Abstract
Background
Coronary flow capacity (CFC), which is a categorical assessment based on the combination of hyperemic coronary flow and coronary flow reserve (CFR), has been introduced as a comprehensive assessment of the coronary circulation to overcome the limitations of CFR alone. The aim of this study was to quantify coronary flow changes after percutaneous coronary intervention in relation to the classification of CFC and the current physiological cutoff values of fractional flow reserve, instantaneous wave‐free ratio, and CFR.
Methods and Results
Using the combined data set from DEFINE FLOW (Distal Evaluation of Functional Performance With Intravascular Sensors to Assess the Narrowing Effect ‐Combined Pressure and Doppler FLOW Velocity Measurements) and IDEAL (Iberian‐Dutch‐English), a total of 133 vessels that underwent intracoronary Doppler flow measurement before and after percutaneous coronary intervention were analyzed. CFC classified prerevascularization lesions as normal (14), mildly reduced (40), moderately reduced (31), and severely reduced (48). Lesions with larger impairment of CFC showed greater increase in coronary flow and vice versa (median percent increase in coronary flow by revascularization: 4.2%, 25.9%, 50.1%, and 145.5%, respectively; P<0.001). Compared with the conventional cutoff values of fractional flow reserve, instantaneous wave‐free ratio, and CFR, an ischemic CFC defined as moderately to severely reduced CFC showed higher diagnostic accuracy with higher specificity to predict a >50% increase in coronary flow after percutaneous coronary intervention. Receiver operating characteristic curve analysis demonstrated that only CFC has a superior predictive efficacy to CFR (P<0.05). Multivariate analysis revealed lesions with ischemic CFC to be the independent predictor of a significant coronary flow increase after percutaneous coronary intervention (odds ratio, 10.7; 95% CI, 4.6–24.8; P<0.001).
Conclusions
CFC showed significant improvement of identification of lesions that benefit from revascularization compared with CFR with respect to coronary flow increase.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02328820.
Keywords: coronary blood flow, coronary flow capacity, coronary flow reserve, fractional flow reserve, percutaneous coronary intervention
Subject Categories: Coronary Circulation, Percutaneous Coronary Intervention, Revascularization, Coronary Artery Disease
Nonstandard Abbreviations and Acronyms
- APV
average peak coronary blood flow velocity
- CFC
coronary flow capacity
- CFR
coronary flow reserve
- Pa
aortic pressure
- FFR
fractional flow reserve
- hAPV
hyperemic average peak coronary blood flow velocity
- iFR
instantaneous wave‐free ratio
- PCI
percutaneous coronary intervention
- R‐hAPV
ratio of hAPV after PCI to before PCI
Clinical Perspective
What Is New?
Coronary flow capacity is a modified flow‐based comprehensive assessment of the coronary circulation that improves the discriminating ability to predict coronary flow increase after revascularization in comparison with conventional coronary flow reserve.
Moderately‐to‐severely reduced coronary flow capacity has a high predictive performance with high sensitivity for a significant increase in coronary flow compared with the conventional cutoff values for fractional flow reserve, instantaneous wave‐free ratio, and coronary flow reserve.
What Are the Clinical Implications?
Coronary flow capacity is a useful index to identify lesions that benefit from revascularization with respect to coronary flow increase and can yield incremental information in the treatment strategies based on the current cutoff values of conventional physiological indices.
The current study provides a physiological rationale for revascularization of lesions guided by coronary flow capacity grading.
Previous studies have shown that the benefit of revascularization in patients with stable coronary artery disease exists along a continuum. 1 , 2 , 3 Pressure‐derived coronary physiological indices like fractional flow reserve (FFR) are commonly used as invasive markers to quantify the functional severity of an epicardial coronary artery lesion and to guide revascularization. However, a complete description of coronary physiology extends beyond epicardial pressure gradients. 4 , 5 , 6 , 7 Therefore, a comprehensive hemodynamic assessment could augment identification of optimal lesions for revascularization.
Coronary flow reserve (CFR) provides a well‐validated index incorporating both epicardial and microcirculatory contributions. 8 This index associates with long‐term adverse events. 9 , 10 However, as a ratio of 2 physiological conditions, CFR alone does not distinguish between alterations in baseline versus hyperemic flow. To overcome these limitations, Johnson and Gould introduced the concept of coronary flow capacity (CFC) as a novel, comprehensive framework for coronary physiology using positron emission tomography. 11 CFC is a categorical assessment based on the combination of hyperemic myocardial blood flow and CFR to classify the impairment of myocardial blood flow. This concept has been subsequently translated into intracoronary physiological assessment and found to offer superior prognostic efficacy for long‐term clinical outcomes versus CFR in deferred coronary artery lesions. 12 This superior capacity for discriminations of adverse cardiovascular events sets the basis for aiming to use CFC as an invasive diagnostic tool. We hypothesized that CFC would also improve the identification of lesions that benefit from percutaneous coronary intervention (PCI) with respect to coronary flow improvement. Thus, the aim of this study was to quantify coronary flow changes after PCI in relation to the classification of CFC and the current cutoff values of conventional physiological indices.
Methods
Study Design and Patient Population
Study data combined 2 studies. One was a subset of the DEFINE FLOW (Distal Evaluation of Functional Performance With Intravascular Sensors to Assess the Narrowing Effect—Combined Pressure and Doppler FLOW Velocity Measurements) study (NCT02328820). 13 Another was the international multicenter pooled database from Amsterdam UMC (The Netherlands), Imperial College of London (United Kingdom), and Hospital Clinico San Carlos (Spain), part of which was from IDEAL study. 14 In brief, the DEFINE FLOW study included patients with coronary artery disease who had at least one epicardial stenosis of ≥50% diameter stenosis; the multicenter pooled database enrolled patients with ≥1 intermediate coronary stenosis (40%–70% diameter stenosis at visual assessment). PCI was performed only when both FFR and CFR values were below the established criteria (FFR ≤0.80 and CFR <2.0) for the DEFINE FLOW study, while PCI was done at the operator’s discretion on the basis of not only FFR values but also impaired CFR (CFR <2.0) or angiographic findings with suspicious symptoms of angina in the pooled database that encompassed the IDEAL study. Subjects from both studies underwent pre‐PCI coronary physiological assessment including intracoronary Doppler flow measurement, but post‐PCI physiological assessment was not mandatory. All subjects with measurements before and after PCI were included in the current analysis. The exclusion criteria of both studies were prior coronary artery bypass grafting, left main coronary artery disease, subtotal or similar high‐grade lesions, and culprit lesions for recent myocardial infarction. The institutional ethics committees for each center approved the parent study, and all subjects gave written informed consent. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Coronary Angiographic and Physiological Assessments
Before angiography, the operator administered intracoronary nitroglycerin as per routine. Coronary angiography was performed by standard techniques. All physiological measurements were performed using a 0.014‐inch dual‐sensor–equipped guidewire (Combowire; Philips‐Volcano, San Diego, CA). For serial or tandem lesions in the same vessel, only a single physiologic study was performed distal to all lesions. Intracoronary physiological measurements including distal coronary pressure and average peak coronary blood flow velocity (APV) were simultaneously recorded during stable conditions at rest and at hyperemia. Hyperemia was induced by intracoronary adenosine administration (20–150 μg) or intravenous adenosine infusion (140 μg/kg per minute) through either a central or peripheral vein. Intracoronary physiological assessment was repeated after PCI.
