Abstract
To evaluate the association between prolonged second stage of labor and the risk of adverse neonatal outcomes with a systematic review and meta-analysis. PubMed, Scopus and EMBASE were searched using the search strategy “Labor Stage, Second” AND (length OR duration OR prolonged OR abnormal OR excessive). Observational studies that examine the relationship between prolonged second stage of labor and neonatal outcomes were selected. Prolonged second stage of labor was defined as 4 h or more in nulliparous women and 3 h or more in multiparous women. The main neonatal outcomes were 5 min Apgar score <7, admission to the Neonatal Intensive Care Unit, neonatal sepsis and neonatal death. Data collection and quality assessment were carried out independently by the three reviewers. Twelve studies were selected including 266,479 women. In nulliparous women, a second stage duration greater than 4 h increased the risk of 5 min Apgar score <7, admission to the Neonatal Intensive Care Unit and neonatal sepsis and intubation. In multiparous women, a second stage of labor greater than 3 h was related to 5 min Apgar score <7, admission to the Neonatal Intensive Care Unit, meconium staining and composite neonatal morbidity. Prolonged second stage of labor increased the risk of 5 min Apgar score <7 and admission to the Neonatal Intensive Care Unit in nulliparous and multiparous women, without increasing the risk of neonatal death. This review demonstrates that prolonged second stage of labor increases the risk of neonatal complications in nulliparous and multiparous women.
Keywords: Apgar score, meta-analysis, Neonatal Intensive Care Unit, neonatal morbidity, newborn care, labor stage, second, systematic review
1. Introduction
The second stage of labor is the period of time between full cervical dilatation and birth of the baby, during which the woman has an involuntary urge to bear down, as a result of expulsive uterine contractions [1].
The description of the onset of the second stage of labor in clinical practice is often not precisely known. If complete dilatation is found on vaginal examination, it remains uncertain how long this cervical status has been present [2].
Multiple observational studies [2,3,4] have observed an increase in maternal complications associated with a prolonged second stage of labor, such as operative vaginal delivery, third-/fourth-degree perineal lacerations, caesarean delivery, urinary retention, postpartum hemorrhage and chorioamnionitis, as well as an increase in neonatal complications like seizures, hypoxic-ischemic encephalopathy, sepsis and increased mortality. However, the criteria these studies used to define the second stage of labor are heterogenous.
Thus, diagnosis and management of prolonged second stage of labor and its complications are difficult and often pose a dilemma to the obstetrician regarding timing and type of intervention [5]. Additionally, evidence on the duration of the second stage of labor is of very low certainty [1] and it is unclear whether there is a point of time from which the risk of perinatal complications increases and at which health professionals should intervene to prevent adverse events [3,6].
Nevertheless, there are professionals involved in childbirth care that try to reduce the duration of the second stage by obstetric interventionism in order to avoid neonatal complications. Paradoxically, these interventions, such as immediate pushing (initiated as soon as complete dilation is identified) [7], instrumental birth [8] or fundal pressure [9], may themselves increase the risk of neonatal morbidity.
In the past, a prolonged second stage of labor had been defined as a period of time that lasted beyond 2 h with epidural analgesia or 1 h without epidural analgesia for multiparous women. For nulliparous women, a prolonged second stage is defined as a period of time that lasted beyond 3 h with epidural analgesia or 2 h without epidural analgesia [10]. Recently, though, the American College of Obstetricians and Gynecologists (ACOG) [11] and the National Institute for Health and Care Excellence (NICE) [12] have allowed longer durations in specific cases. In spite of this, the correct management of the second stage of labor should be individualized according to birth progress, fetal malposition or the use of epidural analgesia [11,12]. For example, the Eunice Kennedy Shriver National Institute of Child Health and Human Development document suggested allowing one additional hour for the use of epidural analgesia. Thus, at least 3 h in multiparous women and 4 h in nulliparous women would be considered to diagnose a prolonged second stage of labor [11].
Thus, our objective was to evaluate the evidence on the association between prolonged second stage of labor (defined as 4 h in nulliparous women and 3 h in multiparous women) and the risk of adverse neonatal outcomes.
2. Materials and Methods
This systematic review with a meta-analysis was done according to PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analyses) [13,14].
2.1. Data Sources and Searches
The adopted search strategy was: “Labor Stage, Second” (Mesh) AND (length OR duration OR prolonged OR abnormal OR excessive). Studies were identified in three main databases: PubMed [15], Scopus [16] and EMBASE [17], from 1 January 1990 to 1 November 2019. As well as published studies, we included non-published studies which had been included in the conference proceedings of the main scientific associations and indexed in the databases consulted. All languages were included. The search results for each database are provided in detail in Table A1.
All members of the research team had prior training in the methodology of systematic reviews, literature reviews and critical reading. AAA and AHM are also experts in meta-analysis.
Studies were included according to four criteria: (I) duration of second stage of labor greater than 4 h in nulliparous women; (II) duration of second stage of labor greater than 3 h in multiparous women; (III) studies reporting neonatal outcomes in relation to duration of second stage of labor; (IV) studies that stratified results by parity. Reference lists from the selected studies were also examined to locate further studies not identified using the search strategy. Two authors (NIT and AAA) independently performed the literature search and excluded any articles that did not meet the established inclusion criteria. A third author (MMA) was consulted to resolve any disagreements or uncertainty regarding inclusion.
2.2. Main Outcomes
The primary outcomes were 5 min Apgar score < 7, admission to the Neonatal Intensive Care Unit, neonatal sepsis and neonatal death. All neonatal outcomes examined by the available studies were included in this review. The definitions of some of the variables included in our study are shown in Table 1.
Table 1.
Definition of variables.
