Abstract
Background
Improving health‐related quality of life is an important goal in the management of patients with heart failure (HF). Defining health‐related quality of life changes over time in patients with HF with preserved (HFpEF) or reduced ejection fraction and showing their association with other important clinical events could support the use of health‐related quality of life as a measure of quantifying HF care.
Methods and Results
In the Alberta HEART (Heart Failure Aetiology and Analysis Team) cohort (n=621), patients were categorized into 4 subgroups: healthy controls (n=98), at risk (n=163), HFpEF (n=191), and HF with reduced ejection fraction (n=169). The change of the Kansas City Cardiomyopathy Questionnaire (KCCQ), EuroQOL 5 dimensions, and Functional Assessment of Cancer Therapy—Anemia over 12 months, and its association with a composite of death or rehospitalization within 3 years were assessed. At baseline, the KCCQ overall summary score was 73 (interquartile range, 53–86) in HFpEF and 78 (interquartile range, 56–90) in HF with reduced ejection fraction (P=0.22). Overall, 30.5% of patients with HF experienced ≥5‐point improvements and 32.4% had ≥5‐point worsening in KCCQ overall summary score at 12 months, which did not differ between HFpEF and HF with reduced ejection fraction (P=0.23). Clinical events were higher in patients with HF who had a decline in KCCQ over 12 months as compared with those with stable KCCQ scores (70.2% versus 52.0%, P=0.012). The results were similar for the Functional Assessment of Cancer Therapy—Anemia and EuroQOL 5 dimensions.
Conclusions
In patients with HF, the KCCQ quantified clinically meaningful changes over time, which were associated with important clinical outcomes in patients with HFpEF. Given the observed variability and prognostication in different patient trajectories, health‐related quality of life measures could be valuable for quantifying the quality of care in healthcare systems.
Keywords: health‐related quality of life, heart failure, heart failure with preserved ejection fraction, Kansas City Cardiomyopathy Questionnaire
Subject Categories: Heart Failure, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- EQ‐5D
EuroQOL 5 dimensions questionnaire
- FACT‐An
Functional Assessment of Cancer Therapy—Anemia
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HRQoL
health‐related quality of life
- IQR
interquartile range
- KCCQ OSSKCCQ
Kansas City Cardiomyopathy Questionnaire
- LVEF
left ventricular ejection fraction
- NT‐proBNP
N‐terminal pro‐B-type natriuretic peptide
- NYHA
New York Heart Association
- TOPCAT
Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist
Clinical Perspective
What Is New?
In the Alberta HEART (Heart Failure Aetiology and Analysis Team) cohort, we investigated the change of health‐related quality of life (HRQoL) and its correlation with outcomes in patients across the risk spectrum of heart failure (HF).
Patients with HF with preserved ejection fraction had numerically lower Kansas City Cardiomyopathy Questionnaire scores compared with patients with HF with reduced ejection fraction.
After adjustment for the baseline Kansas City Cardiomyopathy Questionnaire level and clinical variables, a decrease in Kansas City Cardiomyopathy Questionnaire over time was associated with adverse clinical outcomes, and there was a stronger relationship between the change in HRQoL and clinical outcomes in patients with HF with preserved ejection fraction than those with HF with reduced ejection fraction.
What Are the Clinical Implications?
Improving HRQoL is important for patients with HF and as end point in HF trials.
The relationship between HRQoL and quality of care for patients with HF has been unclear.
Given the observed variability and prognostication in different patient trajectories, HRQoL measures could be valuable measures for quantifying the quality of care in healthcare systems.
Improving patient‐reported health status, their symptoms, function, and quality of life is an important goal in the management of patients with heart failure (HF). Many patients weigh quality of life improvement greater than prolonging survival. 1 , 2 , 3 Moreover, patients with HF may report their functioning, symptoms, and quality of life differently from that assessed by a clinician, underscoring the importance of assessing health status directly from patients themselves. 4 , 5
The longitudinal assessment of patients with HF via clinical exam, biomarkers, imaging, and formally assessed health‐related quality of life (HRQoL) is important for the management of patients. 6 Similar symptom burden and quality of life impairments have been observed in patients with HF with preserved (HFpEF) or reduced ejection fraction (HFrEF) in clinical trials. 7 , 8 , 9 However, there is a paucity of studies 10 comparing the change of HRQoL over time in patients with HFpEF and HFrEF or as compared with at‐risk or control patients with other causes for their symptoms. Furthermore, fatigue—a common symptom of many patients—is infrequently captured or rigorously assessed in clinical assessments.
