Abstract
The development of the induced pluripotent stem cells (iPSCs) technology has revolutionized the world on the establishment of pluripotent stem cells (PSCs) across a great variety of animal species. Generation of iPSCs from domesticated animals would provide unrestricted cell resources for the study of embryonic development and cell differentiation of these species, for screening and establishing desired traits for sustainable agricultural production, and as veterinary and preclinical therapeutic tools for animal and human diseases. Induced PSCs from domesticated animals thus harbor enormous scientific, economical, and societal values. Although much progress has been made toward the generation of PSCs from these species, major obstacles remain precluding the exclamation of the establishment of bona fide iPSCs. The most prominent of them remain the inability of these cells to silence exogenous reprogramming factors, the obvious reliance on exogenous factors for their self-renewal, and the restricted development potential in vivo. In this review, we summarize the history and current progress in domestic farm animal iPSC generation, with a focus on swine, ruminants (cattle, ovine, and caprine), horses, and avian species (quails and chickens). We also discuss the problems associated with the farm animal iPSCs and potential future directions toward the complete reprogramming of somatic cells from farm animals.
Keywords: avian, differentiation, induced pluripotent stem cells, livestock, pluripotency, reprogramming
Introduction
Pluripotent stem cells (PSCs) were mainly referring to embryonic stem cells (ESCs) derived from the inner cell mass (ICM) of animal embryos (Figure 1), which can differentiate into any cell type of the three-germ layers and can propagate almost infinitely in a laboratory setting (Martin, 1981; Bradley et al., 1984; Thomson et al., 1998; Gilbert, 2013). These properties of PSCs are fundamental for the application in regenerative medicine, animal reproduction, and modeling diseases. Since 2006, another type of PSCs, the induced pluripotent stem cells (iPSCs) rapidly emerged. These cells were initially reprogrammed from mouse and human somatic cells by the exogenous expression of OCT4 (also known as POU5F1), SOX2, KLF4, and c-MYC (OSKM), or OCT4, SOX2, NANOG, and LIN28 (OSNL) (Takahashi and Yamanaka, 2006; Okita et al., 2007; Yu et al., 2007). Compared with ESCs, iPSCs have multiple advantages in terms of generation and applications, including the unnecessity of expensive instrumentation and labor-intensive process, the ability to be mass produced in the laboratory environment within short timeframes, the convenience of autologous iPSCs derivation from host somatic cells (host-specific iPSCs), and the circumvention of the ethical dilemmas underlying destruction of human and animal embryos. Since the initial introduction by Yamanaka’s group, the iPSCs technology has been successfully applied to many species from rodents to primates (Liu et al., 2008; Esteban et al., 2009; Li et al., 2009, 2011b; Liao et al., 2009; Wu et al., 2009; Honda et al., 2010; Tomioka et al., 2010; Bao et al., 2011; Nagy et al., 2011; Breton et al., 2013).
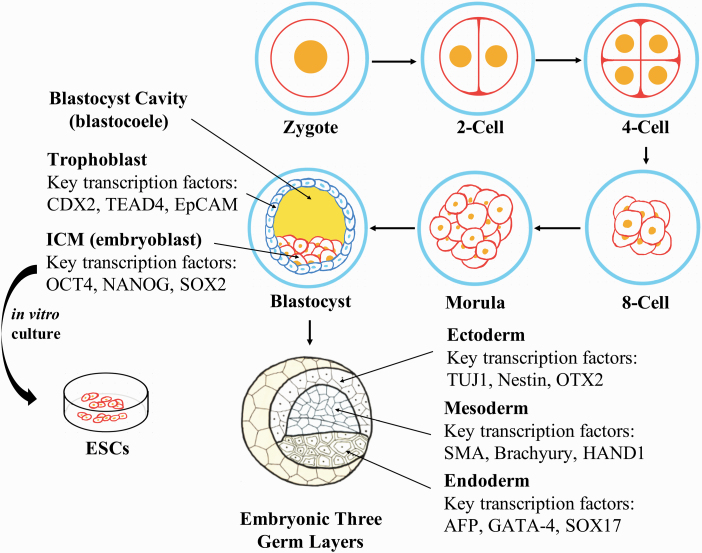
Figure 1.
The development of early mammalian embryos. The embryo development starts from a zygote. Then, it enters the cleavage process and divides into two cells, four cells, eight cells, etc., and to morula stage. After that, it will form a blastocyst, consisting of trophoblasts, blastocyst cavity, and the ICM. Blastomeres isolated from the ICM of mammalian embryos could be cultured in vitro to generate pluripotent ESCs. Upon implantation, ICM from the blastocyst will differentiate into primitive endoderm and epiblasts, with the latter further developing into the three-germ layers of an embryo, while the trophoblasts will become the placenta.
Pluripotency is classified into at least three different stages. The postimplantation pluripotent state is represented by primed pluripotency (Brons et al., 2007; Tesar et al., 2007; Nichols and Smith, 2009; Najm et al., 2011) as seen in human ESCs/iPSCs and mouse epiblast stem cells. These cells have flat, monolayered colony morphology (Figure 2), rely on basic fibroblast growth factor (bFGF)/Activin A signaling for self-renewal, are refractory to single-cell colonization, and have gone through X chromosome inactivation in females (XaXi) (Thomson et al., 1998; Vallier et al., 2004; James et al., 2005; Camus et al., 2006). The preimplantation pluripotent state is represented by naïve pluripotency (Nichols and Smith, 2009) as seen in mouse ESCs/iPSCs, with dome-shaped, three-dimensional compact colony morphology (Figure 2), dependence on leukemia inhibitory factor (LIF) signals for self-renewal, and the activation of both X chromosomes in females (XaXa). Compared with the primed state, naïve pluripotent ESCs are capable of single-cell colonization, a highly valued property for gene targeting and genetic screening (Smith et al., 1988; Niwa et al., 1998). In addition, naïve ESCs exhibit greater differentiation capacity in vivo, especially for chimera formation when injected into early embryos (Brons et al., 2007; Tesar et al., 2007; Bao et al., 2009; Najm et al., 2011). While the differentiation capacities of both primed and naïve-state PSCs are restricted to all cell types of the embryonic origin, recently, another type of PSCs, called extended PSCs (EPSCs), derived from mice and humans was found to exhibit additional developmental bipotential, to give rise to cells of not only embryonic but also extraembryonic origins including the trophectoderm cells (Yang et al., 2017a, 2017b).
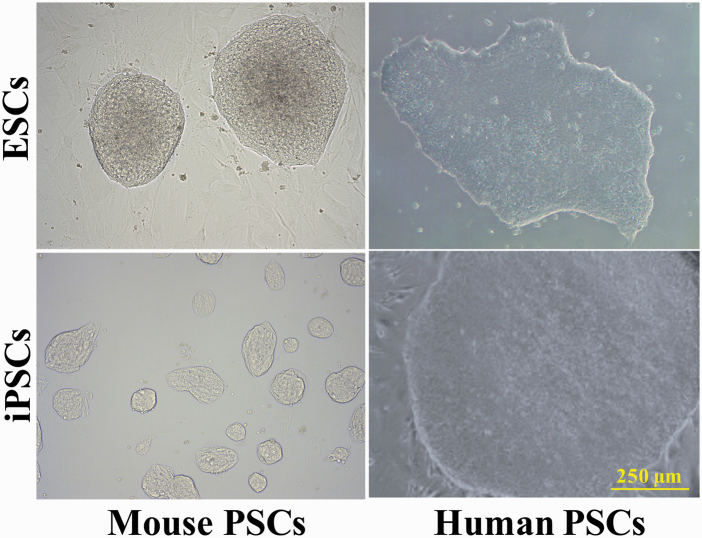
Figure 2.
Morphology differences between mouse and human PSC colonies. The typical colony morphologies of mouse and human PSCs in culture, including both ESCs and iPSCs. Note the dome-shaped, three-dimensional, multilayered morphology of mouse PSC colonies vs. the flat, two-dimensional, and monolayered human PSC colonies. Scale bar = 250 µm.
The generation of iPSCs from agricultural animal species holds great potential for applications ranging from improving farm animal reproduction, animal welfare, and disease resistance, to meat and dairy product quality, as well as for a basic understanding of the mammalian embryonic development and differentiation. These areas form the critical knowledge and technological basis for establishing a sustainable agriculture system to accommodate the need of the increasing global population. However, compared with the thriving advancement in rodent and human iPSC studies, it has been challenging to generate bona fide iPSCs from farm animals. The reported farm animal iPSCs exhibit major issues, for example, limited cell propagation, reliance on the expression of exogenous factors for self-renewal, restricted differentiation capacity, low reprogramming efficiency, the production of trophectoderm-like cells, and limited in vivo developmental potential.
Porcine iPSCs
Pigs are regarded as a perfect model for bridging the gap of preclinical experiments in humans because of the similarity in size and physiology between them (Brevini et al., 2007). Thus, the investigation of porcine iPSC (piPSCs) not only has the potential to improve animal production but also benefits the development of therapeutics for many human diseases. The earliest studies on the generation of piPSCs were reported in 2009 (Esteban et al., 2009; Ezashi et al., 2009; Wu et al., 2009). These cells were reprogrammed from fetal or postnatal fibroblasts and bone marrow cells using a human or mouse version of OSKM or the six-factor (OCT4, SOX2, KLF4, c-MYC, NANOG, and LIN28 [OSKMNL]) cocktail. They resembled human PSC colony morphology in general and were capable of forming embryoid bodies (EBs) in vitro (Ezashi et al., 2009; Wu et al., 2009) and teratomas in vivo (Esteban et al., 2009; Ezashi et al., 2009; Wu et al., 2009). Importantly, the piPSCs showed continued passaging capacity ranging from 25 to 48 times without losing their PSC-like colony morphology. However, two out of the three studies reported continued transgene expression in the piPSCs (Esteban et al., 2009; Ezashi et al., 2009). The third study found spontaneous piPSC differentiation upon withdrawing the Doxycycline (Dox)-induced transgene expression (Wu et al., 2009). The dependence of Dox-induced transgene expression was also found in a later study (Zhang et al., 2014). The reported efficiency of piPSC colony generation ranges from 0.3% to 3.8% of the starting cells, as estimated by the early-stage reprogramming marker—alkaline phosphatase (AP) staining (Ruan et al., 2011; Cheng et al., 2012; Ma et al., 2014; Zhang et al., 2014, 2015a), and is comparable to the mouse and human iPSC generation.
While many groups reported the generation of piPSC colonies with human ESC-like morphology (Esteban et al., 2009; Ezashi et al., 2009; Wu et al., 2009; Montserrat et al., 2011, 2012; Ruan et al., 2011; Kues et al., 2013; Zhang et al., 2015b; Li et al., 2018), efforts were also taken to establish naïve-state characteristics similar to mouse ESCs (Liu et al., 2012b; Rodriguez et al., 2012; Fujishiro et al., 2013; Kwon et al., 2013, 2016; Gu et al., 2014; Zhang et al., 2014, 2015b; Du et al., 2015; Chakritbudsabong et al., 2017; Mao et al., 2017; Xu et al., 2019) (Table 1). Most of the studies were able to achieve single-cell colonization following enzyme treatment (Liu et al., 2012b; Fujishiro et al., 2013; Kwon et al., 2013; Gu et al., 2014; Zhang et al., 2014, 2015b; Du et al., 2015; Chakritbudsabong et al., 2017; Mao et al., 2017; Xu et al., 2019), a key feature of naïve pluripotency. These piPSCs were derived mainly from the media adopting “2i” condition with inhibitors for extracellular signal-regulated kinases 1/2 and glycogen synthase kinase 3 (Ying et al., 2008) for the establishment of the ground-state pluripotent mouse PSCs, with the addition of LIF and/or other chemicals to inhibit histone methylation/deacetylation, transforming growth factor-beta (TGF-ß), or FGF signals. These cells formed dome-shaped colony morphology with characteristics of mouse PSCs and their naïve-like features prompted the study of piPSC chimeric capacity. Several groups reported that early porcine embryos injected with these piPSCs could develop into blastocyst stage in vitro and the injected piPSCs contributed efficiently to both ICM and trophectoderm (Cheng et al., 2012; Fujishiro et al., 2013; Du et al., 2015; Zhang et al., 2015b; Fukuda et al., 2017; Li et al., 2018; Xu et al., 2019).
Table 1.
