Abstract
Nicotinamide adenine dinucleotide (NAD) kinases are essential and ubiquitous enzymes involved in the tight regulation of NAD/nicotinamide adenine dinucleotide phosphate (NADP) levels in many metabolic pathways. Consequently, they represent promising therapeutic targets in cancer and antibacterial treatments. We previously reported diadenosine derivatives as NAD kinase inhibitors with bactericidal activities on Staphylococcus aureus. Among them, one compound (namely NKI1) was found effective in vivo in a mouse infection model. With the aim to gain detailed knowledge about the selectivity and mechanism of action of this lead compound, we planned to develop a chemical probe that could be used in affinity-based chemoproteomic approaches. Here, we describe the first functionalized chemical probe targeting a bacterial NAD kinase. Aminoalkyl functional groups were introduced on NKI1 for further covalent coupling to an activated SepharoseTM matrix. Inhibitory properties of functionalized NKI1 derivatives together with X-ray characterization of their complexes with the NAD kinase led to identify candidate compounds that are amenable to covalent coupling to a matrix.
Keywords: antibiotics, chemical probe, diadenosine derivatives, NAD kinase, nucleoside, Sonogashira coupling, staphylococci
1. Introduction
Nicotinamide adenine dinucleotide (NAD) kinases (NADK) are ubiquitous enzymes, which catalyze the phosphorylation of NAD to nicotinamide adenine dinucleotide phosphate (NADP), which is subsequently reduced to NADPH [1,2,3]. Since it is the only known enzyme producing NADP de novo, NAD kinase plays a crucial role in controlling the intracellular balance of NAD(H) and NADP(H) in many cellular metabolic pathways [4,5]. While the NADK enzymatic activity has been known for decades, their genes were identified more recently, leading to the discovery of orthologs in nearly all living organisms. The essentiality of the NADK gene has been experimentally validated in several bacteria, including Escherichia coli [6], Salmonella enterica, Bacillus subtilis [7,8], Mycobacterium tuberculosis [9] and Staphylococcus aureus [10,11,12]. Moreover, it was shown that human NAD kinase displays kinetic and structural features that differ considerably from that of prokaryotes [13,14]. Therefore, NADK represents a promising target for the development of novel antibiotics with an original mode of action.
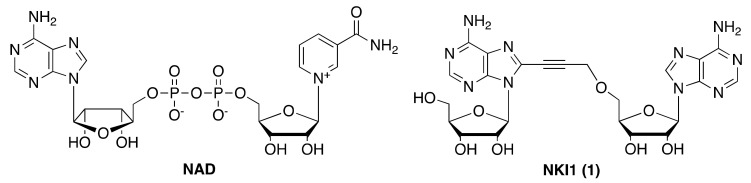
Previously we solved the crystal structures of NADK from Listeria monocytogenes (LmNADK1) in the free state and bound to NAD and NADP, and described the first non-natural inhibitor of this enzyme [15]. Following a fragment-based approach, we identified a series of diadenosine derivatives with low micromolar inhibitory potencies against recombinant LmNADK1 and S. aureus NADK [16,17]. We subsequently discovered the first NAD kinase inhibitor (namely NKI1, Figure 1) active in mice infected with S. aureus, including antibiotic-resistant strains [18].
Figure 1.
Chemical structures of nicotinamide adenine dinucleotide (NAD) and NAD kinase inhibitor NKI1 (1).
To get more information about the selectivity of our lead compound and its mechanism of action, we aimed at immobilizing NKI1 derivatives on a SepharoseTM matrix that could be further used in chemical proteomics [19]. Affinity chromatography is one of the most powerful chromatographic methods to analyze and purify proteins from complex mixtures or crude extracts. Various immobilized-nucleos(t)ides and cofactors are now commercially available. However specific affinity matrices may need to be developed by coupling the suitable ligand onto the matrix. The attachment chemistry and the spacer between the ligand and the matrix are important factors to be considered [20].
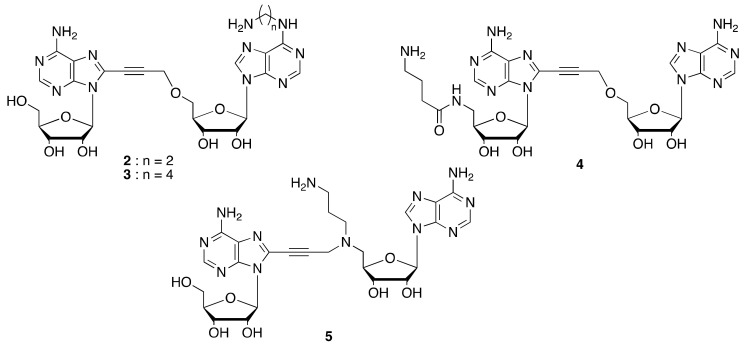
Here, we describe the synthesis of a series of functionalized diadenosine derivatives based on the chemical structure of NKI1 [18]. Short aminoalkyl chains were introduced at each flanking region or middle region of the diadenosine motif (Figure 2) that could be used for coupling with a NHS-activated matrix. NADK binding properties of functionalized NKI1 were analyzed by X-ray crystallography and by measuring NADK activity, allowing selection of the candidate ligands for further covalent attachment to a matrix.
Figure 2.
Chemical structures of the NKI1 analogues 2–5.
2. Results
2.1. Chemical Synthesis of Functionalized Diadenosine Derivatives
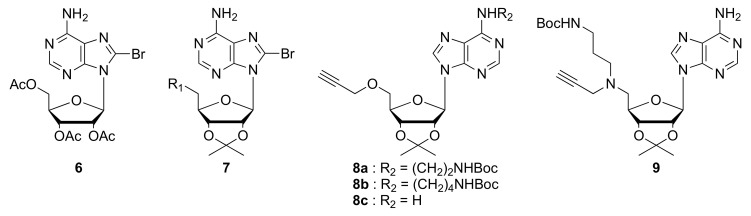
The key step for synthesizing derivatives 2–5 is a Sonogashira cross-coupling reaction between a bromide (6 or 7) and an alkyne (8 or 9) (Figure 3). The appropriate building blocks were easily prepared according to classical methodology.
Figure 3.
Key building blocks involved in Sonogashira cross-coupling reaction.
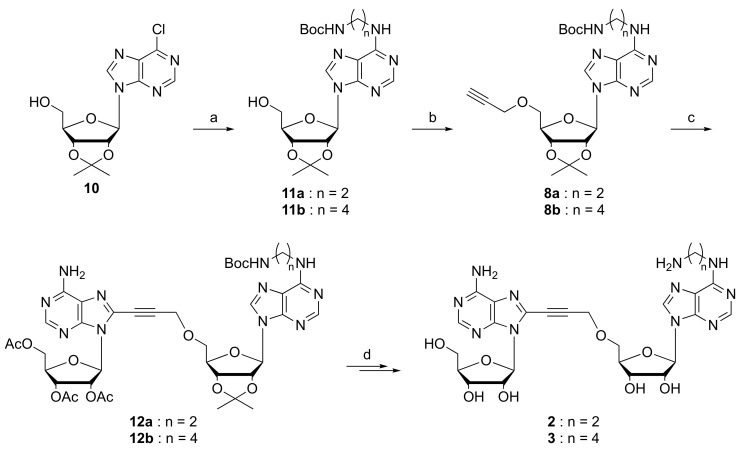
The synthetic route to the N6-functionalized NKI1 derivatives 2 and 3 is depicted in Scheme 1. An aminoalkyl side chain (2 or 4 carbon) was introduced at the 6-position of adenosine by SNAr from the 6-chloropurine derivative 10. Selective 5’-O-propargylation of 11a and 11b in the presence of propargyl bromide and NaH in DMF afforded the building blocks 8a and 8b, respectively, which were involved in Sonogashira cross-coupling reaction with bromide 6 to give 12a and 12b. Finally, removal of all protecting groups provided the desired compounds 2 and 3.
Scheme 1.
Reagents and conditions: (a) BocNH(CH2)nNH2, DME, NEt3, 80 °C, 66% (n = 2) or 91% (n = 4); (b) NaH, propargylbromide, THF, 4 °C, 60% or 68%; (c) 6, Pd(PPh3)4, CuI, NEt3, THF, 60 °C, 37% or 40%; (d) 28% NH4OH/MeOH, rt; TFA/H2O, 4 °C, 42% or 61% in two steps.
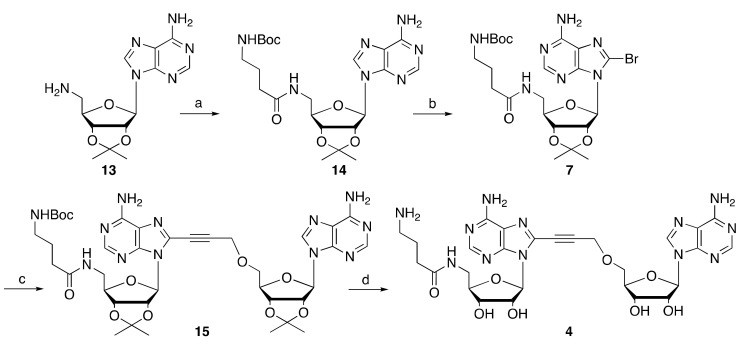
The synthesis of compound 4 was achieved by reaction of 4-(N-Boc-amino)butyric acid (Boc-GABA-OH) with the 5’-amino derivative 13 in the presence of PyBOP and DIEA in DMF, followed by C8 bromination of the resulting amide 14 to give the key intermediate 7 (Scheme 2). Sonogashira coupling of bromide 7 and alkyne 8c [16], followed by acidic treatment of the coupling product 15 gave the desired derivative 4 (Scheme 2).
Scheme 2.
Reagents and conditions: (a) Boc-GABA, PyBOP, DIEA, DMF, 74%; (b) Br2, acetate buffer/dioxane, 69%; (c) 8c, Pd(PPh3)4, CuI, NEt3, THF, 60 °C, 25%; (d) TFA/H2O, 4 °C, 45%.
