Abstract
Background
Neuromuscular blockade (NMB) agents are often administered to control shivering during targeted temperature management following cardiac arrest. In this study, we hypothesized that early, continuous NMB would result in a greater reduction in serum lactate levels among comatose patients after cardiac arrest.
Methods and Results
Randomized trial of continuous NMB for 24 hours versus usual care following cardiac arrest conducted at 5 urban centers in the United States. Adult patients who achieved return of spontaneous circulation, remained unresponsive, and underwent targeted temperature management after cardiac arrest were included. The primary outcome was change in lactate over 24 hours. A total of 83 patients were randomized, and 80 were analyzed (37 and 43 in the NMB and usual care arms, respectively). There was no significant interaction between time and treatment group with respect to change in lactate over 24 hours (median lactate change from 4.2 to 2.0 mmol/L [−2.2 mmol/L] in the NMB arm versus 4.0 to 1.7 mmol/L [−2.3 mmol/L] in the usual care arm; geometric mean difference, 1.3 [95% CI, 1.0–1.8]; P=0.07 for the interaction term). There was no difference in hospital survival (38% [NMB] versus 33% [usual care]; P=0.63) or survival with good functional outcome (30% [NMB] versus 21% [usual care]; P=0.35). There were no adverse events in either arm attributed to study interventions.
Conclusions
Continuous NMB compared with usual care did not reduce lactate over the first 24 hours after enrollment compared with usual care. There was no difference in overall hospital survival, hospital survival with good neurologic outcome, or adverse events.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02260258.
Keywords: biomarkers, cardiopulmonary resuscitation, clinical trial, heart arrest, hypothermia, neuromuscular blockade
Subject Categories: Cardiopulmonary Arrest, Cardiopulmonary Resuscitation and Emergency Cardiac Care, Clinical Studies
Nonstandard Abbreviations and Acronyms
- ICU
intensive care unit
- NMB
neuromuscular blockade
- NSE
neuron‐specific enolase
- TTM
targeted temperature management
Clinical Perspective
What Is New?
On the basis of promising observational data, continuous neuromuscular blockade has been suggested as an adjunctive therapy to targeted temperature management for patients who remain comatose following cardiac arrest.
In this multicenter, randomized trial of early, continuous neuromuscular blockade versus usual care for patients after cardiac arrest who remained comatose and were receiving targeted temperature management, there was no effect of neuromuscular blockade with respect to the primary outcome of serum lactate change over 24 hours.
What Are the Clinical Implications?
This trial does not support the routine use of continuous neuromuscular blockade for comatose patients after cardiac arrest who are receiving targeted temperature management.
Cardiac arrest is a devastating event that affects >500 000 individuals per year in the United States. 1 Although survival rates following cardiac arrest have improved, mortality remains high and many survivors experience long‐term neurologic sequelae. 2 , 3 A key link in the cardiac arrest chain of survival is the provision of post–cardiac arrest critical care and neuroprotective strategies. Targeted temperature management (TTM) is a central component of critical care post–cardiac arrest care, and current guidelines from the American Heart Association suggest all initial survivors of cardiac arrest who remain unresponsive receive TTM. 4 Beyond TTM and early coronary angiography in suspected acute coronary syndrome, there are few evidence‐based specific therapies in the post–cardiac arrest setting.
The administration of continuous neuromuscular blockade (NMB) in combination with TTM has been proposed as an additional therapeutic intervention for initial survivors of cardiac arrest. NMB may improve post–cardiac arrest outcomes through several mechanisms, including reduction of global oxygen consumption, prevention of patient‐ventilator dyssynchrony, reduction of metabolic demand, reduction of inflammation, and shorter time to target temperature. 5 Multicenter observational data found that continuous NMB following cardiac arrest was associated with more rapid lactate reduction and reduced mortality. 6
This randomized trial tested the efficacy and safety of continuous NMB compared with usual care following cardiac arrest. We tested the hypothesis that continuous NMB would increase lactate clearance over the first 24 hours after cardiac arrest.
Methods
Data Sharing and Disclosure
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the corresponding author.
Trial Setting and Design
This was a phase 2, multicenter, randomized, superiority trial of continuous NMB using rocuronium versus usual care in patients who had sustained return of spontaneous circulation but who remained unresponsive after cardiac arrest. Enrolling sites were 5 urban tertiary care centers in the United States (Table S1). Legally authorized representatives of all patients provided written informed consent.
The trial was registered at clinicaltrials.gov (NCT02260258) before the first enrollment, and institutional review boards of all enrolling institutions approved the protocol. A Data Safety and Monitoring Board evaluated and monitored the trial for safety.