Data Analysis
Physiological data were extracted from the digital archive (ComboMap, Philips‐Volcano, San Diego, CA) and analyzed offline using a custom software package designed with MATLAB (Mathworks, Inc, Natick, MA, USA) for the IDEAL data set and in core laboratory using custom software Studymanager for the DEFINE FLOW data set by experienced analysts blinded to the coronary angiogram and clinical characteristics. FFR was calculated as the ratio of mean distal coronary pressure to aortic pressure (Pa) during hyperemia. instantaneous wave‐free ratio (iFR) is the ratio of distal coronary pressure/Pa measured during a prespecified period in mid‐to‐late diastole without hyperemia. CFR was calculated as the ratio of hyperemic average peak coronary blood flow velocity (hAPV) to basal APV. The conventional cutoff values of physiological indices: 0.80 for FFR, 0.89 for iFR, and 2.0 for CFR, which have been recommended in decision making in the current treatment guidelines of revascularization, 15 , 16 , 17 were applied. CFC categorizes vessels into 4 grades based on hAPV and CFR. In the present study, CFC classification was applied according to the criteria developed in a previous study as follows 12 : Normal CFC was defined as a CFR ≥2.8 with corresponding hAPV of ≥49.0 cm/s. Mildly reduced CFC was defined as a CFR <2.8 and >2.1 and corresponding hAPV of <49.0 and >33.0 cm/s, respectively. Moderately reduced CFC was defined as CFR ≤2.1 and >1.7 with corresponding hAPV of ≤33.0 and >26.0 cm/s, respectively. Finally, severely reduced CFC was defined as a CFR ≤1.7 and corresponding hAPV of ≤26.0 cm/s. According to the design of CFC grading established in the previous study, 12 a grading of moderately or severely reduced CFC was considered “ischemic” CFC in the present study. The change in hyperemic coronary flow was assessed by the ratio of post‐PCI hAPV to pre‐PCI hAPV. A previous positron emission tomography study showed an average increase in coronary flow after PCI of 46%. 18 Accordingly, we defined a >50% increase of peak coronary flow velocity after PCI (ratio of hAPV after PCI to before PCI [R‐hAPV] ≥150%) as a significant improvement. For 2 subjects with multivessel disease evaluated before and after PCI, the single vessel with the lower FFR value was included in this study to maintain 1 vessel/subject.
Statistical Analysis
Normality of the distribution was assessed by Shapiro‐Wilk statistics, and the homogeneity of variances was assessed by Levene’s test. Continuous variables are expressed as mean±standard deviation when normally distributed and as median with first and third quartiles (Q1, Q3) when nonnormally distributed. Categorical variables are presented as counts and percentages. Overall differences of clinical, physiological, and angiographic parameters in the classification of CFC grading (4 categories) and grading of CFR based on CFC criteria were compared with Kruskal–Wallis, or chi‐squared tests, followed by post hoc Mann–Whitney U, chi‐squared, or Fisher’s exact tests with Bonferroni adjustment. Receiver operating characteristic curve analysis was performed to compare the predictive efficacies of physiological indices to predict lesions with R‐hAPV ≥150%. To evaluate the incremental predictive ability of each physiological cutoff value added to angiographic stenosis severity as a baseline model, the concordance index was assessed. The discriminatory ability of ischemic CFC added to binary conventional physiological indices was assessed by the reclassification performance of each model using the integrated discrimination improvement and net reclassification improvement values. Predictive factors for 50% increase in coronary flow (R‐hAPV ≥150%) were assessed by using univariate and multivariate logistic regression analyses. FFR and iFR showed a significant relationship in the present study (Spearman’s rho=0.87). Therefore, 2 multivariate models based on either FFR or iFR were made for clinical use. Associated variables in univariate analysis (P≤0.10) were entered into the final multivariate model. The statistical analysis was performed by using SPSS version 22.0 (SPSS, Inc., Chicago, IL) and R version 3.5.3. A P value of <0.05 was considered statistically significant, using 2‐tailed testing when applicable.
Results
Baseline Characteristics
A total of 133 vessels were analyzed in this study (33 vessels from DEFINE FLOW and 100 from the pooled database). Nine subjects were missing either pre‐PCI or post‐PCI iFR. Clinical, angiographic, and physiological characteristics of this study are shown in Table 1. The median values of FFR, iFR, and CFR before PCI were 0.70 (Q1–Q3: 0.56–0.80), 0.82 (Q1–Q3: 0.61–0.90), and 1.6 (Q1–Q3: 1.3–2.1). There were no significant differences between pre‐ and post‐PCI blood pressure (pre‐PCI Pa at rest: 98±16 versus post‐PCI Pa at rest: 99±16; P=0.30; pre‐PCI Pa at hyperemia: 91±16 versus post‐PCI Pa at hyperemia: 90±17; P=0.23). The median value of hAPV changed from 26.8 to 42.6 cm/s after PCI. The median value of R‐hAPV was 149.4% (Q1–Q3: 112.6%–255.4%) and a R‐hAPV ≥150% was observed in 66 vessels (49.6%).
Table 1.
