Abstract
Background
To date, there is no cumulative evidence supporting the association of atrial fibrillation (AF) noninducibility after ablation and freedom from AF. We performed a systematic review and meta‐analysis to determine whether AF noninducibility by burst pacing after catheter ablation is associated with reduced AF recurrence.
Methods and Results
We searched PubMed, Embase, Web of Science, and Cochrane Library databases through July 2019 to identify studies that evaluated AF noninducibility versus inducibility by burst pacing after catheter ablation for freedom from AF. A fixed effects model was used to estimate relative risk (RR) with 95% CIs. Twelve prospective cohort studies with AF noninducibility (n=1612) and inducibility (n=1160) were included. Compared with AF inducibility, AF noninducibility by burst pacing after ablation was associated with a reduced risk of AF recurrence (RR, 0.68; 95% CI, 0.60–0.77). Subgroup analysis showed that different AF types (paroxysmal AF and nonparoxysmal AF), different follow‐up times (≤6, 6–12, and >12 months), and different degrees of burst pacing (mild, moderate, severe) had no significant impact on the RRs. However, different cut‐off times for AF inducibility had a significant impact on the RR (P interaction=0.009), and only the cut‐off time of 1 minute showed a significant correlation (RR, 0.54; 95% CI, 0.45–0.66).
Conclusions
AF noninducibility by burst pacing after catheter ablation is associated with reduced clinical recurrence of AF. Induction protocols with a different cut‐off time for AF inducibility have a significant impact on the correlation, and the AF ≥1 minute for AF inducibility is recommended.
Keywords: association, atrial fibrillation, induction protocol, noninducibility, recurrence
Subject Categories: Atrial Fibrillation, Catheter Ablation and Implantable Cardioverter-Defibrillator, Electrophysiology
Nonstandard Abbreviations and Acronyms
- AF
atrial fibrillation
- GRADE
grading of recommendations assessment, development and evaluation
- MOOSE
Meta‐analysis of Observational Studies in Epidemiology
- NOS
Newcastle‐Ottawa Scale
- PAF
paroxysmal atrial fibrillation
- PRISMA
preferred reporting items for systematic reviews and meta‐analyses
- RR
relative risk
Clinical Perspective
What Is New?
This systematic review and meta‐analysis shows that atrial fibrillation (AF) noninducibility by burst pacing after ablation is significantly associated with freedom from AF, compared with AF inducibility.
Different AF types (paroxysmal AF and nonparoxysmal AF), and different follow‐up times (≤6, 6–12, and >12 months) have no significant impact on the relative risks, and all show a correlation.
Induction protocols with different cut‐off times (1, 2, and 5–10 minutes) for AF inducibility have a significant impact on the correlation, and AF ≥1 minute for AF inducibility is recommended.
What Are the Clinical Implications?
AF noninducibility by burst pacing after ablation is a prognostic factor of freedom from AF, which can be employed as a main procedural end point in AF ablation.
For the AF induction test, electrophysiologists should pay more attention to the cut‐off time as AF inducible, rather than the degrees of burst pacing; “AF ≥1 minute (cut‐off time) for AF inducibility” is recommended.
In the AF ablation procedure, persistent AF inducibility suggests a higher risk of recurrence and thus a potential need for additional ablation to render AF noninducibility; patients with paroxysmal AF with AF noninducibility after pulmonary vein isolation may not require additional ablation, such as substrate or linear ablation, which is technically challenging for completely transmural injury and is potentially proarrhythmic.
Atrial fibrillation (AF) is one of the most common arrhythmias and affects ≈33.5 million people worldwide. Catheter ablation is an effective method for the treatment of symptomatic AF and an important alternative to pharmacological therapy, with the advantages of maintaining a longer duration of sinus rhythm, improving quality of life, and reducing hospitalizations. 1 Although the techniques of catheter ablation of AF have been greatly developed, and its efficacy has been definitely established, the recurrence of AF after ablation remains a major concern. 2 Over the past decade, electrophysiologists have attempted to find better ablation strategies and prognostic factors to improve the success rate of AF ablation.
AF noninducibility by burst pacing is defined as the inability to induce AF with a prespecified electrophysiological induction protocol. AF noninducibility by burst pacing after ablation has been adopted as one of the common electrophysiological end points for guiding ablation strategies to improve clinical outcomes in both patients with paroxysmal AF (PAF) 3 , 4 , 5 , 6 , 7 and those without PAF. 3 , 8 More recently, studies targeting rotors or AF drivers responsible for AF maintenance have employed AF noninducibility as a main procedural end point. 9 Is AF noninducibility after ablation really a prognostic factor of freedom from AF? The cumulative evidence supporting the AF noninducibility after catheter ablation as a prognostic factor is largely inconclusive. 10 , 11 , 12 , 13 , 14
Hence, the primary objective of this study was to compare the postoperative recurrence of AF between AF noninducibility and AF inducibility by burst pacing after catheter ablation in patients with symptomatic AF. The secondary objective is to determine which induction protocol is desirable for AF induction testing.
Methods
All supporting data are available within the article and its online supplementary files.
This systematic review and meta‐analysis was performed according to the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) guidelines 15 and MOOSE (Meta‐analysis of Observational Studies in Epidemiology) for the reporting of our study. 16 There was no registered protocol.
Search Strategy and Selection Criteria
We conducted a comprehensive systematic literature search of online databases including PubMed, Embase, Web of Science, and Cochrane Library from inception through July 2019. We conducted electronic searches using MeSH terms and corresponding key words (Data S1). The reference lists of all included studies and relevant review articles were further scrutinized to identify additional citations that may fit our inclusion criteria.
The inclusion criteria were as follows: study population: patients with symptomatic AF underwent catheter ablation; intervention: noninducibility versus inducibility by burst pacing after catheter ablation; study design: prospective cohort studies (randomized controlled trial not available because of the peculiarity of the objective that patients with AF noninducibility and AF inducibility could not be randomly assigned); and outcome measures: recurrence of AF or freedom from AF.
