Abstract
Background
The study investigated sex differences in cognitive outcomes at 90 days after first‐ever stroke using data from a population‐based sample.
Methods and Results
The study sample consisted of 1227 participants from the 2009–2016 Brain Attack Surveillance in Corpus Christi project (south Texas, United States) who had first‐ever ischemic stroke or intracerebral hemorrhage and survived 90 days after stroke. Poststroke cognitive function was assessed by the Modified Mini‐Mental State Examination (3MSE) (range: 0–100; dementia: <78). The associations of sex with dichotomized and continuous outcomes were examined using logistic regression and tobit regression, respectively. Inverse probability weighting and multiple imputation were used to deal with missing data. The study sample was evenly distributed by sex, and primarily composed of Mexican Americans (59.1%) and non‐Hispanic whites (34.1%). Women scored 2.96 points worse on the 3MSE than men at 90 days poststroke (95% CI, −3.99 to −1.93). The prevalence of dementia was 27.6% for men (95% CI, 23.5%–31.6%) and 35.6% for women (95% CI, 31.5%–39.7%), and the unadjusted odds ratio (OR) of dementia comparing women with men was 1.45 (95% CI, 1.24–1.69). The association was attenuated after adjustment for sociodemographic, stroke, and prestroke characteristics (OR, 0.82; 95% CI, 0.61–1.09).
Conclusions
Women had worse cognitive outcomes than men at 90 days poststroke. The differences were attributable to sociodemographic and prestroke characteristics, especially widowhood status. Potential mechanisms linking widowhood to dementia in the acute poststroke stage warrant further investigation to inform interventions addressing the unique care needs of women stroke survivors with dementia and cognitive dysfunction.
Keywords: cognition, epidemiology, sex, stroke
Subject Categories: Cerebrovascular Disease/Stroke, Epidemiology, Women, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- 3MSE
Modified Mini‐Mental State Examination
- BASIC
Brain Attack Surveillance in Corpus Christi
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- MMSE
Mini‐Mental State Examination
- NIHSS
National Institutes of Health Stroke Scale
Clinical Perspective
What Is New?
The study provides contemporary, population‐based data on sex differences in poststroke cognitive outcomes, and shows that the higher prevalence of being widowed among women may be a contributing factor to worse poststroke cognitive outcomes in women compared with men.
What Are the Clinical Implications?
Women stroke survivors in special social circumstances, such as widowhood, may have greater unmet needs for social support, which is critical for postacute rehabilitation care and stroke recovery.
Stroke is a major vascular contributor to cognitive impairment and dementia, which increases the risk for disability and poor quality of life.1, 2 Compared with men, women have worse poststroke functional and quality‐of‐life outcomes, because of their older age at stroke onset, greater stroke severity, worse prestroke functioning and higher prevalence of risk factors for cognitive impairment.3 Sex differences in cognitive function and age‐related cognitive decline exist as a result of biological and social factors throughout the life course,4 and female sex is strongly associated with prestroke dementia.5 However, sex differences in cognitive function and dementia after stroke remain unclear.
Existing studies on sex differences in poststroke cognitive outcomes are few and were heterogeneous in their designs and methods making comparisons across studies challenging.3, 5 In particular, there has been a lack of population‐based studies, which limits the generalizability of the findings. Because previous studies were rarely designed to specifically investigate sex differences, residual confounding may exist because of inadequate covariate adjustment. Most importantly, older age and cognitive impairment, well‐recognized risk factors for attrition,6 are differentially distributed between men and women stroke survivors. Ignoring this informative missingness may have resulted in biased estimates of the sex difference in poststroke cognitive function such that the sex difference was underestimated.
The present study aimed to contribute to existing literature by examining potential sex differences in cognitive outcomes at 90 days poststroke and contributing factors to the sex differences, using data from a population‐based stroke study and methods to minimize selection bias caused by sex difference in attrition.
Methods
Study Population
Data were obtained from the BASIC (Brain Attack Surveillance in Corpus Christi) project, a population‐based stroke surveillance study that captures all stroke cases among residents aged 45 years and older in the nonimmigrant biethnic community of Nueces County in south Texas, United States.7 Because of the sensitive nature of the data collected for this study, data will not be publicly available. Possible stroke cases are ascertained through active and passive surveillance methods, and then validated by stroke fellowship trained physicians.8 Identified stroke patients are invited to participate in the baseline interview shortly after stroke onset, and those who complete the baseline interview are followed up at ≈ 90 days poststroke for an outcome interview. For participants who are unable to complete the interviews, proxy interviews are completed by informants on behalf of the patients. Details of the study have been described elsewhere.7
The present study drew participants from all stroke cases occurring between January 1, 2009, and December 31, 2016. Included participants were those who survived 90 days poststroke, had first‐ever ischemic stroke or intracerebral hemorrhage, and completed both baseline and outcome interviews. During the specified period, 1708 of 2278 (75.0%) 90‐day survivors of first‐ever ischemic or hemorrhagic stroke completed the baseline interview, and 1227 of them (71.8%) subsequently completed the outcome interview, which constitutes the final sample for the primary analysis (Figure 1). Data for patients who did not participate in baseline or outcome interviews were used in the analysis to account for potential differential attrition and minimize selection bias.
