionally adjusted kit components. Manufacturer protocol was then followed. Samples were diluted 1:5 and ran in duplicate. Absorbance was measured at 405 nm after 60 min of development (SpectraMax i3; Molecular Devices; San Jose, CA). The average concentration of the sample used to determine repeatability across plates was 27.8 pg/mL. The intra- and interassay coefficient of variation was 16.4% and 19.3%, respectively.
Pig-major acute phase protein (pig-MAP) determination
Serum samples collected at preprocedure (baseline) and 0.5 hr postcastration were analyzed for pig-MAP using a commercially available pig-MAP ELISA kit (Acuvet Biotech catalog no. AC/PME01; Zaragoza, Spain). Each sample was diluted 1:1,000 and ran in duplicate. Absorbance was read at 450 nm (SpectraMax i3; Molecular Devices; San Jose, CA). The average concentrations used to determine repeatability across plates were 1.6 and 0.8 μg/mL. The intra- and inter-assay coefficient of variation was 7.5% and 28.5%, respectively.
Statistical analysis
The total duration of behaviors was converted into proportion of time a piglet engaged in each behavior prior to analysis. This was to account for periods of time when a piglet was not in view and could not be scored. Data were analyzed using a generalized linear mixed model with a beta distribution, including treatment, time, litter, and time × treatment interaction in SAS (Statistical Analysis System 9.4, SAS Institute Inc., NC). Litter was included as a random effect, and time was a repeated measure with piglet as the experimental unit. Post hoc tests were conducted using the Tukey–Kramer adjustment. Statistical significance was set a P < 0.05.
Cortisol was log-transformed for normality prior to analysis. Wound scores, temperature of the surgical site (from IRT images), cortisol, PGEM, and pig-MAP were analyzed using a mixed model in SAS, including litter, time, treatment, and time × treatment interaction. Litter was included as a random effect, and time was a repeated measure with piglet as the experimental unit. A post hoc Tukey’s test was conducted for significant outcomes.
Results
Behavioral observations
Piglets were in-view and able to be scored for 90.7 ± 0.05% of the observation period in this study. Four individual behaviors (agonistic: P = 0.004, desynchronized: P = 0.045, tail wagging: P = 0.026, and trembling: P = 0.049) and 1 grouped behavior (pain: P = 0.026) were affected by treatment across the observation period (Table 3). Laser-castrated piglets trembled significantly more than scalpel-castrated piglets (P = 0.041) and engaged in more desynchronized behaviors (P = 0.039). Laser-castrated piglets also wagged their tails significantly more than sham piglets (P = 0.027). Agonistic behavior was displayed significantly more by laser-castrated piglets than both scalpel-castrated (P = 0.005) and sham (P = 0.037) piglets. Laser-castrated piglets demonstrated significantly more pain behaviors than piglets that were scalpel-castrated (P = 0.049).
Table 3.
Proportion of time piglets was engaged in specific behaviors (n = 10 piglets per treatment group) postcastration
| Postcastration | ||||
|---|---|---|---|---|
| Behavior1 | Treatment P-value | CO2 laser | Scalpel | Sham |
| Tail wagging | 0.0257 | 0.02 ± 0.00a | 0.00 ± 0.00ab | 0.00 ± 0.00b |
| Trembling | 0.0493 | 0.07 ± 0.07a | 0.02 ± 0.02b | 0.04 ± 0.05ab |
| Desynchronized | 0.0446 | 0.18 ± 0.07a | 0.05 ± 0.03b | 0.16 ± 0.06ab |
| Agonistic | 0.0038 | 0.01 ± 0.00a | 0.00 ± 0.00b | 0.00 ± 0.00b |
| Pain2 | 0.0257 | 0.06 ± 0.01a | 0.02 ± 0.00b | 0.03 ± 0.01ab |
1Only significant behavior variables are presented.
2Pain behaviors include: scratching, stiffness, trembling, and tail wagging.
a,bValues within a row with different superscripts are significantly different (P < 0.05).
Eleven individual behaviors and both grouped behaviors (active and pain) were significantly affected by time across the observation period (Table 4): lying (P = 0.011), nosing (P = 0.001), nosing udder (P < 0.0001), sleeping (P < 0.0001), standing (P < 0.0001), suckling (P < 0.0001), walking (P < 0.0001), sitting (P < 0.0001), tail wagging (P = 0.003), desynchronized (P = 0.009), agonistic (P = 0.003), active (P < 0.0001), and pain (P = 0.032). Irrespective of treatment group, at 1, 2, 6, and 7 hr postcastration, piglets were significantly less active, spending more time lying and sleeping and less time standing and engaged in active behaviors compared with piglets at 0, 8, and 24 hr postprocedure (P < 0.05). Piglets also spent significantly more time nosing the sow’s udder at 8 hr postcastration compared with all other time points (P < 0.001). They spent significantly more time nosing other substrates at 24 hr postcastration than at 0 and 8 hr (P < 0.05). Piglets also spent significantly more time suckling preprocedure compared with all postcastration time points (P < 0.0001). Tail wagging and pain behaviors were not significant after the Tukey–Kramer adjustment. There were no significant behavioral differences between any of the treatment groups precastration (P > 0.05), and no significant time × treatment effects were found.
Table 4.