| Definitions | Authors | ||||||
|---|---|---|---|---|---|---|---|
| 1995; Menticoglou [18] |
2007; Cheng [19] | 2009; Allen [4] | 2009; Rouse [20] | 2012; Bleich [21] | 2017; Sandström [22] | 2019; Infante [23] | |
| Acidosis | NR | NR | NR | NR | NR | A pH value <7.05 and base excess <−12 in the umbilical artery. | NR |
| Birth depression | NR | NR | Delay in initiating and maintaining respirations after birth requiring resuscitation by mask or endotracheal tube for at least 3 min, a 5 min Apgar score of 3 or less, or neonatal seizures due to hypoxic-ischemic encephalopathy. | NR | NR | NR | NR |
| Intubation | NR | NR | NR | Intubation in delivery room. | NR | NR | NR |
| Heart compressions | NR | NR | NR | NR | NR | Resuscitation in delivery room with heart compressions and/or intubation. | NR |
| Advanced neonatal resuscitation | NR | NR | NR | NR | NR | NR | Type III: Oxygen therapy with positive intermittent pressure. Type IV: Endotracheal intubation, Type V: Cardiac massage and/or using drugs |
| Admission to Neonatal Intensive Care Unit | Need for admission to the Neonatal Intensive Care Unit for any reason at all or with a 5 min Apgar score < 7 or arterial cord pH < 7.20. | NR | Neonatal intensive care unit admission with duration of stay longer than 24 h. | Admission to a neonatal intensive care unit for >48 h. | NR | NR | NR |
| Prolonged neonatal stay | NR | Neonatal stay >2 d for vaginal delivery and >4 d for caesarean delivery. | NR | NR | NR | NR | NR |
| Neonatal seizures | NR | NR | NR | NR | Seizures in the first 24 h of life. | NR | NR |
| Sepsis | NR | NR | Positive blood culture, septicemia or systematic infection. | NR | Positive blood culture. | NR | NR |
| Minor trauma | NR | NR | One or more of the following neonatal traumas: linear skull fracture, other fractures (clavicle, ribs, numerus, or femur), facial palsy, or cephalohematoma. | NR | NR | NR | NR |
| Major trauma | NR | NR | One or more of the following neonatal traumas: depressed skull fracture, intracranial hemorrhage, or brachial plexus palsy. | NR | NR | NR | NR |
| Composite neonatal morbidity | NR | Composite variable for 5 min Apgar <7, UA pH <7.0, UA base excess ≥12, shoulder dystocia, NICU stay, and birth trauma (which includes brachial plexus injury, facial nerve palsy, clavicular fracture, skull fracture, head laceration, and cephalohematoma defined and diagnosed by the attending pediatrician). | Composite of any of the other neonatal outcomes. | Any of the following occurrences: a 5 min Apgar score <4, an umbilical artery pH <7.0, seizures, intubation, stillbirth, neonatal death, or admission to a NICU. | NR | NR | Composite of any of the other neonatal outcomes. |
| Neonatal death | Death during the second stage of labor or in the first 28 d of life | NR | NR | NR | NR | NR | NR |
NR: not reported.
2.3. Data Extraction and Quality Assessment
Data collection and quality assessment were carried out independently by the three reviewers (NIT, AHM and JRA). We tried to contact the authors of several studies to provide us with data that did not appear in their manuscripts.
We used the Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews to assess the risk of bias in each included study [24]. Eleven domains were assessed to appraise the methodological quality of a study and to determine the extent to which a study had addressed the possibility of bias in its design, conduct and analysis.
2.4. Data Synthesis
For the categorical results, the odds ratio (OR) was used along with its 95% confidence intervals (95% CI). To calculate the OR, either the Mantel–Haenszel fixed-effects or Der Simonian–Laird random-effects models were used, depending on whether there was heterogeneity between the studies. Heterogeneity was assessed using the I2 and the statistical Cochran’s Q tests. I2 values of < 25%, 25–50 and >50% normally correspond to small, medium and large heterogeneity, respectively [14,25,26]. Publication bias was also evaluated using the Egger asymmetry test and funnel plots [14,27]. Statistical significance was defined at the ≤0.05 level.
All calculations were done with the StatsDirect statistical software, version 2.7.9. (Stats Direct Ltd., Cheshire, England) [14].
3. Results
3.1. Study Selection
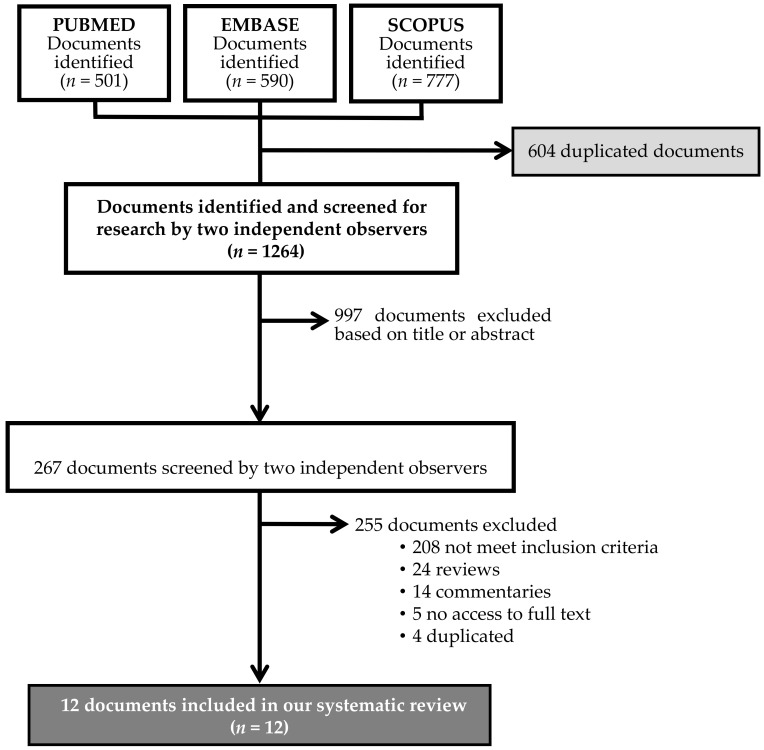
A total of 1868 studies were selected from the literature search. After removing any duplicated articles, 267 were selected by title and abstract. After applying the inclusion/exclusion criteria, twelve articles were selected for the qualitative and quantitative analyses (meta-analysis) (Figure 1).
Figure 1.
PRISMA flow diagram of the literature reviewing process.
3.2. Study Characteristics
The description of the studies included in this systematic review are shown in Table 2. The sample included 268,624 women. The selected studies were conducted in Canada [4,18,28], United States [19,20,21,29,30], China [31], Sweden [22,32] and Spain [23]. The sample size of these studies ranged from 307 [31] to 121,490 [4]. All studies were restricted to singleton infants with cephalic presentation. Eight of these articles studied nulliparous women [18,20,21,22,28,30,31,32], two studied multiparous women [19,23] and two studied both (nulliparous and multiparous women) [4,29]
Table 2.
Characteristics of the studies analyzed.
