Abstract
Background
Decreased extracellular matrix formation and few vascular smooth muscle cells (VSMCs) in cerebral vascular walls are the main characteristics of intracranial aneurysm (IA) pathogenesis. Recently, osteoprotegerin was reported to activate collagen biosynthesis and VSMC proliferation via the TGF‐β1 (transforming growth factor‐β1) signaling. This study aimed to investigate whether osteoprotegerin can prevent IA progression in rats through enhanced collagen expression and VSMC proliferation.
Methods and Results
IAs were surgically induced in 7‐week‐old male Sprague–Dawley rats; at 1‐week post‐operation, recombinant mouse osteoprotegerin or vehicle control was continuously infused for 4 weeks into the lateral ventricle using an osmotic pump. In the osteoprotegerin‐treatment group, the aneurysmal size was significantly smaller (37.5 μm versus 60.0 μm; P<0.01) and the media of IA walls was thicker (57.1% versus 36.0%; P<0.01) than in the vehicle‐control group. Type‐I and type‐III collagen, TGF‐β1, phosphorylated Smad2/3, and proliferating cell nuclear antigen were significantly upregulated in the IA walls of the osteoprotegerin group than that in the control group. No significant difference was found in the expression of proinflammatory genes between the groups. In mouse VSMC cultures, osteoprotegerin treatment upregulated the expression of collagen and TGF‐β1 genes, and activated VSMC proliferation; the inhibition of TGF‐β1 signaling nullified this effect.
Conclusions
Osteoprotegerin suppressed the IA progression by a unique mechanism whereby collagen biosynthesis and VSMC proliferation were activated via TGF‐β1 without altering proinflammatory gene expression. Osteoprotegerin may represent a novel therapeutic target for IAs.
Keywords: cell proliferation, collagen, intracranial aneurysm, osteoprotegerin, transforming growth factor‐β1, vascular smooth muscle cell
Subject Categories: Cerebral Aneurysm
Nonstandard Abbreviations and Acronyms
- α‐SMA
α‐smooth muscle actin
- AAA
abdominal aortic aneurysm
- CSF
cerebrospinal fluid
- IA
intracranial aneurysm
- PCNA
proliferating cell nuclear antigen
- qRT‐PCR
quantitative reverse transcription polymerase chain reaction
- TGF‐β1
transforming growth factor‐β1
- TRAIL
TNF‐related apoptosis‐inducing ligand
- VSMC
vascular smooth muscle cell
Clinical Perspective
What Is New?
This study provides novel in vivo and in vitro evidence suggesting that osteoprotegerin administration may prevent intracranial aneurysm (IA) progression.
Osteoprotegerin may protects against IA progression in the unique mechanism whereby activating collagen biosynthesis and vascular smooth muscle cells proliferation via transforming growth factor‐β1 without altering proinflammatory gene expression.
What Are the Clinical Implications?
Using experimentally induced IAs in rat, this study showed the potential contribution of osteoprotegerin to the pathogenesis of IA.
These novel findings about osteoprotegerin provide insight into IA pathogenesis and on the pathways that are crucial for protective therapies against IA in human.
Intracranial aneurysms (IAs) are the major cause of devastating subarachnoid hemorrhages and are a relatively common disease with a prevalence ranging from 1% to 5%. 1 Although IAs >7 mm in size are more prone to rupture, 2 the high prevalence of IAs and the life‐threatening severity of the resulting subarachnoid hemorrhages indicate that treating unruptured IAs is of utmost importance. Although surgical clipping and endovascular coil embolization are well‐established therapies for IAs, 1 non‐invasive medical treatments that reduce the risk of IA development and rupture have not been developed, especially for patients that are at high risk for intervention.
The prominent histological features of IAs are mainly described as the thinning extracellular matrix and the few vascular smooth muscle cells (VSMCs) in cerebral vascular walls. 3 , 4 , 5 Recently, we described the transcription factor nuclear factor‐κB, which is a pivotal mediator given its transcriptional regulation of various proinflammatory genes in IA walls in response to chronic stimulation via hemodynamic stress. 6 , 7 We further found that collagen biosynthesis in IA walls was significantly inhibited because of upregulated interleukin‐1β expression and nuclear factor‐κB pathway activation. 8 Furthermore, expression of inflammatory cytokines such as interleukin‐1β and tumor necrosis factor‐α, can promote VSMC loss in IA walls. 3 , 4 , 5 However, the pathophysiology of IA formation, development, and rupture remain poorly elucidated.
Osteoprotegerin, a member of the tumor necrosis factor receptor superfamily, was identified in 1997 as an essential regulator of bone metabolism. 9 This soluble glycoprotein is broadly expressed in various tissues, such as the bone and vasculature, including VSMCs and endothelial cells. 10 , 11 In recent years, numerous studies have revealed that osteoprotegerin is significantly associated with several vascular diseases, such as atherosclerosis, 12 vascular calcification, 13 and abdominal aortic aneurysm. 14 Moreover, osteoprotegerin can enhance the expression of collagen genes via TGF‐β1 (transforming growth factor‐β1) and promote VSMC proliferation in vivo and in vitro. 15 , 16 Despite these accumulating findings, the functional correlation between osteoprotegerin and IAs is unclear. However, a few patients diagnosed with Juvenile Paget Disease (a rare disorder involving homozygous mutations of TNFRSF11B, which encodes osteoprotegerin) have been reported to have bilateral giant IAs. 17 , 18
Based on these findings, we hypothesized that osteoprotegerin treatment could prevent IA progression, thereby promoting collagen biosynthesis and VSMC proliferation in IA walls via TGF‐β1 signaling. Thus, in the present study, we examined the effects of osteoprotegerin on collagen biosynthesis and VSMC proliferation in IA walls using rats with experimentally induced IA and primary mouse aortic VSMC cultures.
Methods
All animal experiments were approved by the institutional animal care and use committees and the ethics committee of Kyoto University, and were conducted in accordance with the guidelines of Japan's Act on Welfare and Management of Animals. This study is reported in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The authors (M.M. and H.K.) declare that all supporting data are available within the article and its online supplementary files.
Induction of Experimental IAs in Rats
All surgical procedures were performed under local anesthesia (dexmedetomidine, 0.15 mg/kg, Nippon Zenyaku Kogyo, Fukushima, Japan; midazolam, 2 mg/kg, Astellas Pharma, Tokyo, Japan and butorphanol, 2.5 mg/kg, Meiji Seika Pharma, Tokyo, Japan; intraperitoneal administration). Maximum efforts were made to minimize suffering. IAs were induced in rats as previously described. 19 Briefly, to induce hypertension, the left renal artery and the left common carotid artery were ligated at the same time using 10‐0 nylon in 7‐week‐old male Sprague–Dawley rats (Japan SLC, Shizuoka, Japan; n=86, Figure 1A). Sham‐operated rats (n=6) underwent this surgery without any ligation. Rats were fed a high‐salt diet containing 8% sodium chloride (F‐2, Oriental Yeast, Tokyo, Japan) and 0.12% β‐aminopropionitrile (Tokyo Chemical, Tokyo, Japan), an inhibitor of lysyl oxidase. The blood pressure of each rat was measured 3 times at each time point, using a tail‐cuff blood pressure‐analysis system (BP‐98A; Softron, Tokyo, Japan) without anesthesia, and the average values were recorded.
Figure 1. Experimental procedures, systemic blood pressure, and osteoprotegerin concentrations in rat cerebrospinal fluid.
