Abstract
Background
As transcatheter aortic valve replacement (TAVR) is expected to progress into younger patient populations, valve‐in‐TAVR (ViTAVR) may become a frequent consideration. Data on ViTAVR, however, are limited. This study investigated the outcome of ViTAVR in comparison to valve in surgical aortic valve replacement (ViSAVR), because ViSAVR is an established procedure for higher‐risk patients requiring repeated aortic valve intervention.
Methods and Results
Clinical and procedural data of patients who underwent ViTAVR at 3 sites in the United States and Germany were retrospectively compared with data of patients who underwent ViSAVR at Cedars‐Sinai Medical Center, according to Valve Academic Research Consortium‐2 criteria. A total of 99 consecutive patients, 52.5% women, with a median Society of Thoracic Surgeons score of 7.2 were identified. Seventy‐four patients (74.7%) underwent ViSAVR, and 25 patients (25.3%) underwent ViTAVR. Balloon‐expandable devices were used in 72.7%. ViSAVR patients presented with smaller index devices (21.0 versus 26.0 mm median true internal diameter; P<0.001). Significantly better postprocedural hemodynamics (median prosthesis mean gradient, 12.5 [interquartile range, 8.8–16.2] versus 16.0 [interquartile range, 13.0–20.5] mm Hg; P=0.045) were observed for ViTAVR compared with the ViSAVR. Device success, however, was not different (79.2% and 66.2% for ViTAVR and ViSAVR, respectively; P=0.35), as were rates of permanent pacemaker implantation (16.7% versus 5.4%; P=0.1). One‐year‐mortality was 9.4% and 13.4% for ViTAVR and ViSAVR, respectively (log‐rank P=0.38).
Conclusions
Compared with ViSAVR, ViTAVR provides acceptable outcomes, with slightly better hemodynamics, similar device success rates, and similar 1‐year mortality.
Keywords: aortic surgery, aortic valve replacement, structural valve deteriotation, transcutaneous aortic valve implantation, valve‐in‐valve
Subject Categories: Valvular Heart Disease, Aortic Valve Replacement/Transcather Aortic Valve Implantation, Catheter-Based Coronary and Valvular Interventions, Treatment, Cardiovascular Surgery
Nonstandard Abbreviations and Acronyms
- AR
aortic regurgitation
- AV
aortic valve
- PVL
paravalvular leakage
- SAVR
surgical aortic valve replacement
- TAVR
transcatheter aortic valve replacement
- ViSAVR
valve‐in‐SAVR
- ViTAVR
valve‐in‐TAVR
- ViV
valve‐in‐valve
Clinical Perspective
What Is New?
This is the first study comparing valve in transcatheter aortic valve replacement (TAVR) with valve in surgical aortic valve replacement.
Although valve‐in‐TAVR seems to result in slightly superior hemodynamics, rates of residual gradients >19 mm Hg, and thus device success, as well as 1‐year mortality do not differ compared with valve in surgical aortic valve replacement.
What Are the Clinical Implications?
Considering the increasing number of patients treated using TAVR, valve‐in‐TAVR may potentially serve as a valuable treatment option for degenerated TAVR devices.
In vitro experiments that address hemodynamic aspects of valve‐in‐TAVR should be promoted.
Isolated redo surgery for degenerated surgical bioprosthetic aortic valves (AV) accounts for ≈6.9% of all AV procedures.1 Redo surgery in intermediate‐risk patients can be performed with acceptable clinical outcomes. Compared with primary surgical aortic valve replacement (SAVR), however, redo SAVR is associated with increased 30‐day mortality, postoperative stroke, and pacemaker implantation.2 Because of increased age and risk profile, as well as adhesions related to the previous procedure, redo SAVR patients are generally at higher surgical risk and may therefore be denied for repeated intervention. Following conceptualization using an animal model in 2007, transcatheter valve‐in‐valve (ViV) for failed surgical bioprosthetic AVs (valve‐in‐SAVR [ViSAVR]) has gained attention as a therapeutic option for patients at high or prohibitive surgical risk.3 ViSAVR is considered safe, effective, and reproducible.4, 5, 6, 7
Bioprostheses are prone to structural valve degeneration, resulting in limited long‐term durability.8 Although yet unproved, similar processes are also likely to occur in transcatheter aortic valve replacement (TAVR) prostheses. As TAVR has proved to provide at least similar clinical outcomes compared with SAVR for a range of patients, including younger and lower‐risk populations, structural valve degeneration will also be more frequently observed in TAVR patients.9, 10 As infective endocarditis following TAVR is rare (incidences of 1.1%–1.8% per patient‐year),11, 12 valve‐in‐TAVR (ViTAVR) might become a frequent consideration for failed TAVR devices. However, limited data exist for TAVR as ViV for patients with degenerated TAVR valves.13 Furthermore, to the best of our knowledge, no data exist to compare ViTAVR against ViSAVR with regard to clinical and hemodynamic outcome.
Methods
The analytical methods used in this study are available from the first author on request.
Study Population
For this study, baseline, procedural, and clinical data of patients who underwent repeated intervention for degenerated TAVR or SAVR between November 4, 2009, and May 10, 2018, were retrospectively collected. Data on ViTAVR were collected at 3 sites in the United States and Germany (Cedars‐Sinai Medical Center, Los Angeles, CA; Heart Center Leipzig, Leipzig, Germany; and Heart Center Dresden, Dresden, Germany). Within this study period, n=15 patients underwent ViTAVR for paravalvular leakage (PVL). These patients are excluded from this analysis. For the ViSAVR group, data of 2 widely used SAVR devices were retrospectively collected for consecutive patients at Cedars‐Sinai Medical Center (Carpentier Edwards Perimount, Edwards Lifesciences, CA: n=56 [75.7%]; and Medtronic Mosaic, Medtronic, Minneapolis, MN: n=18 [24.3%]).