| YEAR OF PUBLICATION AUTOR |
COUNTRY | STUDY DESIGN | POPULATION UNDER STUDY |
DURATION OF SECOND STAGE OF LABOUR n (%) | DELIVERY MODE N (%) | USE OF EA N (%) |
INCLUSION/EXCLUSION CRITERIA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–1 h | 1–2 h | 2–3 h | 3–4 h | >4 h | Spontaneous Vaginal Delivery | Operative Vaginal Delivery | Caesarean Section | |||||||
| NULLIPAROUS |
1995/ Menticoglou [18] |
Canada | Cohort study | 6041 | 2622 (43.4) | 1805 (29.9) | 927 (15.3) | 379 (6.3) | 308 (5.1) | 4942 (81.8) | 932 (15.5) | 167 (2.7) | NR |
|
|
2009/ Rouse [20] |
United States | Secondary analysis of a clinical trial | 4126 | 1901 (46.1) | 1251 (30.3) |
614 (14.9) | 217 (5.2) | 143 (3.5) | 3054 (74.0) | 765 (18.5) | 307 (7.5) | 3916 (95.0) |
|
|
|
2011/ Li [31] |
China | Case-control study | 307 | 206 (67.1) | 29 (9.4) | 60 (19.5) | 12 (4.0) | NR | NR | NR | NR |
|
||
|
2012/ Bleich [21] |
United States | Cohort study | 21,991 | 13,736 (62.5) | 4933 (22.4) |
1833 (8.3) |
1062 (4.8) |
427 (2.0) | 19,326 (87.9) | 1367 (6.2) | 1298 (5.9) | 13,676 (62.2) |
|
|
|
2015/ Altman [32] |
Sweden | Cohort study | 32,796 | 10,731 (32.7) | 9491 (29.0) |
5856 (17.8) |
3898 (11.9) |
2820 (8.6) | NR | 6728 (20.5) | NR | 19,417 (59.2) |
|
|
|
2015/ Hunt [28] |
Canada | Cohort study | 1515 | NC | NC | NC | 629 (41.5) | 886 (58.5) | 615 (40.6) | 662 (43.7) | 238 (15.7) | NR |
|
|
|
2017/ Sandström [22] |
Sweden | Cohort study | 42,539 | 13,558 (31.9) | 12,225 (28.7) | 7710 (18.1) | 5238 (12.3) | 3808 (9.0) | NR | NR | NR | NR |
|
|
|
2018/ Souter [30] |
United States | Cohort study in a poster session | 20,029 | 16,682 (83.3) | 3347 (16.7) | 14,942 (74.6) | 3015 (15.0) | 2072 (10.4) | 20,029 (100) |
|
||||
| MULTIPAROUS |
2007/ Cheng [19] |
United States | Cohort study | 5158 | 4112 (79.7) | 550 (10.7) | 239 (4.6) | 257 (5.0) | 4480 (86.8) | 414 (8.1) | 263 (5.1) | 2274 (44.1) |
|
|
|
2019/ Infante [23] |
Spain | Cohort study | 2145 | 1589 (74.1) | 327 (15.2) | 165 (7.7) | 64 (3.0) | 2070 (96.5) | 75 (3.5) | NR | 1675 (78.1) |
|
||
| NULLIPAROUS AND MULTIPAROUS |
2009/ Allen [4] |
Canada | Cohort study | 55,936 nulliparous | 38,790 (69.3) | 7832 (14.0) | 4406 (7.9) | 4908 (8.8) | 101,897 (83.8) | 15,865 (13.1) | 3734 (3.1) | 61,077 (50.3) |
|
|
| 65,554 multiparous | 59,227 (90.3) | 4171 (6.3) | 1188 (1.8) | 968 (1.6) | ||||||||||
|
2017/ Ogunyemi [29] |
United States | Poster session | 10,487 * | NC | NC | NC | NC | NC | NR | NR | NR | NR |
|
|
NC: not calculated, NR: not reported, EA: epidural analgesia, BW: birthweight, WG: weeks gestation, *: no data on nulliparous/multiparous.
3.3. Study and Data Quality
The included studies had a low risk of bias, except for three studies that did not identify confounding factors [18,28,31] and four studies that did not include strategies to deal with confounding factors [18,28,30,31] (Table A2).
With regard to the selection of subjects, all studies except one [31] specified inclusion and exclusion criteria, selecting all women (nulliparas and/or multiparas) with singleton cephalic presentation that reached second stage of labor within a specific period of time.
Seven of the studies included in the meta-analysis [4,18,20,21,23,28,32] correctly defined prolonged second stage of labor (in this case, second stage of labor longer than 4 h in nulliparas and longer than 3 h in multiparas). Conversely, only three of them [18,21,28] established the maneuver used once prolonged second stage of labor was diagnosed (instrumental birth, continuing maternal pushing, caesarean, etc.).
As for data and information collection, five studies [4,22,23,30,32] included missing or incomplete data as exclusion criteria, so they were not included in the analysis.
3.4. Main Outcomes and Meta-Analysis
3.4.1. Nulliparous Women
5 min Apgar score <7
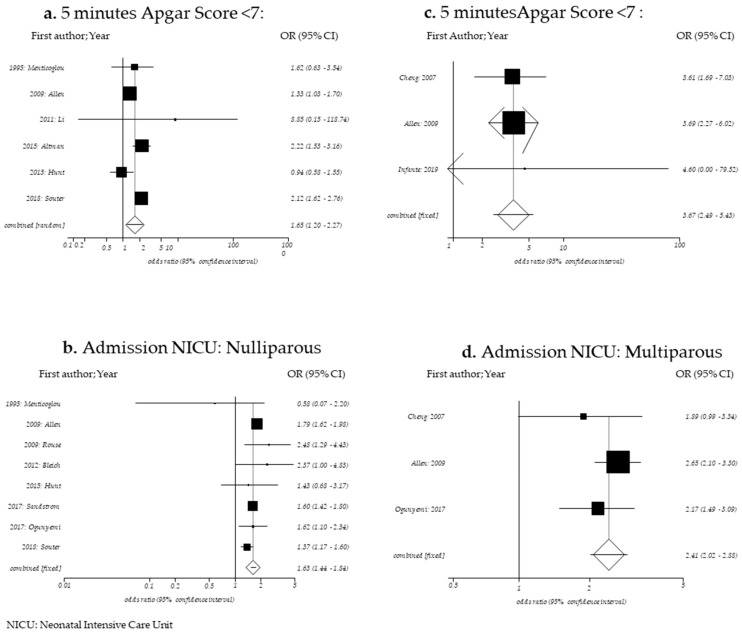
To determine the relation between prolonged second stage of labor in nulliparous women (Table A3) and risk of low 5 min Apgar score (<7), six studies were included (n = 116,624) [4,18,28,30,31,32]. A significant increase in low 5 min Apgar score was observed when the second stage of labor lasted more than 4 h with respect to when the second stage of labor was ≤ 4 h. (OR = 1.65; 95% CI: 1.20–2.27). For this analysis, a random-effects model was used since heterogeneity was observed (Cochran’s Q p-value = 0.0041; I2 = 71.0) (Figure 2a; Table 3).
Figure 2.
Forest plot for 5 min Apgar Score < 7 in nulliparous women (a), admission to NICU in nulliparous women (b), 5 min Apgar Score < 7 in multiparous women (c) and (d) admission to NICU in multiparous women.
Table 3.
Summary of results obtained following meta-analysis of all variables studied in nulliparous women. Summary of results obtained following meta-analysis of all variables studied in multiparous women.