Summary of piPSC reprogramming conditions
| Publications | Starting cell types | Induction method | Reprogramming factors (h, human; m, mouse; p, porcine) | Basal media | Ligands/chemicals |
|---|---|---|---|---|---|
| Ezashi et al. (2009) | Fetal fibroblasts | Lentiviral transduction | hOCT4, hSOX2, hKLF4, and hc-MYC | Knockout DMEM + 20% KSR | bFGF |
| Esteban et al. (2009) | Embryonic fibroblasts | Retroviral transduction | hOCT4, hSOX2, hKLF4, hc-MYC, and mOCT4, mSOX2, mKLF4, and mc-MYC | High-glucose DMEM + 10% FBS | bFGF2 |
| Wu et al. (2009) | Primary ear fibroblasts | DOX-induced lentiviral transduction | hOCT4, hSOX2, hKLF4, hc-MYC, hNANOG, and hLIN28 | High-glucose DMEM + 15% FBS | LIF, DOX |
| Primary bone marrow cells | DMEM/F12 + 20% KSR | ||||
| West et al. (2010) | Mesenchymal cells | Lentiviral transduction | hOCT4, hSOX2, hKLF4, hc-MYC, hNANOG, and hLIN28 | DMEM/F12 + 20% KSR or mTeSR1 medium | bFGF |
| Telugu et al. (2010) | Fetal fibroblasts | Retroviral transduction | hOCT4, hSOX2, hKLF4, and hc-MYC | DMEM/F12 + 20% KSR | PD0325901, CHIR99021, LIF; VPA |
| Montserrat et al. (2011) | Ear fibroblast | Retroviral transduction | hOCT4, hSOX2, hKLF4, and hc-MYC | Knockout DMEM + 20% KSR and high-glucose DMEM + 15% FBS media (1:1) | bFGF, LIF |
| Ruan et al. (2011) | Embryonic fibroblasts | Retroviral transduction | hOCT4, hSOX2, hKLF4, and hc-MYC | high-glucose DMEM + 15% FBS media or DMEM/F-12 + 20% KSR | LIF, bFGF |
| Hall et al. (2012) | Embryonic fibroblasts | DOX-induced lentiviral transduction | hOCT4, hNANOG, hSOX2, hKLF4, and hc-MYC | Knockout DMEM + 15% KSR | bFGF, DOX |
| Rodriguez et al. (2012) | Fetal fibroblasts | DOX-induced lentiviral transduction | hOCT4, hSOX2, hKLF4, and hc-MYC | From DMEM + 10% FBS to N2B27 medium | LIF, DOX, BSA, PD0325901, CHIR99021, PD173074 |
| Montserrat et al. (2012) | Adult fibroblast | Retroviral transduction | mSOX2, mKLF4, and mc-MYC | Knockout DMEM + 20% KSR + 20% FBS and high-glucose DMEM + 15% FBS media (1:1) | bFGF, LIF |
| Cheng et al. (2012) | Embryonic fibroblasts | Retroviral transduction | mOCT4, mSOX2, mKLF4, and mc-MYC | Knockout DMEM + 20% FBS | LIF, bFGF, VPA |
| Liu et al. (2012b) | Mesenchymal cells | Retroviral transduction | pOCT4 and pKLF4 | DMEM/F12 + 20% KSR or FBS | LIF, PD0325901, CHIR99021, Forskolin, SB431542, sodium butyrate |
| Kues et al. (2013) | Fetal fibroblasts | Sleeping beauty transposon | mOCT4, mSOX2, mKLF4, and mc-MYC | DMEM/F12 + 20% KSR | bFGF |
| Yang et al. (2013) | Dermal fibroblasts | Lentiviral transduction | hOCT4, hSOX2, hKLF4, hc-MYC, hNANOG, and hLIN28 | DMEM/F12 + 20% KSR or mTeSR1 medium | bFGF |
| Fujishiro et al. (2013) | Embryonic fibroblasts | Retroviral transduction | hOCT4, hSOX2, hKLF4, and hc-MYC | DMEM + 15% FBS or DMEM + 20% KSR | Forskolin, LIF |
| Kwon et al. (2013) | Ear fibroblasts | Lentiviral transduction | hOCT4, hSOX2, hKLF4, hc-MYC, hNANOG, and hLIN28 | DMEM/F12 + 10% KSR + 10% FBS | LIF, bFGF |
| Ma et al. (2014) | Fetal fibroblasts | Retroviral transduction | mOCT4, mSOX2, mKLF4, miR-302a, miR-302b, and miR-200c | Knockout DMEM + 20% KSR | LIF |
| Park et al. (2013) | Embryonic fibroblasts | Episomal plasmid | mOCT4, mSOX2, mKLF4, and mc-MYC | 50:50 mixture of low glucose DMEM and Ham’s F10 medium + 15% FBS | SCF, bFGF |
| Gu et al. (2014) | Embryonic fibroblasts | Retroviral transduction | mOCT4, mSOX2, mKLF4, and mc-MYC | Knockout DMEM + 20% KSR | bFGF, PD0325901, CHIR99021, SB431542, VPA |
| Zhang et al. (2014) | Adipose-derived stem cells | DOX-induced lentiviral transduction | hOCT4, hSOX2, hKLF4, and hc-MYC | DMEM/F12 + 20% KSR | DOX, N2, B27, PD0325901, CHIR99021, LIF |
| Zhang et al. (2015a) | Embryonic fibroblasts | Retroviral transduction | mOCT4, mSOX2, mKLF4, and mc-MYC | DMEM + 15% FBS | LIF, bFGF, BMP4, CHIR99021, SB431542 |
| Zhang et al. (2015b) | Embryonic fibroblasts | Retroviral transduction | pOCT4, pSOX2, pKLF4, and pMYC | DMEM/F12 + 20% KSR or DMEM + 15% FBS | bFGF or N2, B27, LIF, CHIR99021, PD0325901 |
| Du et al. (2015) | Fetal fibroblasts | Episomal plasmid | hOCT4, hSOX2, hKLF4, hc-MYC, hNANOG, hLIN28, hNR5A2, and miR302/367 cluster | DMEM/F12 + neurobasal medium (1:1) + 0.5% N2 + 1% B27 | LIF, CHIR99021, PD0325901 |
| Fukuda et al. (2017) | Embryonic fibroblasts | Lentiviral transduction | mOCT4, mSOX2, mKLF4, mc-MYC, mNANOG, and mLIN28 | KSR media | LIF, PD0325901, CHIR99021, thiazovivin, GF109203X(GFx) |
| Mao et al. (2017) | Embryonic fibroblasts | Retroviral transduction | mOCT4, mSOX2, mKLF4, mc-MYC, mTet1/3, and mKdm3a | Knockout DMEM + 10% KSR + 10% FBS | Sodium butyrate |
| Kwon et al. (2016) | Embryonic fibroblasts | Lentiviral transduction | hOCT4, hSOX2, hKLF4, hc-MYC, hNANOG, and hLIN28 | DMEM/F12 + 10% KSR + 10% FBS | bFGF, LIF |
| Chakritbudsabong et al. (2017) | Embryonic fibroblasts | Retroviral transduction | hOCT4, hSOX2, hKLF4, hc-MYC, and hLIN28 | piPSC media | |
| Li et al. (2018) | Fetal fibroblasts | Episomal plasmid | hOCT4, hSOX2, hKLF4, hc-MYC, hLIN28, and shRNA against p53 | DMEM/F12 + 20% KSR | bFGF, PD0325901, CHIR99021 |
| Xu et al. (2019) | Embryonic fibroblasts or Meninges microvascular pericyte cells | Retroviral transduction | pOCT4, pSOX2, pKLF4, and pMYC | Modified LCDM medium (50% neurobasal medium, 50% DMEM/F12 + 5% KSR) or 50% mTeSR1 + 50% modified LCDM medium | N2, B27, LIF, CHIR99021, (S)-(+)-dimethindene maleate, minocycline hydrochloride, Vitamin C |
| Setthawong et al. (2019) | Sertoli cells | Retroviral transduction | hOCT4, hSOX2, hKLF4, and hc-MYC | DMEM/F12 + 10% KSR + 10% FBS | bFGF, LIF |
However, the postimplantation development of these chimeric embryos varied substantially between studies. One study (Fujishiro et al., 2013) reported significantly decreased piPSC incorporation along with the porcine fetal development (up to day 65). Others, including a study with extensive in vivo investigation, reported that no chimeric embryos yielded live births (Du et al., 2015; Zhang et al., 2015b). Using the culture condition for EPSCs (Yang et al., 2017b; Gao et al., 2019), the generation of EPSC-like piPSCs that contributed to both embryonic and extraembryonic tissues in mouse and pig blastocysts and postimplantation conceptuses was reported, although the percentage of piPSC incorporation was very low (Xu et al., 2019). To date, only a single report details the production of live chimeric piglets of which 69% had detectable signals in ears and tails (West et al., 2010), and with germline transmission of the donor nuclei in young pigs (West et al., 2011).
Retroviral vectors are transcriptionally silent in human and mouse PSCs, which is evidence for fully reprogrammed iPSCs (Maherali et al., 2007; Okita et al., 2007; Wernig et al., 2007). A number of studies tried to overcome the issues of viral genome integration and the transgene dependency for piPSC self-renewal, by testing a variety of starting somatic cell types, expressing vectors, and additional reprogramming factors (Esteban et al., 2009; Ezashi et al., 2009; Wu et al., 2009; West et al., 2010, 2011; Montserrat et al., 2011, 2012; Ruan et al., 2011; Cheng et al., 2012; Hall et al., 2012; Liu et al., 2012b, 2014; Rodriguez et al., 2012; Fujishiro et al., 2013; Kues et al., 2013; Kwon et al., 2013, 2016; Park et al., 2013; Yang et al., 2013; Gu et al., 2014; Ma et al., 2014; Zhang et al., 2014, 2015a, 2015b; Du et al., 2015; Chakritbudsabong et al., 2017; Fukuda et al., 2017; Mao et al., 2017; Li et al., 2018; Setthawong et al., 2019; Xu et al., 2019) (Table 1). However, only a single study reported silenced retroviral transgene expression (Zhang et al., 2015a). The non-integrative episomal plasmid vector was also used to deliver reprogramming factors into somatic cells (Park et al., 2013; Du et al., 2015; Li et al., 2018). However, two of the studies found genome integration of the plasmid DNA into piPSC genome (Park et al., 2013; Du et al., 2015). The third study also reported persistent exogenous gene expression in most of the piPSC lines, with only one line without detectable plasmid expression after extensive passaging and subcloning (Li et al., 2018). The pluripotent gene DNA methylation status was also evaluated. The promoter DNA regions of OCT4 and NANOG were partially or highly methylated in some piPSCs (Fujishiro et al., 2013; Gu et al., 2014; Du et al., 2015), while several others reported highly demethylated OCT4 promoter in piPSCs (Wu et al., 2009; Cheng et al., 2012; Ma et al., 2014), with unmethylated Nanog promoter reported in one study (Zhang et al., 2014).
Nuclear transfer (NT) technology was also used to test the ability of piPSCs in animal cloning. No obvious difference was found between the use of initial somatic cells and piPSCs as donors for the successful generation of NT embryos (Cheng et al., 2012; Gu et al., 2014; Zhang et al., 2014). However, NT embryos using piPSCs as the donor either failed to develop to term or produce any live piglets, in contrast to the NT embryos derived from fibroblasts as the nuclear donor (Du et al., 2015). One possible cause of this low success rate for NT embryo development is likely the persistent transgene expression in these piPSCs. This is supported by a study where several cloned piglets developed to term when differentiated piPSCs harboring silenced transgenes were used as the nuclei donor, although none survived postnatally (Fan et al., 2013).
Overall, although with similar issues to the other livestock iPSCs, piPSCs are less problematic to establish and propagate. The reported piPSCs could be generated and stably propagated for a long period while maintaining normal karyotype for the majority of the cell population. However, piPSCs still have the major drawback of an inability to fully replace the exogenous reprogramming factors by the endogenous pluripotent network for continued self-renewal. The infrequently observed incomplete demethylation of the pluripotent genomic loci also indicates an epigenome not yet fully converted to pluripotent state in these cells.
Bovine iPSCs
Bona fide bovine iPSCs (biPSCs) are expected to have great potential for agriculture and biotechnology applications. These pluripotent, renewable cell resources will enable a better understanding of the embryonic differentiation in ruminants, and facilitate the generation of genetically superior cattle for improved products and disease resistance, through techniques including in vitro genetic selection, gametes differentiation, and in vitro fertilization in laboratory settings (Bogliotti et al., 2018; Yuan, 2018). The generation of biPSCs could also promote veterinary and stem cell-based preclinical therapeutic development in bovine disease models, such as citrullinemia and leukocyte adhesion deficiency (LAD; Kehrli et al., 1992; Casal and Haskins, 2006). The former is a rare genetic cattle disorder leading to early postnatal death (Dennis et al., 1989; Healy et al., 1990), and cattle with the latter usually die within 1 yr of age (Shuster et al., 1992; Nagahata, 2004; Meydan et al., 2010). Although controllable with genetic screening before the artificial insemination, the carrier prevalence of these diseases is still noticeable global wise (Nagahata, 2004; Meydan et al., 2010; Li et al., 2011a; Patel and Patel, 2014). In humans, both diseases are life-threatening genetic disorders (Quinonez and Thoene, 2004; Elhasid and Rowe, 2010).
