Abstract
Background
Coronavirus disease 2019 (COVID‐19) is spreading widely around the world. We conducted this meta‐analysis to explore the prevalence of cardiovascular comorbidities in COVID‐19, severe acute respiratory syndrome (SARS), and Middle East respiratory syndrome (MERS) cases.
Methods and Results
Relevant reports updated to April 17, 2020, were searched from PubMed, Embase, Web of Science, and the Cochrane Library with no restriction on language. A random‐effects model was used in this meta‐analysis to obtain pooled proportions of cardiovascular comorbidities in COVID‐19, SARS, and MERS. A total of 22 studies (12 for COVID‐19, 4 for SARS, and 6 for MERS) were included in this analysis, and the average age of patients with COVID‐19, SARS, and MERS was 46.41±1.79, 39.16±2.25, and 52.51±4.64 years, respectively. Proportions of cardiovascular comorbidities in coronavirus diseases were as follows: COVID‐19: proportion of hypertension was 17.1% (95% CI, 13.2%–20.9%), proportion of cardiac disease was 4.5% (95% CI, 3.6%–5.5%) and proportion of diabetes mellitus was 8.5% (95% CI, 5.5%–11.4%); SARS: proportion of hypertension was 4.5% (95% CI, 2.0%–7.0%), proportion of cardiac disease was 2.1% (95% CI, 0.6%–3.7%) and proportion of diabetes mellitus was 3.7% (95% CI, 1.0%–6.4%); MERS: proportion of hypertension was 30.3% (95% CI, 18.3%–42.2%), proportion of cardiac disease was 20.9% (95% CI, 10.7%–31.1%), and proportion of diabetes mellitus was 45.4% (95% CI, 27.3%–63.5%).
Conclusions
The prevalence of cardiovascular comorbidities varies among different coronavirus‐associated diseases. With the development of time, proportions of cardiovascular comorbidities in COVID‐19 need further attention.
Keywords: cardiovascular comorbidities, COVID‐19, Middle East respiratory syndrome, pooled analysis, prevalence, severe acute respiratory syndrome
Subject Categories: Hypertension, Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- 2019‐nCoV
novel coronavirus 2019
- ACE2
angiotensin‐converting enzyme 2
- COVID‐19
coronavirus disease 2019
- MERS
Middle East respiratory syndrome
- SARS
severe acute respiratory syndrome
Clinical Perspective
What Is New?
The prevalence of cardiovascular comorbidities varies among different coronavirus‐associated diseases.
The proportions of hypertension, cardiac disease, and diabetes mellitus in Middle East respiratory syndrome were all higher than those in coronavirus disease 2019 and severe acute respiratory syndrome. Coronavirus disease 2019 had an apparently higher prevalence of hypertension than severe acute respiratory syndrome.
What Are the Clinical Implications?
Knowing the prevalence of cardiovascular comorbidities in coronavirus disease 2019, severe acute respiratory syndrome, and Middle East respiratory syndrome would be helpful in realizing not only the connection between cardiovascular comorbidities and coronavirus‐associated diseases but also the discrepancies among different coronavirus‐associated diseases.
Conclusions about coronavirus disease 2019 could provide some assistance in fighting the epidemic.
The outbreak of novel coronavirus‐infected pneumonia (coronavirus disease 2019 [COVID‐19]) attributable to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection has affected all 31 provinces in China and 43 countries and territories around the world. 1 , 2 , 3 The absolute number of new cases and severe cases have been increasing rapidly daily because of enhanced transmissibility of the virus. The World Health Organization declared COVID‐19 a public health emergency of international concern on February 1, 2020. The novel coronavirus 2019 (2019‐nCoV) was isolated from biologic samples and identified as genus Betacoronavirus, placing it alongside other severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). 4 2019‐nCoV is a new strain of coronavirus not previously identified in humans. Coronaviruses are zoonotic and are a large family of viruses that cause illness ranging from the common cold to more severe diseases, such as MERS and SARS. 5 In 2002, the SARS epidemic originated from an animal market in South China and then affected more than 8000 people, with 916 deaths in 29 countries, 6 and the mortality was nearly 11%. After the emergence of SARS, MERS was the second coronavirus resulting in a major global public health crisis. It first emerged in 2012 in Saudi Arabia when a 60‐year‐old man presented with severe pneumonia. 7 Since 2012, 2494 laboratory confirmed cases of MERS have been reported, and 858 associated deaths have occurred (34.4% case‐fatality ratio). 8 COVID‐19 appears to have greater infectivity and a lower case fatality rate when compared with SARS and MERS. 9 Between countries, case fatality rates of COVID‐19 vary significantly, but it could range from about 0.17% to 15.34%. 10
The present study was undertaken to provide a systematic evaluation and detailed estimate of the prevalence of cardiovascular comorbidities (hypertension, cardiac disease, and diabetes mellitus) in SARS, MERS and COVID‐19 cases. Lessons learned from the MERS and SARS outbreaks can provide valuable insight into how to handle the current epidemic. This assessment may aid the public health sector while formulating policies for surveillance, preparedness, and response to COVID‐19.
Methods
The authors declare that all supporting data are available within the article.
Search Strategy
We searched MEDLINE via PubMed, Embase, Web of Science, and the Cochrane Library, with no restriction on language for articles published until April 17, 2020. The keywords of the search included “COVID‐19,” “2019 novel coronavirus,”“SARS,” and “MERS.”
