Abstract
Atrial fibrillation is a common clinical manifestation in hospitalized patients with coronavirus disease 2019 (COVID‐19). Medications used to treat atrial fibrillation, such as antiarrhythmic drugs and anticoagulants, may have significant drug interactions with emerging COVID‐19 treatments. Common unintended nontherapeutic target effects of COVID‐19 treatment include potassium channel blockade, cytochrome P 450 isoenzyme inhibition or activation, and P‐glycoprotein inhibition. Drug‐drug interactions with antiarrhythmic drugs and anticoagulants in these patients may lead to significant bradycardia, ventricular arrhythmias, or severe bleeding. It is important for clinicians to be aware of these interactions, drug metabolism changes, and clinical consequences when choosing antiarrhythmic drugs and anticoagulants for COVID‐19 patients with atrial fibrillation. The objective of this review is to provide a practical guide for clinicians who are managing COVID‐19 patients with concomitant atrial fibrillation.
Keywords: antiarrhythmic drug, atrial fibrillation, COVID‐19
Subject Categories: Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- AAD
antiarrhythmic drug
- AF
atrial fibrillation
- CHF
congestive heart failure
- COVID‐19
coronavirus disease 2019
- CYP
cytochrome P
- IK
potassium channel
- INa
sodium channel
Coronavirus disease 2019 (COVID‐19) is caused by severe acute respiratory syndrome coronavirus 2, which was first reported in 2019 in Wuhan, Hubei, China. 1 , 2 It infects host cells through angiotensin‐converting enzyme 2 membrane receptors, which are highly expressed in heart and lungs, especially in patients with underlying cardiovascular disease. 3 COVID‐19 patients with underlying cardiovascular disease have increased risk of morbidity and mortality from complicated myocardial injury, myocarditis, congestive heart failure (CHF), thromboembolism, and arrhythmias. 4 Atrial fibrillation (AF) is the most common arrhythmia seen in critically ill patients. 5 Several potential pharmacotherapies are emerging. 4 , 6 Risks of arrhythmogenic effects of COVID‐19 pharmacotherapies have been noted 4 , 7 (Table). The overall objective of this review is to provide practical guidance for clinicians who are managing COVID‐19 patients with concomitant AF recommendations (Figure 1) based on the pharmacological characteristics of COVID‐19 pharmacotherapies, anticoagulants, and antiarrhythmic drugs (AADs) in the setting of limited clinical data.
Figure 1.
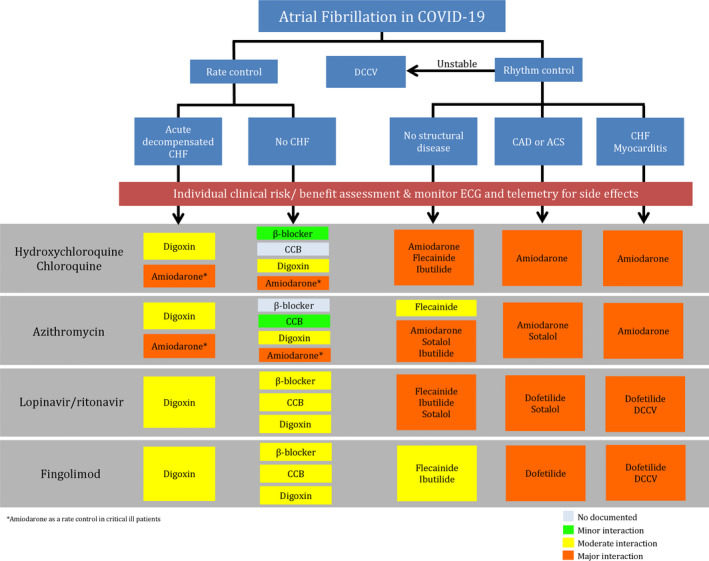
Management guidance algorithm for atrial fibrillation and concomitant coronavirus disease 2019 (COVID‐19).
ACS indicates acute coronary syndrome; CAD, coronary artery disease; CCB, calcium channel blocker; CHF, congestive heart failure; and DCCV, direct current cardioversion.
Table 1.
Mechanisms, Pharmacokinetics, and Dynamics of the Emerging COVID‐19 Pharmacotherapy
| Pharmacotherapy | Mechanism of Action | Metabolism | Mechanism of Interaction | Clinical Manifestation | |
|---|---|---|---|---|---|
| Interaction With AADs | Interaction With Anticoagulants | ||||
| Chloroquine/hydroxychloroquine |
|
CYP2C8, CYP3A4, CYP2D6, and CYP1A1 |
|
|
None |
| Azithromycin |
|
MRP2 and ABCB (minimally by CYP3A4) |
|
|
|
| Lopinavir/ritonavir |
|
CYP3A4 |
|
|
|
| Remdesivir |
|
Mainly by phosphorylation in nonspecific organ and partially by CYP2C8, CYP2D6, and CYP3A4 |
|
None | None |
| Ribavirin |
|
Mainly by hydrolase and partially by CYP2C8, CYP2D6, and CYP3A4 | None | None | None |
| Favipiravir |
|
Oxidase and renally excreted | None | None | None |
| Umifenovir |
|
CYP3A4 |
|
|
None |
| Interferon |
|
Proteolytic degradation by lysosomal enzymes, which are excreted in urine | None | None | None |
| Tocilizumab |
|
Unknown (expected to undergo catabolism) |
|
None |
|
| Sarilumab |
|
Unknown (expected to undergo catabolism) |
|
None |
|
| Pirfenidone |
|
CYP1A2 and renally excreted | None | Amiodarone, a CYP1A2 inhibitor, may increase pirfenidone plasma concentration | None |
| Bevacizumab |
|
The reticuloendothelial system | None | None | None |
| Eculizumab |
|
The reticuloendothelial system | None | None | None |
| Lenzilumab |
|
The reticuloendothelial system | None | None | None |
| Methylprednisolone |
|
CYP3A4 |
|
|
|
| Fingolimod |
|
CYP4F2 |
|
|
None |
AAD indicates antiarrhythmic drug; ABCB, ATP binding cassette subfamily B; ACE2, angiotensin‐converting enzyme 2; COVID‐19, coronavirus disease 2019; CYP, cytochrome P; DOAC, direct oral anticoagulant; GM‐CSF, granulocyte‐macrophage colony‐stimulating factor; ICaL, L‐type calcium channel; IKach, acetylcholine‐dependent potassium channels; IKr, potassium channel; LMWH, low‐molecular‐weight heparin; MRP2, multidrug resistance‐associated protein 2; TdP, torsade de pointes; and VEGF, vascular endothelial growth factor.