Efforts for establishing biPSCs have been reported since 2011. A number of groups reprogrammed bovine fetal or adult cells using OSKM or OSKM plus NANOG and/or LIN28 (OSKMN/L), or plus large T antigen factors expressed in either retroviral, lentiviral, or PiggyBac vectors (Han et al., 2011; Sumer et al., 2011; Cao et al., 2012; Deng et al., 2012; Kawaguchi et al., 2015; Talluri et al., 2015; Malaver-Ortega et al., 2016). These bovine iPSCs generally resemble primed-pluripotent human iPSCs in morphology, with a low induction efficiency (0.01% to 0.77%) based on AP staining (Sumer et al., 2011; Cao et al., 2012; Deng et al., 2012). Further study of these cells identified some pluripotent characters, including activation of endogenous pluripotent genes, EB differentiation in vitro, and in vivo teratoma formation upon injection into immunodeficient mice. However, only a few studies reported bovine iPSCs capable of continued propagation beyond 20 passages (Cao et al., 2012; Kawaguchi et al., 2015; Talluri et al., 2015). Also, except for one study (Deng et al., 2012), nearly all other groups reported continued expression of exogenous transgenes in the bovine iPSCs, which appeared to be needed for the self-renewal of these cells. In addition to primed pluripotency-like bovine iPSCs, naïve-like biPSCs were also reportedly generated from bovine fibroblasts, testicular cells, or amnion cells using medium conditions similar to those for naïve-state mouse PSC derivation (Wang et al., 2013; Lin et al., 2014; Heo et al., 2015; Kawaguchi et al., 2015; Zhao et al., 2017). These biPSCs resemble mouse PSCs in morphology and showed the capacity of single-cell colonization. However, most of these biPSCs exhibited limited activation of endogenous OCT4, SOX2, and NANOG (Wang et al., 2013; Lin et al., 2014; Heo et al., 2015; Zhao et al., 2017). Also, others reported the dependence of biPSCs on continued transgene expression for self-renewal, with a failure of teratoma formation upon injecting in nude mice (Kawaguchi et al., 2015).
Another issue during the bovine cell reprogramming process is the concomitant generation of trophectoderm-like cells, which also express some pluripotent genes including OCT4. Using the same reprogramming conditions and starting somatic cells reported to generate biPSCs, bovine trophectoderm cells were derived (Kawaguchi et al., 2015, 2016). An expanded six-factor or eight-factor (OSKMNL plus large T antigen and telomerase reverse transcriptase) reprogramming cocktail resulted in the exclusive induction of trophectoderm cell colonies with no biPSCs (Talbot et al., 2017). Thus, conditions for human/mouse iPSC derivation tend to be more compatible with the induction of porcine iPSCs than biPSCs. For instance, microRNA mir302/367 cluster (Du et al., 2015) or mir302a/b and mir200 (Ma et al., 2014) were found to significantly enhance the generation of piPSC colonies, but with no apparent effect for bovine cell reprogramming (Pillai et al., 2019). In addition, bovine somatic cells appear more susceptible to reprogramming with bovine-specific factors (Zhao et al., 2017; Pillai et al., 2019), by comparison to piPSCs that can be stably generated using reprogramming factors of either human or mouse origin.
Although the biPSCs derived in all the studies acquired obviously a certain level of pluripotency, their reprogramming status is incomplete. The problems of persistent transgene activity in established biPSCs (Canizo et al., 2018), and the limited propagation capacity of the biPSCs (Pillai et al., 2019), provided evidence that the majority of reported biPSCs were partially reprogrammed cells or trophectoderm-lineage stem cells with features similar to those reported in pigs (Ezashi et al., 2011). The yet unidentified reprogramming hurdles that exist within bovine somatic cells and/or during the reprogramming process appear to prevent the complete conversion of somatic bovine cell nuclei into either the primed or naïve-pluripotent state. Overall, the establishment of completely reprogrammed, chimerism-compatible biPSCs with prolonged self-renewal capacity and silenced transgenes still represents a major challenge in the field that warrants further attention.
Ovine and Caprine iPSCs
Ovine and caprine are both important small ruminants for agricultural production and biomedical modeling. In 2011, two studies reported the development of iPSCs from sheep (siPSCs) by reprogramming ovine fetal fibroblasts using Dox-inducible lentiviral vector system (Bao et al., 2011; Li et al., 2011b). While one group (Li et al., 2011b) found that exogenous OSKM factors were sufficient to generate primed PSC-like AP and the PSC surface marker stage-specific embryonic antigen 4 (SSEA-4)-positive siPSCs capable of maintenance in bFGF and fetal bovine serum (FBS)-based medium, a second group (Bao et al., 2011) reported that OSKM or OSKMNL-induced siPSCs were unstable. An eight-factor cocktail and knockout serum replacement (KSR)-based medium were used to generate expandable naïve PSC-like siPSCs, which were positive for AP and PSC surface marker SSEA1, TRA-1-60, and TRA-1-81 (Bao et al., 2011). Both types of siPSCs showed the activation of some key endogenous pluripotent genes and formed EBs in vitro and teratomas in vivo with three-germ layer development. These cells were capable of being propagated for more than 20 times. However, the maintenance of these siPSCs was dependent upon continuous transgene expression, as removing of Dox from culture media caused cell differentiation (Bao et al., 2011; Li et al., 2011b).
Other studies (Liu et al., 2012a; Sartori et al., 2012) reported the generation of siPSCs from ovine embryo fibroblasts using retroviral vector-based OSKM expression. Naïve PSC-like siPSCs formed when cultured in LIF and FBS-based medium (Liu et al., 2012a), while a primed PSC-like siPSC was created following culture in bFGF and KSR-based medium (Sartori et al., 2012). The siPSCs in both studies showed AP-positive staining and were capable of forming EBs in vitro and teratoma formations in vivo. Interestingly, the stem cell surface markers were not present in siPSCs in this study, but these cells were able to form chimeric ICMs in 2N and 4N blastocysts upon injection into early ovine embryos (Liu et al., 2012a). Another study took the chimeric siPSC ovine embryos to term to produce 17 live lambs that demonstrated very little siPSC integration into the host tissue (less than 0.1%) (Sartori et al., 2012).
A later study (German et al., 2015) explored the potential of using siPSCs as a nuclear donor for animal cloning. They found that the preimplantation development rate of ovine NT embryos injected with siPSCs was significantly lower than those using the ovine fibroblasts as a donor. The reason for the poor development rate of NT embryos might stem from the continued transgene expression, an absence of endogenous NANOG in the siPSCs, or both (German et al., 2015). Only two studies reported the silencing of all transgenes in the induced siPSCs (Liu et al., 2012a; Shi et al., 2015) but so far with no follow-up evaluation of the development potential for the NT embryos.
The establishment of naïve PSC-like goat iPSCs (giPSCs) was initially reported in 2011 from primary goat ear fibroblasts using the Dox-inducible eight-factor cocktail (Ren et al., 2011). Similar giPSCs were also induced later from goat fetal fibroblasts using Dox-inducible mouse OSKM (Tai et al., 2015). These giPSCs resembled mouse PSCs in colony morphology, stained positive for AP, SSEA1, TRA-1-60, and TRA-1-81, and were capable of colonization after trypsinization for at least 15 to 30 passages (Ren et al., 2011; Tai et al., 2015). However, although these giPSCs showed the capacity of EB and teratoma differentiation, they still were dependent on exogenous factors for self-renewal, and removal of Dox resulted in their differentiation. In addition, microarray analysis revealed that compared with the goat ESCs, many pluripotent genes were still underexpressed in the siPSCs (Tai et al., 2015).
Primed PSC-like giPSCs were also reportedly reprogrammed from fetal ear fibroblasts with lentiviral expression of human OSKM and cultured in bFGF and KSR-based reprogramming medium (Song et al., 2013). These cells were found to need mechanical dissociation early (before passage 5) and thereafter could be passaged by partial trypsinization. Although endogenous NANOG was under-stimulated, these giPSCs still showed EB and teratoma differentiation capacities. Interestingly, the viral transgenes were silenced at passage 15 (Song et al., 2013). A follow-up study by the same group reported no significant difference for the fusion and cleavage rates between NT embryos using these giPSCs or goat fibroblasts as donors (Song et al., 2016). Using the bovine version of lentiviral vector expressing OSKM factors and the mir302/367 cluster, primed or naïve-like, AP-positive giPSCs could be induced in the KSR-based medium supplemented with 2i/LIF or bFGF (Sandmaier et al., 2015; Tai et al., 2015). These cells formed teratoma in vivo and differentiated to extra-embryonic endoderm and neuronal cells under directed differentiation in vitro. However, none of these giPSCs silenced their transgene expression (Sandmaier et al., 2015).
Epigenetic modifications were tested to improve the reprogramming efficiency of ovine and caprine iPSCs. The efficiency of siPSCs induction varied from 0.002% to 0.5% (Bao et al., 2011; Liu et al., 2012a). The additional p53 knockdown and exogenous expression of the conserved histone 3 and histone 4 chaperone—anti-silencing function 1 (ASF1) further increased reprogramming efficiency to 1% (Shi et al., 2015). The addition of valproic acid (VPA) and vitamin C (Tai et al., 2015) or the expression of the protein arginine methyltransferases (PRMT5) (Chu et al., 2015) was found to improve the reprogramming efficiency of giPSCs. Notably, giPSCs were also reprogrammed by OSKM messenger RNAs transfection of goat fetal fibroblasts, with a calculated reprogramming efficiency of nearly 1% (Chen et al., 2017). Overall, the trends for ovine and caprine iPSC generation resembled those in porcine iPSC generation, with the issue of persistent transgene expression still observed in most of the established iPSC lines. This likely represents a major hurdle that limits their potentials in NT and chimeric embryo development.
Equine iPSCs
As companion and sporting animals, horses face many musculoskeletal problems (Smith et al., 2014). Therefore, equine iPSCs (eiPSCs) are considered to be a promising therapeutic tool for musculoskeletal injuries both for horses and as preclinical study for human diseases. Different from the cellular reprogramming of other farm animals, all reported eiPSCs were generated by mouse or human OSKM expression with no additional exogenous factors (Nagy et al., 2011; Khodadadi et al., 2012; Breton et al., 2013; Sharma et al., 2014; Whitworth et al., 2014; Lee et al., 2016; Quattrocelli et al., 2016; Moro et al., 2018; Pessôa et al., 2019), and in one study without c-Myc (Khodadadi et al., 2012). The culture media for these eiPSCs were also standard KSR or FBS-based conditions with the addition of LIF and/or bFGF as described for mouse or human PSCs. Only two studies applied additional chemicals for eiPSC induction including 2i, A83-01 (TGFß inhibitor), Thiazovivin (Rho-associated protein kinase [ROCK] inhibitor), SB431542 (anaplastic lymphoma kinase receptor inhibitor) (Nagy et al., 2011) and LY294002 (PI3K/AKT inhibitor; Whitworth et al., 2014; Table 2). Various fetal, foal, and adult horse fibroblasts were used in reprogramming (Nagy et al., 2011; Khodadadi et al., 2012; Breton et al., 2013; Whitworth et al., 2014; Moro et al., 2018; Pessôa et al., 2019), and the eiPSC generation efficiency ranged from 0.028% to 0.18% of the starting cells (Nagy et al., 2011; Sharma et al., 2014; Pessôa et al., 2019). One study found that compared with fibroblast and mesenchymal stem cells (MSCs), adipose-derived stem cells had the greatest reprogramming efficiency, while equine bone marrow cells failed to be reprogrammed (Pessôa et al., 2019). Data from this study also indicate that the human version of OSKM factors seemed to be more efficient for equine cell reprogramming compared with those of the mouse origin (Pessôa et al., 2019). Also, there was no reported issue for the self-renewal of eiPSCs. Most of the generated eiPSC lines could be passaged over 25 times (Nagy et al., 2011; Khodadadi et al., 2012; Breton et al., 2013; Whitworth et al., 2014; Pessôa et al., 2019) and displayed normal chromosome numbers (Nagy et al., 2011; Khodadadi et al., 2012; Breton et al., 2013; Whitworth et al., 2014; Quattrocelli et al., 2016).
Table 2.