Selection of Articles
Articles were eligible for inclusion if they met the following criteria: (1) those that were clinical studies or consecutive cases about human; (2) required clinical data could be extracted from articles; and (3) at least 3 cases were reported in an article. Articles that met the following criteria were excluded: (1) reports published as review articles, letters, editorials, conference abstracts, vaccination trials, or family‐based studies; (2) studies that did not contain data of specific comorbidities; and (3) articles containing data that were also included by other larger studies (eg, if data of article A were also included in article B, which contained more patients, article A would be excluded, while article B would be included).
Data Extraction
Two researchers (Y.L., S.W.) extracted data independently for each identified article, and any disagreements were resolved by discussion. The following data were recorded: first author's name, year of publication; screening time; number, age, and sex of participants; and number of cardiovascular comorbidities, referring to hypertension, cardiac disease (mainly referred to coronary heart disease), and diabetes mellitus.
Quality Assessment
We used the Newcastle‐Ottawa Scale to assess the quality of included articles. 11 Assessment scores of 0 to 3, 4 to 6, and 7 to 9 indicated poor, fair, and good studies, respectively.
Statistical Analysis
Weighted average was used to calculate the average age. Publication bias was assessed by Egger's test. The meta‐analysis of proportions (and 95% CIs) of cardiovascular comorbidities was calculated for the identified studies. Meta‐analysis was conducted by using the Stata version 15.0 (Stata Corporation, College Station, TX). Since it was assumed that the relationship between the comorbidities and coronavirus‐associated disease varies across populations, a random‐effects model (I‐V heterogeneity) was used, unless there was no significant heterogeneity among studies. Heterogeneity of results across studies was assessed by a standard chi‐square test with significance set at P<0.10 and an I2 statistic with significance set at I2 >50%. (I2 statistic is heterogeneity's quantitative evaluation. It is calculated by the formula I2=[(Q−df)/Q]×100%, where Q is the chi‐square statistic and df is the degrees of freedom).
Results
We identified 1654 reports; 85 full‐text articles were retrieved for in‐depth review. A total of 22 studies that reported the prevalence of cardiovascular comorbidities in COVID‐19 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 (n=51 268 cases from 12 studies) and SARS 24 , 25 , 26 , 27 (n=1608 cases from 4 studies) and MERS 28 , 29 , 30 , 31 , 32 , 33 (n=597 cases from 6 studies) were selected. All cases included in this meta‐analysis were laboratory‐confirmed cases. Figure 1 shows the selection of articles. All 22 articles were available as full reports (all in English). The assessment of the quality of reports is given in Table 1. Characteristics of included studies are given in Table 2. The average age of SARS cases (39.16±2.25 years; range, 37.04–42.3 years) was significantly younger than COVID‐19 cases (46.41±1.79 years; range: 44.0–57.8 years; t test P<0.0001), and the average age of COVID‐19 cases was significantly younger than MERS cases (52.51±4.64 years; range, 35.92–64.5 years; t test P<0.0001). Certainly, the average age of SARS cases was significantly younger than MERS cases (t test P<0.0001).
Figure 1. Flowchart diagram of the systematic search and study selection strategy.
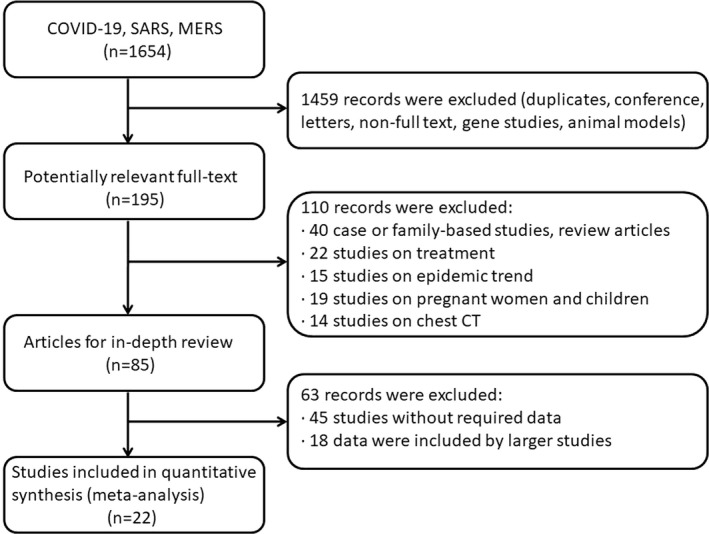
COVID‐19, coronavirus disease 2019; CT, computed tomography; MERS, Middle East respiratory syndrome; and SARS, severe acute respiratory syndrome.
Table 1.