Figure 1. Flow diagram of the study sample, Brain Attack Surveillance in Corpus Christi project, United States, 2009–2016.
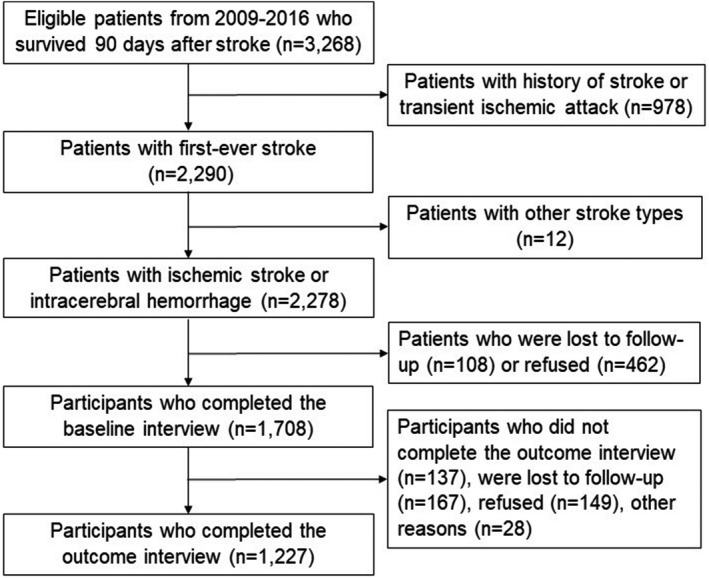
The BASIC project was approved by the institutional review boards at the University of Michigan and the local hospital systems. Written informed consent was obtained from patients or their surrogate.
Measures
The outcome measure was cognitive function at 90 days poststroke, as measured by the Modified Mini‐Mental State Examination (3MSE) among participants who were able to complete the outcome interview in‐person.9 The 3MSE has been widely used in research and clinical settings to detect decline in cognitive function, with good validity and reliability in the stroke population.10 It measures a variety of cognitive domains across a range of difficulty levels, including orientation, memory, language, reasoning, and executive function.9 The total score ranges from 0 to 100, with higher scores indicating better cognitive function. We classified participants scoring <78 as having dementia based on previous validation evidence.11, 12 Both continuous and dichotomized cognitive outcome measures were used in the analyses.
The 3MSE was administered in English or Spanish depending on participants’ preference. Approximately 5.3% of the study sample (n=52) completed the assessment in Spanish. Participants with proxy outcome interviews did not have 3MSE assessment, and therefore had missing data on cognitive outcomes. A total of 249 of 1227 participants (20.3%) had missing cognitive outcome data, among which 213 (85.5%) were because of having proxy interviews and 36 did not compete the assessment because of other reasons such as refusal or telephone interviews.
Sex (men, women), the primary independent variable, and other covariates were ascertained at baseline from interviews or medical records. Potential confounders were sociodemographic, stroke and prestroke characteristics. Sociodemographic characteristics included age, race/ethnicity (non‐Hispanic white, Mexican American, other), education (below high school, high school, vocational/some college, college or more), marital status (married/partnered, single, widowed, separated/divorced), and health insurance status (insured, uninsured). Stroke characteristics included stroke type (ischemic stroke, intracerebral hemorrhage) and initial stroke severity assessed by the National Institutes of Health Stroke Scale (NIHSS).13 Prestroke characteristics included prestroke cognitive function, prestroke disability, prestroke depression status, number of medical conditions, current smoking, and obesity. Prestroke cognitive function was measured by the Informant Questionnaire on Cognitive Decline in the Elderly,14 a widely used screening tool for dementia based on proxy‐report. Informants of the study participants were asked to rate changes in participants’ cognitive functioning before stroke, upon which participants were classified as having normal cognitive function (≤3); cognitive impairment, no dementia (>3 and <3.44); or dementia (≥3.44) before stroke.15 Prestroke disability was measured by the modified Rankin Scale, and categorized as no symptoms/disability (0 or 1), slight/moderate disability (2 or 3), and moderately severe/severe disability (4 or 5).16 Prestroke depression status was based on self‐report. Participants were asked whether they had ever been told they had depression by a doctor, and whether they were currently taking or had ever taken medications for depression. The 3 mutually exclusive categories included no history of depression, history of depression, and on medication for depression at stroke onset. The number of medical conditions was calculated as the total count of the following medical diagnoses ascertained from medical records: coronary artery disease, Alzheimer's disease or dementia, atrial fibrillation, cancer, chronic obstructive pulmonary disease, congestive heart failure, diabetes mellitus, hypertension, high cholesterol, end‐stage renal disease, epilepsy, and Parkinson's disease. Obesity was classified based on body mass index, which was calculated using measured height and weight ascertained from medical records (normal: <25; overweight: ≥25 and <30; obese: ≥30).
