Abstract
The clinical spectrum of the perinatal COVID-19 and prospective data on neonatal outcomes remains largely unexplored. Most of the existing literature is in the form of case series or single-centre experience. In this review, we aim to summarize available literature on the clinical spectrum of COVID-19 in neonates and mothers and suggest a practical approach towards management of clinical scenarios. This review explores the clinical characteristics and outcomes of COVID-19 in neonates born to mothers who were detected with the virus during the pregnancy. We conducted a comprehensive search of PubMed, Google Scholar and Cochrane Database of Systematic Review between November 2019 and June 2020 and screened articles related to perinatal COVID-19. This review included 786 mothers, among which 64% (504) were delivered by caesarian section. There were 3 still births and 107 (14%) were delivered preterm. Out of 793 neonates born, 629 neonates (79%) were tested after birth. The commonest symptom in neonates was respiratory distress. Respiratory support was needed in 60 neonates (7.6%), with 14 babies needing mechanical ventilation (1.8%), 25 needing non-invasive ventilation and 21 needing nasal oxygen. Only 35 of the 629 tested neonates (5.5%) were positive for COVID-19. Of the 35 positive neonates, 14 (40%) were symptomatic. The COVID-19 seems to have favourable neonatal outcomes. Majority of neonates are asymptomatic. Respiratory distress is the most common manifestation.
|
What is known: •COVID-19 affects all ages. •Neonatal disease is usually mild. | |
|
What is new: •Vertical transmission is a possible route of infection in neonates. •Breast milk and skin-to-skin contact are safe in COVID-19-infected mothers if performed with appropriate use of precautions such as hand and breast hygiene and masking. |
Supplementary Information
The online version contains supplementary material available at 10.1007/s00431-020-03866-3.
Keywords: COVID-19, Perinatal COVID, SARS-COV-2, Neonatal Sepsis
Introduction
Coronaviruses are positive sense single strand RNA viruses of zoonotic origin surrounded by an envelope. The Coronaviridae family has Orthocoronavirinae subfamily which is further divided into four genera, namely, Alpha, Beta, Gamma, and Delta coronavirus [1, 2]. So far, seven human coronaviruses (HCoVs) have been identified, all of which fall within the Alpha and Beta coronavirus genera. The severe acute respiratory distress syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome-related coronavirus (MERS CoV) and novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) belong to the genus Beta coronavirus [2–5].
In early December 2019, an outbreak of respiratory illness of unknown origin was reported in Wuhan, Hubei Province, China, which was later isolated and reported as SARS-CoV-2 virus [6, 7]. Since the beginning of this epidemic, SARS-CoV-2 has now spread to all continents and was declared as pandemic by WHO on March 11, 2020 (https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10) [8–11]. SARS-CoV-2 infection has also been reported in pregnant women and neonates, but the data is scattered and limited [12].
Methodology
We conducted a comprehensive review of literature describing perinatal SARS-CoV-2 infection which included articles published between November 2019 and June 2020. To identify relevant articles, we systematically searched PubMed, Google Scholar and Cochrane Database of Systematic Review. For searching PubMed, we combined the population terms (“Infant”[Mesh] OR “premature birth”[MeSH Terms] OR newborn OR neonat* OR perinat*) and the outcome terms (“COVID-19” [Supplementary Concept] OR “SARS-CoV” OR “coronavirus” OR “COVID-19” OR “SARS-CoV-2” OR “novel coronavirus” OR “neonatal covid” OR “2019-nCOV”). The initial search yielded 873 articles, of which 824 were in English. We found 6 meta-analyses, of which 3 were related to perinatal COVID-19. No randomized control trials were found. After excluding duplicates, the rest of the articles comprising of observational studies (single and multi-centre) and case reports were screened by 2 researchers independently. Similarly, the Cochrane Database of Systematic Review and Google Scholar were searched. All relevant prospective/retrospective studies (including case reports) and review articles were included. Further, a rapid review was conducted to include more recent articles up to August 2020. Guidelines and recommendations of relevant national and international organizations were also reviewed. In this article, we discuss important aspects of perinatal–neonatal COVID-19 infection, systematically summarize current evidence (updated to June 2020) and propose a practical approach towards its management and prevention in neonates.
Transmission of COVID-19
The SARS-CoV-2 enters the host cell by attaching to the SARS-coronavirus receptor, angiotensin-converting enzyme 2 (ACE-2). The entry is facilitated by the spike (S) protein. ACE-2 is a surface molecule expressed in alveolar type 2 cells of lung, oesophageal upper epithelial cells and absorptive enterocytes from ileum and colon [13]. The novel SARS-CoV-2 transmits between humans through the respiratory droplets and aerosols from coughing and sneezing usually up to 6 ft. It can also be transmitted through direct or indirect contact with mucous membranes of the eyes, mouth or nose [14]. Possibility of infection through gastrointestinal tract has also been reported in a study by invasion of ACE-2 expressing enterocytes [15]. According to the initial available literature, the risk of vertical transmission is 5–30% but may be underreported due to lack of early diagnosis. ACE2 receptors and TMPRSS2 molecules are highly expressed in placenta and the expression peaks at term and the virus often invade the placenta and rarely cause miscarriage [16–19]. From two different case series, 4 infants had positive nasopharyngeal SARS-CoV-19 RT-PCR within 2 days of life, but cord blood, amniotic fluid and cord blood samples were negative in these infants [20, 21]. Serological testing with SARS-CoV-2-specific IgM and IgG showed positive IgM in serum of 3 out of 7 newborns in two different studies with negative nasopharyngeal swab and serum RT-PCR in the same infants [22, 23]. Serum IgM can give both false positive and false negative results depending on the technique used. It is very difficult to differentiate early postnatal transmission/colonization versus vertical transmission in neonates. There are cases of neonatal detection for SARS-CoV-2 within the first 24 h of life. An infant born to a 41-year-old pregnant woman with severe COVID-19 infection requiring mechanical ventilation tested positive at 16 h age. The patient underwent a caesarean delivery, and the neonate was isolated immediately without delayed cord clamping or skin-to-skin contact. The serology tests IgM and IgG for SARS-CoV-2 were negative [24]. Another recent case by Kirstman et al. reports a term male infant born to a 40-year-old COVID-19-positive mother by caesarian section. The infant did not require resuscitation at birth but was admitted to NICU at 37 h for temperature instability, hypoglycaemia and feeding difficulty. The infant had positive RT-PCR from nasopharyngeal swab on day of birth, day 2 and day 7. Also, this is the first report where samples of placenta, maternal vaginal swab and breast milk were positive for the virus [25]. Another study tested multiple breast milk samples in two COVID-19-positive mothers. Authors reported positive RT-PCR in one mother on days 10, 12 and 13. Further breast milk samples repeated later were negative. [26] In a case report of preterm infant born to mother with COVID-19, infant had fever after birth and was treated with intravenous antibiotics. Maternal vaginal secretions, umbilical cord blood, neonatal nasal and throat swabs at birth were negative for SARS-CoV-2. But amniotic fluid sample was positive. The second nasal swab test at 24 h was positive for SARS-CoV-2. Third and fourth neonate’s PCR test was positive 1 week later. In this case, the neonate was separated from mother at birth and was fed with formula [27]. These findings raise the uncertainty regarding transmission of SARS-CoV-2 by placenta and human milk and highlight the importance of measures to prevent horizontal transmission. Previous case series have not detected COVID-19 virus in breast milk or placenta [28–31]. Recently there have been few case reports suggestive of secretion of coronavirus in breast milk [32]. However, in a case series of 18 mothers, data suggests that the presence of SARS-CoV-2 RNA in breast milk does not represent replication-competent virus and that breast milk may be safe for the infant. Furthermore, pasteurization by Holders technique has shown to completely destroy the viral RNA present in the breast milk [33]. Neonates may also acquire infection postnatally from close contact with other COVID-19-positive people such as healthcare workers and family members.
Clinical presentation
The mean incubation period of COVID-19 is 5.2 days (95% CI: 4.1–7 days) with over 90% infected individuals developing symptoms within the initial 10 days. Based on these findings, 14-day monitoring period is advised after contact with a COVID-19-confirmed case. The reproduction number (R0), defined as average number of secondary infections caused by one infected person in a naïve population, ranges from 1.4 to 6.49 with the mean of 3.28, which is higher compared to H1N1 influenza (R0 of 1.2–1.6) and SARS-CoV (R0 of 2–5) [34–36].
According to the published literature, the clinical characteristics of pregnant women with COVID-19 are similar to non-pregnant COVID-19 adult patients [36–44]. In our review that included 786 mothers, we found that 64% (504) delivered by caesarian section. There were 3 still births, and 107 (14%) were delivered preterm. The gestation was not specified in 33% of cases, and the rest were term neonates. A recently conducted systematic review and meta-analysis on outcomes of coronavirus spectrum infections in pregnancy included 19 studies with 79 women affected with SARS, MERS and SARS-CoV-2 infections. The authors reported miscarriage in 39.1%, preterm birth (< 37 weeks) in 24.3%, premature pre-labour rupture of membranes (pPROM) in 20.7%, preeclampsia in 16% and foetal growth restriction in 11.7% of COVID-19 mothers [29]. On subgroup analysis consisting of 41 pregnancies with COVID-19 (SARS-CoV-2 infections), the most common adverse pregnancy outcome was preterm birth. It was reported in 56% of patients, of which 15% delivered before 34 weeks. The perinatal mortality was 7%, including one still birth and one neonatal death [29]. Furthermore, pPROM was observed in 18.8% of patients [29]. However, it is important to emphasize these data reflect small numbers in early COVID-19 era, and the perinatal issues may not be directly related to maternal or neonatal infection with coronavirus.