AF in COVID‐19
The prevalence of cardiac arrhythmias was up to 16.7% in hospitalized patients and as high as 44.4% in the intensive care unit in Wuhan. 8 In New York City, arrhythmias were reported in 24 of 130 patients (18.5%) who required invasive mechanical ventilation, of whom 23 of 24 patients (95.8%) had atrial arrhythmias. 9 In an Italian cohort of 99 patients hospitalized with COVID‐19, AF was found in 19% of all cases and in 36% of patients with underlying cardiovascular disease, and AF was more common in patients who did not survive (42.1% versus 32.5%). 10 In a small Italian report, 75% of hospitalized COVID‐19 geriatric patients in Italy had a medical history of AF. 11
Evidence of myocardial injury was noted in 19.7% of 416 hospitalized COVID‐19 patients, leading to a higher mortality rate compared with those without myocardial injury (51.2% versus 4.5%, respectively). 12 Viral activated cytokine storm syndrome in fulminant myocarditis could play a major role in COVID‐19 mortality. 13 CHF was present in up to 23.0% of COVID‐19 patients and was more prevalent in nonsurvivors compared with survivors (52% versus 12%; P<0.0001). 14 AF is associated with in‐hospital mortality and morbidity in patients with acute myocarditis. 15 Inflammatory cytokines, including CRP (C‐reactive protein), interleukin‐6, and tumor necrosis factor‐α, may have an important role in AF pathogenesis. 16 Guidelines for the management of AF in various conditions and populations have been published 17 , 18 ; however, guidance for managing AF in COVID‐19 patients is currently not available.
Overview of Mechanisms and Pharmacokinetics and Dynamics of the Emerging COVID‐19 Pharmacotherapy
A list of emerging COVID‐19 pharmacotherapies is summarized in Table. Pharmacokinetics and pharmacodynamics of AADs (Figure 2) and anticoagulants (Figure 3), focused on known and plausible drug‐drug interactions with emerging COVID‐19 pharmacotherapies, are discussed. Major interactions are illustrated in Figure 4.
Figure 2.
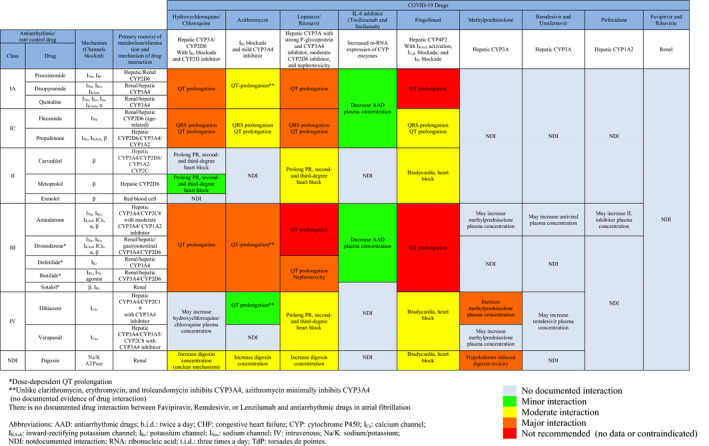
AADs Used in AF and Their Drug Interaction With COVID‐19 Therapy.
AF indicates atrial fibrillation; and COVID‐19, coronavirus disease 2019.
Figure 3.
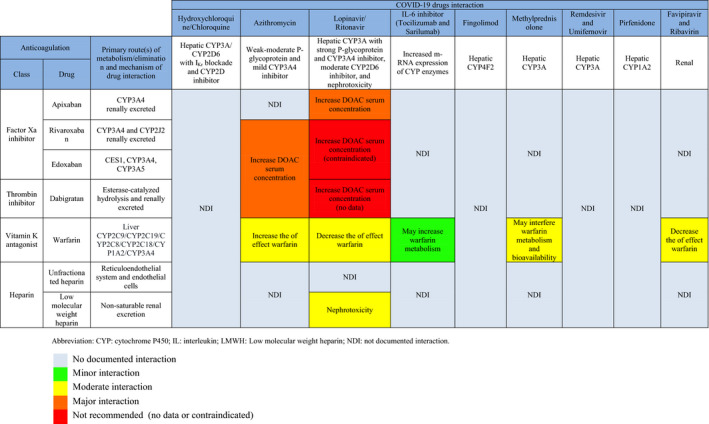
Anticoagulation Drugs Used in AF and Potential Drug‐Drug Interactions With COVID‐19 Therapy.
AF indicates atrial fibrillation; and COVID‐19, coronavirus disease 2019.
Figure 4.
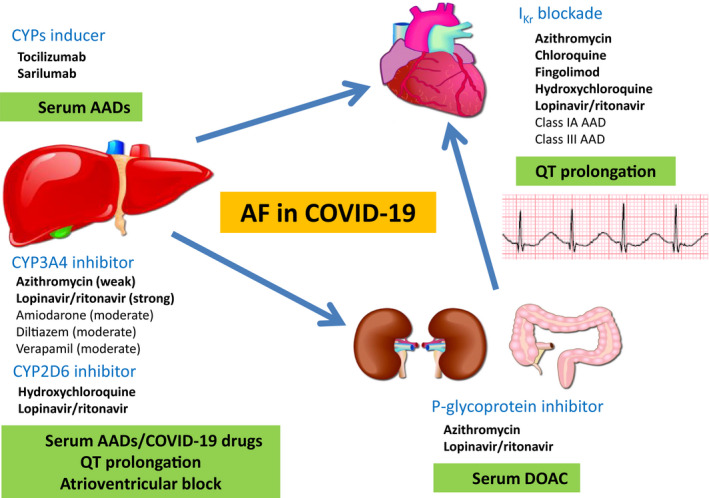
Drug‐drug interactions between antiarrhythmic drugs/anticoagulants in atrial fibrillation (AF) and emerging coronavirus disease 2019 (COVID‐19) pharmacotherapies. AAD indicates antiarrhythmic drug; CYP, cytochrome P; DOAC, direct oral anticoagulant; and IKr, potassium channel.