| Variable | Number of Studies | Number of Subjects | Egger Bias (p-Value) |
I2 95% CI | Cochran’s Q (p-Value) |
OR 95% CI |
|---|---|---|---|---|---|---|
| 1 min Apgar Score <7 | 1 | 307 | NC | NC | NC | NC |
| 5 min Apgar score <7 | 6 | 116,624 | 0.7861 | 71.0 (2,1–85,6) | 0.0041 | 1.65 (1.20–2.27) |
| 5 min Apgar score <4 | 2 | 36,922 | NC | NC | 0.7026 | 2.27 (1.08–4.74) |
| 5 min Apgar Score <3 | 1 | 21,991 | NC | NC | NC | NC |
| Umbilical artery pH <7 | 2 | 29,117 | 0.8132 | NC | 0.8132 | 2.30 (0.94–5.69) |
| Umbilical artery pH <7.10 | 0 | 0 | NR | NR | NR | NR |
| Umbilical artery base excess >−12 | 0 | 0 | NR | NR | NR | NR |
| Acidosis | 1 | 33,429 | NC | NC | NC | NC |
| Birth depression | 1 | 55,936 | NC | NC | NC | NC |
| Resuscitation at delivery | 2 | 42,020 | NC | NC | <0.001 | 2.60 (0.81–8.63) |
| Intubation | 2 | 46,665 | NC | NC | 0.681 | 2.19 (1.23–3.90) |
| Heart compressions | 1 | 42,539 | NC | NC | NC | NC |
| ANR | 0 | 0 | NR | NR | NR | NR |
| Meconium aspiration | 1 | 42,539 | NC | NC | NC | NC |
| Meconium-stained amniotic fluid | 1 | 4487 | NC | NC | NC | NC |
| Admission to Neonatal Intensive Care Unit | 8 | 156,650 | 0.8326 | 48.8 (0.0–75.4) | 0.0573 | 1.63 (1.44–1.84) |
| Prolonged neonatal stay | 0 | 0 | NR | NR | NR | NR |
| Neonatal seizures | 3 | 70,571 | NC | 92.3 (78.6–95.8) | <0.001 | 4.67 (0.78–27.78) |
| Neonatal sepsis | 3 | 82,053 | NC | 0.0 (0–72.9) | 0.7962 | 1.57 (1.07–2.29) |
| Birth trauma | 1 | 4064 | NC | NC | NC | NC |
| Minor trauma | 1 | 55,936 | NC | NC | NC | NC |
| Major trauma | 1 | 55,936 | NC | NC | NC | NC |
| Shoulder dystocia | 1 | 20,029 | NC | NC | NC | NC |
| Brachial plexus injury | 1 | 4126 | NC | NC | NC | NC |
| Erb’s palsy | 1 | 21,991 | NC | NC | NC | NC |
| Hypoxic ischemic encephalopathy | 1 | 42,539 | NC | NC | NC | NC |
| Hypothermia treatment | 1 | 42,539 | NC | NC | NC | NC |
| Composite neonatal morbidity | 1 | 4126 | NR | NR | NR | NR |
| Any perinatal morbidity | 0 | 0 | NR | NR | NR | NR |
| Neonatal death | 2 | 28,032 | NC | NC | NC | 7.21 (0.37–139.71) |
| Variable | Number of Studies | Number of Subjects |
Egger Bias
(p-Value) |
I2 95% CI |
Cochran’s Q
(p-Value) |
OR 95% CI |
| 1 min Apgar Score < 7 | 0 | 0 | NR | NR | NR | NR |
| 5 min Apgar score < 7 | 3 | 72,857 | NC | 0.0 (0.0–72.9) | 0.987 | 3.67 (2.48–5.43) |
| 5 min Apgar score < 4 | 0 | 0 | NR | NR | NR | NR |
| 5 min Apgar Score ≤ 3 | 0 | 0 | NR | NR | NR | NR |
| Umbilical artery pH < 7 | 1 | 5158 | NC | NC | NC | NC |
| Umbilical artery pH < 7.10 | 1 | 1912 | NC | NC | NC | NC |
| Umbilical artery base excess > −12 | 1 | 5158 | NC | NC | NC | NC |
| Acidosis | 0 | 0 | NR | NR | NR | NR |
| Birth depression | 1 | 65,554 | NC | NC | NC | NC |
| Resuscitation at delivery | 0 | 0 | NR | NR | NR | NR |
| Intubation | 0 | 0 | NR | NR | NR | NR |
| Heart compressions | 0 | 0 | NR | NR | NR | NR |
| ANR | 1 | 2145 | NC | NC | NC | NC |
| Meconium aspiration | 0 | 0 | NR | NR | NR | NR |
| Meconium-stained amniotic fluid | 2 | 11,193 | NC | NC | 0.121 | 1.29 (1.01–1.66) |
| Admission to Neonatal Intensive Care Unit | 3 | 76,692 | NC | 0.0 (0.0–72.9) | 0.417 | 2.41 (2.02–2.88) |
| Prolonged neonatal stay | 1 | 5158 | NC | NC | NC | NC |
| Neonatal seizures | 0 | 0 | NR | NR | NR | NR |
| Neonatal sepsis | 0 | 0 | NR | NR | NR | NR |
| Birth trauma | 0 | 0 | NR | NR | NR | NR |
| Minor trauma | 1 | 65,554 | NC | NC | NC | NC |
| Major trauma | 1 | 65,554 | NC | NC | NC | NC |
| Shoulder dystocia | 1 | 5158 | NC | NC | NC | NC |
| Brachial plexus injury | 0 | 0 | NR | NR | NR | NR |
| Erb’s palsy | 0 | 0 | NR | NR | NR | NR |
| Hypoxic ischemic encephalopathy | 0 | 0 | NR | NR | NR | NR |
| Hypothermia treatment | 0 | 0 | NR | NR | NR | NR |
| Composite neonatal morbidity | 2 | 7303 | NC | NC | 0.330 | 1.97 (1.39–2.80) |
| Any perinatal morbidity | 1 | 65,554 | NC | NC | NC | NC |
| Neonatal death | 0 | 0 | NR | NR | NR | NR |
ANR: advanced neonatal resuscitation; NC: not calculated; NR: not reported.
Admission to Neonatal Intensive Care Unit
To assess the risk of admission to the Neonatal Intensive Care Unit, eight studies were employed (n = 156,650) [4,18,20,21,22,28,29,30].
The risk significantly increased when the second stage of labor lasted more than 4 h with respect to when the second stage of labor was ≤ 4 h (OR, 1.63; 95%CI 1.44–1.84). For this analysis, a random-effects model was used since medium heterogeneity was observed (Cochran’s Q p-value = 0.057; I2 = 48.8) (Figure 2B; Table 3).
Neonatal Sepsis
By combining three studies (n = 82,053) [4,20,21], we found that the risk of neonatal sepsis increased when the duration of the second stage of labor was longer than 4 h with respect to when the second stage of labor was ≤ 4 h (OR, 1.57; 95% CI 1.07–2.29). For this analysis, a fixed-effects model was used since no heterogeneity was observed (Cochran’s Q p-value = 0.7962; I2 = 0.0) (Table 3).
Neonatal Death
Two studies (n = 28,032) [18,21] were employed to determine the relationship between prolonged second stage of labor and risk of neonatal death, and no differences were found (OR, 7.21; 95% CI 0.37–139.71) (Table 3).
Other Neonatal Outcomes
No significant associations were reported between prolonged second stage in nulliparous women and 1 min Apgar score < 1, 5 min Apgar score ≤ 3, umbilical artery pH < 7, acidosis, meconium-stained amniotic fluid, meconium aspiration, birth depression, minor or major trauma, birth trauma, shoulder dystocia, brachial plexus injury, Erb’s palsy, resuscitation at birth, heart compressions, hypoxic ischemic encephalopathy, hypothermia treatment or composite neonatal morbidity. When the results of two studies were combined [20,22], only an increased risk of neonatal intubation in women with a second stage of labor > 4 h was observed (OR, 2.19; 95% CI 1.23–3.90) (Table 3).
3.4.2. Multiparous Women
5 min Apgar Score < 7
To determine the relation between prolonged second stage of labor in multiparous women (Table A4) and risk of low 5 min Apgar score (< 7), three studies were included (n = 72,857) [4,19,23]. A significant increase in low 5 min Apgar score was observed when the second stage of labor lasted more than 3 h with respect to when the second stage of labor was ≤ 3 h (OR, 3.67; 95% CI 2.49–5.43). For this analysis, a fixed-effects model was used since no heterogeneity was observed (Cochran’s Q p-value = 0.987; I2 = 0.0) (Figure 2C; Table 3).
