Abstract
Background
Increases in heart rate are thought to result in incomplete left ventricular (LV) relaxation and elevated filling pressures in patients with heart failure with preserved ejection fraction (HFpEF). Experimental studies in isolated human myocardium have suggested that incomplete relaxation is a result of cellular Ca2+ overload caused by increased myocardial Na+ levels. We tested these heart rate paradigms in patients with HFpEF and referent controls without hypertension.
Methods and Results
In 22 fully sedated and instrumented patients (12 controls and 10 patients with HFpEF) in sinus rhythm with a preserved ejection fraction (≥50%) we assessed left‐sided filling pressures and volumes in sinus rhythm and with atrial pacing (95 beats per minute and 125 beats per minute) before atrial fibrillation ablation. Coronary sinus blood samples and flow measurements were also obtained. Seven women and 15 men were studied (aged 59±10 years, ejection fraction 61%±4%). Patients with HFpEF had a history of hypertension, dyspnea on exertion, concentric LV remodeling and a dilated left atrium, whereas controls did not. Pacing at 125 beats per minute lowered the mean LV end‐diastolic pressure in both groups (controls −4.3±4.1 mm Hg versus patients with HFpEF −8.5±6.0 mm Hg, P=0.08). Pacing also reduced LV end‐diastolic volumes. The volume loss was about twice as much in the HFpEF group (controls −15%±14% versus patients with HFpEF −32%±11%, P=0.009). Coronary venous [Ca2+] increased after pacing at 125 beats per minute in patients with HFpEF but not in controls. [Na+] did not change.
Conclusions
Higher resting heart rates are associated with lower filling pressures in patients with and without HFpEF. Incomplete relaxation and LV filling at high heart rates lead to a reduction in LV volumes that is more pronounced in patients with HFpEF and may be associated with myocardial Ca2+ retention.
Keywords: Ca2+ cycling/excitation-contraction coupling, heart failure, hypertension, ion channels/membrane transport, mechanisms
Subject Categories: Cardiomyopathy, Heart Failure, Hypertrophy, Ion Channels/Membrane Transport
Nonstandard Abbreviations and Acronyms
- AF
atrial fibrillation
- BP
blood pressure
- bpm
beats per minute
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HR
heart rate
- LA
left atrial
- LV
left ventricular
- SR
sarcoplasmic reticulum
Clinical Perspective
What Is New?
Moderate heart rate elevations by atrial pacing results in left ventricular volume loss that is much more pronounced in patients who have heart failure with preserved ejection fraction than in controls without hypertension and is associated with myocardial Ca2+ retention.
This is the first in vivo observation that links myocardial Ca2+ handling with a functionally relevant hemodynamic‐mechanical effect in heart failure with preserved ejection fraction.
What Are the Clinical Implications?
Higher heart rates in both controls and patients who have heart failure with preserved ejection fraction are associated with lower and not higher filling pressures. This physiological response may present a therapeutic target.
Hypertensive heart disease with associated diastolic dysfunction is a common substrate for both atrial fibrillation (AF) and heart failure (HF) with preserved ejection fraction (HFpEF). 1 , 2 It is commonly assumed that heart rate (HR) lowering should benefit patients with HFpEF because it provides more time for ventricular filling, whereas higher HRs are detrimental because of an increase in filling pressures. 3 , 4 In light of this, it is not surprising that ≈80% of patients with HFpEF in recent clinical trials were noted to be taking β‐blockers. 5
Changes in HR and stroke volume are critical components of the heart’s capacity to adapt to a change in demand. With exercise, HR rises along with an increase in myocardial contractility, ventricular filling rate, and arterial vasodilation, which combine to increase stroke volume and cardiac output. 6 , 7 , 8 These changes are in part attributable to a rise in adrenergic tone and the action of skeletal muscle pumps to increase systemic venous return. In healthy individuals, exercise is not associated with a significant rise in left ventricular (LV) filling pressures. In patients with HFpEF, however, a pronounced rise in LV filling pressures at higher HRs is an important determinant of exercise intolerance. 9 , 10 , 11 This unfavorable hemodynamic response is further compounded by an intrinsic prolongation of myocardial relaxation. 12 , 13 In isolated human myocardium from patients with HFpEF, higher stimulation rates result in incomplete relaxation with a rise in diastolic force production. 14 It is in this context that HR lowering is commonly believed to reduce filling pressures and improve LV filling, as it provides more time for relaxation. 12 , 13
Translational studies have revealed that incomplete relaxation at higher HRs is in part caused by impaired cardiomyocyte Ca2+ handling. 15 , 16 , 17 In myocardium from patients with hypertensive heart disease and HFpEF, diastolic Ca2+ levels cannot be restored to normal (Ca2+ overload) at higher HRs, which results in diastolic myofilament activation manifested as incomplete relaxation. 12 The mechanism commonly offered to explain Ca2+ overload is an increase in cardiac myocyte Na+ levels that lowers the driving force for cellular Ca2+ extrusion via the Na+/Ca2+ exchanger. 16 , 17 , 18
Demonstration of elevated filling pressures and incomplete relaxation at higher HRs along with evidence for their underlying mechanisms have been derived from exercise studies and in vitro studies of maximally loaded isolated myocardium. We have recently raised the concern that inferences from these studies have been incorrectly applied to patients with HFpEF and proposed that moderate HR elevations, and not HR lowering, would result in lower filling pressures in patients with HFpEF. 19 Based on clinical observations we also propose that a major effect of moderate elevation of resting HRs is a marked reduction in LV diastolic volume that could be the result of an Na+‐ and Ca2+‐dependent residual diastolic myocardial activation. 20 In the present study, we tested this in fully sedated patients with HFpEF who had underlying hypertensive heart disease and in controls without hypertension undergoing radiofrequency ablation for paroxysmal AF.
We studied patients who met the enrollment criteria only if they were in sinus rhythm. The instrumentation routinely employed in AF ablation procedures allowed us to make invasive measurements with minimal excess procedural risks. Patients without hypertension who were deemed to have “vagally mediated” paroxysmal AF and with no other significant comorbidities were enrolled as referent controls.
METHODS
The authors declare that all supporting data are available within the article. Requests for details of the analytic methods and study materials should be made to the corresponding author.
Study Population
The study was conducted between December 2015 and April 2019 and was approved by the University of Vermont’s (UVM’s) institutional review board. A total of 523 patients scheduled to undergo AF ablation were screened for the inclusion/exclusion criteria specified below. Twenty‐six patients met the criteria and provided informed consent. Three of these patients could not be studied for procedural reasons, and 1 patient was found to have a pulmonary mass before the procedure, resulting in a total of 22 studied patients (12 controls and 10 patients with HFpEF).
