Abstract
Background
Left ventricular assist device (LVAD) thrombosis is clinically devastating and impacts the cost effectiveness of LVAD therapy for advanced heart failure. Anticoagulation and antiplatelet therapies represent the standard of care to mitigate LVAD thrombosis. Phosphodiesterase type 5 inhibitors (PDE‐5is) exhibit hemodynamic, antiplatelet, and antithrombotic effects. Using a national registry, we examined the relationship of PDE‐5i use on thrombotic events in patients with continuous‐flow LVADs.
Methods and Results
We obtained data from 13 772 patients with continuous flow LVADs participating in a national registry. Patients implanted with primary LVADs from 2012 to 2017 were included in the analysis. The primary end point was a composite of LVAD thrombosis and ischemic stroke. Patients were analyzed according to any use of PDE‐5i after LVAD implantation (PDE‐5i group) versus no use after LVAD implantation (no PDE‐5i group). The primary end point was significantly lower in the PDE‐5i group compared with the no PDE‐5i group (hazard ratio [HR], 0.84; 95% CI, 0.77–0.91; P<0.001) at 48 months. The components of the primary end point (LVAD thrombosis: HR, 0.82; 95% CI, 0.74–0.90; P<0.001; and ischemic stroke: HR, 0.85; 95% CI, 0.75–0.97; P=0.019), as well as the secondary end point all‐cause mortality (HR, 0.86; 95% CI, 0.79–0.93; P<0.001) were lower in the PDE‐5i group versus the no PDE‐5i at 48 months post LVAD. The favorable results observed with postimplant PDE‐5i use were consistent with both axial and centrifugal flow devices.
Conclusions
The postimplant use of PDE‐5i was associated with fewer thrombotic events and improved survival in LVAD patients. A randomized clinical trial is warranted to confirm these findings.
Keywords: complications, heart failure, pharmacology, sildenafil
Subject Categories: Heart Failure, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- HR
hazard ratio
- INTERMACS
Interagency Registry for Mechanically Assisted Circulatory Support
- LDH
lactate dehydrogenase
- LVAD
left ventricular assist device
- PDE‐5i
phosphodiesterase type 5 inhibitors
Clinical Perspective
What Is New?
Postimplant use of phosphodiesterase 5 inhibitors is associated with a lower incidence of thrombotic events (device thrombosis or ischemic stroke) and improved survival in patients with implanted continuous flow left ventricular assist devices.
• Phosphodiesterase 5 inhibitors seem to be beneficial regardless of the continuous flow–left ventricular assist device type (axial or centrifugal).
Benefit with phosphodiesterase 5 inhibitor use in patients with continuous flow–left ventricular assist devices is obtained at the expense of an increased risk for gastrointestinal bleeding.
What Are the Clinical Implications?
A randomized controlled trial is urgently needed to confirm the apparent benefit with the use of phosphodiesterase 5 inhibitors in the current centrifugal left ventricular assist device era.
The advent of left ventricular assist device (LVAD) therapy was paradigm shifting in the management of patients with advanced heart failure.1, 2, 3 However, device support remains fraught with hemocompatibility‐related adverse events including thrombosis and bleeding, which account for nearly half of all adverse events.4, 5 The introduction of an LVAD in the circulatory system results in an altered hematologic balance due to blood‐pump interfaces and changes in hemodynamics and rheology, necessitating the use of anticoagulation.6 Significant adverse events occur in both centrifugal‐ and axial flow–type LVAD devices.
Pump thrombosis and stroke are major complications following LVAD implantation previously reported in the primary clinical trials.2, 3, 7 Subsequently, an increase in the rate of device thrombosis among patients who received the HeartMate II was observed in a study including 837 patients from 3 institutions.8 Hemocompatibility‐related complications remain, despite the improvements in the design of the third‐generation LVAD.9
Phosphodiesterase type 5 inhibitors (PDE‐5is) are known to enhance nitric oxide–mediated vasodilation by inhibiting degradation of cGMP10 and exhibit antiplatelet and antithrombotic effects.11, 12, 13, 14 Hence, sildenafil, the prototypical agent in the class of PDE‐5is, which has been used frequently to unload the right ventricle in patients with LVADs,15, 16 may impact not only hemodynamics but hemostasis as well.10, 14 Recognizing the potential favorable effects of PDE‐5is on thrombotic events in patients with LVAD, importantly to date, only a few small single‐center studies have examined this association reporting conflicting results.17, 18, 19
In the present study, we examined whether the postimplant use of a PDE‐5i is associated with a lower incidence of thrombotic events in a population of 13 772 patients with implanted continuous‐flow LVADs included in a national registry.
Methods
Primary LVAD from 2012 to 2017 were included in the analysis.
Study Data
The authors declare that all supporting data are available within the article and its online supplementary files. The INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) Data Access, Analysis, and Publications Committee approved the investigational protocol. The INTERMACS Data and Clinical Coordinating Center and each participating institution have received Institutional Review Board/Ethics Review Board approval for active informed consent or a waiver of consent to enroll participants, collect data, and perform analytic studies. All procedures are Health Insurance Portability and Accountability Act compliant, and INTERMACS has received a Federal Certificate of Confidentiality and other protection for the identities of patients and devices identified within the registry. Patients were classified into 2 groups: those who received PDE‐5i after implantation (n=4950) and those who did not (n=8822). Baseline characteristics of patients included in the study are presented in Table 1.
