Abstract
The genus Chryseobacterium in the family Weeksellaceae is known to be polyphyletic. Amino acid identity (AAI) values were calculated from whole-genome sequences of species of the genus Chryseobacterium, and their distribution was found to be multi-modal. These naturally-occurring non-continuities were leveraged to standardise genus assignment of these species. We speculate that this multi-modal distribution is a consequence of loss of biodiversity during major extinction events, leading to the concept that a bacterial genus corresponds to a set of species that diversified since the Permian extinction. Transfer of nine species ( Chryseobacterium arachidiradicis , Chryseobacterium bovis, Chryseobacterium caeni, Chryseobacterium hispanicum, Chryseobacterium hominis, Chryseobacterium hungaricum,, Chryseobacterium pallidum and Chryseobacterium zeae ) to the genus Epilithonimonas and eleven ( Chryseobacterium anthropi , Chryseobacterium antarcticum , Chryseobacterium carnis , Chryseobacterium chaponense , Chryseobacterium haifense, Chryseobacterium jeonii, Chryseobacterium montanum , Chryseobacterium palustre , Chryseobacterium solincola , Chryseobacterium treverense and Chryseobacterium yonginense ) to the genus Kaistella is proposed. Two novel species are described: Kaistella daneshvariae sp. nov. and Epilithonimonas vandammei sp. nov. Evidence is presented to support the assignment of Planobacterium taklimakanense to a genus apart from Chryseobacterium, to which Planobacterium salipaludis comb nov. also belongs. The novel genus Halpernia is proposed, to contain the type species Halpernia frigidisoli comb. nov., along with Halpernia humi comb. nov., and Halpernia marina comb. nov.
Keywords: Chryseobacterium, extinction, genus delineation, taxonomy
The history of the genus Chryseobacterium is complicated by multiple instances in which species from other genera have been transferred into or removed from the genus. The genus was proposed in 1994 to contain many of the species that were at that time considered to be members of the genus Flavobacterium but were dissimilar both phenotypically and genetically to Flavobacterium aquatile (type and only strain: ATCC 11947T), which is the type species of the genus Flavobacterium [1]. In the same report, the genus Bergeyella was created to contain the generically misclassified species Weeksella zoohelcum [2] and the genus Empedobacter was revived to accommodate the species Empedobacter brevis , which had been given a variety of names over the years, including ‘Empedobacter breve’ [3, 4]. A decade later, the genus Kaistella was created with a single species, Kaistella koreensis , to accommodate three strains with 16S rRNA sequences quite distant from the type strains of the species of the genus Chryseobacterium that had been published at that time [5]. The next year (2005) the genus Elizabethkingia was created based on both 16S rRNA gene sequence and phenotypic differences to accommodate the species previously described as Chryseobacterium meningosepticum (reclassified as Elizabethkingia meningoseptica ), as well as the previously unnamed species Elizabethkingia miricola [6]. The genus Sejongia was created for Sejongia antarctica and Sejongia jeonii [7], and Sejongia marina was added shortly thereafter [8]. The members of the genus Sejongia were later recognised as having a 16S rRNA gene sequence similar to those of Chryseobacterium haifense and Chryseobacterium hominis and were transferred to the genus Chryseobacterium [9] in the same year that the single species in the genus Kaistella ( Kaistella koreensis ) was reclassified as a member of the genus Chryseobacterium [10]. The genus Planobacterium was created with the description of Planobacterium taklimakanense [11], which was later proposed to belong to the genus Chryseobacterium on the basis of 16S rRNA gene sequence similarity [12]. The genus Epilithonimonas was created to accommodate the species Epilithonimonas tenax [13], and by 2015 the genus had grown to contain four more species [14–17]. The species of the genus Epilithonimonas were subsequently transferred to the genus Chryseobacterium [18], despite the recognition that this would cause the genus Chryseobacterium to contain three separate lineages. This was considered to be the more conservative reclassification approach as it would result in minimal changes.
There are several genera the members of which are phenotypically similar to members of the genus Chryseobacterium but have historically been considered distinct. In 1986, the genus Weeksella was created with a single species ( Weeksella virosa ) to contain strains previously named as ‘CDC group IIf’ from human clinical specimens [19]. After the 1994 transfer of Weeksella zoohelcum to the genus Bergeyella , it remained a single-species genus until 2015, when Weeksella massiliensis was described [20]. The genus Riemerella was created to accommodate Riemerella anatipestifer , named to honour O.V. Riemer, who in 1904 first described the disease septicemia anserum exsudativa in geese infected by Riemerella anatipestifer [21]. A related bacterium causing respiratory infections in pigeons was later described as Riemerella columbina [22]. This species also causes illness in ostriches [23]. Riemerella columbipharyngis was found in apparently healthy pigeons [24]. The novel genus and species Wautersiella falsenii were created to accommodate clinical isolates that were similar phenotypically to members of the genera Chryseobacterium and Empedobacter [25], but it was later proposed that the single species in this genus be incorporated into the genus Empedobacter [26]. The genera Soonwooa [27], Cruoricaptor [28] and Daejeonia [29] were each established to contain a single species. The genus Chryseobacterium and all of these related genera had been previously described as belonging to the Chryseobacterium/Riemerella branch of the family Flavobacteriaceae but results from a whole genome sequence (WGS) analysis of 1000 type strain genomes from the phylum Bacteroidetes [30] recently indicated that they are distinct from the Flavobacteriaceae, and the family name Weeksellaceae was proposed for them.
WGS analysis has revolutionised the identification and taxonomic classification of bacterial isolates. High-coverage contigs from draft genome assemblies of short sequence reads (e.g., Illumina, with at least 50× coverage) have been shown to produce results indistinguishable from those produced using complete circularised genomes when values such as the average nucleotide identity using blast (ANIb) and genome-to-genome distance calculation (GGDC) formula 2 predicted DNA–DNA hybridization (DDH) are calculated [31–33]. Consequently, the WGS analyses described here are based on high-coverage draft genomes. The ANIb 95 % cut-off value for species delimitation [34] was calculated to be equivalent to a GGDC-predicted DDH value of slightly less than 65%, lower than the DDH value of approximately 70 % which has long been used for species delimitation [32]. This might support the 96.5 % whole-genome ANI (gANI) species threshold that has been suggested [35], or it might simply be an artefact of the inexactness of traditional DDH measurements. The use of WGS data in the naming of species is supplanting 16S rRNA sequence analysis [36], and some have argued for allowing the naming of a species based solely on WGS data, with the genome assembly itself serving as the type material [37].
A set of 182 isolates (primarily of clinical origin) belonging to, or resembling phenotypically, the members of the genera Chryseobacterium , Elizabethkingia and Empedobacter was previously analysed using DNA–DNA hybridization and comparative 16S rRNA gene sequence analysis, resulting in the description of four novel species that each contained between two and six strains. The majority of the other isolates could be assigned to already-described species, but 15 strains each appeared distinct from all others and almost certainly represented novel species [12]. We refrained from proposing names for these, as per recommendations that multiple strains be used in describing a species [38]. The current study continues that previous work with a comprehensive whole-genome sequence analysis of those isolates. Over the course of this collaboration we have added 19 complete type strain genomes to the 85 genomes attributed to the type strains of species of the genus Chryseobacterium that were previously available and 20 additional genomes, some of which are the sole representative of yet-unnamed species. In the process we compared these to other species from the genus Chryseobacterium and its nearest neighbours, specifically those which were characterised in the Bergey’s chapter on Chryseobacterium and related genera [39] and came to the conclusion that a taxonomic realignment was necessary. Distribution of AAI values calculated from type strain comparisons was found to be multimodal, which led us to propose a re-organization based on average amino acid identity (AAI) that separates the Chryseobacterium genus into four different genera. These correspond to the multiple lineages apparent in previous publications [18] and also recognised as separate genera in the Genome Taxonomy Database [40]. This aligns genus assignments so that the type strain for each species in each genus has ≥76 % AAI values compared with the type strain of the type species of its genus.
Twenty-eight strains whose genome sequence has not previously been reported are listed in Table 1. This includes the type strains of Chryseobacterium lactis , Chryseobacterium carnis, Chryseobacterium bernardetii and Chryseobacterium nakagawai that had been described in the earlier study [12], along with one or two additional strains from the same DNA–DNA hybridization group. Of the DNA–DNA hybridization groups that were assigned in that paper to existing species (based on 16S rRNA gene sequence), a representative strain was selected for sequencing. Additional strains that might be taxonomically informative were identified by reviewing records of 16S rRNA gene sequences from isolates in the Special Bacteriology Reference Laboratory (SBRL) strain collection. Several members of the DNA–DNA hybridization group that we believed to be Chryseobacterium taklimakanense , additional representatives of several species of the genus Chryseobacterium that were well-represented in the collection, and strains of several suspected novel species were selected for sequencing. These 28 genomes were compared with genomes available in the public domain, including genomes from the type strains of all species that were considered to belong to the genus Chryseobacterium (n=76), genomes of selected strains considered to be members of the genus Chryseobacterium and representative of their respective species, but not type strains (n=4), and genomes of the type strains of species from the ‘nearest neighbour’ genera discussed in the introduction (n=20) (Table S1, available in the online version of this article). There remain more than 30 species of the genus Chryseobacterium with validly published names that have no type strain genome sequenced.
Table 1.
Details on newly sequenced strains
tDDH, traditional DNA–DNA hybridization; nd, not determined.
|
Strain number sequenced |
Other strain numbers |
Previously described as: |
Proposed as: |
Geographic location |
Source |
Date isolated |
tDDH group |
DDH_# |
Accession Number |
|---|---|---|---|---|---|---|---|---|---|
|
G0081 |
CL88/78; Hayes B19/1 |
Kaistella carnis |
Unknown |
Beef |
1973 |
95 |
95 |
||
|
F5649 |
CCUG 73498, CIP 111693 |
Epilithonimonas vandammei |
Iowa, USA |
Testicle |
1984 |
224 |
224 |
||
|
H6466 |
CCUG 73281 |
Epilithonimonas vandammei |
Indianapolis, Indiana, USA |
Leg wound |
July, 2013 |
nd |
|||
|
H3001 |
CCUG 73276; CIP 111694 |
Unidentified |
Kaistella daneshvariae |
New York, USA |
Peritoneal cavity |
January, 2004 |
nd |
||
|
F9257 |
CL712/92 |
Florida, USA |
Blood |
1987 |
212 |
212 |
|||
|
H4753 |
CCUG 73278 |
Michigan, USA |
Blood |
2008 |
nd |
||||
|
H5297 |
NCTC 13453; CCUG 52711; CIP 109415; DSM 22165; NF802 |
Epilithonimonas hominis |
La Louvière, Belgium |
Blood |
1998 |
nd |
|||
|
G0240 |
CL278/82; CCUG 73500 |
‘123 group’ |
Chryseobacterium sp. nov. |
Darlington, England |
Urine |
1982 |
|
153 |
|
|
G0235 |
CL542/79; CCUG 73274 |
‘71 group’ |
London, England |
Green lizard |
1979 |
71 |
148 |
||
|
H4373 |
KC_2159; CPW406; KCTC 12088; NBRC 102008; DSM 15235 |
Lake Daecheong, Korea |
Freshwater lake sediment |
|
nd |
||||
|
F4391 |
CL720/92 |
Kaistella haifensis |
Indiana, USA |
Lung |
1983 |
|
236 |
||
|
H3056 |
CCUG 73499 |
Unidentified |
Kaistella daneshvariae |
New Mexico, USA |
Blood |
2004 |
nd |
||
|
G0079 |
CL311/80; A16/80; CCUG 73267 |
Kaistella haifensis |
Midlothian, Scotland |
Calf |
1980 |
93 |
93 |
||
|
G0229 |
CL318/82 |
Doncaster, England |
Sputum |
1982 |
142 |
142 |
|||
|
G0211 |
CL97/78; Hayes S10/1; CCUG 73273 |
Unknown |
Soil |
Unknown |
140 |
||||
|
G0197 |
A139/68; CCUG 73271 |
Paisley, Scotland |
Milk bottle rinse |
Unknown |
63 |
128 |
|||
|
KC_1864 |
NCTC 11390, A140/68, F68 |
Paisley, Scotland |
Milk bottle rinse, |
Unknown |
63 |
63 |
|||
|
G0041 |
F91 |
Gloucester, England |
Kidney abscess |
Late 1960's |
78 |
78 |
|||
|
G0162 |
CL192/74; CCUG 73268 |
‘78 group’ |
Chryseobacterium sp. nov. |
Newcastle-upon-Tyne, England |
Urine |
1974 |
78 |
117 |
|
|
F9942 |
A103/68; CCUG 73266 |
‘125 group’ |
Paisley, Scotland |
Milk swab |
Unknown |
125 |
64 |
||
|
G0188 |
A104/68; CCUG 73270 |
‘125 group’ |
Paisley, Scotland |
Milk swab |
Unknown |
125 |
125 |
||
|
G0186 |
A86/68; CCUG 73269 |
‘123 group’ |
Chryseobacterium sp. nov. |
London, England |
Human |
Unknown |
123 |
123 |
|
|
G0201 |
CL381/78 |
‘132 group’ |
Chryseobacterium sp. nov. |
Dublin, Irish Republic |
Drinking water |
1978 |
132 |
132 |
|
|
G0207 |
CL189/78; CCUG 73272 |
‘132 group’ |
Nuneaton, England |
Zimmer water bag |
1978 |
132 |
136 |
||
|
G0239 |
CL141/82; CCUG 73275 |
‘132 group’ |
London, England |
Cryoprecipitate, transfusion reaction |
1982 |
nd |
152 |
||
|
H4638 |
CCUG 73277 |
Pennsylvania, USA |
Larynx |
2008 |
nd |
||||
|
H5559 |
CCUG 73280 |
Wailuku, Hawaii, USA |
Blood |
2010 |
nd |
||||
|
H5143 |
CCUG 73279 |
‘132 group’ |
Tennessee, USA |
Cerebrospinal fluid (CSF) |
2009 |
nd |
DNA–DNA hybridization was performed as described previously [41]. All strains were biochemically characterised in all or most of a range of 68 conventional biochemical tests by methods described previously [42] and whole-genome sequences were generated as described previously [32, 43–47].