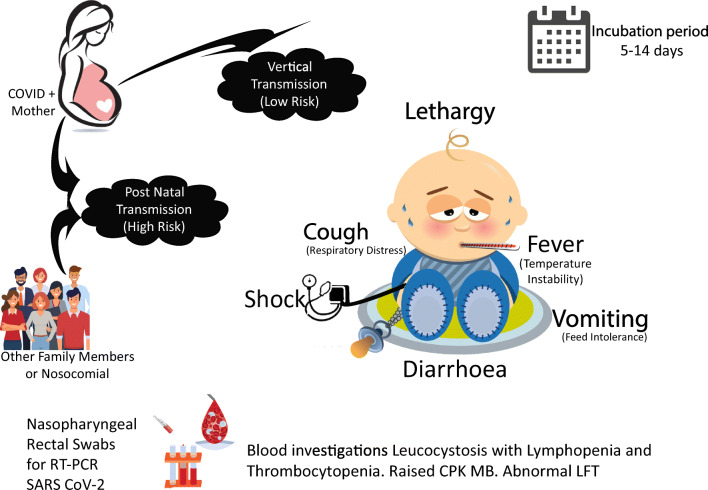
The transmission and symptom profile of COVID-19 in neonates are described in Fig. 1. As per our compiled data, out of 793 neonates born, 629 neonates (79%) were tested after birth. The commonest symptom in this group was respiratory distress. Respiratory support was needed in 60 neonates, with 14 babies needing mechanical ventilation, 25 needing non-invasive ventilation and 21 needing nasal oxygen. The respiratory symptoms are more likely related to prematurity, transient tachypnoea, and respiratory distress syndrome rather than COVID-19 pneumonia. Only 35 of the 629 tested neonates (5.5%) were positive for COVID-19. However, it is difficult to rule out false negative cases, as the timing and protocol for testing was variable and the optimal protocol is currently unclear. Of the 35 positive neonates, 14 (40%) were symptomatic. The commonly described symptoms were respiratory (tachypnoea, respiratory distress, cough), gastrointestinal (diarrhoea, vomiting, feed intolerance), lethargy and temperature instability. It is also difficult to assume if these symptoms are attributable to COVID-19 disease as few infants who tested negative also developed symptoms. [30] The potential severity of SARS-CoV-2 late onset sepsis has been discussed in the case report by Munoz et al. which describes manifestation of hypotension, need of mechanical ventilation with high settings and occurrence of pneumothorax [45]. Although the extent of disease severity is difficult to describe given the limited literature, majority of neonates have had mild disease. The available case reports and case series on neonates born to COVID-19 pregnant women are summarized in Table 1.
Fig. 1.
Common neonatal symptoms of COVID-19
Table 1.
Literature search
| Sl. no. | Study name | Place | Number of delivered pregnant women and infants | Maternal outcomes | Neonatal findings |
|---|---|---|---|---|---|
| Infants with positive testing and had clinical features (can be attributable to SARS-CoV-2 infection) | |||||
| 1 | Kamali Aghdam M et al. [62] | Mousavi Hospital, Zanjan, Iran | 1 mother and neonate pair |
Delivered by caesarean section. Mother and father reported symptoms postnatally with fever and cough Parents were mildly symptomatic so not tested as per Iran’s protocol |
Term AGA neonate, on day 15, had fever, lethargy, tachycardia, tachypnoea, respiratory distress. Chest x-ray was normal Pharyngeal swab RT-PCR – positive; blood, urine, stool tests - negative Recovered in 2 days with supportive care Likely postnatal horizontal transmission |
| 2 | Diaz CA et al. [63] | Madrid, Spain | 1 infant and mother pair |
Delivered by caesarean section due to severe PE Father: fever, gastrointestinal symptoms on the day of delivery, positive for SARS-Cov-2 Mother: low-grade fever on day 3 postdelivery, respiratory symptoms on day 5 with positive nasopharyngeal swab RT-PCR |
Term SGA, had TTN required CPAP support for 2 h Neonate’s nasopharyngeal RT-PCR - negative on day 6 and repeat on day 7 - positive On day 9, neonate developed respiratory distress with pneumonia improved with supportive care. Chest X-ray: ground glass opacities in right perihilar region |
| 3 | Zhang ZJ et al. [64] | Searched data from central Govt of China and local hospitals | 4 neonates with positive NAAT tests | 3 mothers had symptoms before delivery, 1 mother had after the delivery with SARS-Cov-2 positive in all 4 mothers. All 4 mothers underwent caesarean section |
3 neonates were separated from mother, and one neonate was with mother and was on direct breast feeding till the neonate had symptoms on day 16 Time of presentation was 30 h of life, two infants on day 5 and 17 days Two neonates had fever, one neonate had respiratory distress, another neonate had cough, and one neonate was asymptomatic Two infants tested positive with nasopharyngeal swab, other two infants were tested positive with anal swabs 3 neonates CT Chest: increased lung markings |
| 4 | Zeng L et al. [20] | Wuhan children Hospital, China | 33 mother and neonate pairs |
All 33 COVID-19 mothers had pneumonia 26 underwent caesarean delivery 4 had preterm delivery |
Two infants had asphyxia Three infants had positive nasopharyngeal (and rectal) swab PCR on first on day 2 and day 4, negative on day 6 of life Clinical features: lethargy, fever, vomiting, pneumonia on X-ray Lab: Leucocytosis, lymphopenia, elevated CKMB Preterm newborn had critical course due to asphyxia, sepsis |
| 5 | Yu N et al. [21] | Tongji Hospital, Wuhan, China | 7 Mother and neonate pairs |
All 7 women had caesarean delivery None of them admitted to ICU |
All neonates - term 3 tested, one positive Positive neonate had mild respiratory symptoms |
| 6 | Ferrazzi E et al. [65] | Italy | 42 mother and neonate pairs |
In 5 cases, diagnosis was confirmed postnatally 24 mothers: vaginal delivery 10 mothers: caesarean section due to COVID related causes 8 mothers: caesarean section for obstetric reasons |
11 neonates were preterm. Two very preterm neonates had APGAR score < 7 at 5 min. In 10 cases, breast feeding was allowed with mask. Two neonates, in whom mother’s COVID-19 was diagnosed postnatally, breast feeding and skin-to-skin contact were allowed without mask initially. In these neonates, SARS-CoV-2 was positive on day 1 and 3, respectively. Another neonate who was immediately separated, within a few hours, developed gastrointestinal symptoms. After 3 days, same neonate developed respiratory symptoms and required 1 day of mechanical ventilation and recovered. First test for SARS-CoV-2 was doubtful few hours after delivery and positive 3 days later. Vertical transmission cannot be ruled out in these cases |
| 7 | Coronado Munoz A et al. [45] | Houston, Texas, USA | 1 neonate |
Mother was group B streptococcus carrier and neonate was treated for suspected sepsis for 48 h and discharged One of eight household contacts of the patient, a 49-year-old woman, was symptomatic, but not tested |
At 3 weeks of life, presented with a 2-day history of nasal congestion, tachypnoea and reduced feeding with hypotension. Baby was treated with fluid boluses, ventilation, antibiotics and vasopressors. Chest X-ray: bilateral infiltrates and partial collapse of the right upper lobe. ICD was inserted for pneumothorax. He had leukopenia with elevated CRP and procalcitonin. Bacterial cultures were sterile. Nasal swabs RT-PCR for SARS-Cov-2 at admission was positive and was treated with azithromycin and hydroxychloroquine. Recovered gradually and discharged on day 9 of admission |
| 8 | Salvatori G et al. [66] | Bambino Gesu`” Children’s Hospital, Rome, Italy | 2 mother and infant pairs | Postnatally two mother, and term infants pairs were admitted on day 18, day 10, respectively. Both mother and neonate were probably infected by a third person. Mothers were asymptomatic |
First neonate was asymptomatic and the second neonate had cough, diarrhoea and poor feeding and needed intravenous fluids for 5 days None of them required respiratory support. Neonatal nasopharyngeal swabs - positive on admission Both mothers breast milk samples - negative |
| 9 | Zamaniyan M et al. [27] | Mazandaran University of Medical Sciences, Sari, Iran | 1 mother and neonate pair | Mother was confirmed case of SARS-CoV-2, had pneumonia and required oxygen through nasal mask. Caesarean delivery was done due maternal condition at 32 weeks of gestation |
APGAR scores were normal. Neonate was isolated and fed with formula milk Neonate had fever after birth and was treated with intravenous antibiotics Vaginal secretions, umbilical cord blood, neonatal nasal and throat swabs at birth - negative. But amniotic fluid sample – was positive. The second nasal swab test at 24 h – positive. Third and fourth neonate’s PCR test was positive 1 week later. Possibility of vertical transmission present |
| 10 | Nie R et al. [67] | Hubie province, China | 27 mothers and 28 neonates (1 twin pair) |
Twenty-two women: caesarean section, 5 women: vaginal deliveries 4 neonates had foetal distress |
10 neonates - preterm. One late preterm newborn had ARDS and later recovered. Twenty-six newborns were tested by throat swabs One newborn tested positive for SARS-CoV-2 infection and had fever with pneumonia, but the cord blood and placental samples were negative. Retesting on days 4, 8 and 15 after birth – negative Pneumonia was resolved gradually |
| Infants with positive testing and had clinical features (may or may not be attributable to SARS-CoV-2 infection) | |||||
| 11 | Kirtsman M et al. [25] | Mountsinai Hospital, Toronto, Canada | 1 mother and neonate pair | Neutropenia, gestational diabetes and history of frequent bacterial infections. Presented at 35 + 5 weeks with fever and cough and confirmed for SARS-CoV-2 and underwent caesarean delivery due to maternal condition |
Late preterm AGA, did not require resuscitation. DCC was not done. No skin to skin contact was performed before first test Neonate’s nasopharyngeal swabs at birth, on day 2 and day 7 were positive; Neonatal plasma on day 4, and stool on day 7 - positive. Placental tissue, maternal vaginal swab on day 1 and on day 7, expressed breast milk swab on day 2 positive. Cord blood tissue was tested negative. Placental histopathology - widespread infarction The mother and newborn were roomed in a negative- pressure room and was breast- fed with appropriate precautions The neonate had mild hypothermia, feeding difficulties and intermittent hypoglycaemic episodes and neutropenia so admitted to NICU at 37 h of age for intravenous administration of glucose. Recovered and discharged home with his mother on day 4 after birth. The clinical features of the baby in our case were compatible with the course of a late pre- term neonate except for elevated liver enzyme levels This case represents a probable case of congenital SARS- CoV-2 infection |