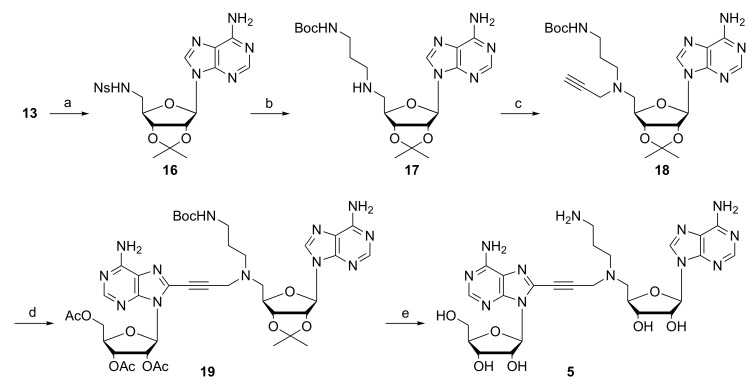
Finally, introduction of an aminoalkyl side chain at the middle position of the ligand was achieved from 5’-amino derivative 13 via the Ns strategy [21]. Nosylation of 13, followed by alkylation of the N-sulfonamide 16 and removal of Ns group afforded the N-monosubstituted amine 17 (Scheme 3). Alkylation of the secondary amine using propargyl bromide afforded the key N-alkylated intermediate 9 in good overall yield. Sonogashira reaction between 9 and 6 led to the coupling product 19. Finally, two-step removal of protecting groups gave the desired derivative 5.
Scheme 3.
Reagents and conditions: (a) NsCl, pyridine, 88%; (b) BocNH(CH2)3Br, K2CO3, DMF, 50 °C; then PhSH, 79%; (c) Propargylbromide, DIEA, DMF, 82%; (d) 6, Pd(PPh3)4, CuI, NEt3, THF, 60 °C, 63%; (e) 28% NH4OH, MeOH; TFA/H2O, 61% in two steps.
2.2. X-ray Structures of LmNADK1 Bound to Compounds 2–5
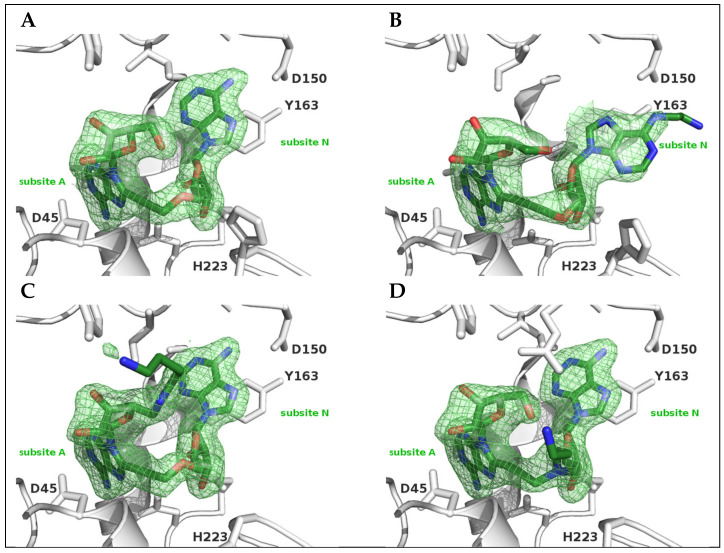
A prerequisite to use a chemical probe is that it retains its binding affinity for its target. The binding properties of the four synthesized derivatives 2–5 were analyzed by X-ray crystallography. We checked the binding properties of these new di-adenosine derivatives by determining their complex with crystallized LmNADK1. The soaking procedure described previously for inhibitor 1 [18] was used to obtain crystals of the target in complex with compounds 2-5. The X-ray structures were solved by molecular replacement using PDB6RGC [18] as a starting template and refined to 2.2–2.4 Å resolution. The resulting structures showed the same overall mode of binding to LmNADK1, the ligands occupying both sub-sites A and N of the NAD binding pocket (Figure 4).
Figure 4.
X-ray structures of LmNADK1 in complex with ligands NKI1 (6RG9), 2 (6Z61), 4 (6Z65) and 5 (6Z64). A zoomed in view of the NAD binding site is shown in the image. Important residues discussed in the text are shown in stick and labeled. Compounds NKI1 (A), 2 (B), 4 (C) and 5 (D) are shown in sticks color according to cpk code with carbon in green. The figures were drawn using Pymol (www.pymol.org).
While compound 3 failed to yield complex with the crystallized target, compound 2 soaking led to a complex with the target. The structure (Figure 4B) showed a clear change in the orientation of the adenine moiety located in the subsite N (syn vs anti conformation), while that of the adenine moiety in the subsite A was maintained. The purine base flipping positioned the amino end out of the target, while the imidazole ring of the adenine still stacked on Tyr163. However, the N6 spacer arm appears to prevent the usual orientation of the ligand in the subsite N due to van der Waals clashes with neighboring residues, especially Asp150 which is normally hydrogen bonded to the N6 atom. The binding modes of compounds 4 and 5 were very similar to that of NKI1, the spacer arm having little impact on the orientation of the ligands. When the 4-carbon aminoalkyl chain is grafted at the 5’-end of the di-adenosine derivative (compound 4), no particular interaction of this added spacer arm with any residues from the protein could be observed (Figure 4C) and the amino functional group appeared readily amenable to derivatization. Similarly, when the spacer arm was introduced between the two adenosine residues via the N-propargyl atom (compound 5), the aminoalkyl chain pointed outside the NAD binding site (Figure 4D). In all three cases, the spacer arms appeared flexible in the crystal structures (weak electron densities; see Figure 4B–D) suggesting they are perfectly amenable to column grafting.
2.3. Effects of Compounds 2–5 on LmNADK1 Activity
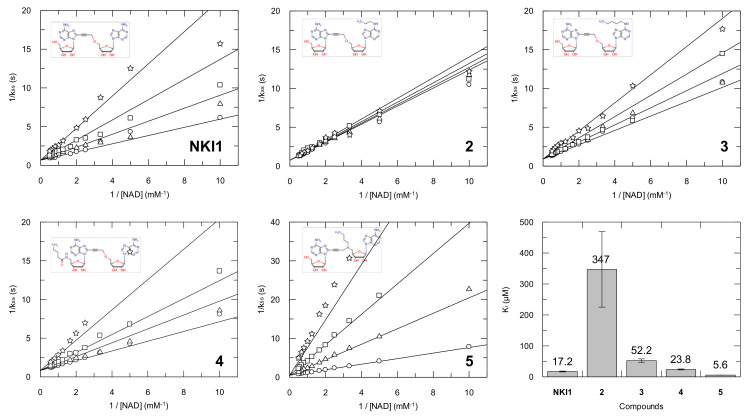
Another important parameter to select the chemical probe is that it retains its binding properties. The potency of ligands 2–5 as inhibitors of recombinant LmNADK1 in vitro were determined using the coupling assay described previously [15].
The introduction of an aminoalkyl chain at position 6 of the adenosine residue located in the N subsite (compounds 2 and 3) led to sharp decrease in inhibitory potency (Figure 5). This finding is consistent with our previous work describing the affinity drop induced by the introduction of a N6-cyclopropylamino group on the adenosine residue located in the subsite N [18]. Introduction of the spacer arm at the 5’-end of the diadenosine derivative (compound 4) resulted in a slightly reduced inhibitory potency as compared to that of inhibitor 1 (NKI1). In contrast, when the spacer arm was attached in the middle part of the ligand, between the two adenosine residues via the N propargyl atom, inhibitory potency of compound 5 was slightly increased.
Figure 5.
Determination of inhibition constants of compounds 1–5. Inhibitor concentrations were 0 (circles), 10 (triangles), 25 (squares) and 50 (stars) µM. Secondary plots (kss as a function of initial NAD concentration) were fitted assuming a competitive inhibition using GraFit software (v 7.0.3) and are presented here as double reciprocal plots. Ki values determined are represented as bars (values are displayed on top of the bars).
3. Discussion
By taking advantage of the substrate recognition characteristics of NADK [15,16], we designed four ligand derivatives based on the structure of our lead NADK inhibitor NKI1 [18]. Successful synthesis of the four diadenosine derivatives bearing short aminoalkyl chains to be coupled to NHS-activated SepharoseTM matrix allowed investigation of their specific properties towards NADK. NADK from L. monocytogenes was selected as representative target for this study, as the recombinant enzyme is easily produced and more amenable to subsequent crystallization trials than NADK from S. aureus. Additionally, we showed that SaNADK shares 57% of sequence identity with its listerial counterpart [14,18]. While compound 3 failed to bind to LmNADK, the other three derivatives yielded complexes that diffracted with 2.2–2.4 Å resolution. Compounds 4 and 5 in complex with LmNADK1 showed a binding mode highly similar to that of NKI1. In contrast, compound 2 showed a different orientation which could explain its increased inhibition constant. In line with their binding properties, compounds 4 and 5 had inhibition potentials in the range of NKI1 Ki. Compound 5 had the highest apparent affinity for the purified enzyme of all derivatives tested in our study.
Our functionalized diadenosine derivative 5, and in a lesser extent compound 4, have the required features to develop a specific affinity chromatography column, which could be applied to bacterial NADKs. Ultimately, this chemical probe will help to decipher the selectivity characteristics of our lead compound.
4. Materials and Methods
4.1. General Information
Commercially available reagents and solvents, unless otherwise stated, were used without purification. Anhydrous reactions were carried out under an argon atmosphere. Analytical thin-layer chromatography (TLC) was performed on TLC plates pre-coated with silica gel 60 F254. Compounds were visualized with UV light (254 nm) and by spraying with a mixture of ethanol/anisaldehyde/sulfuric acid/acetic acid (90/5/4/1), followed by heating. Reactions were also monitored using an HPLC system (Agilent (Agilent Technologies, France, Les Ulis) 1100 equipped with a C18 reverse phase column) coupled to a mass spectrometer (ESI source). Flash chromatography was performed with silica gel 60 (230–400 mesh). HPLC purification was carried out on an Agilent system (1100 Series) equipped with a diode array detector using a C18 reverse phase column (Kromasil, 5 μm, 100 Å, 150 × 10 mm) and a linear gradient of acetonitrile in 10 mM triethylammonium acetate (TEAA) buffer over 15 or 20 min at a flow rate of 4 mL/min. 1H and 13C NMR spectra were recorded on Bruker Avance 400 (Bruker, France, Wissembourg) at 400.13 MHz and 100.62 MHz, respectively. Chemical shifts (δ) are reported in parts per million (ppm) relative to the solvent signals. Coupling constants (J values) are reported in Hz. The complete assignment of 1H and 13C signals was performed by analysis of the correlated homonuclear 1H,{1H}–COSY and heteronuclear 1H,{13C}–HMBC, 1H,{13C}–HSQC spectra (see copies of 1H and 13C NMR spectra of prepared compounds in the Supplementary Materials). High-resolution mass spectra (HRMS) were recorded with a Q-Tof Micro mass spectrometer under electrospray ionization (ESI) using 0.1% formic acid in acetonitrile/water in positive ion mode. The purity of all tested compounds was greater than 97% (HPLC analysis). Retention time (tR) and gradient are specified.