Sequence alignments were performed using the ClustalW module in BioEdit v7.0.5.3 [48]. For analysis of 16S rRNA genes, sequences were trimmed to match the start and end of the near full-length sequence JX100817 (deposited as Chryseobacterium carnis strain G0081T). All pairwise comparisons with a 16S rRNA gene sequence identity greater than >98.65 % were considered as potentially representing the same species, as this limit has been recommended for species demarcation [49]. The rpoB gene sequences were extracted from WGS data as described previously [32]. Phylogenetic trees were reconstructed using MEGAX [50] with the Jukes-Cantor substitution model.
Proteomes from each genome were generated by Prodigal v2.6.2 [51]. For each pairwise comparison, an all-versus-all search of all proteins was carried out using BLASTp v2.4.0+ [52] in both directions. If both directions of BLASTp searches resulted in the same protein match (pair) and exceeded 40 % in amino acid identity and 50 % in coverage length, we included the protein sequences for computing the arithmetic mean amino acid sequence identity (AAI). Percentage of conserved proteins (POCP) scores were calculated as previously described [53]. GGDC formula 2 was used to generate the predicted DDH values. The Jspecies software package version 1.2.1 was used to calculate average nucleotide identity with BLASTn alignments (ANIb) [31, 34]. Additional data visualizations were produced using JMP v11 (SAS Institute).
The gene pairings identified during the AAI pairwise comparisons described above were used to generate a list of 488 loci present in a single copy in all of the strains listed in Table S1. The maximum sequence length for each orthologous group was computed, and groups with members having a length ≥90 % of this maximum were selected for alignment. These 148 were aligned (as protein sequences) using ClustalW in BioEdit v7.0.5.3 [48], and manual refinements were made to align start and stop codons. Unusually divergent (<25 % nucleotide identity) loci were filtered out, and the remaining 119 gene sequences from each genome were concatenated. The most highly conserved of these loci were selected using a criterion that they had to have at least 25 % identity at the nucleic acid level. Gaps and invariant positions were masked, which left 68,272 core variable nucleotide sites. Maximum-likelihood analysis using the Jukes-Cantor substitution model was performed in RAxML ver 8.2.12 [54]. The best scoring topology of 250 maximum-likelihood trees had 100 bootstrap replicates overlaid. The extended majority-rule consensus indicated convergence (1.54 % weighted Robinson–Foulds mean) after 100 replicates [55].
Historically, there have been no unambiguous criteria for creation of a novel genus, and mis-classification of species has occurred frequently. The addition of 16S rRNA gene sequence analysis to the phenotypic characterization of strains, which was originally used for taxonomic classification, represented a marked improvement but for several reasons is not ideal. Firstly, 16S rRNA gene sequences can differ at their various loci within a strain’s genome. This has been observed in the genus Elizabethkingia [32] as well as the genera Pseudomonas [56], Prevotella [57] and Neiserria [58]. Secondly, while a 16S rRNA gene sequence similarity below 98.5 % can ensure that two organisms will have less than 70 % DDH and therefore be separate species [59], the converse is not true. Organisms may have 16S rRNA gene sequences over 99 % similar or even identical and still belong to different species, with DDH values below 70 % and ANI values less than 95 %. For example, the type strains of Chryseobacterium shigense [60] and Chryseobacterium carnipullorum [61] share 99.3 % identity in their 16S rRNA gene sequences, which could indicate they belong to the same species, but genome comparisons suggest otherwise (DNA–DNA hybridization <44 % [62, 63] and ANIb <91 %). The 16S rRNA gene sequences of Chryseobacterium jejuense are also very similar to those of Chryseobacterium nakagawai and Chryseobacterium lactis (98.9 and 98.8% identical, respectively), but the ANIb values from genome comparisons (averaging <87 and<82 %, respectively) confirm that all are separate species.
There are several species that have been included in the genus Chryseobacterium on the basis of the results of 16S rRNA gene sequence analysis whose inclusion is not supported by the additional evidence of whole-genome sequence and/or reconstruction of a phylogenetic tree containing sequences from all of the closely related species. The proposal to include Planobacterium taklimakanense within the genus Chryseobacterium was made based on a 16S rRNA phylogenetic tree [12], and consequently the species Chryseobacterium frigidum was included in the genus Chryseobacterium in large part due to its similarity with Chryseobacterium taklimakanense [64], but an analysis that included all of the species of the genus Chryseobacterium and additional species of the family Weeksellaceae (Fig. S1), indicates it is more closely related to the members of the genus Cruoricaptor . The 16S rRNA gene sequence for the effectively but not yet validly published species ‘Chryseobacterium chengduensis’ is consistent with it being outside the genus Chryseobacterium (possibly in the genus Daejeonia ) when all of the closely related species are included. The assignment of this species to the genus Chryseobacterium was made based on an incomplete analysis that lacked an outgroup and included only 18 species of the genus Chryseobacterium , including several that we classify as belonging to the genus Kaistella [65]. The species Chryseobacterium reticulitermitis was described as a member of the genus Chryseobacterium based on a neighbour-joining tree that used the distantly related species Gramella echinicola as the outgroup. Our more extensive neighbour-joining tree (Fig. S1) located the 16S rRNA gene sequence of Chryseobacterium reticulitermitis near to the base of the tree. To include it as a member of the genus Chryseobacterium would require the undesirable inclusion of other well-established genera, such as Riemerella and Cloacibacterium, into the genus Chryseobacterium also. It is clear that genus assignment based on 16S rRNA gene sequencing has serious limitations, and that an objective and reproducible method for genus determination is needed.
Initially, a proposed method for genus delineation based on Percentage of Conserved Proteins (POCP) [53] was investigated for its utility in distinguishing species belonging to the genus Chryseobacterium and related genera. Calculation of AAI values based on the specified parameters was an intermediate step in generating the protein comparisons for POCP, but we found AAI values themselves to be more informative than POCP. This was fortunate, as the use of AAI values for genus determination has inherent advantages over POCP, particularly for incomplete genomes. Reciprocal AAI values are limited to the range of 0 to 100 regardless of protein quantities in each genome and are symmetric, so completeness of the genome will have less of an effect on this metric.
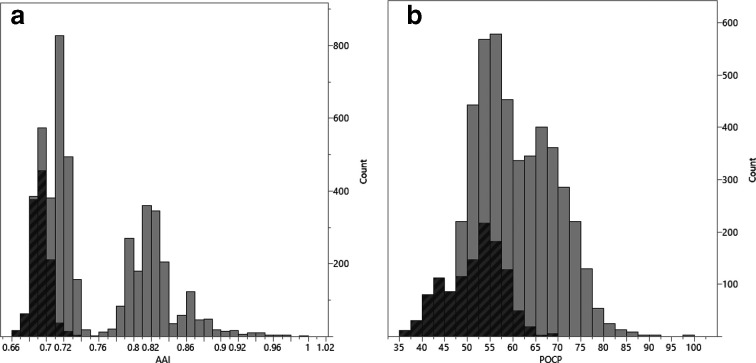
When AAI was calculated for type strain comparisons between species of the genus Chryseobacterium and species from related genera using the parameters specified for POCP, all AAI values were >65 %, and the distribution was bi-modal (Fig. 1). Out of 8646 AAI comparisons, only 44 (0.5 %) were between 74 and 76 %. This suggested a natural cut-off value between 74 and 76 %. Comparisons using complete genomes produced the same AAI results as when draft genomes were used (data not shown), which was expected for genomes that meet or exceed the minimum standards [66]. Most comparisons between strains that had an AAI of ≥76 % were categorised as representing members of the same genus, while most that had an AAI≤74 % represented members of different genera. Of the strain comparisons that deviated from this general rule, many contained strains that had been originally categorised as belonging to one of the genera that were later subsumed into the genus Chryseobacterium . We have formulated a strategy of genus delineation based on the criterion that the type strain of a species must have an AAI value greater than 76 % when compared with the type strain of the type species of its genus in order to represent a member of the same genus, and that all type strains must have an AAI value greater than 74 % when compared with each other. Exact AAI values calculated are parameter-dependant, so any application of this 74–76 % cut-off for genus delineation must use AAI values calculated with the parameters of 40 % amino acid identity and 50 % coverage length. The discontinuities of AAI distributions used to realign the polyphyletic genus Chryseobacterium were thus used in a manner similar to taxonomic redistributions of the genera Rhodococcus [67] and Mycobacterium [68].
Fig. 1.
Distribution of (A) AAI% and (B) POCP, for type strain comparisons where both strains are listed in LSPN as members of the genus Chryseobacterium (which includes the strains described in this paper as members of the genera Epilithonimonas , Halpernia, Kaistella or Planobacterium ), or one of its closest relatives ( Bergeyella , Cloacibacterium , Cruoricaptor , Elizabethkingia , Riemerella or Soonwooa ). Dark grey indicates comparisons between strains already considered to belong to different genera. Note that few comparisons yield an AAI between 74 and 76 %, and that unlike POCP distribution, AAI distribution is bi-modal.
Just like the distribution of AAI values, the distribution of ANI values is discontinuous [69]. A dearth of ANI values in the low 70 % s among Enterococci [70] was attributed to the Permian extinction approximately 252 million years ago (MYA) which is believed to have eliminated 90–95 % of all species on the planet [71, 72]. The Permian was the third of five recognised major extinction events in the history of our planet [73–76]. A major extinction is characterised by a loss of biodiversity, followed by a re-diversification of the survivors [77]. This process pertains not only to multi-cellular fossil-forming organisms, but also to organisms for which there is no good fossil record, as evidenced by studies of microbialites [78] and of lichen-forming fungi [79]. In order to assess whether the observed AAI gap between 74 and 76% among members of the family Weeksellaceae would correspond to this gap in ANI values due to the Permian extinction, we charted AAI data for each strain comparison against average nucleotide identity blastn (ANIb) data for the same set of strains (data available on request), and found that a quadratic fit could be used to derive a formula that calculates the expected AAI from the ANIb (Fig. S2, part A). The gap in AAI values from 74 to 76 % does indeed correspond to an expected gap in ANIb values between 72.5 and 74 %, and examination of the distribution of ANIb scores shows a similar gap in this range (Fig. S2, part B).
We reasoned that if the gap in AAI values between 74 and 76 % represented the Permian extinction, then the second, less distinct valley in the distribution histogram that appears at an ANIb value of approximately 80 % might be an artefact of the Triassic extinction, which ended approximately 201 MYA [80]. Linear regression using these values, and a value of 100 % ANI for the present time, predicts that divergence of strains with the 95 % ANI that is used for species delineation [34] would have occurred approximately 65 MYA. This coincides with the timing of the Cretaceous–Paleogene (K-pg) extinction (notable for elimination of the non-avian dinosaurs [81–83]). If our calculations are correct that would mean that a bacterial species has a common ancestor which survived the K-pg extinction, and we could think of a genus as having a common ancestor that survived the Permian extinction (Fig. S3).
None of this actually proves our speculation that the multi-modal distribution of AAI (and ANIb) observed when large numbers of strain comparisons are examined is a consequence of extinction events. Regardless of their etiology, these naturally-occurring ‘gaps’ in distribution of AAI values can be utilised in a standardised taxonomic strategy [67].