Devices
In the ViTAVR group, valve models at index procedure included the following: CoreValve (Medtronic; n=5 [20%]); Sapien XT (Edwards Lifesciences Corp, Irvine, CA; n=6 [24%]); Sapien (n=10 [40%]); Acurate neo (Boston Scientific, Marlborough, MA; n=1 [4%]); Sapien 3 (Edwards Lifesciences; n=1); JenaValve (JenaValve Technology GmbH, Munich, Germany; n=1); and Ventor (Medtronic; n=1).
Indication for ViV was categorized on the basis of the mode of prosthesis dysfunction, as follows: (1) aortic stenosis: mean prosthesis gradient >19 mm Hg, aortic regurgitation (AR) < moderate, and PVL < moderate; (2) AR: transvalvular AR > mild, mean prosthesis gradient <20 mm Hg, PVL < moderate; (3) PVL > mild; (4) combined: mean prosthesis gradient >19 mm Hg, AR > mild. Prosthesis stenosis was defined as being severe for mean prosthesis gradient >40 mm Hg.
Valve sizes were defined on the basis of true internal diameter.14
End Point Definitions
The primary outcome was all‐cause 1‐year mortality, and the composite end points of device success and early safety were based on the Valve Academic Research Consortium‐2 criteria.15 The study hypothesis was that ViTAVR is superior to ViSAVR in terms of the primary outcome. Device success was defined as follows: (1) absence of procedural mortality; (2) correct valve positioning without requirement for second ViV; and (3) adequate prosthesis performance on the basis of mean postprocedural gradient <20 mm Hg and grade of prosthetic regurgitation < moderate. Early safety at 30 days was assessed using all‐cause mortality, stroke (disabling and nondisabling), life‐threatening bleeding, acute kidney injury (stage 2 or 3; including renal replacement therapy), coronary artery obstruction requiring intervention, major vascular complication, and valve‐related dysfunction requiring repeated procedure. Conduction disturbances and arrhythmia were summarized using a combined end point of new arrhythmia based on Valve Academic Research Consortium‐2 criteria or new permanent pacemaker implantation within 30 days. Hemodynamic outcome was assessed at 1 and 30 days and was 96% and 66.7% complete, respectively. Retrospective data collection was approved by the ethic committees of the respective sites, and need for individual patient consent was waived.
Statistical Analysis
Normality of data was evaluated using Shapiro‐Wilk tests. Continuous variables of normal distribution were compared using Student t test and are shown as mean±SD. Continuous variables without normal distribution were compared using Wilcoxon rank‐sum or Kruskal‐Wallis test and are shown as median±interquartile range (IQR). Categorical variables were compared using χ2 or exact Fisher test (for expected frequencies <5) and are shown as frequencies and percentages. Survival curves were analyzed and presented using the Kaplan‐Meier algorithm. Difference between survival was analyzed using log‐rank test. Data analysis followed recommendations of reproducible research using RStudio version 1.1.453 (RStudio Team [2016]; RStudio: Integrated Development for R; RStudio, Inc, Boston, MA; http://www.rstudio.com/).16 The following packages were included: compareGroups, DiagrammeR, dplyr, ggplot2, gridExtra, lubridate, survival, and rmarkdown. 17
Results
Baseline Characteristics
Study Population
During this study period, a total of 8541 patients underwent native TAVR at the 3 centers. A total of 100 ViV patients were identified for this study; 99 patients (52 [52.5%] women) with a median Society of Thoracic Surgeons score of 7.2% (IQR, 4.3%–13.4%) were included. One ViTAVR patient was excluded because of early ViTAVR 3 days following the index procedure. Of 99 ViV patients, 74 (74.7%) underwent ViSAVR and 25 (25.3%) underwent ViTAVR (Figure 1).
Figure 1. Study population.
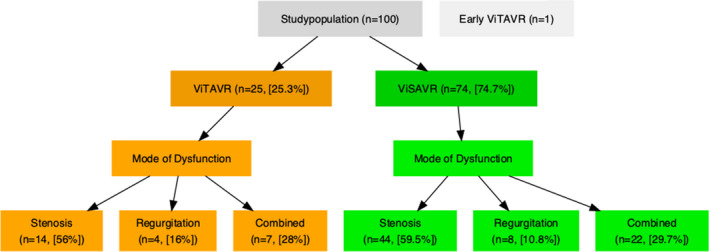
Ninety‐nine individuals were included to compare hemodynamic and clinical outcome of valve in transcatheter aortic valve replacement (ViTAVR; orange) against valve in surgical aortic valve replacement (ViSAVR; green).
ViTAVR patients were, on average, 6 years older (P=0.058) and presented with higher rates of chronic obstructive lung disease (P=0.006; Table 1). Seventeen patients (14.9%) presented with small (ie, true internal valve diameter <19 mm) index devices, all of which were in the ViSAVR group (23% of all valves in the ViSAVR group). Coronary artery disease and prior coronary interventions were common in both groups (combined frequency of percutaneous coronary intervention and coronary artery bypass surgery in ViTAVR and ViSAVR of 48% and 52.7%, respectively).