Characteristics and Quality Assessment of Included Studies
| Study | Year | Study Design | Selection | Comparability | Outcome | Quality Score (0–9) |
|---|---|---|---|---|---|---|
| COVID‐19 | ||||||
| NCPERET et al 12 | 2020 | Retrospective | ★★★ | ★ | ★★★★ | 8 |
| Di Qi et al 13 | 2020 | Retrospective | ★★★ | ★ | ★★★ | 7 |
| Guyi Wang et al 14 | 2020 | Retrospective | ★★★ | ★ | ★★★★ | 8 |
| Min Cao et al 15 | 2020 | Retrospective | ★★★ | ★ | ★★★★ | 8 |
| Songqiao Liu et al 16 | 2020 | Retrospective | ★★★ | ★ | ★★★★ | 8 |
| Xu Chen et al 17 | 2020 | Retrospective | ★★★ | ★ | ★★★★ | 8 |
| Zhibing Lu et al 18 | 2020 | Retrospective | ★★★ | ★ | ★★★★ | 8 |
| Yucai Hong et al 19 | 2020 | Retrospective | ★★★ | ★ | ★★★★ | 8 |
| Sijiao Wang et al 20 | 2020 | Retrospective | ★★★ | ★ | ★★★★ | 8 |
| Yongtao Zheng et al 21 | 2020 | Retrospective | ★★★ | ★ | ★★★★ | 8 |
| Bo Yuan et al 22 | 2020 | Retrospective | ★★★ | ★ | ★★★★ | 8 |
| Christopher M. Petrilli et al 23 | 2020 | Retrospective | ★★★ | ★ | ★★★★ | 8 |
| SARS | ||||||
| Hsiao‐Ling Chang et al 25 | 2006 | Retrospective | ★★★ | ★ | ★★★★ | 8 |
| Hai‐Ying Lu et al 24 | 2005 | Retrospective | ★★★ | ★ | ★★★★ | 8 |
| Ping Tim Tsui et al 27 | 2003 | Retrospective | ★★★ | ★ | ★★★★ | 8 |
| Nelson Lee et al 26 | 2003 | Retrospective | ★★★ | ★ | ★★★ | 7 |
| MERS | ||||||
| Khalid A. Alburikan et al 28 | 2020 | Retrospective | ★★★ | ★ | ★★★★ | 8 |
| Yaseen M. Arabi et al 32 | 2014 | Retrospective | ★★★ | ★ | ★★★★ | 8 |
| Ziad A. Memish et al 33 | 2014 | Retrospective | ★★★ | ★ | ★★★ | 7 |
| Jaffar A. Al‐Tawfiq et al 30 | 2014 | Retrospective | ★★★ | ★ | ★★★ | 7 |
| WHO 31 | 2013 | Retrospective | ★★★ | ★ | ★★★ | 7 |
| Abdullah Assiri et al 29 | 2013 | Retrospective | ★★★ | ★ | ★★★★ | 8 |
COVID‐19 indicates coronavirus disease 2019; MERS, Middle East respiratory syndrome; NCPERET, Novel Coronavirus Pneumonia Emergency Response Epidemiology Team; SARS, severe acute respiratory syndrome; and WHO, World Health Organization.
Table 2.
Characteristics of Included Studies
| Study | Year | Patients (N) | Age (y) | Hypertension (N) | Cardiac Disease (N) | Diabetes Mellitus (N) | ||
|---|---|---|---|---|---|---|---|---|
| All | Male | Female | ||||||
| COVID‐19 | ||||||||
| NCPERET et al 12 , * | 2020 | 44 672 | 22 981 | 21 691 | 45.9 | 2683/20 812 | 873/20 812 | 1102/20 812 |
| Di Qi et al 13 | 2020 | 267 | 149 | 118 | 48.0 | 20 | NA | 26 |
| Guyi Wang et al 14 | 2020 | 242 | 119 | 123 | 45.0 | 36 | 9 | 15 |
| Min Cao et al 15 | 2020 | 198 | 101 | 97 | 50.1 | 42 | 12 | 15 |
| Songqiao Liu et al 16 | 2020 | 620 | 326 | 294 | 44.48 | 96 | 13 | 40 |
| Xu Chen et al 17 | 2020 | 291 | 145 | 146 | 46.0 | 39 | 12 | 22 |
| Zhibing Lu et al 18 | 2020 | 123 | 61 | 62 | 57.8 | 41 | 15 | 14 |
| Yucai Hong et al 19 | 2020 | 140 | 71 | 69 | 45.66 | 36 | 4 | 12 |
| Sijiao Wang et al 20 | 2020 | 165 | 92 | 73 | 44.0 | 24 | 8 | 12 |
| Yongtao Zheng et al 21 | 2020 | 30 | 13 | 17 | 44.5 | 3 | 1 | 3 |
| Bo Yuan et al 22 | 2020 | 417 | 198 | 219 | 45.4 | 63 | 28 | 32 |
| Christopher M. Petrilli et al 23 | 2020 | 4103 | 2072 | 2031 | 52.0 | 983 | 235 | 614 |
| Total/weighted average (SD) | 51 268 | 26 328 | 24 940 | 46.41 (1.79) | ||||
| SARS | ||||||||
| Hsiao‐Ling Chang et al 25 ) | 2006 | 346 | 128 | 218 | 42.3 | 23 | 19 | 30 |
| Hai‐Ying Lu et al 24 | 2005 | 801 | 389 | 412 | 37.04 | 21 | 8 | 9 |
| Ping Tim Tsui et al 27 | 2003 | 323 | 127 | 196 | 41 | 16 | 3 | 8 |
| Nelson Lee et al 26 | 2003 | 138 | 66 | 72 | 39.3 | NA | 4 | 5 |
| Total/weighted average (SD) | 1608 | 710 | 898 | 39.16 (2.25) | ||||
| MERS | ||||||||
| Khalid A. Alburikan et al 28 | 2020 | 348 | 216 | 132 | 52 | 73 | 24 | 76 |
| Yaseen M. Arabi et al 32 | 2014 | 12 | 8 | 4 | 59 | 6 | 4 | 8 |
| Ziad A. Memish et al 33 | 2014 | 12 | 6 | 6 | 35.92 | 4 | NA | 5 |
| Jaffar A. Al‐Tawfiq et al 30 | 2014 | 17 | 11 | 6 | 60.7 | NA | 11 | 13 |
| WHO 31 | 2013 | 161 | 104 | 57 | 50.0 | NA | 12 | 16 |
| Abdullah Assiri et al 29 | 2013 | 47 | 36 | 11 | 64.5 | 16 | 13 | 32 |
| Total/weighted average (SD) | 597 | 381 | 216 | 52.51 (4.64) | ||||
COVID‐19 indicates coronavirus disease 2019; MERS, Middle East respiratory syndrome; NA, not available; NCPERET, Novel Coronavirus Pneumonia Emergency Response Epidemiology Team; SARS, severe acute respiratory syndrome; and WHO, World Health Organization.