Published studies meeting the following criteria were excluded: (1) AF inducibility using a pharmacological protocol, such as isoproterenol induction; (2) burst pacing not performed after catheter ablation; (3) without specific outcome or sufficient data for extraction; (4) fewer than 30 study patients; and (5) obvious bias in patient selection: only selected patients with repeat procedures of AF ablation or patients screened after the pharmacological protocol, which would affect the reliability and accuracy of the results.
Data Extraction and Quality Assessment
Three investigators (H.‐L.L., P.Y., X.Z.) independently performed the initial search, screened the titles and abstracts for relevance, deleted duplicate records, and identified records as included, excluded, or uncertain. In case of uncertainty, the full‐text article was acquired to determine eligibility. Any discrepancies were resolved through discussion with 2 additional investigators (J.‐Z.H., K.H.). Collected data included the following: first author, year of publication, country, study type, number of patients in each group, number of events, clinical characteristics of patients, ablation lesions, induction protocols (degree of burst pacing and defined time as AF inducible), definition of recurrence, antiarrhythmics before and after ablation, and follow‐up time.
The Newcastle‐Ottawa Scale (NOS) was used for quality assessment of included cohort studies. A maximum of 9 stars was awarded to each study: selection (4 stars), comparability (2 stars), and outcome (3 stars). The scores of 0 to 3, 4 to 6, and 7 to 9 were assigned for low, moderate, and high quality of studies, respectively.
Statistical Analysis
The pooled relative risks (RRs) with 95% CIs were estimated for the dichotomous outcome of clinical recurrence of AF. Heterogeneity among studies was quantified using the Cochran chi‐square test and I 2, which described the percentage of total variation across studies that was attributable to heterogeneity rather than chance. A value of 0% indicated no observed heterogeneity, and larger values showed increasing heterogeneity. I 2 >50% indicated significant heterogeneity. We pooled outcome data using a fixed and random effects model. Publication bias was assessed using a visually inspected funnel plot and was also evaluated by Harbord test and Peter test. A 2‐sided P<0.05 was considered as statistically significant.
The analyses were conducted using Review Manager (RevMan) version 5.3 (The Nordic Cochrane Center, http://ims.cochrane.org/revman) and STATA version 12.0 (StataCorp LLC). Quality of evidence was assessed using GRADE (Grading of Recommendations Assessment, Development and Evaluation) tools and manual guidelines, which are available online (https://gradepro.org/).
Results
Study Selection
According to the search strategy, the processes of literature screening, study selection, and reasons for exclusion are shown on the PRISMA statement flowchart (Figure 1). Our initial search obtained 516 records. After removing duplicates and screening the titles and abstracts, 82 articles were assessed for eligibility. After reviewing the full texts, 12 prospective cohort studies were ultimately included. The following studies that Essebag 2005 3 accord with exclusion criteria (1), Jaïs 2006 17 accord with exclusion criteria (3), Katritsis 2007 18 accord with exclusion criteria (4), Crawford 2010 19 and Fiala 2015 20 accord with exclusion criteria (5), were not included.
Figure 1.
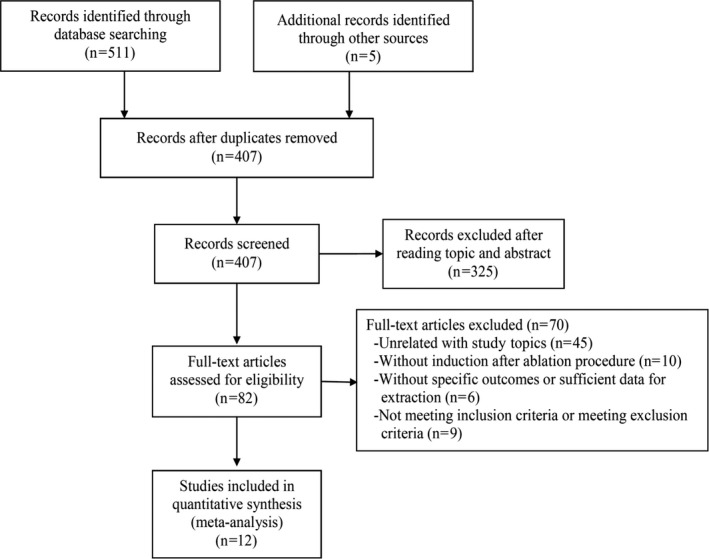
Flow diagram of the study selection process.
Studies Characteristics and Quality Assessment
The main characteristics of the included studies are summarized in the Table. The studies were published between 2004 and 2019. Because of the peculiarity of the objective, a randomized controlled trial design was not available. All of the included studies were prospective cohort studies. Seven studies included only patients with PAF, 4 , 5 , 6 , 7 , 13 , 14 , 21 1 study included only patients without PAF, 10 and 4 studies had a mix of patients with and those without PAF. 8 , 11 , 12 , 22 Population sizes ranged from 60 to 1141, with a total of 2772 patients. The average age was older than 50 years, and the majority of patients were men (not available in 1 study: Skala et al13). The average left atrial diameter ranged from 37±4.8 to 46±8 mm. AF with structural heart disease accounted for 0% to 43% of cases (not available in 3 studies: Santangeli et al12, Liu et al,7 and Oral et al4). Ablation lesions were different, depending on the type of AF and the operators. Pulmonary vein isolation was included in every study. The follow‐up times ranged from 5 to 42.5±9.3 months. The definition of AF recurrence in most studies was the same (>30 seconds). Subgroup analysis was performed according to the AF type, follow‐up time, and the induction protocol (degree of burst pacing and defined time for AF inducibility)
Table 1.
