Abstract
Background
Loss to follow‐up (LTFU) is common in randomized controlled trials. However, its potential impact on primary outcomes from cardiovascular randomized controlled trials is not known.
Methods and Results
We conducted a prospective systematic review (PROSPERO: CRD42019121959) for randomized controlled trials published in 8 leading journals over 5 years from January 2014 to December 2018. Extent, reporting, and handling of LTFU data were recorded, and the proportion of a trial's primary outcome results that lose statistical significance was calculated after making plausible assumptions for the intervention and control arms. These assumptions could drive differential treatment effects between the groups considering relative event incidence between LTFU participants and those included in the primary outcome. We identified 117 randomized controlled trials of which 91 (78%) trials reported LTFU, 23 (20%) reported no LTFU, and 3 (3%) trials did not report on whether LTFU occurred. The median percentage of study participants lost to follow‐up was 2% (interquartile range, 0.33%–5.3%). Only 10 trials (9%) had a low cluster of risk factors for impairment in trial quality. The percentage of trials losing statistical significance varied from 2% when the relative event incidence for LTFU between the randomized groups was 1 for the intervention arm and 1.5 for the control arm to 16% when the relative event incidence was 3 for the intervention arm and 1 for the control arm.
Conclusions
Almost 1 in 6 (16%) cardiovascular randomized trials published in leading journals may have a change in the primary outcome if plausible assumptions are made about differential event rates of participants lost to follow up. There is scope for improvement arising from LTFU in randomized trials in cardiovascular medicine.
Registration
URL: https://www.crd.york.ac.uk/prospero; Unique identifier: CRD42019121959.
Keywords: bias, loss to follow‐up, outcome, outcome and process assessment, patient dropout, randomized controlled trials, relative risk
Subject Categories: Mortality/Survival, Statements and Guidelines, Meta Analysis
Nonstandard Abbreviations and Acronyms
- LTFU
loss to follow‐up
- RCT
randomized controlled trial
- RI
relative event incidence among those lost to follow‐up to the event incidence among those followed up
Clinical Perspective
What Is New?
More than three quarters of cardiovascular randomized controlled trials have participants who are lost to follow‐up. Statistical handling of these data vary widely.
Up to 1 in 6 trials may have a change in the primary outcome if plausible assumptions are made about differential event rates of participants lost to follow up.
What Are the Clinical Implications?
In dealing with loss to follow‐up (LTFU), prevention should be prioritized; otherwise, estimation can be made by using the worst assumption.
When reporting LTFU, authors should provide baseline characteristics of LTFU participants, extent of follow‐up before exclusion, and time of dropout and should address implication of LTFU when interpreting results.
Inadequate allocation concealment is an independent factor associated with LTFU and may drive differential treatment effects.
The gold standard assessment of a medical intervention involves assessment in a randomized controlled trial (RCT).1, 2 Randomization balances the distribution of any known or unknown potential confounding factors between treatment arms.2 This mitigates the possibility of selection bias, especially if the participants’ group allocations are concealed.3 Blinding of patients, therapists, and outcome assessors is an additional useful tool to prevent bias.4, 5 Open‐label clinical trials are often unavoidable if blinding of patients and therapists is not possible.6 Clinical guidelines may be influenced by biased clinical evidence leading to undesirable impacts on patients, healthcare providers, and funders.
Up to 80% of contemporary clinical RCTs fail to achieve complete follow‐up.7, 8, 9 This important factor may affect the integrity of study conclusions. If participants are lost and the characteristics of such participants associate with clinical events, then bias can arise. This is particularly relevant in open‐label studies in which assessors know the group allocations of the participants. Loss to follow‐up (LTFU) in this scenario could favor the intervention arm and neutralize the benefit of randomization.10 It is plausible that attrition bias associated with LTFU drives either overestimation or underestimation of treatment effects.11, 12
Classification of LTFU and recommendations for dealing with LTFU have been made.13 Crucially, however, the contemporary prevalence and effects of LTFU within cardiovascular trials is not known. This prospective systematic review and meta‐analysis was designed to analyze the prevalence and potential impact of LTFU in cardiovascular RCTs. The primary aim was to assess the proportion of trials in which the primary efficacy end point would change if plausible assumptions were made about participants who were unaccounted for in the original analysis. In addition, we assessed estimates of treatment effect according to the extent, reporting, and handling of LTFU and trial characteristics associated with LTFU.
Methods
Eligibility
All supporting data are available within the article and its online supplementary files. Ethics approval was not required. We predefined reports as being eligible for inclusion in this analysis if an RCT in cardiovascular disease was described and published in one of the 5 leading general medical journals and 3 cardiology journals with the highest impact factors (Annals of Internal Medicine, BMJ, JAMA, Lancet, New England Journal of Medicine, Circulation, European Heart Journal, and Journal of the American College of Cardiology). A 5‐year publication period was set from 2014 to 2018. An additional inclusion criterion was if a patient‐important binary primary outcome was statistically significant at a 2‐sided α of 0.05. The rationale behind focusing on statistically significant trials in major journals only is that the results of these trials are most likely to influence clinical guidelines. Therefore, a change in significance of a risk ratio due to bias might affect patient care to an important extent. Cluster trials, crossover trials, N‐of‐1 trials, and trials reported in research letters were excluded. Equivalence and noninferiority studies were excluded unless the authors prespecified testing for superiority. Reports describing secondary analyses of randomized trials were excluded.
A patient‐important outcome was defined as an outcome that would be undesirable for a patient to experience and the patient would thus try to prevent it by undergoing an effective treatment. Mortality and morbidity are examples of outcomes that were included. Surrogate outcomes were considered as nonpatient important (Data S1). The protocol was registered on PROSPERO (CRD42019121959).
Literature Search
Reports of RCTs were identified from Medline and Embase using OVID (Data S2). The search was restricted to clinical RCTs in cardiovascular disease published in the selected journals between 2014 and 2018. Trials were considered statistically significant if the 2‐sided 95% CI of an estimate of the relative risk did not include 1.0 or if the 2‐sided P value for superiority was <0.05 when no CI was reported. A calibration exercise was performed before the search. One reviewer identified and reviewed the potentially eligible reports based on an agreed screening form (Data S3). The list of included and excluded reports was provided to the 2‐person reviewer team after screening. Disagreements were resolved by consensus, with the assistance of a third reviewer as required.
Data Collection
Data were extracted based on an agreed data extraction form (Data S4). The primary outcome selected for the review was the one specified within the report. If the report specified both significant primary efficacy and safety outcomes, the primary efficacy outcome was selected. If multiple primary outcomes were specified, the statistically significant outcome in the highest category on the outcome hierarchy was selected (Data S1). If both intention‐to‐treat and per‐protocol analyses were reported, we considered the statistical significance of the former; if both unadjusted and adjusted analyses were reported, the statistical significance of the former was considered. Data on study background, general characteristics, methodological quality,14 the extent of LTFU, its reporting, and its handling in the analysis related to the primary outcome were extracted. Patients were considered as LTFU if they were mistakenly randomized with inappropriate postrandomization exclusion; did not receive the intervention or adhere to treatment, with inappropriate postrandomization exclusion; withdrew consent; crossed over to another arm but were not included in the analysis; or lost contact.15 Trials were categorized by subspecialty focus: electrophysiology, heart failure, interventional cardiology, cardiac surgery, general cardiology, and cardiovascular imaging.
Statistical Analysis
The analysis is explained in more detail in the online supplement (Data S5). Methodological and reporting quality of the included trials was assessed, as suggested by Bikdeli et al14 and the Cochrane risk‐of‐bias assessment tool. 16 The extent of LTFU was calculated as the percentage of LTFU in each trial from each arm (intervention and control). The ratio of LTFU rate to primary event rate was also reported. A univariable random‐effects metaregression analysis was conducted using the log odds of participants lost to follow‐up as the dependent variable and general trial characteristics and methodological characteristics as independent variables.
The potential impact of LTFU on the primary outcome analysis was evaluated by making assumptions about the outcomes in LTFU participants (Data S6). An estimation algorithm proposed by Akl et al17 was adopted with relative incidence of outcomes in LTFU patients compared with patients who were followed‐up (RILTFU/FU), ranging from 1 to 3.16 In addition, the following common assumptions were used for calculations: none of the participants lost to follow‐up had the event; all participants lost to follow‐up had the event; none of those lost to follow‐up in the treatment group had the event, and all those lost to follow‐up in the control group did (best case scenario); all participants lost to follow‐up in the treatment group had the event and none of those in the control group did (worst‐case scenario).
For each trial, 2×2 tables were constructed for the collected data for the calculation of risk ratios associated with each assumption. The percentage of trials with their primary outcome becoming nonsignificant was calculated based on the assumptions and definition of statistical significance reported above. Trials with no LTFU were excluded in the primary analysis but included in a sensitivity analysis. An additional prespecified sensitivity analysis stratified by type of intervention was conducted. Paired differences in proportions between interventional cardiology trials and those of other cardiology subspecialties were also assessed based on different assumptions.