Patients
Between December 2014 and May 2019, we enrolled adult patients (aged ≥18 years) who experienced a cardiac arrest and subsequently had sustained return of spontaneous circulation (≥20 minutes) but remained unresponsive (ie, not following commands) and were undergoing TTM between 32°C and 36°C. We added an additional inclusion criterion of a minimum serum lactate level of ≥2 mmol/L early in study enrollment (Table S2). We excluded patients if they had a traumatic cause of cardiac arrest, were receiving continuous NMB for clinical purposes, were not expected to survive 24 hours, had undergone TTM for ≥6 hours, had a prearrest modified Rankin scale score of ≥4, or were members of a protected population. Patients experiencing in‐hospital or out‐of‐hospital cardiac arrest were eligible, although study teams were primarily located in the emergency departments of participating sites.
Randomization and Intervention
Randomization occurred in blocks of 4 in a 1:1 ratio and was stratified by study site and by the presence of shock. Shock was defined as the use of any vasopressor. An independent statistician generated the random sequence using Power Analysis Sample Size software v13. Research pharmacies at each site maintained site‐specific allocation sequences. Medication was provided open label. Clinical and research teams were not blinded to allocation.
Patients in the continuous NMB arm received a rocuronium bolus of 1.0 mg/kg followed by a continuous infusion of rocuronium for a total of 24 hours titrated to 1 to 2/4 twitches on a train‐of‐4 stimulator. Patients in the usual care arm received 100 mL of normal (0.9% NaCl) saline to mark the 0‐hour time point. Rocuronium was chosen as there was a shortage of cisatracurium in the United States at the time of trial design. All patients, including those who received continuous NMB, were sedated per local site protocol. Use of the Columbia antishivering protocol was recommended to clinical teams, but adherence was not mandated. 7
Sample Size and Outcomes
The primary outcome of the study was change in serum lactate level between enrollment and 24 hours after the receipt of study drug. We chose this outcome because lactate is correlated with hospital survival in patients who experience cardiac arrest. 8 The primary outcome was changed from lactate levels 24 hours after initiation of study drug to better account for any group differences in lactate level at time of enrollment and to allow patients who die before 24 hours to be included in the analysis. This change is reflected in the statistical analysis plan published online before data analysis.
Secondary outcomes for the trial included time from return of spontaneous circulation until target temperature, time to liberation from mechanical ventilation, length of stay in the intensive care unit (ICU), hospital survival, and hospital survival with good functional outcome (as defined by a modified Rankin Scale score of <4). Additional secondary biomarker outcomes included measures of inflammation (interleukin‐1β, interleukin‐6, interleukin‐8, interleukin‐10, and tumor necrosis factor‐ɑ), measures of vascular injury (vascular cell adhesion molecule, intercellular adhesion molecule, E‐selectin, and vascular endothelial growth factor‐A), measures of renal function (kidney injury molecule‐1, cystatin C, and neutrophil‐gelatinase associated lipocalin), and measures of neurologic injury (neuron‐specific enolase [NSE]).
A prespecified safety outcome was muscle weakness, assessed using a modified Medical Research Council scale measured at the time of discharge from the ICU. 9 Trained research staff at each site monitored study patients between enrollment and ICU discharge for any unexpected adverse effects related to study participation.
Data Collection and Blood Sampling
Trained research personnel collected all data according to a detailed, predefined data dictionary, and a physician verified all outcome variables. We entered data into a Research Electronic Data Capture database, a secure, web‐based database tool. 10
We obtained blood samples from patients immediately before study drug administration and then at 12 and 24 hours. The clinical laboratory at each site measured lactate levels. We generally obtained blood samples for lactate measurement from a central venous access catheter. We centrifuged blood not sent to the clinical laboratory, froze it at −80°C, and then sent samples to the coordinating site for storage at −80°C until analysis.
Biomarker Measurement
We used frozen plasma for all biomarker measurements and measured analytes by multiplex analysis using 96‐well multiplex kits. For vascular biomarkers (E‐selectin, vascular cell adhesion molecule, and intercellular adhesion molecule), we diluted plasma samples at either 1:50 or 1:100. For cytokines and vascular endothelial growth factor plasma, samples were not diluted. We measured all samples in duplicate and analyzed mean values from duplicate results.
Statistical Analysis
A detailed statistical analysis plan appears online at clinicaltrials.gov (https://clinicaltrials.gov/ct2/show/NCT02260258) and was published before any analysis being performed. All analyses used a modified intention‐to‐treat population, defined as those subjects receiving random assignment and receiving any study drug (rocuronium or the saline time marker).
Conservative estimates drawn from observational data indicated that a sample size of 80 patients could detect a predicted mean difference in 24‐hour lactate of 2.0 mmol/L (±3.2 mmol/L) with 80% power, assuming a 2‐sided test and an ɑ of 0.05. 6
We describe baseline characteristics by treatment group. We summarize categorical variables by frequencies and percentages and continuous variables using means (SDs) or medians (interquartile ranges [IQRs]).