| 12 | Wang S et al. [52] | Tongji Hospital, Wuhan, China | 1 mother and infant pair |
Delivered by caesarean section Meconium stained amniotic fluid was noted |
Term AGA with normal APGAR scores. Had 1 episode of vomiting after formula feed Had lymphopenia, deranged LFT, increased CKMB Nasopharyngeal swab RT-PCR - positive at 36 h of life. But the placenta, cord blood, breast milk RT-PCR were negative Chest X-ray was normal Chest CT: nodular shadow under the pleura of posterior segment of the upper lobe of right lung |
| 13 | Khan S et al. [68] | Renmin Hospital, Wuhan | 17 mother (12 RT-PCR confirmed, 5 CT confirmed) and infant pairs | All women underwent caesarean section |
Three neonates: preterm and 14 neonates: term. No infant had asphyxia Only 2 neonates had positive throat swab RT-PCR, tested within 24 h of life Total 5 infants had pneumonia, but in these, only one infant had positive test |
| 14 | Alzamora MC et al. [24] | British American Hospital, Lima, Peru | 1 mother and neonate pair | Mother had pneumonia and required ventilation. Caesarean delivery was done due maternal condition at 33 weeks of gestation |
APGAR scores of 6 and 8 at 1 and 5 min, respectively, and required intubation at birth. After 12 h, extubated to CPAP Chest X-ray showed no abnormalities At birth, for both mother and infant, Ig G and IgM titres - negative Nasopharyngeal swab of neonate for SARS-CoV-2 RT-PCR - positive at 16 h of life – shows possibility of vertical transmission. Repeat RT-PCR test at 48 h of life was also positive. Amniotic fluid, cord blood, or placental tissue - not evaluated Seroconversion in mother was identified later |
| 15 | Hantoushzadeh S et al. [69] | Tehran, Iran | 9 pregnant women |
In 9 pregnant women, 7 mothers died and 2 were survived. Delivery was not performed in 2 cases due to borderline viable pregnancy (24 weeks of gestation). One mother delivered by vaginal route and all others underwent caesarean delivery. |
One neonate was term and all other were preterm. 4 cases of IUFD were noted (two 24 weeks, one - 30 weeks, one – 36 weeks) In the remaining six infants, Five were tested negative after birth. A 30 weeks 6 days preterm neonate tested negative by nasopharyngeal swab on day one but presumptively acquired SARS-CoV-2 postnatally and subsequently tested positive on day seven with an accompanying lymphopenia. The neonate was intubated for prematurity, developed a pneumonia on day 2 and stable. One twin pair with gestation 28 weeks died on day 3 with preterm complications |
| 16 | Yan J et al. [70] | 25 hospitals within and outside of Hubei province, china | 99 mothers and 100 neonates (one twin pair) |
An expanded series from four previous small case series (Chen H et al, Liu Y et al, Zhang L et al, Lei D et al) 85 women: caesarean delivery, 14 women vaginal delivery. Caesarean delivery was indicated in 33 for COVID-19 pneumonia, for foetal distress in 9 women |
Among 100 neonates, two were less than 34 weeks, 21 were late preterm One late preterm neonate had severe neonatal asphyxia and died within 2 h after birth (whose mother had severe pneumonia and septic shock after admission and required ICU admission for invasive ventilation). 47 neonates were transferred to NICU for further treatment 86 neonates were tested with nasopharyngeal swab and all were negative In 10 neonates, paired amniotic fluid and cord blood samples negative In six women, vaginal secretion samples tested negative Twelve mothers - breast milk samples tested negative |
| Infants with negative testing and had clinical features (Symptoms may or may not be attributable to SARS-CoV-2 infection) | |||||
| 17 | Zhu H et al. [30] | 5 Hospitals, Wuhan, China | 9 symptomatic mothers and 10 neonates (includes one twin pair) | Onset of clinical symptoms occurred before delivery in 4 cases, on the day of delivery in 2 cases, and after delivery in 3 cases. Seven delivered their infants by caesarean section and two by vaginal delivery |
Six preterm and two SGA. None of the neonates were tested positive Neonates had clinical features noted within 30 min of birth One infant died; another infant had critical course Death: Preterm with respiratory distress. Day 8, he developed refractory shock, multiple organ failure and DIC and died on day 9 |
| 18 | Fan C et al. [71] | Renmin hospital, Wuhan | 2 physician mothers and infant pairs | Both mothers delivered by caesarean section |
Neonate 1: 37 weeks, 3400 g, placenta, cord blood, amniotic fluid, vaginal swab, serial nasopharyngeal and breast milk RT-PCR - negative On day 3, had fever, abdominal distension with lymphopenia and chest X-ray suggestive of diffuse haziness. Recovered and discharged Neonate 2: 36 + 5 weeks, 2890 g, neonate’s nasopharyngeal swab test and tests on products of conception - negative. Mild pneumonia, lymphopenia noted and recovered in 2 days with supportive care |
| Infants with negative testing and had non-specific clinical features (may not be attributable to SARS-CoV-2 infection) | |||||
| 19 | Chen Y et al. [43] | Tongji medical college, Wuhan, China | 4 mother and infant pairs |
Three - caesarean section, one - vaginal delivery One mother required respiratory support |
All had normal APGAR scores Three neonate’s throat swab was negative One infant had facial ulcerations; two had rashes of unknown aetiology at birth One infant had respiratory distress (TTNB) |
| 20 | Xu L et al. [72] | Tongji medical college, Wuhan | 5 mother and neonate pairs |
All five mothers had pneumonia Four mothers: caesarean delivery (two due to COVID-19 and two due to obstetric causes) One mother: vaginal delivery |
Two neonates: Late preterm. None of the neonates had symptoms Infants were isolated and were not breastfed. One term neonate had scattered skin rashes in face and body and gradually disappeared within 7 days The throat swab specimens for SARS-CoV-2 from all five neonates were negative |
| 21 | Lu Zhang et al. [73] | Wuhan, China | 18 mother and neonate pairs | In 18 pregnant women, 8 cases were confirmed, 10 cases were clinically diagnosed. 17 women: caesarean One woman: vaginal delivery. |
Three neonates: preterm. One neonate had mild asphyxia, 5 had bacterial pneumonia. One neonate had gastrointestinal bleeding, and another infant had diarrhoea but reasons were not specified All the newborns tested with throat swab within 24 h of life and were negative All neonates were stable and discharged |
| Infants with negative RT-PCR testing but had positive antibody testing without any clinical features | |||||
| 22 | Zeng H et al. [22] | Zhongnan Hospital, Wuhan, China | 6 pregnant women and infant pairs |
All 6 had caesarean deliveries. 4 mothers had elevated IgM levels at birth |
APGAR scores were normal Negative throat swab in all SARS-CoV-2 specific IgM positive in two infants Elevated IL-6 levels in all infants All asymptomatic |
| 23 | Dong L et al. [23] | Renmin Hospital, Wuhan, China | 1 mother and infant pair |
Underwent caesarean section at term Maternal serum IgM, IgG were elevated, vaginal secretions swab PCR was negative. |
Neonate’s nasopharyngeal swab RT-PCR was negative but serum IgM was positive twice (at 2 h of life and on day 16) Day 6 breast milk test negative Neonate had elevated levels of Serum IL-6, CPK-MB and LDH |
| Infants with negative testing and had no clinical adverse events | |||||
| 24 | Chen L et al. [28] | Wuhan, China | 68 mother and 70 neonate pairs (2 sets of twins) |
5 mothers: vaginal delivery, 38 mothers: caesarean due to COVID-19, 25 mothers: caesarean due to obstetric causes 9 mothers had pneumonia |
14 neonates (21%) premature APGAR scores normal in all Eight new-borns throat swabs and 3 breast milk samples were tested - negative |
| 25 | Liu W et al. [42] | Tongji hospital, Wuhan, China | 19 mothers (10 confirmed cases, 9 with clinical diagnosis) and infant pairs | Eighteen delivered by caesarean section and one by vaginal delivery |
All had normal APGAR scores SARS-CoV-2 RT-PCR test results in throat swab, gastric fluid right after birth, urine and stool of all neonates were negative Virus undetectable in amniotic fluid, umbilical cord blood and breast milk samples All neonates were asymptomatic |
| 26 | Li N et al. [31] | Wuhan, China |
16 confirmed mothers and 17 neonates (1 set of twins) |
14 mothers delivered by caesarean section |
Three (18.8%) neonates were preterm None had birth asphyxia Three tested were negative All asymptomatic |
| 27 | Liu D et al. [37] | Wuhan, China | 11 mother and neonate pairs | All pregnant women had mild pneumonia. 10 women – caesarean, One- vaginal delivery | Three neonates were late preterm. No neonatal death or asphyxia were reported |
| 28 | Chen S et al. [39] | Tongji medical college, Wuhan | 3 mother and neonate pairs | All three mothers delivered by caesarean section. Two mothers had symptoms after the delivery |