Results
After excluding duplicates and screening for eligibility, 117 studies were included from a total of 3668 from the initial search (Figure 1). The list of the 117 studies included in this analysis is provided in Table S1. 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 The mean age of 407 229 study participants was 64.2 years (30% female). The trial subspecialties were electrophysiology (19%) heart failure (3%), interventional cardiology (28%), cardiac surgery (3%), general cardiology (44%), and cardiovascular imaging (3%). Baseline study characteristics of the included trials are reported in Table 1.
Figure 1. Search and screening approach.
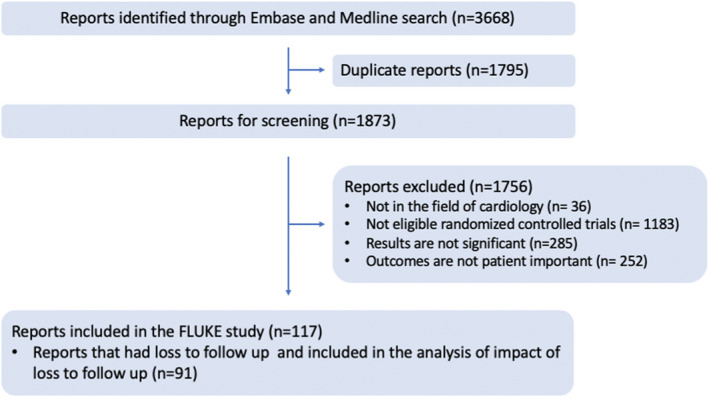
Flow of trial reports identified and screened in this analysis is shown. The search recovered 3668 reports; 1873 reports were screened after removing duplicates; 117 reports were included after screening, and reasons for exclusion are stated in text. FLUKE indicates Follow Up Loss Effect Upon Skewing Evidence.
Table 1.
General Characteristics of 117 Included Trials in the Study (n=117)
| No. (%) | |
|---|---|
| Extent of loss to follow‐up (overall) | |
| <1% | 34 (38) |
| 1% to ≤2.5% | 19 (21) |
| 2.5 to ≤5% | 14 (15) |
| 5% to ≤7.5% | 9 (10) |
| 7.5% to ≤10% | 3 (3) |
| >10% | 12 (13) |
| Cardiology subspecialty | |
| Electrophysiology | 22 (19) |
| Heart failure | 3 (3) |
| Interventional cardiology | 33 (28) |
| Open heart surgery | 4 (3) |
| General cardiologya | 51 (44) |
| Cardiovascular imaging | 4 (3) |
| Control | |
| Standard care | 18 (15) |
| Placebo | 31 (27) |
| Pharmacological | 28 (24) |
| Surgical/interventional | 36 (31) |
| Other | 4 (3) |
| Funding | |
| Private for profit | 58 (50) |
| Private not for profit | 21 (18) |
| Governmental | 24 (20) |
| Not reported | 13 (11) |
| Not funded | 1 (1) |
| Reporting of methods to deal with LTFU | |
| Reported in methods | 100 (86) |
| Reported in results | 1 (1) |
| No | 16 (14) |
| Among the trials that LTFU occurred (n=91)b | |
| Separately reported in 2 arms | 70 (77) |
| Compared the LTFU group baseline characteristics with not LTFU | 0 (0) |
| Implication of LTFU discussed | 6 (7) |
| Analytical method to handle LTFU | |
| No LTFU occurred | 26 (22) |
| Complete case analysisc | 10 (8) |
| Worst‐case scenario | 2 (2) |
| Multiple imputation | 2 (2) |
| Inverse probability weighting | 0 (0) |
| Censored at time of LTFU in time‐to‐event analysis | 75 (64) |
| Assumption that none of the LTFU participants have event | 2 (2) |
| CONSORT diagram | |
| Without the diagram | 32 (27) |
CONSORT indicates Consolidated Standards of Reporting Trials; LTFU, loss to follow‐up.
General cardiology trials in this review referred to pharmacological trials and lifestyle‐changing trials.
Number shown refers to trials that did the following.
Complete case analysis is defined as an analysis that only include patients with complete outcome data. LTFU patients are excluded from the whole analysis.
Assessment of the Methodological Quality of the Trials
The analytical methods that were used for handling LTFU in the primary analysis of the included trials are presented in Table 1. The most commonly used method was censoring at time of LTFU in a time‐to‐event analysis (N=75; 64%). Two trials (2%) assumed that no LTFU participants experienced events, whereas 10 (8%) used complete case analysis and 2 (2%) used a worst‐case scenario in which only the control arm had events. Two trials (2%) used multiple imputation, whereas none reported using inverse probability weighting.
Regarding the reporting of LTFU, 85 (73%) used a Consolidated Standards of Reporting Trials (CONSORT) diagram. Seventy (77%) trials reported that LTFU occurred in the intervention and control arms separately. However, none of the trials compared baseline characteristics of LTFU participants with followed‐up participants. The implications of LTFU are discussed in 6 trials (7%).
Table 2 and Figure 2 demonstrated the number of trials meeting the characteristics (methodological confounders) for impairment in the quality of trial design. Allocation concealment was adequate in 54 trials (46%). Patients were blinded adequately in 41 trials (35%). In 9 trials (8%), enrollment was discontinued prematurely. Twenty‐nine trials used an intention‐to‐treat analysis (25%). Thirty‐one trials (26%) provided a protocol. Forty‐three trials (36%) did not state the status of LTFU explicitly in the report. Only 10 trials (9%) were free from any methodological confounders that might impair the methodological quality.
Table 2.
Methodological and Reporting Quality Assessment of the Included Trials
| Factors | Trials at Risk of Bias (n=117), No. (%) |
|---|---|
| Inadequate allocation sequence concealmenta | 63 (54) |
| No blinding of patientsb | 76 (65) |
| Early stop | 9 (8) |
| Not using intention‐to‐treat analysisc | 29 (25) |
| Absence of protocold | 31 (26) |
| Without explicit statement about status of LTFU | 43 (36) |
LTFU indicates loss to follow‐up.
Allocation concealment defined as to the person enrolling participants does not know in advance which treatment the next person will get which usually involves the use of computer algorithms. It seeks to prevent selection bias by protecting the assignment sequence until allocation, and can always be successfully implemented. 136 It is considered to be adequate according to the definition reported by Jüni et al.3
Blinding defined as to the withholding information about the assigned interventions from people involved in the trial who may potentially be influenced by this knowledge; blinding is performed to prevent performance and ascertainment bias by protecting the sequence after allocation and cannot always be implemented. 136 , 137 It is considered to be adequate only if clearly indicated.
Intention to treat analysis defined as an analysis that included all randomized participants in the analysis who are all retained in the group to which they were allocated. 3 , 136
Consider as absence if the protocol is not published before or is included as appendix beside the main report.
Figure 2. Distribution of trials according to methodological and reporting quality assessments that might impair the outcomes of the trial.
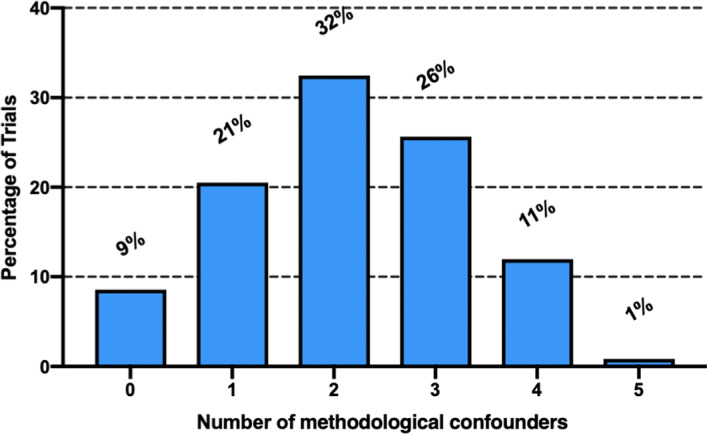
Distribution of trials according to the number of methodological and reporting quality characteristics (methodological confounders) they possess after the assessment: 9% of the trials had none of the methodological confounders (n=10), 21% of the trials possessed 1 methodological confounder (n=24), 32% of the trials possessed 2 major methodological confounders (n=38), and 26% of the trials possessed 3 major methodological confounders (n=30). In addition, 12% of the trials had >3 methodological confounders. This list of methodological confounders analyzed included the following: (1) inadequate allocation sequence concealment, (2) no blinding of patients, (3) early stop of trial, (4) not using intention‐to‐treat analysis, (5) absence of protocol, and (6) no explicit statement about status of loss to follow‐up.
Random‐effects metaregression analysis (Table 3) suggested that inadequate or unclear concealment of allocation was associated with an increase in odds of LTFU (odds ratio, 2.37 [95% CI, 1.42–3.97]; P=0.001). Increasing sample size (P=0.039) and duration of follow‐up (P=0.002) also increased the odds of LTFU. Finally, the odds of LTFU was decreased in nonsurgical or noninterventional trials (odds ratio, 0.50 [95% CI, 0.30–0.85]; P=0.01).
Table 3.