Inclusion Criteria
Adult patients aged >18 years in sinus rhythm with a history of paroxysmal AF undergoing elective radiofrequency ablation with an LV ejection fraction ≥50% by echocardiography, normal regional wall motion, and LV end‐diastolic volume index ≤75 mL/m2 were eligible. Patients who met these inclusion criteria were further categorized as follows: (1) referent controls with no history of hypertension and/or HF (criteria below) and no concentric LV remodeling or hypertrophy (criteria below), and (2) patients with HFpEF with a history of hypertension and a hospitalization for decompensated HF (adjudicated to be primarily caused by HF rather than AF) within the previous 2 years or dyspnea on exertion (New York Heart Association class ≥2) and echocardiographic evidence of concentric LV remodeling or overt hypertrophy (relative wall thickness ≥0.42 or LV mass >115 g/m2 in men/>95 g/m2 in women). The presence of sinus rhythm was confirmed by 12‐lead ECG and continuous ECG recordings at the time of the study. H2FPEF scores were calculated for all patients with HFpEF. 21
Exclusion Criteria
Patients were excluded if they had ≥1 of the following: any LV regional wall motion abnormality, LV ejection fraction <50%, LV end‐diastolic volume index >75 mL/m2, more than moderate valvular disease by echocardiography, the presence of any pulmonary disease that could explain dyspnea, any noncardiac disease or condition known to affect myocardial function, anemia (hemoglobin <11 g/dL in men and <10 g/dL in women), serum creatinine >2.0 mg/dL, history of substance abuse, inability to provide informed consent, active malignancy, severe connective tissue disease, severe liver disease, hypertrophic cardiomyopathy or other restrictive cardiomyopathies, and constrictive pericarditis. Patients with known nonrevascularized coronary artery disease (any stenosis >50%) were also excluded.
Baseline Echocardiogram
Preprocedural transthoracic echocardiograms were performed to assess LV concentric remodeling or hypertrophy using the aforementioned partition values in line with guideline‐recommended linear assessments and calculations from the parasternal long and short axis. 22 Chamber volumes were assessed from an optimized LV apical view that avoided foreshortening. Ejection fraction was determined from LV volumes derived by the modified Simpson equation.
Instrumentation and Ablation Procedure
All patients presented in a fasting state with all medications held. After informed consent was obtained, all patients underwent general anesthesia, preprocedural transesophageal echocardiograms to rule out left atrial (LA) appendage thrombus, and invasive blood pressure (BP) monitoring from the left radial artery. Right and left femoral venous access was obtained by modified Seldinger technique. Following administration of heparin given as a weight‐based bolus to reach a target activated clotting time of >300 seconds, standard multielectrode catheters were placed in the right atrial appendage and coronary sinus or great cardiac vein. An intracardiac ultrasound probe was placed in the right atrium (8F; AcuNav, ACUSON). A double transseptal puncture was performed to insert an irrigated ablation catheter and multielectrode mapping catheter in the left atrium via 2 SL1 sheaths (St. Jude Medical). LA pressure tracings were obtained during transseptal puncture and subsequently with pacing from the right atrial appendage. All of these invasive procedures are routinely performed during AF ablation procedures at the UVM Medical Center.
Experimental Measurements
The following measurements and samples were obtained sequentially (1 through 6) beginning at each patient’s baseline HR (in sinus rhythm), followed by pacing from the right atrial appendage at a rate of 95 beats per minute (bpm) and then at a rate of 125 bpm. Pacing was maintained for 60 to 120 seconds at each rate before measurements were made to establish steady‐state conditions.
Invasive radial artery pressure recorded in sinus rhythm and at 95 bpm and 125 bpm.
Coronary sinus blood samples: The SL1 sheath was advanced into the coronary sinus for blood sampling. The following were measured in sinus rhythm and during and after pacing at 125 bpm: venous blood gases, pH, and serum [Na+], [Ca2+], and [K+]. Two after‐pacing samples were obtained, 1 within 0 to 15 seconds of stopping pacing at 125 bpm and a second 15 to 60 seconds after pacing cessation. All samples were drawn into a 5‐cc heparin‐coated arterial blood gas syringe and placed on ice. Immediately following collection, the samples were analyzed in the clinical pathology laboratory.
LV volumes: Echocardiographic volume measurements were obtained from recorded video clips from the intracardiac ultrasound probe. Volumes were derived by the modified Simpson equation in accordance with echocardiographic guidelines. Individual data points represent the average of 3 measurements at baseline, 95 bpm, and 125 bpm. To illustrate the sequential effects of increasing HRs on LV dimensions, low‐speed M‐mode tracings were obtained while the HR was increased to 95 bpm and 125 bpm within 1 screen.
Coronary sinus blood flow: The coronary sinus cross‐sectional area was obtained using the intracardiac ultrasound probe at rest followed by pulsed‐wave Doppler recordings of blood flow. 23 Mean coronary flow velocities were obtained from the recordings.
LA blood sample: After the transseptal puncture, a blood sample was obtained from the left atrium to determine arterial blood gases and N‐terminal pro‐B‐type natriuretic peptide levels.
LA pressure: Mean LA and LA pressure at the time of LV end‐diastolic pressure (determined at the peak of the R wave of the QRS complex) in sinus rhythm and at 95 and 125 bpm.
Statistical Analysis
Data are presented as means, SDs, and standard errors for graphs or medians and interquartile ranges for pooled composite graphs. Controls and patients with HFpEF were compared using 1‐way fixed effects ANOVA models for continuous items (baseline to 125 bpm). Paired Student t test was used for within‐group comparisons without repeated measures. Multiple comparisons to a baseline control, eg, BP or changes in Ca2+, were evaluated with a Dunnett test to maintain the overall type I error rate. The clinical control and HFpEF baseline characteristics were compared by Student t test for continuous items and a Fisher exact test for categorical variables. Statistical sensitivity analyses were performed using nonparametric testing to explore for potential issues with ANOVA assumptions. Percentage change scores were examined by unpaired t and Mann‐Whitney tests. Formal tests utilized a 5% significance level. Parametric P values confirmed by nonparametric testing are reported. Data analysis was conducted using GraphPad Prism 8.
RESUTLS
Study Population and Baseline Characteristics
The baseline characteristics of the enrolled study participants are listed in Table 1. The average age was 59±10 years, 32% were women, and the average ejection fraction was 0.61±0.04. The mean HR at baseline sinus rhythm was 67±8 bpm. The body mass index was significantly higher in patients with HFpEF versus controls. All but 1 of the patients with HFpEF were receiving >1 BP‐lowering medication. The mean H2FPEF score for enrolled patients with HFpEF was 6.7±0.8 (ranging from 6 to 8), corresponding to an average HFpEF probability of 0.93 (ranging from 0.91 to 0.98). 21
Table 1.