Given the importance to patients and availability of treatments to improve patients’ health status, some organizations, such as the International Consortium for Health Outcomes Measurement, have advocated the use of patient‐reported outcomes as measures of healthcare quality. For outcomes to be embraced as a potential performance measure, it is important to understand the variability in health status over time and that changes over time are associated with other clinically important outcomes. Moreover, it is important to explore whether these characteristics apply similarly to patients with HFrEF and HFpEF to ensure that they could measure the quality of care for all patients with HF. To address these gaps in knowledge, we investigated the change of health status over time, predictors of change, and the association with other clinical outcomes in patients from the Alberta HEART (Heart Failure Aetiology and Analysis Team) study including healthy volunteers, patients at risk of heart failure (with or without symptoms), and patients with HFpEF or HFrEF.
Methods
Alberta HEART Study
The data that support the findings of this study are available upon reasonable request from the Alberta HEART Publication Committee (consisting of JRBD, TA, and JAE). The Alberta HEART study recruited patients with and without cardiovascular disease, healthy controls, and patients with HF from a variety of different clinics and the community at large between 2010 and 2014 in Alberta, Canada. The study was registered on clinicaltrials.gov (NCT02052804) and has been described previously. 11 , 12 Briefly, adult patients across the spectrum of the risk of developing HF were enrolled. Enrolled patients were categorized into 4 subgroups: Group 1: healthy controls; Group 2: at‐risk patients with high risk of developing HF and no clinically overt HF; Group 3: patients with clinical HFpEF; and Group 4: patients with clinical HFrEF. Control patients with no evidence of coronary artery disease, hypertension, diabetes mellitus, organ diseases, inflammatory or autoimmune conditions, or cardiac medication use were recruited by referrals from patients and physicians and through other measures such as public advertising, media event, or other public engagements. Patients with 1 or more of the HF risk factors such as hypertension, diabetes mellitus, atrial fibrillation, coronary artery disease, or obesity but no clinically overt HF formed the at‐risk subgroup. HFpEF was defined as patients with the clinical presentation consistent with HF but with left ventricular ejection fraction ≥45% (with or without right ventricular enrollment). Finally, the HFrEF subgroup consisted of those with clinical diagnosis of HF and systolic HF (ie, ejection fraction <45%). Written informed consent was obtained and the study was approved by the health research ethics boards of the participating institutions.
After enrollment, participants underwent an echocardiogram along with blood tests. Standard baseline demographics, laboratory, and other medical history were collected via direct contact with the patient and with medical record review. Diagnoses were independently adjudicated by 2 cardiologists through reviewing the pertinent information and assessing echo parameters.
Health Status Assessments
Patients directly completed in paper format a set of validated questionnaires including the EuroQOL 5 dimensions questionnaire (EuroQoL‐5D), Functional Assessment of Cancer Therapy—Anemia questionnaire (FACT‐An), and Kansas City Cardiomyopathy Questionnaire (KCCQ) at baseline, and at 6 and 12 months follow‐up during follow‐up visits. This analysis was limited to the data from baseline and 12 months follow‐up visits.
Kansas City Cardiomyopathy Questionnaire
The Kansas City Cardiomyopathy Questionnaire (KCCQ) is a self‐administered HF‐specific instrument that covers a broad spectrum of domains, including physical limitations, symptom frequency and burden, self‐efficacy, social limitation, and quality of life. 13 Scores are transformed into a clinical summary score and overall summary score (OSS), which range from 0 to 100 with higher scores representing better health status. The clinical summary score is derived from the symptom (frequency and severity) and physical limitation domain. The overall summary score is derived from the physical function, symptom (frequency and severity), social function, and quality of life domains. The absolute difference between baseline and 12 months KCCQ OSS and clinical summary score was calculated and categorized into worsened, stable, and improved categories, with an improvement or worsening of ≥5 points, indicating a minimal clinically important difference in health status. 14 , 15
EuroQol 5 dimensions questionnaire
The EQ‐5D is a generic measure of health status designed to be used in clinical trials and economic evaluations of healthcare interventions. It has a 5‐dimension, 5‐level descriptive system, covering the dimensions of mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression. 16 An improvement or worsening of ≥8 points indicates a minimal clinically important difference in health status. 17 , 18
FACT‐An
Patients’ assessment of symptoms such as fatigue is purported to provide further information separate from the physician assessments (eg, New York Heart Association [NYHA] classification) or KCCQ or EQ‐5D. To assess fatigue in greater detail and complement information from the KCCQ, the 47‐item FACT‐An was used, which consists of 5 subscales: physical well‐being (7 items), social/family well‐being (7 items), emotional well‐being (6 items), functional well‐being (7 items), and anemia symptoms (20 items). An improvement or worsening of ≥7 points indicates a minimal clinically important difference in FACT‐An. 19
Clinical Outcomes
Patients had outcomes assessed via linkage to external administrative databases held by Alberta Health Services. Clinical outcomes of interest include all‐cause death, all‐cause death and/or hospitalization for any reason, all‐cause death and/or hospitalization for cardiovascular causes, and hospitalization for cardiovascular causes. The hospitalizations for cardiovascular causes were identified by the presence of International Classification of Diseases 10 codes (Ixx, R000, R001, R570, R931, R943, T821, T817, T820, T825, T827, T828, T86200, Z450, Z452) in the main diagnosis field.