Regression Analysis Exploring the Association Between the Percentage of LTFU Participants and General and Methodological Trial Characteristics
| Trial Characteristic | No. of Trials (n=117) | No. of Patients | Odds Ratio (95% CI) | P for Interaction |
|---|---|---|---|---|
| Number of centers | 0.098 | |||
| 1 | 17 | 57 048 | 1.00 (Reference) | |
| 2–10 | 26 | 19 680 | 1.43 (0.56–3.65) | |
| 11–50 | 34 | 60 940 | 2.24 (0.94–5.37) | |
| >50 | 40 | 259 600 | 1.99 (0.86–4.57) | |
| Sample size | 0.039 | |||
| ≤500 | 43 | 10 971 | 1.00 (Reference) | |
| >500–1000 | 25 | 17 722 | 0.97 (0.45–2.05) | |
| >1000–5000 | 26 | 53 077 | 0.74 (0.36–1.51) | |
| >5000 | 23 | 315 498 | 0.50 (0.24–1.02) | |
| Concealment of allocation | 0.001 | |||
| Yes | 54 | 153 572 | 1.00 (Reference) | |
| No | 63 | 243 696 | 2.37 (1.42–3.97) | |
| Blinding of patients | 0.15 | |||
| Yes | 41 | 288 784 | 1.00 (Reference) | |
| No | 76 | 108 484 | 1.49 (0.86–2.59) | |
| Intention to treat | 0.89 | |||
| Yes | 88 | 347 413 | 1.00 (Reference) | |
| No | 29 | 49 855 | 1.04 (0.57–1.90) | |
| Length of follow‐up, mo | 0.002 | |||
| ≤6 | 27 | 30 234 | 1.00 (Reference) | |
| >6 to 12 | 35 | 50 278 | 1.89 (0.87–4.10) | |
| >12 to 24 | 26 | 67 459 | 2.53 (1.12–5.72) | |
| >24 | 29 | 249 297 | 3.42 (1.57–7.42) | |
| Trial stopped early | 0.75 | |||
| Yes | 9 | 66 577 | 1.00 (Reference) | |
| No | 108 | 330 691 | 1.16 (0.46–2.90) | |
| Surgery or interventional treatment | 0.010 | |||
| Yes | 52 | 51 574 | 1.00 (Reference) | |
| No | 65 | 345 694 | 0.50 (0.30–0.85) | |
| General cardiology | 0.84 | |||
| Yes | 51 | 335 302 | 1.00 (Reference) | |
| No | 66 | 61 966 | 1.06 (0.63–1.77) | |
| Commercial funding | 0.78 | |||
| Yes | 58 | 299 427 | 1.00 (Reference) | |
| No | 59 | 97 841 | 1.08 (0.63–1.83) |
LTFU indicates loss to follow‐up.
Extent of Loss to Follow‐Up
Among the 117 included trials, 91 (78%) reported LTFU. Twenty‐three trials reported no LTFU (20%), and 3 trials did not report whether there was LTFU (3%). Of the trials with LTFU, the median percentage of LTFU was 2% (interquartile range [IQR], 0.3%–4.8%) in the intervention arm, 1.99% (IQR, 0.3%–5.4%) from the control arm, and 1.96% (IQR, 0.33%–5.3%) overall. The median difference between the intervention and the control groups was not significant (P=0.978; Figure 3).
Figure 3. Distribution and difference in proportion of LTFU between the intervention and control arms among 91 trials with LTFU.
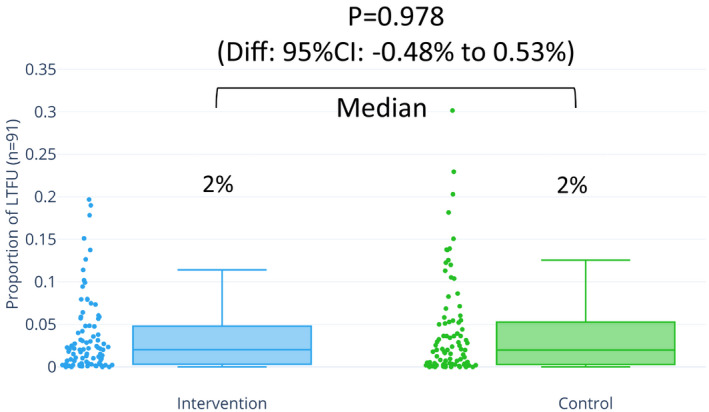
Distribution of LTFU proportions among 91 trials that reported LTFU stratified by intervention and control. A median of 2% LTFU occurred in both the intervention and control arms. The difference is not significant (95% CI, −0.48% to 0.53%; P=0.978). Diff indicates difference; LTFU, loss to follow‐up.
The medians for the ratios of LTFU to events were 0.12 (IQR, 0.03–0.33) in the intervention arm, 0.11 (IQR, 0.02–0.42) in the control arm, and 0.11 (IQR, 0.03–0.41) overall. A value of 0.12 means that ≈1 participant is LTFU when every 10 participants experience the primary outcome. However, the difference between the ratio of the intervention and the control groups was not significant (P=0.473; Figure 4).
Figure 4. Distribution and difference in ratio of LTFU to events between the intervention and control arms among 91 trials with LTFU.
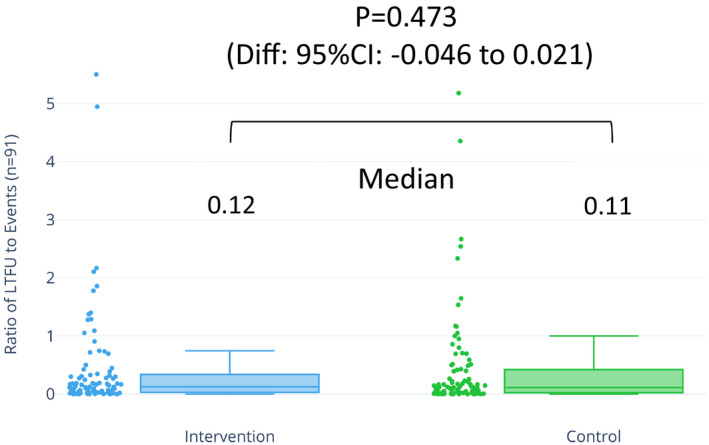
Distribution of ratios across 91 trials with LTFU stratified by intervention and control. Medians of 0.12 from intervention arms and 0.11 from control arms indicate that ≈1 person was lost when 10 experienced events in both intervention and control arms. This shows the relativeness of proportions in between LTFU and events. The difference in ratio was not significant (95% CI, −0.046 to 0.021; P=0.473). Diff indicates difference; LTFU, loss to follow‐up.
Potential Impact of LTFU
Percentage of Trials Losing Significance
For the 4 common assumptions in which all 91 trials were included, the percentages of trials that lost significance were 4% (no participants lost to follow‐up had the event), 11% (all participants lost to follow‐up had the event), 3% (best‐case scenario), and 33% (worst‐case scenario).
Considering the relative event incidence analysis method, Table 4 shows the percentage of eligible trials that lost significance across a range of assumptions for the event incidence among intervention and control arms (Figure 5). The percentage varied from 2% to 16%. Figure 6 shows an inverse‐proportion relationship of the trials losing significance with the percentage of LTFU under the best and worst assumptions made by the relative event incidence analysis method.
Table 4.
Percentage of 91 Trials in Which Results Would Lose Significance Under Different Assumptions on the Outcomes of LTFU Participants in Intervention and Control Arms
| N=91 | RILTFU/FU (Control) a | |||
|---|---|---|---|---|
| 3 | 2 | 1.5 | 1 | |
| RILTFU/FU (intervention) a | ||||
| 1 | 3 | 3 | 2 | 4 |
| 1.5 | 3 | 2 | 3 | 4 |
| 2 | 4 | 3 | 4 | 12 |
| 3 | 3 | 9 | 10 | 16 |
Among the 91 trials, percentages of results that would lose significance under less plausible assumptions: (1) none of the LTFU participants had the event, 4%; (2) all the LTFU participants had the event, 11%; (3) none of those lost to follow‐up in the treatment group had the event, and all those lost to follow‐up in the control group did (best case scenario), 3%; (4) all participants lost to follow‐up in the treatment group had the event, and none of those in the control group did (worst case scenario), 33%. FU indicates follow‐up; LTFU, loss to follow‐up.
RILTFU/FU is the relative event incidence among those with LTFU compared with those followed up.
Figure 5. Bias and loss to follow‐up in randomized controlled trials in cardiovascular medicine.
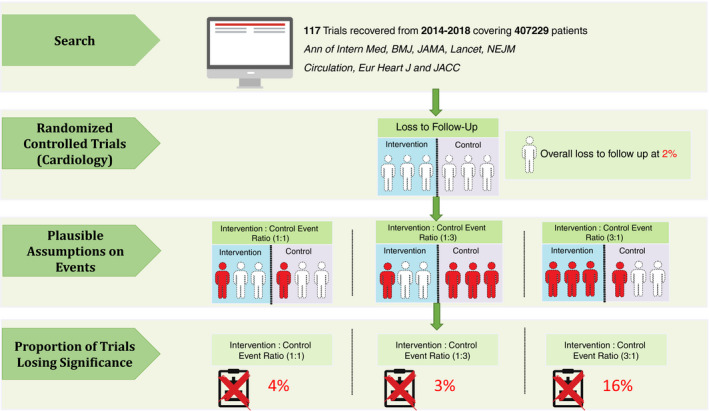
Assumptions being made toward the outcome of LTFU in each trial from the search and the subsequent calculation made. In total, 117 trials from 8 journals covering 407 229 patients from 2014 to 2018 were recovered. Assume participants were randomized to intervention and control, respectively; 3 had events from each arm and 3 dropouts from each arm. From the figure, dotted transparent figures denote LTFU participants, whereas red dotted figures denote LTFU participants being assumed with event. The plausible assumptions being made toward the LTFU was based on relative event incidence and a formula detailed in Data S6. The number of events were assumed based on the reported formula with incidence ranging from 1 to 3. Calculations of how many trials’ relative risks lost significance after making the assumptions were run subsequently. Ann of Intern Med, Annals of Internal Medicine; Eur Heart J, European Heart Journal; JACC, Journal of the American College of Cardiology; LTFU, loss to follow‐up; NEJM, New England Journal of Medicine.