Table 1.
Baseline Demographics and Patient Characteristics Pre‐Implant
| Overall (N=13 772) | PDE‐5 Inhibitor (N=4950) | No PDE‐5 Inhibitor (N=8822) | P Value | |
|---|---|---|---|---|
| Age, y | 57±13 | 56±13 | 58±13 | <0.001 |
| Male, n (%) | 10 834 (78.7) | 3887 (78.5) | 6947 (78.7) | <0.001 |
| Black, n (%) | 3347 (24.3) | 1564 (31.6) | 1783 (20.2) | <0.001 |
| Current smoker, n (%) | 667 (4.8) | 182 (3.7) | 485 (5.5) | <0.001 |
| Body mass index, kg/m2 | 28.6±6.7 | 28.8±6.7 | 28.5±6.6 | 0.044 |
| <35, n (%) | 11 555 (84.6) | 4101 (83.5) | 7454 (85.2) | 0.008 |
| ≥35, n (%) | 2100 (15.4) | 809 (16.5) | 1291 (14.8) | |
| Profile at time of implant, n (%) | ||||
| Critical cardiogenic shock | 2069 (15.0) | 672 (13.6) | 1397 (15.8) | <0.001 |
| Progressive decline | 4789 (34.8) | 1924 (38.9) | 2865 (32.5) | |
| Stable but inotrope dependent | 4757 (34.5) | 1717 (34.7) | 3040 (34.5) | |
| Resting symptoms | 1765 (12.8) | 515 (10.4) | 1250 (14.2) | |
| Exertion intolerant | 272 (2.0) | 84 (1.7) | 188 (2.1) | |
| Exertion limited | 72 (0.5) | 22 (0.4) | 50 (0.6) | |
| Advanced NYHA class III, n (%) | 48 (0.3) | 16 (0.3) | 32 (0.3) | |
| Continuous flow LVAD type, n (%) | ||||
| Axial | 10 183 (73.9) | 3620 (73.1) | 6563 (74.4) | 0.105 |
| Centrifugal | 3589 (26.1) | 1330 (26.9) | 2259 (25.6) | |
| Current device strategy, n (%) | ||||
| Bridge to transplant | 3444 (25.0) | 1247 (25.2) | 2197 (24.9) | 0.708 |
| Destination therapy | 6491 (47.1) | 2301 (46.5) | 4190 (47.5) | |
| Other | 3837 (27.9) | 1372 (27.7) | 2435 (27.6) | |
| Implant year, n (%) | ||||
| 2012 | 1827 (13.3) | 655 (13.2) | 1172 (13.3) | <0.001 |
| 2013 | 2599 (18.9) | 1012 (20.4) | 1587 (18.0) | |
| 2014 | 2700 (19.6) | 1012 (20.4) | 1688 (19.1) | |
| 2015 | 2978 (21.6) | 1084 (21.9) | 1894 (21.5) | |
| 2016 | 2602 (18.9) | 844 (17.1) | 1758 (19.9) | |
| 2017 | 1066 (7.7) | 343 (6.9) | 723 (8.2) | |
| Time on LVAD, mo | 16.5±14.5 | 17.8±14.7 | 15.8±14.4 | <0.001 |
| History of pulmonary hypertension, n (%) | 3065 (22.2) | 1587 (32.1) | 1478 (16.8) | <0.001 |
| History of renal disease, n (%) | 3019 (21.9) | 1317 (26.6) | 1702 (19.3) | <0.001 |
| History of major stroke, n (%) | 507 (3.7) | 187 (3.8) | 320 (3.6) | 0.720 |
| Preimplant inotropes, n (%) | 11 367 (82.5) | 4272 (86.3) | 7095 (80.4) | <0.001 |
| Preimplant INR, n (%) | 1.30±0.39 | 1.31±0.38 | 1.29±0.39 | <0.001 |
| Preimplant LDH >1000 (units per liter) , n (%) | 269/7874 (3.4) | 90/2839 (3.2) | 179/5035 (3.6) | 0.367 |
| Right heart failure, n (%) | 6349/8093 (78.5) | 2488/2912 (85.4) | 3864/5181 (74.5) | <0.002 |
| PDE‐5 inhibitor at baseline, n (%) | 1375 (10.0) | 952 (19.2) | 423 (4.8) | <0.001 |
LDH indicates lactate dehydrogenase; LVAD, left ventricular assist device; INR, international normalized ratio; and NYHA, New York Heart Association; and PDE‐5, phosphodiesterase type 5.
End Points
The primary end point was the composite of pump thrombosis or ischemic stroke. Secondary end points were all‐cause mortality and the composite of all‐cause mortality, pump thrombosis, or ischemic stroke, during LVAD support. Patients not experiencing any of these events were censored at device explant unrelated to either pump thrombosis or ischemic stroke, transplantation, or end of follow‐up on LVAD support. The follow‐up period was 48 months. Pump thrombosis was assigned as “definite or probable thrombosis”; all events were adjudicated and coded by the INTERMACS registry data coordinating center.