Characteristics and Demographics of Included Studies
| Study | Country | Study Type | AF Type | Patients, No. | Age, y | Men, No. (%) | LAD, mm | LVEF | Structural Heart Disease, No. (%) | Ablation Lesions | Burst Pacing | Defined Time as AF Inducible | Defined Time as AF Recurrence | Antiarrhythmics Before Ablation (Ceased Time) | Antiarrhythmics After Ablation (Using Time), mo | Follow‐Up, mo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kawai, 2019 10 | Japan | Prospective, observational | Non‐PAF | 98 | 61±10 | 77 (78.6) | 43.4±7.6 | 63.8±10.1 | 11 (11.2) | PVI±non‐PV triggers | Decremental burst pacing to refractoriness or 187.5 ms (30 beats) | AF/AT ≥5 min | AF/AT >30 s | 2.1±2.5 d | NA | 12 |
| Skala, 2019 13 | Czech Republic | Prospective, observational | PAF | 120 | NA | NA | 42.6±6.7 | NA | 0 (0) | PVI | Decremental burst pacing to 200 ms (5 s) | AF ≥5 min | AF/AT/AFL >30 s | ≥3 d (Amiodarone >3 mo) | NA | 12 |
| Otsuka, 2018 8 | Japan | Prospective, observational | PAF/non‐PAF | 291 | 59.8±10.7 | 249 (85.6) | 39.9±6.1 | 64.6±7.9 | 17 (5.84) | PVI+CTI±CFAE±non‐PV triggers | Decremental burst pacing to 180 ms (5 s) | AF/AT ≥5 min | AF/AT >30 s | ≥5 Half‐lives (except amiodarone) | 1–2 | 42.5±9.3 |
| Santangeli, 2018 12 | United States | Prospective, observational | PAF/non‐PAF | 305 | 55±11 | 242 (79) | 43±7 | 59±8 | NA | PVI±non‐PV triggers | Decremental burst pacing to refractoriness or 180 ms (15 beats) | AF/AT ≥2 min | AF/AT >30 s | ≥5 Half‐lives (except amiodarone) | NA | 19±7 |
| Leong‐Sit, 2013 11 | United States | Prospective, observational | PAF/non‐PAF | 144 | 60 [52–65]* | 114 (79.2) | 46±8 | 57 [53, 62]* | 49 (34.1) | PVI+non‐PV triggers | Decremental burst pacing to 2:1 atrial capture or 180 ms (15 beats) | AF/AFL/AT ≥2 min | AF/AT/AFL >30 s | NA | 1.5–6 (partly continued) | 12 |
| Adlbrecht, 2013 21 | Austria | Prospective, observational | PAF | 121 | 59.5±10.4 | 76 (63) | 44.3±6.9 | 54.2±2.9 | 36 (30) | PVI±CTI | Decremental burst pacing to refractoriness or 200 ms (5 s) | AF >1 min | AF >30 s | Ceased (time NA) | NA | 12.1 [6.5–20.3]* |
| Liu, 2012 7 | China | Prospective, observational | PAF | 1141 | 58.1±11.5 | 730 (64.0) | 37±4.8 | 62.2±6.9 | NA | PVI | NA | NA | AF/AT/AFL >30 s | NA | NA | 12 |
| Satomi, 2008 14 | Germany | Prospective, observational | PAF | 60 | 58.3±10.6 | 45 (75) | 42.9±5.5 | NA | 9 (15) | PVI | Decremental burst pacing to refractoriness (10 s) | AF >10 min | AF/AT/AFL (time NA) | ≥5 Half‐lives (except amiodarone) | 1 | 16.1±8.2 |
| Chang, 2007 5 | Taiwan | Prospective, observational | PAF | 88 | 51±12 | 61 (69.3) | 37±5 | 61±6 | 34 (39) | PVI±LA lines (roofline or MI) | Decremental burst pacing to 150 ms (5–10 s) | AF/AFL >1 min | AF ≥60 s | NA | NA | 12±6 |
| Richter, 2006 22 | Austria | Prospective, observational | PAF/non‐PAF | 234 | 56.7±10.5 | 168 (71.8) | 45±7 | 61.3±7.4 | 52 (22.2) | PVI±LA lines (roofline+MI)±CTI | Decremental burst pacing to refractoriness or 200 ms (5 s) | AF >1 min | AF (time NA) | Partly ceased (time NA) | ≥3 | 5 |
| Haïssaguerre, 2004 6 | France | Prospective, observational | PAF | 70 | 53±9 | 52 (74.3) | 43±7 | 67±12 | 30 (43) | PVI+CTI±MI | Decremental burst pacing to refractoriness (5 s) | AF ≥1 min | AF/AFL (time NA) | ≥5 Half‐lives (except amiodarone) | 0 | 7±3 |
| Oral, 2004 4 | United States | Prospective, observational | PAF | 100 | 55±10 | 80 (80) | 43±6 | 57±9 | NA | PVI+LA lines (septum+roofline+MI±anterior wall) | Burst pacing at refractoriness (≥15 s) | AF >1 min | AF/AFL (time NA) | ≥5 Half‐lives (except amiodarone) | 2–3 | 6 |
AF indicates atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; CFAE, complex fractionated atrial electrograms; CTI, cavotricuspid isthmus; LA, left atrial; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; MI, mitral isthmus; NA, not available; PAF, paroxysmal atrial fibrillation; PV, pulmonary vein; PVI, pulmonary vein isolation; and refractoriness, shortest cycle length with 1:1 atrial capture.
Medians with interquartile range.
Details of the quality and risk of bias assessments of the included studies are outlined in Table S1. The average NOS score was 8.08, and the score for each study was ≥7, indicating that all of the studies were of high quality.