Baseline Characteristics of Enrolled Patients
| Patient Data |
Controls n=12 |
Patients With HFpEF n=10 |
P value |
|---|---|---|---|
| Age, y | 57±9 | 61±10 | 0.33 |
| Women, % | 25 | 40 | 0.65 |
| HR, bpm | 65±7 | 70±9 | 0.21 |
| BMI, kg/m2 | 28±6 | 36±7 | 0.01 |
| BSA, m2 | 2.06±0.31 | 2.12±0.33 | 0.65 |
| sBP, mm Hg | 126±8 | 134±9 | 0.04 |
| DBP, mm Hg | 70±15 | 75±9 | 0.78 |
| ACEI/ARB, No. (%) | 1(8) | 7(70) | 0.027 |
| ccb, No. (%) | 4(33) | 1(10) | 0.32 |
| Diuretic, No. (%) | 0(0) | 0(0) | |
| ß‐Blocker, No. (%) | 4(33) | 8(80) | 0.04 |
| NT‐proBNP, pg/mL | 184±254 | 274±255 | 0.41 |
| CAD, No. (%) | 0(0) | 2(20) | 0.19 |
| Echocardiography | |||
| EF, % | 61±4 | 61±5 | 0.83 |
| Septum , mm | 9.3±0.6 | 12.4±1.8 | <0.001 |
| Posterior wall, mm | 8.5±0.8 | 11.3±1.3 | <0.001 |
| LVEDD, mm | 50±5 | 48±4 | 0.26 |
| LVESD, mm | 34±5 | 30±6 | 0.12 |
| RWT | 0.34±0.3 | 0.48±0.07 | <0.001 |
| LVM, g | 162±40 | 218±35 | 0.002 |
| LVM/BSA | 79±16 | 103±13 | 0.002 |
| LVM/H | 92±21 | 132±20 | <0.001 |
| LVM/H2.7 | 36±8 | 58±17 | <0.001 |
| LA volume, mL | 52±19 | 84±22 | 0.003 |
| LA volume/BSA | 24±7 | 35±12 | 0.008 |
| E, cm/s | 78±19 | 84±15 | 0.52 |
| A, cm/s | 66±17 | 78±33 | 0.40 |
| E/e' med | 7.3±2.6 | 11.3±4.2 | 0.06 |
| E/e' lat | 7.0±3.2 | 8.5±1.97 | 0.36 |
A indicates A‐wave peak velocity; ACEI/ARB, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; BMI, body mass index; bpm, beats per minute; BSA, body surface area; CAD, coronary artery disease; CCB, calcium channel blocker; DBP, diastolic blood pressure; E, E‐wave peak velocity; E/E’med, ratio of mitral peak velocity of early filling (E) to early diastolic septal mitral annular velocity (E'); E/E’lat, ratio of mitral peak velocity of early filling (E) to early diastolic lateral mitral annular velocity; EF, ejection fraction; HFpEF, heart failure with preserved ejection fraction; HR, heart rate; LA, left atrial; LVEDD, left ventricular end‐diastolic diameter; LVESD, left ventricular end‐systolic diameter; LVM, left ventricular mass; LVM/H, left ventricular mass to height ratio; LVM/H2.7, allometric left ventricular mass index; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; RWT, relative wall thickness; and SBP, systolic blood pressure.
Baseline Echocardiographic Characteristics
Baseline echocardiographic characteristics are listed in Table 1. As specified in the inclusion/exclusion criteria, patients with HFpEF met criteria for LV hypertrophy or concentric remodeling. In addition, the LA volume index was significantly higher in patients with HFpEF compared with controls. There was a trend towards a higher medial E/e’.
LA and LV Filling Pressure Response to HR
Figure 1 shows presentative LA tracings from a control patient and a patient with HFpEF at the 3 HRs. From baseline to 125 bpm, the mean LA pressure at LV end‐diastolic pressure decreased on average by 20% and 35%, respectively (mean LA pressure: controls 15.3±5.8 to 11.4±4.1 mm Hg and patients with HFpEF 16.8±6.0 to 12.3±2.6 mm Hg; LV end‐diastolic pressure: controls 15.0±4.4 to 10.2±4.1 mm Hg and patients with HFpEF 16.6±5.4 to 8.9±3.0 mm Hg, all P<0.05). There was a trend towards a greater reduction of LV end‐diastolic pressure in patients with HFpEF (controls −4.3±4.1 mm Hg and patients with HFpEF −8.5±6.0 mm Hg, P=0.08) also shown in Figure 2.
Figure 1. LA Pressures at Baseline Sinus Rhythm and During Atrial Pacing.
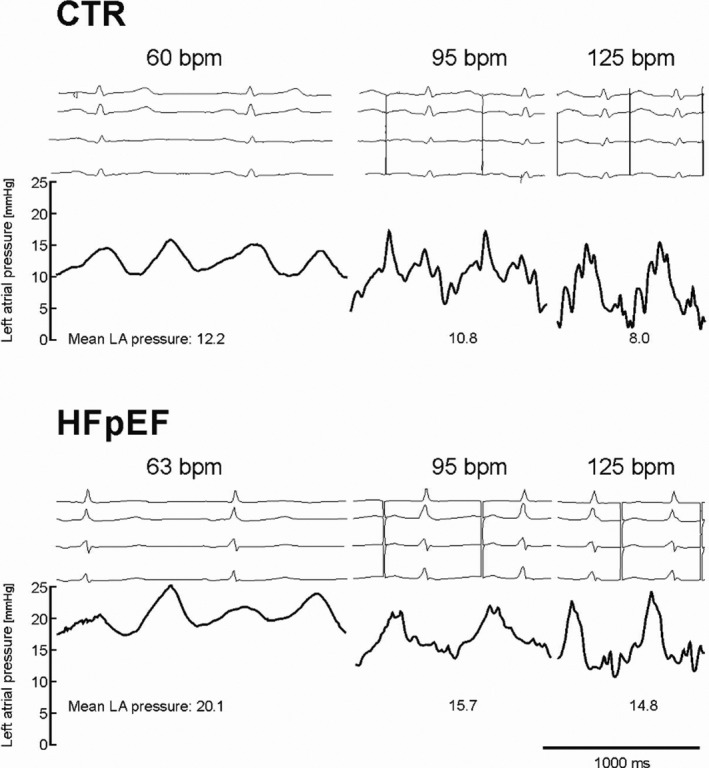
Left atrial (LA) pressures at baseline sinus rhythm and during atrial pacing at 95 beats per minute (bpm) and then 125 bpm in a control patient and in a patient with heart failure with preserved ejection fraction (HFpEF) as demonstrative examples.
Figure 2. LVEDP and Mean LA Pressure at Baseline and During Atrial Pacing.