Statistical Analysis
We compared sample characteristics between men and women using Pearson chi‐square tests for categorical variables and Kruskal–Wallis tests for continuous variables. We examined missing data by comparing sample characteristics between included and excluded patients by baseline and outcome interview participation, respectively; and further compared sample characteristics by availability of the outcome measure in the study sample. We imputed missing values of cognitive outcomes and other covariates primarily attributable to having proxy interviews,17 and then generated inverse probability weights to account for differential attrition due to nonparticipation.18 Variables used in the imputation model included sociodemographic, stroke and prestroke characteristics obtained from the baseline interview and medical records, and poststroke outcomes obtained from the outcome interview, including neurological status assessed by the NIHSS, and disability assessed by a combined measure of activities of daily living and instrumental activities of daily living.
We estimated sex‐specific prevalence of dementia at 90 days poststroke using weighted cross‐tabulations for the dichotomized outcome measure. To examine the sex difference in the prevalence of poststroke dementia, we fit weighted logistic regression models. To examine the influence of each covariate on the sex association, we first fit an unadjusted model, and then fit a series of models by adding each covariate to the unadjusted model. Then, we fit a full model with adjustment for sociodemographic (age, race/ethnicity, education, marital status, and health insurance status), stroke (type and severity), and prestroke (prestroke cognitive function, prestroke depression status, prestroke disability, number of medical conditions, current smoking, and obesity) characteristics. In parallel with the logistic regression models, we fit a set of tobit regression models to examine the sex difference in cognitive scores, with the dependent variable being the continuous outcome measure. We examined collinearity between covariates by computing variance inflation factors.
We conducted 3 sensitivity analyses in relation to missing data. In the first, we repeated the analyses among participants with complete data, and compared them with the main analysis. In the second, we repeated the analyses among participants with normal cognitive function before stroke. In the third, we modeled the probability of missing cognitive outcome data as dependent on the cognitive score itself, because participants without cognitive assessments as a result of proxy interviews may have been more likely to have cognitive deficits.
Statistical analyses were completed with Stata version 14.2 (StataCorp LP) and SAS version 9.4 (SAS Institute Inc). SAS MI and MIANALYZE procedures were used for multiple imputation, and the MNAR statement in the MI procedure was used for the third sensitivity analysis.17 The QLIM procedure was used for tobit regression.
Results
There was no sex difference in baseline interview participation among 2278 patients who survived 90 days after first‐ever ischemic stroke or intracerebral hemorrhage (Table S1). However, women were more likely to participate in the outcome interview than men (Table S2), and more likely to have missing data on the cognitive outcome measure (Table S3). Study participants with missing outcome data had significantly greater initial stroke severity, compared with those with complete outcome data. They were also significantly older, and more likely to be widowed and less educated and have intracerebral hemorrhage and prestroke functional deficits (Table S3). There was no sex difference in missing prestroke cognitive function data (P=0.917).
In the study sample, approximately three fifths were Mexican American, one third were non‐Hispanic white, one third had educational attainment below high school, and nearly one fourth were widowed (Table 1). Men and women were approximately equally distributed; however, women were significantly more likely to be older, widowed, less educated, cognitively and functionally impaired before stroke, and to have greater stroke severity.
Table 1.