Only 20 812 patients were used to calculate percentages of hypertension, cardiac disease, and diabetes mellitus.
Meta‐analysis of the identified studies showed that the proportion of hypertension, cardiac disease, and diabetes mellitus in COVID‐19 was 17.1% (95% CI, 13.2%–20.9%; I2=96.3%; Egger's test P=0.283), 4.5% (95% CI, 3.6%–5.5%; I2=77.4%; Egger's test P=0.583), and 8.5% (95% CI, 5.5%–11.4%; I2=96.2%; Egger's test P=0.177) (Figure 2). For SARS, hypertension was present in 4.5% (95% CI, 2.0%–7.0%; I2=78.7%; Egger's test P=0.128), cardiac disease in 2.1% (95% CI, 0.6%–3.7%; I2=78.8%; Egger's test P=0.165), and diabetes mellitus in 3.7% (95% CI, 1.0%–6.4%; I2=88.4%; Egger's test P=0.137) (Figure 3). MERS exhibited different proportions as follows: hypertension in 30.3% (95% CI, 18.3%–42.2%; I2=59.9%; Egger's test P=0.066), cardiac disease in 20.9% (95% CI, 10.7%–31.1%; I2=89.2%; Egger's test P=0.027), and diabetes mellitus in 45.4% (95% CI, 27.3%–63.5%; I2=95.4%; Egger's test P=0.062) (Figure 4).
Figure 2. Meta‐analysis of the proportion of cardiovascular comorbidities in COVID‐19 cases.
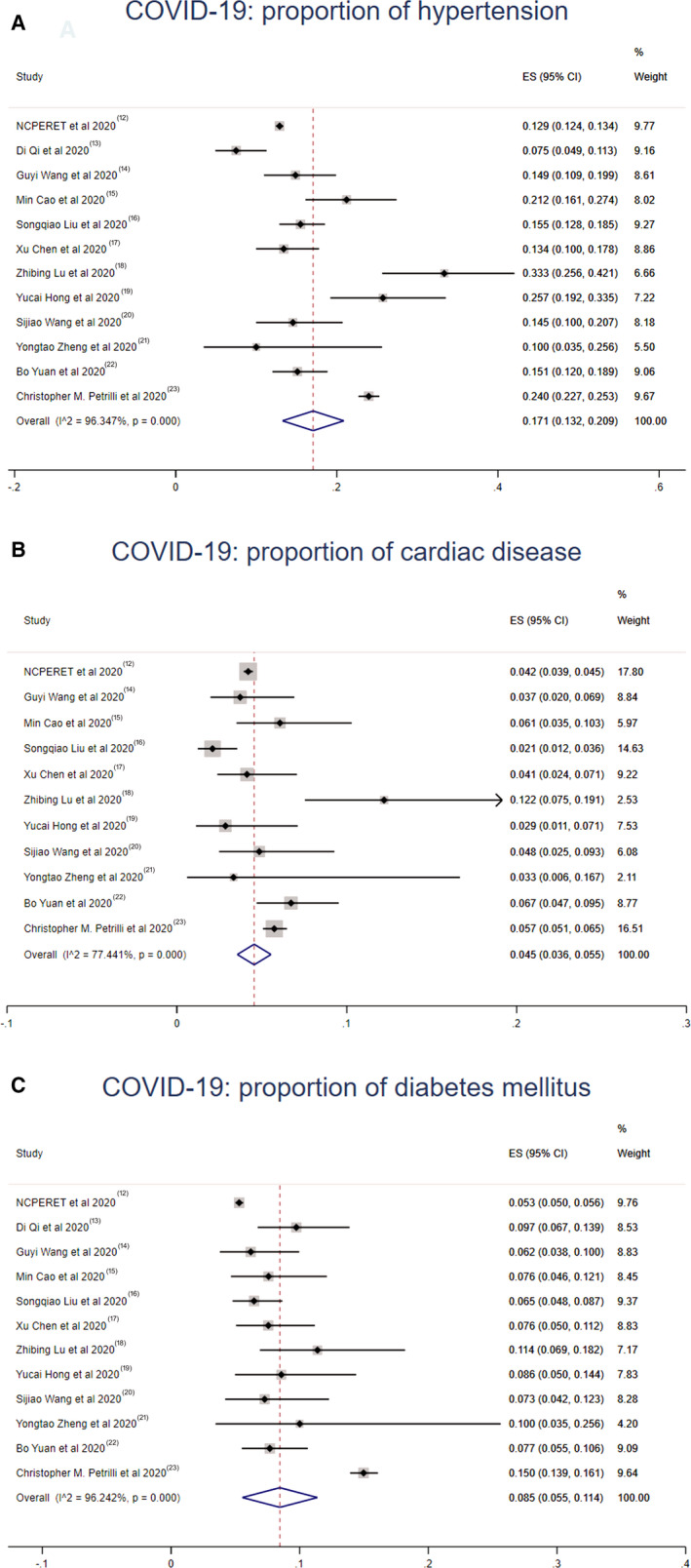
Values represent proportions of hypertension (A), cardiac disease (B), and diabetes mellitus (C) in COVID‐19 cases and the 95% CIs. COVID‐19 indicates coronavirus disease 2019; NCPERET, Novel Coronavirus Pneumonia Emergency Response Epidemiology Team.