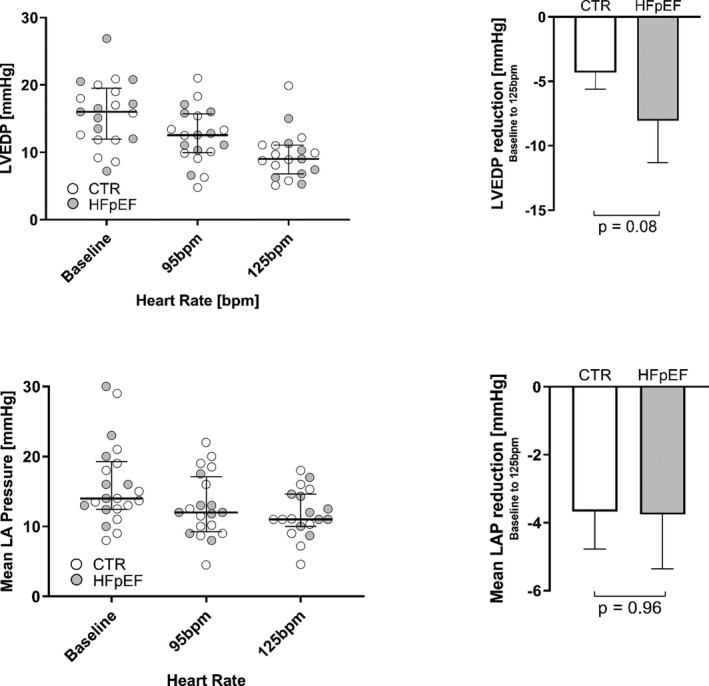
Left ventricular end‐diastolic pressures (LVEDP) and mean left atrial (LA) pressures in controls and patients with heart failure with preserved ejection fraction (HFpEF). bpm indicates beats per minute.
Radial Arterial BP and Coronary Sinus Flow
Systolic BP in controls significantly increased with pacing to 95 bpm (P<0.05) and then plateaued at 125 bpm as shown in Figure 3. There was no change in systolic BP in HFpEF. Diastolic arterial BP increased from baseline with pacing in controls (95 bpm and 125 bpm, both P<0.05) but not in patients with HFpEF. The increase in HR from baseline sinus rhythm to 125 bpm resulted in a near doubling of the coronary sinus blood flow in both groups without changes in oxygen saturation (Table 2).
Figure 3. Arterial BPs at Baseline and During Atrial Pacing.
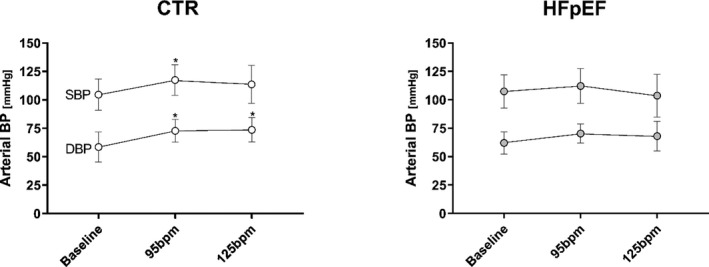
Arterial blood pressures (BPs) in controls and patients with heart failure with preserved ejection fraction (HFpEF). Systolic BP at baseline heart rate vs 95 beats per minute (bpm) (P=0.02). Diastolic BP at baseline heart rate vs 95 bpm (P=0.003) or 125 bpm (P=0.004).
Table 2.
CS and LA Blood Measurements
| Controls | HFpEF | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | Baseline | 125 bpm | P Value | No. | Baseline | 125 bpm | P Value | |
| Oxygen saturation | 9 | 78±12 | 77±11 | ns | 9 | 74±9 | 75±9 | ns |
| CS flow, mL/min | 6 | 248±68 | 452±169 | 0.01 | 9 | 294±152 | 523±179 | 0.002 |
| MVO2, mL/min | 6 | 12±3 | 18±6 | 0.005 | 7 | 19±16 | 28±17 | 0.01 |
| MVO2, mL/min per 100 g | 6 | 7±3 | 11±5 | 0.01 | 7 | 10±8 | 14±9 | 0.01 |
| pH | 9 | 7.33±0.05 | 7.31±0.04 | ns | 9 | 7.30±0.03 | 7.31±0.03 | ns |
Baseline vs 125 beats per minute (bpm): Control patients’ coronary sinus (CS) blood sample measurements compared with those of patients with heart failure with preserved ejection fraction (HFpEF) at baseline heart rate and at paced heart rate of 125 bpm. LA indicates left atrial; MVO2, myocardial oxygen consumption; and ns, nonsignificant.
LV Chamber Size Response to HR
Compared with baseline LV end‐diastolic volumes (left ventricular end‐diastolic volume per body surface area) in sinus rhythm pacing at 125 bpm significantly decreased chamber volumes in both groups as shown in Figure 4 (controls 48.1±10.6 mL/m2 to 41.7±10.5 mL/m2 and patients with HFpEF 37.0±8.9 mL/m2 to 25.3±8.2 mL/m2, both P<0.05). End‐systolic volumes were reduced in HFpEF (controls 19.1±5.7 mL/m2 to 17.9±5.6 mL/m2 [P=0.10] and patients with HFpEF 14.4±4.6 mL/m2 to 10.1±4.6 mL/m2 [P<0.001]). The relative reduction in diastolic LV chamber dimensions was much more pronounced in patients with HFpEF (−32%±11%, versus controls −15%±14%, P<0.01). The pronounced LV volume loss in patients with HFpEF is also demonstrated in the M‐mode tracing (Figure 5).
Figure 4. Left Ventricular End‐Diastolic and End‐Systolic Volume Index at Baseline and During Atrial Pacing.
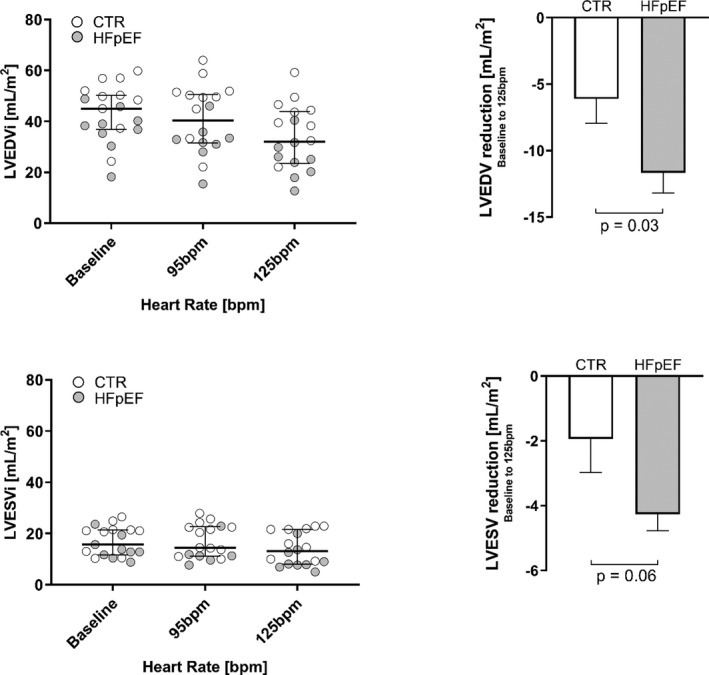
Left ventricular end‐diastolic volume index (LVEDVI) and left ventricular end‐systolic volume (LVESV) index (LVESVI) in controls and patients with heart failure with preserved ejection fraction (HFpEF). Error bars: (left panels) interquartile ranges, (right panels) SEM. bpm indicates beats per minute.