The change in lactate over time was assessed using a linear mixed effects model. We log‐transformed lactate values, which were not approximately normally distributed (log transformation used the natural logarithm). Fixed effects included the allocated treatment (NMB versus usual care), shock stratification, time point (0, 12, and 24 hours), and the interaction between treatment and time point. We included study site and patient within study site as random intercept effects. The primary outcome was the interaction term between allocated treatment and time, which is presented as a ratio of geometric mean differences over 24 hours. 11 Values >1.0 favor the placebo arm.
We present medians and IQRs of lactate over time, by treatment, using longitudinal plots. We compared biomarker outcomes using the above mixed model, using log‐transformed biomarker levels if not normally distributed. Continuous and categorical secondary outcome measures were compared using linear or logistic regression as appropriate, controlling for shock stratification and site. Length of stay in the ICU was compared using negative binomial regression given the right skew of the distribution. ICU length of stay was analyzed (1) including all patients randomized and (2) limiting the analyzed population to those who survived their ICU stay. Muscle weakness scores in ICU survivors were highly left skewed, and we compared these scores using a Mann‐Whitney U test.
We conducted prespecified subgroup analyses based on initial rhythm (shockable versus nonshockable) and shock status. We performed a post hoc analysis by median lactate and an exploratory per‐protocol analysis including only patients who received 24 hours of rocuronium (if so assigned) versus those who survived to 24 hours without receipt of an NMB agent after saline administration (if in the usual care group).
All tests were 2 sided, and the nominal level of statistical significance was 5%. We applied no formal adjustments for multiplicity of testing, but the outcomes were ordered by degree of importance and we interpreted test results in light of the multiple comparisons made. 12 All statistics were performed using STATA, version 15 (StataCorp LP, College Station, TX).
Results
Patients
A total of 818 patients met inclusion criteria, and 83 underwent random assignment (39 allocated to NMB and 44 allocated to usual care). Before study drug administration, 2 patients in the NMB arm and 1 patient in the usual care arm had clinical changes that excluded from receiving study drug. A total of 80 patients (37 in the NMB arm and 43 in the usual care arm) remained in the modified intention‐to‐treat analysis. Figure 1 shows the flow of patients through the trial. Baseline characteristics and cardiac arrest/resuscitation characteristics are in Table 1.
Figure 1. Consolidated Standards of Reporting Trials diagram.
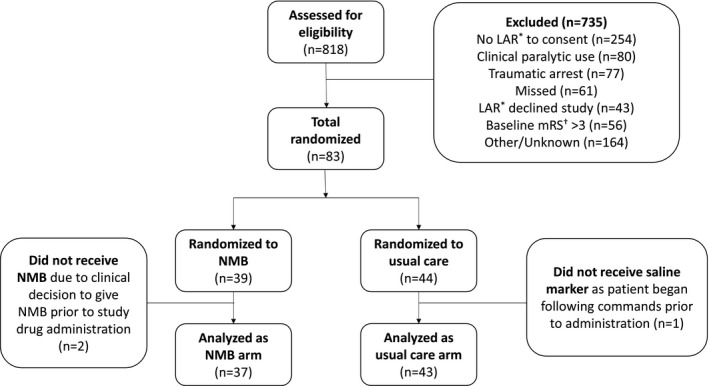
LAR indicates legally authorized representative; mRS, modified Rankin Scale; and NMB, neuromuscular blockade.
Table 1.
Baseline Characteristics
| Variable | Neuromuscular Blockade (n=37) | Usual Care (n=43) |
|---|---|---|
| Demographics | ||
| Age, median (IQR), y | 66 (57–77) | 64 (56–77) |
| Female sex, n (%) | 17 (46) | 14 (33) |
| White race, n (%) | 20 (54) | 22 (51) |
| Medical history, n (%) | ||
| Congestive heart failure | 9 (24) | 12 (28) |
| Atrial fibrillation | 7 (19) | 9 (21) |
| Coronary artery disease | 14 (38) | 9 (21) |
| Prior cardiac arrest | 0 (0) | 0 (0) |
| Chronic pulmonary disease | 12 (32) | 9 (21) |
| Liver cirrhosis | 1 (3) | 2 (5) |
| Kidney disease | 9 (24) | 14 (33) |
| Active malignancy | 3 (8) | 2 (5) |
| Arrest characteristics | ||
| Location (out‐of‐hospital cardiac arrest), n (%) | 35 (95) | 40 (93) |
| Initial rhythm (shockable), n (%) | 17 (46) | 16 (38) |
| Estimated no‐flow time, median (IQR), min | 4 (0–7) | 2 (0–10) |
| Estimated low‐flow time, median (IQR), min | 12 (5–26) | 15 (8–30) |
| Witnessed (yes), n (%) | 27 (73) | 26 (61) |
| Bystander CPR provided (yes), n (%)* | 24 (71) | 26 (67) |
| Arrest cause (cardiac), n (%) | 27 (73) | 30 (70) |
| Characteristics at enrollment | ||
| Time from return of spontaneous circulation to study drug, median (IQR), h | 7.5 (6–8.3) | 6.3 (5–7.5) |
| pH, median (IQR) | 7.3 (7.2–7.3) | 7.3 (7.2–7.4) |
| Pco 2, median (IQR), mmHg | 41.5 (34.5–53.5) | 40.0 (33.5–48.5) |
| Po 2, median (IQR), mmHg | 100.0 (76.0–168.5) | 161.0 (83.0–245.0) |
| Shock stratification (shock), n (%) | 19 (51) | 21 (49) |
| ST‐segment–elevation myocardial infarction present (yes), n (%) | 4 (11) | 6 (14) |
| Target temperature, median (IQR), °C | 35.0 (33.5–36.0) | 34.0 (33.5–35.5) |
CPR indicates cardiopulmonary resuscitation; and IQR, interquartile range.