Figure 6. Distribution of trials by LTFU proportion under the best and worst plausible assumptions made by using the relative event incidence for the control and intervention arms.
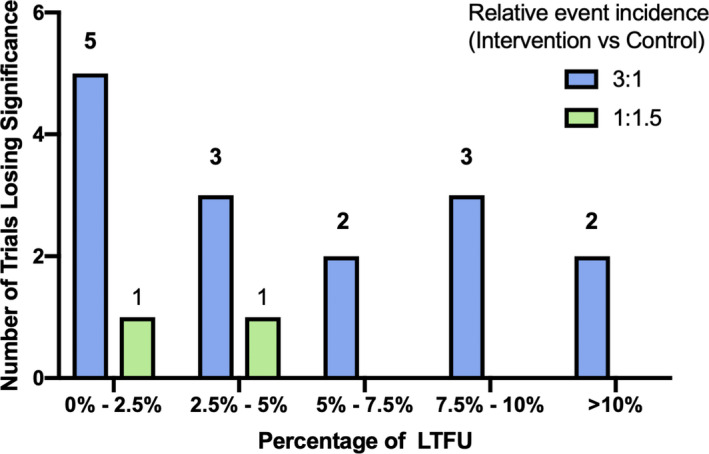
Distribution of trials losing statistical significance stratified by the percentage of LTFU of the individual trial under the best and worst assumptions made by the more plausible relative event incidence method. An inverse‐proportion relationship is shown in the graph, where there is higher number of trials losing significance in trials with lower proportions of LTFU. LTFU indicates loss to follow‐up.
Results of the prespecified sensitivity analysis on the subspecialties are reported (interventional cardiology versus others) in the online Data Supplement. There was a significant difference in the proportion of trials losing significance between interventional cardiology and other subspecialties (difference, 4.35% [95% CI, 0.295%–8.41%]; P=0.0369; Figure S1 and Table S2).
Discussion
We found considerable variation in the extent and reporting of LTFU data in contemporary cardiovascular clinical trials. We observed that certain characteristics of clinical trials—notably, inadequate or unclear allocation concealment, length of follow‐up, sample size, and type of intervention—were associated with increased odds of LTFU. Importantly, the primary result in 1 of 6 trials might change if reasonable assumptions were made about the end point in patients with LTFU.
The inverse‐proportion relationship noted in Figure 6 suggests that the impact of a small proportion of LTFU might be overlooked by investigators. More than one third of trials did not achieve effective blinding among either the participants or the site investigators. This finding is important because ineffective blinding is associated with overestimation of true treatment effects. 135 Allocation concealment was inadequate or unclear in more than half of the trials. Conversely, an intention‐to‐treat analysis was used in 75% of trials, which minimizes the effects of attrition bias.3 Just over half of the trials included an explicit statement about LTFU, and >70% of the trials included a CONSORT flow diagram. Notably, baseline demographics on the LTFU participants were limited. Authors (93%) commonly omitted discussion of the potential impact or reasons for LTFU. We suggest that information on participants with LTFU should be included by authors in an appendix or in a defined column in a table of the trial participants’ characteristics (Table 5). Inverse probability weighting can be a helpful way of handling LTFU participants’ data, but it is not used in any of the included trials. Most trials did not impute data for LTFU participants. We noted a significant association between inadequate or unclear allocation concealment and increased odds of LTFU. This could be explained by less stringently implemented processes in trials with inadequate or unclear allocation concealment, including suboptimal measures for following up participants.
Table 5.
Summary of the Important Common Issues for LTFU and Guidance in Conducting Trials and Reporting Trial Results
| Issues That Should Be Noted | Guidance |
|---|---|
| Inadequate or unclear allocation concealment | If allocation concealment forms part of the trial design, then effective approaches to achieve allocation concealment include using a matched placebo (visually identical to the active treatment); central randomization (performed at a site remote from the trial's location); sequentially numbered, sealed, opaque envelopes3 |
| Large sample size and long follow up duration | LTFU increases with larger trial sample size, hence investigators should be aware and mitigate the number of LTFU for increase in sample size and 1‐y increase in duration |
| Reporting of LTFU | Investigators should strive to reduce the number of LTFU. A CONSORT diagram should be included for readers. When LTFU has occurred, baseline characteristics, and extent of follow up duration before exclusion should be reported in the manuscript or supplement. The implications of LTFU should also be discussed in the manuscript. Time of dropout can be noted on a supplement or in the result paragraph or on the CONSORT diagram for readers to know the extent of follow up before dropout |
CONSORT indicates Consolidated Standards of Reporting Trials; LTFU, loss to follow‐up.
Strengths and Weaknesses of the Study
Our study has several strengths. First, the forms for screening of the trials and related data collection were established before the start of the data collection process. In addition, the calibration exercise was completed upfront as a preparatory step intended to increase accuracy for the screening and data collection. Second, a range of assumptions was made for the participants with LTFU and explored the potential effect of LTFU on the estimate of the effect of the intervention, including whether or not the trial met statistical significance on its primary outcome and the change in the relative risk ratio and number of outcome events. The effect is focused on cardiovascular trials. Our analysis depends on the accuracy and clarity of the included reports. Generalizability is also an issue. We focused our analysis on 8 journals’ publications during a 5‐year period (2014–2018). A wider inclusion strategy with more journals (with lower impact factors) and trials with a nonsignificant primary outcome result might have returned different results. Our findings might underestimate the true effect of LTFU in the effect estimate if a wider range of RCTs were included. Our review included trials with binary data only because of the design of the review analysis, which might further weaken the generalizability of the results. Time of dropout can be a factor influencing the LTFU effect because early dropouts can influence the result to a larger extent than late dropouts. However, exact time of dropout is not noted in the reports, and we are unable to stratify the effect.
Implications
Investigators and sponsors should strive to reduce the number of participants with LTFU. The higher the LTFU, the more uncertainty increases around the treatment effect estimate and the potential for a false result. In the unfortunate event that LTFU happened, its impact can be estimated using the worst assumption (Data S7). As for the reporting of LTFU, editors may consider requiring authors to provide a fully informative and transparent report on participant LTFU including the inclusion and exclusion criteria of patients, which is in line with CONSORT guidelines. Specifically, investigators should provide information on participants with LTFU including their baseline characteristics, reasons for LTFU, and duration of follow‐up before exclusion and then compared with those who completed follow‐up. This information could be published as an appendix. Implications of LTFU should be discussed when LTFU has occurred (Table 5). This review provides estimates of the probability that the primary analysis of cardiovascular trials could lose statistical significance when LTFU events are taken into account by making appropriate estimate of event incidence. Although the 4 less plausible but commonly used assumptions may not eventuate, they can be taken as the upper limit of change in trial significance. Early LTFU has a more influential effect on the analysis than late LTFU near the overall study duration, which highlighted the need for investigators to stratify LTFU by follow‐up extent. Future studies can look at the extent of change in treatment effect in relation to the LTFU proportion and event number and the effect of partial and full LTFU defined as difference in the extent of follow‐up before exclusion. The influence of dropout time on LTFU effect can be explored for assessing the possibility of systemic inclusion of patients accounting for early dropouts.
Conclusions
Almost 1 in 6 (16%) cardiovascular randomized trials published in leading journals may have a change in the primary outcome if plausible assumptions are made about differential event rates of participants lost to follow‐up. There is scope for improvement arising from LTFU in randomized trials in cardiovascular medicine. Bias minimization through mitigation of participants lost to follow‐up offers the opportunity to enhance the value of randomized trials.
Sources of Funding
The study was funded by British Heart Foundation (PG/17/2532884; RE/13/5/30177; RE/18/6134217). The funder had no role in the study design, the writing of manuscript, or the decision to submit this article or future manuscripts for publication.
Disclosures
Berry is employed by the University of Glasgow which holds consultancy and research agreements for his work with companies that have commercial interests in the diagnosis and treatment of angina. The companies include Abbott Vascular, Astra Zeneca, Boehringer Ingelheim, GSK, HeartFlow, Menarini, Novartis, and Siemens Healthcare. Jüni serves as unpaid member of the steering group of trials funded by Astra Zeneca, Biotronik, Biosensors, St. Jude Medical and The Medicines Company, has received research grants to the institution from Astra Zeneca, Biotronik, Biosensors International, Eli Lilly and The Medicines Company, and honoraria to the institution for participation in advisory boards from Amgen, but has not received personal payments by any pharmaceutical company or device manufacturer. The remaining authors have no disclosures to report.