Hydroxychloroquine and Chloroquine
Chloroquine and hydroxychloroquine (less toxic metabolite of chloroquine) block viral entry into cells, reduce cytokines, and inhibit autophagy and lysosomal activity in host cells 19 (Table). They are metabolized by cytochrome P (CYP) 2C8, CYP3A4, CYP2D6, and CYP1A1. They are both CYP2D inhibitors, have a long half‐life, have a large volume of distribution, and are renally excreted (chloroquine, 51%; and hydroxychloroquine, 21%). 20 They also block potassium channel (IKr), potentially prolonging QT, leading to drug‐induced torsade de pointes (Figure 4) in those with concomitant use of drugs prolonging QT and drugs with CYP metabolic inhibition, and patients with reduced repolarization reserve (concealed or manifested congenital long‐QT syndrome). 4
Macrolide
Azithromycin and hydroxychloroquine in combination increase viral clearance compared with hydroxychloroquine monotherapy. 21 The mechanism is likely caused by induction of interferon and interferon‐related genes. 22 Major drug interaction with AADs is QT prolongation from IKr blockade effect of azithromycin 23 (Figure 4). Unlike other macrolides, which are strong CYP3A4 inhibitors, azithromycin minimally inhibits CYP3A4. Azithromycin is a P‐glycoprotein substrate and a weak to moderate P‐glycoprotein inhibitor, which may interact with anticoagulants (Figure 3).
Antiviral Therapy
Lopinavir, a protease inhibitor, is extensively metabolized by CYP3A4 and was therefore combined with ritonavir, a strong CYP3A inhibitor, to enhance its efficacy. Lopinavir/ritonavir has strong drug interactions with AADs (Figure 2). Lopinavir is metabolized by the liver and excreted in the gastrointestinal tract, with minimal renal excretion but significant nephrotoxicity (Table). Ritonavir is also a strong P‐glycoprotein inhibitor and may potentially interact with anticoagulants 24 (Figure 4).
Nucleotide analogs that inhibit RNA‐dependent RNA polymerase, such as remdesivir (adenosine analog), ribavirin (guanosine analog), and favipiravir (purine analog), are being studied in COVID‐19 patients. Remdesivir is mainly metabolized by hydrolase and partially metabolized by CYP2C8, CYP2D6, and CYP3A4. Remdesivir is a CYP3A4 inhibitor in vitro, but no clinically significant drug interaction is documented, likely because of its rapid clearance 25 (Table). Ribavirin is metabolized by non–organ‐specific phosphorylation and renally excreted without CYP metabolism. 4 , 26 Ribavirin does not have documented interaction with AAD but elevates international normalized ratio when used with warfarin by unclear mechanisms. 4 , 27 Favipiravir was approved for influenza treatment in Japan. It is metabolized by oxidase and excreted in the urine. 28 Cardiovascular adverse effects are not yet established. 4
Umifenovir inhibits membrane fusion of viral envelope targeting the S‐protein/angiotensin‐converting enzyme 2 interaction. It is approved in China and Russia. It is mainly metabolized by CYP3A4 and excreted by gastrointestinal tract. 29 There is no documented evidence of drug interaction between AAD and umifenovir. However, given its CYP3A4 substrate, plasma concentration of umifenovir may be increased with amiodarone, diltiazem, and verapamil (Table).
Interferon is a cytokine that has antiviral activity by interfering with viral replication and promoting activation of the adaptive immune system. Interferons undergo proteolytic degradation by lysosomal enzymes and are excreted in urine. 30 The cardiovascular adverse effects include nonspecific arrhythmias, myocardial infarction, cardiomyopathy, and hypotension. Their interaction with AADs is unknown. 4
Interleukin Inhibitor
Critically ill COVID‐19 patients have elevated inflammatory cytokines, including interleukin, granulocyte/macrophage colony‐stimulating factor, interferon, and tumor necrosis factor‐α. 1 Tocilizumab and sarilumab are interleukin‐6 inhibitors. They were found to increase mRNA expression of CYP 450 isoenzymes by reversing the CYP inhibition effect of interleukin‐6. 31 Pirfenidone inhibits interleukin‐β1 and interleukin‐4. Its cardiovascular adverse effects and interaction with AADs are unknown. 4 It is metabolized by CYP1A2 and renally excreted. 32 Amiodarone, a CYP1A2 inhibitor, may increase pirfenidone plasma concentration (Table).
Monoclonal Antibody
Bevacizumab (vascular endothelial growth factor inhibitor), eculizumab (compliment inhibitor), and lenzilumab (granulocyte/macrophage colony‐stimulating factor inhibitor) are under investigation. 33 Mechanism of action includes inhibiting inflammatory cytokines. They are metabolized by the reticuloendothelial system, and a common cardiovascular adverse effect is hypertension. However, their interaction with AADs is unknown. Bevacizumab can cause myocardial toxicity and thromboembolic events, and eculizumab may cause tachycardia and peripheral edema. 4
Immunosuppression
Methylprednisolone is an anti‐inflammatory agent that alters gene expression. It is metabolized by CYP3A4 and renally excreted but does not affect CYP3A4 activity. 34 No significant drug interaction with AADs is reported.
Fingolimod is an immunosuppressive agent through sphingosine 1‐phosphate receptor modulator, which reduces lymphocyte migration. Fingolimod has L‐type calcium channel blockade effect, causing prolongation of PR, RR, and QT intervals. It also activates acetylcholine‐dependent potassium channels in sinoatrial node, causing dose‐dependent bradycardia. 35 Fingolimod is not recommended in patients with recent myocardial infarction, decompensated CHF, stroke, high‐grade atrioventricular block, corrected QT interval >500 ms, or concomitant use of class IA or class III AADs. 36 It is metabolized by hepatic CYP4F2 and excreted in gastrointestinal tract.