Sample Characteristics by Sex, Brain Attack Surveillance in Corpus Christi Project, United States, 2009–2016
| Total (N=1227) | Men (n=626) | Women (n=601) | P Value | |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| Age, y | 67.6±12.3 | 65.7±11.3 | 69.5±13.0 | <0.001 |
| Race/ethnicity | 0.908 | |||
| Non‐Hispanic white | 418 (34.1) | 215 (34.4) | 203 (33.8) | |
| Mexican American | 725 (59.1) | 370 (59.1) | 355 (59.1) | |
| Other | 84 (6.9) | 41 (6.6) | 43 (7.2) | |
| Educationa | 0.002 | |||
| Below high school | 400 (32.8) | 181 (29.1) | 219 (36.6) | |
| High school | 337 (27.6) | 179 (28.8) | 158 (26.4) | |
| Vocational/some college | 304 (24.9) | 150 (24.1) | 154 (25.7) | |
| College or more | 180 (14.7) | 112 (18.0) | 68 (11.4) | |
| Marital statusa | <0.001 | |||
| Married/partnered | 594 (48.5) | 377 (60.3) | 217 (36.1) | |
| Single | 103 (8.4) | 55 (8.8) | 48 (8.0) | |
| Widowed | 282 (23.0) | 64 (10.2) | 218 (36.3) | |
| Separated/divorced | 247 (20.2) | 129 (20.6) | 118 (19.6) | |
| Health insurance statusa | 0.096 | |||
| Insured | 1035 (86.2) | 514 (84.5) | 521 (87.9) | |
| Uninsured | 166 (13.8) | 94 (15.5) | 72 (12.1) | |
| Stroke characteristics | ||||
| Stroke type | 0.057 | |||
| Ischemic stroke | 1076 (87.7) | 538 (85.9) | 538 (89.5) | |
| Intracerebral hemorrhage | 151 (12.3) | 88 (14.1) | 63 (10.5) | |
| Stroke severity (NIHSS)a | 5.6±6.4 | 5.2±6.3 | 6.0±6.6 | 0.031 |
| Prestroke characteristics | ||||
| (IQCODE)a | 0.001 | |||
| Normal | 565 (46.1) | 320 (51.1) | 245 (40.8) | |
| Cognitive impairment no dementia | 338 (27.6) | 159 (25.4) | 179 (29.8) | |
| Dementia | 166 (13.5) | 67 (10.7) | 99 (16.5) | |
| Missing | 158 (12.9) | 80 (12.8) | 78 (13.0) | |
| a | <0.001 | |||
| No history of depression | 640 (52.2) | 383 (61.2) | 257 (42.8) | |
| History of depression | 153 (12.5) | 67 (10.7) | 86 (14.3) | |
| On medication for depression at stroke onset | 166 (13.5) | 55 (8.8) | 111 (18.5) | |
| Missing | 268 (21.8) | 121 (19.3) | 147 (24.5) | |
| Prestroke disability (mRS)a | <0.001 | |||
| No symptoms/disability | 590 (49.2) | 337 (55.0) | 253 (43.2) | |
| Slight/moderate disability | 508 (42.4) | 245 (40.0) | 263 (44.9) | |
| Moderately severe/severe disability | 101 (8.4) | 31 (5.1) | 70 (12.0) | |
| No. of medical conditions | 2.5±1.5 | 2.5±1.5 | 2.5±1.5 | 0.536 |
| Current smokinga | <0.001 | |||
| No | 947 (77.3) | 448 (71.7) | 499 (83.2) | |
| Yes | 278 (22.7) | 177 (28.3) | 101 (16.8) | |
| Obesity (body mass index)a | 0.002 | |||
| Normal | 307 (25.0) | 139 (22.2) | 168 (28.0) | |
| Overweight | 437 (35.6) | 252 (40.3) | 185 (30.8) | |
| Obese | 482 (39.3) | 235 (37.5) | 247 (41.2) | |
Values are expressed as mean±SD or number (percentage).
IQCODE indicates Informant Questionnaire on Cognitive Decline in the Elderly; mRS, modified Rankin Scale; and NIHSS, National Institutes of Health Stroke Scale.
Variables with missing data. The numbers of missing values are 6 for education, 1 for marital status, 26 for health insurance status, 158 for prestroke cognitive function, 268 for prestroke depression status, 28 for prestroke disability, 4 for stroke severity, 2 for current smoking, and 1 for obesity.
The prevalence of poststroke dementia was 27.6% in men (95% CI, 23.5%–31.6%) and 35.6% in women (95% CI, 31.5%–39.7%), after accounting for attrition and missing outcome data. In the unadjusted model, the odds of poststroke dementia were significantly greater in women, compared with men (odds ratio [OR], 1.45; 95% CI, 1.24–1.69). The association was attenuated toward the null after adjustment for marital status and age, respectively (Figure 2, Table S4), and remained nonsignificant after full adjustment for sociodemographic, stroke, and prestroke characteristics (OR, 0.82; 95% CI, 0.61–1.09) (Table 2). Concordantly, results from the unadjusted tobit regression model showed that women had lower 3MSE scores (estimate=−2.96; 95% CI, −3.99 to −1.93) (Table S4), but the difference diminished after covariate adjustment (estimate=0.76; 95% CI, −0.60 to 2.12) (Table 2). Variance inflation factors for testing collinearity (values <2) suggested that correlations among covariates did not have substantial impacts on the results.
Figure 2. Influence of individual covariates on the association between sex and poststroke dementia, Brain Attack Surveillance in Corpus Christi project, United States, 2009–2016.