4.2. Synthesis
2′,3′,5′-Tri-O-acetyl-8-bromoadenosine (6). The title compound was obtained by peracetylation of commercially available 8-bromoadenosine (90% yield).
6-Chloro-9-(2′,3′-O-isopropylidene-ß-D-ribofuranosyl)purine (10). The title compound was obtained by reaction of 6-chloropurine riboside with 2,2-dimethoxypropane in acetone in the presence of toluene-4-sulfonic acid for 3 h at room temperature (91% yield) [22].
N6-(2-tert-Butyloxycarbonyl)aminoethyl-2’,3’-O-isopropylidene-adenosine (11a). To a stirred solution of 6-chloro-9-(2′,3′-O-isopropylidene-ß-d-ribofuranosyl)purine (10) (0.98 g, 3.0 mmol) in anhydrous DME (15 mL) was added NEt3 (1.25 mL, 9.0 mmol), followed by a solution of N-Boc-ethylenediamine (prepared according to reported procedures) [23] (1.44 g, 9.0 mmol) in anhydrous DME (15 mL). The stirred mixture was heated at 80 °C overnight under an argon atmosphere, then concentrated under reduced pressure. The residue was dissolved in dichloromethane (80 mL), washed in turn with water (80 mL) and brine (80 mL). The organic layer was dried over Na2SO4 and concentrated under reduced pressure. The crude product was purified by flash column chromatography (50 g SiO2, 0 to 3% methanol in dichloromethane) to afford compound 11a (0.90 g, 66%) as a white foam. 1H NMR (400 MHz, CDCl3): δ 1.40 (s, 3H, CH3 isop), 1.44 (s, 9H, CH3 Boc), 1.66 (s, 3H, CH3 isop), 3.44 -3.48 (m, 2H, CH2NHBoc), 3.76-3.84 (m, 3H, CH2NH-6 and H-5’), 3.99 (dd, J = 1.4 Hz, J = 12.8 Hz, 1H, H-5”), 4.53-4.56 (m, 1H, H-4’), 5.09 (br s, 1H, NHBoc), 5.13 (dd, J = 5.8 Hz, J = 1.0 Hz, 1H, H-3’), 5.21 (pt, J = 5.5 Hz, 1H, H-2’), 5.87 (d, J = 4.9 Hz, 1H, H-1’), 6.40-6.90 (br s, 2H, NH-6 and OH-5’), 7.84 (s, 1H, H-8), 8.33 (s, 1H, H-2); 13C NMR (100 MHz, CDCl3): δ 25.25 (CH3 isop), 27.66 (CH3 isop), 28.34 (3 CH3 Boc), 40.74 (CH2NH-6 and CH2NHBoc), 63.42 (C-5’), 79.59 (Cq Boc), 81.71 (C-3’), 83.09 (C-2’), 86.15 (C-4’), 94.32 (C-1’), 113.98 (Cq isop), 121.42 (C-5), 139.76 (C-8), 147.61 (C-4), 152.91 (C-2), 155.35 (C-6), 156.30 (CO Boc); HRMS (ESI-TOF): m/z calcd for [C20H30N6O6+H]+ 451.2305, found 451.2293.
N6-(2-tert-Butyloxycarbonyl)aminobutyl-2’,3’-O-isopropylidene-adenosine (11b). Compound 11b was prepared from 10 (0.98 g, 3.0 mmol) and N-Boc-1,4-butanediamine (prepared according to reported procedures) [23] (1.69 g, 9.0 mmol) following the same procedure as for 11a. Purification by flash column chromatography (50 g SiO2, 0 to 3% methanol in dichloromethane) yielded compound 11b (1.31 g, 91%) as a white foam. 1H NMR (400 MHz, CDCl3): δ 1.39 (s, 3H, CH3 isop), 1.45 (s, 9H, CH3 Boc), 1.61 (quint, J = 7.0 Hz, 2H, CH2), 1.66 (s, 3H, CH3 isop), 1.74 (quint, J = 7.0 Hz, 2H, CH2), 3.19 (br q, 2H, CH2NHBoc), 3.68 (br s, 2H, CH2NH-6), 3.80 (br d, J = 12.8 Hz, 1H, H-5’), 3.99 (dd, J = 1.3 Hz, J = 12.8 Hz, 1H, H-5”), 4.53-4.56 (m, 1H, H-4’), 4.78 (br s, 1H, NHBoc), 5.13 (dd, J = 1.0 Hz, J = 5.9 Hz, 1H, H-3’), 5.22 (pt, J = 5.8 Hz, 1H, H-2’), 5.86 (d, J = 4.8 Hz, 1H, H-1’), 6.04 (br s, 1H, OH-5’), 6.67 (br s, 1H, NH-6), 7.79 (s, 1H, H-8), 8.33 (s, 1H, H-2); 13C NMR (100 MHz, CDCl3): δ 25.34 (CH3 isop), 26.97 (CH2), 27.24 (CH2), 27.65 (CH3 isop), 28.42 (CH3 Boc), 40.16 (CH2NH-6 and CH2NHBoc), 63.42 (C-5’), 79.15 (Cq Boc), 81.72 (C-3’), 83.06 (C-2’), 86.14 (C-4’), 94.35 (C-1’), 113.94 (Cq isop), 121.25 (C-5), 139.52 (C-8), 147.31 (C-4), 152.69 (C-2), 155.25 (C-6), 156.01 (CO Boc); HRMS (ESI-TOF): m/z calcd for [C22H34N6O6+H]+ 479.2618, found 479.2614.
N6-(2-tert-Butyloxycarbonyl)aminoethyl-2’,3’-O-isopropylidene-5’-O-propargyl-adenosine (8a). To a stirred suspension of sodium hydride (0.10 g 60% in oil, 2.5 mmol) in anhydrous THF (12 mL) was added at 0 °C a solution of 11a (0.56 g, 1.2 mmol) in THF (12 mL). The reaction mixture was stirred at 0 °C for 1 h, then propargyl bromide (80% in toluene, 0.13 mL, 1.2 mmol) was added dropwise. After 24 h at 4 °C, the reaction was quenched by adding glacial acetic acid (0.14 mL) and stirring for 1 h at 4 °C. After removal of the volatiles under reduced pressure, the crude residue was dissolved ethyl acetate (50 mL) and washed twice with water (60 mL). The organic layer was dried, concentrated under reduced pressure and the residue purified by flash column chromatography (30 g SiO2, 0 to 3% methanol in dichloromethane) affording compound 8a (0.35 g, 60%) as a white foam. 1H NMR (400 MHz, CDCl3): δ 1.41 (s, 3H, CH3 isop), 1.43 (s, 9H, CH3 Boc), 1.64 (s, 3H, CH3 isop), 2.44 (t, J = 2.3 Hz, 1H, HC≡), 3.44 (br q, J = 5.6 Hz, 2H, CH2NHBoc), 3.73 (dd, J = 4.8 Hz, J = 10.3 Hz, 1H, H-5’), 3.78 (dd, J = 3.9 Hz, J = 10.3 Hz, 1H, H-5”), 3.81-3.85 (m, 2H, CH2NH-6), 4.14 (d, J = 2.3 Hz, 2H, CH2C≡), 4.49-4.53 (m, 1H, H-4’), 5.03 (dd, J = 2.7 Hz, J = 6.2 Hz, 1H, H-3’), 5.17 (br s, 1H, NHBoc), 5.34 (dd, J = 2.3 Hz, J = 6.2 Hz, 1H, H-2’), 6.19 (d, J = 2.4 Hz, 1H, H-1’), 6.33 (br s, 1H, NH-6), 8.01 (s, 1H, H-8), 8.39 (s, 1H, H-2); 13C NMR (100 MHz, CDCl3): δ 25.38 (CH3 isop), 27.18 (CH3 isop), 28.35 (3 CH3 Boc), 40.90 (CH2NH-6 and CH2NHBoc), 58.57 (C≡C-CH2), 69.85 (C-5’), 75.24 (C≡C-CH2), 78.80 (C≡C-CH2), 79.44 (Cq Boc), 81.88 (C-3’), 84.76 (C-2’), 85.74 (C-4’), 91.20 (C-1’), 114.23 (Cq isop), 120.16 (C-5), 138.93 (C-8), 148.87 (C-4), 152.91 (C-2), 154.88 (C-6), 156.27 (CO Boc); HRMS (ESI-TOF): m/z calcd for [C23H32N6O6+H]+ 489.2462, found 489.2463.
N6-(2-tert-Butyloxycarbonyl)aminobutyl-2’,3’-O-isopropylidene-5’-O-propargyl-adenosine (8b). Compound 8b was prepared from 11b (0.95 g, 2.0 mmol) following the same procedure as for 8a. Purification by flash column chromatography (70 g SiO2, 0 to 2% methanol in dichloromethane) afforded compound 8b (0.70 g, 68%). 1H NMR (400 MHz, CDCl3): δ 1.36 (s, 3H, CH3 isop), 1.41 (s, 9H, CH3 Boc), 1.51-1.63 (m, 2H, CH2), 1.59 (s, 3H, CH3 isop), 1.69 (quint, J = 7.0 Hz, 2H, CH2), 2.42 (t, J = 2.3 Hz, 1H, HC≡), 3.10-3.19 (m, 2H, CH2NHBoc), 3.60-3.66 (m, 2H, CH2NH-6), 3.69 (dd, J = 4.9 Hz, J = 10.3 Hz, 1H, H-5’), 3.74 (dd, J = 4.0 Hz, J = 10.3 Hz, 1H, H-5”), 4.10 (d, J = 2.3 Hz, 2H, CH2C≡), 4.44-4.49 (m, 1H, H-4’), 4.99 (dd, J = 2.6 Hz, J = 6.1 Hz, 1H, H-3’), 5.32 (dd, J = 2.3 Hz, J = 6.3 Hz, 1H, H-2’), 6.05 (br s, 1H, NH-6), 6.15 (d, J = 2.3 Hz, 1H, H-1’), 7.94 (s, 1H, H-8), 8.34 (s, 1H, H-2); 13C NMR (100 MHz, CDCl3): δ 25.34 (CH3 isop), 27.08 (CH3 isop), 27.14 and 27.19 (2 CH2), 28.40 (3 CH3 Boc), 40.12 (CH2NH-6 and CH2NHBoc), 58.53 (C≡C-CH2), 69.82 (C-5’), 75.30 (C≡C-CH2), 78.83 (C≡C-CH2), 78.99 (Cq Boc), 81.85 (C-3’), 84.68 (C-2’), 85.68 (C-4’), 91.06 (C-1’), 114.17 (Cq isop), 120.07 (C-5), 138.66 (C-8), 148.68 (C-4), 153.23 (C-2), 154.94 (C-6), 156.04 (CO Boc); HRMS (ESI-TOF): m/z calcd for [C25H36N6O6+H]+ 517.2775, found 517.2780.