Antiarrhythmic and Rate Control Drugs for AF and Interactions With COVID‐19 Pharmacotherapy
Antiarrhythmic drug‐drug interactions were reported as the most common cause of drug‐related life‐threatening ventricular arrhythmias, and 84% of these cases were associated with CYP inhibition and 74% were QT prolongation related. 37 Most COVID‐19 pharmacotherapies under investigation have unintended nontherapeutic target effects, including IKr blockade, CYP inhibition, and P‐glycoprotein inhibition (Figure 4). Lopinavir/ritonavir is the only drug that has nephrotoxicity. A summary of AADs used in AF and their interactions with COVID‐19 pharmacotherapies are shown in Figure 2. Dosages and routes of administration are provided in Table S1. A stepwise approach in managing COVID‐19 patients who present with AF is shown in Figure 1.
Class IA
Quinidine is a vagolytic and α‐blocker with use‐dependent sodium channel (INa) blockade and IKr reverse use‐dependent blockade, 38 therefore prolonging the QT interval. 39 It is primarily metabolized by CYP3A4. Plasma concentration of quinidine can be decreased with CYP3A4 inducers (tocilizumab and sarilumab) and can be increased with CYP3A4 inhibitors (lopinavir/ritonavir) 40 (Figure 2).
Disopyramide is also a use‐dependent INa blocker and a reverse use‐dependent IKr blocker. Disopyramide has potent anticholinergic effects and should be avoided in glaucoma, prostatic hyperplasia, and myasthenia gravis patients. 38 Given its negative inotropic effect, disopyramide is not recommended in patients with CHF. 41 Plasma concentration of disopyramide can be decreased through CYP3A4 metabolism 40 (Figure 2).
Procainamide is an INa and IKr blocker. It is available in the intravenous form and can be useful in patients with Wolff‐Parkinson‐White syndrome presenting with preexcited AF. 17 Procainamide is partially metabolized by CYP2D6 42 and mainly (60%) excreted by the kidney. 43 Plasma concentration of procainamide can be decreased with CYP2D6 inducers (tocilizumab and sarilumab) (Figure 2).
Because chloroquine, hydroxychloroquine, azithromycin, and fingolimod have IKr blocking effect, concomitant use of a class IA AAD could potentiate QT prolongation. 39 Interactions of class IA AADs with lopinavir/ritonavir, interleukin‐6 inhibitors, and azithromycin are at the CYP metabolic level. Any class IA AAD should be used with caution when combined with hydroxychloroquine, chloroquine, macrolides, or lopinavir/ritonavir (Figures 1 and 2). Documentation of baseline and sequential QT intervals following initiation of an AAD is recommended. Class IA AAD is not recommended in patients who are treated with fingolimod attributable to QT prolongation and risk of torsade de pointes. 35 , 36 Class IA AADs are not used often in the contemporary management of AF because of less than desired efficacy, cardiac and extracardiac adverse effects, and multiple potential drug‐drug interactions.
Class IC
Flecainide is a use‐dependent INa blocker with moderate negative inotropic effect. Because of specific blocking effect on INa, unlike class IA, flecainide delays depolarization (prolonging QRS duration) with minimal effect on ventricular repolarization (QT interval). 38 However, there are several case reports stating that flecainide can lead to prolonged QT and torsade de pointes. 44 Flecainide is predominantly metabolized by CYP2D6. 45 Flecainide is reasonable for pharmacological cardioversion in the short‐term setting and can be used to maintain sinus rhythm in patients without coronary artery disease or structural heart disease. 17 Because of possible increases in plasma concentration of flecainide when administered with hydroxychloroquine/chloroquine and lopinavir/ritonavir (CYP2D6 inhibitors), we recommend sequential electrocardiographic monitoring before and after drug initiation.
Propafenone is an INa blocker with mild β‐blocking effect (negative inotropic and chronotropic effect) and predominantly metabolized by CYP2D6. Similar to flecainide, propafenone has minimal effect on repolarization. We recommend monitoring for QRS/QT prolongation when administered with hydroxychloroquine/chloroquine and lopinavir/ritonavir (CYP2D6 inhibitors). Given documented prolonged QT from IKr blockade by azithromycin, 23 we also recommend ECG monitoring when azithromycin is used with class IC AADs.
Because myocarditis and CHF are common clinical manifestations in critically ill COVID‐19 patients, the use of class IC AADs in this population needs to be exercised with caution. Their interactions with CYP2D6 inducers (tocilizumab and sarilumab) and CYP2D6 inhibitors (litonavir/ritonavir) need to be recognized by closely monitoring any ECG changes. Fingolimod increases the risk of bradycardia and heart block through L‐type calcium channel and acetylcholine‐dependent potassium channel blockade. ECG before starting AADs, ECG at 6 hours after first dose, and continuous telemetry monitoring are recommended. 36
Class II
β Blockers are recommended as a first‐line therapy for rate control in AF with COVID‐19 infection. 46 All β blockers may have drug interactions with lopinavir/ritonavir, including PR prolongation, second‐degree heart block, and third‐degree heart block. 24 , 47 Lopinavir/ritonavir may increase carvedilol and metoprolol serum concentration through CYP2D6 inhibition, 47 and telemetry should be monitored closely (Figure 1).
Hydroxychloroquine and chloroquine inhibit CYP2D6 and may increase plasma concentration of metoprolol 48 ; a similar effect may be observed in carvedilol but has never been studied.
Carvedilol is a nonselective β blocker. Only immediate release is recommended in AF guidelines and only oral form is available, which limits its utility in acute AF with rapid ventricular response. 17 , 49 Metoprolol and esmolol are cardioselective β blockers. 50 Metoprolol comes in both intravenous and oral forms, 17 whereas esmolol only has intravenous preparation. Both are effective when administered in AF, with rapid ventricular response. 17 β Blockers should also be avoided in acute decompensated CHF and bronchospasm. 49
Class III
Amiodarone blocks multiple ion channels, including INa, IKr, calcium channel, and acetylcholine‐dependent potassium channel, as well as α and β receptors. 38 It prolongs QT but is rarely associated with torsade de pointes (<0.5%). 51 Amiodarone is the most effective and most common AAD used in AF. 52 It is preferred for AF rate and rhythm control in critically ill COVID‐19 patients with significant CHF or borderline blood pressure. 46 However, information on amiodarone and COVID‐19 pharmacotherapy interactions is limited. Surveillance for liver, lung, and thyroid toxicity is recommended for long‐term amiodarone therapy and for short‐term use if clinically indicated. Long‐ and short‐term presentations of amiodarone toxicity have been reported. 53 , 54 Pulmonary toxicity may be a concern for COVID‐19 patients with severe pneumonia who require prolonged mechanical ventilation. Amiodarone should be used with extreme caution when coadministered with lopinavir/ritonavir (QT prolongation).