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | All (N=99) | ViTAVR (N=25) | ViSAVR (N=74) | P Value (Overall) |
|---|---|---|---|---|
| Age, y | 80.0 (73.0–86.0) | 83.0 (80.0–86.0) | 77.0 (69.2–86.0) | 0.058 |
| Women | 0.526 | |||
| No | 47 (47.5) | 10 (40.0) | 37 (50.0) | |
| Yes | 52 (52.5) | 15 (60.0) | 37 (50.0) | |
| STS score | 7.15 (4.31–13.4) | 7.44 (5.65–14.2) | 6.80 (3.74–12.3) | 0.066 |
| NYHA functional class | 0.328 | |||
| II | 3 (3.06) | 1 (4.00) | 2 (2.74) | |
| III | 48 (49.0) | 15 (60.0) | 33 (45.2) | |
| IV | 47 (48.0) | 9 (36.0) | 38 (52.1) | |
| CCS class | 0.118 | |||
| 0 | 78 (80.4) | 18 (72.0) | 60 (83.3) | |
| I | 7 (7.22) | 1 (4.00) | 6 (8.33) | |
| II | 8 (8.25) | 3 (12.0) | 5 (6.94) | |
| III | 3 (3.09) | 2 (8.00) | 1 (1.39) | |
| IV | 1 (1.03) | 1 (4.00) | 0 (0.00) | |
| Diabetes mellitus | 0.115 | |||
| No | 69 (70.4) | 14 (56.0) | 55 (75.3) | |
| Yes | 29 (29.6) | 11 (44.0) | 18 (24.7) | |
| Chronic obstructive lung disease | 0.006 | |||
| No | 64 (64.6) | 10 (40.0) | 54 (73.0) | |
| Yes | 35 (35.4) | 15 (60.0) | 20 (27.0) | |
| Creatinine clearance <60, ml/min | 0.920 | |||
| No | 42 (42.9) | 10 (40.0) | 32 (43.8) | |
| Yes | 56 (57.1) | 15 (60.0) | 41 (56.2) | |
| Previous coronary artery bypass surgery | 0.319 | |||
| No | 61 (61.6) | 18 (72.0) | 43 (58.1) | |
| Yes | 38 (38.4) | 7 (28.0) | 31 (41.9) | |
| Previous PCI | 0.304 | |||
| No | 86 (86.9) | 20 (80.0) | 66 (89.2) | |
| Yes | 13 (13.1) | 5 (20.0) | 8 (10.8) | |
| Previous TIA or stroke | 1.000 | |||
| No | 77 (77.8) | 19 (76.0) | 58 (78.4) | |
| Yes | 22 (22.2) | 6 (24.0) | 16 (21.6) | |
| Permanent aFib | 0.580 | |||
| No | 62 (62.6) | 14 (56.0) | 48 (64.9) | |
| Yes | 37 (37.4) | 11 (44.0) | 26 (35.1) | |
| Permanent pacemaker or defibrillator | 1.000 | |||
| No | 77 (79.4) | 20 (80.0) | 57 (79.2) | |
| Yes | 20 (20.6) | 5 (20.0) | 15 (20.8) | |
| Ejection fraction, % | 60.0 (45.0–65.5) | 59.0 (55.0–69.0) | 60.0 (44.0–65.0) | 0.354 |
| AV mean gradient, mm Hg | 40.0 (26.0–49.5) | 40.0 (24.0–62.0) | 40.0 (26.2–47.8) | 0.939 |
| AV peak gradient, mm Hg | 64.0 (45.2–84.8) | 63.0 (46.0–105) | 66.0 (45.0–84.0) | 0.938 |
| AR > mild | 0.797 | |||
| No | 57 (58.2) | 15 (62.5) | 42 (56.8) | |
| Yes | 41 (41.8) | 9 (37.5) | 32 (43.2) | |
| MR > mild | 0.354 | |||
| No | 61 (62.2) | 18 (72.0) | 43 (58.9) | |
| Yes | 37 (37.8) | 7 (28.0) | 30 (41.1) | |
| Index AVR <19.0 mm true ID | 0.005 | |||
| No | 82 (82.8) | 25 (100) | 57 (77.0) | |
| Yes | 17 (17.2) | 0 (0.00) | 17 (23.0) |
Values are shown as median (interquartile range) for continuous variables and number (percentage) for categorical variables. Group differences are presented as overall P value. aFib indicates atrial fibrillation; AR, aortic regurgitation; AV, aortic valve; AVR, AV replacement; CCS, Canadian Cardiovascular Society; ID, internal diameter; MR, mitral regurgitation; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons; TIA, transient ischemic attack; ViSAVR, valve in surgical AVR; and ViTAVR, valve in transcatheter AVR.
For ViTAVR, 88% of the index devices were either balloon‐expandable Sapien (77.3%; n=17) or self‐expanding CoreValve prostheses (22.7%; n=5).
Indication for and Time to ViV
The median time between procedures was shorter for ViTAVR compared with ViSAVR (3.2 versus 10 years; P<0.001; Table 2). The indication for ViV was almost identical for both groups (Figure S1). Average baseline prosthesis mean gradients were not different between primary AV replacement device categories (Figure 2). Prosthesis stenosis was present in 84.8% (n=84) of the study population. In these patients, the rate of severe prosthesis stenosis (mean prosthesis gradient >40 mm Hg) was 56% (n=47). Two patients presented with low‐flow low‐gradient prosthesis stenosis. In one ViTAVR patient, device dysfunction included both stenosis and PVL. For the remaining patients, indication for ViV could be categorized singularly as aortic stenosis, AR, or combined.
Table 2.