Definitions
Pump thrombosis was identified at the time of intervention or major outcome. The event of pump thrombosis was identified by the following definitions:
Pump exchange that was identified as being due to pump thrombosis (specified in explant reason).
Pump thrombosis was identified (via the device malfunction form) within 60 days before pump exchange.
Pump thrombosis was identified (via the device malfunction form) within 60 days before death, where the death form specified that the device was not functioning normally at the time of death.
Pump thrombosis was identified (via the device malfunction form) within 60 days before transplantation.
Pump thrombosis was identified (via the device malfunction form) within 60 days before pump explant because of “recovery.”
Cardiac transplantation with device removal in which pump thrombosis was identified.
When device thrombosis was not specifically identified at the time of intervention or major outcome, all relevant forms at the time of intervention or major outcome plus the relevant forms within the preceding 60 days were reviewed by 3 INTERMACS steering committee members and the assignment of LVAD thrombosis was adjudicated. The INTERMACS methodology was described for LVAD thrombosis previously.20 A consensus was achieved to determine whether the identified event was associated with no, possible, probable, or definite pump thrombosis. Those events determined to be probable or definite pump thrombosis were coded as pump thrombosis events by INTERMACS and included in the database provided. Ischemic stroke and hemorrhagic stroke were defined and reported separately in the INTERMACS registry (Data S1).
Statistical Analysis
Baseline characteristics are presented as mean±standard deviation for continuous variables or count (percent of total patients) for categorical variables. Differences between PDE‐5i and no PDE‐5i groups were assessed via 2‐tailed t test or chi‐square test, as appropriate. To adjust for differences between patients taking PDE‐5i or not, propensity scores for PDE‐5i treatment and corresponding stabilized inverse probability of treatment weights were calculated using a binary logistic regression model including significant baseline characteristics as covariates (Tables S1 and S2). Improvement in the balance of baseline characteristics was assessed by evaluating a plot of the absolute standardized differences with and without inverse probability of treatment weights (Figure S1). An absolute value in standardized differences of <10% for each variable served to determine adequate covariate balance. Absolute standardized differences close to 0% after weighting indicate excellent covariate balance.
Cox proportional hazards analysis was performed for each outcome and the model weighted by the inverse probability of treatment weights. The proportional hazards assumption for use of PDE‐5i was examined by plotting the Schoenfeld residuals against time and testing the interaction of log‐transformed time with treatment group in the model. Models not meeting the proportional hazards assumption retained the interaction term for log of time and treatment. Multivariable model selection was conducted through the stepwise selection method, incorporating normalization of stabilized inverse probability of treatment weights. All variables collected at baseline (Table S1) were considered for each model, and variables with a P<0.05 were retained in the final model (Table S3). Since the use of PDE‐5i could vary across the 48‐month follow‐up period, with patients coming off treatment or starting treatment throughout the follow‐up period, an additional sensitivity analysis considered PDE‐5i as a time‐dependent covariate in a Cox's model. The relationship between use of PDE‐5i (yes or no) and outcome (yes or no) were examined for each discrete visit interval (1 month, 3 months, 6 months, and every 6 months thereafter until month 48). Cumulative incidence curves graphically present outcomes over time between patients taking a PDE‐5i and those not taking a PDE‐5i. A forest plot for the primary outcome was used to examine predefined subgroups. The relationship between timing of PDE‐5i use (preoperatively or postoperatively) was examined using dummy variables for each timing category compared with no PDE‐5i use as the reference group in a multivariable model. The frequency of aspirin use and all antiplatelet medications were plotted over time by visit interval. A Breslow‐Day test for homogeneity was used to examine differences in treatment group by visit. Mixed models for repeated measurements were used to test for differences in international normalized ratio and lactate dehydrogenase (LDH) values between treatment groups over time. A spline transformation was used for values of LDH at 1 month after implantation to assess the relationship with probability of the primary end point.
All estimates are presented along with 95% CIs and P values. All reported P values are 2‐sided, and a P value of <0.05 was considered to indicate statistical significance. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Study Cohort
Baseline characteristics of the study population are reported in Table 1. Patients on PDE‐5is were younger and more frequently women and black compared with those not on a PDE‐5i. PDE‐5i use after implantation was associated with higher body mass index and more frequent use of preoperative inotropes as well as a history of pulmonary hypertension and renal disease. On the contrary, patients not receiving a PDE‐5i were more often smokers, with less frequent right heart failure and lower preimplant international normalized ratio. Overall median follow‐up (median [interquartile range]) for the entire population was 9.2 months (3.2–19.8); no PDE‐5i group: 7.0 months (3.0–18.6) and PDE‐5i group: 11.3 months (4.2–23.3).
Antithrombotic Treatment
The frequency of aspirin (Figure S2) and antiplatelet agents (including aspirin) use (Figure S3) as well as the international normalized ratio values (Figure S4) with time were not statistically different between the 2 groups after implantation.