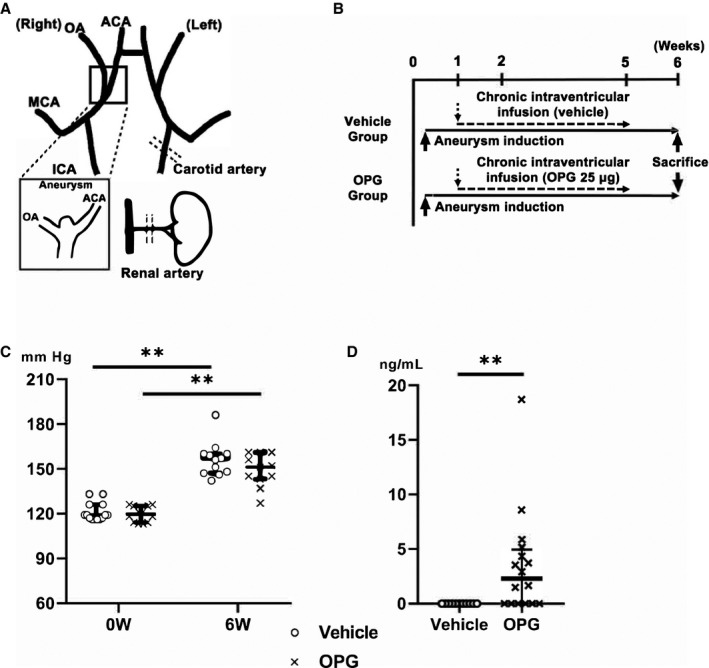
A, Schematic representation of surgical procedures. B, Timeline of the experiments. C, Systemic blood pressure of rats. 0 W: the time before aneurysm induction, 6 W: 6 weeks, the time when the animals were euthanized. D, The concentration of recombinant mouse osteoprotegerin in rat cerebrospinal fluid at 6 weeks post‐aneurysm induction. The results are presented as the median±interquartile range for each group (n=13 in the vehicle group, n=12 in the osteoprotegerin group). ACA indicates anterior cerebral artery; ICA, internal carotid artery; MCA, middle cerebral artery; and OA, olfactory artery. **P<0.01 vs the vehicle group, as determined using the Mann–Whitney U test or Wilcoxon signed‐rank test.
Osmotic Minipump Implantation
Following the timeline for the protocol, rats were anesthetized 5 days after aneurysm‐induction surgery was performed (Figure 1B). Animals were randomly assigned to the osteoprotegerin (n=40) or vehicle (n=40) group. We placed the rats in a stereotaxic frame (SR‐5M‐HT and RA‐6N instruments; Narishige, Tokyo, Japan), shaved their skulls, and drilled a burr hole. Osmotic pumps (Alzet Osmotic Pumps, #2004, 28‐day delivery duration, 0.5 μL/h, Durect, Cupertino, CA, USA) were loaded with 200‐µL PBS (Takara Bio, Kusatsu, Japan) containing 125 μg/mL of recombinant mouse osteoprotegerin (#459‐MO, R&D Systems, Minneapolis, MN) or vehicle (200 μL PBS containing 2% BSA, Sigma‐Aldrich, Tokyo, Japan). The osteoprotegerin dose was based on a previous study on mice. 20 The weight of each rat brain was estimated as 150 to 200 times lower than the corresponding rat's body weight. The pumps were implanted subcutaneously on the back of each animal and were connected via a cannula (Alzet Brain Infusion Kit 2, Durect) and inserted into the lateral ventricle (stereotaxic coordinates of bregma: anteroposterior: 0.8 mm, mediolateral: −1.8 mm [both from bregma], dorsoventral: 4 mm below the skull surface).
ELISA Experiments
Rat cerebrospinal fluid (CSF) samples were collected from the cisterna magna just before euthanasia at 6 weeks post‐IA induction. Recombinant mouse osteoprotegerin concentrations in rat CSF samples were assessed with a sandwich‐type ELISA kit (#KR1020, Immunodiagnostik AG, Bensheim, Germany), following the manufacturer's instructions. All measurements were performed in duplicate.
Evaluation of Morphological IA Changes
Six weeks after IA induction, the rats were deeply anesthetized, euthanized, and perfused transcardially with 4% paraformaldehyde (Wako Pure Chemical Industries, Osaka, Japan). The right anterior cerebral artery/right olfactory artery bifurcation was stripped, and then dipped and frozen in optimal cutting temperature compound (Sakura Finetek Japan, Tokyo, Japan) at −80°C. To analyze the morphological changes in aneurysmal walls, serial 5‐μm frozen sections were prepared with a Cryostat CM 1860 (Leica, Wetzlar, Germany). After Elastica van Gieson staining (Muto Pure Chemicals, Tokyo, Japan), the sections were observed under a light microscope (BZ‐8100, Keyence, Osaka, Japan) to measure the aneurysm size, the media thickness, and the luminal area of the aneurysms. Aneurysm sizes were calculated as the mean of the maximal longitudinal diameter and the maximal transverse diameter of the IAs. The sizes of internal elastic lamina disruption were calculated as the mean of end‐to‐end dimension of degenerated internal elastic lamina plus length of perpendicular line from top of the aneurysm. 21 Aneurysm areas were measured using BZ software (BZ‐II analyzer, Keyence). The media‐thickness ratio was calculated as the ratio of the minimal thickness of media in the IA walls to the thickness of media in the normal arterial walls, near the IAs (Figure 2A).
Figure 2. Chronic intraventricular osteoprotegerin infusion prevented intracranial aneurysm progression.
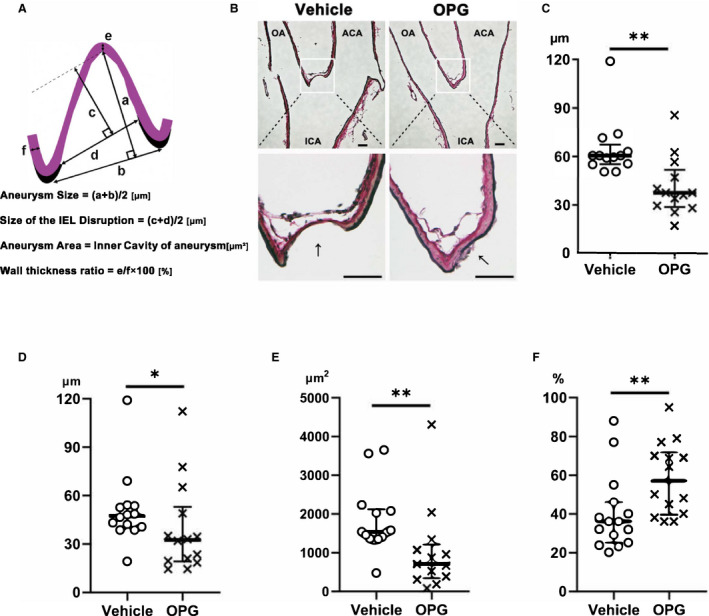
A, Schematic representation of the methods used to measure intracranial aneurysms. B, Representative images of intracranial aneurysms walls observed by Elastica van Gieson staining in rats after vehicle (left) or osteoprotegerin (right) treatment. Morphological measurements of (C) aneurysm size, (D) size of the internal elastic lamina disruption, (E) media‐thickness ratio and (F) aneurysm area. Scale bar=50 μm. The results are presented as the median±interquartile range for each group (n=15 in the vehicle group, n=14 in the osteoprotegerin group). ACA indicates anterior cerebral artery; ICA, internal carotid artery; IEL, internal elastic lamina; OA, olfactory artery; and OPG, osteoprotegerin. **P<0.01 or *P<0.05 vs the vehicle group, as determined using the Mann–Whitney U test.
Immunohistochemistry
Proliferating cell nuclear antigen (PCNA) epitopes were retrieved using sodium citrate buffer (pH 6.0) at 80°C for 5 minutes. After blocking with 2% BSA, 5‐μm frozen sections were incubated with primary antibodies overnight at 4°C, followed by incubation for 2 hours at room temperature (15–25°C) with Alexa Fluor 488‐ and 594‐labeled secondary antibodies (BioLegend, San Diego, CA, USA). Slides covered with Prolong Gold antifade reagent with DAPI (Invitrogen, Waltham, MA, USA) were observed using a fluorescence confocal microscope (FV1000‐IX81, FV10‐ASW version 4.2; Olympus, Tokyo, Japan) with an objective lens (PLANAPO N 60x, NA:1.42, Olympus). Please see Table S1 for a description of the primary antibodies used in the present study.