Summary of eiPSC reprogramming media
| Publications | Basal media | Ligands/chemicals |
|---|---|---|
| Nagy et al. (2011) | High-glucose DMEM + 15% FBS | LIF, bFGF, DOX, CHIR99021, PD0325901, A83-01, Thiazovivin, SB431542 |
| Khodadadi et al. (2012) | Knockout DMEM + 20% KSR | bFGF, LIF |
| Breton et al. (2013) | α-MEM medium + 20% FBS | LIF, bFGF, EGF |
| Whitworth et al. (2014) | Knockout DMEM + 15% ES-FCS | LIF, bFGF, LY294002 |
| Sharma et al. (2014) | Equine fetal fibroblast-conditioned knockout DMEM + 20% KSR) | LIF |
| Quattrocelli et al. (2016) | DMEM/F12 + 20% KSR | bFGF |
| Lee et al. (2016) | High-glucose DMEM + 20% FBS | DOX |
| Moro et al. (2018) | DMEM/F12 + 20% KSR | bFGF, LIF |
| Pessôa et al. (2019) | DMEM/F-12 + 10% KSR | bFGF |
All reported eiPSCs were stained positive for AP, while some studies showed that these cells expressed all (Bavin et al., 2015) or part of the pluripotent surface markers—SSEA1/3/4 and TRA-1-60/81 (Nagy et al., 2011; Khodadadi et al., 2012; Breton et al., 2013; Sharma et al., 2014; Whitworth et al., 2014; Lee et al., 2016). These eiPSCs had activated endogenous pluripotent gene expression, and many of them showed the formation of teratoma with three-germ layer development in vivo (Nagy et al., 2011; Khodadadi et al., 2012; Breton et al., 2013; Sharma et al., 2014; Whitworth et al., 2014; Lee et al., 2016; Quattrocelli et al., 2016). One study also reported that eiPSCs had complete and partial demethylation of NANOG and SOX2 promoters, respectively, which were reversely correlated with the expression of these genes (Hackett et al., 2012). The success of in vitro EB differentiation of eiPSCs is variable and ranges from EBs with tissue derivatives of the three-germ layers in several studies, the only ectoderm commitment in derived EBs (Pessôa et al., 2019), to failure of EB formation (Nagy et al., 2011). Horse NT embryos using eiPSCs as the nuclear donor also failed to develop into blastocysts, in contrast with those using horse fibroblasts as the donor with the development into live foals, even though both types of NT embryos shared similar cleavage rates (Moro et al., 2018). Another major issue similar to the other farm animal iPSCs is the transgene silencing. Only one study reported full silence of all exogenous factors in their eiPSCs (Quattrocelli et al., 2016). Other studies showed persistent expression of all (Khodadadi et al., 2012) or part of the OSKM factors (Breton et al., 2013; Sharma et al., 2014; Whitworth et al., 2014) in eiPSCs.
Primed-state PSCs tend to retain epigenetic memory of their parental cells that favors the differentiation back to the original lineages (Bar-Nur et al., 2011). This was perfectly reflected in the study where two types of initial cells, mesoangioblasts (MABs) and MSCs, were used to generate eiPSC lines cultured in bFGF and KSR-based medium (Quattrocelli et al., 2016). Both eiPSC lines were able to form teratomas in vivo. However, the authors found that the MAB-iPSC-derived teratomas exhibited a significantly greater quantity of immature muscle patches, and the MSC-iPSC-derived teratomas showed significantly larger chondrogenic patches. The in vitro direct myogenic and chondrogenic differentiation assays using these two types of eiPSCs also exhibited similar differentiation bias (Quattrocelli et al., 2016). Naïve pluripotency was reported to resolve problems such as the epigenetic memory and lineage differentiation bias associated with primed human iPSCs (Buecker et al., 2010; Hanna et al., 2010; Gafni et al., 2013). Therefore, it remains interesting to examine if the bias in differentiation would disappear upon converting these eiPSCs into naïve state.
An exciting question regarding eiPSCs is their therapeutic potential in horse diseases, especially their ability to differentiate into tendons and cartilage tissues to treat injuries. Using previously derived eiPSCs, one group successfully generated functional myocytes with electrophysiological response (Amilon et al., 2018). Another group conducted directed neuronal differentiation of eiPSCs and derived electrically active cells with motor neuron-like properties (Sharma et al., 2014). One study found that the eiPSCs could be efficiently differentiated to keratinocytes, with either superior or compatible proliferation and migration capacities compared with primary horse keratinocytes (Aguiar et al., 2016). However, unlike their equine ESC counterparts, eiPSCs failed to generate functional tendon cells and artificial tendon in 3D differentiation assay in vitro (Bavin et al., 2015). For in vivo study, one group found that intradermal injection of allogenic eiPSCs into horses showed only moderate host response without acute rejection, indicating the feasibility of using allogenic eiPSC as therapeutics for injury (Aguiar et al., 2015). In one study, injection of eiPSCs into injured muscles of Rag/mdx mice enhanced muscle regeneration with detected myofibers derived from the eiPSCs (Lee et al., 2016). The same group later reported the differentiation of the eiPSCs to MSCs, which could further differentiate into osteogenic, chondrogenic, and myogenic cells in vitro (Chung et al., 2019). Of note, after inhibiting the major histocompatibility complex class I expression, the derived MSCs were transplanted into lesions of injured horses with positive outcomes indicating alleviated injury (Chung et al., 2019).
Overall, the eiPSCs could be reprogrammed from a variety of horse somatic cells with similar efficiency as the human iPSC induction. These cells demonstrated direct differentiation capacity desired for musculoskeletal injury repair and showed encouraging therapeutic effects in horse injury disease models. However, similar to the other farm animal iPSCs, the transgene silencing still represents a problem for the derived eiPSCs that may limit their further success in therapeutic applications.
Avian iPSCs
Avian embryos including the quail-chick chimera model have been widely used for real-time monitoring of organ and tissue development and functions, developmental patterning, and cell fate determination (Le Douarin, 1984, 2008; Kulesa et al., 2010). Generation of the iPSCs of avian species is expected to facilitate the study of non-mammal vertebrate embryonic development. Quail iPSCs (qiPSCs) were reprogrammed from embryonic fibroblasts with human or mouse version of OSKM factors in two studies (Lu et al., 2012; Rosselló et al., 2013). These cells had compact colony morphology, were positive for AP staining, and could be passaged for 20 to 50 times. These cells could be successfully developed into EBs and neuronal cells in vitro (Lu et al., 2012) or teratomas in vivo (Rosselló et al., 2013). The chimera formation capacity of qiPSCs was also tested upon injection into stage X chicken embryos (Lu et al., 2012). Three-germ layer incorporation and feather chimerism were identified in later stage chick embryos, and two chicks developed to term showed qiPSC differentiation into several tissues, including brain, liver, and gonad, although with no feather chimerism. Also, both studies reported qiPSCs with continued transgene expression (Le Douarin, 1984, 2008; Kulesa et al., 2010).
Chicken iPSCs (ciPSCs) were derived in a number of studies or in another study—the primordial germ cells (PGCs; Lu et al., 2014) from embryonic fibroblasts (Lu et al., 2012, 2014; Rosselló et al., 2013; Dai et al., 2014; Yu et al., 2014; Fuet et al., 2018), feather follicle cells (Kim et al., 2017), or muscle fibroblasts (Katayama et al., 2018) using OSKM factors with or without NANOG and/or LIN28. These cells expressed highly stimulated chicken orthologues of OCT4 (POU5F3) and except for one study (Fuet et al., 2018), chicken SOX2, and NANOG as well. Also, except for the ciPSCs derived from feather follicle cells (Kim et al., 2017), which failed to be passaged beyond 10 times, all other studies reported the ciPSCs to sustain at least 20 to 50 passages (Rosselló et al., 2013; Dai et al., 2014; Yu et al., 2014; Fuet et al., 2018; Katayama et al., 2018). Most of the reported ciPSCs showed the ability to form EBs in vitro (Dai et al., 2014; Yu et al., 2014; Kim et al., 2017; Fuet et al., 2018; Katayama et al., 2018) as well as the direct differentiation into neuronal lineage (Dai et al., 2014), although one study only showed the detection of ectoderm and mesoderm markers in EBs (Fuet et al., 2018). Three studies described the formation of teratomas with three-germ layer detection (Rosselló et al., 2013; Kim et al., 2017; Katayama et al., 2018). Also, although both integrative and non-integrative methods were used in reprogramming, only one study reported the silencing of all transgenes (Rosselló et al., 2013) in ciPSCs generated. Most of the other studies reported persistent expression of viral or transposon transgenes (Lu et al., 2014; Kim et al., 2017; Fuet et al., 2018; Katayama et al., 2018) or the genomic integration of episomal minicircle plasmid DNA (Yu et al., 2014) in reprogrammed chicken cells.
The ciPSCs exhibited chimera capability by injection into stage X chick embryos. One study found that ciPSCs injection resulted in high chimerism of early chick embryos for many tissues. However, greater mortality was also found associated with the ciPSC-injected embryos compared with the fibroblast injection controls (Rosselló et al., 2013). Another study found the contribution of ciPSCs into three-germ layers of the embryonic (Kim et al., 2017) and hatched (Yu et al., 2014) chicken tissues, including the brain, heart, liver, and gonad. The induced chicken PGCs were found to migrate into chicken gonads upon vascular injection at stage 15 chicken embryos (Lu et al., 2014).
Overall, avian iPSCs or PGCs could be generated and be propagated for long in vitro. These cells contributed to live chimeras including gonads, although the germline transmission capacity was not determined. The issue of persistent transgene activation similarly to the other farm animal iPSCs and the seemed incomplete activation of some endogenous pluripotent genes including NONOG in induced ciPSCs may have contributed to the improper chimerism or even tumor-like aggregation upon embryo injection as being noticed (Fuet et al., 2018).
Conclusions and Future Prospects
Numerous efforts have been made to establish bona fide farm animal iPSCs, and during the course of this expedition, a great deal has been achieved. As noted in this review, iPSCs capable of long-term propagation could be established from most of the farm animals except bovine. Although the problem of persistent exogenous gene expression and the reliance on it for the self-renewal of derived iPSCs prevailed in the published studies, still, these iPSCs largely satisfied the criteria for EB and teratoma development, two of the standard tests for PSC pluripotency in vitro and in vivo. Nevertheless, it is obvious that the chimerism capacity and the NT-embryo development potential of these iPSCs remained not optimal. The failed transgene silencing, which is a reflection of inadequate activation of the endogenous pluripotent network in these reprogrammed cells, shall be one of the major problems to be addressed in future studies. There is a sharp contrast between the iPSCs derived from farm animals and humans or mice regarding the silencing of transgenes using similar reprogramming approaches, which indicates that unique and potentially species-specific requirements are yet to be met for the complete reprogramming from farm animal somatic cells. More scrutiny of the experimental conditions in those studies with complete silencing of transgenes, including the differences in starting cell types, the reprogramming factors used and their species origins, the reprogramming media, and signal modifications, may help reveal some critical requirements. On the other hand, conditions used in the recent reports of farm animal ESC derivation, such as those for bovine (Wu et al., 2016; Bogliotti et al., 2018), swine (Gao et al., 2019), and potentially other animals may provide additional clues on key signaling events or biomolecular modifications to improve the reprogramming status of farm animal iPSCs. For instance, increased CDX2 and diminished OCT4 and NANOG expression represents a universal prerequisite for mammalian trophectoderm maintenance (Niwa et al., 2005; Strumpf et al., 2005; Sritanaudomchai et al., 2009; Berg et al., 2011; Ozawa et al., 2012; Fujii et al., 2013; Niakan and Eggan, 2013), and bovine ESCs derived from CDX2-knockout embryos were less prone to trophoblast differentiation compared with the wild-type bESCs (Wu et al., 2016). This might help prevent the concurrent generation of trophoblast-like cells during the reprogramming of bovine and pig somatic cells. Also, the specific pluripotent gene reporters for bovine OCT4 and NANOG are expected to facilitate the identification of completely reprogrammed biPSCs or improve their deriving conditions (Lei et al., 2013; Huang et al., 2018), similar as shown in numerous mouse and human PSC studies.
A detailed understanding of the cellular events and differences in the reprogramming process across human, mouse, and farm animal species would provide the knowledge base essential for the optimization of the farm animal cell reprogramming and the long-term maintenance condition for these iPSCs. One aspect of this is to further understand the roles played by common or species-specific microRNAs in reprogramming. For instance, the expression dynamics of mir302/367 cluster is tightly connected with the fine balance of endogenous OCT4, SOX2, and NANOG expression to maintain the pluripotency of human and mouse PSCs (Card et al., 2008; Marson et al., 2008). The expression of this microRNA cluster was elevated at least during the equine iPSC induction process (Moro et al., 2018; Pessôa et al., 2019), and overexpression of this cluster was found to boost porcine iPSC generation (Ma et al., 2014; Du et al., 2015). Meanwhile, interventions using small molecule inhibitors may be explored more for animal iPSC derivation, as exemplified by the study that treating piPSCs with histone deacetylase inhibitor in NT enabled the establishment of cloned piglets (Fan et al., 2013). Thus far, the generation of bona fide iPSCs from farm animals still remains unachieved. A thorough evaluation of the epigenomic difference between farm animal iPSCs, ESCs, and/or the ICM in embryos, including the DNA and histone methylation and acetylation status for pluripotent and lineage commitment genomic loci would provide valuable insights into the establishment of complete reprogramming.