Patient Characteristics and Physiological Parameters Before and After PCI Across CFC Grading
| Total, N=133 | Normal CFC, N=14 | Mildly Reduced CFC, N=40 | Moderately Reduced CFC, N=31 | Severely Reduced CFC, N=48 | P Value | |
|---|---|---|---|---|---|---|
| Age, y | 63.1±10.1 | 61.6±10.9 | 63.4±10.7 | 63.3±9.9 | 63.3±9.7 | 0.94 |
| Male, n (%) | 101 (76.0) | 13 (92.9) | 29 (72.5) | 25 (80.6) | 34 (70.8) | 0.32 |
| Lesion location RCA/LAD/LCX, n (%) | 35 (26.3)/76 (57.1)/22 (16.5) | 4 (28.6)/9 (64.3)/1 (7.1) | 10 (25.0)/25 (62.5)/5 (12.5) | 11 (35.5)/13 (41.9)/7 (22.6) | 10 (20.8)/29 (60.4)/9 (18.8) | 0.52 |
| Hypertension, n (%) | 74 (55.6) | 7 (50.0) | 21 (52.5) | 19 (61.3) | 27 (56.3) | 0.90 |
| Hyperlipidemia, n (%) | 95 (71.4) | 12 (85.7) | 27 (67.5) | 24 (77.4) | 32 (66.7) | 0.43 |
| Diabetes mellitus, n (%) | 40 (30.1) | 3 (21.4) | 12 (30.0) | 7 (22.6) | 18 (37.5) | 0.46 |
| Medication, n (%) | ||||||
| ACE‐I or ARB | 30 (22.6) | 3 (21.4) | 8 (20.0) | 7 (22.6) | 12 (25.0) | 0.96 |
| β‐blocker | 65 (48.9) | 7 (50.0) | 20 (50.0) | 17 (54.8) | 21 (43.8) | 0.81 |
| CCB | 35 (26.3) | 3 (21.4) | 5 (12.5)* | 13 (41.9) † | 14 (29.2) | 0.042 |
| Statin | 69 (51.9) | 7 (50.0) | 19 (47.5) | 17 (54.8) | 26 (54.2) | 0.91 |
| QCA analyses | ||||||
| Pre‐PCI MLD, mm | 1.00±0.35 | 1.20±0.27 | 1.06±0.37 | 0.95±0.37 | 0.92±0.31 | 0.085 |
| Post‐PCI MLD, mm | 2.41±0.63 | 2.37±0.64 | 2.52±0.60 | 2.30±0.55 | 2.42±0.72 | 0.72 |
| Pre‐PCI RD, mm | 2.60 (2.17–2.86) | 2.35 (2.11–2.73) | 2.65 (2.29–2.89) | 2.43 (1.86–3.08) | 2.61 (2.26–2.83) | 0.77 |
| Post‐PCI RD, mm | 2.80 (2.45–3.28) | 2.67 (2.54–3.03) | 3.07 (2.53–3.47) | 2.60 (2.30–3.38) | 2.96 (2.40–3.27) | 0.68 |
| Pre‐PCI DS, % | 58.5 (48.9–68.3) | 49.1 (44.5–58.5) ‡ | 55.7 (48.6–62.2) ‡ | 62.8 (44.0–72.0) | 66.0 (54.7–71.5) † , § | 0.006 |
| Post‐PCI DS, % | 13.1 (7.5–21.9) | 14.5 (7.3–19.8) | 13.0 (7.3–20.6) | 13.8 (9.0–22.4) | 12.0 (6.0–26.2) | 0.77 |
| Physiologic parameters | ||||||
| Baseline | ||||||
| Pa at rest, mm Hg | 98±16 | 97±15 | 99±15 | 98±17 | 97±1 | 0.98 |
| Pd at rest, mm Hg | 80±20 | 87±15 ‡ | 87±17 ‡ | 83±18 ‡ | 71±22*, † , § | <0.001 |
| Basal APV, cm/s | 16.4 (11.9–20.4) | 27.2 (19.0–38.1)*, ‡ | 18.1 (13.4–23.2) ‡ | 17.6 (10.7–19.6) § | 13.7 (10.9–16.3) † , § | <0.001 |
| Pa at hyperemia, mm Hg | 91±16 | 95±19 | 92±16 | 91±16 | 89±15 | 0.60 |
| Pd at hyperemia, mm Hg | 62±18 | 72±18 ‡ | 68±15 ‡ | 64±17 ‡ | 53±17*, † , § | <0.001 |
| hAPV, cm/s | 26.8 (18.6–38.2) | 57.4 (51.9–81.0)*, † , ‡ | 37.6 (31.5–41.6)*, ‡ , § | 27.4 (20.6–30.1) † , ‡ , § | 18.3 (12.7–22.7)*, † , § | <0.001 |
| FFR | 0.70 (0.55–0.80) | 0.79 (0.70–0.82) ‡ | 0.75 (0.68–0.82) ‡ | 0.73 (0.59–0.79) ‡ | 0.56 (0.45–0.73)*, † , § | <0.001 |
| iFR | 0.82 (0.61–0.90) | 0.86 (0.80–0.95) ‡ | 0.88 (0.81–0.92) ‡ | 0.82 (0.68–0.91) ‡ | 0.57 (0.37–0.85)*, † , § | <0.001 |
| CFR | 1.6 (1.3–2.1) | 2.2 (1.7–3.6)*, ‡ | 2.2 (1.7–2.4)*, ‡ | 1.7 (1.4–1.9) † , ‡ , § | 1.3 (1.0–1.5)*, † , § | <0.001 |
| After PCI | ||||||
| Pa at rest, mm Hg | 99±16 | 98±15 | 99±15 | 100±17 | 98±16 | 0.99 |
| Pd at rest, mm Hg | 94±16 | 93±16 | 94±15 | 95±16 | 95±16 | 0.98 |
| Basal APV, cm/s | 19.1 (14.7–27.0) | 25.8 (16.3–32.6) | 19.0 (14.2–26.9) | 17.0 (13.1–23.8) | 18.7 (14.5–27.3) | 0.22 |
| Pa at hyperemia, mm Hg | 90±17 | 92±15 | 89±18 | 91±17 | 89±16 | 0.91 |
| Pd at hyperemia, mm Hg | 80±17 | 81±17 | 80±18 | 81±17 | 80±16 | 0.99 |
| hAPV, cm/s | 42.6 (36.5–62.3) | 59.3 (47.2–73.5) † | 43.5 (36.7–58.5) § | 39.5 (30.9–67.5) | 41.1 (32.7–66.7) | 0.037 |
| FFR | 0.90 (0.85–0.97) | 0.89 (0.80–0.91) | 0.90 (0.86–0.94) | 0.90 (0.85–0.95) | 0.90 (0.86–0.95) | 0.47 |
| iFR | 0.96 (0.91–0.98) | 0.93 (0.89–0.97) | 0.95 (0.92–0.98) | 0.96 (0.92–0.99) | 0.96 (0.92–0.99) | 0.49 |
| CFR | 2.3 (1.9–2.9) | 2.6 (2.2–3.0) | 2.5 (1.8–3.1) | 2.2 (1.9–3.0) | 2.2 (1.8–2.7) | 0.38 |
| CFC, normal/mild/moderate/severe, n (%) | 71 (53.4)/45 (33.8)/12 (9.0)/5 (3.8) | 11 (78.6)/3 (21.4)/0 (0.0)/0 (0.0) | 23 (57.5)/14 (35.0)/3 (7.5)/0 (0.0) | 13 (41.9)/12 (38.7)/3 (9.7)/3 (9.7) | 24 (50.0)/16 (33.3)/6 (12.5)/2 (4.2) | 0.31 |
| Change in physiological parameters | ||||||
| Absolute change in FFR | 0.21±0.17 | 0.10±0.12 ‡ | 0.16±0.13 ‡ | 0.19±15 ‡ | 0.30±17*, † , § | <0.001 |
| Absolute change in iFR | 0.11 (0.03–0.35) | 0.04 (−0.01 to 0.11) ‡ | 0.08 (0.01 to 0.12) ‡ | 0.11 (0.03–0.30) ‡ | 0.36 (0.11–0.58)*, † , § | <0.001 |
| Absolute change in CFR | 0.6 (0.2–1.2) | 0.2 (−0.4 to 0.7) ‡ | 0.5 (−0.1 to 1.0) ‡ | 0.7 (0.2–1.2) | 1.0 (0.5–1.6) † , § | 0.001 |
| Absolute change in hAPV cm/s | 15.0 (4.7–28.9) | 2.5 (−4.2 to 8.0)*, ‡ | 9.8 (−1.6 to 20.3) ‡ | 14.0 (6.3–36.6) § | 25.3 (12.4–45.7) † , § | <0.001 |
| R‐hAPV, % | 149.4 (112.6–255.4) | 104.2 (91.6 to 115.2)*, ‡ | 125.9 (95.9 to 151.4)*, ‡ | 150.1 (129.4–239.4) † , ‡ , § | 245.5 (168.9–423.8)*, † , § | <0.001 |
| Vessels with R‐hAPV >150%, n (%) | 66 (49.6) | 0 (0.0)*, ‡ | 10 (25.0) ‡ | 16 (51.6) ‡ , § | 40 (83.3)*, † , § | <0.001 |
ACE‐I indicates angiotensin‐converting enzyme inhibitor; APV, average peak coronary flow velocity; ARB, angiotensin receptor blocker; BMI, body mass index; CCB, calcium channel blocker; CFC, coronary flow capacity; CFR, coronary flow reserve; DS, diameter stenosis; FFR, fractional flow reserve; hAPV, hyperemic average peak flow velocity; iFR, instantaneous wave‐free ratio; LAD, left anterior descending coronary artery; LCX, left circumflex artery; MLD, minimal lumen diameter; Pa, aortic pressure; PCI, percutaneous coronary intervention; Pd, distal coronary pressure; QCA, quantitative coronary angiography; RCA, right coronary artery; RD, reference diameter; and R‐hAPV, ratio of post‐PCI hAPV to pre‐PCI.