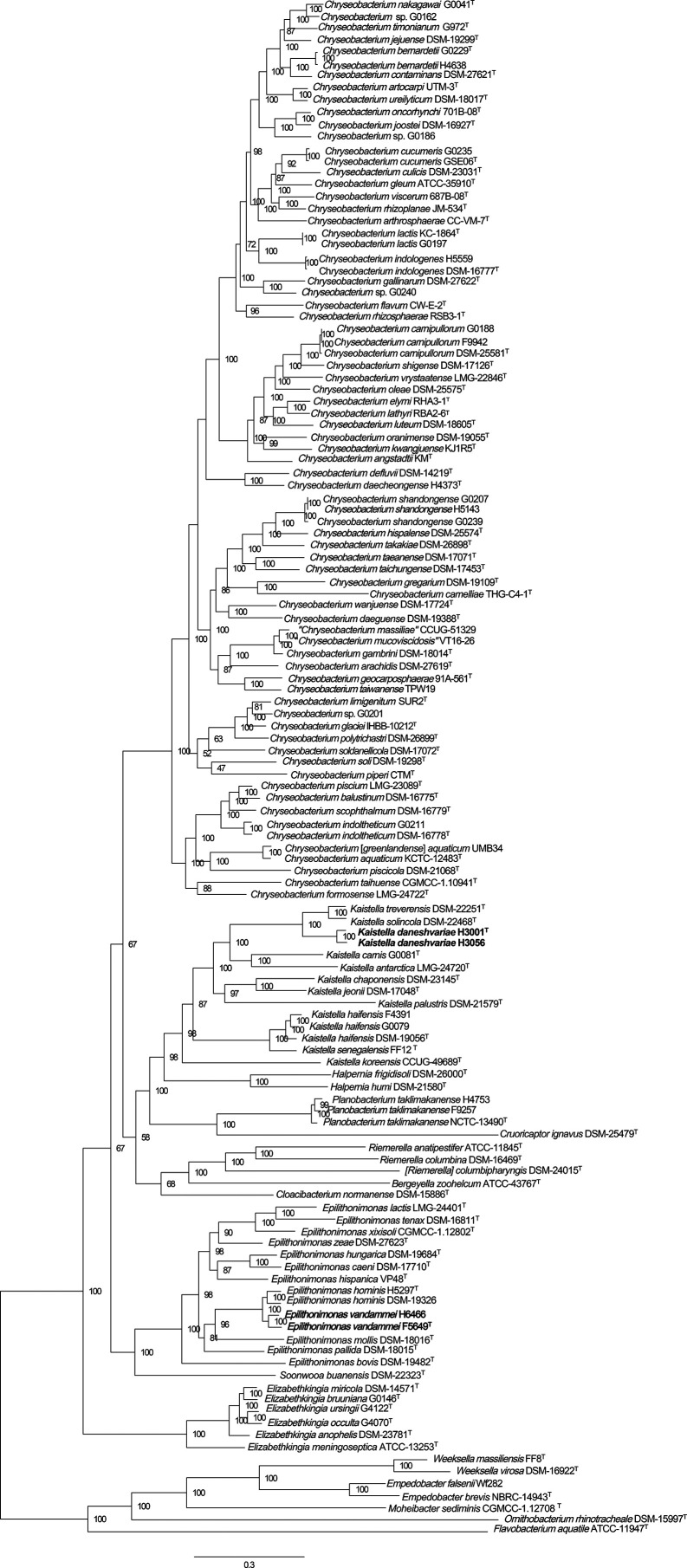
In proposing to subdivide the genus Chryseobacterium , we contradict an earlier proposal [18] to merge these genera despite the evidence from whole-genome sequence data that doing so would result in a polyphyletic genus. The preference to reduce the number of taxonomic changes in the absence of a reliable method of genus delineation was reasonable at the time. Our strategy of genus delineation based on AAI values was developed based on the observation that strain comparisons yielding AAI values between these cut-off points are rare, and we suggest that this rarity is due to the likelihood that most of the bacterial cells whose descendants would have been separated by AAI values in this range were instead eliminated in the Permian extinction. Using this criterion places a number of species into the genera Kaistella (including several originally classified as members of the genus Sejongia ), Epilithonimonas or Planobacterium instead of the genus Chryseobacterium, and it reveals a previously unrecognised genus containing three of the species. The core genome analysis of these species, shown in Fig. 2, supports these divisions, and they can be readily distinguished by rpoB sequence analysis (Fig. S4).
Fig. 2.
Maximum likelihood phylogenetic analysis of core genome loci from the members of the genus Chryseobacterium and closely related genera. The scale bar indicates substitutions per core variable site (n=68,272).
The newly-sequenced strains NCTC 13454T ( Chryseobacterium joostei ), NCTC 10796T ( Chryseobacterium indologenes ), and NCTC 11390T ( Chryseobacterium lactis ) were confirmed as members of the genus Chryseobacterium based on AAI values of >76 % when compared with strain ATCC 35910T of Chryseobacterium gleum, the type species of the genus Chryseobacterium . In contrast, AAI values comparing the type strain of Epilithonimonas tenax (the type species of the genus Epilithonimonas ) to other strains originally described as representing members of the genus Epilithonimonas were all >76 %, and those strains all have AAI values >76 % when compared with each other, but <74 % (range 71.34–71.97 %, mean=71.70 %) when compared with Chryseobacterium gleum ATCC 35910T. Similarly, most strains that had originally been described as members of the genera Sejongia or Kaistella had AAI values >76 % (mean=80 %) when compared with each other or with the strain that was originally described as Kaistella koreensis, but <74 % (mean=72 %) when compared with Chryseobacterium gleum ATCC 35910T.
The genus Epilithonimonas would thus comprise all its original species ( Epilithonimonas lactis , Epilithonimonas ginsengisoli , Epilithonimonas psychrotolerans and Epilithonimonas xixisoli , and Epilithonimonas tenax as the type species) with the addition of Chryseobacterium arachidiradicis renamed as Epilithonimonas arachidiradicis comb. nov., Chryseobacterium bovis as Epilithonimonas bovis comb. nov., Chryseobacterium caeni as Epilithonimonas caeni comb. nov., Chryseobacterium hispanicum as Epilithonimonas hispanica comb. nov., Chryseobacterium hominis as Epilithonimonas hominis comb. nov., Chryseobacterium hungaricum as Epilithonimonas hungarica comb. nov., Chryseobacterium molle as Epilithonimonas mollis comb. nov., Chryseobacterium pallidum as Epilithonimonas pallida comb. nov. and Chryseobacterium zeae as Epilithonimonas zeae comb. nov. The genus Kaistella would consist of its original species ( Kaistella koreensis ) as the type species, and the species Chryseobacterium antarcticum (formerly the type species of the genus Sejongia ) would be named as Kaistella antarctica comb. nov., Chryseobacterium carnis as Kaistella carnis comb. nov., Chryseobacterium chaponense as Kaistella chaponensis comb. nov., Chryseobacterium haifense as Kaistella haifensis comb. nov., Chryseobacterium jeonii as Kaistella jeonii comb. nov., Chryseobacterium montanum as Kaistella montana comb. nov., Chryseobacterium palustre as Kaistella palustris comb. nov., Chryseobacterium solincola as Kaistella solincola comb. nov., Chryseobacterium treverense as Kaistella treverensis comb. nov., and Chryseobacterium yonginense as Kaistella yonginensis comb. nov. The effectively published Chryseobacterium senegalense, which has not yet been added to the validation lists, would also be placed into the genus Kaistella .
Two species do not represent members of any of the genera proposed above, but instead have AAI values indicating that they comprise a separate novel genus. We propose to name this genus after Dr. Malka Halpern, Professor at University of Haifa in Israel. Consequently, the novel genus Halpernia contains Chryseobacterium frigidisoli renamed as the type species Halpernia frigidisoli comb. nov., and Chryseobacterium humi renamed as Halpernia humi comb. nov. On the basis of the results of 16S rRNA sequence analysis (Fig. S1), it appears that strain IMCC3228, originally designated as Sejongia marina and later as Chryseobacterium marinum, also represents a member of this genus, so we propose to rename it as Halpernia marina comb. nov.
This study is an extension of DNA–DNA hybridization studies performed on NCTC and CDC strains which were originally named as belonging to CDC groups IIc, IIe, IIh and IIi [84]. While many of these strains had been described as representing novel species or assigned to already-described species [12], other DNA–DNA hybridization groups could not be assigned for various reasons. WGS analysis was applied to resolve these lingering questions.
Strain G0235 can be assigned to the species Chryseobacterium cucumeris as its genome has an ANIb of 98.41 % when compared with the type strain of that species. It is the only one of the ‘71 group’ strains for which we have a sequenced genome, but the rpoB gene was sequenced for strains F9971 and F9973. The gene sequences for all three strains clustered with the rpoB sequence for the type strain of Chryseobacterium cucumeris (see Fig. S4). The ‘71 group’ can therefore be considered to be Chryseobacterium cucumeris .
G0079, the representative strain of the ‘93 group’, had been attributed to Chryseobacterium anthropi on the basis of its 16S rRNA gene sequence [12], but its whole-genome sequence revealed that it actually belongs to the species Kaistella haifensis based on ANIb similarity of 95.56 % with Kaistella haifensis strain DSM19056T. Kaistella haifensis can be included among the clinically relevant species, as many of the ‘93 group’ members were derived from human clinical specimens. There are currently no available sequence data for the type strain of Chryseobacterium anthropi, CCUG 52764, but we do have whole genome sequence data for our strain F4391 (=CCUG 15260) which was used in the species description for Chryseobacterium anthropi . Strain F4391 was also confirmed to be a strain of Kaistella haifensis by ANIb results of 96.14 %/96.18% and 95.68 %/95.50%, when compared with strains G0079 and DSM 19056T, respectively. Chryseobacterium anthropi was described as distinguishable from Chryseobacterium haifense [85] by a lack of acid production from fructose, lactose and sucrose and by a negative ONPG test (β-galactosidase) [10], all of which were positive for Chryseobacterium haifense DSM 19056T. All ‘93 group’ strains are negative for β-galactosidase, and for production of acid from lactose and sucrose; most, however, produce acid from fructose. Taken together, these findings cast doubt on whether Chryseobacterium anthropi is actually a separate species. As this question cannot be answered definitively without a genome sequence of the type strain of Chryseobacterium anthropi , we propose the name Kaistella anthropi to accommodate strain CCUG 52764.
Strains F9942 and G0188 belonged to the ‘125 group’, which was assigned to Chryseobacterium shigense on the basis of 16S rRNA gene sequence similarity and a DDH value of 77 % at 55 °C [12]. 16S rRNA gene sequence analysis does not distinguish between Chryseobacterium shigense and the later-named Chryseobacterium carnipullorum , but the paper describing Chryseobacterium carnipullorum found the two species to differ by a DDH value of 56.1 % [61]. The ANIb value of strains F9942 and G0188 when compared with each other was 99.98%, and when compared with the type strain of Chryseobacterium carnipullorum were 98.17 and 97.96 %, respectively. The ANIb values of any of these strains when compared with the type strain of Chryseobacterium shigense were less than 91 %. Thus, members of the ‘125 group’ do not belong to Chryseobacterium shigense, instead they belong to the species Chryseobacterium carnipullorum . The two species are readily distinguished by rpoB gene sequence.
The results of our previous 16S rRNA gene sequence analysis indicated that strains G0201 and G0207 represented different species despite being both assigned to the ‘132 group’ in DNA–DNA hybridization studies. The 16S rRNA sequence of G0201 is most similar to that of Chryseobacterium polytrichastri , while the 16S rRNA sequences of strains G0207, G0239, and H5143 are most similar to that of Chryseobacterium shandongense , differing at a single nucleotide. Long-read (PacBio) sequencing was done on all four strains. ANIb comparisons using these assemblies confirms that strains G0207, G0239, and H5143 represent a single species, and differ from G0201 and any other species whose genome has been sequenced thus far, which would be expected as the type strain of Chryseobacterium shandongense has not yet been sequenced. There are phenotypic differences between the type strain of Chryseobacterium shandongense and the sequenced ‘132 group’ strains G0207, G0239, and H5143; the latter all grow on MacConkey agar, and none are mucoid. The species description [86] is emended to include the phenotypes of Chryseobacterium shandongense strains from our ‘132 group’.
The ‘224 group’, represented by strain F5649, was previously attributed to Chryseobacterium hominis on the basis of 16S rRNA gene sequence analysis and DNA–DNA hybridization data [12]. Further confirmation of this species assignment was sought by sequencing the original strain F5649, and a modern isolate (H6466) that had been reported by our lab as Chryseobacterium hominis . Strains F5649 and H5466 represented the same species, as expected. Their genomes were compared with Chryseobacterium hominis strains NCTC 13453T and DSM 19326. The latter was the type strain of ‘ Chryseobacterium arothri ’, a validly published species later determined by 16S rRNA sequence analysis, DNA–DNA hybridization, and phenotypic similarity to represent a member of Chryseobacterium hominis [87, 88]. NCTC 13453T has an ANIb >95 % when compared with Chryseobacterium DSM 19326, providing confirmation that ‘Chryseobacterium arothri’ is indeed a junior synonym for Chryseobacterium hominis , but both strains had an ANIb <94 % when compared with ‘224 group’ strains. The rpoB gene sequence was not useful in distinguishing among the four strains, as the rpoB sequence of Epilithonimonas hominis NCTC 13453T was 96.5 % identical with that of the other Epilithonimonas hominis strain (DSM 19326), but comparisons between Epilithonimonas hominis strains and '224 group' strains ranged from 95.4 to 98 %. The ‘224 group’ strains differed from Chryseobacterium hominis in that they did not produce a DNase and did not produce acid from salicin. Characteristics that were consistently positive or negative are listed in the species description, but there was considerable variability among the strains. Most strains gave positive results (those giving negative results are given in parentheses) for acid production (in ammonium salt medium) from glycerol (CL263/70, CL205/78, CL213/83), casein digestion (CL205/78), gelatinase production (stab method: CL205/78, CL213/83; plate method: CL205/78), hydrolysis of starch (CL263/70, CL184/75, CL187/75, CL205/78), of Tween 20 (CL205/78) and of Tween 80 (CL263/70, CL195/76, CL205/78, CL373/79, CL213/83) and were oxidative according to the Hugh and Leifson O-F test (CL195/76).