Statistical Analysis
Categorical variables were presented as frequencies and percentages and compared using Pearson chi‐square or Fisher's exact tests, as appropriate, and continuous variables were reported as medians with 25th and 75th percentiles (interquartile range [IQR]) and compared using the Kruskal–Wallis test.
For the main analysis, only available HRQoL measurements were used without any imputation. The baseline to 12 months changes were described over time in HRQoL measures across the 4 subgroups (controls, at risk, HFpEF, and HFrEF). Patients were categorized based on their change in HRQoL into 3 categories with worsened, stable, and improved HRQoL. For KCCQ, ≥5 increase and ≥5 decrease in KCCQ score were defined as worsened and improved KCCQ, respectively, and other patients were considered stable. As previously mentioned, the cut‐points of 7 and 8 were used for categorizing the change over time of the FACT‐An and EQ‐5D measures, respectively. Baseline characteristics (demographics, comorbidities, physical symptoms, biomarkers, echocardiographic measurements, and medications) and changes in NYHA class were compared among subgroups with worsened, stable, and improved HRQoL over time. Baseline and changes in NYHA class were compared with the changes in HRQoL measures over time among patients with HF.
Multivariable analysis was performed for the association between changes in HRQoL measures and predictors in patients with HF. As predictors, we included patient characteristics at baseline (demographics, comorbidities, physical symptoms, biomarkers, left ventricle measurements, and health status scores) and changes in health status (biomarkers and left ventricle measurements). We used 2 types of estimation models: (1) a linear regression model with change in HRQoL measures between baseline and 12 month as outcome, and (2) a linear mixed effects regression model with HRQoL measures as outcome. The latter modeled HRQoL measurements as function of time (baseline and 12 month) and a random intercept for all measures of the same patient in order to account for correlations between repeated observations. The first model used the data of participants with HF with available HRQoL measures at baseline and 12 months (N=259); the second model used the data of participants with HF with HRQoL measures at baseline (N=336). To minimize the impact of missing data and to use all possible KCCQ records, we used multiple data imputation repeated 25 times (using SAS proc mi procedure with fully conditional specification method) for missing predictor values whenever KCCQ measures where available at baseline and follow‐up in HF participants. The parameters of the mixed effects regression model were estimated for each of the 25 imputed data sets and combined (using SAS proc mianalyze) to reflect the uncertainty due to missing values. Variables included in the imputation procedure were age, sex, ethnicity, body mass index, physical symptoms, systolic blood pressure, NYHA class, laboratory measurements (natriuretic peptides, hemoglobin, creatinine), and echocardiographic measurements.