One neonate was premature All three neonatal throat swabs, placental PCR and histopathology were negative No adverse neonatal outcomes noted |
| 29 | Wang X et al. [41] | Suzhou, China | 1 mother and neonate pair | Caesarean section was performed for maternal indication | Neonate was preterm (30 weeks). Amniotic fluid, placenta, cord blood, gastric aspirate and throat swab were negative. Neonate did not have any clinical signs of COVID-19 |
| 30 | Liu W et al. [42] | Tongji Medical college, Wuhan, China | 3 Mother and New-born pair |
Two - caesarean section and one- vaginal delivery. Foetal distress, meconium stained amniotic fluid and chorioamnionitis were noted in one delivery |
All three neonates were term All 3 neonates placental, cord blood, throat swabs are negative Two infants were tested negative for urine, faeces also In two mothers, breast milk and vaginal mucus, and in one mother placenta - negative |
| 31 | Yang P et [74] | Zhongnan Hospital, Wuhan, China | 7 mother and infant pairs |
One mother – symptoms 2 days after delivery All seven women - caesarean section (5 for COVID-19) |
Four neonates - late preterm In 5 infants amniotic fluid and cord blood and in 6 infants pharyngeal swab - negative 2 late preterm infants had mild RDS and improved with nasal CPAP |
| 32 | Li Y et al. [75] | Zhejiang University, Hangzhou, China | 1 mother and infant pair | Mother had mild symptoms and undergone caesarean delivery due to COVID-19 |
Late preterm. RT-PCR on Placenta, amniotic fluid, cord blood, breast milk and serial tests on neonate’s oropharyngeal swab, blood, faeces and urine - negative Infant asymptomatic |
| 33 | Lee DH et al. [76] | Daegu Fatima Hospital, Korea | 1 Mother and infant pair | Mother was delivered by caesarean section due to obstructed labour |
Term AGA with normal APGAR scores Neonate was isolated (NICU) and did not have any adverse clinical events. Placenta, amniotic fluid, cord blood and neonate’s nasopharyngeal RT-PCR - negative |
| 34 | Gidlof S et al. [77] | Stockholm south general Hospital, Sweden | 1 mother and 2 infants (twin gestation) | Emergency caesarean was performed due to severe preeclampsia |
Late preterm, vaginal secretions, breast milk and both twins nasopharyngeal swab - negative Twin 1 had respiratory distress and supported with CPAP. On day2, had cyanosis on feeding, improved with supportive care Twin-2 had no symptoms |
| 35 | Lowe B et al. [78] | Queensland Australia | 1 mother and neonate pair | Vacuum delivery was performed for non-reassuring foetal CTG. Mother was started on triple antibiotics for possible chorioamnionitis |
40 weeks AGA with normal APGAR scores. Baby was breastfed and was not separated from mother from birth Baby was asymptomatic At 24 h of life, COVID-19 test - negative |
| 36 | Song L et al. [79] | Tongji medical college, Wuhan | 1 mother and infant pair | Mother had pneumonia, managed. Caesarean section performed for maternal indication. |
Late preterm neonate did not have any clinical features Neonate’s throat swab’s RT-PCR at day 3, day 7 were negative |
| 37 | Chen S et al. [80] | Wuhan, China | 5 mother and neonate pairs |
All mothers had low-grade fever after birth 3 mothers: vaginal delivery 2 mothers: caesarean section |
All 5 infants were term AGA Neonate’s throat swab RT-PCR was negative and did not have any clinical features |
| 38 | Sharma K A et al. [81] | Delhi, India | 1 mother and neonate pair | Mother was asymptomatic and underwent caesarean delivery as per her wish |
Neonate was term and kept at mother’s side and breast fed Tested on day 7: negative for SARS-CoV-2 Neonate did not have any clinical abnormality |
| 39 | Khan S et al. [82] | Renmin Hospital, Wuhan | 3 mother and neonate pairs | All three mothers confirmed for SARS-CoV-2 and delivered vaginally | One preterm (34 + 6 days) and another two term deliveries. Neonates were isolated. Three newborns tested negative for SARS-CoV-2 by nasopharyngeal swab |
| 40 | Khasawneh W et al. [83] | Jordan | 1 mother and neonate pair | Mother underwent caesarean delivery due to preterm labour and previous caesarean section at 36+ 3 weeks of gestation | Neonate was AGA and required supportive oxygen for less than an hour for a possible transient tachypnoea. Infant was isolated. Amniotic fluid, breast milk and neonate’s nasopharyngeal swab at birth - negative |
| 41 | Lyra J et al. [84] | Porto, Portugal. | 1 mother and neonate pair | Mother underwent caesarean delivery due to PPROM and previous caesarean delivery. | Neonate was term AGA and placed in a single-patient negative pressure room. Newborn’s nasal and oropharyngeal swabs at birth, 48 h and at day 7, all negative for SARS-CoV-2 |
| 42 | Kalafat E et al. [85] | Ankara university, Anakara, Turkey. | 1 mother and neonate pair | Mother had pneumonia and undergone caesarean delivery due to maternal condition | Preterm AGA neonate with normal APGAR Scores. Cord blood, placental, breast milk and neonatal swabs negative |
| 43 | Xiong X et al. [86] | Beijing, China | 1 mother and neonate | Mother was diagnosed to have COVID-19 37 days before the delivery at 33 week of gestation and recovered. Vaginal delivery occurred at 38 weeks | Amniotic fluid, neonatal throat swab, and rectal swab RT-PCR at birth - negative. SARS-CoV-2-specific IgG and IgM were positive in maternal sera suggesting mother had been convalescing from infection. But the neonatal IgG and IgM antibodies to SARS-CoV-2 were both negative, suggesting that lack of intrauterine transmission in this case. Placenta did not show any infection and negative for SARS-CoV-2 N protein |
| Data from national registries | |||||
| 44 | NPC-19 registry update [87] | AAP, USA | 304 mother and infant dyads | 230 mothers were confirmed cases of COVID-19 and 74 mothers were PUI (Patient under investigation).206 (68%) were asymptomatic, 85 (28%) were symptomatic and 12 (4%) were admitted for COVID-19 treatment. 191 (63%) women underwent vaginal delivery and 112 (37%) women underwent caesarean section | 52.8% infants were separated to nursery or NICU at birth while remaining 47.2% were roomed in with mothers. At birth, 44 infants required PPV and 8 infants required intubation.262 infants not requited any respiratory support while 19 required oxygen alone, 19 required CPAP and 11 required ventilation. Nine neonates had fever, 33 had respiratory distress, 4 had vomiting and diarrhoea, 2 had hypotonia, and 1 had cough.121 (36.7%) neonates were started on direct breast feeding, 14 (4.2%) were on expressed breast milk by mother, 63 (19.1%) infants were fed expressed breast milk by other person, 6 (1.8%) infants were fed by donor milk and 126 (38.2%) neonates were fed by formula. 249 infants were tested, and 12 were positive for SARS-CoV-2 |
| 45 | Nederland’s Registry (https://www.nvog.nl/actueel/registratie-van-covid-19-positieve-zwangeren-in-nethoss/) | Nederland | 47 mother and 48 neonates (1 twin pair) | 16 mothers delivered by caesarean section, others delivered by vaginal delivery |
One preterm birth No neonate had positive test for COVID-19 31 neonates were breastfed In 8 cases, vaginal cultures, cultivation of the amniotic fluid or the placenta was performed, and one vaginal culture was positive for COVID-19 |
Laboratory diagnosis
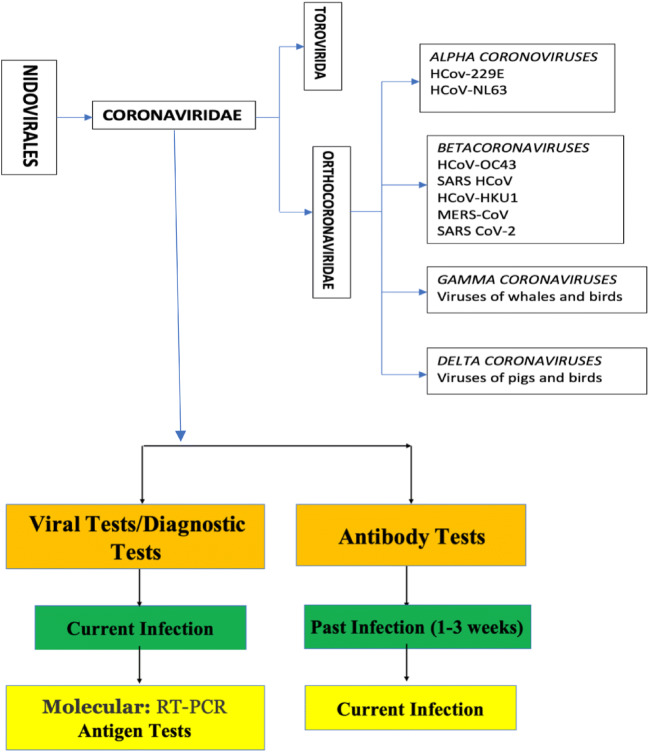
Detection of viral nucleic acid by reverse transcriptase polymerase chain reaction (RT-PCR) is the current gold standard and confirmatory test for COVID-19 [46]. The sensitivity of RT-PCR depends on the precise RT-PCR assay, the type of specimen obtained, the quality of specimen and the duration of the illness at the time of testing. RT-PCR may be false negative in low viral load states like very early or late phase of disease [47]. Nasopharyngeal swab and oropharyngeal swab are recommended for screening or diagnosis of early infection (https://www.aappublications.org/news/2020/07/22/newbornguidance072220). In a study on the sensitivity of RT-PCR for various specimens, positivity rates were 93% for broncho-alveolar lavage,72% for sputum, 63% for nasal swab, 46% for bronchoscopic brush biopsy, 32% for pharyngeal swabs, 29% for faeces, 1% for blood and 0% for urine specimens. Lower respiratory tract specimens have higher positive rates probably due to higher viral loads [17, 47, 48] (https://www.aappublications.org/news/2020/07/22/newbornguidance072220). American Academy of Paediatrics (AAP) recommends testing with molecular assay on swabs of nasopharynx and throat (oropharynx) [one single swab to sample throat first and then the nasopharynx], initially at 24 h of age and repeat testing at 48 h of age. In mechanically ventilated neonates, tracheal aspirate should be tested. In the neonates with initial positive PCR test, follow-up testing with 48–72-h interval should be done until 2 consecutive negative tests (https://www.aappublications.org/news/2020/07/22/newbornguidance072220). The Canadian Paediatric Society recommends testing within 2 h of life after cleansing the face to reduce colonization. For positive tests, repeat testing after 24–48 h is recommended. Optimal precautions should be ensured during sample collection and processing to avoid risk of exposure to virus due to aerosol transmission (https://www.cps.ca/en/documents/position/nicu-care-for-infants-born-to-mothers-with-suspected-or-proven-covid-19).
