The clinical course of patients with heart failure (HF) is generally characterized by periods of clinical stability interrupted by episodes of worsening symptoms. Although sudden clinical deterioration can occur (eg, unstable arrhythmia, “flash” pulmonary edema), most patients with worsening HF experience a gradual worsening over days to weeks and many patients have at least some contact with outpatient clinicians. 1 During this often observed interval between onset of worsening symptoms and hospitalization, clinical experience suggests that management strategies include escalated doses of oral loop diuretics, outpatient intravenous loop diuretics, or addition of thiazide or other adjunctive oral diuretics. Nonetheless, real‐world granular data on the true prevalence of these management strategies, their effectiveness in preventing downstream hospitalization, and the associated prognostic impact are scarce to nonexistent.
See Article by Madelaire et al.
In this context, the article by Madelaire et al in this issue of the Journal of the American Heart Association (JAHA) provides important insights on the association between 2 different types of worsening HF events, hospitalization versus outpatient escalation of oral diuretics, and subsequent 1‐year mortality. 2 The authors retrospectively queried the nationwide Danish administrative registry for all patients with incident HF from 2001 to 2016. The analysis focused on patients who had stabilized and were alive at 4 months following initial HF diagnosis and had been initiated on guideline‐directed medical therapy with an angiotensin‐converting enzyme inhibitor (ACEI)/angiotensin II receptor blocker and β blocker. The investigators then followed this population forward and defined outpatient treatment intensification events as newly prescribed oral loop diuretics of minimum 80 mg/d furosemide equivalent, doubled dosage of furosemide equivalent compared with initial dosage to minimum 160 mg/d, or newly prescribed thiazide in addition to ≥160 mg/d furosemide. Patients were then categorized on the basis of status: no worsening, outpatient intensification event, HF hospitalization, or both types of worsening. Patients with an intensification event or hospitalization were risk set matched to 2 nonworsened HF controls, and absolute and relative 1‐year mortality risks were assessed.
Among 74 990 patients meeting study criteria, median age was 71 years and 36% were women. Although both types of worsening HF events were common, outpatient intensification (9 per 100 person‐years) occurred more frequently than HF hospitalization (7 per 100 person‐years). Compared with patients with HF hospitalization, patients with outpatient diuretic intensification had a similar patient profile with exception of being slightly older, slightly less likely to already be receiving a loop diuretic or mineralocorticoid receptor antagonist therapy, and slightly more likely to be receiving a thiazide diuretic. Only 2% of HF hospitalizations were preceded by an intensification event within 30 days. Compared with matched controls without any clinical worsening, both outpatient diuretic intensification and HF hospitalization were independently associated with higher 1‐year mortality. However, relative risk of death was higher with HF hospitalization, with a >2‐fold increased risk with hospitalization compared with a 75% higher risk following an outpatient intensification event. In terms of absolute risk, the 1‐year mortality rate following HF hospitalization was 22.6% versus 18.0% following outpatient intensification versus 9.8% for patients without either worsening event.
The authors should be congratulated for a timely analysis using a large nationwide real‐world data set. To our knowledge, this is the first study to characterize the frequency and clinical implications of outpatient escalation of oral diuretics in routine clinical practice. Previous studies of outpatient worsening HF have generally been secondary analyses of clinical trials. For example, a post hoc analysis of PARADIGM‐HF (Prospective Comparison of Angiotensin Receptor–Neprilysin Inhibitor With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial) showed that outpatient intensification of HF therapy occurs frequently and with mortality risk similar to HF hospitalization. 3 Likewise, the secondary analysis of MADIT‐CRT (Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy) found outpatient worsening HF to carry high risk of mortality comparable to hospitalization. 4 However, in contrast to the current study, MADIT‐CRT defined outpatient worsening HF as treatment with intravenous diuretics, whereas the PARADIGM‐HF analysis included a combination of intravenous or oral diuretic within a single group. Although outpatient intravenous diuretic therapy may be a viable treatment strategy for worsening HF and increasingly recognized as an end point in HF trials, limited data from clinical trial and real‐world cohorts suggest these events may be relatively rare. 5 , 6 On the other hand, escalation of oral diuretic therapy may be a more feasible management strategy for most clinics and health systems. Thus, the analysis by Madelaire et al2 targets a major gap in the literature on the patterns and clinical implications of oral outpatient diuretic intensification for HF.