Dronedarone shares similar pharmacokinetics with amiodarone 38 ; however, its use is contraindicated in patients with CHF or left ventricular dysfunction. 55 Dronedarone is contraindicated with lopinavir/ritonavir because of dependence on CYP3A4 for clearance and risk of life‐threatening arrhythmia. 47 Therefore, the utility of dronedarone may be limited for AF in COVID‐19 patients.
Amiodarone and dronedarone may cause QT prolongation, particularly when used with hydroxychloroquine, chloroquine, or macrolides. Plasma concentration of amiodarone and dronedarone can be decreased when administered with tocilizumab or sarilumab (CYP3A4 inducers) (Figure 4).
Dofetilide is an IKr blocker and predominantly excreted by the kidney (Figure 2). Hospitalization for ECG monitoring is required for the 3‐day loading period. 38 Dofetilide is more effective than cardioversion in maintaining sinus rhythm 56 and proved to be safe in patients with underlying CHF and myocardial infarction. 57 Information is not available on any long‐term use of dofetilide in patients on COVID‐19 pharmacotherapy.
Ibutilide is an IKr blocker and late inward INa agonist. Ibutilide is used for pharmacological cardioversion (Figure 1) of AF or atrial flutter. QT interval must be monitored for 2 to 6 hours after the infusion. 58 Ibutilide is metabolized by CYP3A4 and CYP2D6.
Dofetilide and ibutilide use may be limited in COVID‐19 patients already receiving hydroxychloroquine, chloroquine, macrolides, or lopinavir/ritonavir because of their intrinsic dose‐dependent QT prolongation. However, the use of ibutilide may be a good option for conversion of acute symptomatic AF (Figure 1).
Sotalol is an IKr blocker (dose dependent) with moderate β‐blockade effect. 38 Sotalol is renally excreted (Figure 2). 38 If sotalol is used in the presence of lopinavir/ritonavir, which is nephrotoxic, QT interval should be followed closely. Sotalol may be used with lopinavir/ritonavir, tocilizumab, or sarilumab, given lack of interaction on CYP3A4 or CYP2D6. However, there are dose‐dependent QT prolongation drug interactions with hydroxychloroquine, chloroquine, and macrolides, so monitoring should be implemented. Class III AADs are generally contraindicated in patients who are treated with fingolimod because of QT prolongation and risk of torsade de pointes. 36
Class IV
Nonhydropyridine calcium channel blockers are used as first‐line rate‐control agents in AF. 17 Diltiazem and verapamil block L‐type calcium channels and are contraindicated in patients with decompensated CHF or significantly reduced ejection fraction because of negative inotropic effect 17 , 49 (Figure 1).
Diltiazem is metabolized by CYP2C19 and CYP3A4. The use of diltiazem (P‐glycoprotein inhibitor) with azithromycin (P‐glycoprotein substrate) has been reported to prolong QT interval, leading to torsade de pointes. 59 Lopinavir/ritonavir may cause prolonged PR and second‐ or third‐degree heart block when used with calcium channel blockers. 24 Lopinavir/ritonavir can increase calcium channel blocker plasma concentration through CYP3A4 inhibition and should be closely monitored 47 , 60 (Figure 1).
Digoxin
Digoxin is a sodium/potassium ATPase pump inhibitor. It could be considered in CHF patients given lack of negative inotropic effect 17 and in patients with marginal blood pressure who do not tolerate calcium channel blockers or β blocker.
Lopinavir/ritonavir increases serum digoxin concentration and toxicity through inhibition of P‐glycoprotein. 61 Lopinavir/ritonavir may cause prolonged PR and second‐ or third‐degree heart block when used with digoxin. 24 Routine monitoring of digoxin levels should be considered.
COVID‐19 patients treated with both fingolimod and digoxin should receive ECG before medication initiation, sequential ECG, and telemetry monitoring for bradycardia and QT prolongation. 36
Concomitant Use of Anticoagulation Therapy and COVID‐19 Drug Therapy
Coagulopathy has been reported in critically ill COVID‐19 patients, and anticoagulation with heparin has shown some mortality benefit. 62 The prevalence of drug‐drug interactions from anticoagulation was reported as up to 26.3% in the AF population. 63 The interaction increased risk of bleeding up to 7‐fold 63 and is expected to be higher in COVID‐19 patients. The guiding principles for anticoagulation in COVID‐19 patients with AF are the same as in patients without COVID‐19, although little is known about potential drug‐drug interactions with COVID‐19 pharmacotherapy. We aim to review the choice of anticoagulation on the basis of possible drug interactions with COVID‐19 pharmacotherapy, as summarized in Figure 3.
