The advent and widespread uptake of antiretroviral therapy (ART) has transformed HIV infection into a chronic disease. 1 With the dramatic decline in AIDS‐related deaths, the life expectancy of people living with HIV (PLHIV) has increased significantly, 1 , 2 and with this, chronic diseases prevalent in the elderly general populations, including cardiovascular disease (CVD), have become important causes of morbidity and mortality in PLHIV. 3 Several epidemiological studies have demonstrated that PLHIV have higher incidence of heart failure (HF), myocardial infarction, ischemic stroke, and peripheral vascular disease. 3 , 4 , 5 Studies also indicate that PLHIV have worse clinical outcomes after CVD events, such as HF or myocardial infarction. 6 , 7 These data highlight the need for understanding HIV‐associated CVD and its complications and the need for evaluating both primary and secondary prevention interventions.
In this issue of the Journal of the American Heart Association (JAHA), Boccara et al 8 report detailed CVD outcome data on 105 PLHIV and 195 matching HIV(−) controls who were admitted with acute coronary syndrome (ACS) to 23 coronary intensive care units across France. The participants were recruited in years 2003 to 2006 and were followed up over a period of 36 months. The authors found that, after accounting for potential confounders, HIV status was associated with significantly increased risk of recurrent ACS but not with major adverse cardiac and cerebral events, the study's composite primary outcome composed of cardiac death, ACS, recurrent revascularization, and stroke. These findings are similar to those of a prior report from the same cohort based on a 12‐month point analyses of outcomes, 9 and restriction of the present analyses to events occurring beyond 12 months of follow‐up found no significant association between HIV status and recurrent ACS, suggesting that the observed association was mainly driven by events occurring in the first 12 months after presentation with ACS. There was no significant association with HIV for the other components of the primary end point other than ACS. Breaking down recurrent revascularization outcomes into urgent and nonurgent showed that PLHIV had a trend toward increased rate of urgent revascularization (P=0.08) but not nonurgent revascularization (P=0.22), indicating that ACS was driving repeated revascularization procedures in PLHIV. The rate of cardiovascular death was the same for both groups (3%).
HIV status was also significantly associated with a secondary outcome of hospitalization for HF at 1 year, although the ejection fraction at baselines and 1‐year after ACS was not different by HIV status. A similar finding of increased HF hospitalization associated with HIV has been reported in a prior study based on the French Nationwide Hospital Medical Information Database, involving 608 PLHIV hospitalized with acute myocardial infarction and 1824 matched HIV(−) controls, with a follow‐up of 12 months. 10 Although this latter study reported a higher prevalence of ischemic cardiomyopathy among the HIV patients at baseline, the higher incidence of HF in the HIV group persisted after adjusting for history of ischemic cardiomyopathy. These observations suggest that the increased incidence of HF in PLHIV after myocardial infarction may be because of the high prevalence of underlying subclinical diastolic cardiac dysfunction in this population. 11 The findings are also consistent with current knowledge that HIV status is independently associated with risk of incident HF. 3 , 5
Similar to first CVD events, the incidence of recurrent CVD events may be attributable to traditional (eg, smoking, hypertension, and hyperlipidemia) and novel/HIV‐related (eg, immune dysregulation, inflammation, and ART) factors. 3 , 4 , 12 Boccara et al8 attempted to explore the role of any disparity in medical treatment of CVD and control of traditional risk factors in long‐term residual risk of recurrent CVD. In this cohort, the frequency of statin prescription, aspirin use, and β‐blocker use was comparable between PLHIV and HIV(−) controls at baseline, at discharge, and at follow‐up. These findings contrast to other studies that showed underuse of statins and aspirin in both primary and secondary prevention settings. 13 , 14 However, the study did find that PLHIV with ACS were more likely to have changes in their statin prescription and less likely to achieve lipid goals compared with their HIV(−) counterparts. The statin prescription changes may have been partially driven by considerations of drug‐drug interactions with ART regimens (eg, those that include starting or discontinuing protease inhibitors). In addition to the choice of statin drugs, the statin dose may have been affected by the same concern and may have led to suboptimal lipid concentrations, suggesting the potential importance of addressing these factors in secondary prevention of CVD in PLHIV. Boccara et al8 also found that PLHIV were less likely to achieve smoking cessation. The findings with respect to smoking are certainly not surprising given the high rate of smoking that is observed in PLHIV compared with HIV(−) individuals 15 and highlights the need to develop interventions targeted to this patient population.