Supporting information
Datas S1–S7 Tables S1–S2 Figure S1 References 14, and 17–134
Acknowledgments
Author contributions: Fong, Ford, and Berry were responsible for study conception and design. Fong and Ford acquired the data. Fong, Jüni, and da Costa analyzed the data. Fong drafted the manuscript. All authors critically revised the manuscript and agreed on the final version.
(J Am Heart Assoc. 2020;9:e015361 DOI: 10.1161/JAHA.119.015361.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. McGauran N, Wieseler B, Kreis J, Schüler YB, Kölsch H, Kaiser T. Reporting bias in medical research—a narrative review. Trials. 2010;11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kendall JM. Designing a research project: randomised controlled trials and their principles. Emerg Med J. 2003;20:164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001;323:42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clark L, Fairhurst C, Torgerson DJ. Allocation concealment in randomised controlled trials: are we getting better? BMJ. 2016;355:i5663. [DOI] [PubMed] [Google Scholar]
- 5. Day SJ, Altman DG. Statistics notes: blinding in clinical trials and other studies. BMJ. 2000;321:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manja V, Lakshminrusimha S. Epidemiology and clinical research design, part 1: study types. Neoreviews. 2014;15:e558–e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tierney JF, Stewart LA. Investigating patient exclusion bias in meta‐analysis. Int J Epidemiol. 2005;34:79–87. [DOI] [PubMed] [Google Scholar]
- 8. Baron G, Boutron I, Giraudeau B, Ravaud P. Violation of the intent‐to‐treat principle and rate of missing data in superiority trials assessing structural outcomes in rheumatic diseases. Arthritis Rheum. 2005;52:1858–1865. [DOI] [PubMed] [Google Scholar]
- 9. Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Bürgi E, Scherer M, Altman DG, Jüni P. The effects of excluding patients from the analysis in randomised controlled trials: meta‐epidemiological study. BMJ. 2009;339:b3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joseph R, Sim J, Ogollah R, Lewis M. A systematic review finds variable use of the intention‐to‐treat principle in musculoskeletal randomized controlled trials with missing data. J Clin Epidemiol. 2015;68:15–24. [DOI] [PubMed] [Google Scholar]
- 11. Valgimigli M, Garcia‐Garcia HM, Vrijens B, Vranckx P, McFadden EP, Costa F, Pieper K, Vock DM, Zhang M, Van Es GA, et al. Standardized classification and framework for reporting, interpreting, and analysing medication non‐adherence in cardiovascular clinical trials: a consensus report from the Non‐adherence Academic Research Consortium (NARC). Eur Heart J. 2019;40:2070–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Balk EM, Bonis PA, Moskowitz H, Schmid CH, Ioannidis JP, Wang C, Lau J. Correlation of quality measures with estimates of treatment effect in meta‐analyses of randomized controlled trials. JAMA. 2002;287:2973–2982. [DOI] [PubMed] [Google Scholar]
- 13. Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. [DOI] [PubMed] [Google Scholar]
- 14. Bikdeli B, Welsh JW, Akram Y, Punnanithinont N, Lee I, Desai NR, Kaul S, Stone GW, Ross JS, Krumholz HM. Noninferiority designed cardiovascular trials in highest‐impact journals. Circulation. 2019;140:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fergusson D, Aaron SD, Guyatt G, Hébert P. Post‐randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ. 2002;325:652–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, and Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akl EA, Briel M, You JJ, Sun X, Johnston BC, Busse JW, Mulla S, Lamontagne F, Bassler D, Vera C, et al. Potential impact on estimated treatment effects of information lost to follow‐up in randomised controlled trials (LOST‐IT): systematic review. BMJ. 2012;344:e2809. [DOI] [PubMed] [Google Scholar]
- 18. Abdel‐Wahab M, Mehilli J, Frerker C, Neumann FJ, Kurz T, Tölg R, Zachow D, Guerra E, Massberg S, Schäfer U, et al. Comparison of balloon‐expandable vs self‐expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. JAMA. 2014;311:1503–1514. [DOI] [PubMed] [Google Scholar]
- 19. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J Jr, Kleiman NS, et al. Transcatheter aortic‐valve replacement with a self‐expanding prosthesis. N Engl J Med. 2014;370:1790–1798. [DOI] [PubMed] [Google Scholar]
- 20. Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, Aboyans V, Alings M, Kakkar AK, Keltai K, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double‐blind, placebo‐controlled trial. Lancet. 2018;391:219–229. [DOI] [PubMed] [Google Scholar]
- 21. Appelboam A, Reuben A, Mann C, Gagg J, Ewings P, Barton A, Lobban T, Dayer M, Vickery J, Benger J. Postural modification to the standard Valsalva manoeuvre for emergency treatment of supraventricular tachycardias (REVERT): a randomised controlled trial. Lancet. 2015;386:1747–1753. [DOI] [PubMed] [Google Scholar]
- 22. Bermejo J, Yotti R, García‐Orta R, Sánchez‐Fernández PL, Castaño M, Segovia‐Cubero J, Escribano‐Subías P, San Román JA, Borrás X, Alonso‐Gómez A, et al. Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double‐blind, randomized clinical trial. Eur Heart J. 2018;39:1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bernat I, Horak D, Stasek J, Mates M, Pesek J, Ostadal P, Hrabos V, Dusek J, Koza J, Sembera Z, et al. ST‐segment elevation myocardial infarction treated by radial or femoral approach in a multicenter randomized clinical trial: the STEMI‐RADIAL trial. J Am Coll Cardiol. 2014;63:964–972. [DOI] [PubMed] [Google Scholar]
- 24. Bhatia RS, Ivers NM, Yin XC, Myers D, Nesbitt GC, Edwards J, Yared K, Wadhera RK, Wu JC, Kithcart AP, et al. Improving the appropriate use of transthoracic echocardiography: the Echo WISELY Trial. J Am Coll Cardiol. 2017;70:1135–1144. [DOI] [PubMed] [Google Scholar]
- 25. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, et al. Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–1800. [DOI] [PubMed] [Google Scholar]
- 26. Bonnet D, Berger F, Jokinen E, Kantor PF, Daubeney PEF. Ivabradine in children with dilated cardiomyopathy and symptomatic chronic heart failure. J Am Coll Cardiol. 2017;70:1262–1272. [DOI] [PubMed] [Google Scholar]
- 27. Boriani G, Tukkie R, Manolis AS, Mont L, Pürerfellner H, Santini M, Inama G, Serra P, de Sousa J, Botto GL, et al. Atrial antitachycardia pacing and managed ventricular pacing in bradycardia patients with paroxysmal or persistent atrial tachyarrhythmias: the MINERVA randomized multicentre international trial. Eur Heart J. 2014;35:2352–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, Wiviott SD, Cannon CP, Braunwald E, Sammons E, et al. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217–1227. [DOI] [PubMed] [Google Scholar]
- 29. Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529–1539. [DOI] [PubMed] [Google Scholar]
- 30. Brar SS, Aharonian V, Mansukhani P, Moore N, Shen AY, Jorgensen M, Dua A, Short L, Kane K. Haemodynamic‐guided fluid administration for the prevention of contrast‐induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. 2014;383:1814–1823. [DOI] [PubMed] [Google Scholar]
- 31. Brignole M, Pokushalov E, Pentimalli F, Palmisano P, Chieffo E, Occhetta E, Quartieri F, Calò L, Ungar A, Mont L; APAF‐CRT Investigators . A randomized controlled trial of atrioventricular junction ablation and cardiac resynchronization therapy in patients with permanent atrial fibrillation and narrow QRS. Eur Heart J. 2018;39:3999–4008. [DOI] [PubMed] [Google Scholar]
- 32. Calkins H, Willems S, Gerstenfeld EP, Verma A, Schilling R, Hohnloser SH, Okumura K, Serota H, Nordaby M, Guiver K, et al. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med. 2017;376:1627–1636. [DOI] [PubMed] [Google Scholar]
- 33. Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, Maeng M, Merkely B, Zeymer U, Gropper S, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513–1524. [DOI] [PubMed] [Google Scholar]
- 34. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 35. Carrick D, Oldroyd KG, McEntegart M, Haig C, Petrie MC, Eteiba H, Hood S, Owens C, Watkins S, Layland J, et al. A randomized trial of deferred stenting versus immediate stenting to prevent no‐ or slow‐reflow in acute ST‐segment elevation myocardial infarction (DEFER‐STEMI). J Am Coll Cardiol. 2014;63:2088–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen SL, Zhang JJ, Han Y, Kan J, Chen L, Qiu C, Jiang T, Tao L, Zeng H, Li L, et al. Double kissing crush versus provisional stenting for left main distal bifurcation lesions: DKCRUSH‐V randomized trial. J Am Coll Cardiol. 2017;70:2605–2617. [DOI] [PubMed] [Google Scholar]