Procedural Characteristics
| Characteristic | ViTAVR (N=25) | ViSAVR (N=74) | P Value (Overall) | N |
|---|---|---|---|---|
| Time since index procedure, y | <0.001 | 55 | ||
| <5 | 18 (72.0) | 5 (16.7) | ||
| 5–10 | 7 (28.0) | 10 (33.3) | ||
| >10 | 0 (0.00) | 15 (50.0) | ||
| Indication for ViV | 0.754 | 99 | ||
| AR | 4 (16.0) | 8 (10.8) | ||
| AS | 14 (56.0) | 44 (59.5) | ||
| Combined | 7 (28.0) | 22 (29.7) | ||
| ViV access | 0.455 | 98 | ||
| Transapical | 4 (16.7) | 7 (9.46) | ||
| Transfemoral | 20 (83.3) | 67 (90.5) | ||
| ViV predilation | <0.001 | 99 | ||
| No | 15 (60.0) | 73 (98.6) | ||
| Yes | 10 (40.0) | 1 (1.35) | ||
| ViV device | 0.226 | 99 | ||
| CoreValve | 7 (28.0) | 12 (16.2) | ||
| CoreValve Evolut R | 3 (12.0) | 3 (4.05) | ||
| Portico | 0 (0.00) | 2 (2.70) | ||
| Sapien | 3 (12.0) | 17 (23.0) | ||
| Sapien XT | 3 (12.0) | 19 (25.7) | ||
| Sapien3 | 9 (36.0) | 21 (28.4) | ||
| ViV device self‐expandable | 0.164 | 99 | ||
| Yes | 10 (40.0) | 17 (23.0) | ||
| No | 15 (60.0) | 57 (77.0) | ||
| ViV size, mm | 26.0 (26.0–26.0) | 23.0 (23.0–26.0) | <0.001 | 99 |
| Postdilatation | 0.547 | 98 | ||
| No | 16 (66.7) | 56 (75.7) | ||
| Yes | 8 (33.3) | 18 (24.3) | ||
| Postprocedural gradient, mm Hg | 0.349 | 95 | ||
| <20 | 19 (79.2) | 47 (66.2) | ||
| >19 | 5 (20.8) | 24 (33.8) | ||
| Post‐ViV PVL > mild | 0.570 | 97 | ||
| No | 25 (100) | 68 (94.4) | ||
| Yes | 0 (0.00) | 4 (5.56) | ||
| Device success | 0.415 | 95 | ||
| Accomplished | 19 (79.2) | 48 (67.6) | ||
| Not accomplished | 5 (20.8) | 23 (32.4) |
Values are shown as median (interquartile range) for continuous variables and number (percentage) for categorical variables. Group differences are presented as overall P value. AR indicates aortic regurgitation; AS, aortic stenosis; PVL, paravalvular leakage; ViSAVR, valve in surgical aortic valve replacement; ViTAVR, valve in transcatheter aortic valve replacement; and ViV, valve‐in‐valve.
Figure 2. Baseline mean gradient depending on type of index aortic valve replacement (AVR) device.
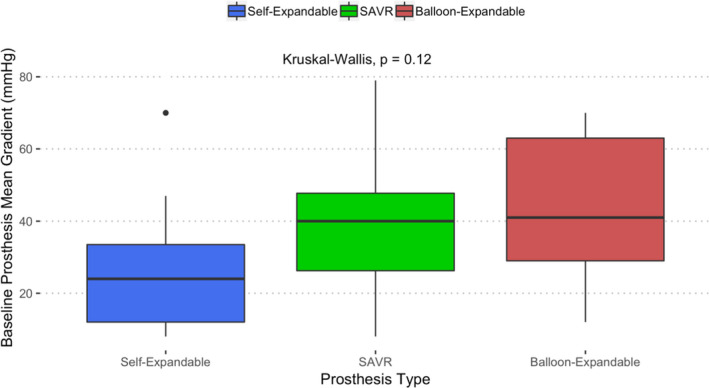
Index AVR devices categorized into self‐expandable (blue), balloon expandable (red), and surgical AVR (SAVR) (green). Kruskal‐Wallis test for between‐group differences.
Procedural Characteristics and Device Success
ViV was performed via the transfemoral route in 87.9% of the patients (Table 2). There was no difference in ViV devices used (Figure S2). The rates of balloon‐ and self‐expandable ViV devices were not different between groups (Figure 3). ViV size was larger in the ViTAVR group (P<0.001), with similar rates of postdilatation. When stratifying for the type of the ViV device used, higher rates of predilation in the ViTAVR group were found only for balloon‐expandable ViV devices (P<0.001).
Figure 3. Type of transcatheter aortic valve replacement device used.
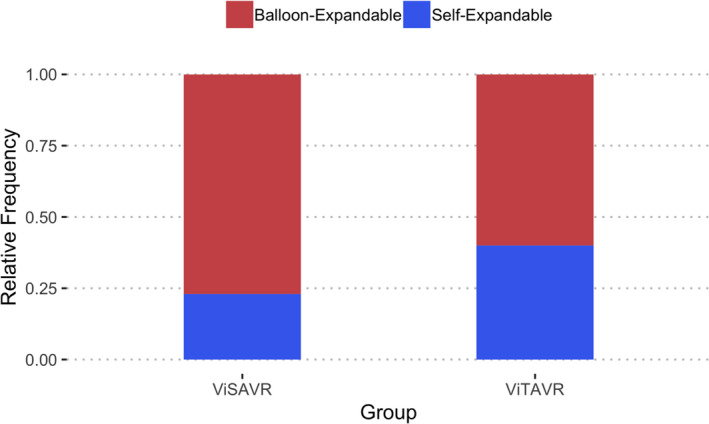
Comparison of valve‐in‐valve devices used in the valve in surgical aortic valve replacement (ViSAVR) (left) and the valve in transcatheter aortic valve replacement (ViTAVR) (right) groups.
Procedural mortality was zero for all procedures. Valve positioning using a single device was accomplished in 100% and 97.3% for ViTAVR and ViSAVR, respectively (P=0.54). Paravalvular regurgitation > mild at day 1 was documented in 2 ViSAVR patients (compared with n=0 in ViTAVR). The overall incidence of residual mean gradients >19 mm Hg at day 1 was 30.5%. The rate of residual gradients was not different between groups. Consequently, device success was not different beween groups.
Early Safety and Clinical Efficacy
Hospital stay was significantly longer for ViTAVR patients (Table 3). Thirty‐day survival for ViTAVR and ViSAVR was 96% and 94.6%, respectively (P=1.0). One ViSAVR patient died because of a procedure‐related ventricular septum defect that could not be closed percutaneously. The remaining 3 ViSAVR patients died because of non–valve‐related causes. One ViTAVR patient died following discharge to a community hospital because of unknown causes. For the survivors, the incidence of acute kidney injury, cerebrovascular event, and major bleeding within 30 days was not different between groups (overall incidences of 1%, 2%, and 3%, respectively).