Figure 5. Left Ventricular M‐Mode Tracing at Baseline and During Atrial Pacing.
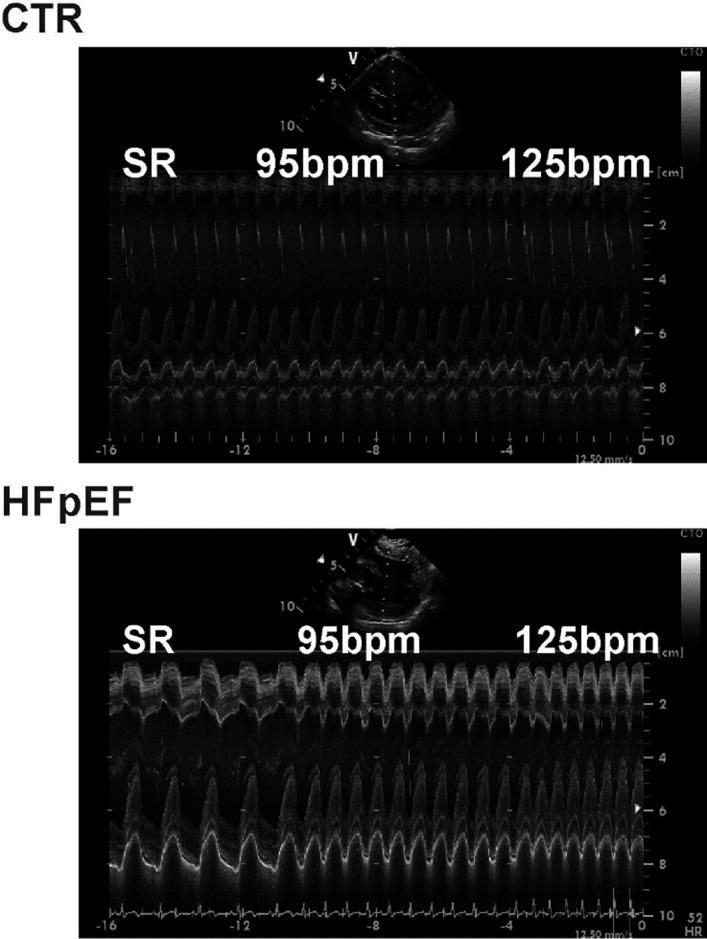
Left ventricular M‐mode tracing recorded with the intracardiac ultrasound probe positioned at the right ventricular septum at baseline sinus rhythm, 95beats per minute (bpm), and 125bpm in controls and in patients with heart failure with preserved ejection fraction (HFpEF). SR indicates sarcoplasmic reticulum.
Ca2+ and Na+ Levels
In controls there were no significant changes in coronary sinus [Ca2+], [Na+], or [K+] immediately following pacing at 125 bpm (Table 3). In contrast, in patients with HFpEF there was a significant increase in [Ca2+] after pacing at 125 bpm (Figure 6) without any significant changes in [Na+] or [K+] levels.
Table 3.
Coronary sinus Ca2+, Na+, and K+ Levels After Pacing
| Controls | HFpEF | |||||
|---|---|---|---|---|---|---|
| 125 bpm |
(n=9) 0 to 15 s |
15 to 60 s | 125 bpm |
Patients With HFpEF (n=8) 0 to 15 s |
15 to 60 s | |
| After Pacing | After Pacing | |||||
| Ca, mg/dL | 8.84±0.45 | 8.86±0.42 | 8.80±0.48 | 8.75±0.30 | 8.83±0.36 | 8.89±0.34* |
| Na, mg/dL | 141.1±2.3 | 141.9±1.9 | 142.0±1.9 | 141.0±1.7 | 140.6±1.3 | 140.6±1.7 |
| K, mg/dL | 4.24±0.23 | 4.22±0.23 | 4.11±0.26 | 4.50±0.61 | 4.44±0.57 | 4.40±0.48 |
Pacing at 125 beats per minute (bpm) versus sinus rhythm after 125 bpm pacing. HFpEF indicates heart failure with preserved ejection fraction.
P=0.03.
Figure 6. Myocardial Calcium Efflux After Pacing at 125 bpm.
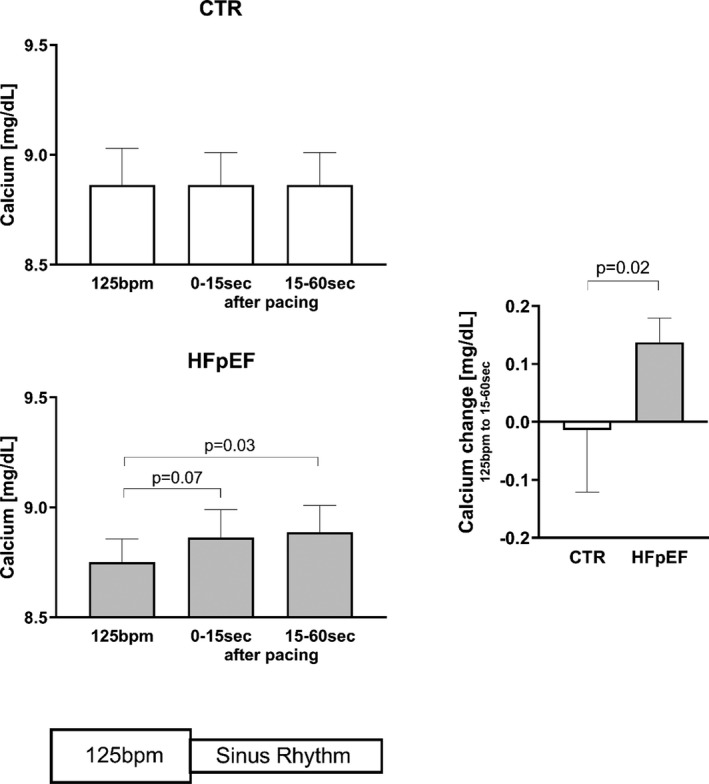
Myocardial calcium efflux after pacing at 125 beats per minute (bpm) in controls and patients with heart failure with preserved ejection fraction (HFpEF)
DISCUSSION
We studied the effects of increased HRs produced by atrial pacing in anesthetized patients with and without hypertensive heart disease–associated HFpEF. A rise in HR had the following effects:
Left‐sided filling pressures decreased in controls and patients with HFpEF.