Figure 3. Meta‐analysis of the proportion of cardiovascular comorbidities in SARS cases.
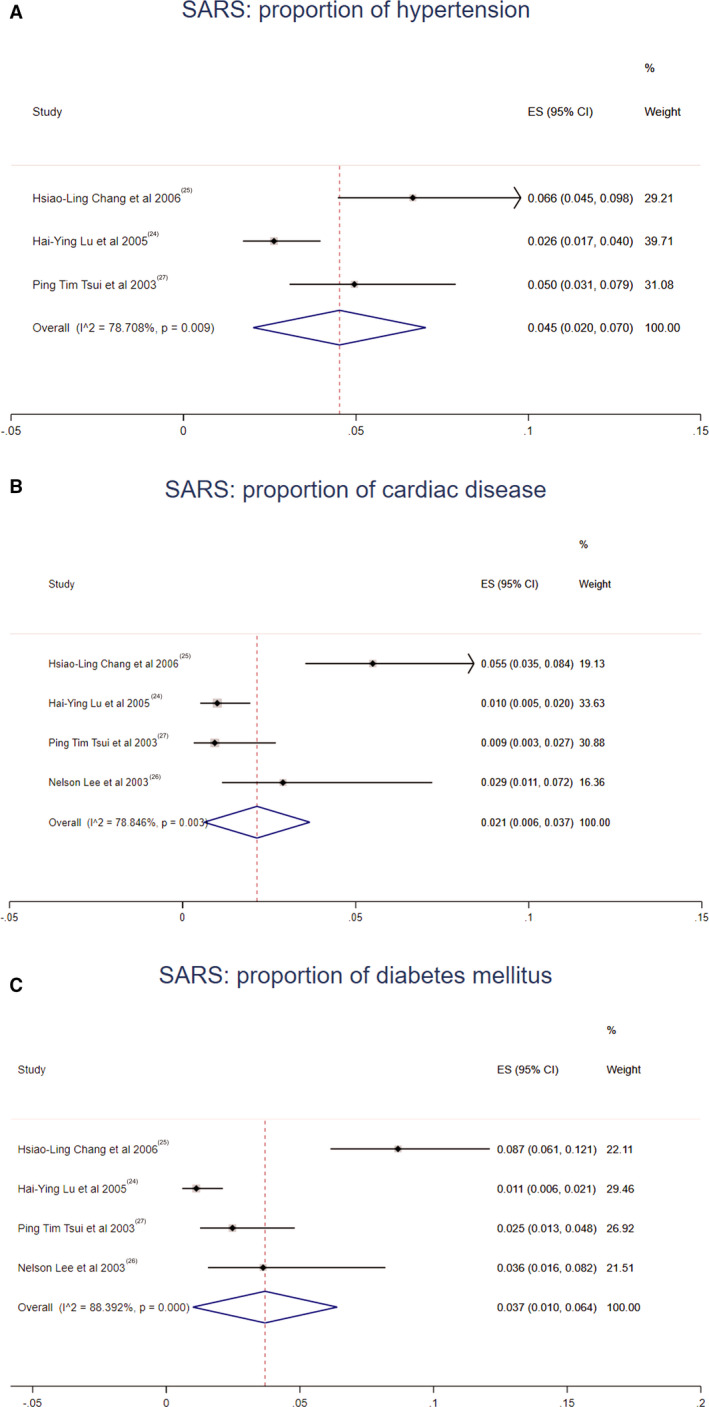
Values represent proportions of hypertension (A), cardiac disease (B), and diabetes mellitus (C) in SARS cases and the 95% CIs. SARS indicates severe acute respiratory syndrome.
Figure 4. Meta‐analysis of the proportion of cardiovascular comorbidities in MERS cases.

Values represent proportions of hypertension (A), cardiac disease (B), and diabetes mellitus (C) in MERS cases and the 95% CIs. MERS indicates Middle East respiratory syndrome; and WHO, World Health Organization.
Discussion
Twenty‐two studies were included in this meta‐analysis, and we found out that the prevalence of cardiovascular comorbidities varies among different coronavirus‐associated diseases. The proportions of hypertension, cardiac disease, and diabetes mellitus in MERS were all higher than those in COVID‐19 and SARS. It could also be obtained from the meta‐analysis that COVID‐19 had an apparently higher prevalence of hypertension than SARS.