Acknowledgments
This work was supported by the Agriculture and Food Research Initiative Competitive Grant (2019-67015-29413) and the Hatch/MultiState W4131 project (1022009) from the USDA National Institute of Food and Agriculture.
Glossary
Abbreviations
- 2i
extracellular signal-regulated kinases 1/2 (PD0325901) and glycogen synthase kinase 3 (CHIR99021) inhibitors
- AP
alkaline phosphatase
- ASF1
anti-silencing function 1
- bFGF
basic fibroblast growth factor
- biPSC
bovine-induced pluripotent stem cell
- BMP
bone morphogenetic protein
- ciPSC
chicken-induced pluripotent stem cell
- DMEM
Dulbecco’s modified Eagle’s medium
- Dox
doxycycline
- EB
embryoid body
- EPSC
extended pluripotent stem cell
- ESC
embryonic stem cell
- FBS
fetal bovine serum
- giPSC
goat-induced pluripotent stem cell
- ICM
inner cell mass
- iPSC
induced pluripotent stem cell
- KSR
knockout serum replacement
- LAD
leukocyte adhesion deficiency
- LCDM
Human LIF, CHIR99021, (S)-(+)- dimethindene maleate, minocycline hydrochloride
- LIF
leukemia inhibitory factor
- MAB
mesoangioblast
- MSC
mesenchymal stem cell
- NT
nuclear transfer
- OSKM
OCT4 SOX2 KLF4 and c-MYC
- OSKMNL
OCT4 SOX2 KLF4 c-MYC NANOG and LIN28
- OSNL
OCT4 SOX2 NANOG and LIN28
- PGC
primordial germ cell
- piPSC
porcine-induced pluripotent stem cell
- PSC
pluripotent stem cell
- qiPSC
quail-induced pluripotent stem cell
- ROCK
rho-associated protein kinase
- SCF
stem cell factor
- siPSC
sheep-induced pluripotent stem cell
- SSEA
stage-specific embryonic antigen
- TGF-ß
transforming growth factor-beta
- VPA
valproic acid
Conflict of interest statement
The authors declare that there is no conflict of interest.
Literature Cited
- Aguiar, C., C. Theoret, O. Smith, M. Segura, P. Lemire, and L. C. Smith. . 2015. Immune potential of allogeneic equine induced pluripotent stem cells. Equine Vet. J. 47:708–714. doi: 10.1111/evj.12345 [DOI] [PubMed] [Google Scholar]
- Aguiar, C., J. Therrien, P. Lemire, M. Segura, L. C. Smith, and C. L. Theoret. . 2016. Differentiation of equine induced pluripotent stem cells into a keratinocyte lineage. Equine Vet. J. 48:338–345. doi: 10.1111/evj.12438 [DOI] [PubMed] [Google Scholar]
- Amilon, K. R., Y. Cortes-Araya, B. Moore, S. Lee, S. Lillico, A. Breton, C. L. Esteves, and F. X. Donadeu. . 2018. Generation of functional myocytes from equine induced pluripotent stem cells. Cell Reprogram. 20:275–281. doi: 10.1089/cell.2018.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, L., L. He, J. Chen, Z. Wu, J. Liao, L. Rao, J. Ren, H. Li, H. Zhu, L. Qian, . et al. 2011. Reprogramming of ovine adult fibroblasts to pluripotency via drug-inducible expression of defined factors. Cell Res. 21:600–608. doi: 10.1038/cr.2011.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, S., F. Tang, X. Li, K. Hayashi, A. Gillich, K. Lao, and M. A. Surani. . 2009. Epigenetic reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature 461:1292–1295. doi: 10.1038/nature08534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Nur, O., H. A. Russ, S. Efrat, and N. Benvenisty. . 2011. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell 9:17–23. doi: 10.1016/j.stem.2011.06.007 [DOI] [PubMed] [Google Scholar]
- Bavin, E. P., O. Smith, A. E. Baird, L. C. Smith, and D. J. Guest. . 2015. Equine induced pluripotent stem cells have a reduced tendon differentiation capacity compared to embryonic stem cells. Front. Vet. Sci. 2:55. doi: 10.3389/fvets.2015.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg, D. K., C. S. Smith, D. J. Pearton, D. N. Wells, R. Broadhurst, M. Donnison, and P. L. Pfeffer. . 2011. Trophectoderm lineage determination in cattle. Dev. Cell 20:244–255. doi: 10.1016/j.devcel.2011.01.003 [DOI] [PubMed] [Google Scholar]
- Bogliotti, Y. S., J. Wu, M. Vilarino, D. Okamura, D. A. Soto, C. Zhong, M. Sakurai, R. V. Sampaio, K. Suzuki, J. C. Izpisua Belmonte, . et al. 2018. Efficient derivation of stable primed pluripotent embryonic stem cells from bovine blastocysts. Proc. Natl. Acad. Sci. U. S. A. 115:2090–2095. doi: 10.1073/pnas.1716161115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley, A., M. Evans, M. H. Kaufman, and E. Robertson. . 1984. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature 309:255–256. doi: 10.1038/309255a0 [DOI] [PubMed] [Google Scholar]
- Breton, A., R. Sharma, A. C. Diaz, A. G. Parham, A. Graham, C. Neil, C. B. Whitelaw, E. Milne, and F. X. Donadeu. . 2013. Derivation and characterization of induced pluripotent stem cells from equine fibroblasts. Stem Cells Dev. 22:611–621. doi: 10.1089/scd.2012.0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevini, T. A., S. Antonini, F. Cillo, M. Crestan, and F. Gandolfi. . 2007. Porcine embryonic stem cells: facts, challenges and hopes. Theriogenology 68:S206–213. doi: 10.1016/j.theriogenology.2007.05.043 [DOI] [PubMed] [Google Scholar]
- Brons, I. G., L. E. Smithers, M. W. Trotter, P. Rugg-Gunn, B. Sun, S. M. Chuva de Sousa Lopes, S. K. Howlett, A. Clarkson, L. Ahrlund-Richter, R. A. Pedersen, . et al. 2007. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448:191–195. doi: 10.1038/nature05950 [DOI] [PubMed] [Google Scholar]
- Buecker, C., H. H. Chen, J. M. Polo, L. Daheron, L. Bu, T. S. Barakat, P. Okwieka, A. Porter, J. Gribnau, K. Hochedlinger, . et al. 2010. A murine ESC-like state facilitates transgenesis and homologous recombination in human pluripotent stem cells. Cell Stem Cell 6:535–546. doi: 10.1016/j.stem.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camus, A., A. Perea-Gomez, A. Moreau, and J. Collignon. . 2006. Absence of nodal signaling promotes precocious neural differentiation in the mouse embryo. Dev. Biol. 295:743–755. doi: 10.1016/j.ydbio.2006.03.047 [DOI] [PubMed] [Google Scholar]
- Canizo, J. R., C. Vazquez Echegaray, D. Klisch, J. F. Aller, D. A. Paz, R. H. Alberio, R. Alberio, and A. S. Guberman. . 2018. Exogenous human OKSM factors maintain pluripotency gene expression of bovine and porcine iPS-like cells obtained with STEMCCA delivery system. BMC Res. Notes 11:509. doi: 10.1186/s13104-018-3627-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, H., P. Yang, Y. Pu, X. Sun, H. Yin, Y. Zhang, Y. Zhang, Y. Li, Y. Liu, F. Fang, . et al. 2012. Characterization of bovine induced pluripotent stem cells by lentiviral transduction of reprogramming factor fusion proteins. Int. J. Biol. Sci. 8:498–511. doi: 10.7150/ijbs.3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card, D. A., P. B. Hebbar, L. Li, K. W. Trotter, Y. Komatsu, Y. Mishina, and T. K. Archer. . 2008. Oct4/Sox2-regulated miR-302 targets cyclin D1 in human embryonic stem cells. Mol. Cell. Biol. 28:6426–6438. doi: 10.1128/MCB.00359-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal, M., and M. Haskins. . 2006. Large animal models and gene therapy. Eur. J. Hum. Genet. 14:266–272. doi: 10.1038/sj.ejhg.5201535 [DOI] [PubMed] [Google Scholar]
- Chakritbudsabong, W., L. Sariya, S. Pamonsupornvichit, R. Pronarkngver, S. Chaiwattanarungruengpaisan, J. N. Ferreira, P. Setthawong, P. Phakdeedindan, M. Techakumphu, T. Tharasanit, . et al. 2017. Generation of a pig induced pluripotent stem cell (piPSC) line from embryonic fibroblasts by incorporating LIN28 to the four transcriptional factor-mediated reprogramming: VSMUi001-D. Stem Cell Res. 24:21–24. doi: 10.1016/j.scr.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Chen, H., Q. Zuo, Y. Wang, J. Song, H. Yang, Y. Zhang, and B. Li. . 2017. Inducing goat pluripotent stem cells with four transcription factor mRNAs that activate endogenous promoters. BMC Biotechnol. 17:11. doi: 10.1186/s12896-017-0336-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, D., Y. Guo, Z. Li, Y. Liu, X. Gao, Y. Gao, X. Cheng, J. Hu, and H. Wang. . 2012. Porcine induced pluripotent stem cells require LIF and maintain their developmental potential in early stage of embryos. PLoS One. 7:e51778. doi: 10.1371/journal.pone.0051778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, Z., B. Niu, H. Zhu, X. He, C. Bai, G. Li, and J. Hua. . 2015. PRMT5 enhances generation of induced pluripotent stem cells from dairy goat embryonic fibroblasts via down-regulation of p53. Cell Prolif. 48:29–38. doi: 10.1111/cpr.12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, M. J., S. Park, J. Y. Son, J. Y. Lee, H. H. Yun, E. J. Lee, E. M. Lee, G. J. Cho, S. Lee, H. S. Park, . et al. 2019. Differentiation of equine induced pluripotent stem cells into mesenchymal lineage for therapeutic use. Cell Cycle 18:2954–2971. doi: 10.1080/15384101.2019.1664224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, R., R. Rossello, C. C. Chen, J. Kessler, I. Davison, U. Hochgeschwender, and E. D. Jarvis. . 2014. Maintenance and neuronal differentiation of chicken induced pluripotent stem-like cells. Stem Cells Int. 2014:182737. doi: 10.1155/2014/182737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Y., Q. Liu, C. Luo, S. Chen, X. Li, C. Wang, Z. Liu, X. Lei, H. Zhang, H. Sun, . et al. 2012. Generation of induced pluripotent stem cells from buffalo (Bubalus bubalis) fetal fibroblasts with buffalo defined factors. Stem Cells Dev. 21:2485–2494. doi: 10.1089/scd.2012.0018 [DOI] [PubMed] [Google Scholar]
- Dennis, J. A., P. J. Healy, A. L. Beaudet, and W. E. O’Brien. . 1989. Molecular definition of bovine argininosuccinate synthetase deficiency. Proc. Natl. Acad. Sci. U. S. A. 86:7947–7951. doi: 10.1073/pnas.86.20.7947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, X., T. Feng, D. Yu, Y. Wu, H. Zou, S. Ma, C. Feng, Y. Huang, H. Ouyang, X. Hu, . et al. 2015. Barriers for deriving transgene-free pig iPS cells with episomal vectors. Stem Cells 33:3228–3238. doi: 10.1002/stem.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhasid, R., and J. M. Rowe. . 2010. Hematopoetic stem cell transplantation in neutrophil disorders: severe congenital neutropenia, leukocyte adhesion deficiency and chronic granulomatous disease. Clin. Rev. Allergy Immunol. 38:61–67. doi: 10.1007/s12016-009-8129-y [DOI] [PubMed] [Google Scholar]