Cell Counting
Macrophages and VSMCs were defined by immunohistochemistry as ionized calcium‐binding adapter molecule 1 (a macrophage marker 22 , 23 )‐positive and α‐smooth muscle actin (α‐SMA)‐positive cells, respectively. Macrophages infiltrating into IA walls were counted within a 100‐μm‐square field around the dome of induced IAs chosen from 5 to 15 sections of cerebral arteries per individual animals. The PCNA‐ and α‐SMA‐positive cells in the media of IA walls were counted in the same manner. The ratio of α‐SMA/PCNA double‐positive cells to α‐SMA‐positive cells was also calculated. Cells were counted 3 times at each time point and the average values were recorded.
In Situ DNA Fragmentation Assay
Terminal deoxynucleotidyl transferase‐mediated dUTP‐biotin nick end labeling assays were performed using VasoTACS In Situ Apoptosis Detection kit (R&D Systems, Minneapolis, MN) according to Manufacturer's instructions. The sections were observed under a light microscope (BZ‐8100, Keyence). Apoptotic cells were quantified as blue stained cells within a 100‐μm‐square field around the dome of induced IAs chosen from 5 to 15 sections of cerebral arteries per individual animals.
RNA Isolation and Quantitative Reverse Transcription‐Polymerase Chain Reaction Analysis
RNA was extracted from whole rat Willis arterial rings or cultured cells using an RNeasy Micro Kit (Qiagen, Hilden, Germany). The cDNA was transcribed using a High‐Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA), following the manufacturer's instructions. To quantify gene expression, a quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) was performed using the Applied Biosystems 7300 Real‐Time PCR System (Applied Biosystems, Waltham, MA, USA) and THUNDERBIRD SYBR qPCR Mix (TOYOBO, Osaka, Japan), with β‐actin expression as the internal control. The qRT‐PCR was performed in 40 cycles of 95°C at 15 seconds for denaturation and 60°C at 60 seconds for extension. Quantitative expression was acquired from the cycle threshold value, and the data were calculated by the comparative cycle threshold method. The primer sets used in these experiments are described in Table S2.
Cell Culture and Treatment
Primary mouse aortic VSMCs isolated from the aorta of wild‐type C57/BL6 mice (Japan SLC, Shizuoka, Japan) using collagenase (Wako Pure Chemical Industries) were incubated in DMEM (Wako Pure Chemical Industries) containing 10% fetal bovine serum (Biosera, Nuaillé, France) at 37°C and 5% CO2. After 12 hours of serum starvation, 1 ng/mL of lipopolysaccharide (Wako Pure Chemical Industries), with or without 100 ng/mL mouse recombinant osteoprotegerin, was added to the medium and incubated for 12 hours in the presence or absence of SB431542 (Cayman Chemicals, Ann Arbor, MI, USA)—a selective TGF‐β receptor I inhibitor that specifically blocks Smad signaling. The cells used for all experiments were subcultured for 3 to 6 passages.
Cell‐Proliferation Assay
Cell proliferation was analyzed using the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Fitchburg, WI, USA), following the manufacturer's instructions. Cell suspensions (100 μL/well) were seeded in a 96‐well plate. After 12 hours of serum starvation, vehicle or mouse recombinant osteoprotegerin (100 ng/mL) was added to medium with or without SB431542, and the cells were incubated for 24, 36, or 48 hours.
Statistical Analysis
Barring the data on mRNA levels, the experimental data have been expressed in terms of median±interquartile range (IQR). The quantitative mRNA expression levels have been expressed in terms of mean±SD. As the experimental sample size for the 2‐group comparisons was small and the continuous variables may not have followed normal distribution, non‐parametric Mann–Whitney U test or Wilcoxon signed rank test were performed. Differences among multiple groups were analyzed by Kruskal–Wallis 1‐way ANOVA, followed by a Tukey–Kramer post‐hoc test. P<0.05 was considered to reflect statistical significance. All statistical analyses were performed using JMP 14 software (SAS Institute Inc., Cary, NC, USA).
Results
Osteoprotegerin Expression in IA Walls
To investigate the role of osteoprotegerin in IA development, we measured its expression in IA walls. According to the immunohistochemistry results, osteoprotegerin was more strongly expressed in IA walls of rats that underwent aneurysm‐induction surgery than in the cerebral arterial walls of sham‐operated rats (Figure S1A). In particular, osteoprotegerin was mainly expressed in the VSMCs of IA walls. The qRT‐PCR analysis revealed that osteoprotegerin mRNA expression was significantly upregulated in IA walls compared with that in normal cerebral arterial walls of sham‐operated rats at 6 weeks after aneurysm induction (P<0.01, n=5, respectively; Figure S1B).
Blood Pressures, Mouse Recombinant Osteoprotegerin Concentrations in Rat CSF, and Effects of Chronic Osteoprotegerin Intraventricular Infusion on IA Progression
To confirm the involvement of osteoprotegerin in IA pathogenesis, we evaluated the effects of chronic intraventricular osteoprotegerin infusion on IA development in rats. We used mouse recombinant osteoprotegerin for rats. Mouse osteoprotegerin shares 94% and 86% amino acid sequence identity with rat and human osteoprotegerin, respectively. Moreover, many previous studies have concluded that human osteoprotegerin is effective in various experimental rat or mouse models. 24 , 25 At 6 weeks post‐aneurysm induction, no differences in blood pressure were observed (osteoprotegerin group, 151 mm Hg [IQR, 143–161 mm Hg], n=13; vehicle group, 157 mm Hg [IQR, 147–160 mm Hg], n=14; Figure 1C). Mouse osteoprotegerin was assessed by ELISA to investigate whether the osteoprotegerin concentration was maintained in rat CSF in the osteoprotegerin group. Mouse osteoprotegerin concentrations in rat CSF samples from the osteoprotegerin group were significantly higher than in those from the vehicle group (osteoprotegerin group, 3.2 ng/mL [IQR, 0.01–5.3 ng/mL], n=13; vehicle group, 0 ng/mL, n=11; P<0.01; Figure 1D).
Aneurysms were significantly smaller in the osteoprotegerin group than in the vehicle group (osteoprotegerin group, 37.5 μm [IQR, 28.8–51.8 µm], n=13; vehicle group, 60 μm [IQR, 53.9–65.1 µm], n=14; P<0.01; Figure 2C). The sizes of internal elastic lamina disruption were significantly smaller in the osteoprotegerin group than in the vehicle group (osteoprotegerin group, 32.5 μm [IQR, 19.3–53 µm], n=13; vehicle group, 47.3 μm [IQR, 40–53.6 µm], n=14; P<0.05; Figure 2D). The aneurysm areas were significantly narrower in the osteoprotegerin group than in the vehicle group (osteoprotegerin group, 700 μm2 [IQR, 342–1204 µm2] n=13; vehicle group, 1526 μm2 [IQR, 1352–2112 µm2] n=14; P<0.01; Figure 2E). The media‐thickness ratio was significantly higher in the osteoprotegerin group than in the vehicle group (osteoprotegerin group, 57.1% [IQR, 39.5%–71.8%], n=13; vehicle group, 36% [IQR, 25%–46%]; n=14; P<0.01; Figure 2F). No ruptured aneurysms at the right anterior cerebral artery/right olfactory artery bifurcation were found between both groups.
Effects of Osteoprotegerin on Type‐I and Type‐III Collagen, and Phosphorylated Smad2/3 Expression in Rat IA Walls
To determine whether osteoprotegerin affects collagen gene expression and TGF‐β1 signaling in IA walls, we investigated type‐I and type‐III collagen, TGF‐β1, and phosphorylated Smad2/3 levels in IA walls. Type‐I and type‐III collagen and phosphorylated Smad2/3 were upregulated in rat IA walls in the osteoprotegerin group versus the vehicle group, as determined by immunohistochemistry (Figure 3A). The qRT‐PCR showed that type‐I and type‐III collagen, and TGF‐β1‐mRNA expression in rat IA walls in the osteoprotegerin group was significantly increased versus that in the vehicle group (type‐I collagen, P=0.028, n=14; type‐III collagen, P=0.034, n=14; TGF‐β1, P=0.014, n=14; Figure 3B through 3D).