Proportion of time piglets were engaged in specific behaviors (n = 30 piglets total) pre- and postcastration
| Precastration | Postcastration | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Behavior1 | Baseline | Time P-value |
0 hr | 1 hr | 2 hr | 6 hr | 7 hr | 8 hr | 24 hr |
| Lying | 0.71 ± 0.03 | 0.0107 | 0.47 ± 0.04a | 0.84 ± 0.03bc | 0.94 ± 0.03bc | 0.79 ± 0.03c | 0.93 ± 0.02b | 0.52 ± 0.03a | 0.42 ± 0.04a |
| Nosing | 0.00 ± 0.00 | 0.0006 | 0.02 ± 0.01a | 0.03 ± 0.01ab | — | 0.00 ± 0.03ab | 0.02 ± 0.02ab | 0.02 ± 0.00a | 0.10 ± 0.02b |
| Nosing udder | 0.07 ± 0.02 | <0.0001 | 0.27 ± 0.05ab | 0.15 ± 0.04a | 0.13 ± 0.04a | 0.18 ± 0.04a | 0.11 ± 0.04a | 0.41 ± 0.06b | 0.20 ± 0.04a |
| Sleeping | 0.44 ± 0.05 | <0.0001 | 0.23 ± 0.05a | 0.68 ± 0.05bc | 0.82 ± 0.04c | 0.67 ± 0.05b | 0.79 ± 0.04bc | 0.32 ± 0.05a | 0.24 ± 0.04a |
| Standing | 0.17 ± 0.04 | <0.0001 | 0.44 ± 0.04ac | 0.14 ± 0.03b | 0.02 ± 0.02bc | 0.19 ± 0.03b | 0.09 ± 0.02b | 0.32 ± 0.03c | 0.50 ± 0.03a |
| Suckling | 0.52 ± 0.11 | <0.0001 | 0.03 ± 0.02a | 0.03 ± 0.02ab | — | 0.08 ± 0.03ab | — | 0.11 ± 0.04bc | 0.17 ± 0.06c |
| Walking | 0.05 ± 0.02 | <0.0001 | 0.15 ± 0.02a | 0.06 ± 0.02bc | 0.00 ± 0.01abc | 0.02 ± 0.00c | 0.05 ± 0.01b | 0.07 ± 0.01ab | 0.10 ± 0.02ab |
| Sitting | 0.13 ± 0.02 | <0.0001 | 0.12 ± 0.02ac | 0.04 ± 0.02ab | 0.07 ± 0.03abc | 0.03 ± 0.01b | 0.06 ± 0.02abc | 0.17 ± 0.02c | 0.09 ± 0.02abc |
| Tail wagging | 0.00 ± 0.00 | 0.00305 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.03 ± 0.00 |
| Desynchronized | 0.12 ± 0.13 | 0.0086 | 0.26 ± 0.07a | 0.04 ± 0.02b | 0.40 ± 0.16a | 0.13 ± 0.09ab | 0.01 ± 0.03ab | 0.18 ± 0.07ab | 0.10 ± 0.02ab |
| Agonistic | — 4 | 0.0029 | 0.00 ± 0.00ac | 0.01 ± 0.01abc | — | 0.00 ± 0.00a | 0.00 ± 0.00bc | 0.00 ± 0.00a | 0.00 ± 0.00b |
| Active2 | 0.29 ± 0.03 | <0.0001 | 0.53 ± 0.04a | 0.16 ± 0.03bc | 0.06 ± 0.03bc | 0.21 ± 0.03c | 0.07 ± 0.02b | 0.48 ± 0.04a | 0.57 ± 0.04a |
| Pain3 | 0.02 ± 0.02 | 0.03205 | 0.05 ± 0.02 | 0.11 ± 0.03 | 0.02 ± 0.02 | 0.02 ± 0.01 | 0.05 ± 0.02 | 0.00 ± 0.00 | 0.05 ± 0.01 |
1Only significant behavior variables are presented.
2Active behaviors include: nosing, suckling, walking, chewing, playing, and running.
3Pain behaviors include: scratching, stiffness, trembling, and tail wagging.
4Dash indicates behavior was not observed.
5Not significant after Tukey–Kramer adjustment.
a–cValues within a row with different superscripts are significantly different (P < 0.05).
Wound healing
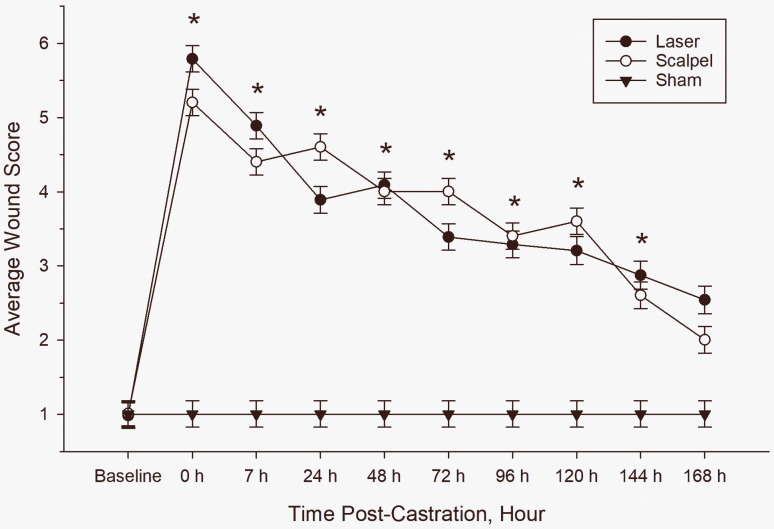
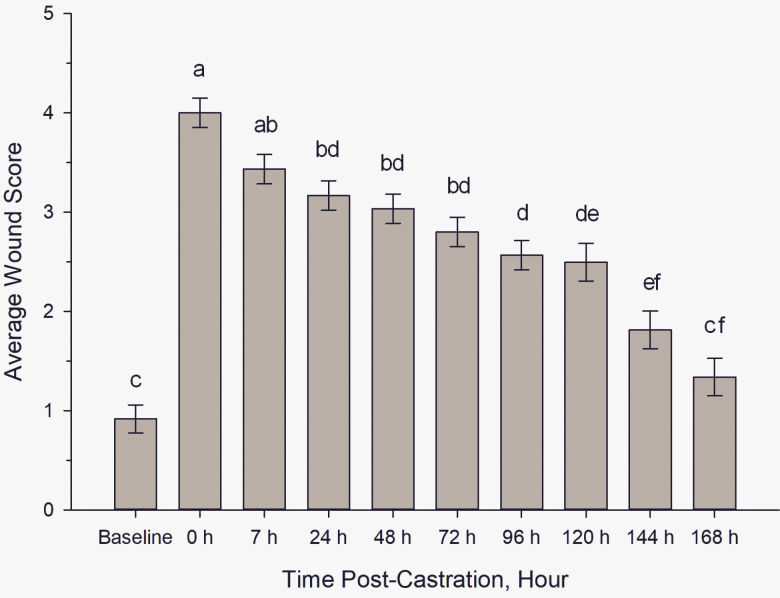
There were significant time and treatment effects on wound scores (P <0.0001 for both). Both castrated-treatment groups had significantly higher wound scores than sham piglets (P <0.0001); however, there was no significant difference found in wound score between laser-castrated and scalpel-castrated piglets (Figure 1; P = 0.988). Wound scores significantly decreased over time (Figure 2). In castrated piglets, similar wound scores were noted during the following postsurgical time ranges: 0 to 7, 7 to 72, 24 to 120, 120 to 144, and 144 to 168 hr (P > 0.05 for all time ranges). Castration wound scores did not reach baseline levels until 168 hr (7 d) postcastration.