Table 3.
Clinical Outcome
| Variable | ViTAVR (N=25) | ViSAVR (N=74) | P Value (Overall) | N |
|---|---|---|---|---|
| Day 1 ejection fraction, % | 60.0 (50.0–65.0) | 60.0 (47.0–65.0) | 0.631 | 96 |
| Day 1 PVL > mild | 1.000 | 97 | ||
| No | 25 (100) | 70 (97.2) | ||
| Yes | 0 (0.00) | 2 (2.78) | ||
| Day 1 ViV mean gradient, mm Hg | 15.0 (11.8–17.5) | 16.0 (11.0–21.0) | 0.370 | 95 |
| Day 1 ViV peak gradient, mm Hg | 26.5 (19.8–36.2) | 29.0 (20.5–38.0) | 0.249 | 95 |
| Postprocedural gradient category | 0.572 | 95 | ||
| Normal | 19 (79.2) | 52 (73.2) | ||
| 20–24 mm Hg | 4 (16.7) | 9 (12.7) | ||
| 25–29 mm Hg | 1 (4.17) | 4 (5.63) | ||
| >29 mm Hg | 0 (0.00) | 6 (8.45) | ||
| Hospital stay, d | 9.00 (4.00–16.0) | 3.00 (2.00–6.00) | <0.001 | 93 |
| Death (hospital) | 1.000 | 99 | ||
| No | 24 (96.0) | 70 (94.6) | ||
| Yes | 1 (4.00) | 4 (5.41) | ||
| New permanent pacemaker implantation or major arrhythmia | 0.011 | 78 | ||
| No | 13 (68.4) | 55 (93.2) | ||
| Yes | 6 (31.6) | 4 (6.78) | ||
| Day 30 ejection fraction, % | 59.0 (51.0–65.5) | 61.0 (54.0–66.0) | 0.810 | 67 |
| Day 30 PVL > mild | 1.000 | 69 | ||
| No | 16 (100) | 51 (96.2) | ||
| Yes | 0 (0.00) | 2 (3.77) | ||
| Day 30 ViV mean gradient, mm Hg | 12.5 (8.75–16.2) | 16.4 (13.0–20.2) | 0.043 | 68 |
| Day 30 ViV peak gradient, mm Hg | 24.0 (17.2–29.8) | 29.5 (23.8–36.0) | 0.059 | 66 |
| Postprocedural gradient at 30 d, mm Hg | 0.201 | 68 | ||
| <20 | 14 (87.5) | 35 (67.3) | ||
| >19 | 2 (12.5) | 17 (32.7) |
Values are shown as median (interquartile range) for continuous variables and number (percentage) for categorical variables. Group differences are presented as overall P value. PVL indicates paravalvular leakage; ViSAVR, valve in surgical aortic valve replacement; ViTAVR, valve in transcatheter aortic valve replacement; and ViV, valve‐in‐valve.
There were no incidences of coronary obstruction requiring reintervention, major vascular complication, and valve‐related dysfunction requiring repeated procedure. Consequently, early safety was 91.9% in the entire cohort (91.9% and 92% for ViSAVR and ViTAVR, respectively; P=1.0). There were higher rates of the combined end point of new major arrhythmia or new permanent pacemaker implantation within 30 days in the ViTAVR group (31.6% versus 6.8% for ViTAVR and ViSAVR, respectively; P=0.01). All but one pacemaker implantation in the ViTAVR patients was because of high‐degree AV block; one patient received a pacemaker following ViTAVR because of preexisting sick‐sinus syndrome; one patient showed new, hemodynamically significant atrial fibrillation. Considering only pacemaker implantation for higher‐degree AV block group, rates of pacemaker implantation for ViTAVR were not statistically different compared with ViSAVR (16.7% versus 5.4%; P=0.1). When stratifying for the type of the ViV device used, higher rates of permanent pacemaker implantation in the ViTAVR group (42.9% versus 0%; P=0.026) were only observed for self‐expandable ViV devices.
Mean transvalvular gradients at 30 days were lower for ViTAVR compared with ViSAVR (12.5 [IQR, 8.75–16.2] versus 16.4 [IQR, 13.0–20.2] mm Hg; P=0.043). Differences in peak transvalvular gradients did not reach statistical significance (24.0 [IQR, 17.2–29.8] versus 29.5 [IQR, 23.8–36.0] mm Hg; P=0.059) (Figure 4). Repeated measures analysis revealed the following: (1) no differences between groups, (2) a significant decrease of transvalvular gradients over time (P<0.001 for both mean and peak AV gradient), (3) a significant change in depression of transvalvular gradients within subjects over time (P<0.001 for both mean and peak AV gradient), and (4) no significant interaction between the variables time and primary AV replacement device.
Figure 4. Hemodynamic outcome.
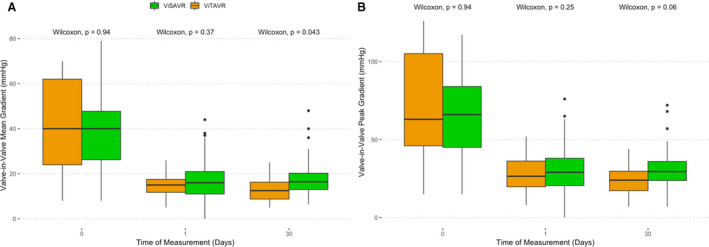
A, Valve‐in‐valve mean gradients. B, Valve‐in‐valve peak gradients. Green=valve in surgical aortic valve replacement (ViSAVR), and orange=valve in transcatheter aortic valve replacement (ViTAVR). See Results section with regard to repeated‐measures analysis.