2’,3’,5’-Tri-O-acetyl-8-[3-(N6-(2-tert-butyloxycarbonyl)aminoethyl-2’,3’-O-isopropylidene-5’-O-adenosinyl)propargyl]adenosine (12a). To an argon-degassed solution (three times) of 8a (0.20 g, 0.42 mmol) and bromide 6 (0.31 g, 0.63 mmol) in anhydrous THF (8 mL) in the presence of NEt3 (0.18 mL, 1.26 mmol) were added CuI (8 mg, 0.042 mmol) and Pd(PPh3)4 (24 mg, 0.021 mmol). The stirred mixture was degassed 3 times, then heated at 60 °C under argon atmosphere for 5 h. The volatiles were removed under reduced pressure and the residue purified by flash column chromatography (20 g SiO2, 0 to 4% methanol in dichloromethane) affording compound 12a (0.15 g, 40%) and the homocoupling product (63 mg, 8%). 1HNMR (400 MHz, CDCl3): δ 1.41 (s, 3H, CH3 isop), 1.43 (s, 9H, CH3 Boc), 1.64 (s, 3H, CH3 isop), 2.05 (s, 3H, COCH3), 2.10 (s, 3H, COCH3), 2.14 (s, 3H, COCH3), 3.39-3.47 (m, 2H, CH2NHBoc) 3.77 (br, 2H, CH2NH-6), 3.84 (dd, J = 5.1 Hz, J = 10.2 Hz, 1H, H-5’B), 3.92 (dd, J = 4.0 Hz, J = 10.2 Hz, 1H, H-5”B), 4.32 (dd, J = 6.0 Hz, J = 11.8 Hz, 1H, H-5’A), 4.41 (td, J = 6.0 Hz, J = 11.8 Hz, 1H, H-4’A), 4.47 (br, 2H, CH2C≡), 4.50-4.55 (m, 2H, H-4’B and H-5”A), 5.07 (dd, J = 3.0 Hz, J = 6.2 Hz, 1H, H-3’B), 5.23 (br s, 1H, NHBoc), 5.35-5.41 (br s, 1H, H-2’B), 5.96 (t, J = 6.1 Hz, 1H, H-3’A), 6.19 (d, J = 2.2 Hz, 1H, H-1’B), 6.22 (d, J = 4.2 Hz, 1H, H-1’A), 6.24 (br s, 2H, NH2), 6.30 (dd, J = 4.2 Hz, J = 6.1 Hz, 1H, H-2’A), 6.46 (br s, 1H, NH-6B), 8.02 (s, 1H, H-8B), 8.37 (s, 1H, H-2A), 8.42 (s, 1H, H-2B); 13CNMR (100 MHz, CDCl3): δ 20.43 (COCH3), 20.49 (COCH3), 20.64 (COCH3), 25.36 (CH3 isop), 27.16 (CH3 isop), 28.36 (3 CH3 Boc), 40.71 (CH2NHBoc and CH2NH-6), 59.04 (C≡C-CH2), 63.05 (C-5’A), 70.49 (C-5’B), 70.54 (C-3’A), 72.52 (C-2’A), 76.69 (C≡C-CH2), 79.49 (Cq Boc), 79.86 (C-4’A), 81.80 (C-3’B), 84.70 (C-2’B), 85.74 (C-4’B), 87.37 (C-1’A), 91.12 (C-1’B), 91.80 (C≡C-CH2), 114.36 (Cq isop), 119.78 (C-5A), 120.20 (C-5B), 133.26 (C-8A), 138.88 (C-8B); 148.90 (C-4B); 149.50 (C-4A), 153.23 (C-2B), 154.26 (C-2A), 155.00 (C-6B), 155.52 (C-6A), 156.31 (CO Boc), 169.42 (CO), 169.50 (CO), 170.58 (CO); HRMS (ESI-TOF): m/z calcd for [C39H49N11O13+H]+ 880.3589, found 880.3582.
2’,3’,5’-Tri-O-acetyl-8-[3-(N6-(4-tert-butyloxycarbonyl)aminobutyl-2’,3’-O-isopropylidene-5’-O-adenosinyl)propargyl]adenosine (12b). Compound 12b was prepared from 8b (206 mg, 0.4 mmol) and bromide 6 (184 mg, 0.26 mmol) following the same procedure as for 12a. Purification by chromatography on silica gel (1 to 4% methanol in dichloromethane) gave compound 12b (88 mg, 37%). 1H NMR (400 MHz, CDCl3): δ 1.41 (s, 3H, CH3 isop), 1.46 (s, 9H, CH3 Boc), 1.56-1.66 (m, 2H, CH2), 1.64 (s, 3H, CH3 isop), 1.67-1.79 (m, 2H, CH2), 2.05 (s, 3H, COCH3), 2.10 (s, 3H, COCH3), 2.15 (s, 3H, COCH3), 3.15-3.25 (m, 2H, CH2NHBoc), 3.60-3.70 (m, 2H, CH2NH-6), 3.85 (dd, J = 5.2 Hz, J = 10.1 Hz, 1H, H-5’B), 3.92 (dd, J = 4.2 Hz, J = 10.1 Hz, 1H, H-5”B), 4.33 (dd, 1H, J = 6.0 Hz, J = 11.8 Hz, H-5”A), 4.38-4.44 (m, 1H, H-4”A), 4.48 (d, J = 1.0 Hz, 2H, CH2C≡), 4.50-4.55 (m, 2H, H-4’B and H-5”A), 5.10 (dd, J = 3.0 Hz, J = 6.2 Hz, 1H, H-3’B), 5.41 (dd, J = 2.2 Hz, J = 6.2 Hz, 1H, H-2’B), 5.93 (br, 2H, NH2), 5.96 (pt, 1H, J = 6.0 Hz, H-3’A), 6.18 (d, J = 2.2 Hz, 1H, H-1’B), 6.23 (d, 1H, J = 4.2 Hz, H-1’A), 6.30 (dd, J = 4.2 Hz, J = 6.0 Hz, 1H, H-2’A), 7.96 (s, 1H, H-8B), 8.39 (s, 1H, H-2A), 8.43 (s, 1H, H-2B); 13C NMR (100 MHz, CDCl3): δ 20.44 (COCH3), 20.51 (COCH3), 20.65 (COCH3), 25.36 (CH3 isop), 27.04 (CH2), 27.16 (CH3 isop), 27.26 (CH2), 28.44 (3 CH3 Boc), 40.03 (CH2NH-6 and CH2NHBoc), 59.05 (C≡C-CH2), 63.05 (C-5’A), 70.50 (C-3’A), 70.56 (C-5’B), 72.50 (C-2’A), 75.62 (C≡C-CH2), 79.16 (Cq Boc), 79.88 (C-4’A), 81.86 (C-3’B), 84.59 (C-2’B), 85.79 (C-4’B), 87.41 (C-1’A), 91.05 (C-1’B), 91.91 (C≡C-CH2), 114.37 (Cq isop), 118.13 (C-5A); 119.80 (C-5B), 133.42 (C-8A), 138.71 (C-8B), 148.92 (C-4B); 149.40 (C-4A); 153.43 (C-2B); 154.27 (C-2A); 154.96 (C-6B), 155.38 (C-6A), 156.05 (CO Boc), 169.37 (CO), 169.48 (CO), 170.55 (CO); HRMS (ESI-TOF): m/z calcd for [C41H53N11O13+H]+ 908.3803, found 908.4268.
8-[3-(N6-2-Aminoethyl-5’-O-adenosinyl)propargyl]adenosine (2). To a solution of 12a (46 mg, 0.05 mmol) in MeOH (5 mL) was added 28% NH4OH (1 mL). After stirring overnight at room temperature, the reaction mixture was concentrated under reduced pressure, and the crude product (37 mg) was used in the next step without further purification. To the foam (37 mg, 0.05 mmol) was added at 0 °C a 70% aqueous solution of TFA (1 mL). After stirring for 2 h, water was added and the mixture was lyophilized. Purification by reverse phase HPLC (0 to 20% acetonitrile in 10 mM TEAA buffer over 15 min, tR = 13.3 min) afforded compound 2 as acetate salt (13 mg, 42% in two steps). 1H NMR (400 MHz, DMSO-d6): δ 1.88 (s, 1.3H, CH3 Ac), 2.81-2.86 (m, 2H, CH2NH2), 3.54 (d, J = 4.2 Hz, J = 12.2 Hz, 1H, H-5’A), 3.59 (br, 2H, CH2NH-6), 3.68 (d, J = 3.9 Hz, J = 12.2 Hz, 1H, H-5’’A), 3.76 (d, J = 5.5 Hz, J = 10.6 Hz, 1H, H-5’B), 3.85 (dd, J = 3.6 Hz, J = 10.6 Hz, 1H, H-5”B), 3.97-4.02 (m, 1H, H-4’A), 4.08-4.13 (m, 1H, H-4’B), 4.19-4.23 (m, 2H, H-3’A and H-3’B), 4.60 (s, 2H, CH2C≡), 4.63 (t, 1H, J = 5.1 Hz, H-2’B), 5.01 (dd, J = 5.3 Hz, J = 6.6 Hz, 1H, H-2’A), 5.93 (d, 1H, J = 5.1 Hz, H-1’B), 5.95 (d, J = 6.6 Hz, 1H, H-1’A), 7.63 (br s, 2H, NH2), 7.73 (br s, 2H, NH2), 8.17 (s, 1H, H-2B), 8.24 (s, 1H, H-2A), 8.33 (s, 1H, H-8B); 13C NMR (100 MHz, DMSO-d6): δ 21.83 (CH3 Ac), 40.94 (CH2NH2 and CH2NH-6), 58.76 (C≡C-CH2), 62.63 (C-5’A), 70.74 (C-5’B), 71.00 (C-3’B), 71.43 (C-3’A), 72.11 (C-2’A), 73.71 (C-2’B), 75.55 (C≡C-CH2), 83.33 (C-4’B), 87.16 (C-4’A), 87.98 (C-1’B), 89.92 (C-1’A), 92.60 (C≡C-CH2), 119.83 (C-5A and C-5B), 133.45 (C-8A), 139.76 (C-8B), 148.89 (C-4A and C-4B), 153.07 (C-2A), 153.90 (C-2B), 156.63 (C-6A and C-6B), 172.70 (CO Ac); HRMS (ESI-TOF): m/z calcd for [C25H31N11O8+H]+ 614.2435, found 614.2433.