Includes immediate CPR provided by trained advanced cardiac life support responders. If unknown, bystander CPR coded as not performed. In‐hospital cardiac arrest excluded.
Trial and Concomitant Therapies
Between return of spontaneous circulation and study enrollment, 6 (16%) patients in the NMB arm and 2 (5%) patients in the usual care arm received a bolus dose of neuromuscular blocking agent. Between enrollment and the 12‐hour time point, 6 (14%) patients in the usual care arm received some neuromuscular blocking agent. Between the 12‐ and 24‐hour time points, 8 (19%) patients in the usual care arm received a neuromuscular blocking agent. Nine (21%) patients in the usual care arm received some neuromuscular blocking agent between enrollment and the 24‐hour time point, with 2 (22%) of these receiving only bolus dosing. Neuromuscular blocking agents given in the usual care arm after enrollment were given in response to shivering (89%) or worsening respiratory failure (11%). No patient in the NMB arm had NMB stopped early with the exception of those who died in the first 24 hours (2 in the NMB arm and 3 in the usual care arm) (Table S3).
The median cardiovascular sequential organ failure assessment score at enrollment was 3.0 (IQR, 0.0–4.0) in the NMB arm and 1.0 (IQR, 0.0–4.0) in the usual care arm. At 12 hours, the median scores were 2.5 (IQR, 0.0–3.0) and 1.5 (0.0–4.0) in the NMB and usual care arms, respectively. At 24 hours, the median cardiovascular sequential organ failure assessment scores were 2.0 (IQR, 0.0–4.0) and 3.0 (IQR, 0.0–3.0) in the NMB and usual care arms, respectively.
Primary and Key Secondary Outcomes
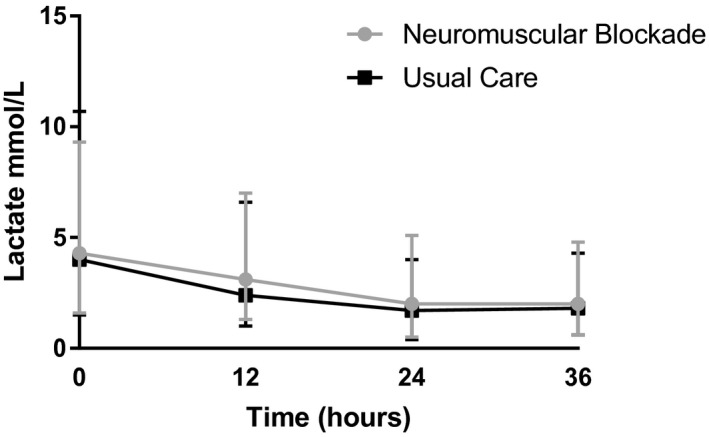
The median lactate level at enrollment was 4.2 mmol/L (IQR, 2.5–5.4 mmol/L), and was similar between groups (median, 4.2 mmol/L [IQR, 2.6–5.0 mmol/L] in the NMB group versus 4.0 mmol/L [IQR, 2.5–6.7 mmol/L] in the usual care group). By 12 hours after enrollment, lactate levels fell in both groups to a median of 3.1 mmol/L (IQR, 1.7–3.7 mmol/L) in the NMB group and 2.4 mmol/L (IQR, 1.4–4.2 mmol/L) in the usual care group. At the 24‐hour time point, lactate was 2.0 mmol/L (IQR, 1.5–3.1 mmol/L) and 1.7 mmol/L (IQR, 1.3–2.3 mmol/L) in the NMB and usual care arms, respectively. There was no detectable between‐group difference in lactate change over time (ratio of geometric mean difference, 1.3; 95% CI, 1.0–1.8; P=0.07 for the interaction between study arm and time). There was additionally no difference in median lactate level at 24 hours after enrollment (P=0.12). Median lactate levels over time are represented graphically in Figure 2.
Figure 2. Longitudinal plot of lactate over time.