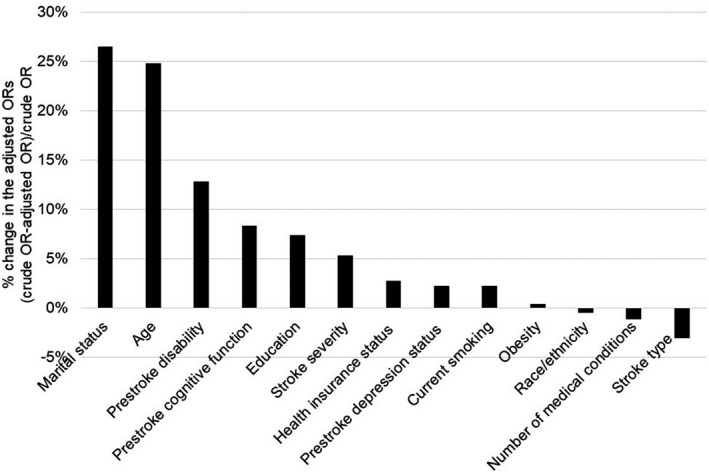
Table 2.
Results From Fully Adjusted Regression Models of the Association Between Sex and Poststroke Cognitive Outcomes, Brain Attack Surveillance in Corpus Christi Project, United States, 2009–2016
| Dichotomized Outcome | Continuous Outcome | |
|---|---|---|
| Odds Ratio (95% CI) | Estimate (95% CI) | |
| Sex | ||
| Men | Reference | Reference |
| Women | 0.82 (0.61–1.09) | 0.76 (−0.60 to 2.12) |
| Age | 1.08 (1.07–1.09) | −0.44 (−0.48 to −0.40) |
| Race/ethnicity | ||
| Non‐Hispanic white | Reference | Reference |
| Mexican American | 1.85 (1.49–2.30) | −4.07 (−5.33 to −2.80) |
| Other | 2.30 (1.70–3.10) | −4.49 (−5.97 to −3.01) |
| Education | ||
| Below high school | Reference | Reference |
| High school | 0.35 (0.30–0.41) | 7.91 (7.05–8.76) |
| Vocational/some college | 0.22 (0.16–0.31) | 10.34 (9.09–11.59) |
| College or more | 0.18 (0.14–0.23) | 12.52 (10.93–14.12) |
| Marital status | ||
| Married/partnered | Reference | Reference |
| Single | 1.47 (1.19–1.81) | −2.84 (−4.16 to −1.51) |
| Widowed | 1.36 (1.07–1.74) | −1.22 (−2.38 to −0.07) |
| Separated/divorced | 0.86 (0.67–1.12) | −0.37 (−1.43 to 0.68) |
| Health insurance status | ||
| Insured | Reference | Reference |
| Uninsured | 0.81 (0.62–1.06) | −0.21 (−1.13 to 0.72) |
| Stroke type | ||
| Ischemic stroke | Reference | Reference |
| Intracerebral hemorrhage | 2.00 (1.56–2.58) | −3.78 (−4.73 to −2.83) |
| Stroke severity (NIHSS) | ||
| Linear term | 1.16 (1.12–1.19) | −3.70 (−4.64 to −2.76) |
| Quadratic term | 1.00 (1.00–1.00) | Not applicable |
| Prestroke cognitive function (IQCODE) | ||
| Normal | Reference | Reference |
| Cognitive impairment no dementia | 1.19 (0.92–1.55) | −0.62 (−1.66 to 0.43) |
| Dementia | 2.86 (1.83–4.46) | −5.95 (−9.05 to −2.86) |
| Prestroke depression status | ||
| No history of depression | Reference | Reference |
| History of depression | 1.08 (0.75–1.54) | 0.01 (−2.33 to 2.35) |
| On medication for depression at stroke onset | 1.02 (0.63–1.66) | −0.39 (−3.21 to 2.42) |
| Prestroke disability (mRS) | ||
| No symptoms/disability | Reference | Reference |
| Slight/moderate disability | 1.24 (1.01–1.51) | −0.97 (−1.84 to −0.11) |
| Moderately severe/severe disability | 1.85 (1.10–3.12) | −3.87 (−6.68 to −1.07) |
| No. of medical conditions | 1.02 (0.95–1.10) | −0.16 (−0.56 to 0.24) |
| Current smoking | ||
| No | Reference | Reference |
| Yes | 1.46 (1.19–1.81) | −1.14 (−1.86 to −0.43) |
| Obesity | ||
| Normal | Reference | Reference |
| Overweight | 0.96 (0.71–1.29) | −0.21 (−1.87 to 1.44) |
| Obese | 0.80 (0.60–1.05) | 0.91 (−0.85 to 2.67) |
The sample size for all models was 1227.
IQCODE indicates Informant Questionnaire on Cognitive Decline in the Elderly; mRS, modified Rankin Scale; and NIHSS, National Institutes of Health Stroke Scale.