For ViTAVR, there were no differences with respect to hemodynamic outcome between balloon‐ and self‐expandable ViV or primary AV replacement prostheses. Also, similar postprocedural gradients were observed in the ViSAVR group for small compared with large index devices (P=0.12; Figure S3). All‐cause 1‐year‐mortality was 9.4% and 13.4% for the ViTAVR and ViSAVR groups, respectively (log‐rank P=0.42; Figure 5). All but one death following discharge were non–cardiac related. One cardiac death was observed, which was not valve related.
Figure 5. One‐year‐survival.
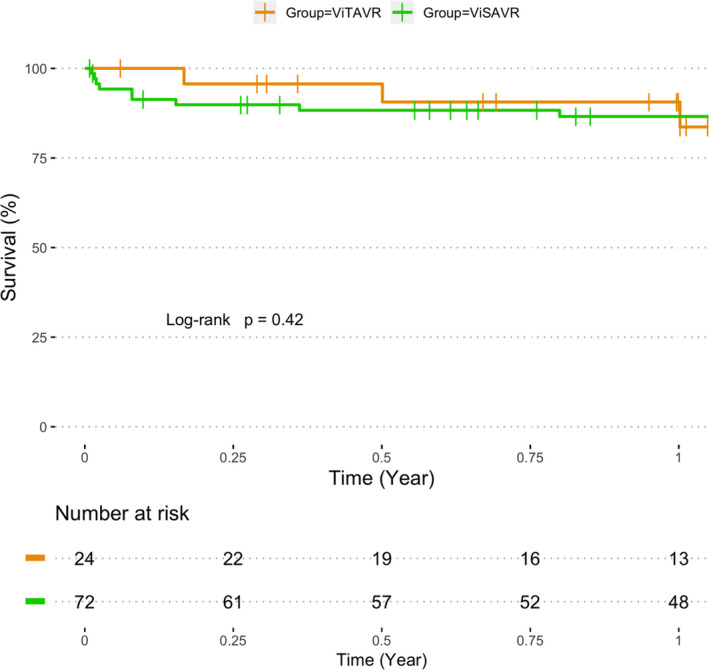
Survival curve of cumulative 1‐year all‐cause mortality for valve in surgical aortic valve replacement (ViSAVR) (green) and valve in transcatheter aortic valve replacement (ViTAVR) (orange).
Discussion
This is the first study to compare ViV for degenerated surgical aortic bioprostheses in patients at high risk for repeated surgery against ViV for degenerated TAVR prostheses. According to Valve Academic Research Consortium‐2 clinical end points, the main findings are the following: (1) ViTAVR is safe, with low incidence of periprocedural complications; (2) ViTAVR is effective in improving AV function, with low incidences of PVL; (3) ViTAVR provides slightly superior hemodynamic outcome, but similar rates of residual gradients >19 mm Hg; and (4) ViTAVR provides similar 1‐year all‐cause mortality compared with ViSAVR, despite increased surgical risk.
ViTAVR and ViSAVR Were Performed on Comparbale Study Populations
Data on ViSAVR demonstrate a decline of predicted surgical risk from 11.6% in 2011 to 7.4% in 2017. 4 , 5 , 7 , 19 , 20 , 21 , 22 This is in line with our study that reports on intermediate‐ to high‐risk patients. By nature of the index procedure, the gap of predicted risk between ViTAVR and ViSAVR is likely going to persist, because both TAVR and bioprosthetic SAVR shift toward younger patients. 23
Significant AR following TAVR is associated with impaired survival, but is relatively infrequent in current TAVR practice. 24 PVL has been shown to be more frequent for self‐ compared with balloon‐expandable prostheses. 25 This disadvantage for self‐expandable valves seems to diminish for newer‐generation devices (presented by Dr Holger Thiele at the Transcatheter Cardiovascular Therapeutics meeting [TCT 2018], San Diego, CA, September 23, 2018). Consequently, PVL as an indication for ViTAVR may become relatively rare in the future, leading to ViTAVR and ViSAVR being performed in comparable patient populations.
The rate of PVL patients in our initial cohort of ViTAVR patients was close to numbers reported previously.13 The exclusion of PVL patients showed that the indication for ViV becomes almost identical to ViSAVR, which, in turn, is in line with registry data.5, 7 Therefore, TAVR devices scheduled for ViV because of valve deterioration present similar hemodynamic compromises compared with their surgical counterparts. Pure prosthesis regurgitation as the indication for ViV is uncommon, and ≈75% of patients present with prosthesis stenosis.
ViTAVR Provides Slightly Superior Hemodynamics
Increased postprocedural gradients remain the Achilles heel of ViSAVR. It occurs in 26.8% to 37.0% of patients following ViV and is associated with increased mortality at 1 year.7 No data exist for increased gradients following ViTAVR. Thus, our study is the first to demonstrate similar rates of increased postprocedural gradients following ViTAVR. This result is surprising because both the design of TAVR devices and the size of TAVR devices that presented for ViTAVR favored no incidence of residual gradients. However, 3 considerations have to be noted. First, the definition of increased gradients is based on data obtained by ViV for failed surgical aortic bioprosthesis. It is unclear whether a similar cutoff should be applied also for ViTAVR with respect to an association with mortality. Second, the variable of increased gradients categorizes a continuous variable into a binary one, with no further differentiation for even higher gradients. Third, leaflet abnormalities, including thickening and subclinical thrombosis, have been shown to be more frequent in transcatheter compared with surgical aortic bioprostheses. 26 , 27 This could possibly contribute to decreased prosthesis opening area following ViTAVR despite greater index valve sizes and the lack of a surgical sewing ring.
Thus, our study shows a tendency toward improved hemodynamics at 30 days for the ViTAVR compared with the ViSAVR group. As device success depends on the absence of increased residual gradients, however, further in vitro studies are required to deepen our understanding of the causes and contributors of increased gradients following both ViSAVR and ViTAVR.