Primary End Point—Composite Pump Thrombosis or Ischemic Stroke
The results at 48 months are shown in Table 2. PDE‐5i use was associated with a reduction in the event rate (adjusted hazard ratio [HR], 0.84; 95% CI, 0.77–0.91; P<0.001; Figure 1A). The components pump thrombosis (adjusted HR, 0.82; 95% CI, 0.74–0.90; P<0.001; Figure 1B) and ischemic stroke (adjusted HR, 0.85; 95% CI, 0.75–0.97; P=0.019; Figure 1C) both were significantly reduced in the PDE‐5i cohort. Figure S5 depicts centrifugal and axial flow LVAD demonstrating a reduction in events with both pump types with PDE‐5i use. Table S4 provides an analysis of the end points based on “use” or “no use” of PDE‐5i based on exposure <6 months after implantation and ≥6 months after implantation. The median percentage of reported PDE‐5i use out of the total number of visits attended was 75% (interquartile range, 33%–100%).
Table 2.
End Points Through 48 Months
| End Point | PDE‐5 Inhibitor, n (%) | No PDE‐5 Inhibitor, n (%) | PDE‐5 Inhibitor vs No PDE‐5 Inhibitor | P Value |
|---|---|---|---|---|
| Adjusted Hazard Ratio* (95% CI) | ||||
| Primary end point | 921 (18.6) | 1750 (20.5) | 0.84 (0.77–0.91) | <0.001 |
| Pump thrombosis | 652 (13.2) | 1260 (14.8) | 0.82 (0.74–0.90) | <0.001 |
| Ischemic stroke | 349 (7.1) | 645 (7.6) | 0.85 (0.75–0.97) | 0.019 |
| All‐cause mortality | 1066 (21.5) | 1943 (22.4) | 0.86 (0.79–0.93) | <0.001 |
| All‐cause mortality, pump thrombosis, or ischemic stroke | 1770 (35.8) | 3308 (38.1) | 0.84 (0.79–0.89) | <0.001 |
PDE‐5 indicates phosphodiesterase type 5.
Each end point model is adjusted for the significant variables listed in Table S3.
Figure 1. Cumulative incidence curves through 48 months.
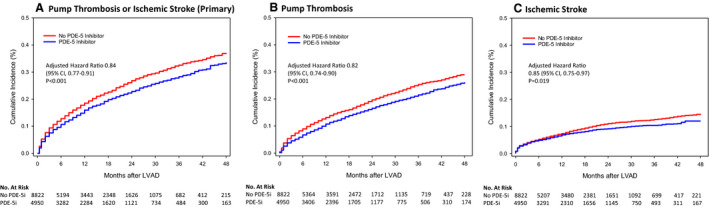
A, Primary end point. B, Pump thrombosis. C, Ischemic stroke. LVAD indicates left ventricular assist device.
Exploration of the relationship between the timing of PDE‐5i use (preimplant, postimplant) and the primary end point demonstrated that the benefit was evident only in patients taking a PDE‐5i after implantation. There was no association between the preimplant PDE‐5i use and the primary outcome (P=0.478; Table S5).
Secondary End Points
There was a reduction in all‐cause mortality with PDE‐5i use (HR, 0.86; 95% CI, 0.79–0.93; P<0.001). However, the benefit appeared to be greatest within the first 6 months (Table S4 and Figure S6). Similar were the results of the PDE‐5i use regarding the combined secondary end point of all‐cause mortality, pump thrombosis, or ischemic stroke (Table S4 and Figure S7).
Adjustments and Sensitivity Analyses
Table 3 shows the impact of PDE‐5i use as a time‐varying covariate with adjustments for the variables in Table S3. Since the use of PDE‐5i could vary across the follow‐up period with patients coming off treatment or starting treatment throughout the follow‐up period, an additional sensitivity analysis considered PDE‐5i as a time‐dependent covariate in a Cox's model. The relationship between use of PDE‐5i (yes or no) and outcome (yes or no) was examined for each discrete visit interval (1 month, 3 months, 6 months, and every 6 months thereafter until month 48). The prespecified primary and secondary end points all demonstrated a significant and more pronounced reduction in hazard with PDE‐5i.
Table 3.
Sensitivity Analysis Using PDE‐5 Inhibitor as a Time‐Varying Covariate
| End Point | PDE‐5 Inhibitor vs No PDE‐5 Inhibitor | P Value |
|---|---|---|
| Adjusted Hazard Ratioa (95% CI) | ||
| Pump thrombosis or ischemic stroke (primary end point) | 0.54 (0.45–0.66) | <0.001 |
| Pump thrombosis | 0.45 (0.35–0.57) | <0.001 |
| Ischemic stroke | 0.70 (0.51–0.96) | 0.028 |
| All‐cause mortality | 0.43 (0.35–0.53) | <0.001 |
| All‐cause mortality, pump thrombosis, or ischemic stroke | 0.50 (0.44–0.58) | <0.001 |
PDE‐5 indicates phosphodiesterase type 5.
Each end point model is adjusted for the significant variables listed in Table S3.
Heterogeneity of the Primary End Point
Based on the existing literature,17, 18 we specifically analyzed the LDH level <400, 400 to 700, and >700 (Figure 2) to examine the impact of hemolysis and use of PDE‐5i and thrombotic events. Sixty‐eight percent of patients had LDH data available at 1 month. The effect of PDE‐5i on outcome appeared to vary depending on the LDH level. In those with LDH <400, PDE‐5i significantly lowered thrombotic events, while the risk remained neutral for patients with LDH ≥400. Interestingly, patients on a PDE‐5i exhibited lower levels of LDH with time than those not on the drug (Figures S8 and S9). The timing of occurrence of the primary end point to both groups stratified by LDH at 1 month after LVAD implantation is depicted in Table S6.