Figure 1.
Average wound score (±SE) of piglets in each treatment group over time. Asterisks represent a significant difference (P < 0.05) between the castrated piglets (laser and scalpel; n = 20) and sham piglets (n = 10).
Figure 2.
Average wound score (±SE) of castrated piglets (n = 20) over time. Different letters indicate significance (P < 0.05).
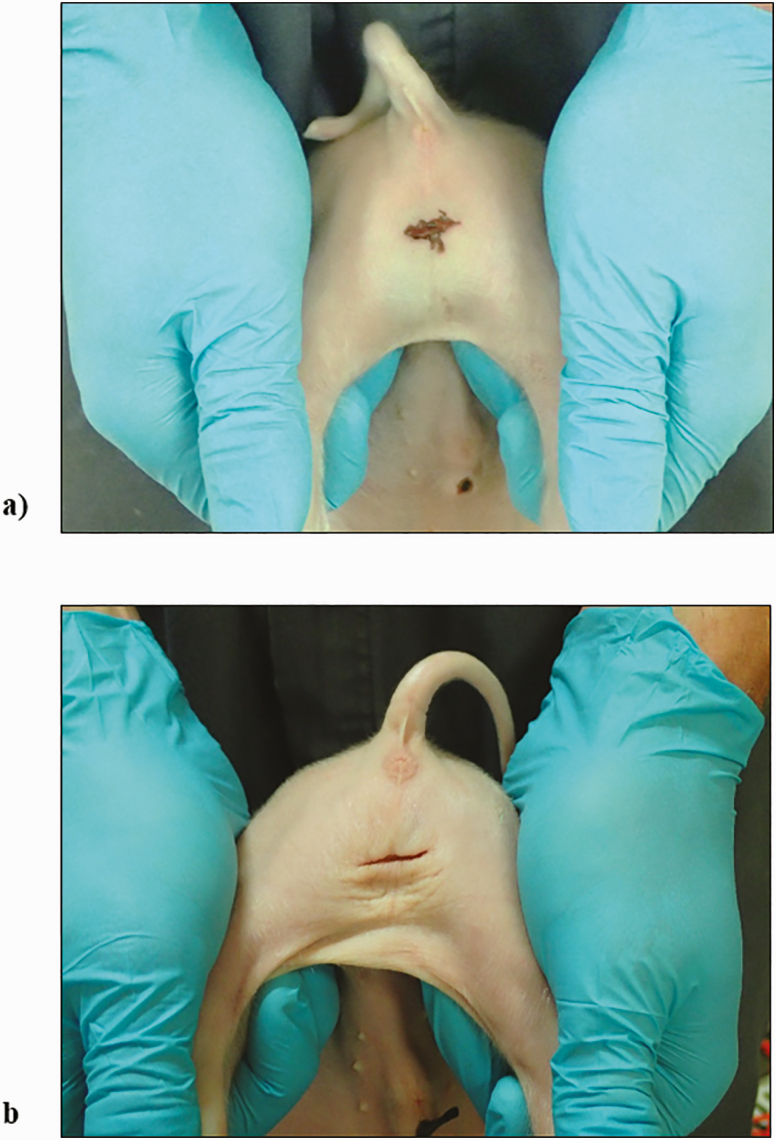
A researcher involved in this study, who did not score castration wounds, noted evidence of scrotal tissue burns and bruising of the surrounding tissues in a number of the still images. Once unblinded to treatment, the images were assessed and both tissue burns and bruising were only observed in piglets that had been castrated using the CO2 laser (Figure 3).
Figure 3.
A comparison of the surgical wound at 24 hr postcastration for a piglet undergoing the procedure using (a) the CO2 laser or (b) a scalpel. Evidence of tissue burning at the incision site and bruising of the surrounding tissues is clear in the laser-castrated piglet.