- Esteban, M. A., J. Xu, J. Yang, M. Peng, D. Qin, W. Li, Z. Jiang, J. Chen, K. Deng, M. Zhong, . et al. 2009. Generation of induced pluripotent stem cell lines from Tibetan miniature pig. J. Biol. Chem. 284:17634–17640. doi: 10.1074/jbc.M109.008938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezashi, T., H. Matsuyama, B. P. Telugu, and R. M. Roberts. . 2011. Generation of colonies of induced trophoblast cells during standard reprogramming of porcine fibroblasts to induced pluripotent stem cells. Biol. Reprod. 85:779–787. doi: 10.1095/biolreprod.111.092809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezashi, T., B. P. Telugu, A. P. Alexenko, S. Sachdev, S. Sinha, and R. M. Roberts. . 2009. Derivation of induced pluripotent stem cells from pig somatic cells. Proc. Natl. Acad. Sci. U. S. A. 106:10993–10998. doi: 10.1073/pnas.0905284106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, N., J. Chen, Z. Shang, H. Dou, G. Ji, Q. Zou, L. Wu, L. He, F. Wang, K. Liu, . et al. 2013. Piglets cloned from induced pluripotent stem cells. Cell Res. 23:162–166. doi: 10.1038/cr.2012.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuet, A., G. Montillet, C. Jean, P. Aubel, C. Kress, S. Rival-Gervier, and B. Pain. . 2018. NANOG is required for the long-term establishment of avian somatic reprogrammed cells. Stem Cell Reports 11:1272–1286. doi: 10.1016/j.stemcr.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii, T., N. Sakurai, T. Osaki, G. Iwagami, H. Hirayama, A. Minamihashi, T. Hashizume, and K. Sawai. . 2013. Changes in the expression patterns of the genes involved in the segregation and function of inner cell mass and trophectoderm lineages during porcine preimplantation development. J. Reprod. Dev. 59:151–158. doi: 10.1262/jrd.2012-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishiro, S. H., K. Nakano, Y. Mizukami, T. Azami, Y. Arai, H. Matsunari, R. Ishino, T. Nishimura, M. Watanabe, T. Abe, . et al. 2013. Generation of naive-like porcine-induced pluripotent stem cells capable of contributing to embryonic and fetal development. Stem Cells Dev. 22:473–482. doi: 10.1089/scd.2012.0173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, T., T. Tani, S. Haraguchi, K. Donai, N. Nakajima, H. Uenishi, T. Eitsuka, M. Miyagawa, S. Song, M. Onuma, . et al. 2017. Expression of six proteins causes reprogramming of porcine fibroblasts into induced pluripotent stem cells with both active X chromosomes. J. Cell. Biochem. 118:537–553. doi: 10.1002/jcb.25727 [DOI] [PubMed] [Google Scholar]
- Gafni, O., L. Weinberger, A. A. Mansour, Y. S. Manor, E. Chomsky, D. Ben-Yosef, Y. Kalma, S. Viukov, I. Maza, A. Zviran, . et al. 2013. Derivation of novel human ground state naive pluripotent stem cells. Nature 504:282–286. (Comparative Study Research Support, Non-U.S. Gov’t) doi: 10.1038/nature12745 [DOI] [PubMed] [Google Scholar]
- Gao, X., M. Nowak-Imialek, X. Chen, D. Chen, D. Herrmann, D. Ruan, A. C. H. Chen, M. A. Eckersley-Maslin, S. Ahmad, Y. L. Lee, . et al. 2019. Establishment of porcine and human expanded potential stem cells. Nat. Cell Biol. 21:687–699. doi: 10.1038/s41556-019-0333-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- German, S. D., K. H. Campbell, E. Thornton, G. McLachlan, D. Sweetman, and R. Alberio. . 2015. Ovine induced pluripotent stem cells are resistant to reprogramming after nuclear transfer. Cell Reprogram. 17:19–27. doi: 10.1089/cell.2014.0071 [DOI] [PubMed] [Google Scholar]
- Gilbert, S. F. 2013. Developmental biology. 10 illustrated ed. Sunderland: Sinauer Associates, Inc. . [Google Scholar]
- Gu, Q., J. Hao, T. Hai, J. Wang, Y. Jia, Q. Kong, J. Wang, C. Feng, B. Xue, B. Xie, . et al. 2014. Efficient generation of mouse ESCs-like pig induced pluripotent stem cells. Protein Cell 5:338–342. doi: 10.1007/s13238-014-0043-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett, C. H., L. Greve, K. D. Novakofski, and L. A. Fortier. . 2012. Comparison of gene-specific DNA methylation patterns in equine induced pluripotent stem cell lines with cells derived from equine adult and fetal tissues. Stem Cells Dev. 21:1803–1811. doi: 10.1089/scd.2011.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, V. J., M. Kristensen, M. A. Rasmussen, O. Ujhelly, A. Dinnyés, and P. Hyttel. . 2012. Temporal repression of endogenous pluripotency genes during reprogramming of porcine induced pluripotent stem cells. Cell. Reprogram. 14:204–216. doi: 10.1089/cell.2011.0089 [DOI] [PubMed] [Google Scholar]
- Han, X., J. Han, F. Ding, S. Cao, S. S. Lim, Y. Dai, R. Zhang, Y. Zhang, B. Lim, and N. Li. . 2011. Generation of induced pluripotent stem cells from bovine embryonic fibroblast cells. Cell Res. 21:1509–1512. doi: 10.1038/cr.2011.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna, J., A. W. Cheng, K. Saha, J. Kim, C. J. Lengner, F. Soldner, J. P. Cassady, J. Muffat, B. W. Carey, and R. Jaenisch. . 2010. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc. Natl. Acad. Sci. U. S. A. 107:9222–9227. doi: 10.1073/pnas.1004584107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy, P. J., P. A. Harper, and J. A. Dennis. . 1990. Bovine citrullinaemia: a clinical, pathological, biochemical and genetic study. Aust. Vet. J. 67:255–258. doi: 10.1111/j.1751-0813.1990.tb07780.x [DOI] [PubMed] [Google Scholar]
- Heo, Y. T., X. Quan, Y. N. Xu, S. Baek, H. Choi, N. H. Kim, and J. Kim. . 2015. CRISPR/Cas9 nuclease-mediated gene knock-in in bovine-induced pluripotent cells. Stem Cells Dev. 24:393–402. doi: 10.1089/scd.2014.0278 [DOI] [PubMed] [Google Scholar]
- Honda, A., M. Hirose, M. Hatori, S. Matoba, H. Miyoshi, K. Inoue, and A. Ogura. . 2010. Generation of induced pluripotent stem cells in rabbits: potential experimental models for human regenerative medicine. J. Biol. Chem. 285:31362–31369. doi: 10.1074/jbc.M110.150540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, D., L. Wang, N. C. Talbot, C. Huang, L. Pu, X. Zhao, X. Tian, M. Zhang, and Y. Tang. . 2018. Analyzing bovine OCT4 and NANOG enhancer activity in pluripotent stem cells using fluorescent protein reporters. PLoS One 13:e0203923. doi: 10.1371/journal.pone.0203923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, D., A. J. Levine, D. Besser, and A. Hemmati-Brivanlou. . 2005. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development 132:1273–1282. (Research Support, Non-U.S. Gov't Research Support, U.S. Gov’t, P.H.S.) doi: 10.1242/dev.01706 [DOI] [PubMed] [Google Scholar]
- Katayama, M., T. Hirayama, T. Tani, K. Nishimori, M. Onuma, and T. Fukuda. . 2018. Chick derived induced pluripotent stem cells by the poly-cistronic transposon with enhanced transcriptional activity. J. Cell. Physiol. 233:990–1004. doi: 10.1002/jcp.25947 [DOI] [PubMed] [Google Scholar]
- Kawaguchi, T., D. Cho, M. Hayashi, T. Tsukiyama, K. Kimura, S. Matsuyama, N. Minami, M. Yamada, and H. Imai. . 2016. Derivation of induced trophoblast cell lines in cattle by doxycycline-inducible piggyBac vectors. PLoS One 11:e0167550. doi: 10.1371/journal.pone.0167550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi, T., T. Tsukiyama, K. Kimura, S. Matsuyama, N. Minami, M. Yamada, and H. Imai. . 2015. Generation of naïve bovine induced pluripotent stem cells using piggyBac transposition of doxycycline-inducible transcription factors. PLoS One 10:e0135403. doi: 10.1371/journal.pone.0135403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrli, M. E. Jr, M. R. Ackermann, D. E. Shuster, M. J. van der Maaten, F. C. Schmalstieg, D. C. Anderson, and B. J. Hughes. . 1992. Bovine leukocyte adhesion deficiency. Beta 2 integrin deficiency in young Holstein cattle. Am. J. Pathol. 140:1489–1492. Availbale from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1886535/pdf/amjpathol00090-0212.pdf [PMC free article] [PubMed] [Google Scholar]
- Khodadadi, K., H. Sumer, M. Pashaiasl, S. Lim, M. Williamson, and P. J. Verma. . 2012. Induction of pluripotency in adult equine fibroblasts without c-MYC. Stem Cells Int. 2012:429160. doi: 10.1155/2012/429160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. M., Y. H. Park, J. M. Lim, H. Jung, and J. Y. Han. . 2017. Technical Note: Induction of pluripotent stem cell-like cells from chicken feather follicle cells. J. Anim. Sci. 95:3479–3486. doi: 10.2527/jas.2017.141 [DOI] [PubMed] [Google Scholar]
- Kues, W. A., D. Herrmann, B. Barg-Kues, S. Haridoss, M. Nowak-Imialek, T. Buchholz, M. Streeck, A. Grebe, I. Grabundzija, S. Merkert, . et al. 2013. Derivation and characterization of sleeping beauty transposon-mediated porcine induced pluripotent stem cells. Stem Cells Dev. 22:124–135. doi: 10.1089/scd.2012.0382 [DOI] [PubMed] [Google Scholar]
- Kulesa, P. M., C. M. Bailey, C. Cooper, and S. E. Fraser. . 2010. In ovo live imaging of avian embryos. Cold Spring Harb. Protoc. 2010:pdb.prot5446. doi: 10.1101/pdb.prot5446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, D. J., I. S. Hwang, H. R. Kim, Y. R. Kim, K. B. Oh, S. A. Ock, G. S. Im, J. W. Lee, and S. Hwang. . 2016. Aberrant methylation of Meg3 in alpha1,3-galactosyltransferase knockout pig induced pluripotent stem cells. Anim. Cells Syst. 20:130–139. doi: 10.1080/19768354.2016.1191543 [DOI] [Google Scholar]
- Kwon, D. J., H. Jeon, K. B. Oh, S. A. Ock, G. S. Im, S. S. Lee, S. K. Im, J. W. Lee, S. J. Oh, J. K. Park, . et al. 2013. Generation of leukemia inhibitory factor-dependent induced pluripotent stem cells from the Massachusetts General Hospital miniature pig. Biomed Res. Int. 2013:140639. doi: 10.1155/2013/140639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin, N. M. 1984. Ontogeny of the peripheral nervous system from the neural crest and the placodes. A developmental model studied on the basis of the quail-chick chimaera system. Harvey Lect. 80:137–186. [PubMed] [Google Scholar]
- Le Douarin, N. M. 2008. Developmental patterning deciphered in avian chimeras. Dev. Growth Differ. 50 (Suppl 1):S11–S28. doi: 10.1111/j.1440-169X.2008.00989.x [DOI] [PubMed] [Google Scholar]
- Lee, E. M., A. Y. Kim, E. J. Lee, J. K. Park, S. I. Park, S. G. Cho, H. K. Kim, S. Y. Kim, and K. S. Jeong. . 2016. Generation of equine-induced pluripotent stem cells and analysis of their therapeutic potential for muscle injuries. Cell Transplant. 25:2003–2016. doi: 10.3727/096368916X691691 [DOI] [PubMed] [Google Scholar]
- Lei, L., L. Li, F. Du, C. H. Chen, H. Wang, and C. L. Keefer. . 2013. Monitoring bovine fetal fibroblast reprogramming utilizing a bovine NANOG promoter-driven EGFP reporter system. Mol. Reprod. Dev. 80:193–203. doi: 10.1002/mrd.22147 [DOI] [PubMed] [Google Scholar]
- Li, Y., M. Cang, A. S. Lee, K. Zhang, and D. Liu. . 2011b. Reprogramming of sheep fibroblasts into pluripotency under a drug-inducible expression of mouse-derived defined factors. PLoS One 6:e15947. doi: 10.1371/journal.pone.0015947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, D., J. Secher, P. Hyttel, M. Ivask, M. Kolko, V. J. Hall, and K. K. Freude. . 2018. Generation of transgene-free porcine intermediate type induced pluripotent stem cells. Cell Cycle 17:2547–2563. doi: 10.1080/15384101.2018.1548790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., H. Wang, Y. Zhang, M. Hou, J. Zhong, and Y. Zhang. . 2011a. Identification of BLAD and citrullinemia carriers in Chinese Holstein cattle. Anim. Sci. Pap. Rep. 29:37–42. [Google Scholar]
- Li, W., W. Wei, S. Zhu, J. Zhu, Y. Shi, T. Lin, E. Hao, A. Hayek, H. Deng, and S. Ding. . 2009. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell 4:16–19. doi: 10.1016/j.stem.2008.11.014 [DOI] [PubMed] [Google Scholar]