P<0.05 vs moderately reduced CFC.
P<0.05 vs mildly reduced CFC.
P<0.05 vs severely reduced CFC.
P<0.05 vs normal CFC.
Lesions classified by CFC before PCI were as follows: 14 normal CFC, 40 mildly reduced CFC, 31 moderately reduced CFC, and 48 severely reduced CFC. Figure 1 shows the distribution plot of CFC grading. There were no significant differences in clinical variables among the groups with different CFC grading except for the use of calcium channel blockers and baseline percent diameter stenosis. Values of pre‐PCI FFR and iFR showed considerable overlap among CFC classifications. After PCI, there were no significant differences in FFR, iFR, CFR, and CFC grading across the groups.
Figure 1.
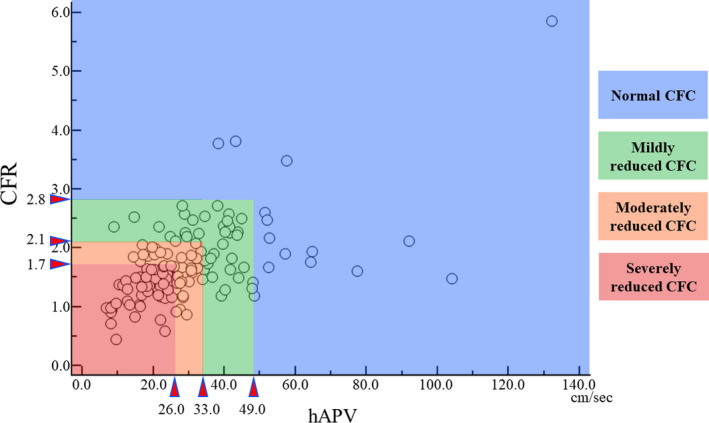
The distribution of CFC grading.
CFC indicates coronary flow capacity; CFR, coronary flow reserve; and hAPV, hyperemic average peak flow velocity.
The Discriminating Efficacy of CFC for the Coronary Flow Increase in Comparison With CFR
Figure 2 shows the relationship between increase in coronary flow and pre‐PCI CFR or CFC classification, and the individual coronary flow changes in each CFC grade are shown in Figure S1. Lesions with a greater impairment of CFC showed a larger increase in coronary flow (R‐hAPV: 104.2% for normal CFC; 125.9% for mildly reduced; 150.1% for moderately reduced; 245.5% for severely reduced; P<0.001; Figure 2A). Using the same CFR distribution based on CFC criteria showed that only lesions with CFR corresponding to severely reduced CFC (CFR ≤1.7) had a significantly higher coronary flow increase after PCI (R‐hAPV: 97.4% for CFR in the normal zone; 126.6% for CFR in the mildly reduced zone; 133.3% for CFR in the moderately reduced zone; 181.7% for CFR in the severely reduced zone; P=0.001; Figure 2B).
Figure 2.
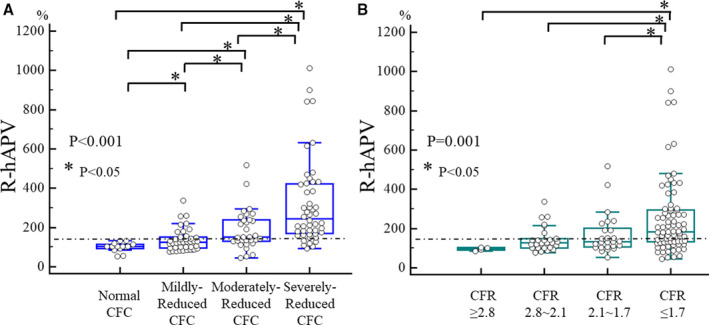
Change in coronary flow after PCI across CFC and CFR classification.
A, classification according to CFC grading and (B) classification according to CFR grading based on the CFC criteria. CFC indicates coronary flow capacity; CFR, coronary flow reserve; and R‐hAPV, ratio of post‐PCI hyperemic average peak flow velocity to pre‐PCI.
Diagnostic Efficacy Compared Between Conventional Physiological Indices and CFC Classification for the Vessels With Significant Coronary Flow Increase After PCI
Figure 3 shows the relationship between the pre‐PCI values of conventional indices and hyperemic velocity change after PCI. All indices showed a significant linear relationship with change in coronary flow. However, almost 40% of patients who underwent revascularization in the present study did not achieve a significant increase in coronary flow after PCI even in lesions below the current cutoff values (Figure 4). Compared with conventional cutoff values of FFR (≤0.80), iFR (≤0.89), and CFR (<2.0), the “ischemic” CFC (moderately to severely reduced CFC) showed numerically higher diagnostic accuracy with higher specificity to predict over 50% increase in coronary flow after PCI (accuracy and specificity: 75.2% and 65.7% for ischemic CFC; 63.9% and 37.3% for FFR ≤0.80; 67.7% and 42.9% for iFR ≤0.89; 63.2% and 40.3% for CFR <2.0, Figure 4). Receiver operating characteristic curve analysis showed that a significantly high predictive efficacy of CFC was detected in comparison with CFR, although there is no statistical difference between the conventional physiological indices (Figure 5). The concordance index of adding physiological cutoff values to angiographic stenosis severity revealed the incremental predictive efficacy of all physiological indices, although only CFR did not show statistical significance (Figure 6). Table 2 shows the results of the reclassification efficacy of ischemic CFC grading to predict the occurrence of significant coronary flow increase. Compared with conventional physiological indices‐based classifications, all of the models integrated with CFC grading showed further increases in their incremental reclassification ability.