Increased Rates of Pacemaker Implantation With Similar 1‐Year Survival
ViTAVR is a safe procedure with zero procedural mortality and no incidence of valve malpositioning. Furthermore, it is safe with respect to hospital death, major vascular or cerebrovascular events, acute kidney injury, and major bleeding. However, a combined end point of new arrhythmia or new pacemaker implantation occurred frequently. New pacemaker implantation following native TAVR remains a common procedure even for new‐generation devices. Compared with native TAVR, ViSAVR shows lower rates of permanent pacemaker implantation, in part likely because of a protective ability of the sewing ring toward conductive structures. The incidence of new permanent pacemaker of 5.4% for ViSAVR in our study is in line with numbers previously reported; the incidence of new pacemaker implantation following valve‐deteriorated ViTAVR of 16.7% is higher than reported by Barbanti et al 13 and slightly higher compared with current data on native trileaflet TAVR. Given that ViTAVR places a device into 2 calcified structures (ie, native calcified AV and deteriorated TAVR device), greater amounts of calcified elements compared with ViSAVR pushed radially may be a contributor.
Despite a higher predicted surgical risk, 1‐year survival and clinical outcome were not different for ViTAVR, although longer duration of follow‐up is required in future studies.
Limitations
The main limitation of this study is the retrospective, observational character. Data collection was based on clinical documentation and review of selected variables, leading to inconsistencies with respect to data collection and quality. Second, only 2 surgical aortic bioprostheses were used for comparison collected at a single center. Thus, institutional bias cannot be excluded. Finally, although our study provides the largest series of deteriorated TAVR devices scheduled for ViV compared with ViSAVR, the sample size is still small and our study may therefore be underpowered to detect differences to a statistically significant degree.
Conclusions
For intermediate‐ to high‐risk patients, ViTAVR provides acceptable clinical outcome compared with ViSAVR. Hemodynamic outcome seems slightly better, but device success rates and 1‐year all‐cause mortality may not be different.
Sources of Funding
This study was funded by the Leipzig Heart Institute, Leipzig, Germany, and the Cedars‐Sinai Smidt Heart Institute, Los Angeles, CA.
Disclosures
Dr Chakravarty is proctor and consultant for Edwards LifeSciences. Dr Mangner reports speaker′s honoraria from Edwards and Medtronic and consultant honoraria from Biotronik, outside the submitted work. Dr Borger has received speakers’ honoraria and/or consultation fees from Edwards Lifesciences, Medtronic, Abbott (previous St. Jude Medical), and CryoLife. Dr Makkar has received grant support from Edwards Lifesciences Corporation; is a consultant for Abbott Vascular, Cordis, and Medtronic; and holds equity in Entourage Medical. The remaining authors have no disclosures to report.
Supporting information
Figures S1–S3
Acknowledgments
The authors thank the clinical staff at Heart Center Leipzig and Heart Center Dresden for their help with data collection. The team around Jasminka Stegic is recognized for their dedicated patient care at Cedars‐Sinai Smidt Heart Institute. Finally, the R community is appreciated for providing open‐source tools and voluntary support that enable reproducible research.
(J Am Heart Assoc. 2020;9:e013973 DOI: 10.1161/JAHA.119.013973.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. Kaneko T, Vassileva CM, Englum B, Kim S, Yammine M, Brennan M, Suri RM, Thourani VH, Jacobs JP, Aranki S. Contemporary outcomes of repeat aortic valve replacement: a benchmark for transcatheter valve‐in‐valve procedures. Ann Thorac Surg. 2015;100:1298–1304; discussion 1304. [DOI] [PubMed] [Google Scholar]
- 2. Onorati F, Biancari F, De Feo M, Mariscalco G, Messina A, Santarpino G, Santini F, Beghi C, Nappi G, Troise G, et al. Mid‐term results of aortic valve surgery in redo scenarios in the current practice: results from the multicentre European RECORD (REdo Cardiac Operation Research Database) initiative. Eur J Cardiothorac Surg. 2015;47:269–280. [DOI] [PubMed] [Google Scholar]
- 3. Walther T, Falk V, Dewey T, Kempfert J, Emrich F, Pfannmüller B, Bröske P, Borger MA, Schuler G, Mack M, et al. Valve‐in‐a‐valve concept for transcatheter minimally invasive repeat xenograft implantation. J Am Coll Cardiol. 2007;50:56–60. [DOI] [PubMed] [Google Scholar]
- 4. Dvir D, Webb J, Brecker S, Bleiziffer S, Hildick‐Smith D, Colombo A, Descoutures F, Hengstenberg C, Moat NE, Bekeredjian R, et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve‐in‐valve registry. Circulation. 2012;126:2335–2344. [DOI] [PubMed] [Google Scholar]
- 5. Dvir D, Webb JG, Bleiziffer S, Pasic M, Waksman R, Kodali S, Barbanti M, Latib A, Schaefer U, Rodés‐Cabau J, et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312:162–170. [DOI] [PubMed] [Google Scholar]
- 6. Webb JG, Wood DA, Ye J, Gurvitch R, Masson J‐B, Rodés‐Cabau J, Osten M, Horlick E, Wendler O, Dumont E, et al. Transcatheter valve‐in‐valve implantation for failed bioprosthetic heart valves. Circulation. 2010;121:1848–1857. [DOI] [PubMed] [Google Scholar]