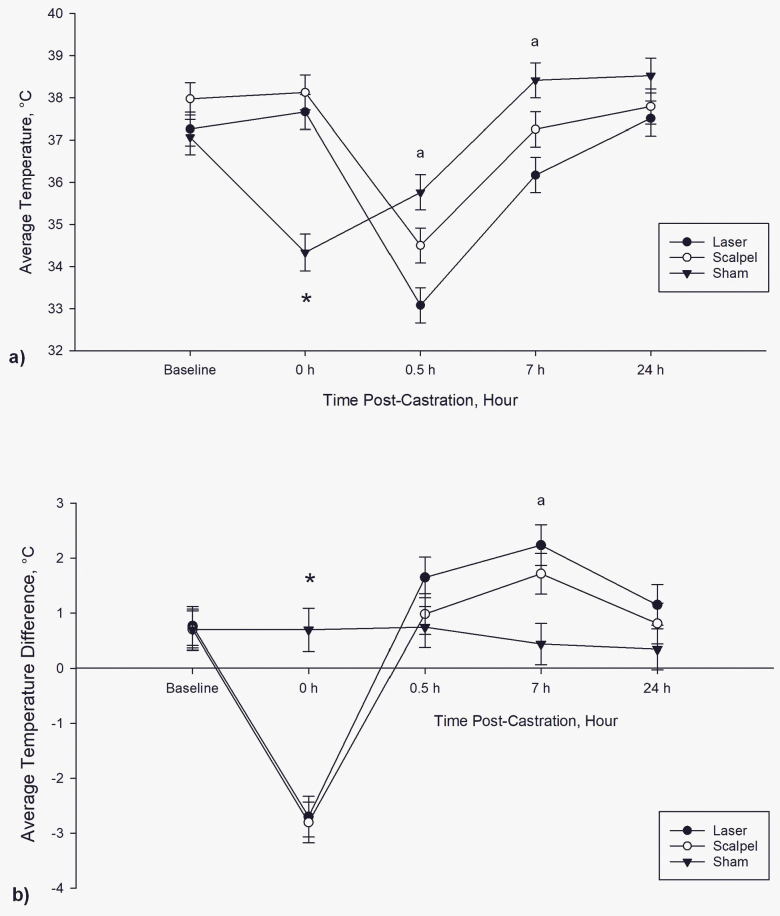
IRT imaging
There were significant time (P < 0.0001), treatment (P = 0.010), and time × treatment interactions (P < 0.0001) found for temperature at the incision site. Zero hour postcastration, laser-castrated and scalpel-castrated piglets had significantly higher incision site temperatures compared with sham piglets (Figure 4a; P < 0.0001). At 0.5 and 7 hr postcastration, laser-castrated piglets had significantly lower temperatures at the site of incision compared with sham piglets (P = 0.0005 and 0.010, respectively). Across the assessment period, laser-castrated piglets had significantly lower incision site temperatures compared with scalpel-castrated piglets (P = 0.008). Irrespective of treatment, piglets had significantly lower incisional temperatures at 0.5 hr postcastration compared with all other time points (P < 0.0001).
Figure 4.
Average temperature (°C) (a) at the incision site (±SE) and (b) temperature difference (±SE) between the incision site and the surrounding tissues of the scrotum of piglets in each treatment group over time. Asterisk represents a significant difference (P < 0.05) between the castrated piglets (n = 20) and sham piglets (n = 10); letter represents a significant difference between laser-castrated piglets (n = 10) and sham piglets.
There was no significant difference in temperature of the surrounding tissues on the scrotum between treatment groups (P = 0.081); however, there was a significant effect of time (P <0.0001), with piglets having lower temperatures at 0 and 0.5 hr postcastration compared with baseline, 7 and 24 hr.
There were significant time (P < 0.0001) and time × treatment interactions (P < 0.0001) found for the temperature difference between the 2 focal sites on the scrotum. The difference in temperature between the 2 sites was significant at 0 hr postcastration, where both laser-castrated and scalpel-castrated piglets had a 2.8 ± 0.1 °C increase in temperature at the site of incision compared with the surrounding tissues (Figure 4b). At 7 hr postcastration, scalpel- and laser-castrated piglets had a 2.0 ± 0.3 °C increase in temperature of the surrounding tissues compared with the site of incision. Temperatures of the 2 sites did not differ significantly between the “incision” and the surrounding tissues on the scrotum of sham piglets.
Cortisol concentration
There were no treatment (P = 0.19) or treatment × time interactions (P = 0.59) found for plasma cortisol concentrations. There was a significant time effect observed (P = 0.01). Cortisol concentrations were elevated for both surgical castration groups at 0.5 hr. Mean plasma cortisol concentrations precastration were 447.0 ± 55.7, 389.9 ± 63.2, and 582.1 ± 137.1 ng/mL for the laser-castrated, scalpel-castrated, and control piglets, respectively (Table 5). At 0.5 hr after castration, the cortisol concentrations were 659.2 ± 69.0, 540.7 ± 67.5, and 588.9 ± 83.1 ng/mL for the laser-castrated, scalpel-castrated, and control piglets, respectively.
Table 5.
Mean (95% confidence interval) analyte concentration of cortisol, pig-MAP1, and PGEM2 analyzed for piglets undergoing surgical castration by CO2 laser, scalpel, or sham (n = 10 piglets per treatment group).
| Treatment | P-value | |||||
|---|---|---|---|---|---|---|
| CO2 laser | Scalpel | Sham | Treatment | Time | Treatment × time | |
| Cortisol, ng/mL | ||||||
| Baseline | 447.0 321.1 to 573.0 |
389.9 246.9 to 532.9 |
582.1 271.9 to 892.4 |
0.19 | 0.01 | 0.59 |
| 0.5 hr | 695.2 534.8 to 855.7 |
540.7 380.2 to 701.2 |
588.9 428.4 to 749.3 |
|||
| Pig-MAP, mg/mL1 | ||||||
| Baseline | 0.97 0.71 to 1.23 |
0.94 0.69 to 1.20 |
0.88 0.62 to 1.14 |
0.73 | 0.62 | 0.92 |
| 0.5 hr | 0.96 0.71 to 1.22 |
0.91 0.65 to 1.16 |
0.87 0.61 to 1.13 |
|||
| PGEM, pg/mL1 | ||||||
| Baseline | 157.1 114.6 to 199.7 |
153.1 110.5 to 195.7 |
139.7 97.0 to 182.3 |
0.62 | 0.61 | 0.84 |
| 0.5 hr | 157.8 115.2 to 200.3 |
129.8 87.2 to 172.4 |
135.5 92.9 to 178.1 |
|||
1Pig-MAP, pig-major acute phase protein; PGEM, prostaglandin E metabolite.