Figure 3. Histological and mRNA expression analyses of intracranial aneurysms demonstrating that osteoprotegerin promoted collagen expression and transforming growth factor‐β1‐Smad2/3 signaling in intracranial aneurysm walls.
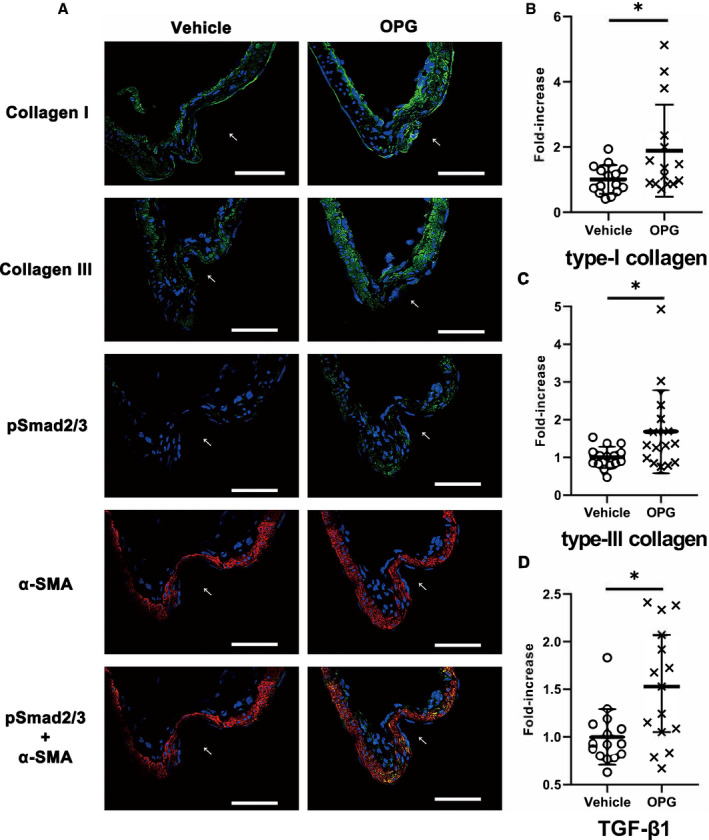
A, Immunohistochemical staining of type‐I and type‐III collagen (green), phosphorylated Smad2/3 (green), and α‐smooth muscle actin (red) in intracranial aneurysm walls (left, vehicle treatment; right, osteoprotegerin treatment). All samples were co‐stained with DAPI (blue). The arrows indicate the luminal sides of intracranial aneurysms. Scale bar: 50 μm. Type‐I collagen (B), type‐III collagen (C), and transforming growth factor‐β1 (D) mRNA‐expression levels were evaluated by quantitative reverse transcription‐polymerase chain reaction analysis, and the data were normalized to β‐actin expression. Expression levels are presented as fold‐increases over the expression levels in the vehicle group. The results are presented as the mean±SD (n=15) for each group. pSmad2/3 indicates phosphorylated Smad2/3; TGF‐β1, transforming growth factor‐β1; and α‐SMA, α‐smooth muscle actin. *P<0.05 vs the vehicle group, as determined using the Mann–Whitney U test.
Effects of Osteoprotegerin on Cell Proliferation and PCNA Expression in VSMCs in Rat IA Walls
To determine whether osteoprotegerin might promote VSMC proliferation in IA walls, we investigated PCNA and α‐SMA expression. Immunohistochemical staining revealed significantly more α‐SMA/PCNA double‐positive cells in the IA walls of osteoprotegerin‐treated rats than in vehicle‐treated rats (osteoprotegerin group, 54.5% [IQR, 49.3%–57.1%], n=13; vehicle group, 32.5% [IQR, 28.4%–39%], n=14; P<0.01; Figure 4A and 4B).
Figure 4. Histological analysis of intracranial aneurysms demonstrating that osteoprotegerin promoted vascular smooth muscle cell proliferation in intracranial aneurysm walls.
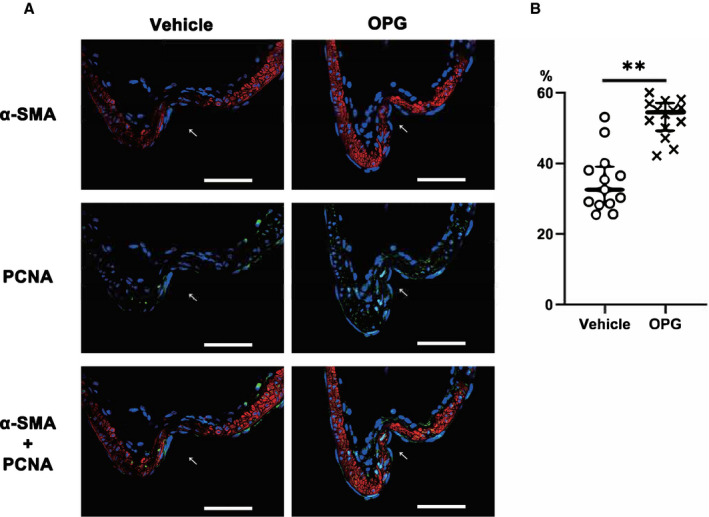
A, Immunohistochemical staining of α‐smooth muscle actin (red) and proliferating cell nuclear antigen (green) in intracranial aneurysm walls (left, vehicle treatment; right, osteoprotegerin treatment). All samples were co‐stained with DAPI (blue). The arrows indicate the luminal sides of the intracranial aneurysms. Scale bar=50 μm. B, The ratios of α‐smooth muscle actin/proliferating cell nuclear antigen double‐positive cells to α‐smooth muscle actin‐positive cells within a 100‐μm2 field around the dome of induced intracranial aneurysms are shown. The results are presented as the median±interquartile range for each group (n=15). PCNA indicates proliferating cell nuclear antigen; and α‐SMA, α‐smooth muscle actin. **P<0.01 vs the vehicle group, as determined using the Mann–Whitney U test.
Effects of Osteoprotegerin on Apoptosis of VSMCs in Rat IA Walls
To determine whether osteoprotegerin might affect apoptotic event in cells within IA walls, we investigated terminal deoxynucleotidyl transferase‐mediated dUTP‐biotin nick end labeling staining. There was no significant difference in apoptotic cells in the IA walls between both groups (Figure S2).
Effects of Osteoprotegerin on Gene Expression Related to Phenotypic Modulation of VSMCs in Rat IA Walls
To determine whether osteoprotegerin might alter the genes expression related to phenotypic modulation of VSMCs, we investigated the expression of α‐SMA, smooth muscle 22 alpha (SM‐22α), embryonic form of myosin heavy chain (SMemb), and Kruppel like factor 4. No significant differences were found in such genes expression between both groups, according to the qRT‐PCR results (Figure S3).
Effects of Osteoprotegerin on Inflammation‐Related Gene Expression in VSMCs in Rat IA Walls
To determine whether osteoprotegerin might alter the expression of inflammation‐related cytokine genes, we investigated the expression of MCP‐1 (monocyte chemotactic protein 1), MMP‐2 (matrix metalloproteinase‐2), MMP‐9 (matrix metalloproteinase‐9) intercellular adhesion molecule 1, and ionized calcium‐binding adapter molecule 1 (a macrophage marker 22 , 23 ) in addition to TRAIL (tumor necrosis factor‐related apoptosis‐inducing ligand) and Syndecan‐1, as molecular partners of osteoprotegerin. 26 , 27 Immunohistochemistry revealed no differences in ionized calcium‐binding adapter molecule 1 expression, or in the number of macrophages infiltrating into the IA walls, between the vehicle and osteoprotegerin groups (Figure 5A and 5B). Similarly, no significant differences were found in MCP‐1, MMP‐2, MMP‐9, intercellular adhesion molecule 1, TRAIL, or Syndecan‐1 mRNA expression between both groups, according to the qRT‐PCR results (Figure 5C through 5H). Although RANKL (receptor activator of nuclear factor kappa‐Β ligand) is known to be a binding partner of osteoprotegerin, we could not detect the significant RANKL mRNA expression in cerebral vessels of rats at all as in previous reports. 28
Figure 5. Histological and mRNA expression analyses of intracranial aneurysms demonstrating that osteoprotegerin did not alter the expression of inflammation‐related genes in intracranial aneurysm walls.