Serological tests are less likely to be reactive in the first several days of infection so are less useful in diagnosis of acute infection. Sensitivity of ELISA is 87% compared to 82% by immunochromatographic card test [49]. Similarities in genetic sequence with other viruses cause cross-reactivity and high false positive rates for serological tests. In a recent study, the positive detection rate was significantly increased (98.6%) when IgM ELISA assay was combined with PCR for each patient compared to qPCR test alone (51.9%) [50] (Fig. 2). Serological surveys can aid in investigation of an ongoing outbreak and in retrospective assessment of the attack rate or extent of an outbreak (https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19). A recent report on placental pathology in 16 women with SARS-CoV-2 infection showed features of decidual arteriopathy and maternal vascular malperfusion [51]. These findings are also seen in hypertensive disorders of pregnancy and may reflect an inflammatory state affecting placental physiology.
Fig. 2.
Common tests available for coronavirus family
Complete blood count may show normal or low total while cell counts, decreased lymphocyte count and low platelet count. Various enzymes levels including creatinine kinase, lactate dehydrogenase, alanine aminotransferase and inflammatory markers (CRP, ferritin) have shown non-specific elevation [20, 23, 30, 52]. Chest X-ray findings can vary from normal in the initial phases to unilateral or bilateral patchy or ground glass involvement [53]. There are no characteristic X-ray and CT features of neonatal COVID-19 till date because of limited data. USG chest could emerge to be a useful tool as it can be done at bedside and does not cause radiation exposure.
Testing of pregnant women
Based on resource availability, it may be useful to promote a general SARS-CoV-2 screening for pregnant women admitted to the hospital in order to prevent NICU outbreak. Universal screening of neonates, parents and healthcare workers can prevent spread of SARS-CoV-2 infection among the neonates admitted to a NICU even in an area with a high incidence of SARS-CoV-2 [54].
Many centres currently practise universal testing approach for pregnant women to determine optimal hospital isolation practices, use of personal protective equipment and neonatal care. However, the application of this strategy may not possible in resource limited settings or in cases of emergency delivery [55]. All pregnant women with clinical features attributable to COVID-19 should be tested by sending nasopharyngeal and/or oropharyngeal swab for RT-PCR testing. If symptoms persist and test reports are negative, ongoing isolation and repeat testing after 2–5 days is recommended. No emergency procedure or delivery should be delayed pending test results. All such cases should be considered suspect cases.
Testing of the neonate
Neonates born to mothers with COVID-19 infection within 14 days of delivery or up to 28 days after birth and neonates exposed to close contacts with COVID-19 infection should be tested [48]. If symptomatic, specimens should be collected as soon as possible. Asymptomatic neonates born to mothers with confirmed COVID-19 infection should be tested between 2 and 12 h after cleansing the face (https://www.cps.ca/en/documents/position/nicu-care-for-infants-born-to-mothers-with-suspected-or-proven-covid-19). Repeat testing after 2–3 days for positive cases and after 3–5 days for negative cases is recommended. If testing is not readily available, clinical monitoring should be done (https://www.aappublications.org/news/2020/07/22/newbornguidance072220). For diagnosing vertical transmission, it is important to follow a universally accepted definition for comparison and collation of data. One such classification system and case definition for SARS-CoV infection in pregnant women, foetuses and neonates has been proposed recently [49] (Table 2).
Table 2.
Suggested case definition for neonatal and maternal COVID-19 infection (adapted from Shah PS, Diambomba Y, Acharya G, Morris SK, Bitnun A. Classification system and case definition for SARS-CoV-2 infection in pregnant women, foetuses and neonates. Acta Obstet Gynecol Scand. 2020;99 [5]:565–568. doi:10.1111/aogs.13870)
| C0VID-19 | Mother | Congenital infection in foetus/perinatal period | Acquired neonatal infection postnatal period |
|---|---|---|---|
| Confirmed | PCR – Positive in a respiratory sample irrespective of symptoms | PCR – Positive in sample collected within first 12 h of birth or amniotic fluid collected prior to rupture of membrane | PCR – Positive at birth AND at 24–48 h of age AND alternate explanation for clinical features excluded |
| Probable | PCR – Positive in nasopharyngeal swab at birth (AND) placental swab from foetal side of placenta | PCR – Positive at birth but not at 24–48 h of age AND alternate explanation for clinical features excluded | |
| Possible | Symptomatic but no testing done | PCR – Negative AND presence of anti-SARS-CoV-2 IgM Ab – Positive within first 12 h of birth | PCR – Neonatal sample negative at birth AND PCR – positive in any of maternal vaginal/placental/skin swab at birth AND alternate explanation for clinical features excluded |
| Unlikely | No detection of the virus by PCR in a respiratory sample and no other cause identified | No detection of the virus by PCR AND antibody testing not done | PCR – Negative in nasopharyngeal swab at birth OR in any of maternal vaginal/placental/cord/neonatal nasopharyngeal/skin swab at birth AND alternate explanation for clinical features not identified |
| Not Infected | No detection of the virus by PCR in a respiratory sample and other cause identified | No detection of the virus by PCR AND antibody testing negative | PCR – Negative in nasopharyngeal swab at birth OR in any of maternal vaginal/placental/cord/neonatal nasopharyngeal/skin swab at birth AND alternate explanation for clinical features identified |
Recommended samples
Mother: nasopharyngeal/ nasal/broncho-alveolar lavageFoetus: foetal or placental tissue
Neonate: umbilical cord blood or neonatal blood, amniotic fluid, nasopharyngeal swab
Guidelines from Chinese expert consensus, AAP/American College of Obstetricians and Gynaecologists (ACOG)/Society for Maternal and Fetal Medicine (SMFM)/Centers for Disease Control and Prevention (CDC) and RCOG/RCPCH are compared in Supplementary Table 1.
Management
Standardized testing protocol, prevention of spread from mother to child and providing optimal treatment to symptomatic neonates are the key areas in perinatal management of neonates with suspect/positive for COVID-19. It is advisable to cohort pregnant women who are positive for SARS-CoV-2 or as person under investigation (PUI) with ILI (influenza-like illness) (https://www.aappublications.org/news/2020/07/22/newbornguidance072220, https://www.cps.ca/en/documents/position/nicu-care-for-infants-born-to-mothers-with-suspected-or-proven-covid-19, https://www.rcog.org.uk/globalassets/documents/guidelines/2020-04-17-coronavirus-covid-19-infection-in-pregnancy.pdf, https://www.cdc.gov/coronavirus/2019-ncov/hcp/inpatient-obstetric-healthcare-guidance.html, https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/novel-coronavirus-2019) [48, 55, 56]. Ideally this should be done prior to hospital admission through telephonic or video consultation or at hospital entry. Antenatal steroids and magnesium sulphate for neuroprotection should be administered as per current guidelines. The pregnant women should wear mask to prevent the spread through droplet transmission. Neonatology team should discuss with the parents prior to delivery regarding delayed cord clamping, separation of the neonate from mother versus rooming-in and feeding options using a shared decision-making approach. The practices of rooming-in, PPE and mother-neonate separation are extremely variable according to different countries/setting and guidelines from scientific societies or institutions. Thus, choices must be adapted to the local needs and situation. What is more important is to have a complete, open information to the family and a shared choice post-counselling. In real life, ‘zero risk’ does not exist, and decision should be based after evaluating the risk-benefit ratio, resource availability, feasibility and parental preference [55, 56].
Precautions and PPE
In the current context of pandemic, triaging pregnant women as suspect, positive or negative cases will optimize utilization of resources.
Contact and droplet precautions are recommended for routine encounters with confirmed or suspect neonates with COVID-19 infection. These include gown, gloves, standard procedural mask and eye protection (face shield or goggles). Airborne, contact and droplet precautions are recommended for procedures including bag and mask ventilation, intubation, suctioning and neonates on any form of respiratory support. The PPE includes gown, gloves, N95 respiratory mask with eye protection or air-purifying respirator (powered air-purifying respiratory (PAPR) (https://www.aappublications.org/news/2020/07/22/newbornguidance072220).
Delivery room
Resuscitation of neonates born to mothers with confirm or suspected COVID-19 disease should be in accordance with the latest NRP guidelines (https://www.aappublications.org/news/2020/07/22/newbornguidance072220). Delayed cord clamping (DCC) has significant benefits in term and preterm neonates, but the small risk of viral transmission through cord blood does exist. As per existing practice, DCC may be considered unless contraindicated, after discussion with families [48]. Resuscitation should be in a separate room and overcrowding should be avoided. If separate room is not available, the neonatal resuscitation corner should be at least 6 ft away from the delivery cot. Resuscitation may involve suctioning, providing PPV or intubation; hence, the providers should don airborne, contact and droplet level-PPE [48]. DR resuscitative manoeuvres are at high risk of aerosolization; also there is risk of transmission from maternal blood, amniotic fluid and other body fluids. Non-invasive ventilation provided during resuscitation may significantly increase this risk like in the NICU as neonatal CPAP and NIV are almost always invariably associated with 40–50% leaks through the mouth [57]. Therefore, PPE should be a norm in DR in the present pandemic era for all the personnel attending to the mother and her neonate. Based on adult data, the risk of acquiring a viral infection is higher during endotracheal intubation compared with manual ventilation (bag-mask ventilation) [58]. The use of bacterial/viral filters in the exhalation limb of T-piece may be effective in reducing viral dispersion and is recommended by many scientific societies. With close monitoring, it may be reasonable to use smaller filters (approximately 10 mL of dead space) in neonates with birth weight > 2 kg.
Infants needing NICU care
The neonates born to positive mothers should be nursed in an incubator and transported to the NICU by a dedicated team wearing PPE. Ideally, the neonate should be kept in negative pressure isolation rooms or in rooms with high efficiency particulate air filters (HEPA). Staff should handle the baby with isolation precautions and PPE, and such staff should not move to other sections of the NICU. If the NICU does not have a single-room design, open-bay NICU’s should have at least 3 sections, first room for babies born to COVID-19 positive/suspect mothers, second for babies who are born to COVID-19 negative asymptomatic mothers and third room for babies who were in room one but have tested negative twice. The staff and workforce should also follow the same flow, with dedicated team of healthcare workers (HCW) for each section. It is important to remember that NICU should be reserved only for those neonates needing intensive care. A general NICU admission policy due to positive/suspect COVID status may lead to shortage of beds and will also contribute to false epidemiological data and overestimation of the disease severity in neonatal population [59].