AF Recurrence in Total Patients
Twelve studies with a total of 2772 patients provided data evaluating the effect of AF noninducibility versus inducibility by burst pacing after catheter ablation on the recurrence of AF. 4 , 5 , 6 , 7 , 8 , 10 , 11 , 12 , 13 , 14 , 21 , 22 Compared with AF inducibility, AF noninducibility by burst pacing after ablation was associated with a significantly reduced risk of AF recurrence (RR, 0.68; 95% CI, 0.60–0.77 [P<0.00001]) (Figure 2). No significant heterogeneity was revealed (I 2=32%, P heterogeneity=0.13), which indicated the consistency of results among these studies.
Figure 2.
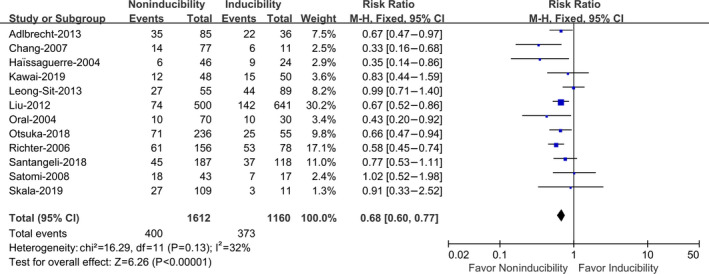
Atrial fibrillation (AF) noninducibility vs AF inducibility by burst pacing after catheter ablation on the recurrence of AF in total patients.
Subgroup Analysis
AF Recurrence in Different AF Types
A subgroup analysis was performed by dividing patients with AF into PAF and non‐PAF subgroups. Ten studies with a total of 2254 patients provided data on AF recurrence with PAF and 4 studies with a total of 374 patients provided data on AF recurrence with non‐PAF. 4 , 5 , 6 , 7 , 8 , 10 , 12 , 13 , 14 , 21 , 22 Different AF types (PAF and non‐PAF) had no significant impact on the RR and showed a correlation (P interaction=0.28) (Figure 3A). For PAF, AF noninducibility by burst pacing after ablation showed a significantly reduced risk of AF recurrence compared with AF inducibility (RR, 0.64; 95% CI, 0.55–0.75 [P<0.00001]), with no significant heterogeneity (I 2=11%, P heterogeneity=0.34) (Figure 3A). For non‐PAF, AF noninducibility was also associated with reduced AF recurrence (RR, 0.75; 95% CI, 0.59–0.96 [P=0.02]), with no heterogeneity (I 2=0%, P heterogeneity=0.91) (Figure 3A).
Figure 3.
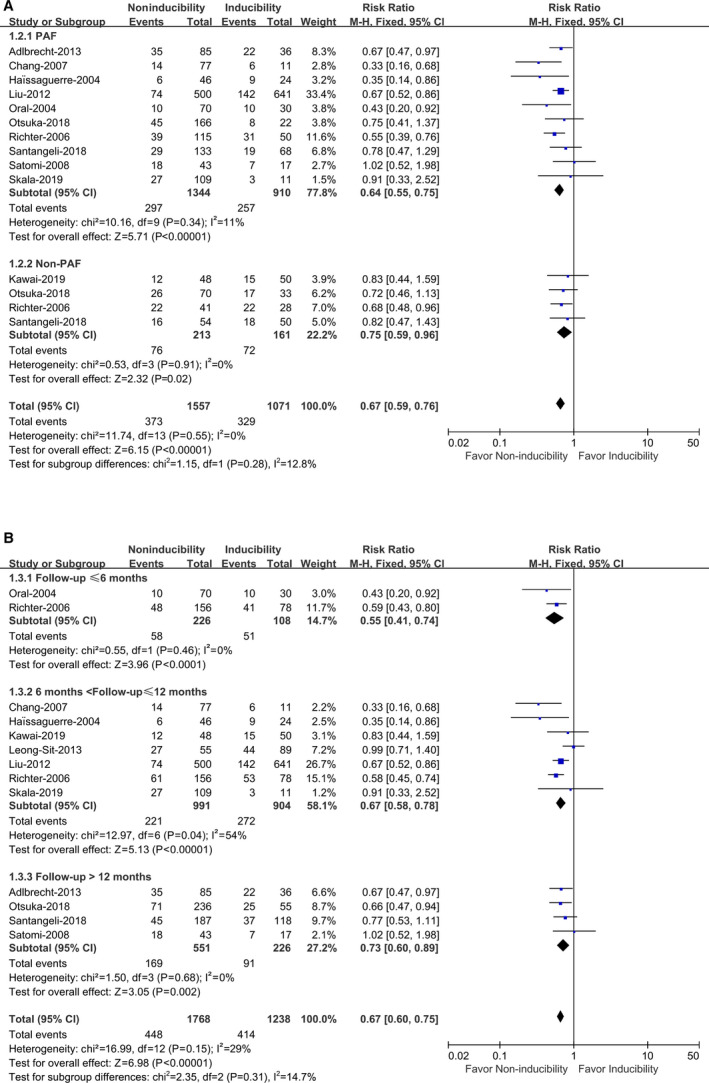
Atrial fibrillation (AF) noninducibility vs AF inducibility by burst pacing after catheter ablation on the recurrence of AF in different AF types (A) and different follow‐up time (B).
PAF indicates paroxysmal atrial fibrillation.