Direct Oral Anticoagulation
Direct Factor Xa Inhibitor
Apixaban is predominantly metabolized by CYP3A4 with a minor (27%) component of renal elimination. Lopinavir/ritonavir is a strong CYP3A4 and P‐glycoprotein inhibitor. Coadministration is generally not recommended, but if necessary, the dose can be reduced by 50% (discontinue if on 2.5 mg twice a day) (Table S1). 4
Rivaroxaban is metabolized in the liver by CYP3A4, with 30% excreted in the urine. It also has a drug interaction with lopinavir/ritonavir (strong CYP3A4 and P‐glycoprotein inhibitor), and coadministration is not recommended. 4 However, rivaroxaban can be used in patients who receive azithromycin (weak CYP3A4 inhibitor) with caution (Figure 3). 64
Edoxaban is mainly excreted in the urine, with <4% metabolized by liver CYP3A4. The dose should be reduced with concomitant use of azithromycin (P‐glycoprotein inhibitor) (Table S1). 65 Concurrent treatment with ritonavir/lopinavir (strong P‐glycoprotein) and edoxaban is not recommended, given lack of adequate data on interaction. 66
Direct Thrombin Inhibitor
Dabigatran is hydrolyzed and 80% renally excreted. However, there are no data on any interaction with azithromycin or ritonavir. 67
Vitamin K Antagonist
Azithromycin increases the effect of warfarin, 68 whereas ribavirin and lopinavir/ritonavir diminish warfarin's effect by unclear mechanisms. In several case reports, the dose of warfarin needed to be increased up to 40% with ribavirin and 125% with ritonavir to maintain international normalized ratio goal. 27 , 69 Tocilizumab and sarilumab can potentially increase warfarin metabolism through induced CYPs. Steroids may increase the bioavailability of warfarin, 70 affecting international normalized ratio. We recommend regular international normalized ratio monitoring in patients who are treated with COVID‐19 pharmacotherapy.
Heparin
Unfractionated heparin and enoxaparin potentiate antithrombin III activity. They have no documented drug interaction with COVID‐19 pharmacotherapy. Unfractionated heparin is metabolized by the reticuloendothelial system and endothelial cells, and enoxaparin metabolites are renally excreted. 71
Therefore, we recommend heparin as an anticoagulant of choice in COVID‐19 patients with AF in the short‐term setting. Direct oral anticoagulants can be used in AF patients with COVID‐19 after hospital discharge without lopinavir/ritonavir.
Clinical Approach
Stepwise approach in COVID‐19 patients with AF is summarized in Figure 1.
General Considerations
QT interval should be monitored closely when class IA, IC, and III AADs are administered with hydroxychloroquine/chloroquine, macrolide, or lopinavir/ritonavir.
Classes IA and IC are contraindicated in COVID‐19 patients on fingolimod.
For patients on fingolimod, we recommend obtaining an ECG before AAD initiation, ECG at 6 hours after first dose, periodic ECG, and continuous telemetry monitoring.
β Blockers are recommended as a first‐line therapy for rate control strategy.
Calcium channel blockers are not recommended in myocarditis patients with acute decompensated CHF.
Digoxin can be used in CHF or in combination with β blockers or calcium channel blockers to achieve rate control.
Heparin is the anticoagulant of choice in hospitalized COVID‐19 patients with AF, especially if patients are being treated with lopinavir/ritonavir.
Direct oral anticoagulants can be used in AF patients with COVID‐19 after discharge without lopinavir/ritonavir.
Rhythm Control
Amiodarone can be useful for rhythm and rate control in critically ill COVID‐19 patients with CHF. Close monitoring with caution is recommended with concurrent lopinavir/ritonavir use. It is not recommended in patients undergoing treatment with fingolimod.
Flecainide is a preferred option for pharmacological cardioversion in patients with no structural heart disease or coronary artery disease who are on fingolimod.
Ibutilide can be used for cardioversion in the patient is not on fingolimod.
Conclusions
The use of AADs or anticoagulants in AF may have significant adverse effects because of drug‐drug interactions with emerging COVID‐19 pharmacotherapy, including IKr blockade, CYP inhibition or enhanced activity, and P‐glycoprotein inhibition. Drug‐drug interactions may lead to increased propensity for bradycardia, tachyarrhythmias, or severe bleeding. The choice of AADs and anticoagulants for COVID‐19 patients with AF should be individualized on the basis of drug interactions, mechanisms of drug metabolism, and potential consequences. A baseline ECG before the initiation of AAD in addition to continuous rhythm monitoring after AAD initiation are recommended. If anticoagulation is started, extra caution should be exercised when used in combination with azithromycin or lopinavir/ritonavir. It is crucial for clinicians to be aware of the indications, contraindications, and key drug‐drug interactions between AADs, anticoagulants, and emerging COVID‐19 pharmacotherapies in this challenging cohort of patients to achieve optimal clinical outcomes.
Sources of Funding
This research is partially supported by Mayo Clinic Cardiovascular Research Publishing Grant.
Disclosures
None.
Supporting information
Table S1
(J Am Heart Assoc. 2020;9:e017529 DOI: 10.1161/JAHA.120.017529.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25:291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Bondi‐Zoccai G, Brown TS, Nigoghossian C, Zidar DA, Haythe J, et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID‐19) pandemic. J Am Coll Cardiol. 2020;75:2352–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bosch NA, Cimini J, Walkey AJ. Atrial fibrillation in the ICU. Chest. 2018;154:1424–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review 2020. JAMA. 2020;323:1824–1836. [DOI] [PubMed] [Google Scholar]
- 7. Giudicessi JR, Noseworthy PA, Friedman PA, Ackerman MJ. Urgent guidance for navigating and circumventing the QTc‐prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID‐19). Mayo Clin Proc. 2020;95:1213–1221. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR Jr, Nahid M, Ringel JB, et al. Clinical characteristics of Covid‐19 in New York City. N Engl J Med. 2020;382:2372–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Inciardi RM, Adamo M, Lupi L, Cani DS, Di Pasquale M, Tomasoni D, Italia L, Zaccone G, Tedino C, Fabbricatore D, et al. Characteristics and outcomes of patients hospitalized for COVID‐19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41:1821–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fumagalli S, Salani B, Gabbani L, Mossello E, Ungar A. Covid‐19 cases in a no‐Covid‐19 geriatric acute care setting: a sporadic occurrence? Eur J Intern Med. In press. ISSN 0953‐6205, 10.1016/j.ejim.2020.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. Published online March 25, 2020. DOI: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Subahi A, Akintoye E, Yassin AS, Abubakar H, Adegbala O, Mishra T, Abdelrahman M, Shokr M, Afonso L. Impact of atrial fibrillation on patients hospitalized for acute myocarditis: insights from a nationally‐representative United States cohort. Clin Cardiol. 2019;42:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hadi HA, Alsheikh‐Ali AA, Mahmeed WA, Suwaidi JM. Inflammatory cytokines and atrial fibrillation: current and prospective views. J Inflamm Res. 2010;3:75–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 18. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 19. Zhou D, Dai SM, Tong Q. COVID‐19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. Journal of Antimicrobial Chemotherapy. 2020. dkaa114, 10.1093/jac/dkaa114. Published online March 20, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schrezenmeier E, Dorner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–166. [DOI] [PubMed] [Google Scholar]
- 21. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. In press. ISSN 0924‐8579, 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Gielen V, Johnston SL, Edwards MR. Azithromycin induces anti‐viral responses in bronchial epithelial cells. Eur Respir J. 2010;36:646–654. [DOI] [PubMed] [Google Scholar]
- 23. Zhang M, Xie M, Li S, Gao Y, Xue S, Huang H, Chen K, Liu F, Chen L. Electrophysiologic studies on the risks and potential mechanism underlying the proarrhythmic nature of azithromycin. Cardiovasc Toxicol. 2017;17:434–440. [DOI] [PubMed] [Google Scholar]
- 24. U.S. Food and Drug Administration . KALETRA safely and effectively. November 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021251s052_021906s046lbl.pdf/. Accessed April 19, 2020.