Figure 2. Adjusted hazard ratios and 95% CIs comparing use of phosphodiesterase type 5 inhibitors (vs no use) for the primary end point of pump thrombosis or ischemic stroke, by various subgroups.
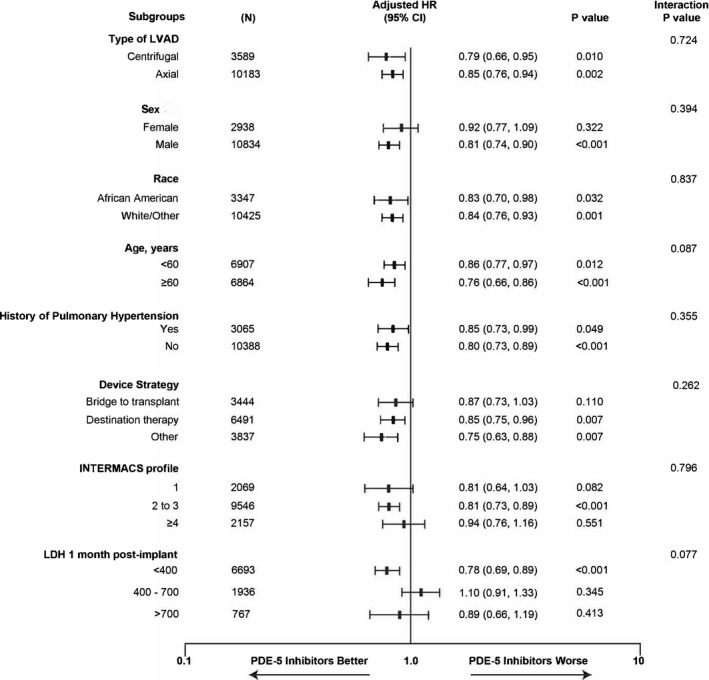
INTERMACS indicates Interagency Registry for Mechanically Assisted Circulatory Support; LDH, lactate dehydrogenase; and LVAD, left ventricular assist device.
Hemorrhagic Adverse Events
No significant difference was observed between the 2 groups (PDE‐5i versus no PDE‐5i) regarding the rates of hemorrhagic stroke (adjusted HR, 0.89; 95% CI, 0.78–1.02; P=0.09; Figure S10). However, the use of a PDE‐5i was associated with 14% increased risk for gastrointestinal bleeding compared with those not on a PDE‐5i (adjusted HR, 1.14; 95% CI, 1.06–1.23; P<0.01; Figure S11).
Discussion
The principle observation from this INTERMACS registry analysis is a reduction in thrombotic events (LVAD thrombosis and or ischemic stroke) associated with the postimplant use of a PDE‐5i. The reduction in thrombotic events was present with both axial and centrifugal‐flow LVAD. Importantly, the association of PDE‐5i use with the reduction in thrombotic events occurred in the setting of similar and conventional antithrombotic treatment (antiplatelets, warfarin) between the 2 groups (PDE‐5i versus no PDE‐5i). The secondary end point all‐cause mortality was significantly reduced in patients with a continuous‐flow LVAD with PDE‐5i use. The reduction in mortality was primarily in the early (<6 months after implantation) period (Table S4).
There is a plausible theoretical framework to support the PDE‐5i–induced reduction of thrombotic events in patients with LVADs.14, 21 The nitric oxide/cGMP signaling cascade participates in the inhibition of platelet adhesion and aggregation. In platelets, cGMP synthesis is catalyzed by soluble guanylyl cyclase, whereas several phosphodiesterases are responsible for cGMP degradation.22 PDE‐5is potentiate nitric oxide–mediated inhibition of platelet aggregation through blockade of cGMP degradation.11, 12, 13, 14, 23
Two recent, retrospective, single‐center studies reported that sildenafil was associated with a reduced risk of thrombosis in Heart Mate II axial flow LVAD recipients with LDH indicative of low‐level hemolysis (ie, LDH 400–700).17, 18 The investigators hypothesized that under these conditions, plasma free hemoglobin acts as a nitric oxide scavenger, potentially promoting a prothrombotic state17, 18, 24 and that sildenafil, by inhibiting phosphodiesterase‐5 may prevent the breakdown of cGMP reactivating the preceding inhibitory pathway for platelet activation and aggregation.17 We have previously reported the importance of monitoring LDH as a harbinger of LVAD thrombosis.8 However, the present analysis does not confirm that PDE‐5i use is associated with reduced thrombotic events in patients with LDH in the previously reported range (ie, LDH 400–700). We observed that PDE‐5is were effective in patients with LDH <400 both in centrifugal and axial flow LVADs (Figure 2). The 1‐month LDH was reported in only 68% of patients, and because of differing platforms for the LDH assay, we were unable to convert the reported LDH levels to equivalent values.