Immune dysregulation and chronic inflammation are considered important mechanisms in pathogenesis of CVD in PLHIV. 16 , 17 Agents that block specific inflammatory pathways have demonstrated effectiveness in preventing CVD in HIV(−) individuals, and preliminary studies suggest similar effect in PLHIV, highlighting the importance of investigating inflammation‐related factors in secondary prevention of CVD in PLHIV. 16 Inflammatory markers, such as CRP (C‐reactive protein) and interleukin‐6 concentrations, were not reported in the present study, as they are not usually included in the routine evaluation of patients admitted with ACS. In addition, in contrast to prior studies showing the role of HIV‐specific factors, such as cluster of differentiation 4 count and viral load, being associated with incidence and prognosis of coronary events, 4 , 18 the present report did not find significant association between these HIV biomarkers and recurrent cardiac events.
This study is one of only a handful of studies 7 , 10 , 12 , 19 , 20 , 21 , 22 specifically investigating CVD outcomes in HIV after a first ACS event (Table). The authors investigated a wide range of CVD outcomes, offering deeper insight into the impact of HIV on prognosis of CVD events and potential mechanisms. They also studied secondary prevention strategies that might influence these outcomes. By contrast, most prior studies 7 , 10 , 12 , 21 evaluated and reported on more limited numbers of post‐ACS CVD outcomes and did not generally look at secondary prevention strategies. Two publications 19 , 20 that reported on a wider range of recurrent CVD outcomes in PLHIV studied only patients who received percutaneous coronary intervention and included patients who received percutaneous coronary intervention for stable angina. This study, on the other hand, investigated PLHIV admitted with ACS, rather than stable angina, which has different pathological mechanism to ACS, and did not limit participation to those who received percutaneous coronary intervention.
Table 1.
Summary of Studies Investigating Cardiovascular Outcomes After Acute Coronary Event or Coronary Revascularization
| Study, Publication Year | Population | % ACS | Baseline | Follow‐Up, mo | % ART (PLHIV) | Age, y | Recurrent Event* | No. of PLHIV | No. of HIV(−) | Matching Variables for HIV(−) Controls |
|---|---|---|---|---|---|---|---|---|---|---|
| Current study 8 | ACS | 100 | 2003–2006 | 36 | 93 | 49 | Cardiovascular death, MI, ACS, TLR, TVR, stroke, MACCE, HF hospitalization | 103 | 195 | Age, sex, ACS type, center, event date |
| Badr, 2015 19 | PCI | 59 | 2003–2011 | 24 | NR | 58 | Cardiac death, MI, TLR, TVR, MACE | 112 | 112 | Age, sex, diabetes mellitus, PCI |
| Carballo, 2015 7 | MI | 100 | 2005–2011 | 12 | 80 | 51 | Cardiovascular death, MI | 133 | 4934 | MI, study period |
| Lorgis, 2013 10 | MI | 100 | 2005–2009 | 12 | NR | 50 | MI, revascularization, HF hospitalization | 435 | 945 | Age, sex |
| Ren, 2009 20 | PCI | 81 | 2000–2007 | 37 | 81 | 53 | Cardiovascular death, MI, TVR, TLR, CABG, MACE | 97 | 97 | Age, sex, PCI, PCI period |
| Boccara, 2006 21 | PCI | 98 | 2001–2003 | 20 | 96 | 43 | MI, restenosis, MACE | 50 | 50 | Age, sex, event period, PCI |
| Hsue, 2004 12 | ACS | 100 | 1993–2003 | NR | 53 | 50 | Restenosis | 68 | 68 | Event date |
| Matetzky, 2003 22 | MI | 100 | 1998–2000 | 15 | 92 | 47 | Cardiac death, MI, ACS, revascularization, HF hospitalization | 24 | 48 | Age, sex, AMI type, event date |
ACS indicates acute coronary syndrome; ART, antiretroviral therapy; CABG, coronary artery bypass graft; HF, heart failure; MACCE, major adverse cardiac and cerebrovascular event; MACE, major adverse cardiac event; MI, myocardial infarction; NR, not reported; PCI, percutaneous coronary intervention; PLHIV, people living with HIV; TLR, target lesion revascularization; and TVR, target vessel revascularization.