- 25. European Medicine Agency . Remdesivir Gilead. April 2020. https://www.ema.europa.eu/en/documents/other/summary‐compassionate‐use‐remdesivir‐gilead_en.pdf. Accessed April 19, 2020.
- 26. Dixit NM, Perelson AS. The metabolism, pharmacokinetics and mechanisms of antiviral activity of ribavirin against hepatitis C virus. Cell Mol Life Sci. 2006;63:832–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schulman S. Inhibition of warfarin activity by ribavirin. Ann Pharmacother. 2002;36:72–74. [DOI] [PubMed] [Google Scholar]
- 28. Du YX, Chen XP. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019‐nCoV infection. Clin Pharmacol Ther. 2020. DOI: 10.1002/cpt.1844. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 29. Deng P, Zhong D, Yu K, Zhang Y, Wang T, Chen X. Pharmacokinetics, metabolism, and excretion of the antiviral drug arbidol in humans. Antimicrob Agents Chemother. 2013;57:1743–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wills RJ. Clinical pharmacokinetics of interferons. Clin Pharmacokinet. 1990;19:390–399. [DOI] [PubMed] [Google Scholar]
- 31. Kim S, Ostor AJ, Nisar MK. Interleukin‐6 and cytochrome‐P450, reason for concern? Rheumatol Int. 2012;32:2601–2604. [DOI] [PubMed] [Google Scholar]
- 32. U.S. Food and Drug Administration . ESBRIET® (pirfenidone) capsules and film‐coated tablets, for oral use. January 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208780s000lbl.pdf/. Accessed April 19, 2020.
- 33. Hamilton JA. GM‐CSF as a target in inflammatory/autoimmune disease: current evidence and future therapeutic potential. Exp Rev Clin Immunol. 2015;11:457–465. [DOI] [PubMed] [Google Scholar]
- 34. Villikka K, Varis T, Backman JT, Neuvonen PJ, Kivisto KT. Effect of methylprednisolone on CYP3A4‐mediated drug metabolism in vivo. Eur J Clin Pharmacol. 2001;57:457–460. [DOI] [PubMed] [Google Scholar]
- 35. Pilote S, Simard C, Drolet B. Fingolimod (Gilenya((R)) ) in multiple sclerosis: bradycardia, atrioventricular blocks, and mild effect on the QTc interval: something to do with the L‐type calcium channel? Fundam Clin Pharmacol. 2017;31:392–402. [DOI] [PubMed] [Google Scholar]
- 36. Vargas WS, Perumal JS. Fingolimod and cardiac risk: latest findings and clinical implications. Ther Adv Drug Saf. 2013;4:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Coughtrie AL, Behr ER, Layton D, Marshall V, Camm AJ, Shakir SAW. Drugs and life‐threatening ventricular arrhythmia risk: results from the DARE study cohort. BMJ Open. 2017;7:e016627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zimetbaum P. Antiarrhythmic drug therapy for atrial fibrillation. Circulation. 2012;125:381–389. [DOI] [PubMed] [Google Scholar]
- 39. Wyse KR, Ye V, Campbell TJ. Action potential prolongation exhibits simple dose‐dependence for sotalol, but reverse dose‐dependence for quinidine and disopyramide: implications for proarrhythmia due to triggered activity. J Cardiovasc Pharmacol. 1993;21:316–322. [DOI] [PubMed] [Google Scholar]
- 40. Nielsen TL, Rasmussen BB, Flinois JP, Beaune P, Brosen K. In vitro metabolism of quinidine: the (3S)‐3‐hydroxylation of quinidine is a specific marker reaction for cytochrome P‐4503A4 activity in human liver microsomes. J Pharmacol Exp Ther. 1999;289:31–37. [PubMed] [Google Scholar]
- 41. Podrid PJ, Schoeneberger A, Lown B. Congestive heart failure caused by oral disopyramide. N Engl J Med. 1980;302:614–617. [DOI] [PubMed] [Google Scholar]
- 42. Lessard E, Fortin A, Belanger PM, Beaune P, Hamelin BA, Turgeon J. Role of CYP2D6 in the N‐hydroxylation of procainamide. Pharmacogenetics. 1997;7:381–390. [DOI] [PubMed] [Google Scholar]
- 43. Klotz U. Antiarrhythmics: elimination and dosage considerations in hepatic impairment. Clin Pharmacokinet. 2007;46:985–996. [DOI] [PubMed] [Google Scholar]
- 44. Oguayo KN, Oyetayo OO, Costa SM, Mixon TA. An unusual case of flecainide‐induced QT prolongation leading to cardiac arrest. Pharmacotherapy. 2014;34:e30–e33. [DOI] [PubMed] [Google Scholar]
- 45. Doki K, Homma M, Kuga K, Aonuma K, Kohda Y. CYP2D6 genotype affects age‐related decline in flecainide clearance: a population pharmacokinetic analysis. Pharmacogenet Genomics. 2012;22:777–783. [DOI] [PubMed] [Google Scholar]
- 46. Lang JP. Brigham and Women's Hospital COVID‐19 clinical guidelines. April 2020. https://covidprotocols.org. Accessed April 11, 2020.