Overall, 14 (38%) patients in the NMB arm survived to hospital discharge compared with 14 (33%) patients in the usual care arm (odds ratio [OR], 1.3 [95% CI, 0.5–3.3]; P=0.63). Eleven (30%) of patients survived with favorable functional outcome in the NMB arm compared with 9 (21%) in the usual care arm (OR, 1.7 [95% CI, 0.6–4.7]; P=0.35). Among patients who survived to ICU discharge, there was no between‐group difference in muscle weakness score, ICU length of stay, or the duration of mechanical ventilation (Table 2). There were no unexpected adverse events related to study drug.
Table 2.
Median Lactate Levels and Key Secondary Outcomes
| Outcome | Neuromuscular Blockade (n=37) | Usual Care (n=43) | Effect Estimate (95% CI) | P Value |
|---|---|---|---|---|
| Primary outcome* | ||||
| Lactate, median (IQR), mmol/L | ||||
| Enrollment (0 h) | 4.2 (2.6–5.0) | 4.0 (2.5–6.7) | … | … |
| 12 h | 3.1 (1.7–3.7) | 2.4 (1.4–4.2) | 1.3 (1.0–1.6) | 0.05 |
| 24 h | 2.0 (1.5–3.1) | 1.7 (1.3–2.3) | 1.3 (1.0–1.8) | 0.07 |
| Secondary outcomes | ||||
| Time from ROSC to target temperature, median (IQR), h † | 6.8 (5.3–9.4) | 8.3 (4.7–11.2) | 1.0 (0.7–1.4) | 0.82 |
| ICU LOS, median (IQR), d ‡ | ||||
| ICU survivors | 9.0 (6.0–16.0) | 5.0 (4.0–12.0) | 1.3 (0.8–2.0) | 0.35 |
| All patients | 6.0 (3.0–11.0) | 4.0 (3.0–7.0) | 1.4 (1.0–1.9) | 0.09 |
| Mechanical ventilation duration, median (IQR), h § | ||||
| Survivors to extubation | 126.3 (76.1–280.6) | 66.9 (55.6–172.7) | 1.4 (0.7–2.9) | 0.32 |
| All patients | 102.0 (64.3–206.4) | 82.7 (47.4–160.7) | 1.3 (0.9–1.9) | 0.18 |
| Hospital survival, n (%) ‖ | 14 (38) | 14 (33) | 1.3 (0.5, 3.3) | 0.63 |
| Discharge mRS ≤3, n (%) ‖ | 11 (30) | 9 (21) | 1.7 (0.6, 4.7) | 0.35 |
| Muscle weakness score, median (IQR) ¶ | 30 (28–30) | 30 (27–30) | n/a | 0.58 |
ICU indicates intensive care unit; IQR, interquartile range; LOS, length of stay; mRS, modified Rankin Scale; and ROSC, return of spontaneous circulation.
Effect estimate represents the ratio of geometric mean differences in change over time. Values >1.0 favor the placebo arm. P value is for the interaction between randomization arm and time.
Time variable for time ROSC to target temperature was log transformed and compared using linear regression, controlling for shock stratification and site. Effect estimate represents geometric mean difference. Values >1.0 favor longer duration in the neuromuscular blockade arm.
LOS truncated at 28 days and compared using negative binomial regression, controlling for stratification and site. Fourteen patients in each arm survived to ICU discharge (n=14 in each arm). Effect estimates represent incidence rate ratios. Values >1.0 favor longer duration to target temperature in the neuromuscular blockade arm.
Duration log transformed and compared using linear regression, controlling for shock stratification and site. Includes patients surviving to discontinuation of mechanical ventilation (n=14 in each group). Two patients discharged from the hospital on mechanical ventilation have duration truncated at time of discharge and are considered survivors to extubation. Effect estimates represent geometric mean difference. Values >1.0 favor longer duration in the neuromuscular blockade arm.
Comparison made using logistic regression, controlling for shock stratification and site. Effect estimates represent odds ratios.
Of 30 possible points.
Results of the exploratory per‐protocol analysis were similar to those in the intention‐to‐treat cohort with respect to the primary outcome of lactate at 24 hours.
Subgroup Analyses
There was no effect modification according to initial rhythm (P=0.43 for the interaction), presence of shock (P=0.85), or median lactate (P=0.20) (Figures S1 through S3).
Biomarker Analyses
Overall, 62 patients (78%) had blood collected and frozen for biomarker analysis. Of these patients, 29 (47%) were in the NMB arm and 33 (53%) were in the usual care arm. The median baseline NSE levels were 9.7 mg/mL (IQR, 6.0–12.8 mg/mL) and 9.2 mg/mL (IQR, 5.9–13.5 mg/mL) in the NMB and usual care arms, respectively. At 24 hours, the median NSE had increased in both groups to 16.2 mg/mL (IQR, 5.3–33.4 mg/mL) and 19.1 mg/mL (IQR, 8.2–35.6 mg/mL) in the NMB and usual care arms, respectively. At baseline, the median S100 levels were 497.2 pg/mL (IQR, 148.2–1694.7 pg/mL) and 567.0 pg/mL (IQR, 303.4–1034.5 pg/mL) in the NMB and usual care arms, respectively. S100 levels decreased in both groups over the first 24 hours to a median of 102.1 pg/mL (IQR, 33.3–1312.0 pg/mL) and 246.9 pg/mL (IQR, 82.0–755.5 pg/mL) in the NMB and usual care arms, respectively. We did not detect any between‐group difference in change in NSE or S100 levels between enrollment and 24 hours (Figure 3).