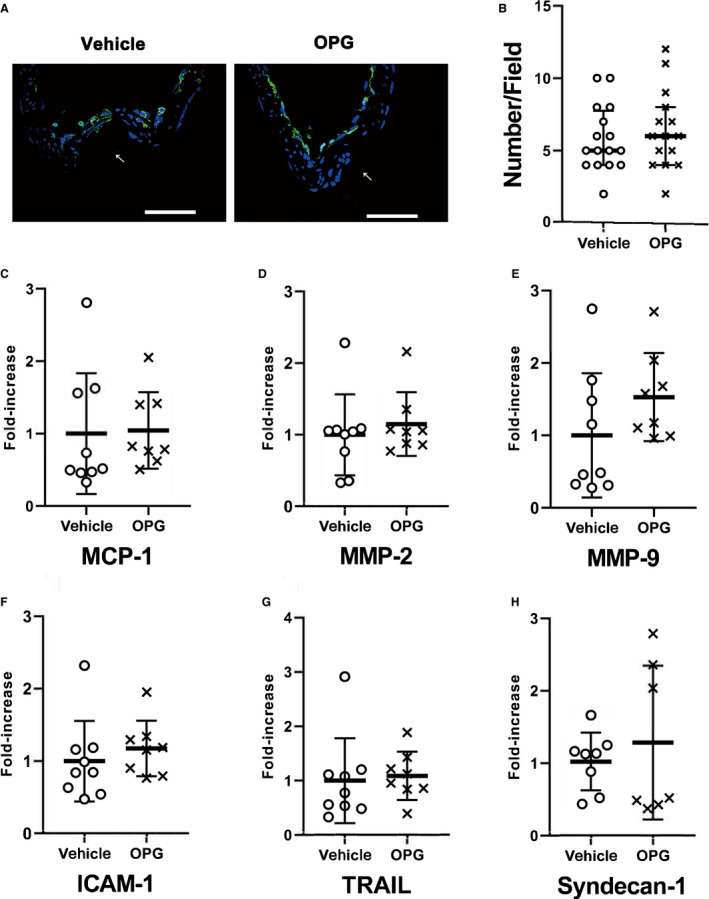
A, Immunohistochemical staining of ionized calcium‐binding adapter molecule 1 in intracranial aneurysm walls (left; vehicle treatment; middle, osteoprotegerin treatment). All samples were co‐stained with DAPI (blue). The arrows indicate the luminal sides of the intracranial aneurysms. Scale bar=50 μm. B, The number of ionized calcium‐binding adapter molecule 1 positive cells infiltrating the intracranial aneurysm walls per 100‐μm2 area (right). The results are presented as the median± interquartile range for each group (n=14 in the vehicle group, n=15 in the osteoprotegerin group). C through H, The mRNA‐expression levels of (C) monocyte chemoattractant protein 1, (D) matrix metalloproteinase‐2, (E) matrix metalloproteinase‐9, (F) intercellular adhesion molecule‐1, (G) tumor necrosis factor‐related apoptosis‐inducing ligand, and (H) Syndecan‐1 were evaluated by quantitative reverse transcription‐polymerase chain reaction analysis, and the data were normalized to β‐actin expression. Expression levels are presented as fold‐increases over the expression levels in the vehicle group. The results are presented as the mean±SD for each group (n=9 in the vehicle group, n=8 in the osteoprotegerin group). The data shown were statistically analyzed using the Mann–Whitney U test. ICAM‐1 indicates intercellular adhesion molecule 1; MCP‐1, monocyte chemotactic protein 1; MMP, matrix metalloproteinase; and TRAIL, tumor necrosis factor‐related apoptosis‐inducing ligand.
Effects of Osteoprotegerin and a TGF‐β1 Inhibitor on Type‐I and Type‐III Collagen, and TGF‐β1 Expression in Mouse Aortic VSMCs In Vitro
To investigate whether osteoprotegerin might upregulate collagen biosynthesis and enhance TGF‐β1 signaling, we evaluated primary mouse aortic VSMCs treated with several combination of lipopolysaccharide (1 ng/mL, 12 hours), recombinant mouse osteoprotegerin (100 ng/mL), and SB431542 (a selective TGF‐β receptor I inhibitor that specifically blocks Smad signaling, 20 μmol/L) because VSMCs have been described as the major source of osteoprotegerin in arterial walls. 11 The qRT‐PCR revealed that mRNA expression of type‐I and ‐III collagen, and TGF‐β1 were significantly upregulated in osteoprotegerin‐treated VSMCs versus vehicle‐treated VSMCs under lipopolysaccharide stimulation (type‐I collagen, P<0.01, n=8; type‐III collagen, P<0.01, n=8; TGF‐β1, P=0.021, n=8; Figure 6A through 6C). Furthermore, SB431542 significantly inhibited such effects of osteoprotegerin on these gene expression levels (lipopolysaccharide+osteoprotegerin group versus lipopolysaccharide+osteoprotegerin+SB435142 group: type‐I collagen, P<0.01, n=8; type‐III collagen, P<0.01, n=8; TGF‐β1, P<0.01, n=8).
Figure 6. mRNA expression and cell proliferation analyses demonstrating that osteoprotegerin stimulated collagen expression in mouse aortic vascular smooth muscle cells via transforming growth factor‐β1 signaling.
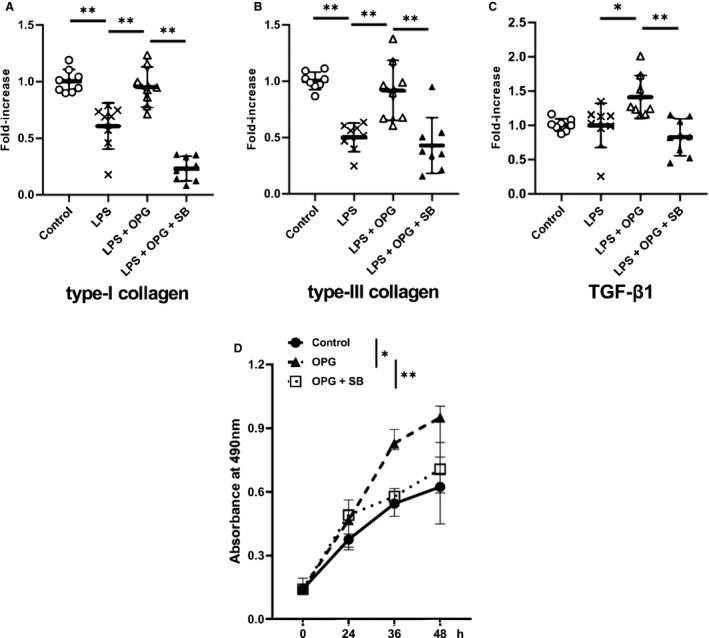
The vascular smooth muscle cells cultures were exposed to lipopolysaccharide (1 ng/mL) for 12 hours, with or without recombinant mouse osteoprotegerin (100 ng/mL), either in the presence or absence of the specific transforming growth factor‐β1 inhibitor, SB431542 (20 μmol/L). The mRNA‐expression levels of (A) type‐I collagen, (B) type‐III collagen, and (C) transforming growth factor‐β1 were evaluated by quantitative reverse transcription‐polymerase chain reaction analysis, and the data were normalized to β‐actin expression. Expression levels are presented as fold‐increases over the control cultures (n=8 for each group). The results are presented as the mean±SD for each group. D, The effect of recombinant osteoprotegerin on mouse vascular smooth muscle cells proliferation (n=7 for each group) was evaluated by performing the cell proliferation assay with or without SB431542 (20 μmol/L). The results are presented as the median±interquartile range for each group. LPS indicates lipopolysaccharide; OPG, osteoprotegerin; SB, SB431542; and TGF‐β1 transforming growth factor‐β1. **P<0.01 or *P<0.05, as determined via Kruskal–Wallis 1‐way ANOVA with the Tukey–Kramer post‐hoc test.