Most strains gave negative results (those giving positive results are shown in parentheses) for acid production (in ammonium salt medium) from ethanol (CL309/73, CL184/75, CL187/75, CL205/78, CL445/80), fructose (CL263/70, CL195/76, CL373/79, CL445/80), mannitol (CL263/70), rhamnose (CL263/70) and sucrose (CL184/75, CL187/75, CL205/78, CL445/80), from glucose in peptone water medium (CL195/76, CL205/78) and from 10 % (w/v) glucose (CL445/80, CL213/83), growth on MacConkey agar (CL195/76, CL205/78, CL373/79), nitrate reduction (CL205/78, CL373/79, CL213/83), nitrite reduction (CL373/79, CL445/80), production of extracellular deoxyribonuclease (CL263/70, CL309/73, CL524/73, CL195/76) and of a brown melanin-like pigment on tyrosine agar (CL373/79).
On the basis of both its phenotypic differences from Chryseobacterium hominis , and whole genome sequence results, the ‘224 group’ thus represents a novel species which we propose to name Epilithonimonas vandammei after Peter Vandamme, first author on the paper that originally described Chryseobacterium as a novel genus.
Strain G0162 was one of two strains originally assigned to the ‘78 group’ (the representative of this group was G0041, later designated as the type strain of Chryseobacterium nakagawai ). The assignment of G0162 to the ‘78 group’ was marginal as its hybridization was only 69 % at 55 °C; its ANIb of 94 % compared with G0041 indicates that it does not represent a member of the same species. Its 16S rRNA sequence is most similar to that of the type strain of Chryseobacterium rhizoplanae JM-534T, but ANIb comparisons between this strain’s genome and those of assemblies of two Chryseobacterium rhizoplanae SRA data sets (SRX3054699 and SRX3054698) were in the range of 81–82 % (data not shown). Thus G0162 is currently the only known representative of its species. The description of Chryseobacterium nakagawai is emended below to reflect removal of strain G0162 from the species.
Strain G0186 was the representative strain for the ‘123 group’, along with strain G0240 and was identified as Chryseobacterium ureilyticum on the basis of a 16S rRNA gene sequence identity of 99.2 % [12]. However, its ANIb compared with Chryseobacterium ureilyticum strain DSM 18017T was less than 83 % and it did not match any other species. Furthermore, strain G0240 was found to have an ANIb <86 % when compared with all other strains of members of the genus Chryseobacterium , including G0186. Hence, the ‘123 group’ is not a group at all, and each isolate is currently the sole representative of its species.
Two strains in the SBRL collection, H3001 and H3056, had identical 16S rRNA gene sequences that were also a close match with the 16S rRNA gene sequences from the type strains of Chryseobacterium treverensis (99.3%) and Chryseobacterium solincola (99.0%), both of which are proposed herein to represent members of the genus Kaistella . Their ANIb results of >96 % when compared with each other, but between 86.6 and 86.94% when compared with Chryseobacterium treverense or Chryseobacterium solincola, indicate that they represent a novel species. We propose below the name Kaistella daneshvariae for this species, in recognition of Maryam Daneshvar. Dr. Daneshvar led the SBRL team during a phase of extraordinary productivity that culminated in the 1996 publication of the second edition of the Manual for Identification of Unusual Pathogenic Gram-Negative Aerobic and Facultatively Anaerobic Bacteria.
In 2013, we proposed to move the species Planobacterium taklimakanense into the genus Chryseobacterium , primarily on the basis of its 16S rRNA gene sequence [12]. On the basis of the WGS information now available, we consider our original proposal to have been premature. Since the 16S rRNA gene from Chryseobacterium salipaludis JC490T clusters with the Planobacterium taklimakanense 16S rRNA gene sequences (Fig. S1)but has sequence identity <95 %, this species is probably a second species in the genus Planobacterium , for which we propose the name Planobacterium salipaludis comb. nov.
Planobacterium taklimakanense is a rare example of agenus assignment – we had to consider whether or not to classify it as a member of the genus Kaistella . The AAI between Planobacterium taklimakanense NCTC 13490T and CCUG 49689T, the type strain of Kaistella koreensis (the type species of the genus Kaistella ) was intermediate at 75.1 %. The highest AAI that the type strain of Planobacterium taklimakanense, NCTC 13490T, shared with any other type strain was 76.25 % (Kaistella haifensis), and it had an AAI >76 % when compared to three of the type strains of species of the genus Kaistella , but it also had an AAI < 74 % when compared with the type strains of nine other species of the genus Kaistella . Several characteristics are shared among members of the proposed genus Kaistella , such as small genome size, presence of a carotenoid biosynthetic gene cluster (accessions numbers SNV33133–33186) including lycopene cyclase (SNV33175), absence of the darA gene (Accession number EFK37140 in Chryseobacterium gleum ) and a corresponding absence of the flexirubin pigments that its encoded protein would produce, and a fatty acid composition with >10 % C15 : 0 anteiso. However, unlike the strains belonging to the genus Kaistella , Planobacterium taklimakanense lacks the nosZ gene (accession number KMQ71079 in Kaistella koreensis ) and associated maturase genes necessary to produce nitrous oxide reductase. Core genome analysis placed the genus Halpernia between Kaistella and Planobacterium . Taken together, these findings are sufficient evidence to consider Planobacterium as a separate genus. The SBRL at CDC receives several specimens of this species every year for identification, and it should be recognised as a potential human pathogen.
Phylogenetic analysis of the 16S rRNA gene sequences (Fig. S1) of all species described as members of the genus Chryseobacterium , along with most other members of the family Weeksellaceae and Flavobacterium aquatile as the outgroup, places the recently published Chryseobacterium reticulitermitis near the base of the tree, near the genus Soonwooa . The effectively, but not validly, published ‘Chryseobacterium chengduensis’ clusters with Daejongia ginsengosidivorans. Chryseobacterium frigidum appears to be most closely related to Cruicaptor ignavus, but divergent phenotypically, both in cell shape and in major fatty acid composition. No whole-genome sequences exist for any of these species, so out of an abundance of caution we make no proposals for them at this time. In contrast, the 16S RNA gene species Chryseobacterium salipaludis is clustered with several fully sequenced strains of Planobacterium taklimakanense , and is phenotypically similar to them, providing sufficient evidence for our proposal of this species as Planobacterium salipaludis comb. nov. Similarly, both 16S rRNA gene sequence and phenotype of Chryseobacterium marinum were similar to those of the proposed species of the genus Halpernia, prompting us to designate this species as Halpernia marina comb. nov. All species of the genus Kaistella cluster together apart from any other genus, as do the species of the genera Planobacterium and Halpernia but there is some intermingling among the species of the genera Epilithonimonas and Chryseobacterium . In contrast, phylogenetic analysis of rpoB gene sequences (Fig. S3) provides a much clearer separation of the genera, and is in agreement with the core genome maximum likelihood analysis (Fig. 2) and with the genus assignments based on AAI.
Available phenotypic data from the literature for each of the 109 species listed as members of the genus Chryseobacterium in the LSPN as of September 2018, along with phenotypic data for two novel species described herein, has been tabulated (Table S2). Phenotypic differentiation of members of the family Flavobacteriaceae has long been recognised as difficult and unreliable, as variation within each species is substantial, and certain assays produce different results depending on the method used. For example, strains of members of the genus Elizabethkingia that were unable to grow on MacConkey agar when initially isolated gained the ability to do so after several passages [89]. Table S2 Despite these limitations, certain trends can be discerned and are discussed here. Because some data were not available, we describe the results as a fraction with the denominator representing the number of species in a particular genus that have data available.
Almost half of species of the genus Chryseobacterium tested (27 out of 66) degraded urea, but only one species of the genus Kaistella did, and none of the species of the genera Halpernia or Epilithonimonas . Most species of the genera Chryseobacterium (73/75), Halpernia (2/2), and Epilithonimonas (11/11) degraded aesculin, but almost half (6/13) of the species of the genus Kaistella did not. The only strains capable of growing on cetrimide agar were among the species of the genus Chryseobacterium , although only a minority of those (13 out of 31) were able to do so.
A few species of the genus Chryseobacterium (7/55) produced acid from mannitol while no species of the genera Halpernia (0/1), or Kaistella (0/11) did, and only a single species of the genus Epilithonimonas (1/8) produced acid from mannitol, but results varied for different laboratories. No species of the genus Halpernia (0/1) and only a single species of the genus Kaistella (1/11) produced acid from arabinose, but over 20 % of species of the genera Chryseobacterium (14/61) and Epilithonimonas (3/10) did. No species of the genus Kaistella (0/11) produced acid from trehalose, but half or more of species of the genera Chryseobacterium (33/52), Epilithonimonas (5/9), and Halpernia (1/2) did.
A novel phospholipid fatty acid, anteiso-C17 : 2 ω3,7, has been recently identified in the cell membrane of the type strain of Halpernia frigidisoli [90]. The fact that this fatty acid is predominant when the bacterium is grown at 0°C indicates its importance for cell membrane adaptation. All species of the genus Halpernia grew at 5 °C, and none grew at 42 °C. Both of the available genomes of members of the genus Halpernia contain proteorhodopsin and beta-carotene monoxygenase, neither of which were present in any genome from a member of the genera Epilithonimonas, Chryseobacterium or Kaistella .
Christensen et al. [38] recommended that multiple strains be used to describe a novel species, rather than a single individual strain, but publication of single-strain species descriptions continues. There have even been suggestions made that a single complete sequenced genome could be used as the type material for publication of a novel species, regardless of whether it can be cultured [37]. The advantage to this approach is that once a novel species is named, additional strains (particularly metagenome assembled genomes) can be assigned to it based on genome sequence similarity, thereby increasing the diversity of habitats known to be occupied by that particular species and improving our understanding of its biology. Unfortunately, single-strain species naming has led to a proliferation of species names, and there is a danger that if this trend continues, the list of validly published species names will become essentially a list of well-characterised named isolates. This could be avoided by limiting the naming of a species to those that have already been isolated more than once or encountered in more than one metagenomics sample. For these reasons, we have elected to release the genomes of several strains that are the sole known representative of their species, without naming them, in hopes of facilitating future collaborations.
Emended description of the genus Chryseobacterium Vandamme et al. 1994
The description of the genus Chryseobacterium is as stated by Vandamme et al. [1] and emended by Kämpfer et al. [10], Wu et al. [91], and Chen et al. [92], with the following emendments: All species tested produce non-diffusible flexirubin type pigments. Most species do not reduce nitrate or nitrite, and most are not capable of growth at 42 °C. Most do not produce H2S. Tween-80 and starch are usually degraded. Acid production is common from glucose, maltose, and trehalose, but rare from lactose or mannitol. The DNA G+C content ranges from 28.8 to 49.3 mol%.
The type species is Chryseobacterium gleum Vandamme et al. (1994). Whole genome analysis of the type strain of each species produces AAI comparison values, using proteins that share 40 % amino acid identity and 50 % coverage length, of ≥76 % when compared with Chryseobacterium gleum strain F93T, and ≥74 % when compared with the type strain of each of the other species in the family. Species that have already been named as members of the genus Chryseobacterium but have not yet their type stain’s genome sequenced at this time and are not otherwise discussed in this manuscript can be assumed to remain in the genus Chryseobacterium .
Emended description of Chryseobacterium bernardetii Holmes et al. 2013
The description is as given by Holmes et al. [12] and Kim et al. [64] with the following emendments: Different strains give different results for H2S production (lead acetate paper method) and nitrate reduction. The DNA G+C content of type strain G0229T was calculated to be 36.3 mol%.
Emended description of Chryseobacterium indoltheticum Campbell and Williams 1951 Vandamme et al. 1994
The description is as given by Campbell and Williams [93] and discussed by Bernardet et al. [94] with the following emendment: The DNA G+C content was 34.3 mol% for both strains G0141T and G0211.
Emended description of Chryseobacterium lactis Holmes et al. 2013
The description is as given by Holmes et al. [12] with the following emendment: The DNA G+C content of the type strain KC1864T was calculated to be 36.1 mol%.