Figure 3. Biomarkers of neurologic injury over time.
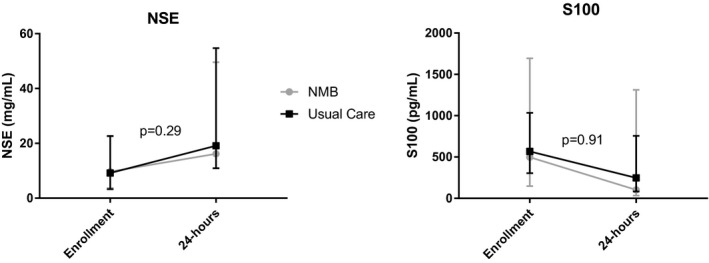
Change in biomarker levels over time assessed via mixed model, controlling for shock stratification as a fixed effect and study site as a random effect. Biomarker values log transformed for the analysis as their distributions visually deviated from normal. P values reflect the interaction between randomization group and time. NMB indicates neuromuscular blockade; and NSE, neuron‐specific enolase.
Kidney injury molecule‐1 levels increased more steeply in the NMB arm compared with the usual care arm. We did not detect between‐group differences over time in any other measured inflammatory, endothelial, vascular, or kidney injury biomarkers (Figures S1 through S3).
Discussion
In this randomized trial of patients after cardiac arrest, there was no difference in lactate at 24 hours in the NMB compared with the usual care arm. Survival and survival with good functional outcome at hospital discharge were not different between study arms. We did not identify any difference in muscle weakness score between groups, and there were no serious, unexpected adverse events related to study drug. There was no difference in the change over time in most measured biomarkers, although kidney injury molecule‐1 increased more steeply in the NMB arm.
NMB may reduce global oxygen consumption, prevent patient‐ventilator dyssynchrony, attenuate inflammation, and shorten time to target temperature. 5 In addition, NMB may have been an unrecognized confounder in early major randomized clinical trials of TTM in the HACA (Hypothermia After Cardiac Arrest) trial, in which patients randomized to mild hypothermia received bolus pancuronium every 2 hours for the prevention of shivering. 13 , 14 In the trial by Bernard et al, patients in the mild hypothermia arm received boluses of vecuronium as required to prevent shivering. 13 , 14 Neuromuscular blocking agents were not routinely given to patients randomized to normothermia in either study. Nevertheless, the 2010 American Heart Association guidelines recommended minimizing the use of NMB in patients after cardiac arrest because of concerns about masking seizure activity and limiting neurologic assessment. 15 Studies of routine NMB use in other critical illnesses, in particular the acute respiratory distress syndrome, have had mixed results, with the most recent major randomized trial having failed to find any benefit with routine NMB administration. 5 , 16 Substantial variability exists with respect to NMB use during TTM. 17
The primary outcome of lactate change correlates with mortality after cardiac arrest. 8 , 18 , 19 The lack of difference in lactate levels in this study may have resulted from several factors. First, it may reflect that there is no effect of continuous NMB on lactate clearance. Alternatively, patients randomized to NMB may have received different doses and durations of sedating medications. Also, although randomization was stratified by shock status, median cardiovascular sequential organ failure assessment scores were higher at enrollment in the NMB arm. An additional contributing factor may have been different target temperatures for TTM in the 2 groups, although existing evidence suggests lower temperatures to be associated with higher lactate levels. 20
Many randomized trials conducted in critically ill populations compare 2 fixed treatment arms, as opposed to comparing a single intervention against “usual care.” 21 When 2 fixed treatment arms are compared, there is the possibility for harmful deviations from usual care in the comparator arm, the inability to compare the 2 arms adequately because of confounding by mismatched covariates, or both, which can arise when the true comparator would be a group with titrated care. In addition, the results are less generalizable to real‐world practice. 22 In the present trial, the comparator arm was “usual care,” and crossover occurred in a substantial minority of subjects (21%). Although this level of crossover likely best reflects current practice in a condition with titrated care, it may have biased the results toward the null. Also, patients enrolled in this trial were those for whom the clinical team had already decided not to treat with continuous NMB. Of those patients screened for inclusion, 10% were excluded because of a clinical decision to administer NMB. Therefore, our trial evaluates those for whom a perceived clinical indication for NMB (ie, severely compromised oxygenation) was not present at enrollment. These factors are important considerations when interpreting our results in the context of current clinical practice.