- Liao, J., C. Cui, S. Chen, J. Ren, J. Chen, Y. Gao, H. Li, N. Jia, L. Cheng, H. Xiao, . et al. 2009. Generation of induced pluripotent stem cell lines from adult rat cells. Cell Stem Cell 4:11–15. (Letter Research Support, Non-U.S. Gov’t) doi: 10.1016/j.stem.2008.11.013 [DOI] [PubMed] [Google Scholar]
- Lin, Y. C., K. K. Kuo, K. Wuputra, S. H. Lin, C. C. Ku, Y. H. Yang, S. W. Wang, S. W. Wang, D. C. Wu, C. C. Wu, . et al. 2014. Bovine induced pluripotent stem cells are more resistant to apoptosis than testicular cells in response to mono-(2-ethylhexyl) phthalate. Int. J. Mol. Sci. 15:5011–5031. doi: 10.3390/ijms15035011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., D. Balehosur, B. Murray, J. M. Kelly, H. Sumer, and P. J. Verma. . 2012a. Generation and characterization of reprogrammed sheep induced pluripotent stem cells. Theriogenology 77:338–46.e1. doi: 10.1016/j.theriogenology.2011.08.006 [DOI] [PubMed] [Google Scholar]
- Liu, K., G. Ji, J. Mao, M. Liu, L. Wang, C. Chen, and L. Liu. . 2012b. Generation of porcine-induced pluripotent stem cells by using OCT4 and KLF4 porcine factors. Cell Reprogram. 14:505–513. doi: 10.1089/cell.2012.0047 [DOI] [PubMed] [Google Scholar]
- Liu, Y., Y. Ma, J. Y. Yang, D. Cheng, X. Liu, X. Ma, F. D. West, and H. Wang. . 2014. Comparative gene expression signature of pig, human and mouse induced pluripotent stem cell lines reveals insight into pig pluripotency gene networks. Stem Cell Rev. Rep. 10:162–176. doi: 10.1007/s12015-013-9485-9 [DOI] [PubMed] [Google Scholar]
- Liu, H., F. Zhu, J. Yong, P. Zhang, P. Hou, H. Li, W. Jiang, J. Cai, M. Liu, K. Cui, . et al. 2008. Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell 3:587–590. (Letter Research Support, Non-U.S. Govt) doi: 10.1016/j.stem.2008.10.014 [DOI] [PubMed] [Google Scholar]
- Lu, Y., F. D. West, B. J. Jordan, E. T. Jordan, R. C. West, P. Yu, Y. He, M. A. Barrios, Z. Zhu, J. N. Petitte, . et al. 2014. Induced pluripotency in chicken embryonic fibroblast results in a germ cell fate. Stem Cells Dev. 23:1755–1764. doi: 10.1089/scd.2014.0080 [DOI] [PubMed] [Google Scholar]
- Lu, Y., F. D. West, B. J. Jordan, J. L. Mumaw, E. T. Jordan, A. Gallegos-Cardenas, R. B. Beckstead, and S. L. Stice. . 2012. Avian-induced pluripotent stem cells derived using human reprogramming factors. Stem Cells Dev. 21:394–403. doi: 10.1089/scd.2011.0499 [DOI] [PubMed] [Google Scholar]
- Ma, K., G. Song, X. An, A. Fan, W. Tan, B. Tang, X. Zhang, and Z. Li. . 2014. miRNAs promote generation of porcine-induced pluripotent stem cells. Mol. Cell Biochem. 389:209–218. doi: 10.1007/s11010-013-1942-x [DOI] [PubMed] [Google Scholar]
- Maherali, N., R. Sridharan, W. Xie, J. Utikal, S. Eminli, K. Arnold, M. Stadtfeld, R. Yachechko, J. Tchieu, R. Jaenisch, . et al. 2007. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 1:55–70. doi: 10.1016/j.stem.2007.05.014 [DOI] [PubMed] [Google Scholar]
- Malaver-Ortega, L. F., H. Sumer, J. Liu, and P. J. Verma. . 2016. Inhibition of JAK-STAT ERK/MAPK and glycogen synthase kinase-3 induces a change in gene expression profile of bovine induced pluripotent stem cells. Stem Cells Int. 2016:5127984. doi: 10.1155/2016/5127984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, J., Q. Zhang, W. Deng, H. Wang, K. Liu, H. Fu, Q. Zhao, X. Wang, and L. Liu. . 2017. Epigenetic modifiers facilitate induction and pluripotency of porcine iPSCs. Stem Cell Rep. 8:11–20. doi: 10.1016/j.stemcr.2016.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson, A., S. S. Levine, M. F. Cole, G. M. Frampton, T. Brambrink, S. Johnstone, M. G. Guenther, W. K. Johnston, M. Wernig, J. Newman, . et al. 2008. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell 134:521–533. doi: 10.1016/j.cell.2008.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, G. R. 1981. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. U. S. A. 78:7634–7638. doi: 10.1073/pnas.78.12.7634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meydan, H., M. A. Yildiz, and J. S. Agerholm. . 2010. Screening for bovine leukocyte adhesion deficiency, deficiency of uridine monophosphate synthase, complex vertebral malformation, bovine citrullinaemia, and factor XI deficiency in Holstein cows reared in Turkey. Acta Vet. Scand. 52:56. doi: 10.1186/1751-0147-52-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montserrat, N., E. G. Bahima, L. Batlle, S. Häfner, A. M. Rodrigues, F. González, and J. C. Izpisúa Belmonte. . 2011. Generation of pig iPS cells: a model for cell therapy. J. Cardiovasc. Transl. Res. 4:121–130. doi: 10.1007/s12265-010-9233-3 [DOI] [PubMed] [Google Scholar]
- Montserrat, N., L. de Oñate, E. Garreta, F. González, A. Adamo, C. Eguizábal, S. Häfner, R. Vassena, and J. C. Izpisua Belmonte. . 2012. Generation of feeder-free pig induced pluripotent stem cells without Pou5f1. Cell Transplant. 21:815–825. doi: 10.3727/096368911X601019 [DOI] [PubMed] [Google Scholar]
- Moro, L. N., G. Amin, V. Furmento, A. Waisman, X. Garate, G. Neiman, A. La Greca, N. L. Santín Velazque, C. Luzzani, G. E. Sevlever, . et al. 2018. MicroRNA characterization in equine induced pluripotent stem cells. PLoS One 13:e0207074. doi: 10.1371/journal.pone.0207074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahata, H. 2004. Bovine leukocyte adhesion deficiency (BLAD): a review. J. Vet. Med. Sci. 66:1475–1482. doi: 10.1292/jvms.66.1475 [DOI] [PubMed] [Google Scholar]
- Nagy, K., H. K. Sung, P. Zhang, S. Laflamme, P. Vincent, S. Agha-Mohammadi, K. Woltjen, C. Monetti, I. P. Michael, L. C. Smith, . et al. 2011. Induced pluripotent stem cell lines derived from equine fibroblasts. Stem Cell Rev. Rep. 7:693–702. doi: 10.1007/s12015-011-9239-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm, F. J., J. G. Chenoweth, P. D. Anderson, J. H. Nadeau, R. W. Redline, R. D. McKay, and P. J. Tesar. . 2011. Isolation of epiblast stem cells from preimplantation mouse embryos. Cell Stem Cell 8:318–325. (Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t) doi: 10.1016/j.stem.2011.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niakan, K. K., and K. Eggan. . 2013. Analysis of human embryos from zygote to blastocyst reveals distinct gene expression patterns relative to the mouse. Dev. Biol. 375:54–64. doi: 10.1016/j.ydbio.2012.12.008 [DOI] [PubMed] [Google Scholar]
- Nichols, J., and A. Smith. . 2009. Naive and primed pluripotent states. Cell Stem Cell 4:487–492. doi: 10.1016/j.stem.2009.05.015 [DOI] [PubMed] [Google Scholar]
- Niwa, H., T. Burdon, I. Chambers, and A. Smith. . 1998. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 12:2048–2060. doi: 10.1101/gad.12.13.2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa, H., Y. Toyooka, D. Shimosato, D. Strumpf, K. Takahashi, R. Yagi, and J. Rossant. . 2005. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell 123:917–929. doi: 10.1016/j.cell.2005.08.040 [DOI] [PubMed] [Google Scholar]
- Okita, K., T. Ichisaka, and S. Yamanaka. . 2007. Generation of germline-competent induced pluripotent stem cells. Nature 448:313–317. doi: 10.1038/nature05934 [DOI] [PubMed] [Google Scholar]
- Ozawa, M., M. Sakatani, J. Yao, S. Shanker, F. Yu, R. Yamashita, S. Wakabayashi, K. Nakai, K. B. Dobbs, M. J. Sudano, . et al. 2012. Global gene expression of the inner cell mass and trophectoderm of the bovine blastocyst. BMC Dev. Biol. 12:33. doi: 10.1186/1471-213X-12-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. K., H. S. Kim, K. J. Uh, K. H. Choi, H. M. Kim, T. Lee, B. C. Yang, H. J. Kim, H. H. Ka, H. Kim, . et al. 2013. Primed pluripotent cell lines derived from various embryonic origins and somatic cells in pig. PLoS One 8:e52481. doi: 10.1371/journal.pone.0052481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, R. K., and A. K. Patel. . 2014. Comparative review of recessive genetic disorders occurrence in Indian Cattle. Curr. Trends Biotechnol. Pharm. 8:321–335. Available from https://abap.co.in/comparative-review-recessive-genetic-disorders-occurrence-indian-cattle [Google Scholar]
- Pessôa, L. V. F., P. R. L. Pires, M. Del Collado, N. C. G. Pieri, K. Recchia, A. F. Souza, F. Perecin, J. C. da Silveira, A. F. C. de Andrade, C. E. Ambrosio, . et al. 2019. Generation and miRNA characterization of equine induced pluripotent stem cells derived from fetal and adult multipotent tissues. Stem Cells Int. 2019:1393791. doi: 10.1155/2019/1393791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai, V. V., T. G. Kei, S. E. Reddy, M. Das, C. Abratte, S. H. Cheong, and V. Selvaraj. . 2019. Induced pluripotent stem cell generation from bovine somatic cells indicates unmet needs for pluripotency sustenance. Anim. Sci. J. 90:1149–1160. doi: 10.1111/asj.13272 [DOI] [PubMed] [Google Scholar]
- Quattrocelli, M., G. Giacomazzi, S. Y. Broeckx, L. Ceelen, S. Bolca, J. H. Spaas, and M. Sampaolesi. . 2016. Equine-induced pluripotent stem cells retain lineage commitment toward myogenic and chondrogenic fates. Stem Cell Rep. 6:55–63. doi: 10.1016/j.stemcr.2015.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinonez, S., and J. Thoene. . 2004. Citrullinemia type I. In: Adam, M., H. Ardinger, R. Pagon, S. Wallace, L. Bean, K. Stephens, and A. Amemiya, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington; p. 1993–2018. [Google Scholar]
- Ren, J., Y. Pak, L. He, L. Qian, Y. Gu, H. Li, L. Rao, J. Liao, C. Cui, X. Xu, . et al. 2011. Generation of hircine-induced pluripotent stem cells by somatic cell reprogramming. Cell Res. 21:849–853. doi: 10.1038/cr.2011.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, A., C. Allegrucci, and R. Alberio. . 2012. Modulation of pluripotency in the porcine embryo and iPS cells. PLoS One 7:e49079. doi: 10.1371/journal.pone.0049079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosselló, R. A., C. C. Chen, R. Dai, J. T. Howard, U. Hochgeschwender, and E. D. Jarvis. . 2013. Mammalian genes induce partially reprogrammed pluripotent stem cells in non-mammalian vertebrate and invertebrate species. eLife 2:e00036. doi: 10.7554/eLife.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan, W., J. Han, P. Li, S. Cao, Y. An, B. Lim, and N. Li. . 2011. A novel strategy to derive iPS cells from porcine fibroblasts. Sci. China Life Sci. 54:553–559. doi: 10.1007/s11427-011-4179-5 [DOI] [PubMed] [Google Scholar]
- Sandmaier, S. E., A. Nandal, A. Powell, W. Garrett, L. Blomberg, D. M. Donovan, N. Talbot, and B. P. Telugu. . 2015. Generation of induced pluripotent stem cells from domestic goats. Mol. Reprod. Dev. 82:709–721. doi: 10.1002/mrd.22512 [DOI] [PubMed] [Google Scholar]
- Sartori, C., A. I. DiDomenico, A. J. Thomson, E. Milne, S. G. Lillico, T. G. Burdon, and C. B. Whitelaw. . 2012. Ovine-induced pluripotent stem cells can contribute to chimeric lambs. Cell Reprogram. 14:8–19. doi: 10.1089/cell.2011.0050 [DOI] [PubMed] [Google Scholar]