- 37. Connolly SJ, Eikelboom JW, Bosch J, Dagenais G, Dyal L, Lanas F, Metsarinne K, O'Donnell M, Dans AL, Ha JW, et al. Rivaroxaban with or without aspirin in patients with stable coronary artery disease: an international, randomised, double‐blind, placebo‐controlled trial. Lancet. 2018;391:205–218. [DOI] [PubMed] [Google Scholar]
- 38. Cuisset T, Deharo P, Quilici J, Johnson TW, Deffarges S, Bassez C, Bonnet G, Fourcade L, Mouret JP, Lambert M, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070–3078. [DOI] [PubMed] [Google Scholar]
- 39. de Belder A, de la Torre Hernandez JM, Lopez‐Palop R, O'Kane P, Hernandez Hernandez F, Strange J, Gimeno F, Cotton J, Diaz Fernandez JF, Saez PC, et al. A prospective randomized trial of everolimus‐eluting stents versus bare‐metal stents in octogenarians: the XIMA Trial (Xience or Vision Stents for the Management of Angina in the Elderly). J Am Coll Cardiol. 2014;63:1371–1375. [DOI] [PubMed] [Google Scholar]
- 40. De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, Jagic N, Mobius‐Winckler S, Rioufol G, Witt N, et al. Fractional flow reserve‐guided PCI for stable coronary artery disease. N Engl J Med. 2014;371:1208–1217. [DOI] [PubMed] [Google Scholar]
- 41. Devereaux PJ, Duceppe E, Guyatt G, Tandon V, Rodseth R, Biccard BM, Xavier D, Szczeklik W, Meyhoff CS, Vincent J, et al. Dabigatran in patients with myocardial injury after non‐cardiac surgery (MANAGE): an international, randomised, placebo‐controlled trial. Lancet. 2018;391:2325–2334. [DOI] [PubMed] [Google Scholar]
- 42. Dewey M, Rief M, Martus P, Kendziora B, Feger S, Dreger H, Priem S, Knebel F, Böhm M, Schlattmann P, et al. Evaluation of computed tomography in patients with atypical angina or chest pain clinically referred for invasive coronary angiography: randomised controlled trial. BMJ. 2016;355:i5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Di Biase L, Burkhardt JD, Lakkireddy D, Carbucicchio C, Mohanty S, Mohanty P, Trivedi C, Santangeli P, Bai R, Forleo G, et al. Ablation of stable VTs versus substrate ablation in ischemic cardiomyopathy: the VISTA randomized multicenter trial. J Am Coll Cardiol. 2015;66:2872–2882. [DOI] [PubMed] [Google Scholar]
- 44. Di Biase L, Burkhardt JD, Mohanty P, Mohanty S, Sanchez JE, Trivedi C, Güneş M, Gökoğlan Y, Gianni C, Horton RP, et al. Left atrial appendage isolation in patients with longstanding persistent AF undergoing catheter ablation: BELIEF trial. J Am Coll Cardiol. 2016;68:1929–1940. [DOI] [PubMed] [Google Scholar]
- 45. Di Biase L, Burkhardt JD, Santangeli P, Mohanty P, Sanchez JE, Horton R, Gallinghouse GJ, Themistoclakis S, Rossillo A, Lakkireddy D, et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing Catheter Ablation (COMPARE) randomized trial. Circulation. 2014;129:2638–2644. [DOI] [PubMed] [Google Scholar]
- 46. Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, Reddy M, Jais P, Themistoclakis S, Dello Russo A, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637–1644. [DOI] [PubMed] [Google Scholar]
- 47. Douketis JD, Spyropoulos AC, Kaatz S, Becker RC, Caprini JA, Dunn AS, Garcia DA, Jacobson A, Jaffer AK, Kong DF, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 49. Engstrøm T, Kelbæk H, Helqvist S, Høfsten DE, Kløvgaard L, Holmvang L, Jørgensen E, Pedersen F, Saunamäki K, Clemmensen P, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST‐segment elevation myocardial infarction and multivessel disease (DANAMI‐3—PRIMULTI): an open‐label, randomised controlled trial. Lancet. 2015;386:665–671. [DOI] [PubMed] [Google Scholar]
- 50. Estruch R, Ros E, Salas‐Salvadó J, Covas MI, Corella D, Arós F, Gómez‐Gracia E, Ruiz‐Gutiérrez V, Fiol M, Lapetra J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra‐virgin olive oil or nuts. N Engl J Med. 2018;378:e34. [DOI] [PubMed] [Google Scholar]
- 51. Farkouh ME, Domanski M, Dangas GD, Godoy LC, Mack MJ, Siami FS, Hamza TH, Shah B, Stefanini GG, Sidhu MS, et al. Long‐term survival following multivessel revascularization in patients with diabetes: the FREEDOM follow‐on study. J Am Coll Cardiol. 2019;73:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Garot P, Morice MC, Tresukosol D, Pocock SJ, Meredith IT, Abizaid A, Carrié D, Naber C, Iñiguez A, Talwar S, et al. 2‐year outcomes of high bleeding risk patients after polymer‐free drug‐coated stents. J Am Coll Cardiol. 2017;69:162–171. [DOI] [PubMed] [Google Scholar]
- 53. Gershlick AH, Khan JN, Kelly DJ, Greenwood JP, Sasikaran T, Curzen N, Blackman DJ, Dalby M, Fairbrother KL, Banya W, et al. Randomized trial of complete versus lesion‐only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels M, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–2434. [DOI] [PubMed] [Google Scholar]
- 55. Gillinov AM, Gelijns AC, Parides MK, DeRose JJ Jr, Moskowitz AJ, Voisine P, Ailawadi G, Bouchard D, Smith PK, Mack MJ, et al. Surgical ablation of atrial fibrillation during mitral‐valve surgery. N Engl J Med. 2015;372:1399–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, Vaid H, O'Donnell M, Laupacis A, Côté R, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–2477. [DOI] [PubMed] [Google Scholar]
- 57. Greenwood JP, Ripley DP, Berry C, McCann GP, Plein S, Bucciarelli‐Ducci C, Dall'Armellina E, Prasad A, Bijsterveld P, Foley JR, et al. Effect of care guided by cardiovascular magnetic resonance, myocardial perfusion scintigraphy, or NICE guidelines on subsequent unnecessary angiography rates: the CE‐MARC 2 randomized clinical trial. JAMA. 2016;316:1051–1060. [DOI] [PubMed] [Google Scholar]
- 58. Halcox JPJ, Wareham K, Cardew A, Gilmore M, Barry JP, Phillips C, Gravenor MB. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE‐AF study. Circulation. 2017;136:1784–1794. [DOI] [PubMed] [Google Scholar]
- 59. Halliday BP, Wassall R, Lota AS, Khalique Z, Gregson J, Newsome S, Jackson R, Rahneva T, Wage R, Smith G, et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED‐HF): an open‐label, pilot, randomised trial. Lancet. 2019;393:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Han Y, Guo J, Zheng Y, Zang H, Su X, Wang Y, Chen S, Jiang T, Yang P, Chen J, et al. Bivalirudin vs heparin with or without tirofiban during primary percutaneous coronary intervention in acute myocardial infarction: the BRIGHT randomized clinical trial. JAMA. 2015;313:1336–1346. [DOI] [PubMed] [Google Scholar]
- 61. Han Y, Zhu G, Han L, Hou F, Huang W, Liu H, Gan J, Jiang T, Li X, Wang W, et al. Short‐term rosuvastatin therapy for prevention of contrast‐induced acute kidney injury in patients with diabetes and chronic kidney disease. J Am Coll Cardiol. 2014;63:62–70. [DOI] [PubMed] [Google Scholar]
- 62. Hernandez AF, Green JB, Janmohamed S, D'Agostino RB Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double‐blind, randomised placebo‐controlled trial. Lancet. 2018;392:1519–1529. [DOI] [PubMed] [Google Scholar]
- 63. Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A, Brachmann J, Lewalter T, Goette A, Block M, et al. Implant‐based multiparameter telemonitoring of patients with heart failure (IN‐TIME): a randomised controlled trial. Lancet. 2014;384:583–590. [DOI] [PubMed] [Google Scholar]
- 64. Hong SJ, Kim BK, Shin DH, Nam CM, Kim JS, Ko YG, Choi D, Kang TS, Kang WC, Her AY, et al. Effect of intravascular ultrasound‐guided vs angiography‐guided everolimus‐eluting stent implantation: the IVUS‐XPL randomized clinical trial. JAMA. 2015;314:2155–2163. [DOI] [PubMed] [Google Scholar]
- 65. Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, Tang G, Wang B, Chen D, He M, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313:1325–1335. [DOI] [PubMed] [Google Scholar]
- 66. Imazio M, Belli R, Brucato A, Cemin R, Ferrua S, Beqaraj F, Demarie D, Ferro S, Forno D, Maestroni S, et al. Efficacy and safety of colchicine for treatment of multiple recurrences of pericarditis (CORP‐2): a multicentre, double‐blind, placebo‐controlled, randomised trial. Lancet. 2014;383:2232–2237. [DOI] [PubMed] [Google Scholar]
- 67. Imazio M, Brucato A, Ferrazzi P, Pullara A, Adler Y, Barosi A, Caforio AL, Cemin R, Chirillo F, Comoglio C, et al. Colchicine for prevention of postpericardiotomy syndrome and postoperative atrial fibrillation: the COPPS‐2 randomized clinical trial. JAMA. 2014;312:1016–1023. [DOI] [PubMed] [Google Scholar]