Prostaglandin E-metabolite concentration
There were no treatment (P = 0.62), time (P = 0.61), or treatment × time interactions (P = 0.84) for PGEM concentrations. The PGEM concentrations prior to castration were 157.1 ± 20.9, 153.1 ± 36.2, and 139.7 ± 13.4 pg/mL for the laser-castrated, scalpel-castrated, and control piglets, respectively. At 0.5 hr after castration, the PGEM concentrations were 157.8 ± 20.0, 129.8 ± 17.7, and 135.5 ± 16.1 pg/mL for the laser-castrated, scalpel-castrated, and control piglets, respectively.
Pig-major acute phase protein concentration
No treatment (P = 0.73), time (P = 0.62), or treatment × time interactions (P = 0.92) were observed for the pig-MAP concentrations. Mean pig-MAP concentrations precastration were 0.97 ± 0.07, 0.94 ± 0.10, and 0.88 ± 0.09 mg/mL for the laser-castrated, scalpel-castrated, and control piglets, respectively. At 0.5 hr postcastration, pig-MAP concentrations were 0.96 ± 0.10, 0.91 ± 0.11, and 0.87 ± 0.06 mg/mL for the laser-castrated, scalpel-castrated, and control piglets, respectively.
Discussion
This study examined the ability of a CO2 surgical laser to reduce pain and improve wound healing of piglets undergoing surgical castration. Piglets that were castrated using the CO2 laser exhibited significantly more pain behaviors (trembling, spasms, rubbing the rump, tail wagging, and stiffness) than scalpel-castrated piglets, which is contrary to the study hypothesis. Three factors likely contributed to this result: the disinfectant used, piglet restraint, and laser-castration technique. Isopropyl alcohol 70% is a common disinfectant used in human and veterinary medicine. Alcohols are highly flammable in nature (CDC, 2008), and there is evidence that heat produced by the CO2 laser caused a chemical reaction with the alcohol on the piglet’s scrotal surface, resulting in thermal tissue damage. In humans, moderate or severe pain after a thermal burn is common (Perry et al., 1981; McIntyre et al., 2016), and this may explain the increased pain behavior observed in laser-castrated piglets. Based on case reports in the human medicine literature, pooling of alcohol-based skin preparations should be avoided, and the disinfectant used should be dried completely (i.e., allowed to evaporate) when using a surgical laser, to prevent burns (Tooher et al., 2004; Jones et al., 2017). In this study, castration of piglets occurred immediately after disinfecting the surgical site, to limit the stress associated with piglet restraint and separation from the sow. Rather than use an alcohol-based disinfectant and wait the recommended drying time (2 to 3 min) with piglets restrained, a nonalcohol-based solution could be used in future work with a CO2 laser (Vo and Bengezi, 2014; Jones et al., 2017). Difficulty in completely immobilizing conscious piglets resulted in suboptimal laser-castration technique, which also likely contributed to the increase in pain behavior observed. Improving piglet restraint to restrict movement during the castration procedure will allow for a more precise incision to be made with the CO2 laser and prevent poor technique from confounding pain behavior results. Laser-castrated piglets engaged in more agonistic behavior across the observation period than all other study piglets. Pain increases aggressive behavior, irritability, and negatively affects interpersonal relations in animal species, including dogs, cats, rats and horses (Curtis, 2008; Fureix et al., 2010; Camps et al., 2012; Njoku et al., 2015); therefore, it could be anticipated that an increase in pain behavior correlated with an increase in agonistic behavior in this study.
There was no difference in wound score (i.e., healing time) between scalpel- and laser-castrated piglets throughout the study. This is likely related to the burning of the scrotal tissue in laser-castrated piglets. When a surgical laser causes thermal tissue damage, a slower healing time within the first few days or weeks has been observed (dogs: Durante and Kriek, 1993; Mison et al., 2003; pigs: Buell and Schuller, 1983; Molgat et al., 1994; Schoinohoriti et al., 2012). The lack of difference in healing time when comparing a CO2 laser to a scalpel may also be a result of poor laser technique or a power setting that was too low, requiring multiple passes with the laser to make an incision (Reid, 1991; Capon and Mordon, 2003). These sequential passes with the laser at the incisional site increases the amount of thermal injury (Reid, 1991). The power setting selected for this study (15 W, continuous) was determined from the manufacturer’s recommendations and by practicing surgical castration on piglet cadavers prior to study start. The power may need to be increased in future work, as multiple passes with the laser were required to make the scrotal incision.
IRT is a validated tool to measure cutaneous temperature and assess inflammation (Całkosiński et al., 2015; Soroko and Howell, 2018). Laser-castrated piglets had a lower temperature at the incision site across the assessment period (up to 24 hr postcastration) compared with scalpel-castrated piglets, suggesting that there was less inflammation at the surgical site when the CO2 laser was used. While this result is consistent with our study hypothesis, it contradicts the literature regarding thermal tissue injury and inflammation (Strudwick and Cowin, 2017). As well, a decrease in inflammation after piglet surgical castration is generally associated with a reduction in acute pain and pain behaviors (Herskin and Di Giminiani, 2018), yet this was not observed. The temperature of the surrounding scrotal tissues (excluding the incision site) did not differ between laser- and scalpel-castrated piglets, suggesting that inflammation at this location may be more predictive of castration-associated pain. The significant drop in temperature from 0 to 0.5 hr postcastration may be related to activation of the sympathetic nervous system (SNS) in piglets, leading to peripheral vasoconstriction and a decrease in cutaneous temperature. This has been observed in animals that are stressed or in pain (Stewart et al., 2005; Dockweiler et al., 2013; Bates et al., 2014). At 0.5 hr postcastration, piglets were separated from the sow, restrained, and a blood sample was collected via jugular venipuncture, all prior to collecting an IRT image. The amount of handling, restraint, and painful procedures piglets in this study were subjected to within a 30-min period almost certainly caused stress and acute physiological changes. Future work should prioritize noninvasive data collection (e.g., IRT, behavior, etc.) prior to conducting more invasive procedures (e.g., venipuncture) to minimize stress and SNS activation from potentially confounding results.