Admission to the Neonatal Intensive Care Unit
To assess the risk of admission to the Neonatal Intensive Care Unit, three studies were employed (n = 76,692) [4,19,29]. The risk significantly increased when the second stage of labor lasted more than 3 h with respect to when the second stage of labor was ≤ 3 h (OR, 2.41; 95% CI 2.02–2.88). For this analysis, a fixed-effects model was used since no heterogeneity was observed (Cochran’s Q p-value = 0.417; I2 = 0.0) (Figure 2D; Table 3).
Neonatal Sepsis
None of the studies that analyzed multiparous women considered this variable when assessing neonatal morbidity in relation to the duration of the second stage of childbirth (Table 3).
Neonatal Death
None of the studies that analyzed multiparous women considered this variable when assessing neonatal morbidity in relation to the duration of the second stage of childbirth (Table 3).
Other Neonatal Outcomes
No significant associations were reported between prolonged second stage in multiparous women and umbilical artery pH < 7.0, umbilical artery pH < 7.10, umbilical artery base excess ≥12, meconium aspiration, shoulder dystocia, prolonged neonatal stay, advanced neonatal resuscitation, birth depression, minor or major trauma or any perinatal morbidity. After combining two studies [19,29], only an increase in the risk of meconium staining was observed (OR, 1.29; 95%CI, 1.01–1.66), and an increase in composite neonatal morbidity (OR,1.97; 95% CI, 1.39–2.80) was observed after another two studies were combined [19,23] (Table 3).
3.4.3. Publication Bias
We did not observe publication bias for the study in any of the variables studied (Table A3 and Table A4).
We can observe a summary of results obtained following meta-analysis of all variables studied in nulliparous and multiparous women in Table 3.
4. Discussion
4.1. Main Findings
Our meta-analysis results suggested that duration of second stage of labor of more than 4 h in nulliparous women increased the risk of low 5 min Apgar score < 7, admission to the Neonatal Intensive Care Unit, neonatal sepsis and neonatal intubation. In multiparous women, when the second stage of labor was longer than 3 h, the risk of 5 min Apgar score < 7, admission to Neonatal Intensive Care Unit, meconium staining and composite neonatal morbidity increased.
However, a prolonged second stage of labor did not increase the risk of any of the other variables studied, such as umbilical artery pH < 7, birth depression, neonatal death meconium aspiration or shoulder dystocia.
4.2. Comparison with Existing Literature
The literature has very limited data on neonatal outcomes of women with duration of second stage of labor of more than 4 h in nulliparas and of more than 3 h in multiparas. We were only able to locate 12 articles with these durations for this review.
An example of this is a recent systematic review by Gimovksy et al., which evaluated the maternal and fetal morbidities associated with prolonged second stage of labor in nulliparous women with epidurals, in which the authors defined prolonged second stage as greater than three hours [33]. Only two papers were included in this systematic review, and very discordant neonatal outcomes were analyzed, which did not allow the results to be combined in order to establish conclusions that would be useful for decision-making in clinical practice.
Another systematic review studied the influence of prolonged second stage of labor on the risk of adverse maternal and neonatal outcomes from 1980 until 2005 [34]. It did not report associations between prolonged second stage and adverse neonatal outcomes, but most of the studies analyzed in this review defined the prolongation of the second stage as more than 2 h, without differentiating according to parity. In addition, it did not conform to the new recommendations of allowing longer durations.
Only one randomized controlled trial [35] specifically addressed the effect of this change in obstetric practice on maternal and neonatal outcomes. In that trial, a policy of extending the second stage of labor for at least 1 h in nulliparous women with epidural anesthesia with respect to “usual labor” (3 h) decreased the incidence of caesarean birth by more than half compared with the common practice (19.5%, 8 of 41, vs. 43.2%, 16 of 37; RR, 0.45; 95% CI, 0.22–0.93). Maternal or neonatal morbidity were not statistically different between the groups. Unfortunately, the trial was underpowered to detect significant differences in the frequency of adverse maternal or neonatal outcomes between groups because the sample studied was very small (only 78 nulliparous women) (35).
However, Zipori et al. [36] recently published another study comparing maternal and neonatal outcomes over two distinct time periods. In period I, the duration of the second stage of labor was considered prolonged according to ACOG limits, and it was called a “classic labor curve” (10). The “new labor curve” of period II allowed nulliparous and multiparous women to continue the second stage of labor for an additional 1 h before diagnosing second-stage arrest. Primary caesarean deliveries decreased with the new policy of labor management, with a small rise in instrumental deliveries, but it also increased other immediate maternal and neonatal complications, such as higher rate of lower umbilical artery cord pH.
4.3. Strengths and Limitations
One of the strengths of this study is that it is the first systematic review to define prolonged second stage of labor according the most recent recommendations (11), that is, 4 h for nulliparous women and 3 h for multiparous women. Most of the studies had large sample sizes with sufficient numbers of participants in each group to lend power to the findings, and the majority of them used methods to control for potential confounding factors.
Among the limitations of our systematic review is that neonatal outcome measures were discordant in the included studies, meaning it was difficult to combine data to summarize important clinical findings, and that the definition of two variables (admission to NICU and composite neonatal morbidity) differed among included studies. None of the studies considered the pushing duration or pushing techniques employed (delayed pushing or immediate pushing). Finally, since they were observational studies, there is a risk of confounding bias even though many of the studies included techniques to control confounding.
5. Conclusions
In nulliparous women, a prolonged second stage of labor is not related with an increased risk of neonatal death. However, it is related with an increased risk of 5 min Apgar score < 7, admission to the Neonatal Intensive Care Unit, neonatal sepsis or intubation. In multiparous women, a prolonged second stage of labor is related with an increased risk of 5 min Apgar score < 7, admission to the Neonatal Intensive Care Unit, meconium staining and composite neonatal morbidity.
These potential risks associated with a prolonged second stage of labor in both nulliparous and multiparous women should serve as an incentive for professionals involved in childbirth care to increase supervision of mothers who exceed these durations.
More studies are needed, especially clinical studies, to guarantee the safety of newborns when the second stage of labor exceeds 4 h in nulliparous women and 3 h in multiparous women.
Appendix A
Table A1.
Search strategies.
| Search Strategies | ||
|---|---|---|
| Search Strategy | Database | Hits |
| “Labor Stage, Second” (Mesh) AND (length OR duration OR prolonged OR abnormal OR excessive) | PubMed | 501 |
| Scopus | 590 | |
| Embase | 777 | |
| Search Strategy “PICO” | ||
| Population | Nulliparous and Multiparous women | |
| Intervention | Second stage labor > 4 h in nulliparous Second stage labor > 3 h in multiparous |
|
| Comparison | Second stage labor ≤ 4 h in nulliparous Second stage labor ≤ 3 h in multiparous |
|
| Outcome | Neonatal morbidity: 5 min Apgar score < 7, admission to the Neonatal Intensive Care Unit, neonatal sepsis and neonatal death. | |
Table A2.