Emended description of Chryseobacterium nakagawai Holmes et al. 2013
The description is as given by Holmes et al. [12] with the following emendments: strains are positive for acid production (in ammonium salt medium) from glycerol, aesculin hydrolysis, growth on cetrimide agar, hydrolysis of Tween 80, and utilisation of citrate (Christensen’s medium). The DNA G+C content of the type strain, G0041T, was calculated to be 35.4 mol%.
Emended description of Chryseobacterium shandongense Yang et al. 2015
The description is as given by Yang et al. [86], with the following emendments: Acid is produced from glucose, ethanol and maltose but not from adonitol, dulcitol, glycerol, inositol, lactose, mannitol, raffinose, salicin, sorbitol or sucrose. Hydrolysis of starch, gelatin and DNA varies between strains, as does growth on MacConkey agar.
Emended description of the genus Epilithonimonas O'Sullivan et al. 2006
The description is as given by O'Sullivan et al. [13] but with the following emendments:
Colonies are circular, entire and convex and may be non-pigmented or yellow to bright orange. Cannot produce acid from mannitol. Does not reduce nitrite, hydrolyze urea or produce hydrogen sulphide or arginine dihydrolase but does degrade aesculin. Will not grow on cetrimide agar, and cannot tolerate 3 % NaCl. The DNA G+C content is between 33.3 and 39.2 mol%.
The type species is Epilithonimonas tenax . Whole genome analysis of the type strain of each species produces AAI comparison values, using proteins that share 40 % amino acid identity and 50 % coverage length, of ≥76 % when compared with Epilithonimonas tenax strain EP105T, and ≥74 % when compared with the type strain of each of the other species in the genus.
Description of Epilithonimonas arachidiradicis comb. nov.
Epilithonimonas arachidiradicis (a.ra.chi.di.ra′di.cis. N.L. fem. n. Arachis - idis the generic name of the peanut plant; L. fem. n. radix - icis root; N.L. gen. n. arachidiradicis of the root of Arachis).
Basonym: Chryseobacterium arachidiradicis Kämpfer et al. 2015
The description is as given by Kämpfer et al. [95]. The type strain is 91A-612T = LMG 27814T = CCM 8490T= CIP 110647T.
Description of Epilithonimonas bovis comb. nov.
Epilithonimonas bovis (bo′vis. L. gen. n. bovis of a cow, referring to the isolation from raw cow's milk).
Basonym: Chryseobacterium bovis Hantsis-Zacharov et al. 2008
The description is as given by Hantsis-Zacharov et al. [96]. The type strain is H9T=DSM 19482T=LMG 24227T.
Description of Epilithonimonas caeni comb. nov.
Epilithonimonas caeni (cae′ni. L. gen. n. caeni of sludge).
Basonym: Chryseobacterium caeni Quan et al. 2007
The description is as given by Quan et al. [97] as emended by Hahnke et al. [18]. The type strain is N4T = CCBAU 10201T=DSM 17710T=KCTC 12506T.
Description of Epilithonimonas hispanica comb. nov.
Epilithonimonas hispanica (his.pa′ni.ca. L. fem. adj. hispanica from Spain).
Basonym: Chryseobacterium hispanicum Gallego et al. 2006
The description is as given Gallego et al. [98]. The type strain is VP48T=CECT 7129T=CCM 7359T=JCM 13554T.
Description of Epilithonimonas hominis comb. nov.
Epilithonimonas hominis (ho′mi.nis. L. gen. n. hominis of a man, of a human being, named as such because most of the known isolates at the time of description were of human origin).
Basonym: Chryseobacterium hominis Vaneechoutte et al. 2007
The description is as given by Vaneechoutte et al. [99]. The type strain is NF802T=CCUG 52711T=CIP 109415T.
Description of Epilithonimonas hungarica comb. nov.
Epilithonimonas hungarica (hun.ga′ri.ca. N.L. fem. adj. hungarica from Hungary, referring to the country from which the type strain was isolated).
Basonym: Chryseobacterium hungaricum Szoboszlay et al. 2008
The description is as given by Szoboszlay et al. [100]. The type strain is CHB-20pT=NCAIM B2269T=DSM 19684T.
Description of Epilithonimonas mollis comb. nov.
Epilithonimonas mollis (mol′lis. L. fem. adj. mollis pliant, sensitive, referring to the sensitivity to antibiotics).
Basonym: Chryseobacterium molle Herzog et al. 2008.
The description is the same as given by Herzog et al. [101]. The type strain is DW3T=DSM 18016T=CCUG 52547T.
Description of Epilithonimonas pallida comb. nov.
Epilithonimonas pallida (pal′li.da. L. fem. adj. pallida pale).
Basonym: Chryseobacterium pallidum Herzog et al. 2008
The description is the same as given by Herzog et al. [101]. The type strain is 26-3St2bT=DSM 18015T=CCUG 52548T.
Description of Epilithonimonas zeae comb. nov.
Epilithonimonas zeae (ze′ae. L. gen. n. zeae, of spelt, of Zea mays).
Basonym: Chryseobacterium zeae Kämpfer et al. 2014
The description is as given by Kämpfer et al. [102]. The type strain is JM-1085T=LMG 27809T=CCM 8491T.
Description of Epilithonimonas vandammei sp. nov.
Epilithonimonas vandammei (van.dam′me.i. N.L. gen. masc. n. vandammei named in honor of Peter Vandamme in recognition of his many contributions to the study of the genus Chryseobacterium and related genera).
Cells are Gram-stain-negative. Colonies are circular, convex, entire, opaque, shiny, smooth and usually yellow-pigmented. Positive for acid production (in ammonium salt medium) from glucose and maltose, aesculin hydrolysis, catalase production, cytochrome oxidase production, growth at 37 °C, at room temperature (18–22 °C) and on β-hydroxybutyrate. Negative for acid production (in ammonium salt medium) from adonitol, arabinose, cellobiose, dulcitol, inositol, lactose, raffinose, salicin, sorbitol, trehalose and xylose, arginine dihydrolase production, fluorescence on King’s B medium, gas production from glucose in peptone water medium, gluconate oxidation, growth at 5 °C and at 42 °C and on cetrimide agar, hydrolysis of tyrosine, H2S production (by both lead acetate paper and triple sugar iron agar methods), KCN tolerance, lecithinase production, lipid inclusions after growth on β-hydroxybutyrate, lysine decarboxylase production, malonate utilisation, motility (hanging drop preparation at both 37 °C and room temperature), ornithine decarboxylase production, phenylalanine deamination, production of β-galactosidase (ONPG test), reduction of 0.4 % (w/v) selenite, urease production, utilisation of citrate (Christensen’s and Simmons’ media) and 3-ketolactose production.
The type strain is F5649T=CCUG 73498T=CIP 111693T and was derived from a human clinical testicle isolate from Iowa, USA, in 1984. The DNA G+C content of the type strain has been calculated from its genome sequence to be 37 mol%.
Description of Halpernia gen. nov.
Halpernia (Hal.per′ni.a. N.L. fem. n. Halpernia named after Malka Halpern, Professor at University of Haifa, Haifa, Israel, in recognition of her many contributions to the study of the genus Chryseobacterium and related genera.)
Cells are aerobic. Colonies are yellow-pigmented. Capable of growth on 3 % NaCl. Grows at 5 and 25 °C. Starch is hydrolyzed. Does not degrade urea. Positive for acid production from cellobiose, glucose and lactose; weakly positive for acid production from salicin. Whole-genome analysis of the type strain of each species produces AAI comparison values, using proteins that share 40 % amino acid identity and 50 % coverage length, of ≥76 % when compared with Halpernia frigidisoli strain PB4T, and ≥74 % when compared with the type strain of each of the other species in the genus. The DNA G+C content is 33.7–34 mol%.
The type species is Halpernia frigidisoli.
Description of Halpernia frigidisoli comb. nov.
Halpernia frigidisoli (fri.gi.di.so′li. L. adj. frigidus cold, cool, chilled; L. neut. n. solum soil; N.L. gen. n. frigidisoli pertaining to cold soil, as the strain was isolated from a cold Antarctic soil).
Basonym: Chryseobacterium frigidisoli Bajerski et al. 2013
The description is as given by Bajerski et al. [103]. The type strain is PB4T=DSM 26000T=LMG 27025T.
Description of Halpernia humi comb. nov.
Halpernia humi (hu′mi. L. gen. n. humi of earth, soil).
Basonym: Chryseobacterium humi Pires et al. 2010
The description is as given by Pires et al. [104]. The type strain is ECP37T=LMG 24684T=NBRC 104927T.
Description of Halpernia marina comb. nov.
Halpernia marina (ma.ri′na. L. fem. adj. marina of the sea, marine).
Basonyms: Sejongia marina Lee et al. 2007, Chryseobacterium marinum Kämpfer et al. 2009
The description is as given by Lee et al. [8] and as emended by Kämpfer et al. [9] . The type strain is IMCC3228T (=KCCM 42689T=NBRC 103143T).
Emended description of the genus Kaistella Kim et al. 2004
The description is as given by Kim et al. [5], but is emended as follows:
All strains grow at room temperature; Carotenoid pigments are usually produced. Colonies may be opaque, translucent or transparent. Catalase and oxidase are usually, but not always, positive, and arginine dihydrolase and β-galactosidase are usually, but not always, negative. Aesculin hydrolysis is present in some species. Nitrite is not reduced, and most species do not reduce nitrate. Does not grow on cetrimide agar. Acid production from mannitol, trehalose and xylose is negative, acid production from glucose varies between species. The DNA base composition ranges from 31.3 to 41.6 mol% G+C.
The type species is Kaistella koreensis . Whole-genome analysis of the type strain of each species produces AAI comparison values, using proteins that share 40 % amino acid identity and 50 % coverage length, of ≥76 % when compared with Kaistella koreensis strain Chj707 T, and ≥74 % when compared with the type strain of each of the other species in the genus.
Description of Kaistella anthropi comb. nov.
Kaistella anthropi (an′thro.pi. Gr. n. anthropos, a human being; N.L. gen. n. anthropi, of a human being, since all strains so far recovered are from human clinical specimens).
Basonym: Chryseobacterium anthropi Kämpfer et al. 2009
The description is as given by Kämpfer et al. [10]. The type strain is NF 1366T=CCUG 52764T=CIP 109762T.
Description of Kaistella antarctica comb. nov.
Kaistella antarctica (ant.arc′ti.ca. L. fem. adj. antarctica southern, named after Antarctica, the geographical origin of the type strain).
Basonyms: Sejongia antarctica Yi et al. 2005, Chryseobacterium antarcticum Kämpfer et al. 2009
The description is as given by Yi et al. [7] and emended by Kämpfer et al. [9] and Hahnke, et al. [18]. The type strain is AT1013T=IMSNU 14040T=KCTC 12225T=JCM 12381T.
Description of Kaistella carnis comb. nov.
Kaistella carnis (car′nis. L. fem. n. carnis, of flesh)
Basonym: Chryseobacterium carnis Holmes et al. 2013
The description is as given by Holmes et al. [12], with the following emendment: The type strain is G0081T=NCTC 13525T=CCUG 60559T=CL88/78T=Hayes B19/1T. The DNA G+C content of type strain G0081 was calculated to be 36.4 mol%.
Description of Kaistella chaponensis comb. nov.
Kaistella chaponensis (cha.po.nen′sis. N.L. fem. adj. chaponensis pertaining to Lake Chapo, Chile, from which the Atlantic salmon harbouring the original two isolates was obtained)
Basonym: Chryseobacterium chaponense Kämpfer et al. 2011
The description is as given by Kämpfer et al. [105]. The type strain is Sa 1147–06T=DSM 23145T=CCM 7737T.
Description of Kaistella daneshvariae sp. nov.
Kaistella daneshvariae (da.nesh.va′ri.ae. N.L. gen. fem. n. daneshvariae named in honor of Maryam Daneshvar, for her many contributions to the description and characterization of strains from CDC’s collection of clinical bacterial isolates).
Cells are Gram-stain-negative, rod shaped, aerobic and non-motile. Colonies on blood agar with 5 % rabbit’s blood are yellow-pigmented, convex and smooth with no lysis and may appear mucoid or runny. Growth occurs at 25 and 35° but not at 42 °C. Does not require NaCl or tolerate high (6 %) NaCl. Does not grow on MacConkey’s, citrate or cetrimide agars. Catalase- and oxidase-positive, and positive for indole production. Does not hydrolyze urea, aesculin or gelatin, and does not reduce nitrate. Produces H2S on lead acetate paper but not triple sugar iron agar. Acid production may occur from glucose, but not from d-xylose, mannitol, lactose, sucrose or maltose.
The type strain is H3001T (=CCUG 73276T=CIP 111694T), which was isolated from the peritoneal cavity of a patient in the state of New York, USA. The DNA G+C content of the type strain is 39.9 %. A second reference strain is H3056 (=CCUG 73499), which was isolated as a blood culture from a patient in New Mexico, USA.