AF Recurrence in Different Follow‐Up Time
Since the recurrence of AF was associated with follow‐up time, we evaluated whether the correlation was affected by the follow‐up time in a subgroup analysis by different follow‐up times. Two studies with a total of 334 patients provided data with follow‐up ≤6 months, 4 , 22 7 studies with a total of 1895 patients with 6< follow‐up ≤12 months, 5 , 6 , 7 , 10 , 11 , 13 , 22 and 4 studies with a total of 777 patients with follow‐up >12 months. 8 , 12 , 14 , 21 Our results showed that different follow‐up times (≤6, 6–12, and >12 months) had no significant impact on the RR (P interaction=0.31) and showed a correlation (Figure 3B). Compared with AF inducibility, AF noninducibility by burst pacing after ablation significantly reduced the risk of AF recurrence in all 3 subgroups with ≤6 months (RR, 0.55; 95% CI, 0.41–0.74 [P<0.0001]), 6 to 12 months (RR, 0.67; 95% CI, 0.58–0.78 [P<0.00001]), and >12 months (RR, 0.73; 95% CI, 0.60–0.89 [P=0.002]), respectively. There was no significant heterogeneity in any of the 3 subgroups (I 2=0%, P heterogeneity=0.46; I 2=46%, P heterogeneity=0.07; and I 2=0%, P heterogeneity=0.68, respectively) (Figure 3B).
AF Recurrence in Different Induction Protocols
Degrees of burst pacing
To determine which degree of burst pacing was desirable for the AF induction test, we classified it into 3 degrees of mild, moderate, and severe stimulation. The mild stimulation was defined as “burst pacing to refractoriness, 2:1 atrial capture, or 180 to 200 ms (maintaining ≤3 seconds per 15 beats).” Moderate stimulation was defined as “burst pacing to refractoriness, or 180 to 200 ms (maintaining 5 seconds per 30 beats).” Severe stimulation was defined as “burst pacing to refractoriness (maintaining ≥10 seconds), or 150 ms (maintaining 5–10 seconds).” Two studies with a total of 449 patients provided data with mild stimulation, 11 , 12 6 studies with a total of 934 patients with moderate stimulation, 6 , 8 , 10 , 13 , 21 , 22 and 3 studies with a total of 248 patients with severe stimulation 4 , 5 , 14 (Figure 4B). The results showed that different degrees of burst pacing (mild, moderate, and severe stimulation) had no significant impact on the RR (P interaction=0.09), which indicated that the degree of burst pacing was not decisive for the correlation. In the moderate and severe stimulation subgroups, AF noninducibility could significantly reduce the risk of AF recurrence (RR, 0.63; 95% CI, 0.53–0.74 [P<0.00001] and RR, 0.57; 95% CI, 0.38–0.86 [P=0.007], respectively), with heterogeneity (I 2=0% [P heterogeneity=0.61] and I 2=64% [P heterogeneity=0.06]) (Figure 4B). While the mild stimulation subgroup showed the effect size was not statistically significant difference (RR, 0.86; 95% CI, 0.67–1.11 [P=0.31]), which could be explained by the small sample size (only 2 studies) resulting in a false‐negative result (Figure 4B).
Figure 4.
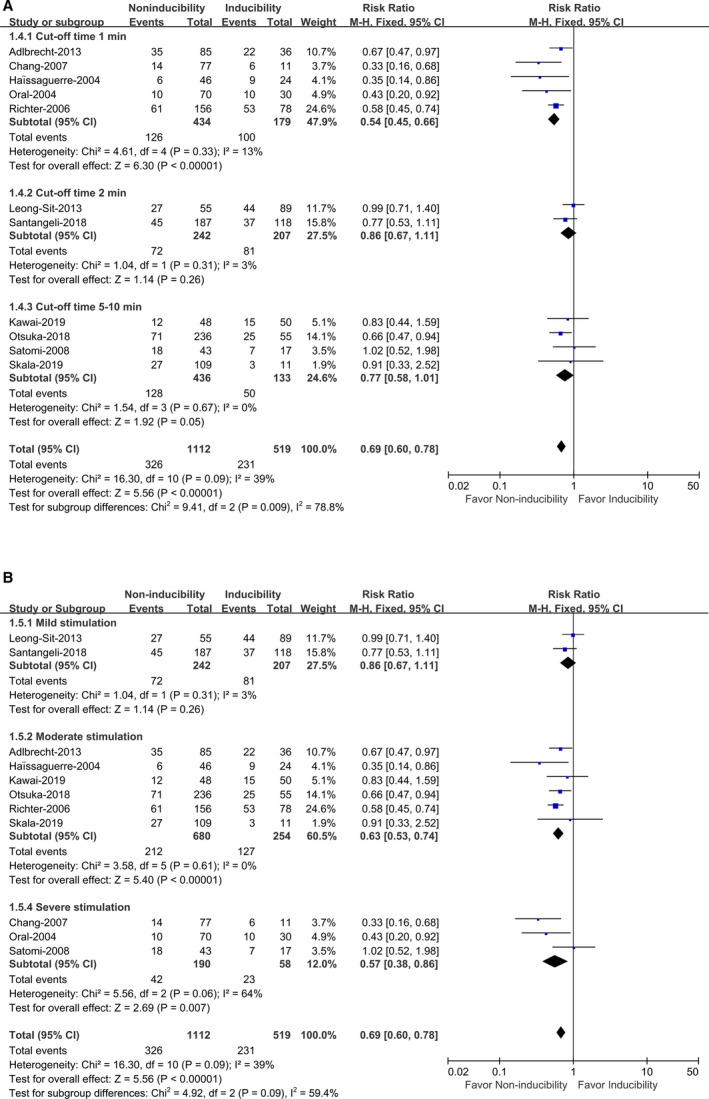
Atrial fibrillation (AF) noninducibility vs AF inducibility by burst pacing after catheter ablation on the recurrence of AF in different induction protocols (cut‐off time [A], degrees of burst pacing [B]). Mild stimulation: burst pacing to refractoriness, 2:1 atrial capture, or 180 to 200 ms (maintaining ≤3 seconds per 15 beats); Moderate stimulation: burst pacing to refractoriness, or 180 to 200 ms (maintaining 5 seconds per 30 beats); severe stimulation: burst pacing to refractoriness (maintaining ≥10 seconds), or 150 ms (maintaining 5–10 seconds).