- 47. U.S. Food and Drug Administration . NORVIR safely and effectively. June 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209512lbl.pdf/. Accessed April 19, 2020.
- 48. Somer M, Kallio J, Pesonen U, Pyykko K, Huupponen R, Scheinin M. Influence of hydroxychloroquine on the bioavailability of oral metoprolol. Br J Clin Pharmacol. 2000;49:549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Van Gelder IC, Rienstra M, Crijns HJ, Olshansky B. Rate control in atrial fibrillation. Lancet. 2016;388:818–828. [DOI] [PubMed] [Google Scholar]
- 50. Tucker WD, Theetha Kariyanna P. Selective Beta‐1‐Blockers. StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK430685/. [PubMed] [Google Scholar]
- 51. Kaufman ES, Zimmermann PA, Wang T, Dennish GW III, Barrell PD, Chandler ML, Greene HL; Atrial Fibrillation Follow‐up Investigation of Rhythm Management Investigators . Risk of proarrhythmic events in the Atrial Fibrillation Follow‐up Investigation of Rhythm Management (AFFIRM) study: a multivariate analysis. J Am Coll Cardiol. 2004;44:1276–1282. [DOI] [PubMed] [Google Scholar]
- 52. Zimetbaum P. Amiodarone for atrial fibrillation. N Engl J Med. 2007;356:935–941. [DOI] [PubMed] [Google Scholar]
- 53. Tanawuttiwat T, Harindhanavudhi T, Hanif S, Sahloul MZ. Amiodarone‐induced alveolar haemorrhage: a rare complication of a common medication. Heart Lung Circ. 2010;19:435–437. [DOI] [PubMed] [Google Scholar]
- 54. Dusman RE, Stanton MS, Miles WM, Klein LS, Zipes DP, Fineberg NS, Heger JJ. Clinical features of amiodarone‐induced pulmonary toxicity. Circulation. 1990;82:51–59. [DOI] [PubMed] [Google Scholar]
- 55. Kober L, Torp‐Pedersen C, McMurray JJ, Gotzsche O, Levy S, Crijns H, Amlie J, Carlsen J; Dronedarone Study Group . Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–2687. [DOI] [PubMed] [Google Scholar]
- 56. Singh S, Zoble RG, Yellen L, Brodsky MA, Feld GK, Berk M, Billing CB Jr. Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: the symptomatic atrial fibrillation investigative research on dofetilide (SAFIRE‐D) study. Circulation. 2000;102:2385–2390. [DOI] [PubMed] [Google Scholar]
- 57. Kober L, Bloch Thomsen PE, Moller M, Torp‐Pedersen C, Carlsen J, Sandoe E, Egstrup K, Agner E, Videbaek J, Marchant B, et al. Effect of dofetilide in patients with recent myocardial infarction and left‐ventricular dysfunction: a randomised trial. Lancet. 2000;356:2052–2058. [DOI] [PubMed] [Google Scholar]
- 58. Stambler BS, Wood MA, Ellenbogen KA, Perry KT, Wakefield LK, VanderLugt JT; Ibutilide Repeat Dose Study Investigators . Efficacy and safety of repeated intravenous doses of ibutilide for rapid conversion of atrial flutter or fibrillation. Circulation. 1996;94:1613–1621. [DOI] [PubMed] [Google Scholar]
- 59. Soubra L, Mroueh A, Kabbani A. QTc prolongation with concurrent use of azithromycin and diltiazem in an old female patient: a case report. Int J Basic Clin Pharmacol. 2013;3:242–246. [Google Scholar]
- 60. Cattaneo D, Formenti T, Astuti N, Meraviglia P, Ridolfo A, Gervasoni C. How relevant are the drug‐drug interactions between antiretroviral boosted‐based regimens and calcium channel blockers in real life? J Antimicrob Chemother. 2018;73:2271–2273. [DOI] [PubMed] [Google Scholar]
- 61. Ding R, Tayrouz Y, Riedel KD, Burhenne J, Weiss J, Mikus G, Haefeli WE. Substantial pharmacokinetic interaction between digoxin and ritonavir in healthy volunteers. Clin Pharmacol Ther. 2004;76:73–84. [DOI] [PubMed] [Google Scholar]
- 62. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Momo K, Kobayashi H, Sugiura Y, Yasu T, Koinuma M, Kuroda SI. Prevalence of drug‐drug interaction in atrial fibrillation patients based on a large claims data. PLoS One. 2019;14:e0225297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. U.S. Food and Drug Administration . XARELTO® (rivaroxaban) safely and effectively. Revised December 2011. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202439s001lbl.pdf/. Accessed April 19, 2020.
- 65. Hokusai VTEI, Buller HR, Decousus H, Grosso MA, Mercuri M, Middeldorp S, Prins MH, Raskob GE, Schellong SM, Schwocho L, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–1415. [DOI] [PubMed] [Google Scholar]
- 66. Bounameaux H, Camm AJ. Edoxaban: an update on the new oral direct factor Xa inhibitor. Drugs. 2014;74:1209–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. U.S. Food and Drug Administration . PRADAXA® (dabigatran etexilate mesylate) safely and effectively. November 2011. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022512s007lbl.pdf/. Accessed April 19, 2020.
- 68. Beckey NP, Parra D, Colon A. Retrospective evaluation of a potential interaction between azithromycine and warfarin in patients stabilized on warfarin. Pharmacotherapy. 2000;20:1055–1059. [DOI] [PubMed] [Google Scholar]
- 69. Rosene AC, Gaspar JL. Inductive effect of a ritonavir‐containing hepatitis C treatment regimen on warfarin in a patient‐A case report. J Pharm Pract. 2019;32:470–473. [DOI] [PubMed] [Google Scholar]
- 70. Peng TR, Lee LL, Wu TW. Interactions between warfarin and prednisolone: a case report. Am J Ther. 2017;24:e494. [DOI] [PubMed] [Google Scholar]
- 71. Boneu B, Caranobe C, Sie P. Pharmacokinetics of heparin and low molecular weight heparin. Baillieres Clin Haematol. 1990;3:531–544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