Blood cortisol has been widely used as a biomarker of stress and pain in animals. In response to surgical castration, cortisol levels increase in piglets and peak 30 to 90 min after processing (Prunier et al., 2005; von Borell et al., 2009; Bates et al., 2014; Tenbergen et al., 2014; Gottardo et al., 2016). The mean plasma cortisol concentration of piglets in this study significantly increased from baseline to 0.5 hr postcastration; however, there was no effect of treatment (i.e., sham piglets who were not castrated also had a significant increase in blood cortisol level). While this contradicts previous work where cortisol levels did not significantly increase in piglets that were handled only and not subjected to a painful procedure (Prunier et al., 2005; Carroll et al., 2006; Marchant-Forde et al., 2014), it is important to note that cortisol is not a specific biomarker of pain. Stress from handling or restraint can cause an increase in plasma cortisol concentration (Llamas Moya et al., 2008). Many piglets in this study were cross-fostered and all were transported to the research facility <24 hr prior to study start. Both of these events are known to be stressful (Sutherland et al., 2010b; Calderón Diaz et al., 2018). Acclimation of research animals to a new environment is ideal, to reduce the risk of stress interfering with the study results. Prostaglandin E and acute phase proteins in the blood increase in response to stress, pain, and inflammation (Piñeiro et al., 2013; Davidson et al., 2014). In this study, there were no treatment or time differences in these outcome measures (PGEM and pig-MAP), which may relate to the high levels of stress piglets were already experiencing due to the recent cross-fostering and transportation events.
Using a CO2 surgical laser instead of a scalpel has the potential to reduce pain, inflammation, and improve animal welfare (Tuncer et al., 2010; López-Jornet and Camacho-Alonso, 2013). In this study, thermal tissue damage caused by the CO2 laser confounded the pain and wound healing results, making it difficult to draw conclusions regarding the utility of this tool for surgical castration of piglets. A nonalcohol-based disinfectant should be used and the laser-castration technique optimized in future work, to more accurately assess pain, inflammation, and wound healing in piglets after processing.
Glossary
Abbreviations
- BW
body weight
- SE
standard error
- ID
identification
- IRT
infrared thermography
- PGEM
prostaglandin E metabolite
- pig-MAP
pig-major acute phase protein
- QC
quality control
- SNS
sympathetic nervous system
Funding
The authors would like to thank personnel at Midwest Veterinary Services for data collection and technical assistance. This project was supported by Agriculture and Food Research Initiative Competitive, grant no. 2020-67015-31540 from the USDA National Institute of Food and Agriculture. Drs Coetzee and Kleinhenz are supported by the Agriculture and Food Research Initiative Competitive, grant nos 2017-67015-27124, 2020-67030-31479, 2020-67015-31540, and 2020-67015-31546 from the USDA National Institute of Food and Agriculture.
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Bates J. L., Karriker L. A., Stock M. L., Pertzborn K. M., Baldwin L. G., Wulf L. W., Lee C. J., Wang C., and Coetzee J. F.. . 2014. Impact of transmammary-delivered meloxicam on biomarkers of pain and distress in piglets after castration and tail docking. PLoS One 9:e113678. doi: 10.1371/journal.pone.0113678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Borell E., Baumgartner J., Giersing M., Jäggin N., Prunier A., Tuyttens F. A., and Edwards S. A.. . 2009. Animal welfare implications of surgical castration and its alternatives in pigs. Animal 3:1488–1496. doi: 10.1017/S1751731109004728 [DOI] [PubMed] [Google Scholar]
- Buell B. R., and Schuller D. E.. . 1983. Comparison of tensile strength in CO2 laser and scalpel skin incisions. Arch. Otolaryngol. 109:465–467. doi: 10.1001/archotol.1983.00800210041009 [DOI] [PubMed] [Google Scholar]
- Calderón Diaz J. A., García Manzanilla E., Diana A., and Boyle L. A.. . 2018. Cross-fostering implications for pig mortality, welfare and performance. Front. Vet. Sci. 5:123. doi: 10.3389/fvets.2018.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Całkosiński I., Dobrzyński M., Rosińczuk J., Dudek K., Chrószcz A., Fita K., and Dymarek R.. . 2015. The use of infrared thermography as a rapid, quantitative, and noninvasive method for evaluation of inflammation response in different anatomical regions of rats. Biomed Res. Int. 2015:972535. doi: 10.1155/2015/972535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps T., Amat M., Mariotti V. M., Le Brech S., and Manteca X.. . 2012. Pain-related aggression in dogs: 12 clinical cases. J. Vet. Behav. 7:99–102. doi: 10.1016/j.jveb.2011.08.002 [DOI] [Google Scholar]
- Capon A., and Mordon S.. . 2003. Can thermal lasers promote skin wound healing? Am. J. Clin. Dermatol. 4:1–12. doi: 10.2165/00128071-200304010-00001 [DOI] [PubMed] [Google Scholar]
- Carroll J. A., Berg E. L., Strauch T. A., Roberts M. P., and Kattesh H. G.. . 2006. Hormonal profiles, behavioral responses, and short-term growth performance after castration of pigs at three, six, nine, or twelve days of age. J. Anim. Sci. 84:1271–1278. doi: 10.2527/2006.8451271x [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention 2008. Chemical disinfectants https://www.cdc.gov/infectioncontrol/guidelines/disinfection/disinfection-methods/chemical.html [accessed April 2, 2020].