Description of Kaistella haifensis comb. nov.
Kaistella haifensis (hai.fen′sis. N.L. fem. adj. haifensis pertaining to Haifa, the name of the university (University of Haifa) where the first isolates were studied.)
Basonym: Chryseobacterium haifense Hantsis-Zacharov and Halpern 2007
The description is the same as for Chryseobacterium haifense [85]. The type strain is H38T=DSM 19056T=LMG 24029T.
Description of Kaistella jeonii comb. nov.
Kaistella jeonii (jeo′ni.i. N.L. gen. n. jeonii named in honour of the late Jae Gyu Jeon, who devoted his life to polar research.)
Basonyms: Sejongia jeonii Yi et al. 2005, Chryseobacterium jeonii Kämpfer et al. 2009
The description is as given by Yi et al. [7] as emended by Kämpfer et al.[9] . The type strain is AT1047T=IMSNU 14049T=KCTC 12226T=JCM 12382T.
Description of Kaistella montana comb. nov.
Kaistella montana sp. nov. (mon.ta′na. L. fem. adj. montana living in the mountains).
Basonym: Chryseobacterium montanum Guo et al. 2016
The description is as given by Guo et al. [106]. The type strain is WG4T=KCTC 52204T=CCTCC AB 2016058T.
Description of Kaistella palustris comb. nov.
Kaistella palustris (pa.lus′tris. L. fem. adj. palustris pertaining to a marsh)
Basonym: Chryseobacterium palustre Pires et al. 2010
The description is as given by Pires et al. [104] as emended by Hahnke, et al. [18]. The type strain is 3A10T (=LMG 24685T=NBRC 104928T).
Description of Kaistella solincola comb. nov.
Kaistella solincola (sol.in′co.la. L. neut. n. solum soil; L. masc. or fem. n. incola an inhabitant; N.L. n. solincola an inhabitant of soil).
Basonym : Chryseobacterium solincola Benmalek et al. 2010
The description is as given by Benmalek et al. [107] as emended by Hahnke, et al. [18]. The type strain is 1YB-R12T=DSM 22468T=CCUG 55604T.
Description of Kaistella treverensis comb. nov.
Kaistella treverensis (tre.ve.ren′sis. N.L. fem. adj. treverensis, pertaining to Augusta Trevirorum, the Latin name of Treves [Trier, West Germany], the city from which the strain was sent for identification).
Basonym: Chryseobacterium treverense Yassin et al. 2010
The description is as given by Yassin et al. [108]. The type strain is IMMIB L-1519T=CCUG 57657T=DSM 22251T.
Description of Kaistella yonginensis comb. nov.
Kaistella yonginensis (yon.gi.nen′sis. N.L. fem. adj. yonginensis of or belonging to Yongin, Korea, from where the type strain was isolated).
Basonym: Chryseobacterium yonginense Joung and Joh, 2011
The description is as given by Joung and Joh [109]. The type strain is HMD1043T=KCTC 22744T=CECT 7547T.
Emended description of the genus Planobacterium Peng et al. 2009
The description is as given by Peng et al. [11].
The type species of the genus is Planobacterium taklimakanense Peng et al. (2009). Despite the naming of the genus based on motility of the first isolate, motility has not been observed in subsequent isolates. The predominant cellular fatty acids are iso-C15 : 0 and anteiso-C15 : 0. Whole-genome analysis of the type strain of each species produces AAI comparison values, using proteins that share 40 % amino acid identity and 50 % coverage length, of ≥76 % when compared with Planobacterium taklimakanense strain X-65T, and ≥74 % when compared with the type strain of each of the other species in the genus.
Emended description of Planobacterium taklimakanense Peng et al. 2009
The description is as given by Peng et al. [11] and Kim et al. [64] but with the following amendment: The DNA G+C content of strain NCTC 13490T is 40.4 mol% based on WGS, and reference strains H4753 and F9257 have DNA G+C contents of 40.8 mol% and 40.7 mol%, respectively.
Description of Planobacterium salipaludis comb. nov.
Planobacterium salipaludis (sa.li.pa.lu′dis. L. n. sal, salt; L. gen. n. paludis, of a swamp; N.L. gen. n. salipaludis, of a salt marsh)
Basonym: Chryseobacterium salipaludis Divyasree et al. 2018
The description is as given by Divyasree et al. [110]. The type strain is JC490T (KCTC 52835T=LMG 30048T).
Supplementary Data
Funding information
CDC research was supported by the Advanced Molecular Detection (AMD) initiative. Research by J. D. N. was supported by Lycoming College Professional Development Grants, NSF Grants 0960114 and 1248096, and a Fulbright Grant from the US State Department. Work by J.-F. B. was supported by institutional funding from French Institut National de la Recherche Agronomique.
Acknowledgements
We thank Aharon Oren for nomenclature advice. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centres for Disease Control and Prevention (CDC). The mention of company names or products does not constitute endorsement by CDC.
Author contributions
This work was conceptualised by A. C. N. and J. D. N., and methodology was developed by A. C. N., C. A. G., and J. D. N. Software development and validation were provided A. C. N., and C. A. G., who also did the data curation with the help of P. K., C. H. and J. F. B., and the formal analysis along with J. D. N., D. B. and L. R. A. C. N. provided the data visualizations and the original draft of the manuscript. All authors participated in performing the experiments and collecting data, and in the review and editing of the final manuscript. Project administration was performed by A. C. N., A. M. W. and J. M., and project resources were provided by B. H. and J. M. J. M. provided supervision and obtained funding for this work.
Conflicts of interest
All authors report that they have no conflicts of interest.
Ethical statement
This report does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Abbreviations: AAI, amino acid identity; ANI, average nucleotide identity; ANIb, average nucleotide identity using blast; CDC, Centers for Disease Control and Prevention; DDH, DNA–DNA hybridization; gANI, whole-genome ANI; GGDC, genome-to-genome distance calculation; K-pg, Cretaceous–Paleogene; MYA, million years ago; NCTC, National Collection of Type Cultures; POCP, Percentage of conserved proteins; rRNA, ribosomal ribonucleic acid; SBRL, Special Bacteriology Reference Laboratory; WGS, whole genome sequence.
Four supplementary figures and two supplementary tables are available with the online version of this article.
References
- 1.Vandamme P, Bernardet JF, Segers P, Kersters K, Holmes B. New perspectives in the classification of the Flavobacteria: description of Chryseobacterium gen. nov., Bergeyella gen. nov., and Empedobacter nom. rev. Int J Syst Bacteriol. 1994;44:827–831. doi: 10.1099/00207713-44-4-827. [DOI] [Google Scholar]
- 2.Holmes B, Steigerwalt AG, Weaver RE, Brenner DJ. Weeksella zoohelcum sp. nov. (Formerly group IIj), from human clinical specimens. Syst Appl Microbiol. 1986;8:191–196. doi: 10.1016/S0723-2020(86)80076-5. [DOI] [Google Scholar]
- 3.Lustig A. Diagnostica Dei Batteri Delle Acque: Con Una Guida Alle Ricerche Batteriologiche E Microscopiche. Rosenberg & Sellier; 1890. [Google Scholar]
- 4.Prévot AR. Traité De Systématique Bactérienne. Paris: Dunod; 1961. [Google Scholar]
- 5.Kim MK, WT I, Shin YK, Kim SH, Lee BC, et al. Kaistella koreensis gen. nov., sp. nov., a novel member of the Chryseobacterium–Bergeyella–Riemerella branch. Int J Syst Evol Microbiol. 2004;54:2319–2324. doi: 10.1099/ijs.0.02998-0. [DOI] [PubMed] [Google Scholar]
- 6.Kim KK, Kim MK, Lim JH, Park HY, Lee ST. Transfer of Chryseobacterium meningosepticum and Chryseobacterium miricola to Elizabethkingia gen. nov. as Elizabethkingia meningoseptica comb. nov. and Elizabethkingia miricola comb. nov. Int J Syst Evol Microbiol. 2005;55:1287–1293. doi: 10.1099/ijs.0.63541-0. [DOI] [PubMed] [Google Scholar]
- 7.Yi H, Yoon HI, Chun J. Sejongia antarctica gen. nov., sp. nov. and Sejongia jeonii sp. nov., isolated from the Antarctic. Int J Syst Evol Microbiol. 2005;55:409–416. doi: 10.1099/ijs.0.63273-0. [DOI] [PubMed] [Google Scholar]
- 8.Lee K, Lee HK, Choi TH, Cho JC. Sejongia marina sp. nov., isolated from Antarctic seawater. Int J Syst Evol Microbiol. 2007;57:2917–2921. doi: 10.1099/ijs.0.65279-0. [DOI] [PubMed] [Google Scholar]
- 9.Kämpfer P, Lodders N, Vaneechoutte M, Wauters G. Transfer of Sejongia antarctica, Sejongia jeonii and Sejongia marina to the genus Chryseobacterium as Chryseobacterium antarcticum comb. nov., Chryseobacterium jeonii comb. nov. and Chryseobacterium marinum comb. nov. Int J Syst Evol Microbiol. 2009;59:2238–2240. doi: 10.1099/ijs.0.009142-0. [DOI] [PubMed] [Google Scholar]
- 10.Kämpfer P, Vaneechoutte M, Lodders N, De Baere T, Avesani V, et al. Description of Chryseobacterium anthropi sp. nov. to accommodate clinical isolates biochemically similar to Kaistella koreensis and Chryseobacterium haifense, proposal to reclassify Kaistella koreensis as Chryseobacterium koreense comb. nov. and emended description of the genus Chryseobacterium . Int J Syst Evol Microbiol. 2009;59:2421–2428. doi: 10.1099/ijs.0.008250-0. [DOI] [PubMed] [Google Scholar]
- 11.Peng F, Liu M, Zhang L, Dai J, Luo X, et al. Planobacterium taklimakanense gen. nov., sp. nov., a member of the family Flavobacteriaceae that exhibits swimming motility, isolated from desert soil. Int J Syst Evol Microbiol. 2009;59:1672–1678. doi: 10.1099/ijs.0.006619-0. [DOI] [PubMed] [Google Scholar]
- 12.Holmes B, Steigerwalt AG, Nicholson AC. DNA–DNA hybridization study of strains of Chryseobacterium, Elizabethkingia and Empedobacter and of other usually indole-producing non-fermenters of CDC groups IIc, IIe, IIh and IIi, mostly from human clinical sources, and proposals of Chryseobacterium bernardetii sp. nov., Chryseobacterium carnis sp. nov., Chryseobacterium lactis sp. nov., Chryseobacterium nakagawai sp. nov. and Chryseobacterium taklimakanense comb. nov. Int J Syst Evol Microbiol. 2013;63:4639–4662. doi: 10.1099/ijs.0.054353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Sullivan LA, Rinna J, Humphreys G, Weightman AJ, Fry JC. Culturable phylogenetic diversity of the phylum 'Bacteroidetes' from river epilithon and coastal water and description of novel members of the family Flavobacteriaceae: Epilithonimonas tenax gen. nov., sp. nov. and Persicivirga xylanidelens gen. nov., sp. nov. Int J Syst Evol Microbiol. 2006;56:169–180. doi: 10.1099/ijs.0.63941-0. [DOI] [PubMed] [Google Scholar]
- 14.Feng H, Zeng Y, Huang Y. Epilithonimonas xixisoli sp. nov., isolated from wetland bank-side soil. Int J Syst Evol Microbiol. 2014;64:4155–4159. doi: 10.1099/ijs.0.065771-0. [DOI] [PubMed] [Google Scholar]
- 15.Hoang V-A, Kim Y-J, Ponnuraj SP, Nguyen N-L, Hwang K-H, et al. Epilithonimonas ginsengisoli sp. nov., isolated from soil of a ginseng field. Int J Syst Evol Microbiol. 2015;65:122–128. doi: 10.1099/ijs.0.065466-0. [DOI] [PubMed] [Google Scholar]
- 16.Ge L, Zhao Q, Sheng H, Wu J, An L. Epilithonimonas psychrotolerans sp. nov., isolated from alpine permafrost. Int J Syst Evol Microbiol. 2015;65:3777–3781. doi: 10.1099/ijsem.0.000489. [DOI] [PubMed] [Google Scholar]
- 17.Shakéd T, Hantsis-Zacharov E, Halpern M. Epilithonimonas lactis sp. nov., isolated from raw cow's milk. Int J Syst Evol Microbiol. 2010;60:675–679. doi: 10.1099/ijs.0.012575-0. [DOI] [PubMed] [Google Scholar]