Despite its novelty and strengths, limitations of the current study should be acknowledged. Most notable, the authors required use of ACEI/angiotensin II receptor blocker and β‐blocker therapy for study eligibility with a goal to enrich the cohort for patients with HF with reduced ejection fraction. However, these medications are frequently used for blood pressure control in patients with HF with preserved ejection fraction, and ejection fraction may be less relevant since both patients with HF with reduced ejection fraction and HF with preserved ejection fraction receive diuretics as a cornerstone of symptomatic management. Furthermore, the mandate for ACEI/angiotensin II receptor blocker and β‐blocker therapy imposed selection bias, particularly when one considers that there are significant gaps in the use of these evidence‐based therapies in the United States and many European countries. 7 , 8 In fact, this mandate for ACEI/angiotensin II receptor blocker and β blocker is reminiscent of a clinical trial (ie, the requirement to be on optimal background therapy for HF with reduced ejection fraction), and may challenge the real‐world nature and generalizability of these data. Second, this study included patients with de novo HF who were subsequently alive 4 months after diagnosis, rather than patients with more established and chronic HF diagnoses. Previous studies have suggested patients with HF with more recent diagnoses carry lower clinical risk. 9 , 10 Although absolute 1‐year mortality rates in the current study were high, inclusion of recently diagnosed patients may have nonetheless shifted the patient profile toward lower risk. Likewise, it is unclear what proportion of patients in this sample subsequently had myocardial recovery and resolution of HF with initiation of therapy. Last, as the authors have acknowledged, residual confounding and less robust statistical adjustment may have been key reasons why the current results (ie, outpatient worsening HF with modestly less risk than HF hospitalization) differ from results of existing PARADIGM‐HF and MADIT‐CRT analyses (ie, outpatient worsening HF with similar risk to HF hospitalization). Indeed, multiple important parameters were not available in the Danish registry for risk adjustment, including vital signs and laboratory values.
Clinical Implications
This work by Madelaire and colleagues2 has several important implications. First, it shows that even a simple increase in oral outpatient diuretics is not a benign event and potentially associated with a 75% higher risk of death. These data highlight outpatient diuretic intensification as a high‐risk feature that should be recognized and put on a pedestal similar to (even if perhaps not “quite as bad”) HF hospitalization. Second, these data provide further evidence that worsening HF is not specific to the hospital setting and that HF hospitalization is unlikely to represent a separate biological entity. 11 These findings are complementary to a recent analysis of BIOSTAT‐CHF (Biology Study to Tailored Treatment in Chronic Heart Failure), which found that although a hospitalized cohort may carry a higher proportion of “high‐risk” patients, high risk is not specific to the in‐hospital location and a large proportion of outpatients face a similarly poor prognosis. 11 , 12 Third, these data have implications for HF clinical trials. Specifically, the recommended definition for the worsening HF clinical end point includes hospitalization or outpatient administration of intravenous diuretics, but does not include escalation of oral diuretics. 13 Acknowledging that the current analysis is limited to patients from a single country, these data suggest that oral diuretic intensification occurs frequently and with clinically significant prognostic implications. These initial data suggest that inclusion of oral diuretic escalation within a broader definition of a worsening HF end point would have the advantage of substantially improved statistical power while further capturing clinically important events. Fourth, the low rates of mineralocorticoid receptor antagonist therapy (32%) in the current study are consistent with prior data demonstrating substantial gaps in guideline‐directed medical therapy upstream of a worsening HF event. 14 As the list of evidence‐based HF with reduced ejection fraction therapies grows and large gaps in implementation remain, distinguishing between “breakthrough worsening” despite target or maximally tolerated doses of quadruple therapy versus “undertreatment worsening” has become increasingly complex. 15 The current data highlight that even if undertreatment “only” comes at the cost of escalated oral diuretics without hospitalization, there is a substantial negative impact on prognosis.