- Curtis T. M. 2008. Human-directed aggression in the cat. Vet. Clin. North Am. Small Anim. Pract. 38:1131–43, vii. doi: 10.1016/j.cvsm.2008.04.009 [DOI] [PubMed] [Google Scholar]
- Davidson S., Copits B. A., Zhang J., Page G., Ghetti A., and Gereau R. W.. . 2014. Human sensory neurons: membrane properties and sensitization by inflammatory mediators. Pain 155:1861–1870. doi: 10.1016/j.pain.2014.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockweiler J. C., Coetzee J. F., Edwards-Callaway L. N., Bello N. M., Glynn H. D., Allen K. A., Theurer M. E., Jones M. L., Miller K. A., and Bergamasco L.. . 2013. Effect of castration method on neurohormonal and electroencephalographic stress indicators in Holstein calves of different ages. J. Dairy Sci. 96:4340–4354. doi: 10.3168/jds.2012-6274 [DOI] [PubMed] [Google Scholar]
- Durante E. J., and Kriek N. P.. . 1993. Clinical and histological comparisons of tissue damage and healing following incisions with the CO2 laser and stainless steel surgical blade in dogs. J. South. Afr. Vet. Assoc. 64:116–120. [PubMed] [Google Scholar]
- Fureix C., Menguy H., and Hausberger M.. . 2010. Partners with bad temper: reject or cure? A study of chronic pain and aggression in horses. PLoS One 5:e12434. doi: 10.1371/journal.pone.0012434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardo F., Scollo A., Contiero B., Ravagnani A., Tavella G., Bernardini D., De Benedictis G. M., and Edwards S. A.. . 2016. Pain alleviation during castration of piglets: a comparative study of different farm options. J. Anim. Sci. 94:5077–5088. doi: 10.2527/jas.2016-0843 [DOI] [PubMed] [Google Scholar]
- Hay M., Vulin A., Genin S., Sales P., and Prunier A.. . 2003. Assessment of pain induced by castration in piglets: behaviour and physiological responses over the subsequent 5 days. Appl. Anim. Behav. Sci. 82:201–218. doi: 10.1016/S0168-1591(03)00059-5 [DOI] [Google Scholar]
- Herskin M. S., and Di Giminiani P.. . 2018. Pain in pigs: characterization, mechanisms and indicators. In: Špinka M., editor, Advances in pig welfare. Cambridge, MA: Elsevier Ltd; p. 325–355. [Google Scholar]
- Jones E. L., Overbey D. M., Chapman B. C., Jones T. S., Hilton S. A., Moore J. T., and Robinson T. N.. . 2017. Operating room fires and surgical skin preparation. J. Am. Coll. Surg. 225:160–165. doi: 10.1016/j.jamcollsurg.2017.01.058 [DOI] [PubMed] [Google Scholar]
- Llamas Moya S., Boyle L. A., Lynch P. B., and Arkins S.. . 2008. Effect of surgical castration on the behavioural and acute phase responses of 5-day-old piglets. Appl. Anim. Behav. Sci. 111:133–145. doi: 10.1016/j.applanim.2007.05.019 [DOI] [Google Scholar]
- López-Jornet P., and Camacho-Alonso F.. . 2013. Comparison of pain and swelling after removal of oral leukoplakia with CO2 laser and cold knife: a randomized clinical trial. Med. Oral Patol. Oral Cir. Bucal. 18:e38–e44. doi: 10.4317/medoral.17960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant-Forde J. N., Lay D. C. Jr, McMunn K. A., Cheng H. W., Pajor E. A., and Marchant-Forde R. M.. . 2014. Postnatal piglet husbandry practices and well-being: the effects of alternative techniques delivered in combination. J. Anim. Sci. 92:1150–1160. doi: 10.2527/jas.2013-6929 [DOI] [PubMed] [Google Scholar]
- McIntyre M. K., Clifford J. L., Maani C. V., and Burmeister D. M.. . 2016. Progress of clinical practice on the management of burn-associated pain: Lessons from animal models. Burns 42:1161–1172. doi: 10.1016/j.burns.2016.01.023 [DOI] [PubMed] [Google Scholar]
- Mison M. B., Steficek B., Lavagnino M., Teunissen B. D., Hauptman J. G., and Walshaw R.. . 2003. Comparison of the effects of the CO2 surgical laser and conventional surgical techniques on healing and wound tensile strength of skin flaps in the dog. Vet. Surg. 32:153–160. doi: 10.1053/jvet.2003.50003 [DOI] [PubMed] [Google Scholar]
- Molgat Y. M., Pollack S. V., Hurwitz J. J., Bunas S. J., Manning T., McCormack K. M., and Pinnell S. R.. . 1994. Comparative study of wound healing in porcine skin with CO2 laser and other surgical modalities: preliminary findings. Int. J. Dermatol. 34:42–47. doi: 10.1111/j.1365-4362.1995.tb04379.x [DOI] [PubMed] [Google Scholar]
- Morales J., Dereu A., Manso A., de Frutos L., Piñeiro C., Manzanilla E. G., and Wuyts N.. . 2017. Surgical castration with pain relief affects the health and productive performance of pigs in the suckling period. Porcine Health Manag. 3:18. doi: 10.1186/s40813-017-0066-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council 2012. Nutrient requirements of swine. 11th rev. ed. Washington, DC: National Academies Press. [Google Scholar]
- Njoku N. U., Omamegbe J. O., Odilichukwu E. O., Ukaha R. O., Jeremiah K. T., and Nwoha R. I. O.. . 2015. Pain evaluation in ovariohysterectomised albino wistar rats using physiological, behavioural and biochemical indices. Nig. Vet. J. 36:1262–1271. [Google Scholar]