Testing of the neonate should be performed between 2 and 12 h after birth. If negative, a repeat test should be performed after 3–5 days. If the neonate is requiring prolonged NICU stay and both tests are negative, the neonate can be shifted to a separate room to prevent ongoing risk of transmission from other babies. Symptomatic and supportive therapy are the main principles of management as no specific antiviral agent is approved for use in neonates.
Respiratory management
Although neonatal COVID can present with varied symptoms involving cardiovascular, GIT or CNS systems, respiratory distress is the commonest system. However, neonates are more likely to need respiratory support due to other conditions such as prematurity, sepsis, respiratory distress syndrome, and transient tachypnoea of newborn rather than due to COVID-19 pneumonia. Epidemiologically, the priority should be to diagnose neonatal ARDS according to Montreux definition and use it to classify disease severity [60].
Respiratory management practices should be evidence based for providing optimal support to the neonate and also protecting HCW from the virus. The main concern related to respiratory support in neonates with suspected or confirmed COVID-19 infection is the generation of aerosol-containing particles that can lead to disease spread. The data related to respiratory management of neonates with COVID-19 infection is limited, as majority of neonates have tested negative or have been asymptomatic. Thus, it seems reasonable to have a practical, physiology-based approach for these neonates as we await more evidence.
Non-invasive support
CPAP remains the standard therapy in managing neonates with respiratory distress in the NICU including those with COVID-19. Heated humidified high flow nasal cannula (HHHFNC) may lead to higher dispersion of virus due to the presence of higher leak around the nose and should be avoided. A study by Simonds et al. showed that air dispersion during NIPPV generates droplets of > 10 μm in size, which are more likely to deposit on local surfaces within a short distance [61]. CPAP with a close circuit provided by the ventilator may be safer as it has lesser degree of aerosol generation, and the same machine and circuit can be used for mechanical ventilation if the patient deteriorates. However infant flow driver, variable flow CPAP should be avoided as one limb is open to the exterior and may be a source of spread. Precautions during use of CPAP for neonates include proper PPE measures for HCW (airborne, droplet and contact-level PPE), optimal fitting of the interface, adequate spacing/ventilation in room and regular disinfection of surfaces/baby environment. The CPAP tubing and water reservoir should be replaced every week and discarded appropriately. Disposable circuits should be preferred, and one should use filters on the expiratory limb (at least) of circuits as is stated by many scientific societies.
Invasive support
Endotracheal intubation is an aerosol generating procedure and should be performed by skilled person wearing adequate PPE. Both cuffed and uncuffed tube may be used for intubation. Low cuff pressure has been demonstrated to be safe in various studies [88, 89]. Video laryngoscope should be preferred as it allows easy access to the airway and avoids close contact. If available, closed in-line suctioning and use of hydrophobic filter at the exhalation port is recommended both during non-invasive and invasive respiratory support. Use of appropriately sized, endotracheal tube is recommended to minimize leak, and oral intubation should be preferred over nasal intubation [90]. Mode of ventilation and strategy for weaning should be based on disease process and current practice.
Stable neonates not requiring NICU admission
Stable neonates born to confirm/suspect COVID-19 mothers who are stable can be roomed-in together in a negative pressure isolation room. The potential benefit of temporary maternal and newborn separation in decreasing neonatal infection versus the issues related to infant separation should be discussed with the family prior to delivery. If mother and baby are roomed-in, an additional caregiver who is non COVID-19 exposed and asymptomatic could be permitted to care for the baby. The mother should wear N-95 mask and practice meticulous hand hygiene. The infant should be placed at least 6 ft from the mother. Breast milk has numerous benefits, and there is little data to suggest that virus transmission occurs via breast milk [25, 26]. Expressed breast milk can be safely fed to the neonate by the designated caregiver after boiling. The breast pump components and paladai/feeding bottles should be thoroughly disinfected after each use. Skin-to-skin contact should be avoided due to risk of transmission of virus. In case the mother wishes to directly breast feed the infant, she should follow strict hand hygiene and wear mask at all times. The neonate should be monitored regularly for vitals and respiratory symptoms. The neonate can be discharged as per routine discharge criteria if asymptomatic and after 2 consecutive negative tests for COVID-19. A comprehensive approach for the perinatal and postnatal management, discharge and follow-up of neonates has been summarized in Fig. 2.
Neonatal transport in suspect or positive case
Transport of a sick infant involves meticulous planning and should be efficient, safe and timely. Data reported by the Neonatal Transport Study Group of the Italian Society of Neonatology showed a significant increase in the median time to complete transport from 120 min previously to 240 min in the current COVID-19 period. The common reasons attributable for this delay include donning and doffing of PPE protocols, disinfection of ambulance, devices and delays in obtaining necessary documentation [91].
It is important to have a clear communication with the referring unit prior to transport regarding the infant’s or mother’s recent COVID status. The shortest route of transport and transit modifications inside referring hospitals should be established (e.g. alternative passage or lifts for transfer of suspect/confirmed cases). The ambulance should be equipped with adequate PPE, and number of team members should be restricted depending on the situation. Members of the transport team must wear protective gear including N95 respirator, disposable gown, visor/goggles, face-shield and shoe cover. The equipment used for stabilization should be placed in air-tight plastic containers for reuse during the transport. The infant must be transported in an incubator, and unnecessary opening of portholes should be avoided. On arriving at the destination, another team from the tertiary hospital can receive the infant from the ambulance (after donning PPE) and escort to the NICU. Once the neonate is safely transferred to the NICU, cleaning of the transport incubator, monitors, syringe pumps and ventilator must be done with disinfectant wipes or paper cloth soaked in 70% ethyl alcohol or other appropriate disinfectant. Other equipment (i.e. masks, laryngoscope, self-inflating bag) should be sterilized according to standard institutional policy. Lastly, the vehicle should be sanitized prior to the next transport. Road transports should be preferred if possible, and the ambulance should not have any open passage/windows between the health compartment and driver. Having a clear institution-based standard operating procedure will minimize delays and ensure safe transport for the infant and the crew [92].
Thus, the overall prognosis of neonates born to COVID-19-positive mothers is favourable. Risk of transmission is low, and most neonates are asymptomatic. Possible protective mechanisms include lower ACE2 expression (SARS-CoV-2 receptor), less robust proinflammatory cytokine response and stronger innate immune system [93, 94].
Limitations
This review is based on mostly retrospective reports, reviews and consensus guidelines whose quality may be suboptimal due to fast-tracked publications considering the unexpected nature of pandemic. These recommendations may change at a later stage, once more robust evidence is available.
Future research considerations
There is a need for large prospective multi-centre international registries of neonatal SARS-CoV-2 infections to better understand the epidemiology, the detail and the possibility of interventions for neonatal cases such as registries by AAP and EPICENTRE Registry [95].
The following key points summarize practical considerations for perinatal COVID-19:
Criteria for testing pregnant women should be same as for general population. Universal testing prior to delivery may help in deciding optimal isolation practices.
Antenatal steroid and magnesium sulphate should be given as per current guidelines.
Mode of delivery should be as per obstetric/foetal indication irrespective of COVID-19 status
Use dedicated delivery room and operating room for confirmed or suspected COVID-19 pregnant women. Perform neonatal resuscitation in a separate room or at least 6 ft away from delivery area as per NRP.
Delayed cord clamping should be done as per current guidelines after discussion with family.
Practice airborne, droplet and contact precautions with PPE for performing deliveries and neonatal resuscitation.
Video laryngoscope should be preferred for intubation, and one should avoid giving medications through endotracheal tube.
Immediate bathing should be avoided because of risk of hypothermia.
Ensure proper disposal of all PPE prior to exiting the room.
Neonates born to positive mothers should be initially tested between 2 and 12 h after cleansing the face.
For infants needing respiratory support, ensure optimal fitting of interface, adequate spacing between beds, use of adequate PPE and frequent disinfection of baby environment every 2–4 hourly.
For infants on mechanical ventilation, use appropriate size endotracheal tube, in-line suctioning, adequate PPE and small hydrophobic filter at exhalation port.
For infants not needing admission, decision to room-in with mother should be based on shared decision-making principle.
Infant can be fed expressed breast milk by healthy caregiver.
If roomed-in, infant crib should be placed at least 6 ft away from the mother. Mother should ensure hand hygiene and wear mask.
Neonatal BCG vaccination can be continued in countries or settings with a high incidence of tuberculosis as per existing practice [61].
Stools and diapers should be considered infectious waste and disposed with all necessary precautions as per protocol.
Mobile phone use should be restricted in clinical areas.
Supplementary Information
(PDF 219 kb)
Acknowledgements
We are grateful to Shivani Bhan for infographics and other technical help.
Abbreviations
- ACE-2
Angiotensin-converting enzyme 2
- HCoVs
Human coronaviruses
- SARS-CoV
Severe acute respiratory distress syndrome coronavirus
- ILI
Influenza like illness
- MERS CoV
Middle East respiratory syndrome-related coronavirus
- PUI
Person under investigation
Authors’ contributors
VV and AP did the literature search, designed and/or debated the series of articles/ studies, made editorial comments and assure the validity of the content. VV, AnP, SB and AP drafted this article. AP, AnP and SB did the critical appraisal of the manuscript. All authors accepted the final manuscript.
Data availability
NA
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
NA
Consent to participate
NA
Consent for publication
Yes we consent for publication.
Code availability
NA
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Venkateshwarlu Vardhelli, Email: venkat959@gmail.com.
Aakash Pandita, Email: aakash.pandita@gmail.com.
Anish Pillai, Email: anishgp1@yahoo.co.in.
Susanta Kumar Badatya, Email: skb48072@gmail.com.