As the public health and financial burden of HF continues to increase, it is vital to recognize the entity of outpatient worsening HF, and to acknowledge an outpatient increase in oral diuretics as a high‐risk clinical scenario. Moreover, although HF hospitalizations have been the traditional focus for researchers, hospital systems, and clinicians, we must appreciate outpatient escalation of oral diuretics as common, potentially occurring more frequently than hospitalization, and a clear marker with poor prognosis. In aggregate, with limited existing data on the burden of outpatient worsening HF, the current study serves as a further call to action to (1) improve the use and dosing of guideline‐directed medical and device‐based therapy to prevent worsening HF and (2) develop effective means to treat worsening HF in the outpatient setting and avoid hospitalization (Figure1). 16 Prior studies and quality measures have emphasized HF hospitalization as an important time for optimizing guideline‐directed care. 17 Madelaire and colleagues2 remind us that this same sense of urgency for maximizing guideline‐directed care and developing new effective therapies should apply to outpatient worsening HF.
Figure 1.
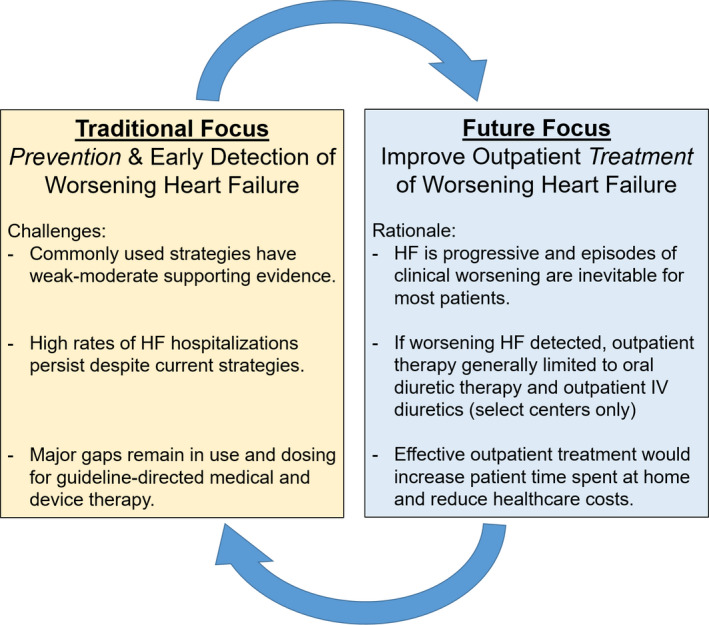
Conceptual framework for reducing the burden of worsening heart failure (HF) and HF hospitalizations. Continued widespread emphasis on generic, empiric, and generally unproven strategies (eg, telemonitoring, salt restriction, and nursing visits) to decrease HF hospitalizations has been largely unsuccessful and expensive. The central goal of these traditional approaches has been to prevent hospitalizations by preventing worsening signs and symptoms. To complement continued efforts of worsening HF prevention, we propose an added focus on developing effective strategies to treat clinical worsening as an outpatient. IV indicates intravenous.
Disclosures
Dr Khan has no disclosures to report. Dr Butler has received research support from the National Institutes of Health, Patient‐Centered Outcomes Research Institute, and the European Union; and serves as a consultant for Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squib, CVRx, G3 Pharmacautical, Innolife, Janssen, Luitpold, Medtronic, Merck, Novartis, Relypsa, StealthPeptide, SC Pharma, Vifor, and ZS Pharma. Dr Greene has received a Heart Failure Society of America/Emergency Medicine Foundation Acute Heart Failure Young Investigator Award, funded by Novartis; has received research support from Amgen, AztraZeneca, Bristol‐Myers Squibb, Merck, and Novartis; has served on advisory boards for Amgen and Cytokinetics; and serves as a consultant for Amgen and Merck.
(J Am Heart Assoc. 2020;9:e017485 DOI: 10.1161/JAHA.120.017485.)
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
For Disclosures, see page 3.