Checklist for Cohort Studies.
| . | 1995; Menticoglou |
2007; Cheng |
2009; Allen |
2009; Rouse |
2011; Li |
2012; Bleich |
2015; Altman |
2015; Hunt |
2017; Sandström |
2017; Ogunyemi | 2018; Souter |
2019; Infante |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Were the two groups similar and recruited from the same population? | Unclear * | No * | No * | Unclear * | Unclear * | Unclear * | Unclear * | Unclear * | Unclear * | Unclear * | Unclear * | Unclear * |
| 2. Were the exposures measured similarly to assign people to both exposed and unexposed groups? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3. Was the exposure measured in a valid and reliable way? | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 4. Were confounding factors identified? | No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes |
| 5. Were strategies to deal with confounding factors stated? | No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes |
| 6. Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 7. Were the outcomes measured in a valid and reliable way? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 8. Was the follow up time reported and sufficient to be long enough for outcomes to occur? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Was follow up complete, and if not, were the reasons to loss to follow up described and explored? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 10. Were strategies to address incomplete follow up utilized? | NA | NA | Yes | NA | NA | NA | Yes | NA | Yes | NA | NA | Yes |
| 11. Was appropriate statistical analysis used? | Unclear | Yes | Yes | Yes | Unclear | Yes | Yes | Unclear | Yes | Yes | Unclear | Yes |
NA: not applicable, *: groups recruited from the same population.
Table A3.
Neonatal morbidity outcomes in nulliparous women (≤4 h versus >4 h second-stage labor).
| 1 min Apgar Score <7 | 5 min Apgar Score <7 | 5 min Apgar Score <4 | 5 min Apgar Score ≤3 | Umbilical Artery pH <7 |
Acidosis | Birth Depression | Resuscitation at Delivery | Intubation | Heart Compressions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | ≤4 h | >4 h | ≤4 h | >4 h | ≤4 h | >4 h | ≤4 h | >4 h | ≤4 h | >4 h | ≤4 h | >4 h | ≤4 h | >4 h | ≤4 h | >4 h | ≤4 h | >4 h | ≤4 h | >4 h | |
| 1995; Menticoglou | NR | NR | 81/5733 | 7/308 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| 2009; Allen | NR | NR | 589/51,028 | 75/4908 | NR | NR | NR | NR | NR | NR | NR | NR | 862/51,028 | 138/4908 | NR | NR | NR | NR | NR | NR | |
| 2009; Rouse | NR | NR | NR | NR | 3/3983 | 0/143 | NR | NR | 15/3983 | 1/143 | NR | NR | NR | NR | NR | NR | 19/3983 | 1/143 | NR | NR | |
| 2011; Li | 17/295 | 8/12 | 3/295 | 1/12 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| 2012; Bleich | NR | NR | NR | NR | NR | NR | 17/21,564 | 2/427 | 83/21,564 | 4/427 | NR | NR | NR | NR | 138/21,564 | 13/427 | NR | NR | NR | NR | |
| 2015; Altman | NR | NR | 188/29,976 | 39/2820 | 32/29,976 | 8/2820 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| 2015; Hunt | NR | NR | 33/629 | 44/886 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| 2017; Sandström | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 330/30,453 | 31/2976 | NR | NR | NR | NR | 58/38,731 | 13/3808 | 36/38,731 | 10/3808 | |
| 2017; Ogunyemi | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| 2018; Souter | NR | NR | 200/16,682 | 84/3347 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 851/16,682 | 248/3347 | NR | NR | NR | NR | |
| Egger Bias (p-value) |
0.7861 | NC | 0.8132 | NC | NC | ||||||||||||||||
| I2 95% CI | 71.0 (2.1–85.6) | NC | NC | NC | NC | ||||||||||||||||
| Q Cochran (p-value) |
0.0041 | 0.7026 | 0.8132 | <0.001 | 0.681 | ||||||||||||||||
| OR 95% CI | 1.65 (1,20–2.27) * | 2.27 (1.08–4.74) * | 2.30 (0.94–5.69) | 2.60 (0.81–8.63) * | 2.19 (1.23–3.90) | ||||||||||||||||
| Meconium Aspiration | Meconium-Stained Amniotic Fluid | Admission to NICU | Neonatal Seizures | Sepsis | Birth Trauma | Minor Trauma | Major Trauma | Shoulder Dystocia | |||||||||||||
| Author | ≤4 h | >4 h | ≤4 h | >4 h | ≤4 h | >4 h | ≤4 h | >4 h | ≤4 h | >4 h | ≤4 h | >4 h | ≤4 h | >4 h | ≤4 h | >4 h | ≤4 h | >4 h | |||
| 1995; Menticoglou | NR | NR | NR | NR | 64/5733 | 2/308 | 5/5733 | 0/308 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |||
| 2009; Allen | NR | NR | NR | NR | 3071/51,028 | 505/4908 | NR | NR | 195/51,028 | 30/4908 | NR | NR | 1311/51,028 | 162/4908 | 78/51,028 | 16/4908 | NR | NR | |||
| 2009; Rouse | NR | NR | NR | NR | 167/3983 | 14/143 | NR | NR | 6/3983 | 0/143 | NR | NR | NR | NR | NR | NR | NR | NR | |||
| 2011; Li | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |||
| 2012; Bleich | NR | NR | NR | NR | 172/21,564 | 8/427 | 41/21,564 | 13/427 | 39/21,564 | 0/427 | NR | NR | NR | NR | NR | NR | NR | NR | |||
| 2015; Altman | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |||
| 2015; Hunt | NR | NR | NR | NR | 12/629 | 24/886 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |||
| 2017; Sandström | 58/38,731 | 10/3808 | NR | NR | 2373/38,731 | 360/3808 | 78/38,731 | 17/3808 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |||
| 2017; Ogunyemi | NR | NR | 309/4215 | 37/272 | 372/4201 | 37/272 | NR | NR | NR | NR | 33/3814 | 1/250 | NR | NR | NR | NR | NR | NR | |||
| 2018; Souter | NR | NR | NR | NR | 817/16,682 | 221/3347 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 367/16,682 | 74/3347 | |||
| Egger Bias (p-value) |
0.8326 | NC | NC | ||||||||||||||||||
| I2 95% CI | 48.8 (0.0–75,4) | 92.3 (78.6–95,8) | 0.0 (0–72.9) | ||||||||||||||||||
| Q Cochran (p-value) |
0.0573 | <0.001 | 0.7962 | ||||||||||||||||||
| OR 95% CI | 1.63 (1.53–1.74) | 4.67 (0,78–27.78) * | 1.57 (1.07–2.29) | ||||||||||||||||||
| Brachial Plexus Injury | Erb’s Palsy | Hypoxic Ischemic Encephalopathy | Hypothermia Treatment | Composite Neonatal Morbidity | Neonatal Death | ||||||||||||||||
| Author | ≤4 h | >4 h | ≤4 h | >4 h | ≤4 h | >4 h | ≤4 h | >4 h | ≤4 h | >4 h | ≤4 h | >4 h | |||||||||
| 1995; Menticoglou | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 0/5733 | 0/308 | |||||||||
| 2009; Allen | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |||||||||
| 2009; Rouse | 10/3983 | 1/143 | NR | NR | NR | NR | NR | NR | 98/3983 | 6/143 | NR | NR | |||||||||
| 2011; Li | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |||||||||
| 2012; Bleich | NR | NR | 82/21,564 | 2/427 | NR | NR | NR | NR | NR | NR | 3/21,564 | 0/427 | |||||||||
| 2015; Altman | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |||||||||
| 2015; Hunt | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |||||||||
| 2017; Sandström | NR | NR | NR | NR | 75/38,731 | 22/3808 | 16/38,731 | 7/3808 | NR | NR | NR | NR | |||||||||
| 2017; Ogunyemi | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |||||||||
| 2018; Souter | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |||||||||
| Egger Bias (p-value) |
NC | ||||||||||||||||||||
| I2 95% CI | NC | ||||||||||||||||||||
| Q Cochran (p-value) |
NC | ||||||||||||||||||||
| OR 95% CI | 7.21 (0.37–139.71) * | ||||||||||||||||||||
NR: not reported; NR: not calculated; CI: confidence interval; * Random effects (DerSimonian–Laird); Bold: Significant results are highlighted.