The 2 previous single‐center studies used the discharge LDH values to define the presence or absence of hemolysis differing from the current analysis, which utilized the LDH values 1 month after implantation. It is known that LDH levels obtained <30 days after HeartMate II implantation may be affected by numerous postimplant variables.25 Our observation that the use of a PDE‐5i after implantation may be associated with the reduction of thrombotic events in the group of patients with LDH <400 U/L suggests that PDE‐5i exposure may be most beneficial before thrombus formation, when the levels of hemolysis are low. As shown in Table S6, LDH >700 at 1 month after implantation is associated with the shortest time to occurrence of the primary end point.
An alternative hypothesis is that the PDE‐5i antithrombotic effects may be attributable to the improvement of hemodynamics in continuous‐flow LVAD recipients.18 Experimental evidence shows that PDE‐5i has the capacity to improve right ventricular contractile function in human and sheep myocardium.26, 27 Theoretically, PDE‐5is may have the ability to augment right ventricular function and improve filling of the left ventricle in patients after LVAD implantation.
It is noteworthy that in the present study the preimplant PDE‐5i use was not associated with thrombotic risk reduction. A retrospective analysis of 11 544 continuous‐flow LVAD recipients from the INTERMACS registry by Gulati et al28 revealed that preimplant PDE‐5i use was associated with a higher incidence of prolonged inotropic support after LVAD implantation, resulting in an increased incidence of severe early right heart failure within 30 days after implantation. In summary, the present and published INTERMACS analyses do not support the use of preimplant PDE‐5i therapy in LVAD candidates.
The time‐related effect of PDE‐5i use may relate to the fact that the risk of pump thrombosis is not constant but changes with time after LVAD implantation. We have previously shown that the hazard of thrombosis is higher early after Heart Mate II LVAD implantation (early risk 3–6 months after implantation) and then follows a descending trajectory with time until it reaches a plateau (later risk) for the duration of LVAD support.29 Accordingly, the benefit from PDE‐5i use appears most impactful early after LVAD (<6 months; Table S4).
Early reports indicate that the rates of thrombotic events are declining with the newest LVAD that is commercially available (HeartMate 3). Despite the significant reduction in pump thrombosis, stroke remains a serious adverse event (9.9% at 2 years after implantation with HeartMate 3).9 The current analysis showed no interaction between the favorable effects of PDE‐5i and the type of LVAD (axial versus centrifugal), denoting a possible beneficial effect of PDE‐5is even in patients on HeartMate 3. Potentially, any current or future LVAD with inherent thrombotic events might benefit from the use of a PDE‐5i. Additionally, the role of PDE‐5is to improve right ventricular function and hence impact the physiology of a continuous‐flow LVAD remains an important adjunctive hypothesis in addition to antiplatelet effects. The pathophysiology of gastrointestinal bleeding in continuous flow LVAD patients is not well established but clearly more prevalent in patients with right ventricular failure.30 Interestingly, digoxin has been reported to reduce gastrointestinal bleeding events in LVAD patients.31 Both digoxin and PDE‐5i may enhance right ventricular contractility.
Although the rates of hemorrhagic strokes were similar between the 2 groups (PDE‐5i versus no PDE‐5i), patients’ use of PDE‐5is after implantation exhibited significantly higher risk of gastrointestinal bleeding, which is a major source of LVAD‐associated morbidity, up to 48 months despite being on the same antiplatelet and antithrombotic treatment as those not on PDE‐5i. The exact mechanism of gastrointestinal bleeding in continuous‐flow LVAD patients is unknown; however, any antiplatelet or anticoagulant drug may potentially augment bleeding from arteriovenous malformations developing as a result of the continuous‐flow LVADs.32 The reduced gastrointestinal bleeding observed with the HeartMate 3 could be related to multiple factors including enhanced pulsatility and the fact that HeartMate 3 patients have more intact high‐molecular‐weight multimers and a higher von Willebrand factor activity during support.33 It is, therefore, anticipated that gastrointestinal bleeding possibly related to PDE‐5is will be significantly lower with the HeartMate 3 LVAD. The HeartMate 3 LVAD, which is not well represented in this analysis, has a lower rate of bleeding as reported in the MOMENTUM (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Suppport Therapy with HeartMate3) trial final analysis compared with the HeartMate II (24.5% versus 30.9% at 24 months, respectively).9 Interestingly, a higher incidence of major bleeding in the first 30 days after LVAD implant was reported in patients receiving a preimplant PDE‐5i in the published INTERMACS study by Gulati et al.28 The net impact of PDE‐5i use after implantation on hemocompatibility in LVAD patients should be carefully evaluated for risk‐benefit in a randomized clinical trial.
Our study has several limitations. This is a registry‐based, nonrandomized, observational study. Despite using robust statistical methods adjusting for >40 variables, residual confounding exists, as it is not possible to adjust for unmeasured variables. Nevertheless, the INTERMACS registry represents the largest database of continuous‐flow LVADs characterized by rigorous data entry, high‐quality monitoring of data, internal adjudication, and quality control. We did not analyze the device variables; pump flow, or pump speed; however, LVAD thrombosis events were defined, adjudicated, and coded by the INTERMACS database physicians. The use of a PDE‐5i was not based on the intention to reduce the risk of thrombotic events but for “right ventricular failure and or pulmonary hypertension.” Dosage, class effect of phosphodiesterase type 5 inhibition, and duration of therapy are important and unanswered by this investigation. The reduction in thrombotic events including ischemic stroke and improved survival observed suggests incremental benefit with PDE‐5i use may apply to all LVAD technology.