Results of the first sensitivity analysis showed that complete case analysis yielded lower prevalence of dementia (men: 19.7% [95% CI, 16.3%–23.4%]; women: 24.7% [95% CI, 20.9%–28.9%]), a weaker unadjusted association between sex and poststroke dementia (OR, 1.34; 95% CI, 0.99–1.81), and a similar adjusted association (OR, 0.75; 95% CI, 0.45–1.27). In the second sensitivity analysis, the prevalence of poststroke dementia among participants with normal cognitive function before stroke was lower than that in the full sample (men: 22.4% [95% CI, 17.8%–27.0%]; women: 27.4% [95% CI, 21.6%–33.3%]), after accounting for attrition and missing outcome data. The unadjusted and adjusted associations between sex and poststroke cognitive outcomes and the most influential factors were consistent with the results of the main analysis (Table S5). The third sensitivity analysis assuming a series of hypothesized relationships between missing values of the cognitive outcomes and the probability of missingness yielded generally consistent results (Table S6).
Discussion
This study examined sex differences in cognitive outcomes at 90 days after stroke among first‐ever stroke patients using data from a population‐based study. We found that women had significantly lower levels of cognition function and a higher unadjusted prevalence of dementia than men; however, the differences were fully attenuated after adjustment for sociodemographic, stroke, and prestroke characteristics. The major contributing factors to the worse poststroke cognitive outcomes in women compared with men were a higher prevalence of being widowed, older age at stroke onset, having worse prestroke functional and cognitive status, and lower educational attainment. The finding that the sex association was influenced by missing outcome data as a result of having a proxy interview, which closely relates to cognitive deficits that were more prevalent in women, highlights the importance of accounting for selection bias in studies of sex disparities in stroke outcomes. Because women have poorer poststroke outcomes than men, the common practice of restricting study participants to only those who are capable of completing cognitive assessment differentially excludes women and may therefore lead to biased estimates.
This study contributes to existing literature by providing contemporary, population‐based data on sex differences in poststroke cognitive outcomes. There has been relatively less research in this area compared with other stroke outcomes, such as function and quality of life, and none of the studies published since 2007 were population‐based.3 Existing findings have been mixed, partially attributable to variations in study design.3 Our results are consistent with the Cognitive Function After Stroke Nigeria Study that the greater odds of poststroke dementia at 90 days in women was reduced after covariate adjustment.3, 19 Although age and stroke characteristics were typically adjusted for in previous studies, some important confounders, such as education and prestroke cognitive and functional status, were often not accounted for,3 which might have resulted in residual confounding as demonstrated in the current study. Consistent with established risk factors for dementia, women stroke survivors in the study sample presented a high‐risk profile compared with men, including older age, lower educational attainment, greater stroke severity and worse prestroke cognitive function. Notably, the proportion of widowed participants among women was over one third, which was more than triple that in men; and marital status showed the greatest influence on the association between sex and poststroke cognitive outcomes, especially among those with normal cognitive function before stroke.
Widowhood has been considered as an older women's issue because of women's higher life expectancy in late life and lower likelihood of remarriage after becoming widowed compared with men.20 Loss of a spouse ranks among the most stressful life events in one's life21 and can cause acute stress from bereavement and chronic stress as a result of reduction in emotional, financial and social support.22 Widowhood‐related stress adversely affects mental, physical and cognitive health, as well as health behaviors, through psychosocial and physiological pathways.22 Consistent with existing findings that widowhood is associated with increased risk for dementia,23, 24 widowed participants in our study sample had a significantly higher prevalence of dementia history and risk factors for dementia before stroke, compared with those in other marital status categories. Stroke may cause additional brain pathology and functional impairments that require substantial informal care and impose difficulties for widowed patients.25 Because widowhood is highly prevalent in women stroke survivors, who have greater cognitive impairment and higher prevalence of dementia, women may have greater unmet needs for social support including emotional, instrumental and informational support, which is critical for intensive rehabilitation care and stroke recovery. Given the high burden of dementia in women and their special social circumstances, there is a need for better understanding of the role of widowhood in postacute care and its outcomes, which could lead to tailored interventions to meet their unique care needs and improve cognitive outcomes.