Figure 3.
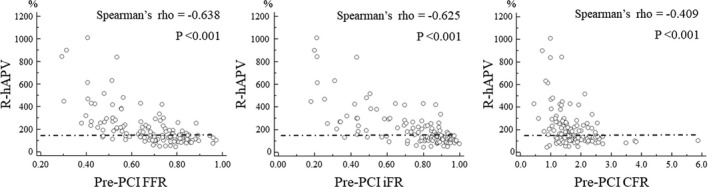
The relationships between pre‐PCI values of conventional physiological indices and change in coronary flow after PCI.
CFC indicates coronary flow capacity; CFR, coronary flow reserve; FFR, fractional flow reserve; iFR, instantaneous wave‐free ratio; PCI, percutaneous coronary intervention; and R‐hAPV, ratio of post‐PCI hyperemic average peak flow velocity to pre‐PCI.
Figure 4.
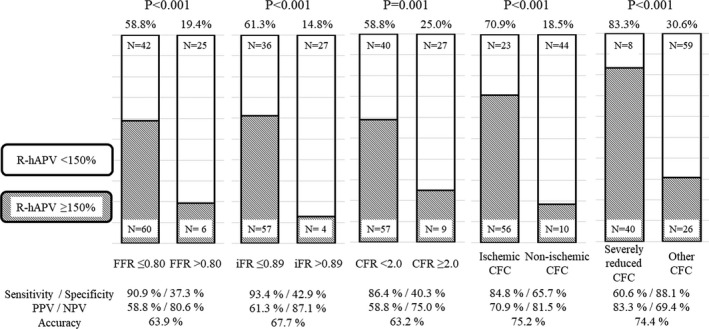
Prevalence of significant increase in coronary flow after PCI in the classification based on the cutoff value of the various physiological indices.
CFC indicates coronary flow capacity; CFR, coronary flow reserve; FFR, fractional flow reserve; iFR, instantaneous wave‐free ratio; NPV, negative predictive value; PPV, positive predictive value; and R‐hAPV, ratio of post‐PCI hAPV to pre‐PCI. R‐hAPV >150% was defined as the significant coronary flow increase after PCI.
Figure 5.
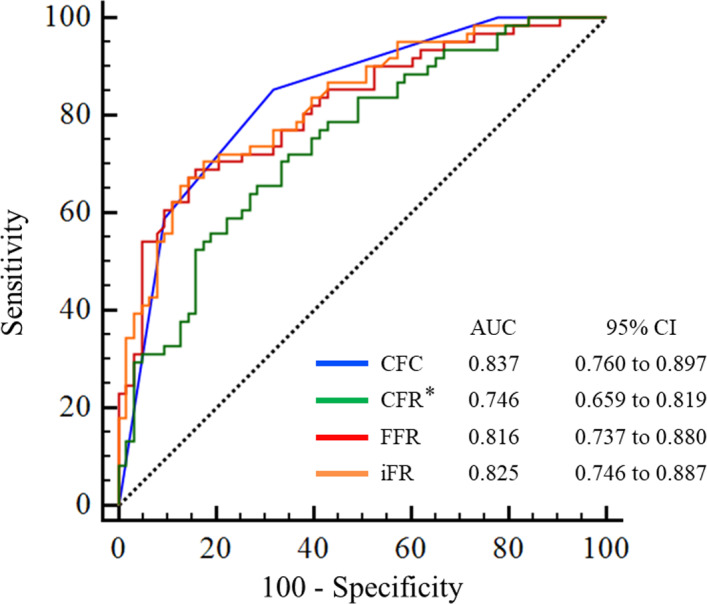
Receiver operating characteristic curve analysis for predicting significant coronary flow increase by intracoronary physiological indices.
*P<0.05 in comparison with CFC. AUC indicates area under the curve; CFC, coronary flow capacity; CFR, coronary flow reserve; FFR, fractional flow reserve; and iFR, instantaneous wave‐free ratio.
Figure 6.
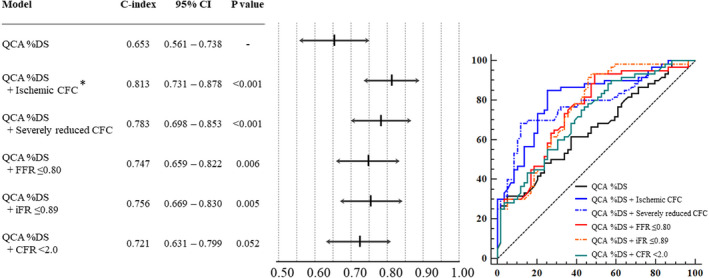
Improvement in C‐index by adding the cutoff value of each physiological index to angiographic stenosis severity. *P<0.05, difference in comparison with QCA %DS+CFR<2.0. %DS indicates percent diameter stenosis; C‐index, concordance index; CFC, coronary flow capacity; CFR, coronary flow reserve; FFR, fractional flow reserve; iFR, instantaneous wave‐free ratio; and QCA, quantitative coronary angiography.
Table 2.
The Incremental Reclassification Ability of CFC to Predict the Significant Coronary Flow Increase
| Prediction Model | NRI | P Value | IDI | P Value |
|---|---|---|---|---|
| FFR | ||||
| FFR+CFC | 1.010 | <0.001 | 0.121 | <0.001 |
| iFR | ||||
| iFR+CFC | 1.070 | <0.001 | 0.118 | <0.001 |
| CFR | ||||
| CFR+CFC | 0.708 | <0.001 | 0.103 | <0.001 |
CFC indicates coronary flow capacity; CFR, coronary flow reserve; FFR, fractional flow reserve; IDI, integrated discrimination improvement; iFR, instantaneous wave‐free ratio; and NRI, net reclassification improvement.
The Predictive Factors for the Significant Increase in Coronary Flow After PCI
The results of univariate and multivariate logistic regression analysis to predict the lesions with R‐hAPV ≥150% are shown in Table 3. Only the factors with P≤0.10 assessed by univariate analysis are listed in the table. The multivariate analysis revealed that the independent predictors of significant coronary flow increase were the lesions with ischemic CFC and FFR ≤0.80 in the FFR model (ischemic CFC: odds ratio [OR], 10.3; 95% CI, 4.3–24.7; P<0.001; and FFR: OR, 5.5; 95% CI, 1.9–16.2; P=0.002) and ischemic CFC and iFR ≤0.89 in the iFR model (ischemic CFC: OR, 14.5; 95% CI, 5.5–38.3; P<0.001; and iFR: OR, 12.5; 95% CI, 3.5–44.3; P<0.001).
Table 3.