The results of this study are similar to those of a recently published and similarly designed randomized trial of post–cardiac arrest NMB conducted in South Korea. 23 In that trial, there was no difference in lactate at 24 hours. Although not statistically significant, survival and survival with good neurologic outcome were higher in the NMB arm. Similarly, a trial conducted in Austria randomizing patients after cardiac arrest to continuous versus intermittently dosed NMB found a higher survival in the continuous NMB arm, although the difference was not significant. 24 No trial in patients who experienced cardiac arrest found significant harms associated with early, continuous postarrest NMB.
Measured biomarkers in this study included markers of neurologic injury, inflammation, cell adhesion, vascular proliferation, and kidney injury. There was no between‐group difference in NSE or S100 levels over time. Similarly, there was no difference in most biomarkers of inflammation, cell adhesion, vascular proliferation, or kidney injury. There was a trend toward a more rapid clearance of tumor necrosis factor‐ɑ in the NMB arm compared with the usual care arm. Patients in the NMB arm had a faster increase in kidney injury molecule‐1 over the first 24 hours. These isolated findings should be considered hypothesis generating.
This study has several limitations. First, the study was underpowered for important secondary outcomes, such as survival. In addition, post–cardiac arrest care and neuroprognostication protocols were not standardized across participating hospitals, which may have increased heterogeneity. Furthermore, this trial was not designed to study whether early NMB facilitates faster time to target temperature, although that is an important question for future study. Rocuronium was selected in this trial as a result of practical considerations. Whether other neuromuscular blocking agents would have been more effective is not clear. Finally, there were a large number of patients who met initial inclusion criteria but were ultimately excluded for a variety of reasons. This limits generalizability and may explain differences between the results of this randomized trial and those of prior observational studies.
Conclusions
In this trial, early, continuous NMB compared with usual care following cardiac arrest was not associated with a decrease in serum lactate levels over the first 24 hours after trial enrollment. There was no between‐group difference in any secondary clinical outcome.
Sources of Funding
The primary study was funded by a Grant‐in‐Aid from the American Heart Association (14GRNT2001002), and additional biomarker substudies were supported by the National Institutes of Health (NIH) (K24HL127101). Dr Moskowitz and Dr Berg are supported by grants from the NIH (K23GM128005 and K23 HL128814, respectively). Dr Grossestreuer receives support from Harvard Catalyst|The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, NIH Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers.
Disclosures
None.
Supporting information
Tables S1–S3
Figures S1–S3
Acknowledgments
We thank Francesca Montillo and Amanda Frias‐Howard for their administrative support. We thank Shaz Shaefi, MD, Matthew Wong, MD, Melanie Pogach, MD, and Ariel Mueller, MA, for their participation on the trial Data Safety and Monitoring Board. Finally, we thank all of the research staff and clinical teams who made this trial possible.
Statistical support: Long Ngo, PhD, and Lakshman Balaji, MPH. Neuromuscular blockade investigators (not listed on main author line): Cameron Dezfulian, MD; Jonathan Elmer, MD; Frank Guyette, MD; Ankur Doshi, MD; Alexandra Weissman, MD; Masa Okubo, MD; Lillian Emlet, MD; Bradley Molyneaux, MD. Research coordinators: Jacob Boise; Varun Konanki; Jocelyn Portmann; Christopher Sulmonte; Alexander Wulff; Sarah Ganley; Julia Balkema; Ying Loo; Thomas Leith; Lethu Ntshinga; Deanna Lee; Garrett Thompson; Gary Theroux, Jr; Ryan Paternoster; Sara DiFiore; Timothy Smith.
(J Am Heart Assoc. 2020;9:e017171 DOI: 10.1161/JAHA.120.017171.)
Supplementary materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017171
For Sources of Funding and Disclosures, see page 8.