Depression affects men and women differently26 and is linked to dementia through biological mechanisms including vascular disease.27 In the study sample, the prevalence of self‐reported history of depression and medication use for depression before stroke tended to be higher in women than in men. However, we did not find a significant association between prestroke depression and poststroke cognitive outcomes, nor an influence of prestroke depression status on the association between sex and poststroke cognitive outcomes (Table S4). This may be because all participants in the study were stroke patients, which is a form of conditioning on stroke, a mediator or confounder of the association between depression and dementia.27
The study has several limitations. First, cognitive function at 90 days poststroke was assessed using the 3MSE, which is a dementia screening tool rather than a comprehensive diagnostic examination according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) or the National Institute on Aging‐Alzheimer's Association guidelines.28, 29 Despite its good sensitivity and specificity for identification of dementia in the stroke population,10 the cognitive scores are subject to measurement errors influenced by noncognitive factors such as cultural differences,30 and the use of a single cutoff score may result in misclassification. Our findings on the prevalence of poststroke dementia are similar to previous population‐based studies that used the Mini‐Mental State Examination (MMSE) to examine the prevalence of dementia within 1 year after stroke including those with prestroke dementia, but higher than estimates using DSM‐based diagnostic criteria.5 Additionally, the cutoff score obtained from existing literature has not been validated in this biethnic population, which may have different optimal cutoff points given their specific sociodemographic and cultural characteristics. Furthermore, the broad measure of cognition used in the present study does not cover the full spectrum of cognitive function and may result in floor and ceiling effects, which was dealt with using tobit regression in the analysis. Evidence suggests differential item functioning by sex in certain items of the MMSE,31 based upon which the 3MSE was developed. However, little is known about differential item functioning by sex of the 3MSE in stroke patients. Because the 3MSE measure was not available for baseline assessment, we cannot eliminate potential item‐response bias by using a difference‐in‐differences approach. Nevertheless, we used the total score of the 3MSE to assess overall cognitive function, which may be less sensitive to item bias than domain‐specific cognitive function. Future research should investigate the metric equivalence of stroke outcome measures across sociodemographic subgroups to enhance accuracy of epidemiological inferences in health disparities research. Second, we examined all‐cause dementia and cognitive impairment, which may be caused by stroke or prestroke neurological disorders, such as Alzheimer's disease, epilepsy, and Parkinson's disease. Sex differences in these preexisting conditions may contribute to the overall sex differences in poststroke cognitive outcomes. However, it may have limited influence on the findings because of their low prevalence in the study sample and nonsignificant sex differences after age adjustment. Consistent results with and without participants with prestroke cognitive impairment or dementia support the robustness of the findings. Third, prestroke depression status and disability were self‐reported and therefore subject to recall bias and measurement errors. Self‐reported medication use for depression may have been influenced by help‐seeking behaviors and social desirability. Fourth, because the study population was mainly composed of nonimmigrant Mexican Americans and non‐Hispanic whites, the results may not be generalized to other racial/ethnic subgroups and recent Mexican immigrants.
Conclusions
The unadjusted prevalence of dementia at 90 days after first‐ever stroke was high, with women having significantly worse cognitive outcomes compared with men. The sex differences were attributable to sociodemographic and prestroke characteristics, especially widowhood status. Potential mechanisms linking widowhood to cognitive impairment and dementia in the acute poststroke stage warrant further investigation. Future research should develop interventions to promote cognitive reserve and resilience of women at high risk for stroke and to address their unique care needs poststroke.
Sources of Funding
This work was supported by the National Institutes of Health (grant numbers R01NS38916, R01NS070941, and U24NS107214).
Disclosures
None.
Supporting information
Tables S1–S6
Acknowledgments
This study was performed in the Corpus Christi Medical Center and CHRISTUS Spohn Hospitals, CHRISTUS Health system, in Corpus Christi, Texas.
(J Am Heart Assoc. 2020;9:e016683 DOI: 10.1161/JAHA.120.016683.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.016683
For Sources of Funding and Disclosures, see page 9.