LV volumes decreased. The end‐diastolic volume loss was approximately twice as pronounced in patients with HFpEF as in controls.
Systolic arterial BPs rose significantly in controls but not in patients with HFpEF.
Based on coronary sinus blood samples, tachycardia‐induced myocardial Ca2+ retention occurs in patients with HFpEF but not in controls. Na+ and K+ are unaffected.
The expectation that high HRs are always associated with incomplete relaxation and a rise in LV filling pressures in patients with HFpEF is derived from exercise studies. During exercise, patients with HFpEF will demonstrate a marked increase in LV filling pressures at higher HRs in contrast to healthy patients. 11 Multivariate physiologic modeling also predicts that a selective increase in HR increases filling pressures. 4 However, the present study demonstrates that an isolated increase in HR has the opposite effect and significantly lowers both LA and LV filling pressures in patients with and without HFpEF. The loss in LV volume at a higher HR is consistent with the reduction in filling pressure, but the much greater loss in patients with HFpEF suggests an abnormality in diastolic properties.
Hemodynamic Effects of Higher HRs at Rest and With Exercise
A review of the literature reveals that a reduction in LV filling pressures with HR elevations caused by atrial pacing has been documented as an ancillary finding in a small number of studies in both large animals and humans, with the latter including patients with HFpEF. 19 Other key findings from these studies are that higher HRs accelerate both contraction and relaxation, reflected in a higher + dP/dt and ‐dP/dt and a shortened time constant of isovolumic pressure decline (tau). End‐systolic elastance, the slope of the end‐systolic pressure‐volume relation, a measure of contractility, increases with higher HRs.
It is important to distinguish between the HR‐induced changes in response to atrial pacing in patients at rest from the much more complex changes that occur during exercise. Principal differences include increased adrenergic tone, which results in larger changes in contractility‐relaxation dynamics; the action of skeletal muscle pumps, which increases systemic venous return; and adjustments to peripheral resistance. During exercise in healthy patients, these additional effects combine to leave filling pressures relatively unaffected, whereas in patients with HFpEF exercise causes filling pressures to markedly increase. 9 , 10 , 11
Bowditch‐Treppe Effect
Higher HRs lead to an increase in contractility in normal myocardium. This intrinsic myocardial property (often referred to as the Treppe effect, or a positive force‐frequency relationship) was first described by Bowditch in 1871. 24 Isolated isometrically contracting normal human myocardium displays an increase in systolic force up to stimulation frequencies of between 120 to 170 per minute; after which force declines. 25 , 26 As the frequency increases, the relaxation rate also increases and relaxation time shortens. Under these fixed loading conditions, diastolic force eventually rises when, despite the increased relaxation rate, the diastolic interval becomes so short that relaxation can no longer reach completion. 12 , 13 , 27 , 28
These cumulative changes in contraction‐relaxation dynamics are largely accounted for by cardiomyocyte Ca2+ handling through an activation of the sarcoplasmic reticulum (SR) Ca2+ ATPase 2a pump. This accelerates cellular Ca2+ sequestration into the SR resulting in shortened relaxation (lusitropic effect of HR) and an increased SR Ca2+ content available for the ensuing contraction, thus increasing contractility (inotropic effect of HR). 17 , 29 This is primarily regulated by protein kinase A–mediated phosphorylation of the SR protein phospholamban, a Ca2+‐dependent modulator of SR Ca2+ ATPase 2a. 30 , 31 Increased contractility also contributes to lower filling pressures by increased torsional deformation and early diastolic recoil (untwisting) that leads to filling by suction. 32 A major reason in vitro findings cannot be directly extrapolated to effects at higher HRs in vivo is because the reductions in filling times and preload—reflected in lower LA pressure—that occur in the intact heart.
HR Effect on LV Volumes, Ca2+, and Clinical Translation
We show that a major effect of pacing is an LV volume loss that is more pronounced in patients with HFpEF. Although a shorter filling time provides a principle explanation for the volume loss, it fails to provide an explanation for the observed differences between controls and patients with HFpEF. Smaller LV volumes at higher HRs are paralleled by increased myocardial Ca2+ levels that shift the left ventricle to a more leftward and steeper end‐systolic pressure volume relationship. With the HR‐dependent increase in cellular Ca2+ load, cytoplasmic Ca2+ levels reach a point where diastolic crossbridge deactivation is progressively incomplete, leaving the myocardium in an activated and energy‐consuming state. 12 , 28 At this stage, compensatory cellular Ca2+ extrusion via the Na+/Ca2+ exchanger is insufficient to restore normal Ca2+ levels in diastole. This is further compounded by a Ca2+ leak from the SR that is more pronounced at high Ca2+ loads. 12 , 13 , 17 The same mechanisms eventually come to play in normal myocardium albeit at significantly higher HRs.
Residual diastolic crossbridge activation leads to shortened myocytes in diastole. At the whole organ level this translates into a reduction in LV diastolic volumes, as observed in this study. It is of clinical relevance that an acceleration of SR Ca2+ uptake by protein kinase A–mediated mechanisms exacerbates the volume loss despite an acceleration of LV relaxation. This is apparent during dobutamine stress testing where comparable HR elevations lead to a much more pronounced LV volume loss than pacing or HR elevations during exercise. 20
The ensuing energetic cost of diastolic myofilament activation at high HRs can be so profound that it exceeds the energy requirement for contraction. 12 , 28 Diastolic myocardial activation is therefore an important but largely unrecognized effect that likely plays a role in tachycardia‐induced myocardial ischemia even in the absence of flow obstructive coronary disease. However, at an HR of 125 bpm, ischemia did not appear to play a role as coronary venous pH and oxygen saturation did not change and LV contractility was normal.
In the HR range studied we found that both systolic and diastolic BPs increase in referent controls but do not significantly change in patients with HFpEF. The trend towards a reduction in BPs between 95 bpm and 125 bpm in patients with HFpEF is likely directly related to the greater reduction in LV volumes observed in these patients, resulting in a blunting or inversion of the HR‐cardiac output relationship. This effect also helps explain the clinical observation that patients with concentrically remodeled left ventricles are more prone to develop hypotension with tachycardia. Because of the loss in volumes, the inflection point of the HR‐mediated increase in cardiac output and BP is moved toward lower HRs in patients with higher degrees of concentric hypertrophy and diastolic dysfunction. At HRs >170 bpm, the associated volume loss can be so profound that stroke volume and cardiac output approach zero. This physiological response is routinely used during transcatheter aortic valve replacement procedures to facilitate the deployment of the aortic valve.