8-[3-(N6-4-Aminobutyl-5’-O-adenosinyl)propargyl]adenosine (3). Compound 3 was obtained from 12b (63 mg, 0.07 mmol) following the same procedure as for 2. Purification by reverse phase HPLC (0 to 30% acetonitrile in 10 mM TEAA buffer over 20 min, tR = 8.7 min) afforded compound 3 as acetate salt (22.6 mg, 50% in two steps). 1H NMR (400 MHz, DMSO-d6): δ 1.48-1.58 (m, 2H, CH2), 1.58-1.69 (m, 2H, CH2), 1.84 (s, 1H, CH3 Ac), 2.73 (t, J = 7.2 Hz, 2H, CH2NH2), 3.50 (br, 2H, CH2NH-6), 3.54 (dd, J = 4.2 Hz, J = 12.1 Hz, 1H, H-5’A), 3.69 (m, J = 3.9 Hz, J = 12.2 Hz, 1H, H-5”A), 3.76 (dd, J = 5.5 Hz, J = 10.5 Hz, 1H, H-5’B), 3.85 (dd, J = 3.8 Hz, J = 10.5 Hz, 1H, H-5”B), 3.98-4.01 (m, 1H, H-4’A), 4.08-4.12 (m, 1H, H-4’B), 4.19-4.23 (m, 2H, H-3’A and H-3’B), 4.60 (s, 2H, CH2C≡), 4.63 (pt, J = 5.3 Hz, 1H, H-2’B), 5.00 (dd, J = 5.3 Hz, J = 6.7 Hz, 1H, H-2’A), 5.93 (d, J = 5.4 Hz, 1H, H-1’B), 5.95 (d, J = 6.7 Hz, 1H, H-1’A), 7.63 (br s, 2H, NH2), 7.79 (br s, 2H, NH2), 8.17 (s, 1H, H-2B), 8.23 (s, 1H, H-2A), 8.32 (s, 1H, H-8B); 13C NMR (100 MHz, DMSO-d6): δ 23.40 (CH3 Ac), 25.81 (CH2), 26.53 (CH2), 39.39 (CH2NH2 and CH2NH-6), 58.73 (C≡C-CH2), 62.49 (C-5’A), 70.66 (C-5’B), 70.83 (C-3’B), 71.29 (C-3’A), 72.12 (C-2’A), 73.56 (C-2’B), 75.51 (C≡C-CH2), 83.33 (C-4’B), 87.07 (C-4’A), 87.82 (C-1’B), 89.88 (C-1’A), 92.72 (C≡C-CH2), 119.71 (C-5A and C-5B), 133.45 (C-8A), 139.76 (C-8B), 148.83 (C-4A and C-4B), 153.18 (C-2A), 153.88 (C-2B), 156.40 (C-6A and C-6B), 173.90 (CO Ac); HRMS (ESI-TOF): m/z calcd for [C27H35N11O8 + H]+ 642.2748, found 642.2748.
5’-[4-(N-tert-Butyloxycarbonyl-amino)butylamido]-5’-deoxy-2’,3’-O-isopropylidene-adenosine (14). To a solution of 5’-amino-5’-deoxy-2’,3’-O-isopropylidene-adenosine (13) [24] (0.31 g, 1.0 mmol) in DMF were added DIEA (0.35 mL, 2.0 mmol), PyBOP (0.52 g, 1.0 mmol) and 4-(tert-butoxycarbonylamino)-butyric acid (0.20 g, 1.0 mmol). After stirring for 1 h at room temperature, the reaction was diluted with water (40 mL) and extracted with ethyl acetate (2x 80 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by flash column chromatography (18 g SiO2, 0 to 5% methanol in dichloromethane) to yield compound 3 (0.36 g, 74%) as a white foam. 1H NMR (400 MHz, DMSO-d6): δ 1.32 (s, 3H, CH3 isop), 1.36 (s, 9H, CH3 Boc), 1.54 (s, 3H, CH3 isop), 1.59 (quint, J = 7.2 Hz, 2H, CH2), 2.09 (td, J = 2.4 Hz, J = 7.2 Hz, 2H, CH2CO), 2.89 (br q, J = 6.6 Hz, 2H, CH2NHBoc), 3.30-3.35 (m, 2H, H-5’ and H5’’), 4.17 (td, J = 3.2 Hz, J = 5.7 Hz, 1H, H-4’), 4.90 (dd, J = 6.3 Hz, J = 3.2 Hz, 1H, H-3’), 5.43 (dd, J = 3.0 Hz, J = 6.3 Hz, 1H, H-2’), 6.12 (d, J = 3.0 Hz, 1H, H-1’), 6.75 (br s, 1H, NHBoc), 7.34 (s, 2H, NH2) 8.06 (br, 1H, NHCO), 8.19 (s, 1H, H-2), 8.32 (s, 1H, H-8); 13C NMR (100 MHz, DMSO-d6): δ 25.70 (CH3 isop), 26.15 (CH2), 27.50 (CH3 isop), 28.70 (3 CH3 Boc), 33.14 (CH2CO), 39.95 (CH2NHBoc), 41.16 (C-5’), 77.85 (Cq Boc), 82,17 (C-3’), 83.30 (C-2’), 84.63 (C-4’), 89.55 (C-1’), 113.99 (Cq isop), 119.80 (C-5), 140.53 (C-8), 149.25 (C-4), 153.19 (C-2), 156.02 (CO Boc), 156.67 (C-6), 172.68 (CONH); HRMS (ESI-TOF): m/z calcd for [C22H33N7O6+H]+ 492.2570, found 492.2582.
8-Bromo-5’-(4-(N-tert-butyloxycarbonyl-amino)butylamido)-5’-deoxy-2’,3’-O-isopropylidene-adenosine (7). To a solution of 14 (0.17 g, 0.35 mmol) in 1,4-dioxane (1.4 mL) and sodium acetate buffer (2.1 mL, 0.5 M, pH = 5.3) was added bromine (36 µL, 0.69 mmol) dropwise. After stirring for 3 h at room temperature, the reaction was quenched by adding a saturated aqueous solution of Na2S2O3 (10 mL). After full discoloration, ethyl acetate (80 mL) was added and the organic layer was washed with water (20 mL) and brine (20 mL). The organic layer was dried over Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by flash column chromatography (10 g SiO2, 0-4% methanol in dichloromethane) to yield compound 7 (0.135 g, 69%) as a pale yellow foam. 1H NMR (400 MHz, CDCl3): δ 1.38 (s, 3H, CH3 isop), 1.42 (s, 9H, CH3 Boc), 1.65 (s, 3H, CH3 isop), 1.83-1.92 (m, 2H, CH2), 2.32-2.40 (m, 2H, CH2CO), 3.18-3.23 (m, 2H, CH2NHBoc), 3.37 (dt, J = 3.0 Hz, J = 14.3 Hz, 1H, H-5’), 3.99 (ddd, J = 4.0 Hz, J = 8.0 Hz, J = 14.3 Hz, 1H, H-5’’), 4.46 (pq, J = 3.2 Hz, 1H, H-4’), 4.86 (br s, 1H, NHBoc), 4.89 (dd, J = 2.6 Hz, J = 6.2 Hz, 1H, H-3’), 5.45 (dd, J = 4.2 Hz, J = 6.2 Hz, 1H, H-2’), 6.09 (d, J = 4.2 Hz, 1H, H-1’), 6.40 (br s, 2H, NH2), 7.64 (br s, 1H, NHCO), 8.35 (s, 1H, H-2); 13C NMR (100 MHz, CDCl3): δ 25.41 (CH3 isop), 26.04 (CH2), 27.46 (CH3 isop), 28.38 (3 CH3 Boc), 33.49 (CH2CO), 39.94 (CH2NHBoc), 40.93 (C-5’), 79.22 (Cq Boc), 81.49 (C-3’), 82.18 (C-2’), 83.95 (C-4’), 91.98 (C-1’), 114.83 (Cq isop), 120.51 (C-5), 127.79 (C-8), 150.17 (C-4), 151.59 (C-2), 153.96 (C-6), 156.23 (CO Boc), 173.01 (CONH); HRMS (ESI-TOF): m/z calcd for [C22H32BrN7O6+H]+ 570.1675, found 570.1683.