The use of a PDE‐5i after LVAD implantation was associated with fewer occurrences of thrombotic events and improved survival in patients with continuous‐flow LVADs in this INTERMACS registry study. The addition of PDE‐5i to the medical regimen of LVAD recipients should be further evaluated in a randomized controlled trial.
Sources of Funding
None.
Disclosures
None.
Supporting information
Data S1 Tables S1–S6 Figures S1–S11
Acknowledgments
The authors thank Drs James Kirklin and Francis Pagani for their support and critical review of the manuscript and the data analysis. The protocol was reviewed and accepted and the database transferred before the transition of the INTERMACS registry to the Society of Thoracic Surgeons National Database.
(J Am Heart Assoc. 2020;9:e015897 DOI: 10.1161/JAHA.119.015897.)
For Sources of Funding and Disclosures, see page 8.
References
- 1. Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, et al. Long‐term use of a left ventricular assist device for end‐stage heart failure. N Engl J Med. 2001;345:1435–1443. [DOI] [PubMed] [Google Scholar]
- 2. Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM III, Long JW, et al. Advanced heart failure treated with continuous‐flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. [DOI] [PubMed] [Google Scholar]
- 3. Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, Boyce SW, Najjar SS, Jeevanandam V, Anderson AS, et al. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med. 2017;376:451–460. [DOI] [PubMed] [Google Scholar]
- 4. Hasin T, Marmor Y, Kremers W, Topilsky Y, Severson CJ, Schirger JA, Boilson BA, Clavell AL, Rodeheffer RJ, Frantz RP, et al. Readmissions after implantation of axial flow left ventricular assist device. J Am Coll Cardiol. 2013;61:153–163. [DOI] [PubMed] [Google Scholar]
- 5. Uriel N, Colombo PC, Cleveland JC, Long JW, Salerno C, Goldstein DJ, Patel CB, Ewald GA, Tatooles AJ, Silvestry SC, et al. Hemocompatibility‐related outcomes in the MOMENTUM 3 trial at 6 months: a randomized controlled study of a fully magnetically levitated pump in advanced heart failure. Circulation. 2017;135:2003–2012. [DOI] [PubMed] [Google Scholar]
- 6. Muslem R, Caliskan K, Leebeek FWG. Acquired coagulopathy in patients with left ventricular assist devices. J Thromb Haemost. 2018;16:429–440. [DOI] [PubMed] [Google Scholar]
- 7. Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, et al. Use of a continuous‐flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. [DOI] [PubMed] [Google Scholar]
- 8. Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano CA, Rame JE, Acker MA, Blackstone EH, Ehrlinger J, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370:33–40. [DOI] [PubMed] [Google Scholar]
- 9. Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, et al. A fully magnetically levitated left ventricular assist device—final report. N Engl J Med. 2019;380:1618–1627. [DOI] [PubMed] [Google Scholar]
- 10. Archer SL, Michelakis ED. Phosphodiesterase type 5 inhibitors for pulmonary arterial hypertension. N Engl J Med. 2009;361:1864–1871. [DOI] [PubMed] [Google Scholar]
- 11. Halcox JP, Nour KR, Zalos G, Mincemoyer RA, Waclawiw M, Rivera CE, Willie G, Ellahham S, Quyyumi AA. The effect of sildenafil on human vascular function, platelet activation, and myocardial ischemia. J Am Coll Cardiol. 2002;40:1232–1240. [DOI] [PubMed] [Google Scholar]
- 12. Lewis GD, Witzke C, Colon‐Hernandez P, Guerrero JL, Bloch KD, Semigran MJ. Sildenafil improves coronary artery patency in a canine model of platelet‐mediated cyclic coronary occlusion after thrombolysis. J Am Coll Cardiol. 2006;47:1471–1477. [DOI] [PubMed] [Google Scholar]
- 13. Villagra J, Shiva S, Hunter LA, Machado RF, Gladwin MT, Kato GJ. Platelet activation in patients with sickle disease, hemolysis‐associated pulmonary hypertension, and nitric oxide scavenging by cell‐free hemoglobin. Blood. 2007;110:2166–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Andersson KE. PDE5 inhibitors—pharmacology and clinical applications 20 years after sildenafil discovery. Br J Pharmacol. 2018;175:2554–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ravichandran AK, LaRue SJ, Novak E, Joseph SA, Schilling JD. Sildenafil in left ventricular assist device is safe and well‐tolerated. ASAIO J. 2018;64:280–281. [DOI] [PubMed] [Google Scholar]
- 16. Sparrow CT, LaRue SJ, Schilling JD. Intersection of pulmonary hypertension and right ventricular dysfunction in patients on left ventricular assist device support: is there a role for pulmonary vasodilators? Circ Heart Fail. 2018;11:e004255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saeed O, Rangasamy S, Selevany I, Madan S, Fertel J, Eisenberg R, Aljoudi M, Patel SR, Shin J, Sims DB, et al. Sildenafil is associated with reduced device thrombosis and ischemic stroke despite low‐level hemolysis on Heart Mate II support. Circ Heart Fail. 2017;10:e004222. [DOI] [PubMed] [Google Scholar]