MACE includes ACS, revascularization, and cardiac death; and revascularization indicates PCI or CABG.
The present publication complements findings from the authors' prior 1‐year report 9 on the same cohort by extending the follow‐up to 3 years and demonstrating that the impact of HIV status on recurrent ACS was mainly within the first year after the index event, pointing to a potential window of opportunity for more aggressive preventive interventions, including addressing the 2 modifiable traditional risk factors (smoking and low‐density lipoprotein cholesterol) that were less adequately treated in their study cohort. Whether these findings and potential interventions are generalizable to other HIV‐ACS populations or more recent cohorts may be tested in future studies. The current study also supplements the prior report by exploring secondary outcomes, including HF hospitalizations, which were higher in PLHIV. Only 2 other studies 10 , 22 of those listed in the Table reported on HF outcome.
Despite the authors' effort to maximize the power of their study by implementing a multicenter collaboration of 23 coronary intensive care units across France, the study may have been powered to detect only strong associations (eg, ≈2‐fold increase in relative risk) between HIV status and the CVD. As indicated by the authors in the article, the study had 80% power to detect a relative risk of 1.8 at a significance level of 0.05 if the incidence of the outcomes among HIV(−) controls was 25% over the 3‐year follow‐up period. The incidence of CVD events, even for the primary composite outcome of major adverse cardiac and cerebral events in the HIV(−) controls, was only 15.1%; hence, the power of the study to detect significant association between HIV status and major adverse cardiac and cerebral events would have been considerably lower, even if the underlying relative risk was 1.8. Therefore, the nonsignificant multivariable hazard ratio of 1.6 (95% CI, 0.67–3.82) may have been because of lack of statistical power. Specifying composite outcomes, such as major adverse cardiac and cerebral events, can be useful to increase the power and relevance of a study; however, that is only the case if the exposure of interest is expected to have comparable effect on the component outcomes, which may, arguably, not be the case for stroke. The power of the study could actually be diluted if some of the component outcomes are not associated with the exposure of interest. Nonetheless, putting the findings of this study in context of prior reports of recurrent CVD outcomes after ACS, percutaneous coronary intervention, or both shows that HIV is associated with certain recurrent CVD outcomes (Figure).
Figure 1. Recurrent cardiovascular event: people living with HIV (PLHIV) vs HIV(−) controls.
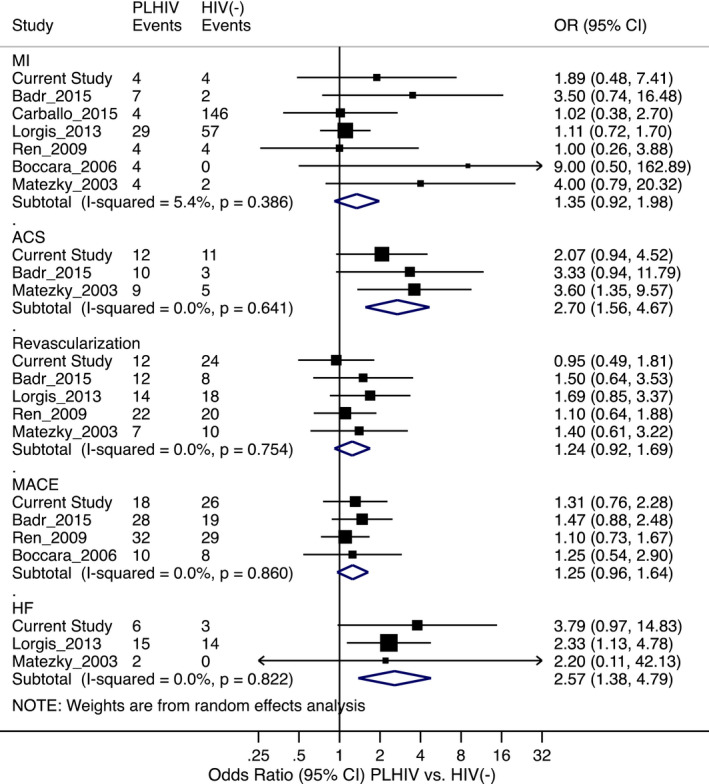
The effect estimates are unadjusted odds ratios (ORs), but cases and controls have been matched on certain variables in most of the studies, as shown in the Table. References: current study 8 ; Badr, 2015 19 ; Carballo, 2015 7 ; Lorgis, 2013 10 ; Ren, 2009 20 ; Boccara, 2006 21 ; Hsue, 2004 12 ; and Matetzky, 2003. 22 ACS indicates acute coronary syndrome; HF, heart failure; MACE, major adverse cardiac event; MI, myocardial infarction; and Revascularization, percutaneous coronary intervention or coronary artery bypass graft.