Other factors clearly play important roles in diastolic dysfunction in HFpEF, including an HR‐dependent reduction in the atrial contribution to LV filling, slowed actomyosin relaxation kinetics, and myocardial modifications that increase stiffness, eg, increased collagen levels and changes in the posttranslational modification of titin. 33 , 34 However, the latter 2 mechanisms are likely mainly at play at resting HRs when sarcomere lengths exceed 2.0 micrometers.
Tachycardia‐Induced Myocardial Ca2+ Retention is Not Na+ Dependent
Direct in vivo assessments of myocardial Na+ and Ca2+ levels are not feasible in patients. Instead we made measurements in the coronary effluent. We hypothesized that Ca2+ and Na+ levels would rise in coronary sinus blood as soon as pacing is stopped, reflecting a restoration of normal cellular ion levels. Indeed, Ca2+ levels increased in the coronary sinus after pacing in patients with HFpEF but not in controls. To our knowledge, these results provide a first in vivo documentation of myocardial Ca2+ overload that very likely contributes to the more pronounced LV volume loss in HFpEF. It is not surprising to find Ca2+ levels elevated for more than 15 seconds after pacing cessation as normalization of the cellular Ca2+ content is an incremental beat‐to‐beat process that is buffered by SR Ca2+ resequestration. 29 This experimental approach also allowed us to test the Na+ paradigm, which contends that HR‐dependent elevations in intracellular Na+ are responsible for Ca2+ overload via the Na+/Ca2+ exchange mechanism as the driving Na+ gradient is narrowed. 17 , 18 In vitro studies in isolated human myocardium have suggested that intracellular Na+ levels rise by about 25% to 30% with stimulation at 120 per minute. 35 Such marked changes in cellular Na+ should be readily detectable in the coronary effluent after pacing cessation. However, Na+ levels were unchanged in both controls and patients with HFpEF, making this an unlikely explanation for the observed differences in [Ca2+]. It is conceivable that HRs >125 bpm would eventually result in an ischemia‐mediated reduction in Na+/K+‐ATPase activity followed by detectable changes in Na+17. In addition, other factors, eg, reduced phospolamban phosphorylation or a leakage of the ryanodine receptor may play a role in our findings.
Therapeutic Implications
Because higher HRs are associated with lower filling pressures in patients with HFpEF, it is possible that higher HRs convey a clinical benefit without raising BP and without adverse effects from higher cellular Ca2+ levels. 14 , 36 , 37 Higher HRs may also benefit patients with AF as chronic diastolic dysfunction with elevated filling pressures is an important driver of this arrhythmia. 38 , 39 However, it remains to be determined whether HR elevation within a clinically acceptable range results in meaningful benefit in patients with HFpEF.
Conversely, we recently demonstrated that pharmacological HR lowering with β‐blockers in patients with HFpEF is associated with higher natriuretic peptide levels and more HF admissions. 40 , 41 The results of the current study provide additional key evidence challenging this paradigm.
Limitations
Our study was relatively small but attempts to characterize previous in vitro findings in vivo. Sampling from the coronary sinus following HR manipulations is an indirect measure of myocardial ion handling. The required duration of pacing and to reach steady state before serum sampling has not been validated. However, this approach can be safely accomplished in humans and has been evaluated for its ability to quantify the release of cardiomyocyte metabolites, which are otherwise undetectable if sampled peripherally. 42 Our study population included only patients with HFpEF who had hypertensive heart disease and paroxysmal AF with predominant obesity. As such, both the presence of obesity and AF could be confounders for symptomatic dyspnea used as enrollment criteria. Likewise, these results may not apply to other HFpEF phenotypes.
Sources of Funding
This research was supported by National Institutes of Health grants R01 HL‐118524 (M. LeWinter) and R01 HL‐122744 (M. Meyer).
Disclosures
UVM and Dr Meyer have licensed patents for the use of pacemakers for the prevention and treatment of HFpEF. The remaining authors have no disclosures to report.
Acknowledgments
We would like to thank the patients for their willingness to participate in this research and the electrophysiology laboratory staff for accommodating the investigators. We also acknowledge the advice and guidance from Peter Callas, PhD, from the UVM Biomedical Statistics Research Core.
(J Am Heart Assoc. 2020;9:e017215 DOI: 10.1161/JAHA.120.017215.)
For Sources of Funding and Disclosures, see page 11.
References
- 1. Reddy YN, Obokata M, Gersh BJ, Borlaug BA. High prevalence of occult heart failure with preserved ejection fraction among patients with atrial fibrillation and dyspnea. Circulation. 2018;137:534–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Messerli FH, Rimoldi SF, Bangalore S. The transition from hypertension to heart failure: contemporary update. JACC Heart failure. 2017;5:543–551. [DOI] [PubMed] [Google Scholar]
- 3. Topol EJ, Traill TA, Fortuin NJ. Hypertensive hypertrophic cardiomyopathy of the elderly. New Engl J Med. 1985;312:277–283. [DOI] [PubMed] [Google Scholar]
- 4. Hay I, Rich J, Ferber P, Burkhoff D, Maurer MS. Role of impaired myocardial relaxation in the production of elevated left ventricular filling pressure. Am J Physiol Heart Circ Physiol. 2005;288:H1203–H1208. [DOI] [PubMed] [Google Scholar]
- 5. Solomon SD, Rizkala AR, Lefkowitz MP, Shi VC, Gong J, Anavekar N, Anker SD, Arango JL, Arenas JL, Atar D, et al. Baseline characteristics of patients with heart failure and preserved ejection fraction in the PARAGON-HF Trial. Circ Heart Fail. 2018;11:e004962. [DOI] [PubMed] [Google Scholar]
- 6. Braunwald E. The control of ventricular function in man. Br Heart J. 1965;27:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harrison DC, Goldblatt A, Braunwald E, Glick G, Mason DT. Studies on cardiac dimensions in intact, unanesthetized man. I. description of techniques and their validation. II. effects of respiration. III. effects of muscular exercise. Circ Res. 1963;13:448–467. [DOI] [PubMed] [Google Scholar]
- 8. Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58:281–291. [DOI] [PubMed] [Google Scholar]
- 9. Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart (British Cardiac Society). 2011;97:964–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. [DOI] [PubMed] [Google Scholar]