- 18.Hahnke RL, Meier-Kolthoff JP, Garcia-Lopez M, Mukherjee S, Huntemann M, et al. Genome-based taxonomic classification of Bacteroidetes . Front Microbiol. 2003;2016:7. doi: 10.3389/fmicb.2016.02003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmes B, Steigerwalt AG, Weaver RE, Brenner DJ. Weeksella virosa gen. nov., sp. nov. (formerly group IIF), found in human clinical specimens. Syst Appl Microbiol. 1986;8:185–190. doi: 10.1016/S0723-2020(86)80075-3. [DOI] [Google Scholar]
- 20.Sankar SA, Lo CI, Fall B, Sambe-Ba B, Mediannikov O, et al. Noncontiguous finished genome sequence and description of Weeksella massiliensis sp. nov. New Microbes New Infect. 2015;8:89–98. doi: 10.1016/j.nmni.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segers P, Mannheim W, Vancanneyt M, De Brandt K, Hinz KH, et al. Riemerella anatipestifer gen. nov., comb. nov., the causative agent of septicemia anserum exsudativa, and its phylogenetic affiliation within the Flavobacterium–Cytophaga rRNA homology group. Int J Syst Bacteriol. 1993;43:768–776. doi: 10.1099/00207713-43-4-768. [DOI] [PubMed] [Google Scholar]
- 22.Vancanneyt M, Vandamme P, Segers P, Torck U, Coopman R, et al. Riemerella columbina sp. nov., a bacterium associated with respiratory disease in pigeons. Int J Syst Bacteriol. 1999;49:289–295. doi: 10.1099/00207713-49-1-289. [DOI] [Google Scholar]
- 23.Bocklisch H, Hühn F, Herold W, Tomaso H, Diller R, et al. Ostrich – a new avian host of Riemerella columbina . Vet Microbiol. 2012;154:429–431. doi: 10.1016/j.vetmic.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Rubbenstroth D, Ryll M, Hotzel H, Christensen H, Knobloch JK-M, et al. Description of Riemerella columbipharyngis sp. nov., isolated from the pharynx of healthy domestic pigeons (Columba livia f. domestica), and emended descriptions of the genus Riemerella, Riemerella anatipestifer and Riemerella columbina . Int J Syst Evol Microbiol. 2013;63:280–287. doi: 10.1099/ijs.0.036798-0. [DOI] [PubMed] [Google Scholar]
- 25.Kämpfer P, Avesani V, Janssens M, Charlier J, De Baere T. Description of Wautersiella falsenii gen. nov., sp. nov., to accommodate clinical isolates phenotypically resembling members of the genera Chryseobacterium and Empedobacter . Int J Syst Evol Microbiol. 2006;56:2323–2329. doi: 10.1099/ijs.0.64393-0. [DOI] [PubMed] [Google Scholar]
- 26.Zhang R-G, Tan X, Liang Y, Meng T-Y, Liang H-Z, et al. Description of Chishuiella changwenlii gen. nov., sp. nov., isolated from freshwater, and transfer of Wautersiella falsenii to the genus Empedobacter as Empedobacter falsenii comb. nov. Int J Syst Evol Microbiol. 2014;64:2723–2728. doi: 10.1099/ijs.0.063115-0. [DOI] [PubMed] [Google Scholar]
- 27.Joung Y, Song J, Lee K, Oh H-M, Joh K, et al. Soonwooa buanensis gen. nov., sp. nov., a member of the family Flavobacteriaceae isolated from seawater. Int J Syst Evol Microbiol. 2010;60:2061–2065. doi: 10.1099/ijs.0.017707-0. [DOI] [PubMed] [Google Scholar]
- 28.Yassin AF, Inglis TJJ, Hupfer H, Siering C, Schumann P, et al. Cruoricaptor ignavus gen. nov., sp. nov., a novel bacterium of the family Flavobacteriaceae isolated from blood culture of a man with bacteraemia. Syst Appl Microbiol. 2012;35:421–426. doi: 10.1016/j.syapm.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Siddiqi MZ, Choi GM, Kim MS, Im W-T. Daejeonia ginsenosidivorans gen. nov., sp. nov., a ginsenoside-transforming bacterium isolated from lake water. Int J Syst Evol Microbiol. 2017;67:2665–2671. doi: 10.1099/ijsem.0.001994. [DOI] [PubMed] [Google Scholar]
- 30.García-López M, Meier-Kolthoff JP, Tindall BJ, Gronow S, Woyke T, et al. Analysis of 1,000 type-strain genomes improves taxonomic classification of Bacteroidetes . Frontiers in Microbiology. 2019;10:2083. doi: 10.3389/fmicb.2019.02083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richter M, Rosselló-Móra R. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci USA. 2009;106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholson AC, Gulvik CA, Whitney AM, Humrighouse BW, Graziano J, et al. Revisiting the taxonomy of the genus Elizabethkingia using whole-genome sequencing, optical mapping, and MALDI-TOF, along with proposal of three novel Elizabethkingia species: Elizabethkingia bruuniana sp. nov., Elizabethkingia ursingii sp. nov., and Elizabethkingia occulta sp. nov. Antonie van Leeuwenhoek. 2018;111:55–72. doi: 10.1007/s10482-017-0926-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auch AF, von Jan M, Klenk H-P, Göker M. Digital DNA–DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand Genomic Sci. 2010;2:117–134. doi: 10.4056/sigs.531120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goris J, Klappenbach JA, Vandamme P, Coenye T, Konstantinidis KT, et al. DNA–DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol. 2007;57:81–91. doi: 10.1099/ijs.0.64483-0. [DOI] [PubMed] [Google Scholar]
- 35.Varghese NJ, Mukherjee S, Ivanova N, Konstantinidis KT, Mavrommatis K, et al. Microbial species delineation using whole genome sequences. Nucleic Acids Res. 2015;43:6761–6771. doi: 10.1093/nar/gkv657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garrity GM. A new genomics-driven taxonomy of bacteria and archaea: are we there yet? J Clin Microbiol. 2016;54:1956–1963. doi: 10.1128/JCM.00200-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitman WB. Genome sequences as the type material for taxonomic descriptions of prokaryotes. Syst Appl Microbiol. 2015;38:217–222. doi: 10.1016/j.syapm.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Christensen H, Bisgaard M, Frederiksen W, Mutters R, Kuhnert P, et al. Is characterization of a single isolate sufficient for valid publication of a new genus or species? proposal to modify recommendation 30b of the Bacteriological Code (1990 revision) Int J Syst Evol Microbiol. 2001;51:2221–2225. doi: 10.1099/00207713-51-6-2221. [DOI] [PubMed] [Google Scholar]
- 39.Hugo C, Bernardet JF, Nicholson A, Kämpfer P. Chryseobacterium. In: Whitman WB, Rainey F, Kämpfer P, Trujillo M, Chun J, et al., . Bergey's Manual of Systematics of Archaea and Bacteria. 2019. In. editors. [Google Scholar]
- 40.Parks DH, Chuvochina M, Waite DW, Rinke C, Skarshewski A, et al. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol. 2018;36:996–1004. doi: 10.1038/nbt.4229. [DOI] [PubMed] [Google Scholar]
- 41.Brenner DJ, McWhorter AC, Knutson JK, Steigerwalt AG. Escherichia vulneris: a new species of Enterobacteriaceae associated with human wounds. J Clin Microbiol. 1982;15:1133–1140. doi: 10.1128/jcm.15.6.1133-1140.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmes B, Lapage SP, Malnick H. Strains of Pseudomonas putrefaciens from clinical material. J Clin Pathol. 1975;28:149–155. doi: 10.1136/jcp.28.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villarma A, Gulvik CA, Rowe LA, Sheth M, Juieng P, et al. Twelve complete reference genomes of clinical isolates in the Capnocytophaga genus. Genome Announc. 2017;5 doi: 10.1128/genomeA.01186-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicholson AC, Whitney AM, Emery BD, Bell ME, Gartin JT, et al. Complete genome sequences of four strains from the 2015-2016 Elizabethkingia anophelis outbreak. Genome Announc. 2016;4 doi: 10.1128/genomeA.00563-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stropko SJ, Pipes SE, Newman JD. Genome-based reclassification of Bacillus cibi as a later heterotypic synonym of Bacillus indicus and emended description of Bacillus indicus . Int J Syst Evol Microbiol. 2014;64:3804–3809. doi: 10.1099/ijs.0.068205-0. [DOI] [PubMed] [Google Scholar]
- 46.Buonaccorsi VP, Boyle MD, Grove D, Praul C, Sakk E, et al. GCAT-SEEKquence: genome consortium for active teaching of undergraduates through increased faculty access to next-generation sequencing data. CBE Life Sci Educ. 2011;10:342–345. doi: 10.1187/cbe.11-08-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buonaccorsi V, Peterson M, Lamendella G, Newman J, Trun N, et al. Vision and change through the genome consortium for active teaching using next-generation sequencing (GCAT-SEEK) CBE Life Sci Educ. 2014;13:1–2. doi: 10.1187/cbe.13-10-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 49.Kim M, Oh H-S, Park S-C, Chun J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol. 2014;64:346–351. doi: 10.1099/ijs.0.059774-0. [DOI] [PubMed] [Google Scholar]
- 50.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hyatt D, Chen G-L, LoCascio PF, Land ML, Larimer FW, et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin QL, Xie BB, Zhang XY, Chen XL, Zhou BC, et al. A proposed genus boundary for the prokaryotes based on genomic insights. J Bacteriol. 2014;196:2210–2215. doi: 10.1128/JB.01688-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pattengale ND, Alipour M, Bininda-Emonds ORP, Moret BME, Stamatakis A. How many bootstrap replicates are necessary? J Comput Biol. 2010;17:337–354. doi: 10.1089/cmb.2009.0179. [DOI] [PubMed] [Google Scholar]
- 56.Bodilis J, Nsigue-Meilo S, Besaury L, Quillet L. Variable copy number, intra-genomic heterogeneities and lateral transfers of the 16S rRNA gene in Pseudomonas . PLoS One. 2012;7:e35647. doi: 10.1371/journal.pone.0035647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen J, Miao X, Xu M, He J, Xie Y, et al. Intra-genomic heterogeneity in 16S rRNA genes in strictly anaerobic clinical isolates from periodontal abscesses. PLoS One. 2015;10:e0130265. doi: 10.1371/journal.pone.0130265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walcher M, Skvoretz R, Montgomery-Fullerton M, Jonas V, Brentano S. Description of an unusual Neisseria meningitidis isolate containing and expressing Neisseria gonorrhoeae-specific 16S rRNA gene sequences. J Clin Microbiol. 2013;51:3199–3206. doi: 10.1128/JCM.00309-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stackebrandt E, Ebers J. Taxonomic parameters revisited: tarnished gold standards. Microbiol Today. 2006;33:152–155. [Google Scholar]
- 60.Shimomura K, Kaji S, Hiraishi A. Chryseobacterium shigense sp. nov., a yellow-pigmented, aerobic bacterium isolated from a lactic acid beverage. Int J Syst Evol Microbiol. 2005;55:1903–1906. doi: 10.1099/ijs.0.63690-0. [DOI] [PubMed] [Google Scholar]
- 61.Charimba G, Jooste P, Albertyn J, Hugo C. Chryseobacterium carnipullorum sp. nov., isolated from raw chicken. Int J Syst Evol Microbiol. 2013;63:3243–3249. doi: 10.1099/ijs.0.049445-0. [DOI] [PubMed] [Google Scholar]
- 62.De Ley J. Reexamination of the association between melting point, buoyant density, and chemical base composition of deoxyribonucleic acid. J Bacteriol. 1970;101:738–754. doi: 10.1128/jb.101.3.738-754.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huss VAR, Festl H, Schleifer KH. Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst Appl Microbiol. 1983;4:184–192. doi: 10.1016/S0723-2020(83)80048-4. [DOI] [PubMed] [Google Scholar]
- 64.Kim T, Kim M, Kang O, Jiang F, Chang X, et al. Chryseobacterium frigidum sp. nov., isolated from high-Arctic tundra soil, and emended descriptions of Chryseobacterium bernardetii and Chryseobacterium taklimakanense . Int J Syst Evol Microbiol. 2016;66:609–615. doi: 10.1099/ijsem.0.000761. [DOI] [PubMed] [Google Scholar]
- 65.Wen C-fang, Xi L-xin, Zhao S, Hao Z-xiang, Luo L, et al. Chryseobacterium chengduensis sp. nov. isolated from the air of captive giant panda enclosures in Chengdu, China. J Zhejiang Univ Sci B. 2016;17:610–618. doi: 10.1631/jzus.B1500214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chun J, Oren A, Ventosa A, Christensen H, Arahal DR, et al. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int J Syst Evol Microbiol. 2018;68:461–466. doi: 10.1099/ijsem.0.002516. [DOI] [PubMed] [Google Scholar]