Defined cut‐off time for AF inducibility
To determine which cut‐off time for AF inducibility was desirable for the AF induction test, 3 subgroups with defined cut‐off times of 1, 2, and 5 to 10 minutes were classified for analysis. Five studies with a total of 613 patients with a cut‐off time of 1 minute, 4 , 5 , 6 , 21 , 22 2 studies with a total of 449 patients with a cut‐off time of 2 minutes, 11 , 12 and 4 studies with a total of 569 patients with a cut‐off time of 5 to 10 minutes were evaluated 8 , 10 , 13 , 14 (Figure 4A). The results showed that different cut‐off times (1, 2, and 5–10 minutes) for AF inducibility had a significant impact on the RR (P interaction=0.009). Only in the subgroup of cut‐off time of 1 minute, AF noninducibility was associated with a significantly reduced risk of AF recurrence (RR, 0.54; 95% CI, 0.45–0.66 [P<0.00001]) (Figure 4A), with no significant heterogeneity (I 2=13%, P heterogeneity=0.33). In contrast, no statistical significance was revealed in the subgroups with cut‐off times of 2 minutes (RR, 0.86; 95% CI, 0.67–1.11 [ P=0.26]) and 5 to 10 minutes (RR, 0.77; 95% CI, 0.58–1.01 [P=0.05]) (Figure 4A). The results indicated that AF ≥1 minute for AF inducibility is the recommended protocol for the AF induction test.
Publication Bias, Sensitivity Analysis, and Quality of Evidence
Inspection of the funnel plot indicated a symmetric distribution of the included 12 studies (Figure 5). Formal statistical tests (Harbord test, P=0.398; Peter test, P=0.702) demonstrated that there was no potential publication bias among studies. Sensitivity analyses have confirmed the robustness of the results (Figure S1). Meanwhile, the random effects model (Figures S2 through S4) was performed and showed almost the same results with the fixed effects model (Figures 2, 3 through 4), which also indicated the robustness of the results. In addition, GRADE ratings of the quality of evidence in the 12 cohort studies are provided in Table S2. According to GRADE system categories, the quality of evidence for the main outcome (AF recurrence in total patients) was moderate.
Figure 5.
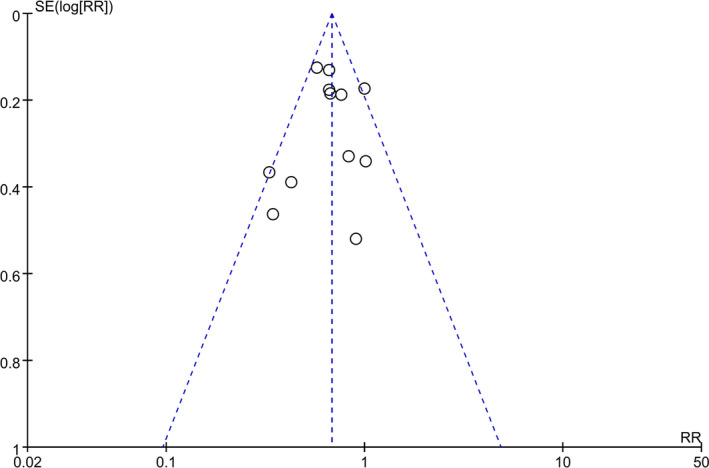
Funnel plot of all of the 12 included studies.
Discussion
Main Findings
Our study comprehensively and systematically reviewed the current available literature, including 12 publications with 2772 (1612 versus 1160) patients, and found that: (1) AF noninducibility by burst pacing after catheter ablation was significantly associated with reduced risk of AF recurrence; (2) different AF types (PAF and non‐PAF) and different follow‐up times (≤6, 6–12, and >12 months) had no significant impact on the RRs, and all showed correlations; (3) induction protocols with different cut‐off times (1, 2, and 5–10 minutes) for AF inducibility had a significant impact on the correlation, and the AF ≥1 minute for AF inducibility is recommended; and (4) different degrees of burst pacing (mild, moderate, and severe stimulation) had no significant impact on the RR and seem not to be decisive for the correlation. To our knowledge, this study is the first systematic review and meta‐analysis reflecting the cumulative evidence for evaluating the association of AF noninducibility by burst pacing after catheter ablation and postoperative AF recurrence. Although randomized controlled trials were not available because of the peculiarity of the objective, all of the included prospective cohort studies were of high quality according to the recommended quality evaluation of NOS. In addition, there was no significant heterogeneity among the main results of the included studies, and the sensitivity analysis also showed that the results were not affected by any individual study. All of these factors indicated the robustness of the results.
Possible Mechanisms for the Findings
The mechanism by which AF noninducibility by burst pacing after ablation is associated with reduced AF recurrence remains unclear. Chang et al 5 found that patients with AF inducibility after ablation had lower left atrial and right atrial voltages compared with those with AF noninducibility, which indicated that the biatrial substrate of perpetuating activity may play a critical role in the outcome of AF induction testing. Patients with AF noninducibility after ablation have fewer substrates capable of maintaining AF, and therefore have a lower risk of AF recurrence. In contrast, patients with AF inducibility have more substrates capable of maintaining AF, and therefore have a higher risk of AF recurrence.
Different AF types (PAF and non‐PAF) had no significant impact on the RRs, and all showed a correlation. However, the non‐PAF shows the decreased tendency of RRs, which can be explained by the complicated multifactorial nature and the faster substrate deterioration of non‐PAF, causing the relatively higher AF recurrence in the subgroup of AF noninducibility in non‐PAF compared with PAF.
In addition, our results also show that different follow‐up times (≤6, 6–12, and >12 months) had no significant impact on the RRs, and all showed a correlation. It is interesting that the RR decreases gradually with the prolongation of follow‐up time, despite the lack of a statistically significant difference and it cannot be distinguished from noise. However, this “tendency” is consistent with clinical practice and has strong external information to support such claims. This phenomenon can be well understood through the mechanism whereby as the time is prolonged, along with atrial remodeling, the substrates capable of maintaining AF progress and deteriorate, resulting in increases in AF recurrence in both groups (AF noninducibility and AF inducibility), which results in a disparity of AF recurrence between the 2 groups that was not as obvious as before, and therefore, the RR decreases.