- 11. Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Selby DE, Palmer BM, LeWinter MM, Meyer M. Tachycardia-induced diastolic dysfunction and resting tone in myocardium from patients with a normal ejection fraction. J Am Coll Cardiol. 2011;58:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Runte KE, Bell SP, Selby DE, Haussler TN, Ashikaga T, LeWinter MM, Palmer BM, Meyer M. Relaxation and the role of calcium in isolated contracting myocardium from patients with hypertensive heart disease and heart failure with preserved ejection fraction. Circ Heart Fail. 2017;10:e004311 10.1161/CIRCHEARTFAILURE.117.004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klein FJ, Bell S, Runte KE, Lobel R, Ashikaga T, Lerman LO, LeWinter MM, Meyer M. Heart rate-induced modifications of concentric left ventricular hypertrophy: exploration of a novel therapeutic concept. Am J Physiol-Heart Circ Physiol. 2016;311:H1031–H1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zile MR, Gaasch WH. Abnormal calcium homeostasis: one mechanism in diastolic heart failure. J Am Coll Cardiol. 2011;58:155–157. [DOI] [PubMed] [Google Scholar]
- 16. Bers DM, Despa S, Bossuyt J. Regulation of Ca2+ and Na+ in normal and failing cardiac myocytes. Ann NY Acad Sci. 2006;1080:165–177. [DOI] [PubMed] [Google Scholar]
- 17. Eisner DA, Caldwell JL, Trafford AW, Hutchings DC. The control of diastolic calcium in the heart: basic mechanisms and functional implications. Circ Res. 2020;126:395–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bers DM, Barry WH, Despa S. Intracellular Na+ regulation in cardiac myocytes. Cardiovasc Res. 2003;57:897–912. [DOI] [PubMed] [Google Scholar]
- 19. Meyer M, LeWinter MM. Heart rate and heart failure with preserved ejection fraction. Circ Heart Fail. 2019;12:e006213 10.1161/CIRCHEARTFAILURE.119.006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meyer M, McEntee RK, Nyotowidjojo I, Chu G, LeWinter MM. Relationship of exercise capacity and left ventricular dimensions in patients with a normal ejection fraction. An exploratory study. PLoS One. 2015;10:e0119432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocard. 2015;28(1–39):e14. [DOI] [PubMed] [Google Scholar]
- 23. Vrublevsky AV, Boshchenko AA, Karpov RS. Simultaneous transesophageal Doppler assessment of coronary flow reserve in the left anterior descending artery and coronary sinus allows differentiation between proximal and non-proximal left anterior descending artery stenoses. Eur J Echocardiogr. 2004;5:25–33. [DOI] [PubMed] [Google Scholar]
- 24. Bowditch H. Uber die Eigenthumlichkeiten der Reizbarkeit, welche die Muskelfasern des Herzens zeigen. Arb Physiol Anstalt Leipzig. 1971;6:139–176. [Google Scholar]
- 25. Mulieri LA, Hasenfuss G, Leavitt B, Allen PD, Alpert NR. Altered myocardial force-frequency relation in human heart failure. Circulation. 1992;85:1743–1750. [DOI] [PubMed] [Google Scholar]
- 26. Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, Holubarsch C, Posival H, Just H, Drexler H. Relation between myocardial function and expression of sarcoplasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circ Res. 1994;75:434–442. [DOI] [PubMed] [Google Scholar]
- 27. Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, Prestle J, Minami K, Just H. Relationship between Na+-Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation. 1999;99:641–648. [DOI] [PubMed] [Google Scholar]
- 28. Meyer M, Keweloh B, Guth K, Holmes JW, Pieske B, Lehnart SE, Just H, Hasenfuss G. Frequency‐dependence of myocardial energetics in failing human myocardium as quantified by a new method for the measurement of oxygen consumption in muscle strip preparations. J Mol Cell Cardiol. 1998;30:1459–1470. [DOI] [PubMed] [Google Scholar]
- 29. Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Boston, MA: Kluwer Academic; 2001. [Google Scholar]
- 30. Meyer M, Bluhm WF, He H, Post SR, Giordano FJ, Lew WY, Dillmann WH. Phospholamban-to-SERCA2 ratio controls the force-frequency relationship. Am J Physiol. 1999;276:H779–H785. [DOI] [PubMed] [Google Scholar]
- 31. Bluhm WF, Kranias EG, Dillmann WH, Meyer M. Phospholamban: a major determinant of the cardiac force-frequency relationship. Am J Physiol Heart Circ Physiol. 2000;278:H249–H255. [DOI] [PubMed] [Google Scholar]
- 32. Bell SP, Nyland L, Tischler MD, McNabb M, Granzier H, LeWinter MM. Alterations in the determinants of diastolic suction during pacing tachycardia. Circ Res. 2000;87:235–240. [DOI] [PubMed] [Google Scholar]
- 33. Donaldson C, Palmer BM, Zile M, Maughan DW, Ikonomidis JS, Granzier H, Meyer M, VanBuren P, LeWinter MM. Myosin cross‐bridge dynamics in patients with hypertension and concentric left ventricular remodeling. Circ Heart Fail. 2012;5:803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, Van Buren P, Meyer M, et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation. 2015;131:1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pieske B, Maier LS, Piacentino V 3rd, Weisser J, Hasenfuss G, Houser S. Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. Circulation. 2002;106:447–453. [DOI] [PubMed] [Google Scholar]
- 36. Yeshwant SC, Zile MR, Lewis MR, Lewinter M, Meyer M. Safety and feasibility of a nocturnal heart rate elevation-exploration of a novel treatment concept. J Cardiac Fail. 2019;25:67–71. [DOI] [PubMed] [Google Scholar]
- 37. Wahlberg K, Arnold ME, Lustgarten D, Meyer M. Effects of a higher heart rate on quality of life and functional capacity in patients with left ventricular diastolic dysfunction. Am J Cardiol. 2019;124:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goff ZD, Laczay B, Yenokyan G, Sivasambu B, Sinha SK, Marine JE, Ashikaga H, Berger RD, Akhtar T, Spragg DD, et al. Heart rate increase after pulmonary vein isolation predicts freedom from atrial fibrillation at 1 year. J Cardiovasc Electrophysiol. 2019;30:2818–2822. [DOI] [PubMed] [Google Scholar]
- 39. Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, Hillege HL, Bergsma-Kadijk JA, Cornel JH, Kamp O, et al. Lenient versus strict rate control in patients with atrial fibrillation. New Eng J Med. 2010;362:1363–1373. [DOI] [PubMed] [Google Scholar]
- 40. Nambiar L, Silverman D, Vanburen P, LeWinter M, Meyer M. Beta‐blocker cessation in stable outpatients with heart failure with a preserved ejection fraction. J Cardiac Fail. 2019;26:281–282. [DOI] [PubMed] [Google Scholar]
- 41. Silverman DN, Plante TB, Infeld M, Callas PW, Juraschek SP, Dougherty GB, Meyer M. Association of beta‐blocker use with heart failure hospitalizations and cardiovascular disease mortality among patients with heart failure with a preserved ejection fraction: a secondary analysis of the TOPCAT trial. JAMA Netw Open. 2019;2:e1916598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Truong QA, Januzzi JL, Szymonifka J, Thai WE, Wai B, Lavender Z, Sharma U, Sandoval RM, Grunau ZS, Basnet S, et al. Coronary sinus biomarker sampling compared to peripheral venous blood for predicting outcomes in patients with severe heart failure undergoing cardiac resynchronization therapy: the BIOCRT study. Heart Rhythm. 2014;11:2167–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]