5’-O-[3-(5’-(4-(N-tert-Butyloxycarbonyl-amino)butylamido)-5’-deoxy-2’,3’-O-isopropylideneadenosine-8-yl)prop-2-yn-1-yl]-2’,3’-O-isopropylideneadenosine (15). To a solution of bromide 7 (0.19 g, 0.34 mmol) and alkyne 8c [18] (0.18 g, 0.51 mmol) in THF (4.0 mL) was added NEt3 (0.14 mL, 1.02 mmol). The stirred mixture was degassed by vacuum/argon purging (3 times) before adding sequentially CuI (7 mg, 0.036 mmol) and Pd(PPh3)4 (20 mg, 0.017 mmol), followed by another 15 min argon degassing. After stirring for 3 h at 60 °C, reaction was incomplete (TLC monitoring), Pd(PPh3)4 (40 mg, 0.034 mmol) was added. After 3 h at 60 °C, volatiles were removed and the residue was purified by flash column chromatography (18 g SiO2, 0 to 7% methanol in dichloromethane) to yield 15 (74 mg, 25 %) as a pale orange foam. 1H NMR (400 MHz, DMSO-d6): δ 1.28 (s, 3H, CH3 isop), 1.34 (s, 3H, CH3 isop), 1.35 (s, 9H, CH3 Boc), 1.48 (s, 3H, CH3 isop), 1.55 (s, 3H, CH3 isop), 1.58 (quint, J = 7.2 Hz, 2H, CH2), 2.04-2.08 (m, 2H, CH2CO), 2.89 (q, J = 6.6 Hz, 2H, CH2CO), 3.30-3.40 (m, 2H, H-5’A and H-5”A), 3.72 (dd, J = 5.0 Hz, J = 10.3 Hz, 1H, H-5’B), 3.80 (dd, J = 5.7 Hz, J = 10.3 Hz, 1H, H-5’’B), 4.14-4.18 (m, 1H, H-4’A), 4.36-4.40 (m, 1H, H-4’B), 4.58 (s, 2H, CH2C≡), 4.95 (dd, J = 3.4 Hz, J = 6.3 Hz, 1H, H-3’A), 5.02 (dd, J = 3.0 Hz, J = 6.2 Hz, 1H, H-3’B), 5.43 (dd, J = 2.6 Hz, J = 6.2 Hz, 1H, H-2’B), 5.51 (dd, J = 2.7 Hz, J = 6.3 Hz, 1H, H-2’A), 6.11 (d, J = 2.7 Hz, 1H, H-1’A), 6.19 (d, J = 2.6 Hz, 1H, H-2’B), 6.73 (br, 1H, NHBoc), 7.54 (br, 2H, NH2), 7.65 (br, 2H, NH2), 8.03 (t, J = 5.7 Hz, 1H, NHCO), 8.22 (s, 1H, H-2B, 8.24 (s, 1H, H-2A), 8.36 (s, 1H, H-8B); 13C NMR (100 MHz, DMSO-d6): δ 25.66 (2 CH3 isop), 26.12 (CH2), 27.50 (2 CH3 isop), 28.69 (3 CH3 Boc), 33.12 (CH2CO), 39.82 (CH2NHBoc), 41.06 (C-5’A), 58.67 (C≡C-CH2), 70.33 (C-5’B), 75.50 (C≡C-CH2), 77.85 (Cq Boc), 81.91 (C-3’B), 82.37 (C-3’A), 82.96 (C-2’A), 83.80 (C-2’B), 84.81 (C-4’B), 85.25 (C-4’A), 89.76 (C-1’A and C-1’B), 92.75 (C≡C-CH2), 113.90 (Cq isop), 114.01 (Cq isop), 119.44 (C-5A and C-5B), 132.57 (C-8A), 140.45 (C-8B), 148.78 (C-4A), 149.24 (C-4B), 152.24 (C-2A), 154.24 (C-2B), 155.76 (C-6B), 156.03 (C-6A), 156.38 (CO Boc), 172.60 (CONH); HRMS (ESI-TOF): m/z calcd for [C38H50N12O10+H]+ 835.3851, found 835.3840.
5’-O-[3-(5’-(4-Aminobutylamido)-5’-deoxy-adenosin-8-yl)prop-2-yn-1-yl]-adenosine (4). Compound 15 (64 mg, 0.08 mmol) was dissolved in an ice-cold solution of 50% aqueous TFA (5.0 mL) and the reaction mixture was allowed to warm to room temperature. After stirring for 3 h at room temperature, water was added and the reaction mixture was lyophilized. Purification of the residue by reverse phase HPLC (5 to 25% acetonitrile in 10 mM TEAA buffer over 15 min) yielded 4 (22.6 mg, 45%) as acetate salt. tR = 10.4 min; 1H NMR (400 MHz, DMSO-d6): δ 1.68 (quint, J = 7.2 Hz, 2H, CH2), 1.79 (s, 1.88 H, CH3 Ac), 2.20 (t, J = 6.2 Hz, 2H, CH2CO), 2.65 (t, J = 6.2 Hz, 2H, CH2NH2), 3.38 (dt, J = 4.8 Hz, J = 14.0 Hz, 1H, H-5’A), 3.47 (dt, J = 5.8 Hz, J = 14.0 Hz, 1H, H-5”A), 3.77 (dd, J = 5.6 Hz, J = 10.6 Hz, 1H, H-5’B), 3.85 (dd, J = 3.7 Hz, J = 10.6 Hz, 1H, H-5”B), 3.98-4.01 (m, 1H, H-4’A), 4.08-4.13 (m, 2H, H-3’A and H-4’B), 4.20 (t, J = 4.7 Hz, 1H, H-3’B), 4.61-4.63 (m, 3H, H-2’B and CH2C≡), 5.01-5.04 (m, 1H, H-2’A), 5.92 (d, J = 5.3 Hz, 1H, H-1’B), 5.94 (d, J = 6.4 Hz, 1H, H-1’A), 7.25 (s, 2H, NH2), 7.62 (s, 2H, NH2), 8.17 (s, 1H, H-2B), 8.24 (s, 1H, H-2A), 8.27 (m, 1H, NHCO), 8.32 (s, 1H, H-8B); 13C NMR (100 MHz, DMSO-d6): δ 23.08 (CH3 Ac), 26.58 (CH2), 33.02 (CH2CO), 40.02 (CH2NH2), 41.27 (C-5’A), 58.74 (C≡C-CH2), 70.77 (C-5’B), 71.02 (C-3’B), 71.71 (C-3’A and C-2’A), 73.69 (C-2’B), 75.70 (C≡C-CH2), 83.28 (C-4’B), 84.55 (C-4’A), 87.96 (C-1’B), 89.67 (C-1’A), 92.56 (C≡C-CH2), 119.50 (C-5B), 119.81 (C-5A), 133.41 (C-8A), 139.84 (C-8B), 149.16 (C-4A), 149.95 (C-4B), 153.18 (C-2B), 154.21 (C-2A), 156.50 (C-6B), 156.59 (C-6A), 172.41 (CONH), 173.60 (CO Ac); HRMS (ESI-TOF): m/z calcd for [C27H34N12O8+H]+ 655.2701, found 655.2708.
5’-Deoxy-5’-(2-nitrobenzenesulfonamido)-2’,3’-O-isopropylidene-adenosine (16). To a solution of 13 (2.30 g, 7.5 mmol) in pyridine (75 mL) was added 2-nitro-benzenesulfonyl chloride (3.82 g, 17.25 mmol). After stirring for 2 h at room temperature, volatiles were removed and the residue was purified by flash column chromatography (240 g SiO2, 3% isocratic methanol in dichloromethane) to yield compound 16 (3.25 g, 88%) as a white solid. 1H NMR (400 MHz, DMSO-d6): δ 1.29 (s, 3H, CH3 isop), 1.52 (s, 3H, CH3 isop), 3.19-3.33 (m, 2H, H-5’ and H-5’’), 4.24 (td, J = 2.9 Hz, J = 5.7 Hz, 1H, H-4’), 4.95 (dd, J = 2.9 Hz, J = 6.2 Hz, 1H, H-3’), 5.37 (dd, J = 2.9 Hz, J = 6.2 Hz, H-2’), 6.12 (d, J = 2.9 Hz, H-1’), 7.38 (s, 2H, NH2), 7.70-7.74 (m, 1H, H-4 Ns), 7.78-7.82 (m, 1H, H-3 Ns), 7.85-7.87 (m, 1H, H-5 Ns), 7.91-7-93 (m, 1H, H-2 Ns), 8.13 (s, 1H, H-8), 8.29 (s, 1H, H-2), 8.60 (br s, 1H, NH-5’); 13C NMR (100 MHz, DMSO-d6): δ 25.60 (CH3 isop), 27.42 (CH3 isop), 45.12 (C-5’), 82.01 (C-3’), 83.33 (C-2’), 84.68 (C-4’), 90.00 (C-1’), 133.94 (Cq isop), 299.82 (C-5), 124.83 (C-3 Ns), 129.90 (C-6 Ns), 132.93 (C-5 Ns), 133.01 (C-1 Ns), 134.50 (C-4 Ns), 140.63 (C-8), 148.00 (C-2 Ns), 148.90 (C-4), 153.05 (C-2), 156.71 (C-6); HRMS (ESI-TOF): m/z calcd for [C19H21N7O7S+H]+ 492.1302 found, 492.1302.
5’-N-(N-tert-Butyloxycarbonyl-aminopropyl)amino-5’-deoxy-2’,3’-O-isopropylidene-adenosine (17). To a solution of 17 (0.80 g, 1.63 mmol) in DMF was added K2CO3 (0.68 g, 4.88 mmol) followed by tert-butyl-3-bromopropylcarbamate (prepared according reported procedures) [25] (0.47 g, 1.95 mmol). After stirring overnight at 50 °C, alkylation was incomplete and tert-butyl-3-bromopropylcarbamate (0.23 g, 0.98 mmol) was further added. After heating at 50 °C for 24 h, thiophenol (0.36 mL, 3.26 mmol) was added and stirring was maintained for 18 h at room temperature. Volatiles were then removed under reduced pressure and the residue purified by flash column chromatography (85 g SiO2 70–230 mesh, 5 to 11% methanol in dichloromethane to yield compound 17 (0.61 g, 79%) as a white foam. 1H NMR (400 MHz, DMSO-d6): δ 1.33 (s, 3H, CH3 isop), 1.36 (s, 9H, CH3 Boc), 1.48 (quint, J = 6.8 Hz, 2H, CH2), 1.54 (s, 3H, CH3 isop), 2.46-2.51 (m, 2H, CH2NH), 2.66 (dd, J = 6.1 Hz, J = 12.2 Hz, 2H, H-5’), 2.73 (dd, J = 5.9 Hz, J = 12.2 Hz, 2H, H-5”), 2.93 (br q, J = 6.8 Hz, 2H, CH2NHBoc), 4.21 (td, J = 2.8 Hz, J = 5.9 Hz, 1H, H-4’), 4.97 (dd, J = 6.3 Hz, J = 2.8 Hz, 1H, H-3’), 5.45 (dd, J = 3.0 Hz, J = 6.3 Hz, 1H, H-2’), 6.09 (d, J = 3.0 Hz, 1H, H-1’), 6.75 (br, 1H, NHBoc), 7.30 (s, 2H, NH2), 8.16 (s, 1H, H-8), 8.35 (s, 1H, H-2); 13C NMR (100 MHz, DMSO-d6): δ 25.72 (CH3 isop), 27.53 (CH3 isop), 28.72 (3 CH3 Boc), 30.08 (CH2), 38.51 (CH2NHBoc), 47.26 (CH2NH), 51.47 (C-5’), 77.81 (Cq Boc), 82.65 (C-3’), 83.20 (C-2’), 85.33 (C-4’), 89.75 (C-1’), 113.68 (Cq isop), 119.71 (C-5), 140.42 (C-8), 149.41 (C-4), 153.15 (C-2), 156.07 (CO Boc), 156.62 (C-6); HRMS (ESI-TOF): m/z calcd for [C21H33N7O5+H]+ 464.2621, found 464.2628.