Univariate and Multivariate Logistic Regression Analyses of FFR and iFR Models to Predict the Significant Increase in Coronary Flow
| Univariate Logistic Regression |
Multivariate Logistic Regression FFR Model |
Multivariate Logistic Regression iFR Model |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Diabetes mellitus | 0.494 | 0.231–1.056 | 0.069 | ||||||
| Use of statin | 1.781 | 0.896–3.543 | 0.100 | ||||||
| QCA RD | 1.862 | 0.937–3.701 | 0.076 | ||||||
| QCA %DS | 1.051 | 1.019–1.083 | 0.002 | ||||||
| FFR ≤0.80 | 5.952 | 2.247–15.771 | <0.001 | 5.497 | 1.860–16.243 | 0.002 | |||
| iFR ≤0.89 | 10.581 | 3.424–32.694 | <0.001 | 12.499 | 3.530–44.262 | <0.001 | |||
| CFR <2.0 | 4.275 | 1.816–10.061 | 0.001 | ||||||
| Moderately to severely reduced CFC | 10.713 | 4.621–24.837 | <0.001 | 10.261 | 4.269–24.665 | <0.001 | 14.514 | 5.502–38.285 | <0.001 |
CFC indicates coronary flow capacity; CFR, coronary flow reserve; DS, diameter stenosis; FFR, fractional flow reserve; iFR, instantaneous wave‐free ratio; MLD, minimal lumen diameter; OR, odds ratio; QCA, quantitative coronary angiography; and RD, reference diameter.
Discussion
The major findings of our study are as follows: (1) CFC grading improved the discriminating ability to predict coronary flow increase after PCI in comparison with conventional CFR; (2) ischemic CFC showed the high predictive performance with high sensitivity for a significant increase in coronary flow compared with the conventional cutoff values for FFR, iFR, and CFR; and (3) CFC provided additional information for predicting which lesions had an increase in coronary flow after PCI when accounting for conventional physiology‐based decision making. These results justify the use of CFC grading in clinical practice.
The Superiority of Coronary Flow Capacity as a Comprehensive Assessment of Coronary Circulation to Predict Coronary Flow Increase After PCI
The ORBITA (Percutaneous Coronary Intervention in Stable Angina) trial raised doubt with respect to the effect of revascularization on angina symptoms in chronic coronary syndrome. 19 Recently, the clinical outcomes of the ISCHEMIA (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches) trial, the latest randomized trial to investigate the further benefit of revascularization compared with optimal medical therapy in patients with chronic coronary syndrome were reported. 20 Compared with an initial medical strategy, an initial revascularization strategy did not demonstrate a risk reduction for adverse cardiovascular events over median 3 years. Based on the insight from the recent randomized trials, a physiologic rationale has been more strongly required before undertaking revascularization for coronary artery disease in patients with chronic coronary syndrome. An increase in coronary flow is the most fundamental rationale behind revascularization. Coronary intervention without corresponding coronary flow increase would not be expected to improve patient outcomes and could even be harmful. 2 , 21 Therefore, we need accurate methods to identify lesions with the best potential for coronary flow increase after PCI.
The previous studies showed that not only reduction in resistance of epicardial artery lesions but also improvement in coronary microcirculation after PCI are important factors for coronary flow increase. 6 , 22 Based on this insight, comprehensive assessment of the whole coronary circulation can be a favorable indicator for “effective” revascularization with respect to coronary flow increase. 7 CFR has been a representative index as a comprehensive assessment for the coronary circulation and has provided predictive efficacy for long‐term adverse events. 9 , 10 , 23 However, the dependence of CFR on resting hemodynamics can diminish its discriminating efficacy. Receiver operating characteristic curve analysis demonstrated that CFR was not superior for identification of vessels with coronary flow increase after PCI compared with FFR or iFR which are regarded as specific assessments for epicardial coronary artery stenosis (Figure 5; FFR versus iFR, P=0.74; FFR versus CFR, P=0.17; iFR versus CFR, P=0.06). In line with our hypothesis, CFC as a modified flow‐based comprehensive assessment of coronary circulation showed higher discriminating efficacy than CFR (P=0.013). Meanwhile, that of CFC was numerically higher but comparable with FFR and iFR (CFC versus FFR, P=0.67; CFC versus iFR, P=0.79). However, compared with the current cutoff values of FFR or iFR, ischemic CFC demonstrated high specificity to predict significant coronary flow increase, which might reduce unnecessary revascularization. Our findings add further rationale for CFC‐guided decision making.
Clinical Implication of CFC Grading in the Current Physiological Treatment Strategy
The cutoff value of FFR=0.80 is commonly used for clinical decision making. However, previous studies revealed that the occurrence of adverse clinical events is associated with FFR values as a continuous variable, and many lesions around the FFR cutoff value do not need revascularization. 2 , 24 In line with this concern, the established FFR cutoff value=0.80 showed rather low specificity but very high sensitivity to predict a significant increase in coronary flow in the present study (Figure 4). This would be attributable to the design of current criterion of FFR‐based decision making to avoid missing the patients who might benefit from revascularization. Thus, a considerable number of unnecessary revascularizations that cannot obtain a significant coronary flow increase might be performed when the treatment decision is made based on the FFR=0.80 cutoff value. In the present study, the best cutoff values of FFR or iFR assessed by receiver operating characteristic curve were much lower than the current clinically used cutoff values (the best cutoff values: ≤0.68 for FFR; <0.80 for iFR). Their predictive efficacies were similar to CFC classification (Figure S2), although their high sensitivities were reduced. The best cutoff value of FFR was close to the estimated cutoff point of FFR for superiority of revascularization to medical therapy (FFR ≤0.67), which was reported by the previous meta‐analysis. 2 These findings might suggest that the current physiological cutoff points could be reconsidered for optimizing revascularization criteria. Of note, current CFC classification, which has already shown the clinical efficacy in deferral patients, 12 provided further discriminating efficacy with high specificity for the lesions with coronary flow increase after PCI. In addition, lesions with severely reduced CFC showed high specificity for predicting improvement (Figure 4). This high predictive value of severely reduced CFC still remained even when limiting the lesions around conventional FFR cutoff values (FFR 0.70–0.85; Figure S3). Furthermore, this study demonstrated an incremental reclassification ability of CFC over conventional indices. These findings suggest that CFC, like additional benefit of CFR in FFR‐based treatment strategy, 23 could yield incremental useful information for our decision making, although further investigations are needed.