References
- 1.Banerjee A, Kulcsar K, Misra V, Frieman M, Mossman K. Bats and coronaviruses. Viruses. 2019;11(1):41. doi: 10.3390/v11010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng VC, Lau SK, Woo PC, Yuen KY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20(4):660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015;386(9997):995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan JF, To KK. Tse H, Jin DY, Yuen KY. Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol. 2013;21(10):544–555. doi: 10.1016/j.tim.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P (2020) A novel coronavirus from patients with pneumonia in China, 2019, New England journal of medicine. 24 [DOI] [PMC free article] [PubMed]
- 7.Gorbalenya AE (2020) Severe acute respiratory syndrome-related coronavirus–the species and its viruses, a statement of the coronavirus study group. BioRxiv. 1
- 8.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam TT, Shum MH, Zhu HC, Tong YG, Ni XB, Liao YS, Wei W, Cheung WY, Li WJ, Li LF, Leung GM. Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins. Nature. 2020;26:1–6. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 11.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KS, Lau EH, Wong JY, Xing X (2020) Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med [DOI] [PMC free article] [PubMed]
- 13.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet (London, England) 2020;395(10224):e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, Cui X, Xiao J, Meng T, Zhou W, Liu J (2020) The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. BioRxiv. 1
- 16.Li M, Chen L, Zhang J, Xiong C, Li X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One. 2020;15(4):e0230295. doi: 10.1371/journal.pone.0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baud D, Greub G, Favre G, Gengler C, Jaton K, Dubruc E, Pomar L. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA. 2020;323(21):2198–2200. doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz DA (2020) An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med 17 [DOI] [PubMed]
- 19.Raschetti R, Vivanti AJ, Vauloup-Fellous C, Loi B, Benachi A, de Luca D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat Commun. 2020;11:5164. doi: 10.1038/s41467-020-18982-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng L, Xia S, Yuan W, Yan K, Xiao F, Shao J, Zhou W (2020) Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr 26 [DOI] [PMC free article] [PubMed]
- 21.Yu N, Li W, Kang Q, Xiong Z, Wang S, Lin X, Liu Y, Xiao J, Liu H, Deng D, Chen S (2020) Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis 24 [DOI] [PMC free article] [PubMed]
- 22.Zeng H, Xu C, Fan J, Tang Y, Deng Q, Zhang W, Long X (2020) Antibodies in infants born to mothers with COVID-19 pneumonia. Jama. 26 [DOI] [PMC free article] [PubMed]
- 23.Dong L, Tian J, He S, Zhu C, Wang J, Liu C, Yang J (2020) Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. Jama 26 [DOI] [PMC free article] [PubMed]
- 24.Alzamora MC, Paredes T, Caceres D, Webb CM, Valdez LM, La Rosa M (2020) Severe COVID-19 during pregnancy and possible vertical transmission [published online ahead of print, 2020 Apr 18]. Am J Perinatol. 10.1055/s-0040-1710050 [DOI] [PMC free article] [PubMed]
- 25.Kirtsman M, Diambomba Y, Poutanen SM, et al. (2020) Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection [published online ahead of print, 2020 May 14]. CMAJ. cmaj.200821. 10.1503/cmaj.200821 [DOI] [PMC free article] [PubMed]
- 26.Groß R, Conzelmann C, Müller JA, et al. (2020) Detection of SARS-CoV-2 in human breastmilk [published online ahead of print, 2020 May 21]. Lancet. S0140–6736(20)31181–8. 10.1016/S0140-6736(20)31181-8
- 27.Zamaniyan M, Ebadi A, Aghajanpoor Mir S, Rahmani Z, Haghshenas M, Azizi S (2020) Preterm delivery in pregnant woman with critical COVID-19 pneumonia and vertical transmission. Prenat Diagn 17 [DOI] [PMC free article] [PubMed]
- 28.Di Mascio D, Khalil A, Saccone G, Rizzo G, Buca D, Liberati M, Vecchiet J, Nappi L, Scambia G, Berghella V, D’Antonio F. Outcome of coronavirus spectrum infections (SARS, MERS, COVID 1-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;25:100107. doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu H, Wang L, Fang C, Peng S, Zhang L, Chang G, Xia S, Zhou W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9(1):51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li N, Han L, Peng M, Lv Y, Ouyang Y, Liu K, Yue L, Li Q, Sun G, Chen L, Yang L (2020) Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. Clin Infect Dis 1 [DOI] [PMC free article] [PubMed]
- 31.Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Jama. 24 [DOI] [PubMed]
- 32.Costa S, Posteraro B, Marchetti S, et al. (2020) Excretion of SARS-CoV-2 in human breast milk [published online ahead of print, 2020 Jun 2]. Clin Microbiol Infect. S1198-743X(20)30304–9. 10.1016/j.cmi.2020.05.027 [DOI] [PMC free article] [PubMed]
- 33.Chambers C, Krogstad P, Bertrand K, et al. (2020) Evaluation for SARS-CoV-2 in Breast Milk From 18 Infected Women [published online ahead of print, 2020 Aug 19]. JAMA. e2015580. 10.1001/jama.2020.15580 [DOI] [PMC free article] [PubMed]
- 34.Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J (2020) The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med 1 [DOI] [PMC free article] [PubMed]
- 35.Chen J (2020) Pathogenicity and transmissibility of 2019-nCoV—a quick overview and comparison with other emerging viruses. Microbes Infect 4 [DOI] [PMC free article] [PubMed]
- 36.Breslin N, Baptiste C, Gyamfi-Bannerman C, Miller R, Martinez R, Bernstein K, Ring L, Landau R, Purisch S, Friedman AM, Fuchs K. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020;9:100118. doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu D, Li L, Wu X, Zheng D, Wang J, Yang L, Zheng C. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. Am J Roentgenol. 2020;18:1–6. doi: 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Chen H, Tang K, Guo Y (2020) Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Inf Secur 4 [DOI] [PMC free article] [PubMed]
- 39.Chen S, Huang B, Luo DJ, Li X, Yang F, Zhao Y, Nie X, Huang BX. Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases. Zhonghua bing li xue za zhi= Chin J Pathol. 2020;49:E005. doi: 10.3760/cma.j.cn112151-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Jiang Y, Wei M, Cheng BH, Zhou XC, Li J, Tian JH, Dong L, Hu RH. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province. Zhonghua fu chan ke za zhi. 2020;55:E009. doi: 10.3760/cma.j.cn112141-20200218-00111. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Zhou Z, Zhang J, Zhu F, Tang Y, Shen X, Shen X (2020) A case of 2019 Novel coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis [DOI] [PMC free article] [PubMed]
- 42.Liu W, Wang Q, Zhang Q, Chen L, Chen J, Zhang B, Lu Y, Wang S, Xia L, Huang L, Wang K. Coronavirus disease 2019 (COVID-19) during pregnancy: a case series
- 43.Chen Y, Peng H, Wang L, Zhao Y, Zeng L, Gao H, Liu Y. Infants born to mothers with a new coronavirus (COVID-19) Front Pediatr. 2020;8:104. doi: 10.3389/fped.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mimouni F, Lakshminrusimha S, Pearlman SA, Raju T, Gallagher PG, Mendlovic J. Perinatal aspects on the covid-19 pandemic: a practical resource for perinatal–neonatal specialists. J Perinatol. 2020;40:820–826. doi: 10.1038/s41372-020-0665-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coronado Munoz A, Nawaratne U, McMann D, Ellsworth M, Meliones J, Boukas K. Late-onset neonatal sepsis in a patient with Covid-19. N Engl J Med. 2020;382(19):e49. doi: 10.1056/NEJMc2010614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention. Interim guidelines for collecting, handling, and testing clinical specimens from persons under investigation (PUIs) for coronavirus disease 2019 (COVID-19). February 14, 2020. https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html (Accessed on March 15, 2020)
- 47.World Health Organization. Laboratory testing for coronavirus disease ( COVID-19) in suspected human cases: interim guidance, 19 March 2020. World Health Organization; 2020
- 48.Trevisanuto D, Moschino L, Doglioni N, Roehr CC, Gervasi MT, Baraldi E. Neonatal resuscitation where the mother has a suspected or confirmed novel coronavirus (SARS-CoV-2) infection: suggestion for a pragmatic action plan. Neonatology. 2020;117(2):133–140. doi: 10.1159/000507935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah PS, Diambomba Y, Acharya G, Morris SK, Bitnun A. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand. 2020;99(5):565–568. doi: 10.1111/aogs.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F, Dela Cruz CS, Wang Y, Wu C, Xiao Y, Zhang L (2020) Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis 21 [DOI] [PMC free article] [PubMed]
- 51.Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA (2020) Placental pathology in COVID-19. medRxiv. 1 [DOI] [PMC free article] [PubMed]
- 52.Wang S, Guo L, Chen L, et al. (2020) A case report of neonatal COVID-19 infection in China. Clin Infect Dis :ciaa225 (e-pub ahead of print). 10.1093/cid/ciaa225
- 53.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C (2020) Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 24 [DOI] [PMC free article] [PubMed]
- 54.Cavicchiolo ME, Trevisanuto D, Lolli E, Mardegan V, Saieva AM, Franchin E, Plebani M, Donato D, Baraldi E (2020) Universal screening of high-risk neonates, parents, and staff at a neonatal intensive care unit during the SARS-CoV-2 pandemic [published online ahead of print, 2020 Aug 7]. Eur J Pediatr 1–7. 10.1007/s00431-020-03765-7 [DOI] [PMC free article] [PubMed]