References
- 1. McGuire LC, Ford ES, Ajani UA. Cognitive functioning as a predictor of functional disability in later life. Am J Geriat Psychiat. 2006;14:36–42. [DOI] [PubMed] [Google Scholar]
- 2. Nys G, Van Zandvoort M, Van Der Worp H, De Haan E, De Kort P, Jansen B, Kappelle L. Early cognitive impairment predicts long‐term depressive symptoms and quality of life after stroke. J Neurol Sci. 2006;247:149–156. [DOI] [PubMed] [Google Scholar]
- 3. Gall S, Phan H, Madsen TE, Reeves M, Rist P, Jimenez M, Lichtman J, Dong L, Lisabeth LD. Focused update of sex differences in patient reported outcome measures after stroke. Stroke. 2018;49:531–535. [DOI] [PubMed] [Google Scholar]
- 4. Li R, Singh M. Sex differences in cognitive impairment and Alzheimer's disease. Front Neuroendocrinol. 2014;35:385–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre‐stroke and post‐stroke dementia: a systematic review and meta‐analysis. Lancet Neurol. 2009;8:1006–1018. [DOI] [PubMed] [Google Scholar]
- 6. Chatfield MD, Brayne CE, Matthews FE. A systematic literature review of attrition between waves in longitudinal studies in the elderly shows a consistent pattern of dropout between differing studies. J Clin Epidemiol. 2005;58:13–19. [DOI] [PubMed] [Google Scholar]
- 7. Smith MA, Risser JM, Moyé LA, Garcia N, Akiwumi O, Uchino K, Morgenstern LB. Designing multi‐ethnic stroke studies: the Brain Attack Surveillance in Corpus Christi (BASIC) project. Ethn Dis. 2004;14:520–526. [PubMed] [Google Scholar]
- 8. Piriyawat P, Šmajsová M, Smith MA, Pallegar S, Al‐Wabil A, Garcia NM, Risser JM, Moyé LA, Morgenstern LB. Comparison of active and passive surveillance for cerebrovascular disease: the Brain Attack Surveillance in Corpus Christi (BASIC) Project. Am J Epidemiol. 2002;156:1062–1069. [DOI] [PubMed] [Google Scholar]
- 9. Teng E, Chui H. The Modified Mini‐Mental State Examination (3MS). Can J Psychiatry. 1987;41:114–121. [PubMed] [Google Scholar]
- 10. Grace J, Nadler JD, White DA, Guilmette TJ, Giuliano AJ, Monsch AU, Snow MG. Folstein vs Modified Mini‐Mental State Examination in geriatric stroke: stability, validity, and screening utility. Arch Neurol. 1995;52:477–484. [DOI] [PubMed] [Google Scholar]
- 11. Woodford H, George J. Cognitive assessment in the elderly: a review of clinical methods. QJM. 2007;100:469–484. [DOI] [PubMed] [Google Scholar]
- 12. Bland RC, Newman SC. Mild dementia or cognitive impairment: the Modified Mini‐Mental State Examination (3MS) as a screen for dementia. Can J Psychiatry. 2001;46:506–510. [DOI] [PubMed] [Google Scholar]
- 13. Williams LS, Yilmaz EY, Lopez‐Yunez AM. Retrospective assessment of initial stroke severity with the NIH stroke scale. Stroke. 2000;31:858–862. [DOI] [PubMed] [Google Scholar]
- 14. Jorm A, Jacomb P. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio‐demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. [DOI] [PubMed] [Google Scholar]
- 15. Jorm AF. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): a review. Int Psychogeriatr. 2004;16:275–293. [DOI] [PubMed] [Google Scholar]
- 16. Rankin J. Cerebral vascular accidents in patients over the age of 60: II. Prognosis. Scott Med J. 1957;2:200–215. [DOI] [PubMed] [Google Scholar]
- 17. Yuan Y. Multiple imputation using SAS software. J Stat Softw. 2011;45:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weuve J, Tchetgen EJT, Glymour MM, Beck TL, Aggarwal NT, Wilson RS, Evans DA, de Leon CFM. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akinyemi RO, Allan L, Owolabi MO, Akinyemi JO, Ogbole G, Ajani A, Firbank M, Ogunniyi A, Kalaria RN. Profile and determinants of vascular cognitive impairment in African stroke survivors: the CogFAST Nigeria Study. J Neurol Sci. 2014;346:241–249. [DOI] [PubMed] [Google Scholar]
- 20. Carr D, Bodnar‐Deren S. Gender aging and widowhood. International Handbook of Population Aging. New York, NY: Springer Publishing; 2009:705–728. [Google Scholar]
- 21. Holmes TH, Rahe RH. The social readjustment rating scale. J Psychosom Res. 1967. [DOI] [PubMed] [Google Scholar]
- 22. Waite LJ. Marital history and well‐being in later life. International Handbook of Population Aging. New York, NY: Springer Publishing; 2009:691–704. [Google Scholar]
- 23. Sommerlad A, Ruegger J, Singh‐Manoux A, Lewis G, Livingston G. Marriage and risk of dementia: systematic review and meta‐analysis of observational studies. J Neurol Neurosurg Psychiatry. 2018;89:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shin SH, Kim G, Park S. Widowhood status as a risk factor for cognitive decline among older adults. Am J Geriat Psychiat. 2018;26:778–787. [DOI] [PubMed] [Google Scholar]
- 25. Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci. 2009;11:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. [DOI] [PubMed] [Google Scholar]
- 27. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA, American Psychiatric Association, 2013. [Google Scholar]
- 29. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dement. 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Black SA, Espino DV, Mahurin R, Lichtenstein MJ, Hazuda HP, Fabrizio D, Ray LA, Markides KS. The influence of noncognitive factors on the Mini‐Mental State Examination in older Mexican‐Americans: findings from the Hispanic EPESE. J Clin Epidemiol. 1999;52:1095–1102. [DOI] [PubMed] [Google Scholar]
- 31. Jones RN, Gallo JJ. Education and sex differences in the mini‐mental state examination: effects of differential item functioning. J Gerontol B: Psychol. 2002;57:P548–P558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6