- 7. Webb JG, Mack MJ, White JM, Dvir D, Blanke P, Herrmann HC, Leipsic J, Kodali SK, Makkar R, Miller DC, et al. Transcatheter aortic valve implantation within degenerated aortic surgical bioprostheses: PARTNER 2 valve‐in‐valve registry. J Am Coll Cardiol. 2017;69:2253–2262. [DOI] [PubMed] [Google Scholar]
- 8. Dvir D, Bourguignon T, Otto CM, Hahn RT, Rosenhek R, Webb JG, Treede H, Sarano ME, Feldman T, Wijeysundera HC, et al. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation. 2018;137:388–399. [DOI] [PubMed] [Google Scholar]
- 9. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. Transcatheter aortic‐valve replacement with a balloon‐expandable valve in low‐risk patients. N Engl J Med. 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 10. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, et al. Transcatheter aortic‐valve replacement with a self‐expanding valve in low‐risk patients. N Engl J Med. 2019;380:1706–1715. [DOI] [PubMed] [Google Scholar]
- 11. Mangner N, Woitek F, Haussig S, Schlotter F, Stachel G, Höllriegel R, Wilde J, Lindner A, Holzhey D, Leontyev S, et al. Incidence, predictors, and outcome of patients developing infective endocarditis following transfemoral transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;67:2907–2908. [DOI] [PubMed] [Google Scholar]
- 12. Regueiro A, Linke A, Latib A, Ihlemann N, Urena M, Walther T, Husser O, Herrmann HC, Nombela‐Franco L, Cheema AN, et al. Association between transcatheter aortic valve replacement and subsequent infective endocarditis and in‐hospital death. JAMA. 2016;316:1083–1092. [DOI] [PubMed] [Google Scholar]
- 13. Barbanti M, Webb JG, Tamburino C, Van Mieghem NM, Makkar RR, Piazza N, Latib A, Sinning J‐M, Won‐Keun K, Bleiziffer S, et al. Outcomes of redo transcatheter aortic valve replacement for the treatment of postprocedural and late occurrence of paravalvular regurgitation and transcatheter valve failure. Circ Cardiovasc Interv. 2016;9:e003930. [DOI] [PubMed] [Google Scholar]
- 14. Bapat VN, Attia R, Thomas M. Effect of valve design on the stent internal diameter of a bioprosthetic valve: a concept of true internal diameter and its implications for the valve‐in‐valve procedure. JACC Cardiovasc Interv. 2014;7:115–127. [DOI] [PubMed] [Google Scholar]
- 15. Kappetein AP, Head SJ, Généreux P, Piazza N, Van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es G‐A, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document. Eur Heart J. 2012;33:2403–2418. [DOI] [PubMed] [Google Scholar]
- 16. Gandrud C. Reproducible Research With R and R Studio. CRC Press Taylor & Fancis Group: Chapman; Hall/CRC; 2013. [Google Scholar]
- 17. Subirana I, Sanz H, Vila J. Building bivariate tables: the compareGroupsPackage for R. J Stat Softw. 2014;57:1–16.25400517 [Google Scholar]
- 18. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer New York; 2000. [Google Scholar]
- 19. Eggebrecht H, Schäfer U, Treede H, Boekstegers P, Babin‐Ebell J, Ferrari M, Möllmann H, Baumgartner H, Carrel T, Kahlert P, et al. Valve‐in‐valve transcatheter aortic valve implantation for degenerated bioprosthetic heart valves. JACC Cardiovasc Interv. 2011;4:1218–1227. [DOI] [PubMed] [Google Scholar]
- 20. Ejiofor JI, Yammine M, Harloff MT, McGurk S, Muehlschlegel JD, Shekar PS, Cohn LH, Shah P, Kaneko T. Reoperative surgical aortic valve replacement versus transcatheter valve‐in‐valve replacement for degenerated bioprosthetic aortic valves. Ann Thorac Surg. 2016;102:1452–1458. [DOI] [PubMed] [Google Scholar]
- 21. Ihlberg L, Nissen H, Nielsen NE, Rück A, Busund R, Klaarborg K‐E, Soendergaard L, Harnek J, Miettinen H, Eskola M, et al. Early clinical outcome of aortic transcatheter valve‐in‐valve implantation in the Nordic countries. J Thorac Cardiovasc Surg. 2013;146:1047–1054; discussion 1054. [DOI] [PubMed] [Google Scholar]
- 22. Spaziano M, Mylotte D, Thériault‐Lauzier P, De Backer O, Søndergaard L, Bosmans J, Debry N, Modine T, Barbanti M, Tamburino C, et al. Transcatheter aortic valve implantation versus redo surgery for failing surgical aortic bioprostheses: a multicentre propensity score analysis. EuroIntervention. 2017;13:1149–1156. [DOI] [PubMed] [Google Scholar]
- 23. Mohr FW. Decade in review–valvular disease: current perspectives on treatment of valvular heart disease. Nat Rev Cardiol. 2014;11:637–638. [DOI] [PubMed] [Google Scholar]
- 24. Pibarot P, Hahn RT, Weissman NJ, Arsenault M, Beaudoin J, Bernier M, Dahou A, Khalique OK, Asch FM, Toubal O, et al. Association of paravalvular regurgitation with 1‐year outcomes after transcatheter aortic valve replacement with the SAPIEN 3 valve. JAMA Cardiol. 2017;2:1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abdel‐Wahab M, Mehilli J, Frerker C, Neumann F‐J, Kurz T, Tölg R, Zachow D, Guerra E, Massberg S, Schäfer U, et al; CHOICE Investigators . Comparison of balloon‐expandable vs self‐expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. JAMA. 2014;311:1503–1514. [DOI] [PubMed] [Google Scholar]
- 26. Chakravarty T, Søndergaard L, Friedman J, De Backer O, Berman D, Kofoed KF, Jilaihawi H, Shiota T, Abramowitz Y, Jørgensen TH, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet. 2017;389:2383–2392. [DOI] [PubMed] [Google Scholar]
- 27. Makkar RR, Fontana G, Jilaihawi H, Chakravarty T, Kofoed KF, De Backer O, Asch FM, Ruiz CE, Olsen NT, Trento A, et al. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med. 2015;373:2015–2024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S3