- 18. Zayat R, Ahmad U, Stoppe C, Khattab MA, Arab F, Moza A, Tewarie L, Goetzenich A, Autschbach R, Schnoering H. Sildenafil reduces the risk of thromboembolic events in HeartMate II patients with low‐level hemolysis and significantly improves the pulmonary circulation. Int Heart J. 2018;59:1227–1236. [DOI] [PubMed] [Google Scholar]
- 19. Roberts KL, Shuster JE, Britt NS, Balsara KR, Graetz TJ, Helwani M, Itoh A, Tellor BR. Evaluation of clinical outcomes with phosphodiesterase‐5 inhibitor therapy for right ventricular dysfunction after left ventricular assist device implantation. ASAIO J. 2019;65:264–269. [DOI] [PubMed] [Google Scholar]
- 20. Kirklin JK, Naftel DC, Kormos RL, Pagani FD, Myers SL, Stevenson LW, Acker MA, Goldstein DL, Silvestry SC, Milano CA, et al. Interagency registry for mechanically assisted circulatory support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2014;33:12–22. [DOI] [PubMed] [Google Scholar]
- 21. Rosenthal JL, Starling RC. Coagulopathy in mechanical circulatory support: a fine balance. Curr Cardiol Rep. 2015;17:114. [DOI] [PubMed] [Google Scholar]
- 22. Walter U, Gambaryan S. CGMP and cGMP‐dependent protein kinase in platelets and blood cells. Handb Exp Pharmacol. 2009;191:533–548. [DOI] [PubMed] [Google Scholar]
- 23. Gudmundsdottir IJ, McRobbie SJ, Robinson SD, Newby DE, Megson IL. Sildenafil potentiates nitric oxide mediated inhibition of human platelet aggregation. Biochem Biophys Res Commun. 2005;337:382–385. [DOI] [PubMed] [Google Scholar]
- 24. Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121:1276–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thenappan T, Stulak JM, Agarwal R, Maltais S, Shah P, Eckman P, Emani S, Katz JN, Gregoric I, Keebler ME, et al. Early intervention for lactate dehydrogenase elevation improves clinical outcomes in patients with the HeartMate II left ventricular assist device: insights from the PREVENT study. J Heart Lung Transplant. 2018;37:25–32. [DOI] [PubMed] [Google Scholar]
- 26. Lawless M, Caldwell JL, Radcliffe EJ, Smith CER, Madders GWP, Hutchings DC, Woods LS, Church SJ, Unwin RD, Kirkwood GJ, et al. Phosphodiesterase 5 inhibition improves contractile function and restores transverse tubule loss and catecholamine responsiveness in heart failure. Sci Rep. 2019;9:6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, St Aubin C, Webster L, Rebeyka IM, Ross DB, et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–248. [DOI] [PubMed] [Google Scholar]
- 28. Gulati G, Grandin EW, Kennedy K, Cabezas F, DeNofrio DD, Kociol R, Rame JE, Pagani FD, Kirklin JK, Kormos RL, et al. Preimplant phosphodiesterase‐5 inhibitor use is associated with higher rates of severe early right heart failure after left ventricular assist device implantation. Circ Heart Fail. 2019;12:e005537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smedira NG, Blackstone EH, Ehrlinger J, Thuita L, Pierce CD, Moazami N, Starling RC. Current risks of HeartMate II pump thrombosis: non‐parametric analysis of interagency registry for mechanically assisted circulatory support data. J Heart Lung Transplant. 2015;34:1527–1534. [DOI] [PubMed] [Google Scholar]
- 30. Sparrow CT, Nassif ME, Raymer DS, Novak E, LaRue SJ, Schilling JD. Pre‐operative right ventricular dysfunction is associated with gastrointestinal bleeding in patients supported with continuous‐flow left ventricular assist devices. JACC Heart Fail. 2015;3:956–964. [DOI] [PubMed] [Google Scholar]
- 31. Vukelic S, Vlismas PP, Patel SR, Xue X, Shitole SG, Saeed O, Sims DB, Chinnadurai T, Shin JJ, Forest SJ, et al. Digoxin is associated with a decreased incidence of angiodysplasia‐related gastrointestinal bleeding in patients with continuous‐flow left ventricular assist devices. Circ Heart Fail. 2018;11:e004899. [DOI] [PubMed] [Google Scholar]
- 32. Kawabori M, Kurihara C, Critsinelis AC, Sugiura T, Kaku Y, Civitello AB, Rosengart TK, Morgan JA. Gastrointestinal bleeding after HeartMate II or HVAD implantation: incidence, location, etiology, and effect on survival. ASAIO J. 2020;66:283–290. [DOI] [PubMed] [Google Scholar]
- 33. Netuka I, Kvasnicka T, Kvasnicka J, Hrachovinova I, Ivak P, Marecek F, Bilkova J, Malikova I, Jancova M, Maly J, et al. Evaluation of von Willebrand factor with a fully magnetically levitated centrifugal continuous‐flow left ventricular assist device in advanced heart failure. J Heart Lung Transplant. 2016;35:860–867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Tables S1–S6 Figures S1–S11