As MERS was prevalent mainly in the Middle East, the varying rates of cardiovascular comorbidities between MERS and COVID‐19 and SARS cases may relate to their different patterns of regional spread. Another reason for this difference may relate to the highest average age of MERS cases given that older age is a known risk factor for hypertension, cardiac disease, and diabetes mellitus. Remarkably, a cardinal difference among SARS, MERS, and COVID‐19 is the frequency of cardiovascular involvement. The viral illness could cause a general consequence of the imbalance between infection‐induced increased metabolic demand and reduced cardiac reserve, along with superimposed pneumonia will directly and indirectly affect the cardiovascular system. The viral illness can also potentially destabilize coronary plaques and deteriorate the heart failure through several mechanisms including systemic inflammatory responses, which have been recently documented with COVID‐19. 34 Both SARS and MERS have been linked to acute myocarditis, acute myocardial infarction, and rapid‐onset heart failure. Reversible, subclinical diastolic left ventricular impairment in acute SARS even among those without underlying cardiac disease appears common, likely the result of systemic inflammatory immune response; however, lower ejection fraction upon admission was predictive of later mechanical ventilation. 35
According to previous research on SARS‐CoV, cardiac disease, and diabetes mellitus increased the risk of death by twice as much as other risk factors. 36 A large study from mainland China indicated that the overall case fatality rate of COVID‐19 was 2.3%, but the mortality reached 10.5% in patients with underlying cardiovascular disease. 37 For patients with underlying cardiovascular disease, including hypertension, coronary heart disease, and cardiomyopathy, viral illness can damage myocardial cells through several mechanisms including direct damage by the virus, systemic inflammatory responses, destabilized coronary plaque, and aggravated hypoxia. 38 Patients with cardiovascular disease are more susceptible to cardiac injury after COVID‐19 infection. Cardiac injury can in turn further aggravate the pneumonia and increase the severity of symptoms. Therefore, patients with cardiovascular disease account for a large proportion of deaths from COVID‐19. 39
Proportion of hypertension was very different between COVID‐19 and SARS according to this analysis. Despite the evidence suggesting an influence of age on proportion of hypertension, the rate of hypertension in COVID‐19 cases (average age 46.41±1.79 years) was still nearly 4‐fold higher than that in the SARS cases (average age 39.16±2.25 years) (17.1% versus 4.5%). In accordance with a report, 40 the prevalence of hypertension at 40 to 49 years of age was 22.1% and that at 35 to 39 years of age was 12.6% in China. The ratio between them was 1.8, which was lower than the ratio of hypertension prevalence between COVID‐19 and SARS (3.8). Therefore, there may be some other reasons contributing to the difference of hypertension prevalence between COVID‐19 and SARS apart from age.
Scientists have confirmed that 2019‐nCoV uses the same cell entry receptor, angiotensin‐converting enzyme 2 (ACE2), as SARS coronavirus. 41 This finding has important implications for our understanding of 2019‐nCoV transmissibility and pathogenesis. ACE2, the receptor for SARS‐CoV‐2 and SARS coronavirus, is a surface molecule localized on arterial and venous endothelial cells, arterial smooth muscle cells, and epithelia of the small intestine and the respiratory tract. 42 In the respiratory tract, ACE2 receptor is expressed on the epithelial cells of alveoli, trachea, and bronchi, bronchial serous glands, and alveolar monocytes and macrophages. 43 Downregulation of ACE2, as occurs during COVID‐19 infection, is believed to contribute to pathological changes in the lung. Expression of the ACE2 receptor is also found in many extrapulmonary tissues including heart, kidney, endothelium, intestine, and liver. SARS‐CoV‐2 could invade cells of the above tissues and injure these organs. 44 , 45 Target cells of the MERS coronavirus infection in the lung include pneumocytes, multinucleated epithelial cells, and bronchial submucosal gland cells. 46 All of these cells express a multifunctional cell surface protein, called dipeptidyl peptidase 4 (also known as CD26), constituting the primary entry receptor of MERS coronavirus. 47 Dipeptidyl peptidase 4, the entry receptor of MERS coronavirus, is widely expressed on epithelial cells in the kidney, alveoli, small intestine, liver, and prostate, suggesting that the range of MERS coronavirus tissue tropism is broader than SARS coronavirus and 2019‐nCoV.
There are several limitations in this meta‐analysis. First, the studies included in this analysis indicated a wide among‐studies variance in the prevalence of comorbidities, which may have facilitated the appearance of significant heterogeneity. Furthermore, the large variation among studies in the sample size (30–44 672 2019‐nCoV cases, 138–801 SARS cases and 12–348 MERS cases) may be additional sources of heterogeneity. Third, different studies may define cardiac disease slightly differently, which may increase heterogeneity to some extent. For example, some studies referred only to coronary heart disease, while other studies included several cardiac diseases. Last but not least, further studies are needed to illustrate the discrepancy of the prevalence of comorbidities among different coronavirus diseases more accurately.
As COVID‐19 is spreading in many regions of the world currently, it is reasonable to advise patients with comorbidities of the potential increased risk and to encourage additional, reasonable precautions in geographies with active COVID‐19 transmission. It is important for patients with comorbidities to remain current with vaccinations, including the pneumococcal vaccine given the increased risk of secondary bacterial infection; it would also be prudent to receive influenza vaccination to prevent another source of fever, which could be initially confused with coronavirus infection. Currently, no specific antiviral treatment is available for 2019‐nCoV, and further research is imperative for identifying appropriate therapeutic targets.
Sources of Funding
This research was supported by Grants from the Nature Science Foundation of China (No. 81770324).
Disclosures
None.
(J Am Heart Assoc. 2020;9:e016812 DOI: 10.1161/JAHA.120.016812.)
For Sources of Funding and Disclosures, see page 9.
Contributor Information
Mu Qin, Email: qinmuae@163.com.
Weifeng Jiang, Email: jiangweifeng83@sina.com.