It has been reported that 26% of patients without a history of AF had positive nonspecific AF inducibility using an aggressive electrophysiological induction protocol. 23 The defined cut‐off time of AF inducibility directly determines the assignment of patients to the noninducible and inducible groups. Obviously, different definitions of cut‐off time produce different assignment of patients (noninducible and inducible). Thus, some patients in one study can be assigned to completely different groups as a result of changes in the induction protocol. Therefore, induction protocols have a potential impact on the RRs. It is particularly important for electrophysiologists to determine which induction protocol is desirable for AF induction testing. Our results show that different cut‐off times for AF inducibility have a significant impact on the RR, and only the AF ≥1 minute for AF inducibility, which presents a significant correlation, is recommended. In contrast, the degrees of burst pacing have no significant impact on the RR and seem not to be decisive for the correlation. These results indicate that the cut‐off time for AF inducibility is more important than the degrees of burst pacing in AF induction testing. Electrophysiologists should pay more attention to the cut‐off time for AF inducibility rather than the degrees of burst pacing.
Implications for Clinical Practice
It is known that pulmonary vein isolation has been recognized as a basic strategy of AF ablation. However, other strategies applied to AF ablation to reduce recurrence of AF have not been well established and remain controversial. 24 Our study shows that AF noninducibility by burst pacing after ablation is significantly associated with freedom from AF, regardless of PAF or non‐PAF, which can be employed as a main procedural end point in AF ablation. In the AF ablation procedure, persistent AF inducibility suggests a higher risk of recurrence and thus a potential need for additional ablation to render AF noninducibility. Attention should be paid to the balance of additional ablation rendering AF noninducibility to improve the outcome, the proarrhythmic potential, and other complications caused by excessive ablation. However, patients with PAF who have AF noninducibility by burst pacing after pulmonary vein isolation may not require additional ablation, such as substrate or linear ablation, which is technically challenging for complete transmural injury and potential proarrhythmia. For the AF induction test, electrophysiologists should pay more attention to the cut‐off time for AF inducibility rather than the degrees of burst pacing. AF ≥1 minute for AF inducibility is the recommended protocol for AF induction after ablation.
Strengths and Limitations
Our study has several strengths. First, to our knowledge, this is the first systematic review and meta‐analysis to investigate the association between AF noninducibility by burst pacing after ablation and postoperative clinical recurrence. Second, although this meta‐analysis does not have a registered review protocol, we conducted this study in compliance with the PRISMA guidelines and MOOSE suggestions. Finally, there was no significant heterogeneity or potential publication bias among the results of the included studies, and the sensitivity analysis also indicated the robustness of the results. However, several limitations should be considered. First, a randomized controlled trial design is not available because of the peculiarity of the objective. Second, although all of the included studies were prospective and of high quality by NOS, the use of observational cohort studies and lack of adjusted models may increase the potential of confounding, which will affect our results.
Conclusions
AF noninducibility by burst pacing after catheter ablation is associated with a favorable clinical outcome of freedom from AF, regardless of a PAF or non‐PAF condition and different follow‐up times. In addition, we found that induction protocols with a different cut‐off time for AF inducibility have a significant impact on the correlation, and the AF ≥1 minute for AF inducibility is the recommended protocol. While the different degrees of burst pacing seem to not be decisive. Electrophysiologists should pay more attention to the cut‐off time for AF inducibility rather than the degrees of burst pacing in the AF induction test.
Sources of Funding
This work was supported in part by the National Natural Science Foundation of China (NSFC, 81860070 and 81400188), the Youth Science Foundation Project of Jiangxi Education Department (14189), and the Science and Technology Project of Jiangxi Public Health Department (20141084).
Disclosures
None.
Supporting information
Data S1
Tables S1–S2
Figures S1–S4
Acknowledgments
Liu, Yuan, and Zhu contributed to the acquisition of data, analysis and interpretation of data, drafting of the article, and final approval of the version to be published. Fu contributed to acquisition of data and analysis and interpretation of data. Hong and Hu contributed to the conception and design of the study, analysis and interpretation of data, revision of the article, and final approval of the version to be published.
(J Am Heart Assoc. 2020;9:e015260 DOI: 10.1161/JAHA.119.015260.)
For Sources of Funding and Disclosures, see page 11.
Contributor Information
Kui Hong, Email: hongkui88@163.com.
Jinzhu Hu, Email: hujinzhu1983@sina.com.