5’-N-(N-tert-Butyloxycarbonyl-aminopropyl)propargylamino-5’-deoxy-2’,3’-O-isopropylidene-adenosine (18). To a solution of 17 (0.60 g, 1.3 mmol) in DMF (13 mL), were added DIEA (1.35 mL, 7.7 mmol) and propargyl bromide (80% in toluene, 0.79 mL, 7.10 mmol). After stirring for 2 h at room temperature, the reaction was diluted with ethyl acetate (100 mL) and washed with water (2 x 50 mL). Organic layer was dried over Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by flash column chromatography (20 g SiO2, 3 to 4% methanol in dichloromethane) to yield compound 18 (0.53 g, 82%) as a white foam. 1H NMR (400 MHz, DMSO-d6): δ 1.33 (s, 3H, CH3 isop), 1.37 (s, 9H, CH3 Boc), 1.46 (quint, J = 6.8 Hz, 2H, CH2), 1.54 (s, 3H, CH3 isop), 2.40 (t, J = 6.8 Hz, 2H, CH2N), 2.51-2.55 (m, 1H, H-5’), 2.67 (dd, J = 7.6 Hz, J = 13.1 Hz, 1H, H-5”), 2.84-2.99 (m, 2H, CH2NHBoc), 3.01 (t, J = 2.2 Hz, 1H, HC≡C), 3.29-3.41 (m, 2H, CH2C≡), 4.22 (td, J = 2.8 Hz, J = 6.9 Hz, 1H, H-4’), 4.99 (dd, J = 2.8 Hz, J = 6.3 Hz, 1H, H-3’), 5.49 (dd, J = 2.4 Hz, J = 6.3 Hz, 1H, H-2’), 6.14 (dd, J = 2.4 Hz, 1H, H-1’), 6.72 (br, 1H, NHBoc), 7.30 (s, 2H, NH2), 8.18 (s, 1H, H-8), 8.31 (s, 1H, H-2); 13C NMR (100 MHz, DMSO-d6): δ 25.67 (CH3 isop), 27.43 (CH3 isop), 27.79 (CH2), 28.73 (3 CH3 Boc), 38.39 (CH2NHBoc), 42.40 (C≡C-CH2), 51.53 (CH2N), 55.32 (C-5’), 76.01 (HC≡C-CH2), 77.84 (Cq Boc), 79.21 (C≡C-CH2), 83.34 (C-3’), 83.43 (C-2’), 84.75 (C-4’), 89.67 (C-1’), 113.64 (Cq isop), 119.69 (C-5), 140.47 (C-8), 149.28 (C-4), 153.18 (C-2), 156.04 (CO Boc), 156.60 (C-6); HRMS (ESI-TOF): m/z calcd for [C24H35N7O5+H]+ 502.2778, found 502.2770.
5’-Deoxy-5’-N-(N-tert-Butyloxycarbonyl-aminopropyl)-3-(2’,3’,5’-tri-O-acetyl-adenosine-8-yl)propargyl amino-2’,3’-O-isopropylidene-adenosine (19). To a solution of bromide 6 (0.26 g, 0.55 mmol) and alkyne 18 (0.41 g, 0.82 mmol) in THF (16.5 mL) was added NEt3 (0.23 mL, 1.65 mmol). The stirred mixture was degassed with argon (3 times) before adding sequentially CuI (10 mg, 0.05 mmol) and Pd(PPh3)4 (32 mg, 0.028 mmol), followed by argon degassing. After stirring for 4 h at 60 °C, a second addition of Pd(PPh3)4 (32 mg, 0.028 mmol) was made. After 18 h at 60 °C, crude was filtered over Celite and volatiles were removed under reduced pressure. The residue was purified by flash column chromatography (31 g SiO2, 0 to 7% methanol in dichloromethane) to yield 7 (0.31 g, 63%) as a white foam. 1H NMR (400 MHz, DMSO-d6): δ 1.34 (s, 3H, CH3 isop), 1.37 (s, 9H, CH3 Boc), 1.55 (s, 3H, CH3 isop), 1.51-1.60 (m, 2H, CH2), 1.95 (s, 3H, COCH3), 1.96 (s, 3H, COCH3), 2.06 (s, 3H, COCH3), 2.51-2.56 (m, 2H, CH2N), 2.66 (dd, J = 6.0 Hz, J = 13.2 Hz, 1H, H-5’B), 2.82 (dd, J = 8.0 Hz, J = 13.2 Hz, 1H, H-5”B), 2.89-3.05 (m, 2H, CH2NHBoc), 3.77-3.88 (m, 2H, CH2C≡), 4.16 (dd, J = 5.6 Hz, J = 12.0 Hz, 1H, H-5’A), 4.28-4.35 (m, 2H, H-4’A and H-4’B), 4.41 (dd, J = 3.7 Hz, J = 12.0 Hz, 1H, H-5”A), 5.04 (dd, J = 2.8 Hz, J = 6.3 Hz, 1H, H-3’B), 5.51 (dd, J = 2.5 Hz, J = 6.4 Hz, 1H, H-2’B), 5.72 (t, J = 6.0 Hz, 1H, H-3’A), 6.11 (d, J = 4.6 Hz, 1H, H-1’A), 6.17 (d, J = 2.5 Hz, 1H, H-1’B), 6.19 (dd, J = 4.6 Hz, J = 6.0 Hz, 1H, H-2’A), 6.77 (br t, J = 5.4 Hz, 1H, NHBoc), 7.31 (br, 1H, NH2), 7.57 (s, 2H, NH2), 8.18 (s, 1H, H-2A), 8.19 (s, 1H, H-2B), 8.33 (s, 1H, H-8B); 13C NMR (100 MHz, DMSO-d6): δ 20.48 (CH3), 20.69 (CH3), 20.84 (CH3), 25.66 (CH3 isop), 27.43 (CH3 isop), 27.90 (CH2), 28.70 (3 CH3 Boc), 38.31 (CH2NHBoc), 43.16 (C≡C-CH2), 51.78 (CH2N), 55.35 (C-5’B), 62.86 (C-5’A), 70.24 (C-3’A), 71.71 (C-2’A), 74.34 (C≡C-CH2), 77.90 (Cq Boc), 79.69 (C-4’A), 83.26 (C-3’B), 83.43 (C-2’B), 84.33 (C-4’B), 87.30 (C-1′A), 89.71 (C-1′B), 93.23 (C≡C-CH2), 113.77 (Cq isop), 119.26 (C-5A), 119.71 (C-5B), 133.28 (C-8A), 140.54 (C-8B), 149.04 (C-4B), 149.24 (C-4A), 153.19 (C-2A), 154.32 (C-2B), 156.09 (CO Boc), 156.43 (C-5B), 156.62 (C-6A), 169.73 (CO), 169.81 (CO), 170.41 (CO); HRMS (ESI-TOF): m/z calcd for [C40H52N12O12+H]+ 893.3906, found 893.3939.
5’-[N-3-Aminopropyl-N-(3-(adenosin-8-yl)propargyl)amino]-5’-deoxyadenosine (5). To a solution of 7 (0.29 g, 0.33 mmol) in methanol (3.3 mL) was added 28% NH4OH (0.80 mL, 13.3 mmol). After stirring for 18 h at room temperature, volatiles were removed under reduced pressure and the crude material was brought to 0 °C before adding an ice-cold solution of TFA (50% in water, 10 mL). After 4 h at room temperature, water was added and the reaction mixture was lyophilized. Purification by reverse phase HPLC (0 to 30% acetonitrile in 10 mM TEAA buffer over 15 min) afforded the fully deprotected compound 5 as acetate salt (0.12 g, 61%) as white powder. tR = 10.8 min; 1H NMR (400 MHz, DMSO-d6): δ 1.65 (quint, J = 6.8 Hz, 2H, CH2), 1.78 (s, 3H, CH3 Ac), 2.65 (t, J = 6.8 Hz, 2H, CH2N), 2.70 (t, J = 6.8 Hz, 2H, CH2NH2), 2.80 (dd, J = 4.8 Hz, J = 13.7 Hz, 1H, H-5’B), 2.90 (dd, J = 6.8 Hz, J = 13.7 Hz, 1H, H-5”B), 3.53 (dd, J = 4.0 Hz, J = 12.2 Hz, 1H, H-5’A), 3.69 (dd, J = 3.6 Hz, J = 12.2 Hz, 1H, H-5”A), 3.80 (s, 2H, CH2C≡), 3.96-4.01 (m, 1H, H-4’A), 4.03-4.08 (m, 1H, H-4’B), 4.14 (pt, J = 5.0 Hz, 1H, H-3’B), 4.20 (dd, J = 2.3 Hz, J = 5.1 Hz, 1H, H-3’A), 4.67 (pt, J = 5.1 Hz, 1H, H-2’B), 4.98 (dd, J = 5.3 Hz, J= 6.0 Hz, 1H, H-2’A), 5.89 (d, J = 5.4 Hz, 1H, H-1’B), 6.00 (dd, J = 6.8 Hz, H-1’A), 7.25 (br, 1H, NH2 B), 7.61 (s, 2H, NH2 A), 8.15 (s, 1H, H-2A), 8.17 (s, 1H, H-2B), 8.36 (s, 1H, H-8B); 13C NMR (100 MHz, DMSO-d6): δ 23.23 (CH3 Ac), 28.50 (CH2N), 38.89 (CH2NH2), 44.80 (C≡C-CH2), 51.78 (CH2N), 56.23 (C-5’B), 62.69 (C-5’A), 71.49 (C-3’A), 72.21 (C-3’B and C-2’A), 73.14 (C-2’B), 74.81 (C≡C-CH2), 83.03 (C-4’B), 87.11 (C-4’A), 88.10 (C-1′B), 90.01 (C-1′A), 92.96 (C≡C-CH2), 119.64 and 119.67 (C-5A and C-5B), 133.94 (C-8A), 140.24 (C-8B), 148.84 (C-4A), 149.90 (C-4B), 153.14 (C-2B), 153.67 (C-2A), 156.52 (C-6B), 156.54 (C-6A), 173.90 (CO Ac); HRMS (ESI-TOF): m/z calcd for [C26H34N12O7+H]+ 627.2751 found, 627.2731.