References
- 1. Yang X, Yu Y, Xu J, Shu H, Ja X, Liu H, Wu Y, Zhang L, Yu Z, Fang M, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, Chen H, Wang D, Liu N, Liu D, et al. Characteristics of COVID‐19 infection in Beijing. J Infect. 2020;80:401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peeri NC, Shrestha N, Rahman MS, Zaki R, Tan Z, Bibi S, Baghbanzadeh M, Aghamohammadi N, Zhang W, Haque U. The SARS, MERS and novel coronavirus (COVID‐19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahmadzadeh J, Mobaraki K, Mousavi SJ, Aghazadeh‐Attari J, Mirza‐Aghazadeh-Attari M, Mohebbi I. The risk factors associated with MERS‐CoV patient fatality: a global survey. Diagn Microbiol Infect Dis. 2020;96:114876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nassar MS, Bakhrebah MA, Meo SA, Alsuabeyl MS, Zaher WA. Middle East respiratory syndrome coronavirus (MERS‐CoV) infection: epidemiology, pathogenesis and clinical characteristics. Eur Rev Med Pharmacol Sci. 2018;22:4956–4961. [DOI] [PubMed] [Google Scholar]
- 9. Yang Y, Lu Q, Liu M, Wang Y, Zhang A, Jalali N, Dean N, Longini I, Halloran M, Xu B, et al. Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. medRxiv. 2020. [posted February 21, 2020]. DOI: 10.1101/2020.02.10.20021675. Accessed March 10, 2020. [Google Scholar]
- 10. Global COVID‐19 Case Fatality Rates [Internet]. UK: Centre for Evidence‐Based Medicine; Available at: https://www.cebm.net/covid-19/global‐covid-19-case‐fatality‐rates/. Accessed April 19, 2020. [Google Scholar]
- 11. Stang A, Jonas S, Poole C. Case study in major quotation errors: a critical commentary on the Newcastle‐Ottawa scale. Eur J Epidemiol. 2018;33:1025–1031. [DOI] [PubMed] [Google Scholar]
- 12. The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China. Chin J Epidemiol. 2020;41:145–151. [PMC free article] [PubMed] [Google Scholar]
- 13. Qi D, Yan X, Tang X, Peng J, Yu Q, Feng L, Yuan G, Zhang A, Chen Y, Yuan J, et al. Epidemiological and clinical features of 2019‐nCoV acute respiratory disease cases in Chongqing municipality, China: a retrospective, descriptive, multiple‐center study. medRxiv. 2020. [posted March 3, 2020]. DOI: 10.1101/2020.03.01.20029397. Accessed March 10, 2020. [Google Scholar]
- 14. Wang G, Wu C, Zhang Q, Wu F, Yu B, Lv J, Li Y, Li T, Wu C, Wu G, et al. Epidemiological and clinical features of corona virus disease 2019 (COVID‐19) in Changsha, China. Lancet. 2020. [posted March 10, 2020]. Available at: https://ssrn.com/abstract=3548770. Accessed March 11, 2020. [Google Scholar]
- 15. Cao M, Zhang D, Wang Y, Lu Y, Zhu X, Li Y, Xue H, Lin Y, Zhang M, Sun Y, et al. Clinical features of patients infected with the 2019 novel coronavirus (COVID‐19) in Shanghai, China. medRxiv. 2020. [posted March 6, 2020]. DOI: 10.1101/2020.03.04.20030395. Accessed March 11, 2020. [Google Scholar]
- 16. Liu S, Luo H, Wang Y, Wang D, Ju S, Yang Y. Characteristics and associations with severity in COVID‐19 patients: a multicentre cohort study from Jiangsu Province, China. Lancet. 2020. [posted March 6, 2020]. Available at: https://ssrn.com/abstract=3548753. Accessed March 11, 2020. [Google Scholar]
- 17. Chen X, Zheng F, Qing Y, Ding S, Yang D, Lei C, Yin Z, Zhou X, Jiang D, Zuo Q, et al. Epidemiological and clinical features of 291 cases with coronavirus disease 2019 in areas adjacent to Hubei, China: a double‐center observational study. medRxiv. 2020. [posted March 6, 2020]. DOI: 10.1101/2020.03.03.20030353. Accessed March 11, 2020. [Google Scholar]
- 18. Lu Z, Chen M, Fan Y, Wu X, Zhang L, Guo T, Deng K, Cao J, Luo H, He T, et al. Characteristics and risk factors for fatal outcome in patients with 2019-coronavirus infected disease (COVID‐19) in Wuhan, China. Lancet. 2020. [posted March 3, 2020]. Available at: https://ssrn.com/abstract=3546069. Accessed March 18, 2020. [Google Scholar]
- 19. Hong Y, Huang J, Chen D, Ye Y, Su F, Dai J, Shi J, Shao C, Zhang Z. Clinical characteristics of coronavirus disease 2019 outside Wuhan and development of early risk stratification tool. Res Sq. 2020. [posted March 25, 2020]. Available at: 10.21203/rs.3.rs-19481/v1. Accessed April 18, 2020. [Google Scholar]
- 20. Wang S, Chen Z, Lin Y, Lin L, Lin Q, Fang S, Shi Y, Zhuang X, Ye Y, Wang T, et al. Clinical characteristics of COVID‐19 in Fujian Province: a multicenter retrospective study. Res Sq. 2020. [posted April 8, 2020]. Available at: 10.21203/rs.3.rs-21268/v1. Accessed April 18, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zheng Y, Jia H, Wang F, Sun X, Yang D, Xu M, Xu D, Lu C, Ge B, Chen S, et al. Clinical course and characteristics of corona virus disease 2019 in Xiaoshan, Hangzhou: a retrospective study. Res Sq. 2020. [posted April 9, 2020]. Available at: 10.21203/rs.3.rs-22080/v1. Accessed April 18, 2020. [Google Scholar]
- 22. Yuan B, An Y‐W, Chen Y‐X, Yang J, Wang J‐C, Li W‐X, Wang C, Song S, Liu H‐Q. Epidemiological characteristics of 417 patients infected with COVID‐19 and 368 discharged cases among them in Shenzhen City, China. Res Sq. 2020. [posted March 26, 2020]. Available at: 10.21203/rs.3.rs-19554/v1. Accessed April 18, 2020. [Google Scholar]