Limitations
The present study assessed the change in coronary flow just after revascularization. Further change in coronary circulation might occur during the long‐term follow‐up. 25 , 26 Therefore, this acute change in coronary flow observed in this study might not show the long‐term effects of revascularization on coronary circulation. In the present study, assessment of coronary flow is derived from not flow itself but velocity. It depends on the assessment location of coronary artery or vessel size. Nonpaired comparison of coronary flow velocity between different patients is apparently limited in its ability to interpret the individual increase in flow volume. Therefore, in the present study, we used serial measurements of coronary flow velocity in each patient and the flow ratio of after PCI to before PCI for evaluating coronary flow change, which might at least partially alleviate this limitation. Despite this, one of the important limitations of this study is the lack of the assessment of absolute increase in coronary flow volume. Although coronary flow volume also depends on the assessment location and downstream myocardial mass. The lesions with greater subtended cardiac mass that could provide the greater increase in flow volume by revascularization would be associated with greater requirement for revascularization. Thus, change in coronary flow volume would be another important marker of effectiveness of revascularization. The lack of this important information might make it difficult for us to understand the true predictive efficacy of CFC including its prognostic value. The present study contained these limitations, and further studies are needed to test including flow volume assessment and if CFC‐based decision making may provide prognostic information in relation to coronary flow increase. In the present study, 31 vessels with pre‐PCI FFR values over 0.80 (23.3%) underwent PCI according to the operator’s discretion, guided by low CFR (19 cases [14.3%]) or clinical and angiographic findings (12 cases [9.0%]), although these vessels are rarely treated in the current physiology‐based treatment strategy. However, CFC remains the strongest predictor even when limiting the analysis to FFR ≤0.8 (Table S1). We defined over 150% R‐hAPV as the threshold of significant increase in coronary flow after PCI. The previous meta‐analysis reported that the FFR value of 0.67 is the threshold for the greater benefit of PCI over that of medication. 2 In the present study, 82% of the lesions with FFR ≤0.67 (n=55 lesions) showed over 50% increase of coronary flow (median R‐hAPV: 239.4% [Q1–Q3: 180.3%–419.8%]). This result would support the appropriateness of our definition of significant increase in coronary flow. However, further studies are required to investigate the relationship between long‐term outcomes and increase in coronary flow after PCI. This study was made of 2 data sets from the studies with slightly different inclusion criteria. Regarding to each intrasubset difference in the prevalence of R‐hAPV ≥150% between ischemic CFC and nonischemic CFC, in line with our main result, ischemic CFC showed a higher prevalence than nonischemic CFC in IDEAL subset (76.3% for ischemic CFC versus 17.1% for nonischemic CFC; P<0.001; Figure S4). In the DEFINE FLOW subset, the prevalence of significant flow increase in vessels with ischemic CFC was higher but not statistically significant compared with that of nonischemic CFC (55.0% for ischemic CFC versus 23.1% for nonischemic CFC, P=0.087). This difference in the prevalence of significant flow increase between the 2 studies might be attributable to the small number of cases in the DEFINE FLOW subset and/or selection bias because the only patients with FFR ≤0.80 in DEFINE FLOW study underwent PCI according to the protocol. However, when limiting the analysis in the lesions with FFR ≤0.80, CFC classification also showed the only independent predictor (Table S1). Intracoronary Doppler flow‐based CFC is purely a flow velocity–based assessment. Therefore, these findings would also lend a rationale to treat the lesions according to CFC grading assessed by noninvasive imaging using myocardial blood flow. However, collateral flow was not taken into consideration, whereas 42 lesions (31.6%) showed FFR <0.60. Therefore, the potential benefit of PCI only by improvement of epicardial coronary flow velocity might be overestimated in such severe stenoses. This might lead to some different outcomes of CFC grading between invasive and noninvasive methods. Finally, although this is a relatively large study of the direct assessment for coronary flow change using intracoronary Doppler flow measurements before and after PCI, the number of cases in the present study might be still underpowered to draw the conclusion.
Conclusions
CFC showed a significant improvement of diagnostic efficacy for identification of lesions with a significant increase in coronary flow after revascularization compared with CFR, and its discriminating ability with high specificity would provide the useful information in the treatment strategy based on the current cutoff values of conventional physiological indices. This study provides a physiological rationale for revascularization of lesions guided by ischemic CFC.
Sources of Funding
The DEFINE FLOW study was funded by Philips Volcano Corporation.
Disclosures
Dr Murai has received consulting fees by Philips‐Volcano. Drs Piek and van de Hoef have served as speakers at educational events organized by Philips Volcano, St. Jude Medical, and Boston Scientific, manufacturers of sensor‐equipped guidewires. Dr Wijntjens is partially supported by a research grant by Philips‐Volcano Corporation. Dr Escaned has received fees from Boston Scientific, Abbott Vascular, and Phillips‐Volcano for educational events. Dr Davies is co‐developer of intellectual property, which is licensed to Philips Volcano by Imperial College London, and receives consultancy and research funding from Philips Volcano. Dr van Royen has received educational grants from Baxter, AstraZeneca, Philips Volcano, Biotronik, St. Jude Medical, and Abbott Vascular. Dr Siebes received institutional research support from the University of Texas Health Science Center at Houston (for DEFINE‐FLOW, NCT02328820) and from the Weatherhead PET Center, Division of Cardiology, Department of Medicine, McGovern Medical School at UTHealth and Memorial Hermann Hospital, Houston, Texas. Drs Johnson, Kirkeeide, and Gould have received internal funding from the Weatherhead PET Center for Preventing and Reversing Atherosclerosis; and have an institutional licensing and consulting agreement with Boston Scientific for the smart minimum FFR algorithm. Dr Johnson has received significant institutional research support from St. Jude Medical (CONTRAST, NCT02184117) and Philips Volcano Corporation (DEFINE‐FLOW, NCT02328820) for studies using intracoronary pressure and flow sensors. Dr Gould is the 510(k) applicant for CFR Quant (K113754) and HeartSee (K143664, K171303) software packages for cardiac positron emission tomography image processing, analysis, and absolute flow quantification. The remaining authors have no disclosures to report.
Supporting information
Table S1
Figures S1–S4
(J Am Heart Assoc. 2020;9:e016130 DOI: 10.1161/JAHA.120.016130.)
For Sources of Funding and Disclosures, see page 11.