- 67.Sangal V, Goodfellow M, Jones AL, Schwalbe EC, Blom J, et al. Next-generation systematics: an innovative approach to resolve the structure of complex prokaryotic taxa. Sci Rep. 2016;6:38392. doi: 10.1038/srep38392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gupta RS, Lo B, Son J. Phylogenomics and comparative genomic studies robustly support division of the genus Mycobacterium into an emended genus Mycobacterium and four novel genera. Front Microbiol. 2018;9:67. doi: 10.3389/fmicb.2018.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jain C, Rodriguez-R LM, Phillippy AM, Konstantinidis KT, Aluru S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat Commun. 2018;9:5114. doi: 10.1038/s41467-018-07641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lebreton F, Manson AL, Saavedra JT, Straub TJ, Earl AM, et al. Tracing the Enterococci from Paleozoic origins to the hospital. Cell. 2017;169:849–861.:e13. doi: 10.1016/j.cell.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song H, Wignall PB, Tong J, Yin H. Two pulses of extinction during the Permian–Triassic crisis. Nat Geosci. 2013;6:52–56. doi: 10.1038/ngeo1649. [DOI] [Google Scholar]
- 72.Clapham ME, Bottjer DJ. Prolonged Permian Triassic ecological crisis recorded by molluscan dominance in late Permian offshore assemblages. Proc Natl Acad Sci USA. 2007;104:12971–12975. doi: 10.1073/pnas.0705280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Erwin DH. Lessons from the past: biotic recoveries from mass extinctions. Proc Natl Acad Sci USA. 2001;98:5399–5403. doi: 10.1073/pnas.091092698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thibodeau AM, Ritterbush K, Yager JA, West AJ, Ibarra Y, et al. Mercury anomalies and the timing of biotic recovery following the end-Triassic mass extinction. Nat Commun. 2016;7:11147. doi: 10.1038/ncomms11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burgess SD, Bowring S, Shen SZ, Shen SZ. High-precision timeline for earth's most severe extinction. Proc Natl Acad Sci USA. 2014;111:3316–3321. doi: 10.1073/pnas.1317692111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raup DM, Sepkoski JJ. Mass extinctions in the marine fossil record. Science. 1982;215:1501–1503. doi: 10.1126/science.215.4539.1501. [DOI] [PubMed] [Google Scholar]
- 77.Hull P. Life in the aftermath of mass extinctions. Current Biology. 2015;25:R941–R952. doi: 10.1016/j.cub.2015.08.053. [DOI] [PubMed] [Google Scholar]
- 78.Mata SA, Bottjer DJ. Microbes and mass extinctions: paleoenvironmental distribution of microbialites during times of biotic crisis. Geobiology. 2012;10:3–24. doi: 10.1111/j.1472-4669.2011.00305.x. [DOI] [PubMed] [Google Scholar]
- 79.Huang J-P, Kraichak E, Leavitt SD, Nelsen MP, Lumbsch HT. Accelerated diversifications in three diverse families of morphologically complex lichen-forming fungi link to major historical events. Sci Rep. 2019;9:8518. doi: 10.1038/s41598-019-44881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marzoli A, Bertrand H, Knight KB, Cirilli S, Buratti N, et al. Synchrony of the Central Atlantic magmatic province and the Triassic–Jurassic boundary climatic and biotic crisis. Geology. 2004;32:973. doi: 10.1130/G20652.1. [DOI] [Google Scholar]
- 81.Vellekoop J, Sluijs A, Smit J, Schouten S, Weijers JWH, et al. Rapid short-term cooling following the Chicxulub impact at the Cretaceous–Paleogene boundary. Proc Natl Acad Sci USA. 2014;111:7537–7541. doi: 10.1073/pnas.1319253111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Le Loeuff J. Paleobiogeography and biodiversity of late Maastrichtian dinosaurs: how many dinosaur species went extinct at the Cretaceous–Tertiary boundary? Bulletin de la Societe Geologique de France. 2012;183:547–559. doi: 10.2113/gssgfbull.183.6.547. [DOI] [Google Scholar]
- 83.Petersen SV, Dutton A, Lohmann KC. End-Cretaceous extinction in Antarctica linked to both Deccan volcanism and meteorite impact via climate change. Nat Commun. 2016;7:12079. doi: 10.1038/ncomms12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weyant R, Moss C, Weaver R, Hollis D, Jordan J, et al. Identification of Unusual Pathogenic Gram-negative Aerobic and Facultatively Anaerobic Bacteria. 2nd ed. Baltimore: Williams & Wilkins; 1995. [Google Scholar]
- 85.Hantsis-Zacharov E, Halpern M. Chryseobacterium haifense sp. nov., a psychrotolerant bacterium isolated from raw milk. Int J Syst Evol Microbiol. 2007;57:2344–2348. doi: 10.1099/ijs.0.65115-0. [DOI] [PubMed] [Google Scholar]
- 86.Yang F, Hong Q, Wang X, Zhang R, Li SP, et al. Chryseobacterium shandongense sp. nov., isolated from soil. Int J Syst Evol Microbiol. 2015;65:1860–1865. doi: 10.1099/ijs.0.000186. [DOI] [PubMed] [Google Scholar]
- 87.Campbell S, Harada RM, Li QX. Chryseobacterium arothri sp. nov., isolated from the kidneys of a pufferfish. Int J Syst Evol Microbiol. 2008;58:290–293. doi: 10.1099/ijs.0.65276-0. [DOI] [PubMed] [Google Scholar]
- 88.Kämpfer P, Vaneechoutte M, Wauters G. Chryseobacterium arothri Campbell et al. 2008 is a later heterotypic synonym of Chryseobacterium hominis Vaneechoutte et al. 2007. Int J Syst Evol Microbiol. 2009;59:695–697. doi: 10.1099/ijs.0.004093-0. [DOI] [PubMed] [Google Scholar]
- 89.King EO. Studies on a group of previously unclassified bacteria associated with meningitis in infants. Am J Clin Pathol. 1959;31:241–247. doi: 10.1093/ajcp/31.3.241. [DOI] [PubMed] [Google Scholar]
- 90.Mangelsdorf K, Bajerski F, Karger C, Wagner D. Identification of a novel fatty acid in the cell membrane of Chryseobacterium frigidisoli PB4 T isolated from an East Antarctic glacier forefield. Org Geochem. 2017;106:68–75. doi: 10.1016/j.orggeochem.2017.01.003. [DOI] [Google Scholar]
- 91.Wu Y-F, Wu Q-L, Liu S-J. Chryseobacterium taihuense sp. nov., isolated from a eutrophic lake, and emended descriptions of the genus Chryseobacterium, Chryseobacterium taiwanense, Chryseobacterium jejuense and Chryseobacterium indoltheticum . Int J Syst Evol Microbiol. 2013;63:913–919. doi: 10.1099/ijs.0.040337-0. [DOI] [PubMed] [Google Scholar]
- 92.Chen XY, Zhao R, Chen ZL, Liu L, Li XD, et al. Chryseobacterium polytrichastri sp. nov., isolated from a moss (Polytrichastrum formosum), and emended description of the genus Chryseobacterium . Antonie van Leeuwenhoek. 2015;107:403–410. doi: 10.1007/s10482-014-0338-6. [DOI] [PubMed] [Google Scholar]
- 93.Campbell LL, Williams OB. A study of chitin-decomposing micro-organisms of marine origin. J Gen Microbiol. 1951;5:894–905. doi: 10.1099/00221287-5-5-894. [DOI] [PubMed] [Google Scholar]
- 94.Bernardet J, Bruun B. Genus X. Chryseobacterium Vandamme, Bernardet, Segers, Kersters and Holmes 1994, 829VP. In: Krieg NS JT, Brown DR, Hedlund BP, Paster BJ, Ward NL, editors. Bergey’s Manual of Systematic Bacteriology. 2nd ed. Vol. 4. New York: Springer; 2011. pp. 180–196. [Google Scholar]
- 95.Kämpfer P, Glaeser SP, McInroy JA, Busse H-J. Chryseobacterium arachidiradicis sp. nov., isolated from the geocarposphere (soil around the peanut) of very immature peanuts (Arachis hypogaea) Int J Syst Evol Microbiol. 2015;65:2179–2186. doi: 10.1099/ijs.0.000237. [DOI] [PubMed] [Google Scholar]
- 96.Hantsis-Zacharov E, Senderovich Y, Halpern M. Chryseobacterium bovis sp. nov., isolated from raw cow's milk. Int J Syst Evol Microbiol. 2008;58:1024–1028. doi: 10.1099/ijs.0.65500-0. [DOI] [PubMed] [Google Scholar]
- 97.Quan Z-X, Kim KK, Kim M-K, Jin L, Lee S-T. Chryseobacterium caeni sp. nov., isolated from bioreactor sludge. Int J Syst Evol Microbiol. 2007;57:141–145. doi: 10.1099/ijs.0.64599-0. [DOI] [PubMed] [Google Scholar]
- 98.Gallego V, Garcia MT, Ventosa A. Chryseobacterium hispanicum sp. nov., isolated from the drinking water distribution system of Sevilla, Spain. Int J Syst Evol Microbiol. 2006;56:1589–1592. doi: 10.1099/ijs.0.64264-0. [DOI] [PubMed] [Google Scholar]
- 99.Vaneechoutte M, Kämpfer P, De Baere T, Avesani V, Janssens M, et al. Chryseobacterium hominis sp. nov., to accommodate clinical isolates biochemically similar to CDC groups II-h and II-c. Int J Syst Evol Microbiol. 2007;57:2623–2628. doi: 10.1099/ijs.0.65158-0. [DOI] [PubMed] [Google Scholar]
- 100.Szoboszlay S, Atzel B, Kukolya J, Toth EM, Marialigeti K, et al. Chryseobacterium hungaricum sp. nov., isolated from hydrocarbon-contaminated soil. Int J Syst Evol Microbiol. 2008;58:2748–2754. doi: 10.1099/ijs.0.65847-0. [DOI] [PubMed] [Google Scholar]
- 101.Herzog P, Winkler I, Wolking D, Kämpfer P, Lipski A. Chryseobacterium ureilyticum sp. nov., Chryseobacterium gambrini sp. nov., Chryseobacterium pallidum sp. nov. and Chryseobacterium molle sp. nov., isolated from beer-bottling plants. Int J Syst Evol Microbiol. 2008;58:26–33. doi: 10.1099/ijs.0.65362-0. [DOI] [PubMed] [Google Scholar]
- 102.Kämpfer P, McInroy JA, Glaeser SP. Chryseobacterium zeae sp. nov., Chryseobacterium arachidis sp. nov., and Chryseobacterium geocarposphaerae sp. nov. isolated from the rhizosphere environment. Antonie van Leeuwenhoek. 2014;105:491–500. doi: 10.1007/s10482-013-0101-4. [DOI] [PubMed] [Google Scholar]
- 103.Bajerski F, Ganzert L, Mangelsdorf K, Padur L, Lipski A, et al. Chryseobacterium frigidisoli sp. nov., a psychrotolerant species of the family Flavobacteriaceae isolated from sandy permafrost from a glacier forefield. Int J Syst Evol Microbiol. 2013;63:2666–2671. doi: 10.1099/ijs.0.046904-0. [DOI] [PubMed] [Google Scholar]
- 104.Pires C, Carvalho MF, De Marco P, Magan N, Castro PML. Chryseobacterium palustre sp. nov. and Chryseobacterium humi sp. nov., isolated from industrially contaminated sediments. Int J Syst Evol Microbiol. 2010;60:402–407. doi: 10.1099/ijs.0.010348-0. [DOI] [PubMed] [Google Scholar]
- 105.Kämpfer P, Fallschissel K, Avendaño-Herrera R. Chryseobacterium chaponense sp. nov., isolated from farmed Atlantic salmon (Salmo salar) Int J Syst Evol Microbiol. 2011;61:497–501. doi: 10.1099/ijs.0.022004-0. [DOI] [PubMed] [Google Scholar]
- 106.Guo W, Li J, Shi M, Yuan K, Li N, et al. Chryseobacterium montanum sp. nov. isolated from mountain soil. Int J Syst Evol Microbiol. 2016;66:4051–4056. doi: 10.1099/ijsem.0.001309. [DOI] [PubMed] [Google Scholar]
- 107.Benmalek Y, Cayol JL, Bouanane NA, Hacene H, Fauque G, et al. Chryseobacterium solincola sp. nov., isolated from soil. Int J Syst Evol Microbiol. 2010;60:1876–1880. doi: 10.1099/ijs.0.008631-0. [DOI] [PubMed] [Google Scholar]
- 108.Yassin AF, Hupfer H, Siering C, Busse HJ. Chryseobacterium treverense sp. nov., isolated from a human clinical source. Int J Syst Evol Microbiol. 2010;60:1993–1998. doi: 10.1099/ijs.0.017327-0. [DOI] [PubMed] [Google Scholar]
- 109.Joung Y, Joh K. Chryseobacterium yonginense sp. nov., isolated from a mesotrophic artificial lake. Int J Syst Evol Microbiol. 2011;61:1413–1417. doi: 10.1099/ijs.0.022590-0. [DOI] [PubMed] [Google Scholar]
- 110.Divyasree B, Suresh G, Sasikala C, Ramana CV. Chryseobacterium salipaludis sp. nov., isolated at a wild ass sanctuary. Int J Syst Evol Microbiol. 2018;68:542–546. doi: 10.1099/ijsem.0.002536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.