Effects of Osteoprotegerin and a TGF‐β1 Inhibitor on VSMCs Proliferation in Vitro
To investigate whether osteoprotegerin might promote VSMCs proliferation, primary mouse aortic VSMCs were treated with recombinant mouse osteoprotegerin (100 ng/mL, 12 hours) with or without SB431542 (20 μmol/L). The osteoprotegerin treatment significantly increased aortic VSMC proliferation versus vehicle treatment at 48‐hours post‐stimulation (osteoprotegerin group, 0.95 [IQR, 0.76–1.00]; vehicle group, 0.62 [IQR, 0.45–0.69]; n=7; P<0.01). Moreover, SB431542 significantly inhibited such effects of osteoprotegerin on the proliferation of mouse aortic VSMCs (osteoprotegerin group, 0.95 [IQR, 0.76–1.00]; osteoprotegerin+SB431542 group, 0.71 [IQR, 0.60–0.83]; n=7; P=0.04; Figure 6D).
Discussion
In the present study, we tested the hypothesis that osteoprotegerin can prevent IA progression in rats by activating collagen gene expression and VSMC proliferation in IA walls via TGF‐β1 signaling. We demonstrated that chronic intraventricular osteoprotegerin infusion suppressed IA progression in rats and promoted type‐I and type‐III collagen, TGF‐β1, phosphorylated Smad2/3, and PCNA production. Furthermore, osteoprotegerin administration induced the proliferation and expression of type‐I and type‐III collagen, and TGF‐β1 genes in VSMC cultures under the inflammatory stimulation via lipopolysaccharide. These effects of osteoprotegerin were blocked by a TGF‐β receptor I inhibitor. Collectively, these results supported our hypothesis.
Previously, osteoprotegerin knockout augmented abdominal aortic aneurysms (AAAs) in experimental CaCl2‐induced AAA model mice. 29 The main difference in IA pathogenesis in relationship to that of AAAs is that IAs are mainly derived from muscular arteries without external elastic lamina, 30 and tend to arise at arterial bifurcation sites. However, IAs are similar to AAAs in terms of external arterial wall bulging and macrophage infiltration into arterial walls 31 in response to chronic stimulation with inflammatory cytokines. Although a few patients with the rare congenital disorder known as Juvenile Paget Disease (or “osteoprotegerin deficiency”) showed giant bilateral cavernous IAs, 17 , 18 the association between osteoprotegerin and IAs has not been clearly elucidated. To our knowledge, our results represent the first detailed evidence suggesting that osteoprotegerin participates in IA pathogenesis.
Excessive extracellular matrix breakdown by various collagenases in cerebral vessels is widely known to result in IA formation and rupture. 3 , 5 , 8 In vascular collagen remodeling, TGF‐β1 is a key regulator that can stimulate collagen biosynthesis and inhibit collagen degradation through the TGF‐β1‐Smad2/3 signaling pathway in various cardiovascular diseases. 32 However, osteoprotegerin was reported to enhance the arterial expression of collagen‐related genes 33 and induce autocrine TGF‐β1 genes expression. 15 Furthermore, osteoprotegerin stimulation increased vessel wall thickness and collagen contents in CaCl2‐induced AAA model mice. 34 In agreement, our results strengthened the hypothesis that osteoprotegerin prevents IA progression by activating collagen biosynthesis in IA walls via TGF‐β1 signaling.
A prominent feature of IAs is the loss of VSMCs in the media, 3 which is associated with apoptosis, 4 necrosis, 35 , 36 and disrupted cell proliferation 37 caused by chronic inflammation (eg, luminal thrombosis, 35 high oxidative stress, 38 or hemodynamic stress 36 ). Because osteoprotegerin can antagonize TRAIL, 39 which activates apoptosis in various tissues including the vessels, 40 osteoprotegerin small‐interfering RNA or osteoprotegerin knockout induced apoptosis by upregulating TRAIL, in vivo and in vitro. 39 However, in the present study, osteoprotegerin administration did not alter TRAIL gene expression or apoptotic event within cells. Nevertheless, osteoprotegerin has been shown to activate VSMC proliferation through TGF‐β1 signaling 15 or integrin αvβ3/FAK/AKT pathway. 16 Several integrins are known to directly or indirectly activate TGF‐β1 signaling and to induce VSMCs proliferation with the synergistic effect of TGF‐β1. 40 , 41 Thus, osteoprotegerin may attenuate IA progression by inducing VSMC proliferation in IA walls in a TRAIL‐independent manner such as the integrin‐TGF‐β1 pathway; however this needs further investigation.
The correlation between IAs and TGF‐β1 has been controversial. Previous reports have demonstrated that mutations in the main TGF‐β1 signaling related genes (TGF‐β1, TGF‐β receptor I, and TGF‐β receptor II) were not the major causes of familial IAs. 42 , 43 However, patients diagnosed with Marfan syndrome or Loeys‐Dietz syndrome (rare congenital connective tissue diseases related to TGF‐β1 signaling abnormalities) were considered prone to aortic aneurysms and IAs. 44 , 45 In an animal model of IA, it was reported that the inhibition of Ets‐1, which strongly suppresses TGF‐β1‐induced expression of type‐I collagen, 46 prevented IA progression and promoted collagen biosynthesis in IA walls with a synergistic effect of nuclear factor‐κB inhibiton. 7 , 47 Intriguingly, coating platinum coils with TGF‐β1 provided early cellular coverage within experimental aneurysms in rabbits after endovascular treatment. 48 Considering these findings, the present results about the osteoprotegerin‐TGF‐β1 axis may elucidate the unknown mechanism related to IA progression.
Numerous studies have defined IA as a chronic inflammatory disease via hemodynamic stress and reported that various anti‐inflammatory drugs inhibited IA progression in experimental animal models. 5 , 6 , 7 , 21 , 49 , 50 Upregulated expression of osteoprotegerin was found in human with atherosclerosis 12 or AAA, 14 In agreement, osteoprotegerin expression was significantly upregulated in IA walls compared with that in normal cerebral arterial walls in the present study. In contrast, osteoprotegerin administration was reported to stabilize atherosclerotic lesions in hypercholesterolemic ApoE‐knockout mice 33 or to increase vessel wall thickness in CaCl2‐induced AAA model mice 34 without altering the expression levels of inflammation‐related genes. In the present study, osteoprotegerin administration prevented IA progression through a unique mechanism without affecting proinflammatory gene expression.
Despite its breakthrough results, the present study has 3 limitations. First, we did not examine whether osteoprotegerin prevents the incidence of rupture in experimentally induced IAs. Second, recombinant mouse osteoprotegerin was administered intraventricularly, but it cannot be administered to human patients with IAs in the same manner. However, in our induced‐IA model, the histopathological findings of induced IAs were quite similar to those of human IAs, considering that the pathophysiological characteristics of human IAs can be well recapitulated in experimentally induced IAs by renal hypertension and excessive hemodynamic stress, using unilateral ligation of the carotid artery. 5 , 19 Therefore, our findings indirectly shed new light on a poorly understood mechanism on the growth of human IAs. Finally, we did not examine how osteoprotegerin administration affect IA progression in female rats. However, younger women aged <50 years had higher serum osteoprotegerin levels than men. 51 Estrogen, as a well‐known protective factor of IA formation and rupture in humans, can also stimulate osteoprotegerin production. 52 , 53 These findings might be associated with the predisposition to harbor IAs in postmenopausal women although further study is needed.