Having been conducted in the period from 2003 to 2006, the study may not capture current practices in cardiovascular care and the impact of contemporary ART and HIV care (eg, the study cannot provide insight into newer ART regimens that could influence lipid profiles and affect selection of statins). Indeed, this cohort had at baseline a high rate of lipodystrophy (49%), a complication of ART that is not seen in similar frequency in more recent ART periods and can affect CVD incidence and outcomes. Future large‐scale prospective studies or collaborative meta‐analyses powered to allow for detection of differences in individual CVD outcomes, with a more detailed analysis of the impact of HIV‐specific risk markers and based on more recent data, could yield further details useful to improve the cardiovascular care of PLHIV and optimize secondary prevention strategies.
Disclosures
Dr Erqou has received funding support from the Rhode Island Foundation and the Providence‐Boston Center for AIDS Research. Drs Erqou and Rodriguez‐Barradas are employees of the Department of Veterans Affairs. The views expressed in this article are those of the authors and do not necessarily reflect the positions or policy of the Department of Veterans Affairs or the US government.
(J Am Heart Assoc. 2020;9:e018140 DOI: 10.1161/JAHA.120.018140.)
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
For Disclosures, see page 4.
See Article by Boccara et al.
References
- 1. High KP, Brennan‐Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, Deren S, Effros RB, Gebo K, Goronzy JJ, et al. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012;e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, Burchell AN, Cohen M, Gebo KA, Gill MJ, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. So‐Armah K, Benjamin LA, Bloomfield GS, Feinstein MJ, Hsue P, Njuguna B, Freiberg MS. HIV and cardiovascular disease. Lancet HIV. 2020;e279–e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alonso A, Barnes AE, Guest JL, Shah A, Shao IY, Marconi V. HIV infection and incidence of cardiovascular diseases: an analysis of a large healthcare database. J Am Heart Assoc. 2019;e012241 DOI: 10.1161/JAHA.119.012241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Erqou S, Jiang L, Choudhary G, Lally M, Bloomfield GS, Zullo AR, Shireman TI, Freiberg M, Justice AC, Rudolph J, et al. Heart failure outcomes and associated factors among veterans with human immunodeficiency virus infection. JACC Heart Fail. 2020;501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carballo D, Delhumeau C, Carballo S, Bähler C, Radovanovic D, Hirschel B, Clerc O, Bernasconi E, Fasel D, Schmid P, et al. Increased mortality after a first myocardial infarction in human immunodeficiency virus‐infected patients; a nested cohort study. AIDS Res Ther. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boccara F, Mary‐Krause M, Potard V, Teiger E, Lang S, Hammoudi N, Chauvet M, Ederhy S, Dufour‐Soulat L, Ancedy Y, et al. HIV infection and long‐term residual cardiovascular risk after acute coronary syndrome. J Am Heart Assoc. 2020;e017578 DOI: 10.1161/JAHA.119.017578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boccara F, Mary‐Krause M, Teiger E, Lang S, Lim P, Wahbi K, Beygui F, Milleron O, Gabriel Steg P, Funck‐Brentano C, et al. Acute coronary syndrome in human immunodeficiency virus‐infected patients: characteristics and 1 year prognosis. Eur Heart J. 2011;41–50. [DOI] [PubMed] [Google Scholar]