References
- 1. Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, Tonino PAL, Engstrøm T, Kääb S, Dambrink JH, Rioufol G, et al.; FAME 2 Investigators . Five‐year outcomes with PCI guided by fractional flow reserve. N Engl J Med. 2018;379:250–259. [DOI] [PubMed] [Google Scholar]
- 2. Johnson NP, Tóth GG, Lai D, Zhu H, Açar G, Agostoni P, Appelman Y, Arslan F, Barbato E, Chen SL, et al. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol. 2014;64:1641–1654. [DOI] [PubMed] [Google Scholar]
- 3. Hachamovitch R, Rozanski A, Shaw LJ, Stone GW, Thomson LE, Friedman JD, Hayes SW, Cohen I, Germano G, Berman DS. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress‐rest myocardial perfusion scintigraphy. Eur Heart J. 2011;32:1012–1024. [DOI] [PubMed] [Google Scholar]
- 4. Verhoeff BJ, Siebes M, Meuwissen M, Atasever B, Voskuil M, de Winter RJ, Koch KT, Tijssen JG, Spaan JA, Piek JJ. Influence of percutaneous coronary intervention on coronary microvascular resistance index. Circulation. 2005;111:76–82. [DOI] [PubMed] [Google Scholar]
- 5. Nijjer SS, Petraco R, van de Hoef TP, Sen S, van Lavieren MA, Foale RA, Meuwissen M, Broyd C, Echavarria‐Pinto M, Al‐Lamee R, et al. Change in coronary blood flow after percutaneous coronary intervention in relation to baseline lesion physiology: results of the JUSTIFY‐PCI study. Circ Cardiovasc Interv. 2015;8:e001715 DOI: 10.1161/CIRCINTERVENTIONS.114.001715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murai T, Kanaji Y, Yonetsu T, Lee T, Matsuda J, Usui E, Araki M, Niida T, Isobe M, Kakuta T. Preprocedural fractional flow reserve and microvascular resistance predict increased hyperaemic coronary flow after elective percutaneous coronary intervention. Catheter Cardiovasc Interv. 2017;89:233–242. [DOI] [PubMed] [Google Scholar]
- 7. Marzilli M, Merz CN, Boden WE, Bonow RO, Capozza PG, Chilian WM, DeMaria AN, Guarini G, Huqi A, Morrone D, et al. Obstructive coronary atherosclerosis and ischemic heart disease: an elusive link!. J Am Coll Cardiol. 2012;60:951–956. [DOI] [PubMed] [Google Scholar]
- 8. Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol. 1974;34:48–55. [DOI] [PubMed] [Google Scholar]
- 9. Chamuleau SA, van Eck‐Smit BL, Meuwissen M, Koch KT, Dijkgraaf MG, Verberne HJ, Tijssen JG, Piek JJ. Long‐term prognostic value of CFVR and FFR versus perfusion scintigraphy in patients with multivessel disease. Neth Heart J. 2007;15:369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsuda J, Murai T, Kanaji Y, Usui E, Araki M, Niida T, Ichijyo S, Hamaya R, Lee T, Yonetsu T, et al. Prevalence and clinical significance of discordant changes in fractional and coronary flow reserve after elective percutaneous coronary intervention. J Am Heart Assoc. 2016;5:e004400 DOI: 10.1161/JAHA.116.004400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson NP, Gould KL. Integrating noninvasive absolute flow, coronary flow reserve, and ischemic thresholds into a comprehensive map of physiological severity. JACC Cardiovasc Imaging. 2012;5:430–440. [DOI] [PubMed] [Google Scholar]
- 12. van de Hoef TP, Echavarría‐Pinto M, van Lavieren MA, Meuwissen M, Serruys PW, Tijssen JG, Pocock SJ, Escaned J, Piek JJ. Diagnostic and prognostic implications of coronary flow capacity: a comprehensive cross‐modality physiological concept in ischemic heart disease. JACC Cardiovasc Interv. 2015;8:1670–1680. [DOI] [PubMed] [Google Scholar]
- 13. Stegehuis VE, Wijntjens GWM, van de Hoef TP, Casadonte L, Kirkeeide RL, Siebes M, Spaan JAE, Gould KL, Johnson NP, Piek JJ. Distal evaluation of functional performance with intravascular sensors to assess the narrowing effect—combined pressure and Doppler FLOW velocity measurements (DEFINE‐FLOW) trial: rationale and trial design. Am Heart J. 2020;222:139–146. [DOI] [PubMed] [Google Scholar]
- 14. Nijjer SS, de Waard GA, Sen S, van de Hoef TP, Petraco R, Echavarría‐Pinto M, van Lavieren MA, Meuwissen M, Danad I, Knaapen P, et al. Coronary pressure and flow relationships in humans: phasic analysis of normal and pathological vessels and the implications for stenosis assessment: a report from the Iberian‐Dutch‐English (IDEAL) collaborators. Eur Heart J. 2016;37:2069–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck‐Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al.; ESC Scientific Document Group . 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 16. Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, Smith PK. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69:2212–2241. [DOI] [PubMed] [Google Scholar]
- 17. Lotfi A, Davies JE, Fearon WF, Grines CL, Kern MJ, Klein LW. Focused update of expert consensus statement: use of invasive assessments of coronary physiology and structure: a position statement of the society of cardiac angiography and interventions. Catheter Cardiovasc Interv. 2018;92:336–347. [DOI] [PubMed] [Google Scholar]
- 18. Johnson NP, Gould KL. Physiological basis for angina and ST‐segment change PET‐verified thresholds of quantitative stress myocardial perfusion and coronary flow reserve. JACC Cardiovasc Imaging. 2011;4:990–998. [DOI] [PubMed] [Google Scholar]
- 19. Al‐Lamee R, Thompson D, Dehbi HM, Sen S, Tang K, Davies J, Keeble T, Mielewczik M, Kaprielian R, Malik IS, et al.; ORBITA Investigators . Percutaneous coronary intervention in stable angina (ORBITA): a double‐blind, randomised controlled trial. Lancet. 2018;391:31–40. [DOI] [PubMed] [Google Scholar]
- 20. Hochman JS. International Study of Comparative Health Effectiveness With Medical and Invasive Approaches (ISCHEMIA): primary report of clinical outcomes. Paper presented at AHA. November 16, 2019. Philadelphia, PA: 2019. [Google Scholar]
- 21. Zimmermann FM, Ferrara A, Johnson NP, van Nunen LX, Escaned J, Albertsson P, Erbel R, Legrand V, Gwon HC, Remkes WS, et al. Deferral vs. performance of percutaneous coronary intervention of functionally non‐significant coronary stenosis: 15‐year follow‐up of the DEFER trial. Eur Heart J. 2015;36:3182–3188. [DOI] [PubMed] [Google Scholar]
- 22. Siebes M, Verhoeff BJ, Meuwissen M, de Winter RJ, Spaan JA, Piek JJ. Single‐wire pressure and flow velocity measurement to quantify coronary stenosis hemodynamics and effects of percutaneous interventions. Circulation. 2004;109:756–762. [DOI] [PubMed] [Google Scholar]
- 23. van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek MA, Chamuleau SA, Voskuil M, Henriques JP, Koch KT, de Winter RJ, et al. Physiological basis and long‐term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv. 2014;7:301–311. [DOI] [PubMed] [Google Scholar]
- 24. Barbato E, Toth GG, Johnson NP, Pijls NH, Fearon WF, Tonino PA, Curzen N, Piroth Z, Rioufol G, Jüni P, et al. A prospective natural history study of coronary atherosclerosis using fractional flow reserve. J Am Coll Cardiol. 2016;68:2247–2255. [DOI] [PubMed] [Google Scholar]
- 25. Murai T, Lee T, Kanaji Y, Matsuda J, Usui E, Araki M, Niida T, Hishikari K, Ichijyo S, Hamaya R, et al. The influence of elective percutaneous coronary intervention on microvascular resistance: a serial assessment using the index of microcirculatory resistance. Am J Physiol Heart Circ Physiol. 2016;311:H520–H531. [DOI] [PubMed] [Google Scholar]
- 26. van Liebergen RA, Piek JJ, Koch KT, de Winter RJ, Lie KI. Immediate and long‐term effect of balloon angioplasty or stent implantation on the absolute and relative coronary blood flow velocity reserve. Circulation. 1998;98:2133–2140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figures S1–S4