In conclusion, this is the first study to analyze whether osteoprotegerin administration prevents IA progression. Our findings provide novel in vivo and in vitro evidence that osteoprotegerin induced the expression of genes encoding extracellular matrix proteins and promoted VSMCs proliferation via TGF‐β1‐Smad2/3 signaling in IA walls. In addition, osteoprotegerin administration did not alter the expression levels of inflammatory genes. These novel findings provide insight into IA pathogenesis and on the pathways that are crucial for protective therapies against IA. In the future, further human studies may elucidate the contribution of osteoprotegerin to the pathogenesis of IAs.
Sources of Funding
This work was partially supported by grants from the Japan Society for the Promotion of Science JSPS KAKENHI (grant numbers 26460338 and 17K08592 to Minami and grant number 15H04952 to Kataoka).
Disclosures
None.
Supporting information
Tables S1–S2
Figures S1–S3
Acknowledgments
We thank Dr Tomohiro Aoki (National Cerebral and Cardiovascular Center Research Institute, Suita, Japan) for sharing his expertise in generating experimental intracranial aneurysms in rats.
(J Am Heart Assoc. 2020;9:e015731 DOI: 10.1161/JAHA.119.015731.)
For Sources of Funding and Disclosures, see page 13.
Contributor Information
Manabu Minami, Email: mminami@kuhp.kyoto-u.ac.jp.
Hiroharu Kataoka, Email: kataoka@kuhp.kyoto-u.ac.jp.
References
- 1. Thompson BG, Brown RD, Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES, Duckwiler GR, Harris CC, Howard VJ, Johnston SCC, et al. Guidelines for the management of patients with unruptured intracranial aneurysms. Stroke. 2015;46:2368–2400. [DOI] [PubMed] [Google Scholar]
- 2. Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, Nakayama T, Sakai M, Teramoto A, Tominari S, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366:2474–2482. [DOI] [PubMed] [Google Scholar]
- 3. Kataoka K, Taneda M, Asai T, Kinoshita A, Ito M, Kuroda R. Structural fragility and inflammatory response of ruptured cerebral aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke. 1999;30:1396–1401. [DOI] [PubMed] [Google Scholar]
- 4. Kondo S, Hashimoto N, Kikuchi H, Hazama F, Nagata I, Kataoka H. Apoptosis of medial smooth muscle cells in the development of saccular cerebral aneurysms in rats. Stroke. 1998;29:181–189; discussion 189. [DOI] [PubMed] [Google Scholar]
- 5. Kataoka H. Molecular mechanisms of the formation and progression of intracranial aneurysms. Neurol Med Chir (Tokyo). 2015;55:214–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aoki T, Kataoka H, Shimamura M, Nakagami H, Wakayama K, Moriwaki T, Ishibashi R, Nozaki K, Morishita R, Hashimoto N. NF-kappaB is a key mediator of cerebral aneurysm formation. Circulation. 2007;116:2830–2840. [DOI] [PubMed] [Google Scholar]
- 7. Aoki T, Kataoka H, Nishimura M, Ishibashi R, Morishita R, Miyamoto S. Regression of intracranial aneurysms by simultaneous inhibition of nuclear factor‐κB and Ets with chimeric decoy oligodeoxynucleotide treatment. Neurosurgery. 2012;70:1534–1543; discussion 1543. [DOI] [PubMed] [Google Scholar]
- 8. Aoki T, Kataoka H, Ishibashi R, Nozaki K, Morishita R, Hashimoto N. Reduced collagen biosynthesis is the hallmark of cerebral aneurysm: contribution of interleukin-1beta and nuclear factor‐kappaB. Arterioscler Thromb Vasc Biol. 2009;29:1080–1086. [DOI] [PubMed] [Google Scholar]
- 9. Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. [DOI] [PubMed] [Google Scholar]
- 10. Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Olesen P, Ledet T, Rasmussen LM. Arterial osteoprotegerin: increased amounts in diabetes and modifiable synthesis from vascular smooth muscle cells by insulin and TNF‐alpha. Diabetologia. 2005;48:561–568. [DOI] [PubMed] [Google Scholar]
- 12. Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, Santer P, Smolen J, Poewe W, Willeit J. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109:2175–2180. [DOI] [PubMed] [Google Scholar]
- 13. Morony S, Tintut Y, Zhang Z, Cattley RC, Van G, Dwyer D, Stolina M, Kostenuik PJ, Demer LL. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(-/-) mice. Circulation. 2008;117:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moran CS, McCann M, Karan M, Norman P, Ketheesan N, Golledge J. Association of osteoprotegerin with human abdominal aortic aneurysm progression. Circulation. 2005;111:3119–3125. [DOI] [PubMed] [Google Scholar]
- 15. Toffoli B, Pickering RJ, Tsorotes D, Wang B, Bernardi S, Kantharidis P, Fabris B, Zauli G, Secchiero P, Thomas MC. Osteoprotegerin promotes vascular fibrosis via a TGF‐β1 autocrine loop. Atherosclerosis. 2011;218:61–68. [DOI] [PubMed] [Google Scholar]
- 16. Jia D, Zhu Q, Liu H, Zuo C, He Y, Chen G, Lu A. Osteoprotegerin disruption attenuates HySu-induced pulmonary hypertension through integrin αvβ3/FAK/AKT pathway suppression. Circ Cardiovasc Genet. 2017;10:e001591 10.1161/CIRCGENETICS.116.001591. [DOI] [PubMed] [Google Scholar]
- 17. Allen CA, Hart BL, Taylor CL, Clericuzio CL. Bilateral cavernous internal carotid aneurysms in a child with juvenile paget disease and osteoprotegerin deficiency. AJNR Am J Neuroradiol. 2008;29:7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Polyzos SA, Cundy T, Mantzoros CS. Juvenile Paget disease. Metabolism. 2018;80:15–26. [DOI] [PubMed] [Google Scholar]
- 19. Nagata I, Handa H, Hashimoto N, Hazama F. Experimentally induced cerebral aneurysms in rats: part VI. Hypertension. Surg Neurol. 1980;14:477–479. [PubMed] [Google Scholar]
- 20. Takayama K, Inoue T, Narita S, Maita S, Huang M, Numakura K, Tsuruta H, Saito M, Maeno A, Satoh S, et al. Inhibition of the RANK/RANKL signaling with osteoprotegerin prevents castration-induced acceleration of bone metastasis in castration-insensitive prostate cancer. Cancer Lett. 2017;397:103–110. [DOI] [PubMed] [Google Scholar]
- 21. Ikedo T, Minami M, Kataoka H, Hayashi K, Nagata M, Fujikawa R, Higuchi S, Yasui M, Aoki T, Fukuda M, et al. Dipeptidyl peptidase-4 inhibitor anagliptin prevents intracranial aneurysm growth by suppressing macrophage infiltration and activation. J Am Heart Assoc. 2017;6:e004777 DOI: 10.1161/JAHA.116.004777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tian Y, Kelemen SE, Autieri MV. Inhibition of AIF-1 expression by constitutive siRNA expression reduces macrophage migration, proliferation, and signal transduction initiated by atherogenic stimuli. Am J Physiol Cell Physiol. 2006;290:C1083–C1091. [DOI] [PubMed] [Google Scholar]
- 23. Haga N, Akaihata H, Hata J, Hiraki H, Honda R, Tanji R, Onagi A, Koguchi T, Hoshi S, Ogawa S, et al. The association between local arteriosclerosis of the prostatic arteries and chronic inflammation in human benign prostatic enlargement. Prostate. 2019;79:574–582. [DOI] [PubMed] [Google Scholar]
- 24. Neumann T, Oelzner P, Petrow PK, Thoss K, Hein G, Stein G, Bräuer R. Osteoprotegerin reduces the loss of periarticular bone mass in primary and secondary spongiosa but does not influence inflammation in rat antigen-induced arthritis. Inflamm Res. 2006;55:32–39. [DOI] [PubMed] [Google Scholar]