References
- 1. Andersen LW, Holmberg MJ, Berg KM, Donnino MW, Granfeldt A. In‐hospital cardiac arrest: a review. JAMA. 2019;321:1200–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perman SM, Stanton E, Soar J, Berg RA, Donnino MW, Mikkelsen ME, Edelson DP, Churpek MM, Yang L, Merchant RM; American Heart Association's Get With the Guidelines®—Resuscitation Investigators . Location of in‐hospital cardiac arrest in the United States‐variability in event rate and outcomes. J Am Heart Assoc. 2016;5:e003638 DOI: 10.1161/JAHA.116.003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan PS, McNally B, Tang F, Kellermann A; CARES Surveillance Group . Recent trends in survival from out‐of‐hospital cardiac arrest in the United States. Circulation. 2014;130:1876–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, Leary M, Meurer WJ, Peberdy MA, Thompson TM, et al. Part 8: post‐cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S465–S482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Papazian L, Forel JM, Gacouin A, Penot‐Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. [DOI] [PubMed] [Google Scholar]
- 6. Salciccioli JD, Cocchi MN, Rittenberger JC, Peberdy MA, Ornato JP, Abella BS, Gaieski DF, Clore J, Gautam S, Giberson T, et al. Continuous neuromuscular blockade is associated with decreased mortality in post‐cardiac arrest patients. Resuscitation. 2013;84:1728–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choi HA, Ko SB, Presciutti M, Fernandez L, Carpenter AM, Lesch C, Gilmore E, Malhotra R, Mayer SA, Lee K, et al. Prevention of shivering during therapeutic temperature modulation: the Columbia anti‐shivering protocol. Neurocrit Care. 2011;14:389–394. [DOI] [PubMed] [Google Scholar]
- 8. Donnino MW, Andersen LW, Giberson T, Gaieski DF, Abella BS, Peberdy MA, Rittenberger JC, Callaway CW, Ornato J, Clore J, et al; National Post‐Arrest Research Consortium . Initial lactate and lactate change in post‐cardiac arrest: a multicenter validation study. Crit Care Med. 2014;42:1804–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand‐Zaleski I, Boussarsar M, Cerf C, Renaud E, Mesrati F, Carlet J, et al; Groupe de Réflexion et d'Etude des Neuromyopathies en Réanimation . Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–2867. [DOI] [PubMed] [Google Scholar]
- 10. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feng C, Wang H, Lu N, Tu XM. Log transformation: application and interpretation in biomedical research. Stat Med. 2013;32:230–239. [DOI] [PubMed] [Google Scholar]
- 12. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 13. Hypothermia After Cardiac Arrest Study Group . Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. [DOI] [PubMed] [Google Scholar]
- 14. Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out‐of‐hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. [DOI] [PubMed] [Google Scholar]
- 15. Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, Gabrielli A, Silvers SM, Zaritsky AL, Merchant R, et al; American Heart Association . Part 9: post‐cardiac arrest care: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S768–S786. [DOI] [PubMed] [Google Scholar]
- 16. National Heart, Lung, and Blood Institute PETAL Clinical Trials Network , Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, Gong MN, Grissom CK, Gundel S, Hayden D, Hite RD, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380:1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coppler PJ, Sawyer KN, Youn CS, Choi SP, Park KN, Kim YM, Reynolds JC, Gaieski DF, Lee BK, Oh JS, et al. Variability of post‐cardiac arrest care practices among cardiac arrest centers: United States and South Korean Dual Network Survey of Emergency Physician Research Principal Investigators. Ther Hypothermia Temp Manag. 2017;7:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Orban JC, Novain M, Cattet F, Plattier R, Nefzaoui M, Hyvernat H, Raguin O, Kaidomar M, Kerever S, Ichai C. Association of serum lactate with outcome after out‐of‐hospital cardiac arrest treated with therapeutic hypothermia. PLoS One. 2017;12:e0173239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Donnino MW, Miller J, Goya N, Loomba M, Sankey SS, Dolcourt B, Sherwin R, Otero R, Wira C. Effective lactate clearance is associated with improved outcome in post‐cardiac arrest patients. Resuscitation. 2007;75:229–234. [DOI] [PubMed] [Google Scholar]
- 20. Bro‐Jeppesen J, Hassager C, Wanscher M, Østergaard M, Nielsen N, Erlinge D, Friberg H, Køber L, Kjaergaard J. Targeted temperature management at 33°C versus 36°C and impact on systemic vascular resistance and myocardial function after out‐of‐hospital cardiac arrest: a sub‐study of the Target Temperature Management Trial. Circ Cardiovasc Interv. 2014;7:663–672. [DOI] [PubMed] [Google Scholar]
- 21. Thompson BT, Schoenfeld D. Usual care as the control group in clinical trials of nonpharmacologic interventions. Proc Am Thorac Soc. 2007;4:577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deans KJ, Minneci PC, Suffredini AF, Danner RL, Hoffman WD, Ciu X, Klein HG, Schechter AN, Banks SM, Eichacker PQ, et al. Randomization in clinical trials of titrated therapies: unintended consequences of using fixed treatment protocols. Crit Care Med. 2007;35:1509–1516. [DOI] [PubMed] [Google Scholar]
- 23. Lee BK, Cho IS, Oh JS, Choi WJ, Wee JH, Kim CS, Kim WY, Youn CS. Continuous neuromuscular blockade infusion for out‐of‐hospital cardiac arrest patients treated with targeted temperature management: a multicenter randomized controlled trial. PLoS One. 2018;13:e0209327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stöckl M, Testori C, Sterz F, Holzer M, Weiser C, Schober A, Nichol G, Frossard M, Herkner H, Kechvar J, et al. Continuous versus intermittent neuromuscular blockade in patients during targeted temperature management after resuscitation from cardiac arrest‐a randomized, double blinded, double dummy, clinical trial. Resuscitation. 2017;120:14–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S3