References
- 1. Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, Daniels MR, Bahnson TD, Poole JE, Rosenberg Y, et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS, Sullivan T, Roberts‐Thomson KC, Sanders P. Long‐term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta‐analysis. J Am Heart Assoc. 2013;2:e004549 DOI: 10.1161/JAHA.112.004549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Essebag V, Baldessin F, Reynolds MR, McClennen S, Shah J, Kwaku KF, Zimetbaum P, Josephson ME. Non‐inducibility post‐pulmonary vein isolation achieving exit block predicts freedom from atrial fibrillation. Eur Heart J. 2005;26:2550–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oral H, Chugh A, Lemola K, Cheung P, Hall B, Good E, Han J, Tamirisa K, Bogun F, Pelosi F Jr, et al. Noninducibility of atrial fibrillation as an end point of left atrial circumferential ablation for paroxysmal atrial fibrillation: a randomized study. Circulation. 2004;110:2797–2801. [DOI] [PubMed] [Google Scholar]
- 5. Chang SL, Tai CT, Lin YJ, Wongcharoen W, Lo LW, Tuan TC, Udyavar AR, Chang SH, Tsao HM, Hsieh MH, et al. The efficacy of inducibility and circumferential ablation with pulmonary vein isolation in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:607–611. [DOI] [PubMed] [Google Scholar]
- 6. Haissaguerre M, Sanders P, Hocini M, Hsu LF, Shah DC, Scavee C, Takahashi Y, Rotter M, Pasquie JL, Garrigue S, et al. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation. 2004;109:3007–3013. [DOI] [PubMed] [Google Scholar]
- 7. Liu Y, Huang H, Huang C, Yang Y, Zhang S, Wu S, Ma C; Investigators A . Noninducibility after circumferential pulmonary vein isolation of paroxysmal atrial fibrillation improves clinical outcome: evidence from the Atrial Fibrillation Clinical Trial (AFCT) in China. Int J Cardiol. 2012;158:332–334. [DOI] [PubMed] [Google Scholar]
- 8. Otsuka T, Sagara K, Arita T, Yagi N, Suzuki S, Ikeda T, Yamashita T. Impact of electrophysiological and pharmacological noninducibility following pulmonary vein isolation in patients with paroxysmal and persistent atrial fibrillation. J Arrhythm. 2018;34:501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Atienza F, Almendral J, Ormaetxe JM, Moya A, Martinez‐Alday JD, Hernandez‐Madrid A, Castellanos E, Arribas F, Arias MA, Tercedor L, et al. Comparison of radiofrequency catheter ablation of drivers and circumferential pulmonary vein isolation in atrial fibrillation: a noninferiority randomized multicenter RADAR‐AF trial. J Am Coll Cardiol. 2014;64:2455–2467. [DOI] [PubMed] [Google Scholar]
- 10. Kawai S, Mukai Y, Inoue S, Yakabe D, Nagaoka K, Sakamoto K, Takase S, Chishaki A, Tsutsui H. Predictive value of the induction test with atrial burst pacing with regard to long‐term recurrence after ablation in persistent atrial fibrillation. J Arrhythm. 2019;35:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leong‐Sit P, Robinson M, Zado ES, Callans DJ, Garcia F, Lin D, Dixit S, Bala R, Riley MP, Hutchinson MD, et al. Inducibility of atrial fibrillation and flutter following pulmonary vein ablation. J Cardiovasc Electrophysiol. 2013;24:617–623. [DOI] [PubMed] [Google Scholar]
- 12. Santangeli P, Zado ES, Garcia FC, Riley MP, Lin D, Frankel DS, Supple GE, Schaller RD, Dixit S, Callans DJ, et al. Lack of prognostic value of atrial arrhythmia inducibility and change in inducibility status after catheter ablation of atrial fibrillation. Heart Rhythm. 2018;15:660–665. [DOI] [PubMed] [Google Scholar]
- 13. Skala T, Tudos Z, Moravec O, Hutyra M, Precek J, Skalova J, Klementova O, Zapletalova J, Taborsky M. Atrial fibrillation inducibility after pulmonary vein isolation under general anaesthesia. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14. Satomi K, Tilz R, Takatsuki S, Chun J, Schmidt B, Bansch D, Antz M, Zerm T, Metzner A, Kokturk B, et al. Inducibility of atrial tachyarrhythmias after circumferential pulmonary vein isolation in patients with paroxysmal atrial fibrillation: clinical predictor and outcome during follow‐up. Europace. 2008;10:949–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 17. Jais P, Hocini M, Sanders P, Hsu LF, Takahashi Y, Rotter M, Rostock T, Sacher F, Clementy J, Haissaguerre M. Long‐term evaluation of atrial fibrillation ablation guided by noninducibility. Heart Rhythm. 2006;3:140–145. [DOI] [PubMed] [Google Scholar]
- 18. Katritsis D, Giazitzoglou E, Korovesis S, Kourlaba G, Voridis E, Camm AJ. Staged circumferential and ostial pulmonary vein ablation for the treatment of paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2007;30:102–108. [DOI] [PubMed] [Google Scholar]
- 19. Crawford T, Chugh A, Good E, Yoshida K, Jongnarangsin K, Ebinger M, Pelosi F Jr, Bogun F, Morady F, Oral H. Clinical value of noninducibility by high‐dose isoproterenol versus rapid atrial pacing after catheter ablation of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:13–20. [DOI] [PubMed] [Google Scholar]
- 20. Fiala M, Bulkova V, Sknouril L, Nevralova R, Toman O, Januska J, Spinar J, Wichterle D. Sinus rhythm restoration and arrhythmia noninducibility are major predictors of arrhythmia‐free outcome after ablation for long‐standing persistent atrial fibrillation: a prospective study. Heart Rhythm. 2015;12:687–698. [DOI] [PubMed] [Google Scholar]
- 21. Adlbrecht C, Gwechenberger M, Richter B, Sipotz J, Kaider A, Gossinger H. Prognostic value of induction of atrial fibrillation before and after pulmonary vein isolation. Int J Cardiol. 2013;164:212–216. [DOI] [PubMed] [Google Scholar]
- 22. Richter B, Gwechenberger M, Filzmoser P, Marx M, Lercher P, Gossinger HD. Is inducibility of atrial fibrillation after radio frequency ablation really a relevant prognostic factor? Eur Heart J. 2006;27:2553–2559. [DOI] [PubMed] [Google Scholar]
- 23. Huang W, Liu T, Shehata M, Zhang K, Yao Y, Niu G, Amorn A, Liu X, Chugh SS, Wang X. Inducibility of atrial fibrillation in the absence of atrial fibrillation: what does it mean to be normal? Heart Rhythm. 2011;8:489–492. [DOI] [PubMed] [Google Scholar]
- 24. Kirchhof P, Calkins H. Catheter ablation in patients with persistent atrial fibrillation. Eur Heart J. 2017;38:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S2
Figures S1–S4