- 68. Jennings C, Kotseva K, De Bacquer D, Hoes A, de Velasco J, Brusaferro S, Mead A, Jones J, Tonstad S, Wood D. Effectiveness of a preventive cardiology programme for high CVD risk persistent smokers: the EUROACTION PLUS varenicline trial. Eur Heart J. 2014;35:1411–1420. [DOI] [PubMed] [Google Scholar]
- 69. Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, Kim AS, Lindblad AS, Palesch YY. Clopidogrel and aspirin in acute ischemic stroke and high‐risk TIA. N Engl J Med. 2018;379:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kaiser C, Galatius S, Jeger R, Gilgen N, Skov Jensen J, Naber C, Alber H, Wanitschek M, Eberli F, Kurz DJ, et al. Long‐term efficacy and safety of biodegradable‐polymer biolimus‐eluting stents: main results of the Basel Stent Kosten‐Effektivitäts Trial‐PROspective Validation Examination II (BASKET‐PROVE II), a randomized, controlled noninferiority 2‐year outcome trial. Circulation. 2015;131:74–81. [DOI] [PubMed] [Google Scholar]
- 71. Kaitani K, Inoue K, Kobori A, Nakazawa Y, Ozawa T, Kurotobi T, Morishima I, Miura F, Watanabe T, Masuda M, et al. Efficacy of antiarrhythmic drugs short‐term use after catheter ablation for atrial fibrillation (EAST‐AF) trial. Eur Heart J. 2016;37:610–618. [DOI] [PubMed] [Google Scholar]
- 72. Kandzari DE, Mauri L, Koolen JJ, Massaro JM, Doros G, Garcia‐Garcia HM, Bennett J, Roguin A, Gharib EG, Cutlip DE, et al. Ultrathin, bioresorbable polymer sirolimus‐eluting stents versus thin, durable polymer everolimus‐eluting stents in patients undergoing coronary revascularisation (BIOFLOW V): a randomised trial. Lancet. 2017;390:1843–1852. [DOI] [PubMed] [Google Scholar]
- 73. Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim JM, Stewart R, Lee YS, Lee HJ, Kim MC, Kim JW, Kang HJ, Bae KY, Kim SW, Shin IS, et al. Effect of escitalopram vs placebo treatment for depression on long‐term cardiac outcomes in patients with acute coronary syndrome: a randomized clinical trial. JAMA. 2018;320:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Layland J, Oldroyd KG, Curzen N, Sood A, Balachandran K, Das R, Junejo S, Ahmed N, Lee MM, Shaukat A, et al. Fractional flow reserve vs. angiography in guiding management to optimize outcomes in non‐ST‐segment elevation myocardial infarction: the British Heart Foundation FAMOUS‐NSTEMI randomized trial. Eur Heart J. 2015;36:100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lee PH, Song JK, Kim JS, Heo R, Lee S, Kim DH, Song JM, Kang DH, Kwon SU, Kang DW, et al. Cryptogenic stroke and high‐risk patent foramen ovale: the DEFENSE‐PFO trial. J Am Coll Cardiol. 2018;71:2335–2342. [DOI] [PubMed] [Google Scholar]
- 77. Leoncini M, Toso A, Maioli M, Tropeano F, Villani S, Bellandi F. Early high‐dose rosuvastatin for contrast‐induced nephropathy prevention in acute coronary syndrome: results from the PRATO‐ACS Study (Protective Effect of Rosuvastatin and Antiplatelet Therapy On contrast‐induced acute kidney injury and myocardial damage in patients with Acute Coronary Syndrome). J Am Coll Cardiol. 2014;63:71–79. [DOI] [PubMed] [Google Scholar]
- 78. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 79. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. [DOI] [PubMed] [Google Scholar]
- 80. Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, Arquizan C, Béjot Y, Vuillier F, Detante O, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377:1011–1021. [DOI] [PubMed] [Google Scholar]
- 81. Matsumoto Y, Mori Y, Kageyama S, Arihara K, Sugiyama T, Ohmura H, Yakushigawa T, Sugiyama H, Shimada Y, Nojima Y, et al. Spironolactone reduces cardiovascular and cerebrovascular morbidity and mortality in hemodialysis patients. J Am Coll Cardiol. 2014;63:528–536. [DOI] [PubMed] [Google Scholar]
- 82. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington‐Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–1016. [DOI] [PubMed] [Google Scholar]
- 83. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 84. Mehra MR, Goldstein DJ, Uriel N, Cleveland JC Jr, Yuzefpolskaya M, Salerno C, Walsh MN, Milano CA, Patel CB, Ewald GA, et al. Two‐year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med. 2018;378:1386–1395. [DOI] [PubMed] [Google Scholar]
- 85. Mehra MR, Naka Y, Uriel N, Goldstein DJ, Cleveland JC Jr, Colombo PC, Walsh MN, Milano CA, Patel CB, Jorde UP, et al. A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med. 2017;376:440–450. [DOI] [PubMed] [Google Scholar]
- 86. Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer‐Westendorf J, Bluhmki E, Bouvaist H, Brenner B, Couturaud F, et al. Fibrinolysis for patients with intermediate‐risk pulmonary embolism. N Engl J Med. 2014;370:1402–1411. [DOI] [PubMed] [Google Scholar]
- 87. Mont L, Bisbal F, Hernández‐Madrid A, Pérez‐Castellano N, Viñolas X, Arenal A, Arribas F, Fernández‐Lozano I, Bodegas A, Cobos A, et al. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study). Eur Heart J. 2014;35:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Montalescot G, Pitt B, Lopez de Sa E, Hamm CW, Flather M, Verheugt F, Shi H, Turgonyi E, Orri M, Vincent J, et al. Early eplerenone treatment in patients with acute ST‐elevation myocardial infarction without heart failure: the Randomized Double‐Blind Reminder Study. Eur Heart J. 2014;35:2295–2302. [DOI] [PubMed] [Google Scholar]
- 89. Morillo CA, Verma A, Connolly SJ, Kuck KH, Nair GM, Champagne J, Sterns LD, Beresh H, Healey JS, Natale A. Radiofrequency ablation vs antiarrhythmic drugs as first‐line treatment of paroxysmal atrial fibrillation (RAAFT‐2): a randomized trial. JAMA. 2014;311:692–700. [DOI] [PubMed] [Google Scholar]
- 90. Notarangelo FM, Maglietta G, Bevilacqua P, Cereda M, Merlini PA, Villani GQ, Moruzzi P, Patrizi G, Malagoli Tagliazucchi G, Crocamo A, et al. Pharmacogenomic approach to selecting antiplatelet therapy in patients with acute coronary syndromes: the PHARMCLO trial. J Am Coll Cardiol. 2018;71:1869–1877. [DOI] [PubMed] [Google Scholar]
- 91. Ortiz M, Martín A, Arribas F, Coll‐Vinent B, Del Arco C, Peinado R, Almendral J. Randomized comparison of intravenous procainamide vs. intravenous amiodarone for the acute treatment of tolerated wide QRS tachycardia: the PROCAMIO study. Eur Heart J. 2017;38:1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Patel AN, Henry TD, Quyyumi AA, Schaer GL, Anderson RD, Toma C, East C, Remmers AE, Goodrich J, Desai AS, et al. Ixmyelocel‐T for patients with ischaemic heart failure: a prospective randomised double‐blind trial. Lancet. 2016;387:2412–2421. [DOI] [PubMed] [Google Scholar]
- 93. Perkins GD, Ji C, Deakin CD, Quinn T, Nolan JP, Scomparin C, Regan S, Long J, Slowther A, Pocock H, et al. A randomized trial of epinephrine in out‐of‐hospital cardiac arrest. N Engl J Med. 2018;379:711–721. [DOI] [PubMed] [Google Scholar]
- 94. Pu J, Ding S, Ge H, Han Y, Guo J, Lin R, Su X, Zhang H, Chen L, He B. Efficacy and safety of a pharmaco‐invasive strategy with half‐dose alteplase versus primary angioplasty in ST‐segment‐elevation myocardial infarction: EARLY‐MYO Trial (Early Routine Catheterization After Alteplase Fibrinolysis Versus Primary PCI in Acute ST‐Segment‐Elevation Myocardial Infarction). Circulation. 2017;136:1462–1473. [DOI] [PubMed] [Google Scholar]
- 95. Reardon MJ, Adams DH, Kleiman NS, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Lee JS, Hermiller JB Jr, Chetcuti S, et al. 2‐year outcomes in patients undergoing surgical or self‐expanding transcatheter aortic valve replacement. J Am Coll Cardiol. 2015;66:113–121. [DOI] [PubMed] [Google Scholar]
- 96. Reddy VY, Sievert H, Halperin J, Doshi SK, Buchbinder M, Neuzil P, Huber K, Whisenant B, Kar S, Swarup V, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988–1998. [DOI] [PubMed] [Google Scholar]
- 97. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 98. Rienstra M, Hobbelt AH, Alings M, Tijssen JGP, Smit MD, Brügemann J, Geelhoed B, Tieleman RG, Hillege HL, Tukkie R, et al. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J. 2018;39:2987–2996. [DOI] [PubMed] [Google Scholar]
- 99. Ringh M, Rosenqvist M, Hollenberg J, Jonsson M, Fredman D, Nordberg P, Järnbert‐Pettersson H, Hasselqvist‐Ax I, Riva G, Svensson L. Mobile‐phone dispatch of laypersons for CPR in out‐of‐hospital cardiac arrest. N Engl J Med. 2015;372:2316–2325. [DOI] [PubMed] [Google Scholar]
- 100. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 101. Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. [DOI] [PubMed] [Google Scholar]