- 10. Lorgis L, Cottenet J, Molins G, Benzenine E, Zeller M, Aube H, Touzery C, Hamblin J, Gudjoncik A, Cottin Y, et al. Outcomes after acute myocardial infarction in HIV‐infected patients: analysis of data from a French nationwide hospital medical information database. Circulation. 2013;1767–1774. [DOI] [PubMed] [Google Scholar]
- 11. Erqou S, Lodebo BT, Masri A, Altibi AM, Echouffo‐Tcheugui JB, Dzudie A, Ataklte F, Choudhary G, Bloomfield GS, Wu WC, et al. Cardiac dysfunction among people living with HIV: a systematic review and meta‐analysis. JACC Heart Fail. 2019;98–108. [DOI] [PubMed] [Google Scholar]
- 12. Hsue PY, Giri K, Erickson S, MacGregor JS, Younes N, Shergill A, Waters DD. Clinical features of acute coronary syndromes in patients with human immunodeficiency virus infection. Circulation. 2004;316–319. [DOI] [PubMed] [Google Scholar]
- 13. Ladapo JA, Richards AK, DeWitt CM, Harawa NT, Shoptaw S, Cunningham WE, Mafi JN. Disparities in the quality of cardiovascular care between HIV‐infected versus HIV‐uninfected adults in the United States: a cross‐sectional study. J Am Heart Assoc. 2017;e007107 DOI: 10.1161/JAHA.117.007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Freiberg MS, Leaf DA, Goulet JL, Goetz MB, Oursler KK, Gibert CL, Rodriguez‐Barradas MC, Butt AA, Justice AC. The association between the receipt of lipid lowering therapy and HIV status among veterans who met NCEP/ATP III criteria for the receipt of lipid lowering medication. J Gen Intern Med. 2009;334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, Grinspoon SK, Levin J, Longenecker CT, Post WS. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation. 2019;e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Currier JS, Hsue PY. The role of inflammation in HIV‐associated atherosclerosis‐one size may not fit all. J Infect Dis. 2020;495–497. [DOI] [PubMed] [Google Scholar]
- 17. Hoel H, Ueland T, Knudsen A, Kjær A, Michelsen AE, Sagen EL, Halvorsen B, Yndestad A, Nielsen SD, Aukrust P, et al. Soluble markers of interleukin 1 activation as predictors of first‐time myocardial infarction in HIV‐infected individuals. J Infect Dis. 2020;506–509. [DOI] [PubMed] [Google Scholar]
- 18. Feinstein MJ, Nance RM, Delaney JAC, Heckbert SR, Budoff MJ, Drozd DR, Burkholder GA, Willig JH, Mugavero MJ, Mathews WC, et al. Mortality following myocardial infarction among HIV‐infected persons: the Center for AIDS Research Network of Integrated Clinical Systems (CNICS). BMC Med. 2019;149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Badr S, Minha S, Kitabata H, Fatemi O, Torguson R, Suddath WO, Satler LF, Pichard AD, Waksman R. Safety and long‐term outcomes after percutaneous coronary intervention in patients with human immunodeficiency virus. Catheter Cardiovasc Interv. 2015;192–198. [DOI] [PubMed] [Google Scholar]
- 20. Ren X, Trilesskaya M, Kwan DM, Nguyen K, Shaw RE, Hui PY. Comparison of outcomes using bare metal versus drug‐eluting stents in coronary artery disease patients with and without human immunodeficiency virus infection. Am J Cardiol. 2009;216–222. [DOI] [PubMed] [Google Scholar]
- 21. Boccara F, Teiger E, Cohen A, Ederhy S, Janower S, Odi G, Di Angelantonio E, Barbarini G, Barbaro G. Percutaneous coronary intervention in HIV infected patients: immediate results and long term prognosis. Heart. 2006;543–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matetzky S, Domingo M, Kar S, Noc M, Shah PK, Kaul S, Daar E, Cercek B. Acute myocardial infarction in human immunodeficiency virus‐infected patients. Arch Intern Med. 2003;457–460. [DOI] [PubMed] [Google Scholar]