4.3. Ki Measurements
Activity was measured at 30 °C in 50 mM Bis-Tris pH 7.0, 2 mM sodium citrate, 1 mM MgCl2. Reaction mixtures contained 0–2 mM NAD, 4 mM MgATP, 5 mM Glucose 6-phosphate, 1 U/mL Glucose 6-phosphate dehydrogenase and 0.1 µM LmNADK1. Steady state rate constants (kss) were calculated by fitting the initial (first 500–1000 s) linear part of the NADP production time courses per NADK. Inhibitors were tested at 4 concentrations: 0, 10, 25 or 50 µM and Ki values were determined using the competitive inhibition equation, kss = kcat × NAD/(KM × (1 + [inhibitor]/Ki) + NAD), with GraFit software (v 7.0.3).
Acknowledgments
We thank Frédéric Bonhomme for assisting with HRMS analysis. We thank the members of the Yersinia Research Unit for helpful discussions.
Supplementary Materials
Supplementary File: Copies of 1H and 13C NMR spectra of prepared compounds.
Author Contributions
Conceptualization, S.P., O.D. and G.L.; investigation, D.A.C., C.L. (Clarisse Leseigneur), M.G., D.C., V.H., G.L. and C.L. (Corinne Lionne); writing, S.P., O.D., G.L., C.L. (Corinne Lionne), D.A.C., C.L. (Clarisse Leseigneur) and M.G.; supervision, S.P., O.D. and G.L.; funding acquisition, S.P., O.D., G.L. and C.L. (Corinne Lionne) All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Agence Nationale de la Recherche (ANR-11-EMMA-019-03; ANR-17-CE18-0011-02) and supported by Institut Pasteur, Université de Paris, CNRS and Inserm. The Yersinia Research Unit is a member of the Laboratory of Excellence Integrative Biology of Emerging Infectious Diseases (ANR-LBX-62-IBEID).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability: Samples of the compounds are not available from the authors
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McGuinness E.T., Butler J.R. NAD+ kinase—A review. Int. J. Biochem. 1985;17:1–11. doi: 10.1016/0020-711X(85)90079-5. [DOI] [PubMed] [Google Scholar]
- 2.Magni G., Orsomando G., Raffaelli N. Structural and functional properties of NAD kinase, a key enzyme in NADP biosynthesis. Mini Rev. Med. Chem. 2006;6:739–746. doi: 10.2174/138955706777698688. [DOI] [PubMed] [Google Scholar]
- 3.Depaix A., Kowalska J. NAD Analogs in aid of chemical biology and medicinal chemistry. Molecules. 2019;24:4187. doi: 10.3390/molecules24224187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawai S., Murata K. Structure and function of NAD kinase and NADP phosphatase: Key enzymes that regulate the intracellular balance of NAD(H) and NADP(H) Biosci. Biotechnol. Biochem. 2008;72:919–930. doi: 10.1271/bbb.70738. [DOI] [PubMed] [Google Scholar]
- 5.Bi J., Wang H., Xie J. Comparative genomics of NAD(P) biosynthesis and novel antibiotic drug targets. J. Cell. Physiol. 2011;226:331–340. doi: 10.1002/jcp.22419. [DOI] [PubMed] [Google Scholar]
- 6.Gerdes S.Y., Scholle M.D., D’Souza M., Bernal A., Baev M.V., Farrell M., Kurnasov O.V., Daugherty M.D., Mseeh F., Polanuyer B.M., et al. From genetic footprinting to antimicrobial drug targets: Examples in cofactor biosynthetic pathways. J. Bacteriol. 2002;184:4555–4572. doi: 10.1128/JB.184.16.4555-4572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi K., Ehrlich S.D., Albertini A., Amati G., Andersen K.K., Arnaud M., Asai K., Ashikaga S., Aymerich S., Bessieres P., et al. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. USA. 2003;100:4678. doi: 10.1073/pnas.0730515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters J.M., Colavin A., Shi H., Czarny T.L., Larson M.H., Wong S., Hawkins J.S., Lu C.H.S., Koo B.-M., Marta E., et al. A Comprehensive, CRISPR-based functional analysis of essential genes in bacteria. Cell. 2016;165:1493–1506. doi: 10.1016/j.cell.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sassetti C.M., Boyd D.H., Rubin E.J. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 2003;48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 10.Bae T., Banger A.K., Wallace A., Glass E.M., Åslund F., Schneewind O., Missiakas D.M. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc. Natl. Acad. Sci. USA. 2004;101:12312. doi: 10.1073/pnas.0404728101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhuri R.R., Allen A.G., Owen P.J., Shalom G., Stone K., Harrison M., Burgis T.A., Lockyer M., Garcia-Lara J., Foster S.J., et al. Comprehensive identification of essential Staphylococcus aureus genes using Transposon-Mediated Differential Hybridisation (TMDH) BMC Genom. 2009;10:291. doi: 10.1186/1471-2164-10-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamachika S., Onodera Y., Hiramatsu K., Takase H. Plasmid integration method: A new tool for analysis of the essentiality and function of genes in S. aureus. J. Microbiol. Methods. 2012;90:250–255. doi: 10.1016/j.mimet.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Lerner F., Niere M., Ludwig A., Ziegler M. Structural and functional characterization of human NAD kinase. Biochem. Biophys. Res. Commun. 2001;288:69–74. doi: 10.1006/bbrc.2001.5735. [DOI] [PubMed] [Google Scholar]
- 14.Labesse G., Douguet D., Assairi L., Gilles A.-M. Diacylglyceride kinases, sphingosine kinases and NAD kinases: Distant relatives of 6-phosphofructokinases. Trends Biochem. Sci. 2002;27:273–275. doi: 10.1016/S0968-0004(02)02093-5. [DOI] [PubMed] [Google Scholar]
- 15.Poncet-Montange G., Assairi L., Arold S., Pochet S., Labesse G. NAD kinases use substrate-assisted catalysis for specific recognition of NAD. J. Biol. Chem. 2007;282:33925–33934. doi: 10.1074/jbc.M701394200. [DOI] [PubMed] [Google Scholar]
- 16.Gelin M., Poncet-Montange G., Assairi L., Morellato L., Huteau V., Dugué L., Dussurget O., Pochet S., Labesse G. Screening and in situ synthesis using crystals of a NAD kinase lead to a potent antistaphylococcal compound. Structure. 2012;20:1107–1117. doi: 10.1016/j.str.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Paoletti J., Assairi L., Gelin M., Huteau V., Nahori M.-A., Dussurget O., Labesse G., Pochet S. 8-Thioalkyl-adenosine derivatives inhibit Listeria monocytogenes NAD kinase through a novel binding mode. Eur. J. Med. Chem. 2016;124:1041–1056. doi: 10.1016/j.ejmech.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Gelin M., Paoletti J., Nahori M.A., Huteau V., Leseigneur C., Jouvion G., Dugue L., Clement D., Pons J.L., Assairi L., et al. From substrate to fragments to inhibitor active in vivo against Staphylococcus aureus. ACS Infect. Dis. 2020;6:422–435. doi: 10.1021/acsinfecdis.9b00368. [DOI] [PubMed] [Google Scholar]
- 19.Katayama H., Oda Y. Chemical proteomics for drug discovery based on compound-immobilized affinity chromatography. J. Chromatogr. B. 2007;855:21–27. doi: 10.1016/j.jchromb.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 20.Urh M., Simpson D., Zhao K. Chapter 26 Affinity chromatography: General methods. In: Burgess R.R., Deutscher M.P., editors. Methods in Enzymology. Volume 463. Elsevier Academic Press; Burlington, VT, USA: 2009. pp. 417–438. [DOI] [PubMed] [Google Scholar]
- 21.Kan T., Fukuyama T. Ns strategies: A highly versatile synthetic method for amines. Chem. Comm. 2004:353–359. doi: 10.1039/b311203a. [DOI] [PubMed] [Google Scholar]
- 22.Amiable C., Paoletti J., Haouz A., Padilla A., Labesse G., Kaminski P.-A., Pochet S. 6-(Hetero)Arylpurine nucleotides as inhibitors of the oncogenic target DNPH1: Synthesis, structural studies and cytotoxic activities. Eur. J. Med. Chem. 2014;85:418–437. doi: 10.1016/j.ejmech.2014.07.110. [DOI] [PubMed] [Google Scholar]
- 23.Morrell A., Placzek M.S., Steffen J.D., Antony S., Agama K., Pommier Y., Cushman M. Investigation of the lactam side chain length necessary for optimal indenoisoquinoline topoisomerase I inhibition and cytotoxicity in human cancer cell cultures. J. Med. Chem. 2007;50:2040–2048. doi: 10.1021/jm0613119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolb M., Danzin C., Barth J., Claverie N. Synthesis and biochemical properties of chemically stable product analogs of the reaction catalyzed by S-adenosyl-L-methionine decarboxylase. J. Med. Chem. 1982;25:550–556. doi: 10.1021/jm00347a014. [DOI] [PubMed] [Google Scholar]
- 25.Donnier-Maréchal M., Carato P., Larchanché P.-E., Ravez S., Boulahjar R., Barczyk A., Oxombre B., Vermersch P., Melnyk P. Synthesis and pharmacological evaluation of benzamide derivatives as potent and selective sigma-1 protein ligands. Eur. J. Med. Chem. 2017;138:964–978. doi: 10.1016/j.ejmech.2017.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.