- 102. Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, Sarrazin JF, Thibault B, Rivard L, Gula L, Leong‐Sit P, et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med. 2016;375:111–121. [DOI] [PubMed] [Google Scholar]
- 103. Sardella G, Lucisano L, Garbo R, Pennacchi M, Cavallo E, Stio RE, Calcagno S, Ugo F, Boccuzzi G, Fedele F, et al. Single‐staged compared with multi‐staged PCI in multivessel NSTEMI patients: the SMILE trial. J Am Coll Cardiol. 2016;67:264–272. [DOI] [PubMed] [Google Scholar]
- 104. Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, Tirschwell DL. Long‐term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377:1022–1032. [DOI] [PubMed] [Google Scholar]
- 105. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 106. Shahzad A, Kemp I, Mars C, Wilson K, Roome C, Cooper R, Andron M, Appleby C, Fisher M, Khand A, et al. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT‐PPCI): an open‐label, single centre, randomised controlled trial. Lancet. 2014;384:1849–1858. [DOI] [PubMed] [Google Scholar]
- 107. Smits PC, Abdel‐Wahab M, Neumann FJ, Boxma‐de Klerk BM, Lunde K, Schotborgh CE, Piroth Z, Horak D, Wlodarczak A, Ong PJ, et al. Fractional flow reserve‐guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376:1234–1244. [DOI] [PubMed] [Google Scholar]
- 108. Sohara H, Ohe T, Okumura K, Naito S, Hirao K, Shoda M, Kobayashi Y, Yamauchi Y, Yamaguchi Y, Kuwahara T, et al. HotBalloon ablation of the pulmonary veins for paroxysmal AF: a multicenter randomized trial in Japan. J Am Coll Cardiol. 2016;68:2747–2757. [DOI] [PubMed] [Google Scholar]
- 109. Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen‐Kudsk JE, Settergren M, Sjöstrand C, Roine RO, Hildick‐Smith D, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377:1033–1042. [DOI] [PubMed] [Google Scholar]
- 110. Steinhubl SR, Waalen J, Edwards AM, Ariniello LM, Mehta RR, Ebner GS, Carter C, Baca‐Motes K, Felicione E, Sarich T, et al. Effect of a home‐based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA. 2018;320:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, et al. Transcatheter mitral‐valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. [DOI] [PubMed] [Google Scholar]
- 112. Taguchi I, Iimuro S, Iwata H, Takashima H, Abe M, Amiya E, Ogawa T, Ozaki Y, Sakuma I, Nakagawa Y, et al. High‐dose versus low‐dose pitavastatin in Japanese patients with stable coronary artery disease (REAL‐CAD): a randomized superiority trial. Circulation. 2018;137:1997–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tang YD, Wang W, Yang M, Zhang K, Chen J, Qiao S, Yan H, Wu Y, Huang X, Xu B, et al. Randomized comparisons of double‐dose clopidogrel or adjunctive cilostazol versus standard dual antiplatelet in patients with high posttreatment platelet reactivity: results of the CREATIVE trial. Circulation. 2018;137:2231–2245. [DOI] [PubMed] [Google Scholar]
- 114. Tegn N, Abdelnoor M, Aaberge L, Endresen K, Smith P, Aakhus S, Gjertsen E, Dahl‐Hofseth O, Ranhoff AH, Gullestad L, et al. Invasive versus conservative strategy in patients aged 80 years or older with non‐ST‐elevation myocardial infarction or unstable angina pectoris (After Eighty study): an open‐label randomised controlled trial. Lancet. 2016;387:1057–1065. [DOI] [PubMed] [Google Scholar]
- 115. Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer‐Saraei R, Nordbeck P, Geisler T, Landmesser U, Skurk C, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377:2419–2432. [DOI] [PubMed] [Google Scholar]
- 116. Tomai F, Ribichini F, De Luca L, Petrolini A, Ghini AS, Weltert L, Spaccarotella C, Proietti I, Trani C, Nudi F, et al. Randomized comparison of Xience V and multi‐link vision coronary stents in the same multivessel patient with chronic kidney disease (RENAL‐DES) study. Circulation. 2014;129:1104–1112. [DOI] [PubMed] [Google Scholar]
- 117. Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrié D, Naber C, Lipiecki J, Richardt G, Iñiguez A, Brunel P, et al. Polymer‐free drug‐coated coronary stents in patients at high bleeding risk. N Engl J Med. 2015;373:2038–2047. [DOI] [PubMed] [Google Scholar]
- 118. Valgimigli M, Frigoli E, Leonardi S, Vranckx P, Rothenbühler M, Tebaldi M, Varbella F, Calabrò P, Garducci S, Rubartelli P, et al. Radial versus femoral access and bivalirudin versus unfractionated heparin in invasively managed patients with acute coronary syndrome (MATRIX): final 1‐year results of a multicentre, randomised controlled trial. Lancet. 2018;392:835–848. [DOI] [PubMed] [Google Scholar]
- 119. Valgimigli M, Gagnor A, Calabró P, Frigoli E, Leonardi S, Zaro T, Rubartelli P, Briguori C, Andò G, Repetto A, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385:2465–2476. [DOI] [PubMed] [Google Scholar]
- 120. Valgimigli M, Patialiakas A, Thury A, McFadden E, Colangelo S, Campo G, Tebaldi M, Ungi I, Tondi S, Roffi M, et al. Zotarolimus‐eluting versus bare‐metal stents in uncertain drug‐eluting stent candidates. J Am Coll Cardiol. 2015;65:805–815. [DOI] [PubMed] [Google Scholar]
- 121. Varenne O, Cook S, Sideris G, Kedev S, Cuisset T, Carrié D, Hovasse T, Garot P, El Mahmoud R, Spaulding C, et al. Drug‐eluting stents in elderly patients with coronary artery disease (SENIOR): a randomised single‐blind trial. Lancet. 2018;391:41–50. [DOI] [PubMed] [Google Scholar]
- 122. Velazquez EJ, Lee KL, Jones RH, Al‐Khalidi HR, Hill JA, Panza JA, Michler RE, Bonow RO, Doenst T, Petrie MC, et al. Coronary‐artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374:1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Verheye S, Jolicœur EM, Behan MW, Pettersson T, Sainsbury P, Hill J, Vrolix M, Agostoni P, Engstrom T, Labinaz M, et al. Efficacy of a device to narrow the coronary sinus in refractory angina. N Engl J Med. 2015;372:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, Merkel PA, Moosig F, Specks U, Cid MC, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376:1921–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 126. Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yang J, Yang L, Yu S, Liu J, Zuo J, Chen W, Duan W, Zheng Q, Xu X, Li J, et al. Transcatheter versus surgical closure of perimembranous ventricular septal defects in children: a randomized controlled trial. J Am Coll Cardiol. 2014;63:1159–1168. [DOI] [PubMed] [Google Scholar]
- 128. Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, Pais P, López‐Jaramillo P, Leiter LA, Dans A, et al. Cholesterol lowering in intermediate‐risk persons without cardiovascular disease. N Engl J Med. 2016;374:2021–2031. [DOI] [PubMed] [Google Scholar]
- 129. Yusuf S, Lonn E, Pais P, Bosch J, López‐Jaramillo P, Zhu J, Xavier D, Avezum A, Leiter LA, Piegas LS, et al. Blood‐pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med. 2016;374:2032–2043. [DOI] [PubMed] [Google Scholar]
- 130. Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, Wolf B, Goebel U, Schwer CI, Rosenberger P, et al. Effect of remote ischemic preconditioning on kidney injury among high‐risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313:2133–2141. [DOI] [PubMed] [Google Scholar]
- 131. Zhang J, Gao X, Kan J, Ge Z, Han L, Lu S, Tian N, Lin S, Lu Q, Wu X, et al. Intravascular ultrasound versus angiography‐guided drug‐eluting stent implantation: the ULTIMATE trial. J Am Coll Cardiol. 2018;72:3126–3137. [DOI] [PubMed] [Google Scholar]
- 132. Zhang XD, Gu J, Jiang WF, Zhao L, Zhou L, Wang YL, Liu YG, Liu X. Optimal rhythm‐control strategy for recurrent atrial tachycardia after catheter ablation of persistent atrial fibrillation: a randomized clinical trial. Eur Heart J. 2014;35:1327–1334. [DOI] [PubMed] [Google Scholar]
- 133. Zhao Q, Zhu Y, Xu Z, Cheng Z, Mei J, Chen X, Wang X. Effect of ticagrelor plus aspirin, ticagrelor alone, or aspirin alone on saphenous vein graft patency 1 year after coronary artery bypass grafting: a randomized clinical trial. JAMA. 2018;319:1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 135. Hróbjartsson A, Emanuelsson F, Skou Thomsen AS, Hilden J, Brorson S. Bias due to lack of patient blinding in clinical trials. A systematic review of trials randomizing patients to blind and nonblind sub‐studies. Int J Epidemiol. 2014;43:1272–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sedgwick P. Allocation concealment versus blinding in randomised controlled trials. BMJ. 2013;347:f5518. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Datas S1–S7 Tables S1–S2 Figure S1 References 14, and 17–134