Table A4.
Neonatal Morbidity Outcomes in Multiparous Women (≤3 h versus >3 h second stage of labour).
| 5 min Apgar Score <7 | Umbilical Artery pH <7.0 | Umbilical Artery pH <7.10 | Umbilical Artery Base Excess>−12 | Birth Depression | Advanced Neonatal Resuscitation |
Meconium Amniotic Fluid or Meconium Staining | NICU Admission | Prolonged Neonatal Stay | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | ≤3 h | >3 h | ≤3 h | >3 h | ≤3 h | >3 h | ≤3 h | >3 h | >3 h | ≤3 h | ≤3 h | >3 h | ≤3 h | >3 h | ≤3 h | >3 h | ≤3 h | >3 h |
| 2007; Cheng | 60/4901 | 11/257 | 17/4901 | 1/257 | NR | NR | 31/4901 | 3/257 | NR | NR | NR | NR | 1061/4901 | 73/257 | 145/4901 | 14/257 | 445/4901 | 34/257 |
| 2009; Allen | 311/64,586 | 17/968 | NR | NR | NR | NR | NR | NR | 553/64,586 | 27/968 | NR | NR | NR | NR | 2409/64,586 | 90/968 | NR | NR |
| 2017; Ogunyemi | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 290/5759 | 12/276 | 410/5700 | 40/278 | NR | NR |
| 2019; Infante | 3/2081 | 0/64 | NR | NR | 37/1858 | 3/54 | NR | NR | NR | NR | 39/2081 | 3/64 | NR | NR | NR | NR | NR | NR |
| Egger Bias (p-value) |
NC | NC | NC | |||||||||||||||
| I2 95% CI | 0% (0.0%–72.9%) | NC | 0% (0.0%–72.9%) | |||||||||||||||
| Q Cochran (p-value) |
0.987 | 0.121 | 0.417 | |||||||||||||||
| OR 95% CI | 3.67 (2.49–5.43) | 1.29 (1.01–1.66) | 2.41 (2.02–2.88) | |||||||||||||||
| Minor Trauma | Major Trauma | Shoulder Dystocia | Composite Neonatal Morbidity | Any Perinatal Morbidity | ||||||||||||||
| Author | >3 h | >3 h | ≤3 h | >3 h | ≤3 h | >3 h | ≤3 h | >3 h | ≤3 h | >3 h | ||||||||
| 2007; Cheng | NR | NR | NR | NR | 117/4901 | 10/257 | 361/4901 | 33/257 | NR | NR | ||||||||
| 2009; Allen | 586/64,586 | 27/968 | 104/64,586 | 2/968 | NR | NR | NR | NR | 3662/64,586 | 128/968 | ||||||||
| 2017; Ogunyemi | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ||||||||
| 2019; Infante | NR | NR | NR | NR | NR | NR | 70/2081 | 6/64 | NR | NR | ||||||||
| Egger Bias (p-value) |
NC | |||||||||||||||||
| I2 95% CI | NC | |||||||||||||||||
| Q Cochran (p-value) |
0.330 | |||||||||||||||||
| OR 95% CI | 1.97 (1.39–2.80) | |||||||||||||||||
NR: not reported; NR: not calculated; CI: confidence interval; Random effects (DerSimonian–Laird); Bold: Significant results are highlighted.
Author Contributions
Conceptualization, N.I.-T. and J.R.-A.; Methodology, M.M.-A. and A.A.-A.; Formal Analysis, A.H.-M. and J.R.-A.; Writing—Original Draft Preparation, N.I.-T. and M.M.-A.; Writing—Review & Editing, N.I.-T. and A.A.-A.; Supervision, J.R.-A. and A.H.-M.; Project Administration, A.H.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Recommendations: Intrapartum Care for a Positive Childbirth Experience. [(accessed on 2 October 2020)]; Available online: https://www.who.int/reproductivehealth/publications/intrapartum-care-guidelines/en/
- 2.Myles T.D., Santolaya J. Maternal and neonatal outcomes in patients with a prolonged second stage of labor. [(accessed on 30 January 2020)];Obstet Gynecol. 2003, 102. doi: 10.1016/s0029-7844(03)00400-9. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12850607. [DOI] [PubMed]
- 3.Cheng Y.W., Hopkins L.M., Caughey A.B. How long is too long: Does a prolonged second stage of labor in nulliparous women affect maternal and neonatal outcomes? Am. J. Obstet. Gynecol. 2004;191:933–938. doi: 10.1016/j.ajog.2004.05.044. [DOI] [PubMed] [Google Scholar]
- 4.Allen V.M., Baskett T.F., O’Connell C.M., McKeen D., Allen A.C. Maternal and Perinatal Outcomes With Increasing Duration of the Second Stage of Labor. Obstet. Gynecol. 2009;113:1248–1258. doi: 10.1097/AOG.0b013e3181a722d6. [DOI] [PubMed] [Google Scholar]
- 5.Singh S., Kohli U.A., Vardhan S. Management of prolonged second stage of labor. Int. J. Reprod. Contracept. Obstet. Gynecol. 2018;7:2527–2531. doi: 10.18203/2320-1770.ijrcog20182855. [DOI] [Google Scholar]
- 6.Le Ray C., Audibert F., Goffinet F., Fraser W. When to stop pushing: Effects of duration of second-stage expulsion efforts on maternal and neonatal outcomes in nulliparous women with epidural analgesia. Am. J. Obstet. Gynecol. 2009;201:361.e1-361.e7. doi: 10.1016/j.ajog.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Simpson K.R., James D.C. Effects of Immediate Versus Delayed Pushing During Second-Stage Labor on Fetal Well-Being. Nurs. Res. 2005;54:149–157. doi: 10.1097/00006199-200505000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Thavarajah H., Flatley C., Kumar S. The relationship between the five minute Apgar score, mode of birth and neonatal outcomes. J. Matern. Neonatal Med. 2017;31:1335–1341. doi: 10.1080/14767058.2017.1315666. [DOI] [PubMed] [Google Scholar]
- 9.Moiety F., Azzam A.Z. Fundal pressure during the second stage of labor in a tertiary obstetric center: A prospective analysis. J. Obstet. Gynaecol. Res. 2014;40:946–953. doi: 10.1111/jog.12284. [DOI] [PubMed] [Google Scholar]
- 10.Dystocia and the augmentation of labor. Int. J. Gynecol. Obstet. 1996;53:73–80. doi: 10.1016/S0020-7292(96)80016-6. [DOI] [PubMed] [Google Scholar]
- 11.American College of Obstetricians and Gynecologists Obstetric Care Consensus No. 1. Obstet. Gynecol. 2014;123:693–711. doi: 10.1097/01.aog.0000444441.04111.1d. [DOI] [PubMed] [Google Scholar]
- 12.NICE Intrapartum Care for Healthy Women and Babies—NICE Guidelines. [(accessed on 2 October 2020)]; Available online: https://www.nice.org.uk/guidance/cg190/resources/intrapartum-care-for-healthy-women-and-babies-pdf-35109866447557.