- Setthawong, P., P. Phakdeedindan, N. Tiptanavattana, S. Rungarunlert, M. Techakumphu, and T. Tharasanit. . 2019. Generation of porcine induced-pluripotent stem cells from Sertoli cells. Theriogenology 127:32–40. doi: 10.1016/j.theriogenology.2018.12.033 [DOI] [PubMed] [Google Scholar]
- Sharma, R., M. R. Livesey, D. J. Wyllie, C. Proudfoot, C. B. Whitelaw, D. C. Hay, and F. X. Donadeu. . 2014. Generation of functional neurons from feeder-free, keratinocyte-derived equine induced pluripotent stem cells. Stem Cells Dev. 23:1524–1534. doi: 10.1089/scd.2013.0565 [DOI] [PubMed] [Google Scholar]
- Shi, H., Q. Fu, G. Li, Y. Ren, S. Hu, W. Ni, F. Guo, M. Shi, L. Meng, H. Zhang, . et al. 2015. Roles of p53 and ASF1A in the reprogramming of sheep kidney cells to pluripotent cells. Cell. Reprogram 17:441–452. doi: 10.1089/cell.2015.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster, D. E., M. E. Kehrli Jr, M. R. Ackermann, and R. O. Gilbert. . 1992. Identification and prevalence of a genetic defect that causes leukocyte adhesion deficiency in Holstein cattle. Proc. Natl. Acad. Sci. U. S. A. 89:9225–9229. doi: 10.1073/pnas.89.19.9225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, R. K., E. R. Garvican, and L. A. Fortier. . 2014. The current ‘state of play’ of regenerative medicine in horses: what the horse can tell the human. Regen. Med. 9:673–685. doi: 10.2217/rme.14.42 [DOI] [PubMed] [Google Scholar]
- Smith, A. G., J. K. Heath, D. D. Donaldson, G. G. Wong, J. Moreau, M. Stahl, and D. Rogers. . 1988. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature 336:688–690. (In Vitro Research Support, Non-U.S. Gov’t) doi: 10.1038/336688a0 [DOI] [PubMed] [Google Scholar]
- Song, H., H. Li, M. Huang, D. Xu, C. Gu, Z. Wang, F. Dong, and F. Wang. . 2013. Induced pluripotent stem cells from goat fibroblasts. Mol. Reprod. Dev. 80:1009–1017. doi: 10.1002/mrd.22266 [DOI] [PubMed] [Google Scholar]
- Song, H., H. Li, M. Huang, D. Xu, Z. Wang, and F. Wang. . 2016. Big animal cloning using transgenic induced pluripotent stem cells: a case study of goat transgenic induced pluripotent stem cells. Cell Reprogram. 18:37–47. doi: 10.1089/cell.2015.0035 [DOI] [PubMed] [Google Scholar]
- Sritanaudomchai, H., M. Sparman, M. Tachibana, L. Clepper, J. Woodward, S. Gokhale, D. Wolf, J. Hennebold, W. Hurlbut, M. Grompe, . et al. 2009. CDX2 in the formation of the trophectoderm lineage in primate embryos. Dev. Biol. 335:179–187. doi: 10.1016/j.ydbio.2009.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumpf, D., C. A. Mao, Y. Yamanaka, A. Ralston, K. Chawengsaksophak, F. Beck, and J. Rossant. . 2005. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development 132:2093–2102. doi: 10.1242/dev.01801 [DOI] [PubMed] [Google Scholar]
- Sumer, H., J. Liu, L. F. Malaver-Ortega, M. L. Lim, K. Khodadadi, and P. J. Verma. . 2011. NANOG is a key factor for induction of pluripotency in bovine adult fibroblasts. J. Anim. Sci. 89:2708–2716. doi: 10.2527/jas.2010-3666 [DOI] [PubMed] [Google Scholar]
- Tai, D., P. Liu, J. Gao, M. Jin, T. Xu, Y. Zuo, H. Liang, and D. Liu. . 2015. Generation of Arbas Cashmere goat induced pluripotent stem cells through fibroblast reprogramming. Cell Reprogram. 17:297–305. doi: 10.1089/cell.2014.0107 [DOI] [PubMed] [Google Scholar]
- Takahashi, K., and S. Yamanaka. . 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676. doi: 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Talbot, N. C., W. O. Sparks, C. E. Phillips, A. D. Ealy, A. M. Powell, T. J. Caperna, W. M. Garrett, D. M. Donovan, and L. A. Blomberg. . 2017. Bovine trophectoderm cells induced from bovine fibroblasts with induced pluripotent stem cell reprogramming factors. Mol. Reprod. Dev. 84:468–485. doi: 10.1002/mrd.22797 [DOI] [PubMed] [Google Scholar]
- Talluri, T. R., D. Kumar, S. Glage, W. Garrels, Z. Ivics, K. Debowski, R. Behr, H. Niemann, and W. A. Kues. . 2015. Derivation and characterization of bovine induced pluripotent stem cells by transposon-mediated reprogramming. Cell Reprogram. 17:131–140. doi: 10.1089/cell.2014.0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telugu, B. P., T. Ezashi, and R. M. Roberts. . 2010. Porcine induced pluripotent stem cells analogous to naïve and primed embryonic stem cells of the mouse. Int. J. Dev. Biol. 54:1703–1711. doi: 10.1387/ijdb.103200bt [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar, P. J., J. G. Chenoweth, F. A. Brook, T. J. Davies, E. P. Evans, D. L. Mack, R. L. Gardner, and R. D. McKay. . 2007. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448:196–199. doi: 10.1038/nature05972 [DOI] [PubMed] [Google Scholar]
- Thomson, J. A., J. Itskovitz-Eldor, S. S. Shapiro, M. A. Waknitz, J. J. Swiergiel, V. S. Marshall, and J. M. Jones. . 1998. Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147. doi: 10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- Tomioka, I., T. Maeda, H. Shimada, K. Kawai, Y. Okada, H. Igarashi, R. Oiwa, T. Iwasaki, M. Aoki, T. Kimura, . et al. 2010. Generating induced pluripotent stem cells from common marmoset (Callithrix jacchus) fetal liver cells using defined factors, including Lin28. Genes Cells 15:959–969. doi: 10.1111/j.1365-2443.2010.01437.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallier, L., D. Reynolds, and R. A. Pedersen. . 2004. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev. Biol. 275:403–421. (Comparative Study Research Support, Non-U.S. Gov’t) doi: 10.1016/j.ydbio.2004.08.031 [DOI] [PubMed] [Google Scholar]
- Wang, S. W., S. S. Wang, D. C. Wu, Y. C. Lin, C. C. Ku, C. C. Wu, C. Y. Chai, J. N. Lee, E. M. Tsai, C. L. Lin, . et al. 2013. Androgen receptor-mediated apoptosis in bovine testicular induced pluripotent stem cells in response to phthalate esters. Cell Death Dis. 4:e907. doi: 10.1038/cddis.2013.420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig, M., A. Meissner, R. Foreman, T. Brambrink, M. Ku, K. Hochedlinger, B. E. Bernstein, and R. Jaenisch. . 2007. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448:318–324. doi: 10.1038/nature05944 [DOI] [PubMed] [Google Scholar]
- West, F. D., S. L. Terlouw, D. J. Kwon, J. L. Mumaw, S. K. Dhara, K. Hasneen, J. R. Dobrinsky, and S. L. Stice. . 2010. Porcine induced pluripotent stem cells produce chimeric offspring. Stem Cells Dev. 19:1211–1220. doi: 10.1089/scd.2009.0458 [DOI] [PubMed] [Google Scholar]
- West, F. D., E. W. Uhl, Y. Liu, H. Stowe, Y. Lu, P. Yu, A. Gallegos-Cardenas, S. L. Pratt, and S. L. Stice. . 2011. Brief Report: Chimeric pigs produced from induced pluripotent stem cells demonstrate germline transmission and no evidence of tumor formation in young pigs. Stem Cells 29:1640–1643. doi: 10.1002/stem.713 [DOI] [PubMed] [Google Scholar]
- Whitworth, D. J., D. A. Ovchinnikov, J. Sun, P. R. Fortuna, and E. J. Wolvetang. . 2014. Generation and characterization of leukemia inhibitory factor-dependent equine induced pluripotent stem cells from adult dermal fibroblasts. Stem Cells Dev. 23:1515–1523. doi: 10.1089/scd.2013.0461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z., J. Chen, J. Ren, L. Bao, J. Liao, C. Cui, L. Rao, H. Li, Y. Gu, H. Dai, . et al. 2009. Generation of pig induced pluripotent stem cells with a drug-inducible system. J. Mol. Cell Biol. 1:46–54. doi: 10.1093/jmcb/mjp003 [DOI] [PubMed] [Google Scholar]
- Wu, X., M. Song, X. Yang, X. Liu, K. Liu, C. Jiao, J. Wang, C. Bai, G. Su, X. Liu, . et al. 2016. Establishment of bovine embryonic stem cells after knockdown of CDX2. Sci. Rep. 6:28343. doi: 10.1038/srep28343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J., L. Yu, J. Guo, J. Xiang, Z. Zheng, D. Gao, B. Shi, H. Hao, D. Jiao, L. Zhong, . et al. 2019. Generation of pig induced pluripotent stem cells using an extended pluripotent stem cell culture system. Stem Cell Res. Ther. 10:193. doi: 10.1186/s13287-019-1303-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., B. Liu, J. Xu, J. Wang, J. Wu, C. Shi, Y. Xu, J. Dong, C. Wang, W. Lai, . et al. 2017b. Derivation of pluripotent stem cells with in vivo embryonic and extraembryonic potency. Cell 169:243–257.e25. doi: 10.1016/j.cell.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. Y., J. L. Mumaw, Y. Liu, S. L. Stice, and F. D. West. . 2013. SSEA4-positive pig induced pluripotent stem cells are primed for differentiation into neural cells. Cell Transplant. 22:945–959. doi: 10.3727/096368912X657279 [DOI] [PubMed] [Google Scholar]
- Yang, J., D. J. Ryan, W. Wang, J. C. Tsang, G. Lan, H. Masaki, X. Gao, L. Antunes, Y. Yu, Z. Zhu, . et al. 2017a. Establishment of mouse expanded potential stem cells. Nature 550:393–397. doi: 10.1038/nature24052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying, Q. L., J. Wray, J. Nichols, L. Batlle-Morera, B. Doble, J. Woodgett, P. Cohen, and A. Smith. . 2008. The ground state of embryonic stem cell self-renewal. Nature 453:519–523. doi: 10.1038/nature06968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, P., Y. Lu, B. J. Jordan, Y. Liu, J. Y. Yang, J. M. Hutcheson, C. L. Ethridge, J. L. Mumaw, H. A. Kinder, R. B. Beckstead, . et al. 2014. Nonviral minicircle generation of induced pluripotent stem cells compatible with production of chimeric chickens. Cell Reprogram. 16:366–378. doi: 10.1089/cell.2014.0028 [DOI] [PubMed] [Google Scholar]
- Yu, J., M. A. Vodyanik, K. Smuga-Otto, J. Antosiewicz-Bourget, J. L. Frane, S. Tian, J. Nie, G. A. Jonsdottir, V. Ruotti, R. Stewart, . et al. 2007. Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920. doi: 10.1126/science.1151526 [DOI] [PubMed] [Google Scholar]
- Yuan, Y. 2018. Capturing bovine pluripotency. Proc. Natl. Acad. Sci. U. S. A. 115:1962–1963. doi: 10.1073/pnas.1800248115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., Y. Guo, Y. Cui, Y. Liu, T. Yu, and H. Wang. . 2015a. Generation of intermediate porcine iPS cells under culture condition favorable for mesenchymal-to-epithelial transition. Stem Cell Rev. Rep. 11:24–38. doi: 10.1007/s12015-014-9552-x [DOI] [PubMed] [Google Scholar]
- Zhang, W., Y. Pei, L. Zhong, B. Wen, S. Cao, and J. Han. . 2015b. Pluripotent and metabolic features of two types of porcine iPSCs derived from defined mouse and human ES cell culture conditions. PLoS One 10:e0124562. doi: 10.1371/journal.pone.0124562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., C. Wei, P. Zhang, X. Li, T. Liu, Y. Pu, Y. Li, Z. Cao, H. Cao, Y. Liu, . et al. 2014. Efficient reprogramming of naive-like induced pluripotent stem cells from porcine adipose-derived stem cells with a feeder-independent and serum-free system. PLoS One 9:e85089. doi: 10.1371/journal.pone.0085089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L., Z. Wang, J. Zhang, J. Yang, X. Gao, B. Wu, G. Zhao, S. Bao, S. Hu, P. Liu, . et al. 2017. Characterization of the single-cell derived bovine induced pluripotent stem cells. Tissue Cell 49:521–527. doi: 10.1016/j.tice.2017.05.005 [DOI] [PubMed] [Google Scholar]