- 25. Candido R, Toffoli B, Corallini F, Bernardi S, Zella D, Voltan R, Grill V, Celeghini C, Fabris B. Human full-length osteoprotegerin induces the proliferation of rodent vascular smooth muscle cells both in vitro and in vivo. J Vasc Res. 2010;47:252–261. [DOI] [PubMed] [Google Scholar]
- 26. Mosheimer BA, Kaneider NC, Feistritzer C, Djanani AM, Sturn DH, Patsch JR, Wiedermann CJ. Syndecan-1 is involved in osteoprotegerin-induced chemotaxis in human peripheral blood monocytes. J Clin Endocrinol Metab. 2005;90:2964–2971. [DOI] [PubMed] [Google Scholar]
- 27. Hao Y, Tsuruda T, Sekita-Hatakeyama Y, Kurogi S, Kubo K, Sakamoto S, Nakamura M, Udagawa N, Sekimoto T, Hatakeyama K, et al. Cardiac hypertrophy is exacerbated in aged mice lacking the osteoprotegerin gene. Cardiovasc Res. 2016;110:62–72. [DOI] [PubMed] [Google Scholar]
- 28. Min H, Morony S, Sarosi I, Dunstan CR, Capparelli C, Scully S, Van G, Kaufman S, Kostenuik PJ, Lacey DL, et al. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med. 2000;192:463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bumdelger B, Kokubo H, Kamata R, Fujii M, Yoshimura K, Aoki H, Orita Y, Ishida T, Ohtaki M, Nagao M, et al. Osteoprotegerin prevents development of abdominal aortic aneurysms. PLoS One. 2016;11:e0147088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Masuoka T, Hayashi N, Hori E, Kuwayama N, Ohtani O, Endo S. Distribution of internal elastic lamina and external elastic lamina in the internal carotid artery: possible relationship with atherosclerosis. Neurol Med Chir (Tokyo). 2010;50:179–182. [DOI] [PubMed] [Google Scholar]
- 31. Thompson RW, Holmes DR, Mertens RA, Liao S, Botney MD, Mecham RP, Welgus HG, Parks WC. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest. 1995;96:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lutgens E, Gijbels M, Smook M, Heeringa P, Gotwals P, Koteliansky VE, Daemen MJAP. Transforming growth factor‐beta mediates balance between inflammation and fibrosis during plaque progression. Arterioscler Thromb Vasc Biol. 2002;22:975–982. [DOI] [PubMed] [Google Scholar]
- 33. Ovchinnikova O, Gylfe A, Bailey L, Nordström A, Rudling M, Jung C, Bergström S, Waldenström A, Hansson GK, Nordström P. Osteoprotegerin promotes fibrous cap formation in atherosclerotic lesions of ApoE-deficient mice—brief report. Arterioscler Thromb Vasc Biol. 2009;29:1478–1480. [DOI] [PubMed] [Google Scholar]
- 34. Vorkapic E, Kunath A, Wågsäter D. Effects of osteoprotegerin/TNFRSF11B in two models of abdominal aortic aneurysms. Mol Med Rep. 2018;18:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marbacher S, Marjamaa J, Bradacova K, von Gunten M, Honkanen P, Abo-Ramadan U, Hernesniemi J, Niemelä M, Frösen J. Loss of mural cells leads to wall degeneration, aneurysm growth, and eventual rupture in a rat aneurysm model. Stroke. 2014;45:248–254. [DOI] [PubMed] [Google Scholar]
- 36. Frösen J, Tulamo R, Paetau A, Laaksamo E, Korja M, Laakso A, Niemelä M, Hernesniemi J. Saccular intracranial aneurysm: pathology and mechanisms. Acta Neuropathol. 2012;123:773–786. [DOI] [PubMed] [Google Scholar]
- 37. Wang Y, Wang Y, Li Y, Wang B, Miao Z, Liu X, Ma Y. Decreased expression of circ_0020397 in intracranial aneurysms may be contributing to decreased vascular smooth muscle cell proliferation via increased expression of miR-138 and subsequent decreased KDR expression. Cell Adh Migr. 2019;13:220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Starke RM, Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Koch WJ, Dumont AS. The role of oxidative stress in cerebral aneurysm formation and rupture. Curr Neurovasc Res. 2013;10:247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sato K, Niessner A, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. J Exp Med. 2006;203:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Munger JS, Sheppard D. Cross talk among TGF‐β signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb Perspect Biol. 2011;3:a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li J-M, Fan LM, Shah A, Brooks G. Targeting alphavbeta3 and alpha5beta1 for gene delivery to proliferating VSMCs: synergistic effect of TGF‐beta1. Am J Physiol Heart Circ Physiol. 2003;285:H1123–H1131. [DOI] [PubMed] [Google Scholar]
- 42. Ruigrok YM, Tan S, Medic J, Rinkel GJE, Wijmenga C. Genes involved in the transforming growth factor beta signalling pathway and the risk of intracranial aneurysms. J Neurol Neurosurg Psychiatry. 2008;79:722–724. [DOI] [PubMed] [Google Scholar]
- 43. Santiago-Sim T, Mathew-Joseph S, Pannu H, Milewicz DM, Seidman CE, Seidman JG, Kim DH. Sequencing of TGF‐β pathway genes in familial cases of intracranial aneurysm. Stroke. 2009;40:1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim ST, Brinjikji W, Kallmes DF. Prevalence of intracranial aneurysms in patients with connective tissue diseases: a retrospective study. AJNR Am J Neuroradiol. 2016;37:1422–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mazzella J-M, Frank M, Collignon P, Langeois M, Legrand A, Jeunemaitre X, Albuisson J. Phenotypic variability and diffuse arterial lesions in a family with Loeys-Dietz syndrome type 4. Clin Genet. 2017;91:458–462. [DOI] [PubMed] [Google Scholar]
- 46. Czuwara-Ladykowska J, Sementchenko VI, Watson DK, Trojanowska M. Ets1 is an effector of the transforming growth factor beta (TGF‐beta ) signaling pathway and an antagonist of the profibrotic effects of TGF‐beta. J Biol Chem. 2002;277:20399–20408. [DOI] [PubMed] [Google Scholar]
- 47. Aoki T, Kataoka H, Nishimura M, Ishibashi R, Morishita R, Miyamoto S. Ets-1 promotes the progression of cerebral aneurysm by inducing the expression of MCP-1 in vascular smooth muscle cells. Gene Ther. 2010;17:1117–1123. [DOI] [PubMed] [Google Scholar]
- 48. de Gast AN, Altes TA, Marx WF, Do HM, Helm GA, Kallmes DF. Transforming growth factor beta-coated platinum coils for endovascular treatment of aneurysms: an animal study. Neurosurgery. 2001;49:690–694. [DOI] [PubMed] [Google Scholar]
- 49. Tada Y, Kitazato KT, Tamura T, Yagi K, Shimada K, Kinouchi T, Satomi J, Nagahiro S. Role of mineralocorticoid receptor on experimental cerebral aneurysms in rats. Hypertension. 2009;54:552–557. [DOI] [PubMed] [Google Scholar]
- 50. Ali MS, Starke RM, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Owens GK, Koch WJ, Greig NH, Dumont AS. TNF‐α induces phenotypic modulation in cerebral vascular smooth muscle cells: implications for cerebral aneurysm pathology. J Cereb Blood Flow Metab. 2013;33:1564–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Khosla S, Arrighi HM, Melton LJ, Atkinson EJ, O’Fallon WM, Dunstan C, Riggs BL. Correlates of osteoprotegerin levels in women and men. Osteoporos Int. 2002;13:394–399. [DOI] [PubMed] [Google Scholar]
- 52. Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–495. [DOI] [PubMed] [Google Scholar]
- 53. Rzewuska-Lech E, Jayachandran M, Fitzpatrick LA, Miller VM. Differential effects of 17β-estradiol and raloxifene on VSMC phenotype and expression of osteoblast-associated proteins. Am J Physiol Endocrinol Metab. 2005;289:E105–E112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S3