- 13.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., A Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernández-Martínez A., Arias-Arias A., Morandeira-Rivas A., Pascual-Pedreño A.I., Ortiz-Molina E.J., Rodríguez-Almagro J. Oxytocin discontinuation after the active phase of induced labor: A systematic review. Women Birth. 2019;32:112–118. doi: 10.1016/j.wombi.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 15.PubMed. [(accessed on 2 October 2020)]; Available online: https://pubmed.ncbi.nlm.nih.gov/
- 16.Scopus. Elsevier. [(accessed on 2 October 2020)]; Available online: https://www.elsevier.com/es-es/solutions/scopus.
- 17.Investigación Biomédica—Embase. [(accessed on 2 October 2020)]; Available online: https://www.elsevier.com/es-es/solutions/embase-biomedical-research.
- 18.Menticoglou S.M., Manning F., Harman C., Morrison I. Perinatal outcome in relation to second-stage duration. Am. J. Obstet. Gynecol. 1995;173:906–912. doi: 10.1016/0002-9378(95)90364-X. [DOI] [PubMed] [Google Scholar]
- 19.Cheng Y.W., Hopkins L.M., Laros R.K., Caughey A.B. Duration of the second stage of labor in multiparous women: Maternal and neonatal outcomes. Am. J. Obstet. Gynecol. 2007;196:585.e1-585.e6. doi: 10.1016/j.ajog.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Rouse D.J., Weiner S.J., Bloom S.L., Varner M.W., Spong C.Y., Ramin S.M., Caritis S.N., Peaceman A.M., Sorokin Y., Sciscione A., et al. Second-stage labor duration in nulliparous women: Relationship to maternal and perinatal outcomes. Am. J. Obstet. Gynecol. 2009;201:357.e1-357.e7. doi: 10.1016/j.ajog.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bleich A.T., Alexander J.M., McIntire D.D., Leveno K.J. An Analysis of Second-Stage Labor beyond 3 Hours in Nulliparous Women. Am. J. Perinatol. 2012;29:717–722. doi: 10.1055/s-0032-1314894. [DOI] [PubMed] [Google Scholar]
- 22.Sandström A., Altman M., Cnattingius S., Johansson S., Ahlberg M., Stephansson O. Durations of second stage of labor and pushing, and adverse neonatal outcomes: A population-based cohort study. J. Perinatol. 2016;37:236–242. doi: 10.1038/jp.2016.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Infante-Torres N., Alarcón M.M., Gómez-Salgado J., Almagro J.J.R., Rubio-Álvarez A., Martínez A.H. Relationship between the Duration of the Second Stage of Labour and Neonatal Morbidity. J. Clin. Med. 2019;8:376. doi: 10.3390/jcm8030376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Joanna Briggs Institute Critical Appraisal for Use in JBI Systematic Reviews. Checklist for Cohort Studies. [(accessed on 2 October 2020)]; Available online: https://joannabriggs.org/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Cohort_Studies2017_0.pdf.
- 25.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 26.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses [Internet] [(accessed on 2 October 2020)];Br. Med. J. 2003 327:557–560. doi: 10.1136/bmj.327.7414.557. Available online: https://pubmed.ncbi.nlm.nih.gov/12958120/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egger M., Smith G.D. meta-analysis bias in location and selection of studies. BMJ. 1998;316:61–66. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt J.C., Menticoglou S.M. Perinatal Outcome in 1515 Cases of Prolonged Second Stage of Labour in Nulliparous Women. J. Obstet. Gynaecol. Can. 2015;37:508–516. doi: 10.1016/S1701-2163(15)30227-9. [DOI] [PubMed] [Google Scholar]
- 29.Ogunyemi D., Friedman P., Jovanovski A., Shah I., Hage N., Whitten A. 890: Maternal and neonatal outcomes by parity and second-stage labor duration. Am. J. Obstet. Gynecol. 2017;216:S508–S509. doi: 10.1016/j.ajog.2016.11.799. [DOI] [Google Scholar]
- 30.Souter V., Chien A.J., Kauffman E., Sitcov K., Caughey A.B. 365: Outcomes associated with prolonged second stage of labor in nulliparas. Am. J. Obstet. Gynecol. 2018;218:S226. doi: 10.1016/j.ajog.2017.10.301. [DOI] [Google Scholar]
- 31.Li W.-H., Zhang H.-Y., Ling Y., Jin S. Effect of prolonged second stage of labor on maternal and neonatal outcomes. Asian Pac. J. Trop. Med. 2011;4:409–411. doi: 10.1016/S1995-7645(11)60114-4. [DOI] [PubMed] [Google Scholar]
- 32.Altman M., Sandström A., Petersson G., Frisell T., Cnattingius S., Stephansson O. Prolonged second stage of labor is associated with low Apgar score. Eur. J. Epidemiol. 2015;30:1209–1215. doi: 10.1007/s10654-015-0043-4. [DOI] [PubMed] [Google Scholar]
- 33.Gimovsky A.C., Guarente J., Berghella V. Prolonged Second Stage in Nulliparous with Epidurals. Obstet. Gynecol. Surv. 2017;72:399–401. doi: 10.1097/01.ogx.0000520557.08379.94. [DOI] [PubMed] [Google Scholar]
- 34.Altman M.R., Lydon-Rochelle M.T. Prolonged Second Stage of Labor and Risk of Adverse Maternal and Perinatal Outcomes: A Systematic Review. Birth. 2006;33:315–322. doi: 10.1111/j.1523-536X.2006.00129.x. [DOI] [PubMed] [Google Scholar]
- 35.Gimovsky A.C., Berghella V. Randomized Controlled Trial of Prolonged Second Stage. Obstet. Gynecol. Surv. 2016;71:385–387. doi: 10.1097/OGX.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 36.Zipori Y., Grunwald O., Ginsberg Y., Beloosesky R., Weiner Z. The impact of extending the second stage of labor to prevent primary cesarean delivery on maternal and neonatal outcomes. Am. J. Obstet. Gynecol. 2019;220:191.e1-191.e7. doi: 10.1016/j.ajog.2018.10.028. [DOI] [PubMed] [Google Scholar]