- 55.Chandrasekharan P, Vento M, Trevisanuto D, Partridge E, Underwood MA, Wiedeman J, Katheria A, Lakshminrusimha S (2020) Neonatal resuscitation and postresuscitation care of infants born to mothers with suspected or confirmed SARS-CoV-2 infection. Am J Perinatol 8 [DOI] [PMC free article] [PubMed]
- 56.Yeo KT, Oei JL, De Luca D, et al. (2020) Review of guidelines and recommendations from 17 countries highlights the challenges that clinicians face caring for neonates born to mothers with COVID-19 [published online ahead of print, 2020 Jul 27]. Acta Paediatr. 10.1111/apa.15495 [DOI] [PubMed]
- 57.Centorrino R, Dell'Orto V, Gitto E, Conti G, De Luca D. Mechanics of nasal mask-delivered HFOV in neonates: a physiologic study. Pediatr Pulmonol. 2019;54(8):1304–1310. doi: 10.1002/ppul.24358. [DOI] [PubMed] [Google Scholar]
- 58.Chan MT, Chow BK, Lo T, Ko FW, Ng SS, Gin T, Hui DS. Exhaled air dispersion during bag-mask ventilation and sputum suctioning-implications for infection control. Sci Rep. 2018;8(1):1–8. doi: 10.1038/s41598-017-17765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Luca D. Managing neonates with respiratory failure due to SARS-CoV-2. Lancet Child Adolesc Health. 2020;4(4):e8. doi: 10.1016/S2352-4642(20)30073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Luca D, van Kaam AH, Tingay DG, et al. The Montreux definition of neonatal ARDS: biological and clinical background behind the description of a new entity. Lancet Respir Med. 2017;5(8):657–666. doi: 10.1016/S2213-2600(17)30214-X. [DOI] [PubMed] [Google Scholar]
- 61.Simonds AK, Hanak A, Chatwin M, Morrell M, Hall A, Parker KH, Siggers JH, Dickinson RJ. Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebuliser treatment and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airborne infections. Health Technol Assess (Winchester, England) 2010;14(46):131–172. doi: 10.3310/hta14460-02. [DOI] [PubMed] [Google Scholar]
- 62.Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15-day-old neonate with clinical signs of sepsis, a case report. Infect Dis Ther. 2020;30:1–3. doi: 10.1080/23744235.2020.1747634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Díaz CA, Maestro ML, Pumarega MT, Antón BF, Alonso CP (2020) First case of neonatal infection due to COVID 19 in Spain. Anales de Pediatría (English Edition). 1 [DOI] [PMC free article] [PubMed]
- 64.Zhang ZJ, Yu XJ, Fu T, Liu Y, Jiang Y, Yang BX, Bi Y (2020) Novel coronavirus infection in newborn babies under 28 days in China. Eur Respir J 1 [DOI] [PMC free article] [PubMed]
- 65.Ferrazzi E, Frigerio L, Savasi V, Vergani P, Prefumo F, Barresi S, Bianchi S, Ciriello E, Facchinetti F, Gervasi MT, Iurlaro E. Mode of delivery and clinical findings in COVID-19 infected pregnant women in Northern Italy [DOI] [PMC free article] [PubMed]
- 66.Salvatori G, De Rose DU, Concato C, Alario D, Olivini N, Dotta A, Campana A (2020) Managing COVID-19-positive maternal–infant dyads: an Italian experience. Breastfeed Med 21 [DOI] [PubMed]
- 67.Nie R, Wang SS, Yang Q, Fan CF, Liu YL, He WC, Jiang M, Liu CC, Zeng WJ, Wu JL, Oktay K (2020) Clinical features and the maternal and neonatal outcomes of pregnant women with coronavirus disease 2019. medRxiv. 1
- 68.Khan S, Jun L (2020) Nawsherwan, et al. Association of COVID-19 infection with pregnancy outcomes in healthcare workers and general women. Clin Microbiol Infect S1198-743X(20) 30180–4 (e-pub ahead of print). 10.1016/j.cmi.2020.03.034 [DOI] [PMC free article] [PubMed]
- 69.Hantoushzadeh S, Shamshirsaz AA, Aleyasin A, Seferovic MD, Aski SK, Arian SE, Pooransari P, Ghotbizadeh F, Aalipour S, Soleimani Z, Naemi M (2020) Maternal death due to COVID-19 disease. Am J Obstet Gynecol 28 [DOI] [PMC free article] [PubMed]
- 70.Yan J, Guo J, Fan C, Juan J, Yu X, Li J, Feng L, Li C, Chen H, Qiao Y, Lei D (2020) Coronavirus disease 2019 (COVID-19) in pregnant women: a report based on 116 cases. Am J Obstet Gynecol 23 [DOI] [PMC free article] [PubMed]
- 71.Fan C, Lei D, Fang C, et al. (2020) Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clin Infect Dis :ciaa226 (e-pub ahead of print). 10.1093/cid/ciaa226
- 72.Xu L, Yang Q, Shi H, Lei S, Liu X, Zhu Y, Wu Q, Ding X, Tian Y, Hu Q, Chen F (2020) Clinical presentations and outcomes of SARS-CoV-2 infected pneumonia in pregnant women and health status of their neonates. Sci Bull 28 [DOI] [PMC free article] [PubMed]
- 73.Lu Zhang, Lan Dong, Lei Ming et al. Severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) infection during late pregnancy: a report of 18 patients from Wuhan, China, 28 April 2020, PREPRINT (Version 2) available at Research Square [+10.21203/rs.3.rs-18247/v2+] [DOI] [PMC free article] [PubMed]
- 74.Yang P, Wang X, Liu P, Wei C, He B, Zheng J, Zhao D. Clinical characteristics and risk assessment of newborns born to mothers with COVID-19. J Clin Virol. 2020;10:104356. doi: 10.1016/j.jcv.2020.104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Y, Zhao R, Zheng S, Chen X, Wang J, Sheng X, Zhou J, Cai H, Fang Q, Yu F, Fan J Early release-lack of vertical transmission of severe acute respiratory syndrome coronavirus 2. China [DOI] [PMC free article] [PubMed]
- 76.Lee DH, Lee J, Kim E, Woo K, Park HY, An J (2020) Emergency caesarean section on severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) confirmed patient. Korean J Anesthesiol (e- pub ahead of print). 10.4097/kja.20116 [DOI] [PMC free article] [PubMed]
- 77.Gidlöf S, Savchenko J, Brune T, Josefsson H (2020) COVID-19 in pregnan- cy with comorbidities: more liberal testing strategy is needed. Acta Obstet Gynecol Scand (e-pub ahead of print). 10.1111/aogs.13862 [DOI] [PubMed]
- 78.Lowe B, Bopp B (2020) COVID-19 vaginal delivery–a case report. Aust N Z J Obstet Gynaecol 15 [DOI] [PMC free article] [PubMed]
- 79.Song L, Xiao W, Ling K, Yao S, Chen X. Anesthetic management for emergent cesarean delivery in a parturient with recent diagnosis of coronavirus disease 2019 (COVID-19): a case report. Transl Perioper Pain Med. 2020;7:234–237. [Google Scholar]
- 80.Chen S, Liao E, Shao Y (2020) Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol 28 [DOI] [PMC free article] [PubMed]
- 81.Sharma KA, Kumari R, Kachhawa G, Chhabra A, Agarwal R, Sharma A, Bhatla N (2020) Management of the first patient with confirmed COVID-19 in pregnancy in India: from guidelines to frontlines. Int J Gynecol Obstet 23 [DOI] [PMC free article] [PubMed]
- 82.Khan S, Peng L, Siddique R, Nabi G, Xue M, Liu J, Han G. Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infect Control Hosp Epidemiol. 2020;19:1–3. doi: 10.1017/ice.2020.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khasawneh W, Khassawneh M, Zaghal LA, Hayajneh W, Abdelal F. The first Jordanian newborn delivered to COVID-19 infected mother with no evidence of vertical transmission: A case report
- 84.Lyra J, Valente R, Rosário M, Guimarães M (2020) Cesarean section in a pregnant woman with COVID-19: first case in Portugal. Acta Medica Port 20;33(13) [DOI] [PubMed]
- 85.Kalafat E, Yaprak E, Cinar G, Varli B, Ozisik S, Uzun C, Azap A, Koc A (2020) Lung ultrasound and computed tomographic findings in pregnant woman with COVID-19. Ultrasound Obstet Gynecol 6 [DOI] [PubMed]
- 86.Xiong X, Wei H, Zhang Z, Chang J, Ma X, Gao X, Chen Q, Pang Q (2020) Vaginal delivery report of a healthy neonate born to a convalescent mother with COVID19. J Med Virol 10 [DOI] [PMC free article] [PubMed]
- 87.National Registry for Surveillance and Epidemiology of Perinatal COVID-19 Infection: NPC-19 registry Info:https://collaborate.aap.org/SONPM/Pages/National-Perinatal-COVID19-Registry.aspx: updated on 15/05/2020
- 88.de Wit M, Peelen LM, van Wolfswinkel L, de Graaff JC. The incidence of postoperative respiratory complications: a retrospective analysis of cuffed vs uncuffed tracheal tubes in children 0-7 years of age. Paediatr Anaesth. 2018;28(3):210–217. doi: 10.1111/pan.13340. [DOI] [PubMed] [Google Scholar]
- 89.Thomas RE, Rao SC, Minutillo C, Hullett B, Bulsara MK. Cuffed endotracheal tubes in infants less than 3 kg: a retrospective cohort study. Paediatr Anaesth. 2018;28(3):204–209. doi: 10.1111/pan.13311. [DOI] [PubMed] [Google Scholar]
- 90.Committee on Fetus and Newborn American Academy of Pediatrics Respiratory support in preterm infants at birth. Pediatrics. 2014;133(1):171–174. doi: 10.1542/peds.2013-3442. [DOI] [PubMed] [Google Scholar]
- 91.Bellini C (2020) COVID-19 outbreak impact on neonatal emergency transport. Pediatr Res 23. 10.1038/s41390-020-1027-y [DOI] [PubMed]
- 92.Cavicchiolo ME, Doglioni N, Ventola MA, Biban P, Baraldi E, Trevisanuto D (2020) Neonatal emergency transport system during COVID-19 pandemic in the Veneto region: proposal for standard operating procedures. Pediatr Res 7. [DOI] [PubMed]
- 93.Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323(23):2427–2429. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Valiathan R, Ashman M, Asthana D. Effects of ageing on the immune system: infants to elderly. Scand J Immunol. 2016;83(4):255–266. doi: 10.1111/sji.12413. [DOI] [PubMed] [Google Scholar]
- 95.De Luca D, Rava L, Nadel S, et al. The EPICENTRE (ESPNIC Covid pEdiatric neonatal registry) initiative: background and protocol for the international SARS-CoV-2 infections registry. Eur J Pediatr. 2020;179(8):1271–1278. doi: 10.1007/s00431-020-03690-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 219 kb)
Data Availability Statement
NA