- 23. Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell LF, Chernyak Y, Tobin K, Cerfolio RJ, Francois F, Horwitz LI. Factors associated with hospitalization and critical illness among 4,103 patients with COVID‐19 disease in New York City. medRxiv. 2020. [posted April 11, 2020]. DOI: 10.1101/2020.04.08.20057794. Accessed April 18, 2020. [Google Scholar]
- 24. Lu H‐Y, Xu X‐Y, Lei Y, Wu Y‐F, Chen B‐W, Xiao F, Xie G‐Q, Han D‐M. Clinical features of probable severe acute respiratory syndrome in Beijing. World J Gastroenterol. 2005;11:2971–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chang H‐L, Chen K‐T, Lai S‐K, Kuo H‐W, Su I‐J, Lin RS, Sung F‐C. Hematological and biochemical factors predicting SARS fatality in Taiwan. J Formos Med Assoc. 2006;105:439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt JM, Ahuja A, Yung MY, Leung CB, To KF, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. [DOI] [PubMed] [Google Scholar]
- 27. Tsui PT, Kwok ML, Yuen H, Lai SK. Severe acute respiratory syndrome: clinical outcome and prognostic correlates. Emerg Infect Dis. 2003;9:1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alburikana KA, Abuelizz HA. Identifying factors and target preventive therapies for Middle East respiratory syndrome susceptible patients. Saudi Pharm J. 2020;28:161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Assiri A, AI‐Tawfiq JA, Al‐Rabeeah AA, Al‐Rabiah FA, Al‐Hajjar S, Al‐Barrak A, Flemban H, AI‐Nassir WN, Balkhy HH, AI‐Hakeem RF, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Al‐Tawfiq JA, Hinedi K, Ghandour J, Khairalla H, Musleh S, Ujayli A, Memish ZA. Middle East respiratory syndrome coronavirus: a case‐control study of hospitalized patients. Clin Infect Dis. 2014;59:160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. WHO Mers‐Cov Research Group . State of knowledge and data gaps of Middle East Respiratory Syndrome Coronavirus (MERS‐CoV) in humans. PLoS Curr. 2013;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arabi YM, Arifi AA, Balkhy HH, Najm H, Aldawood AS, Ghabashi A, Hawa H, Alothman A, Khaldi A, Raiy BA. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389–397. [DOI] [PubMed] [Google Scholar]
- 33. Memish ZA, Cotton M, Watson SJ, Kellam P, Zumla A, Alhakeem RF, Assiri A, AI Rabeeah AA, AI‐Tawfiq JA. Community case clusters of Middle East respiratory syndrome coronavirus in Hafr Al‐Batin, Kingdom of Saudi Arabia: a descriptive genomic study. Int J Infect Dis. 2014;23:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dong J, Zhang D, Chen Z, Xu Z, Zhao P, Zhang M, Zhang L, Cheng G, Wang Y, Yang G, et al. Clinical characteristics predicting progression of COVID‐19. Lancet. 2020. [posted February 20, 2020]. Available at: https://ssrn.com/abstract=3539674 or DOI: 10.2139/ssrn.3539674. Accessed March 11, 2020. [Google Scholar]
- 35. Li SS, Cheng CW, Fu CL, Chan YH, Lee MP, Chan JWM, Yiu SF. Left ventricular performance in patients with severe acute respiratory syndrome—a 30-day echocardiographic follow‐up study. Circulation. 2003;108:1798–1803. [DOI] [PubMed] [Google Scholar]
- 36. Chan JW, Ng CK, Chan YH, Mok TY, Lee S, Chu SY, Law WL, Lee MP, Li PC. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS). Thorax. 2003;58:686–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 38. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lewington S, Lacey B, Clarke R, Guo Y, Kong XL, Yang L, Chen Y, Bian Z, Chen J, Meng J, et al. The burden of hypertension and associated risk for cardiovascular mortality in China. JAMA Intern Med. 2016;176:524–532. [DOI] [PubMed] [Google Scholar]
- 41. Hoffmann M, Kleine‐Weber H, Krüger N, Müller M, Drosten C, Pöhlmann S. The novel coronavirus 2019 (2019‐nCoV) uses the SARS‐coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv. 2020. [posted January 31, 2020]. DOI: 10.1101/2020.01.31.929042. Accessed February 24, 2020. [Google Scholar]
- 42. Li WH, Moore MJ, Vasilieva N, Sui JH, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sun Y, Liu L, Pan X. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus S proteins. J Microbiol. 2004;24:25–30. [Google Scholar]
- 44. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019‐nCoV infection. bioRxiv. 2020. [posted February 4, 2020]. DOI: 10.1101/2020.02.03.931766. Accessed March 19, 2020. [Google Scholar]
- 46. Ng DL, Al Hosani F, Keating MK, Gerber SI, Jones TL, Metcalfe MG, Tong S, Tao Y, Alami NN, Haynes LM, et al. Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Am J Pathol. 2016;186:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meyerholz DK, Lambertz AM, McCray PB Jr. Dipeptidyl peptidase 4 distribution in the human respiratory tract implications for the Middle East respiratory syndrome. Am J Pathol. 2016;186:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]