REFERENCES
- 1. Greene SJ, Mentz RJ, Felker GM. Outpatient worsening heart failure as a target for therapy: a review. JAMA Cardiol. 2018;3:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Madelaire C, Gustafsson F, Stevenson LW, Kristensen SL, Kober L, Andersen J, D'Souza M, Biering‐Sorenson T, Andersson C, Torp‐Pederson C, et al. One‐year mortality after intensification of outpatient diuretic therapy. J Am Heart Assoc. 2020;9:e016010 DOI: 10.1161/JAHA.119.016010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Okumura N, Jhund PS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K, Zile MR, Solomon SD, et al. Importance of clinical worsening of heart failure treated in the outpatient setting: evidence from the prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM‐HF). Circulation. 2016;133:2254–2262. [DOI] [PubMed] [Google Scholar]
- 4. Skali H, Dwyer EM, Goldstein R, Haigney M, Krone R, Kukin M, Lichstein E, McNitt S, Moss AJ, Pfeffer MA, et al. Prognosis and response to therapy of first inpatient and outpatient heart failure event in a heart failure clinical trial: MADIT‐CRT. Eur J Heart Fail. 2014;16:560–565. [DOI] [PubMed] [Google Scholar]
- 5. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 6. Greene SJ, Wilson LE, Abbasi SA, Yusuf AA, Hammill BG. Outpatient intravenous diuretic therapy for heart failure in the United States. J Am Coll Cardiol. 2019;73:1101–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP‐HF registry. J Am Coll Cardiol. 2018;72:351–366. [DOI] [PubMed] [Google Scholar]
- 8. Komajda M, Anker SD, Cowie MR, Filippatos GS, Mengelle B, Ponikowski P, Tavazzi L. Physicians' adherence to guideline‐recommended medications in heart failure with reduced ejection fraction: data from the QUALIFY global survey. Eur J Heart Fail. 2016;18:514–522. [DOI] [PubMed] [Google Scholar]
- 9. Böhm M, Komajda M, Borer JS, Ford I, Maack C, Tavazzi L, Moyne A, Swedberg K. Duration of chronic heart failure affects outcomes with preserved effects of heart rate reduction with ivabradine: findings from SHIFT. Eur J Heart Fail. 2018;20:373–381. [DOI] [PubMed] [Google Scholar]
- 10. Greene SJ, Felker GM. Considering the duration of heart failure: using the past to predict the future. Eur J Heart Fail. 2018;20:382–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greene SJ, Felker GM, Butler J. Outpatient versus inpatient worsening heart failure: distinguishing biology and risk from location of care. Eur J Heart Fail. 2019;21:121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferreira JP, Metra M, Mordi I, Gregson J, Ter Maaten JM, Tromp J, Anker SD, Dickstein K, Hillege HL, Ng LL, et al. Heart failure in the outpatient versus inpatient setting: findings from the BIOSTAT‐CHF study. Eur J Heart Fail. 2019;21:112–120. [DOI] [PubMed] [Google Scholar]
- 13. Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, Solomon SD, Marler JR, Teerlink JR, Farb A, et al. 2017 Cardiovascular and stroke endpoint definitions for clinical trials. Circulation. 2018;137:961–972. [DOI] [PubMed] [Google Scholar]
- 14. Butler J, Yang M, Manzi MA, Hess GP, Patel MJ, Rhodes T, Givertz MM. Clinical course of patients with worsening heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73:935–944. [DOI] [PubMed] [Google Scholar]
- 15. Greene SJ, Fonarow GC, Butler J. Risk profiles in heart failure: baseline, residual, worsening, and advanced heart failure risk. Circ Heart Fail. 2020;13:e007132. Jun 2 [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16. Greene SJ, Felker GM. Innovation in diuretic therapy: the missing ingredient for treating worsening heart failure outside the hospital? JACC Basic Transl Sci. 2018;3:35–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhagat AA, Greene SJ, Vaduganathan M, Fonarow GC, Butler J. Initiation, continuation, switching, and withdrawal of heart failure medical therapies during hospitalization. JACC Heart Fail. 2019;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