- Perry S., Heidrich G., and Ramos E.. . 1981. Assessment of pain by burn patients. J. Burn Care Rehab. 2:322–326. doi: 10.1097/00004630-198111000-00004 [DOI] [Google Scholar]
- Piñeiro M., Morales J., Vizcaíno E., Murillo J. A., Klauke T., Petersen B., and Piñeiro C.. . 2013. The use of acute phase proteins for monitoring animal health and welfare in the pig production chain: the validation of an immunochromatographic method for the detection of elevated levels of pig-MAP. Meat Sci. 95:712–718. doi: 10.1016/j.meatsci.2013.03.013 [DOI] [PubMed] [Google Scholar]
- Prunier A., Mounier A. M., and Hay M.. . 2005. Effects of castration, tooth resection, or tail docking on plasma metabolites and stress hormones in young pigs. J. Anim. Sci. 83:216–222. doi: 10.2527/2005.831216x [DOI] [PubMed] [Google Scholar]
- Rault J.R., Lay D. C. Jr., and Marchant-Forde J. N.. . 2011. Castration induced pain in pigs and other livestock. Appl. Anim. Behav. Sci. 135:214–225. doi: 10.1016/j.applanim.2011.10.017 [DOI] [Google Scholar]
- Reid R. 1991. Physical and surgical principles governing carbon dioxide laser surgery on the skin. Dermatol. Clin. 9:297–316. doi: 10.1016/S0733-8635(18)30418-2 [DOI] [PubMed] [Google Scholar]
- Schoinohoriti O. K., Chrysomali E., Tzerbos F., and Iatrou I.. . 2012. Comparison of lateral thermal injury and healing of porcine skin incisions performed by CO2-laser, monopolar electrosurgery and radiosurgery: a preliminary study based on histological and immunohistochemical results. Int. J. Dermatol. 51:979–986. doi: 10.1111/j.1365-4632.2011.05384.x [DOI] [PubMed] [Google Scholar]
- Schultz W. E. 2013. CO2 laser-assisted castration. The Education Center; Vet. Prac. News. 40–41. Available from https://www.aesculight.com/wp-content/uploads/2013/08/18-09-2013_Schultz.pdf. Accessed April 2, 2020. [Google Scholar]
- Soroko M., and Howell K.. . 2018. Infrared thermography: current applications in equine medicine. J. Equine Vet. Sci. 60:90–96. doi: 10.1016/j.jevs.2016.11.002 [DOI] [Google Scholar]
- Stewart M., Webster J. R., Schaefer A. L., Cook N. J., and Scott S. L.. . 2005. Infrared thermography as a non-invasive tool to study animal welfare. Anim. Welfare. 14:319–325. [Google Scholar]
- Strudwick X. L., and Cowin A. J.. . 2017. The role of the inflammatory response in burn injury. In: Kartal S. P. and Bayramgürler D., editors, Hot topics in burn injuries. London, UK: IntechOpen. doi: 10.5772/intechopen.71330 [DOI] [Google Scholar]
- Sutherland M. A. 2015. Welfare implications of invasive piglet husbandry procedures, methods of alleviation and alternatives: a review. N. Z. Vet. J. 63:52–57. doi: 10.1080/00480169.2014.961990 [DOI] [PubMed] [Google Scholar]
- Sutherland M. A., Bryer P. J., Davis B. L., and McGlone J. J.. . 2010b. A multidisciplinary approach to assess the welfare of weaned pigs during transport at three space allowances. J. Appl. Anim. Welf. Sci. 13:237–249. doi: 10.1080/10888705.2010.483879 [DOI] [PubMed] [Google Scholar]
- Sutherland M., Davis B., Brooks T., and McGlone J.. . 2010a. Physiology and behavior of pigs before and after castration: effects of two topical anesthetics. Animal. 4:2071–2079. doi: 10.1017/S1751731110001291 [DOI] [PubMed] [Google Scholar]
- Taylor A. A., and Weary D. M.. . 2000. Vocal responses of piglets to castration: identifying procedural sources of pain. Appl. Anim. Behav. Sci. 70:17–26. doi: 10.1016/s0168-1591(00)00143-x [DOI] [PubMed] [Google Scholar]
- Tenbergen R., Friendship R., Cassar G., Amezcua M. R., and Haley D.. . 2014. Investigation of the use of meloxicam for reducing pain associated with castration and tail docking and improving performance in piglets. J. Swine Health Prod. 22:64–70. [Google Scholar]
- Tooher R., Maddern G. J., and Simpson J.. . 2004. Surgical fires and alcohol-based skin preparations. ANZ J. Surg. 74:382–385. doi: 10.1111/j.1445-1433.2004.02997.x [DOI] [PubMed] [Google Scholar]
- Tuncer I., Ozçakir-Tomruk C., Sencift K., and Cöloğlu S.. . 2010. Comparison of conventional surgery and CO2 laser on intraoral soft tissue pathologies and evaluation of the collateral thermal damage. Photomed. Laser Surg. 28:75–79. doi: 10.1089/pho.2008.2353 [DOI] [PubMed] [Google Scholar]
- Viscardi A. V., and Turner P. V.. . 2018. Use of meloxicam or ketoprofen for piglet pain control following surgical castration. Front. Vet. Sci. 5:299. doi: 10.3389/fvets.2018.00299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo A., and Bengezi O.. . 2014. Third-degree burns caused by ignition of chlorhexidine: a case report and systematic review of the literature. Plast. Surg. (Oakv). 22:264–266. doi: 10.4172/plastic-surgery.1000893 [DOI] [PMC free article] [PubMed] [Google Scholar]