Kaplan–Meier estimator for survival from death and all‐cause hospitalization beyond 12‐month follow‐up visit, stratified by KCCQ OSS change over 12 months, was examined in the total cohort and in patients with HFpEF and HFrEF. Cox proportional hazards and Fine and Gray models were used to assess the association between change in HRQoL scores and rate of subsequent clinical outcomes. To examine the robustness of the association, we employed 3 nested adjustment models: Model 1—unadjusted; Model 2—adjusted for baseline KCCQ score; and Model 3—adjusted for baseline KCCQ score and clinical covariates that are all incorporated into the Meta‐Analysis Global Group In Chronic heart failure risk score 20 and are known to predict clinical outcomes including age, sex, body mass index, blood pressure, serum creatinine, left ventricular ejection fraction, NYHA functional class, current smoker, history of diabetes mellitus, chronic obstructive pulmonary disease, recent HF diagnosis, and treatment with beta‐blockers, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers. Missing predictor values needed to calculate Meta‐Analysis Global Group In Chronic heart failure risk score were imputed as explained previously. Models for cardiovascular hospitalizations included death as competing risk. Hazard ratio was provided with 95% CI, and a 2‐sided P<0.05 was considered as significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
The Alberta HEART cohort enrolled 621 patients. Patient characteristics in the total cohort and different subgroups are provided in Table S1. Patients with HF had a higher rate of comorbidities compared with patients at risk or controls. Data availability on HRQoL measures is provided in Tables S2 and S3. Baseline, and 12‐month KCCQ was available for 555 (89.3%) and 415 (66.8%) Alberta HEART participants, respectively. Of those 555 participants with available KCCQ at baseline, 64/78 (82.0%) controls, 70/141 (49.6%) at risk, 22/177 (12.4%) participants with HFpEF, abd 22/159 (13.8%) participants with HFrEF had baseline KCCQ OSS >95 and were subject to a ceiling effect.
Patients HFpEF or HFrEF had lower (ie, worse) HRQoL scores (including KCCQ, FACT‐An, and EQ‐5D) compared with those at risk of HF (regardless of symptom status) and controls (Table 1). At baseline, the median KCCQ OSS was 73 (IQR, 53, 86) for patients with HFpEF, 78 (IQR, 56, 90) for patients with HFrEF, 95 (IQR, 85, 99) for the at‐risk patients, and 100 (IQR, 98, 100) for the controls. In the comparison between the population with HFpEF and HFrEF, the 12‐month KCCQ OSS were numerically, but not significantly, lower in the population with HFpEF (71 [IQR, 53, 89] versus 79 [IQR, 56, 91], P=0.15) (Table 1 and Table S4).
Table 1.
Health Status Measurements at Baseline and 12 Months and the Change Over Time Using KCCQ, EQ‐5D, and FACT‐An
| Controls | At‐Risk | HFpEF | HFrEF | P Value | |
|---|---|---|---|---|---|
| N* | 98 | 163 | 191 | 169 | |
| KCCQ CSS, median (IQR) | |||||
| Baseline | 100 (97, 100) | 95 (85, 100) | 73 (55, 90) | 82 (61, 92) | <0.0001 |
| 12‐mo | 100 (100, 100) | 96 (84, 100) | 71 (56, 88) | 80 (64, 93) | <0.0001 |
| Δ KCCQ CSS, n (%) | |||||
| Decrease | 1 (3.8) | 23 (21.5) | 49 (34.8) | 33 (28.0) | <0.0001 |
| No change | 24 (92.3) | 64 (59.8) | 50 (35.5) | 53 (44.9) | |
| Increase | 1 (3.8) | 20 (18.7) | 42 (29.8) | 32 (27.1) | |
| KCCQ OSS, median (IQR) | |||||
| Baseline | 100 (98, 100) | 95 (85, 99) | 73 (53, 86) | 78 (56, 90) | <0.0001 |
| 12‐mo | 100 (100, 100) | 96 (84, 100) | 71 (53, 89) | 79 (56, 91) | <0.0001 |
| Δ KCCQ OSS, n (%) | |||||
| Decrease | 1 (3.8) | 19 (17.8) | 52 (36.9) | 32 (27.1) | <0.0001 |
| No change | 22 (84.6) | 71 (66.4) | 50 (35.5) | 46 (39.0) | |
| Increase | 3 (11.5) | 17 (15.8) | 39 (27.7) | 40 (33.9) | |
| FACT‐An, median (IQR) | |||||
| Baseline | 172 (160, 177) | 158 (137, 168) | 133 (110, 154) | 137 (112, 157) | <0.0001 |
| 12‐mo | 178 (167, 183) | 157 (137, 171) | 135 (112, 153) | 140 (117, 160) | <0.0001 |
| Δ FACT‐An, n (%) | |||||
| Decrease | 2 (25.0) | 22 (29.0) | 40 (32.8) | 22 (21.2) | 0.38 |
| No change | 4 (50.0) | 32 (42.0) | 45 (36.9) | 39 (37.5) | |
| Increase | 2 (25.0) | 22 (29.0) | 37 (30.3) | 43 (41.3) | |
| EQ‐5D, median (IQR) | |||||
| Baseline | 90 (80, 95) | 80 (70, 90) | 70 (55, 80) | 70 (50, 80) | <0.0001 |
| 12‐mo | 90 (85, 95) | 80 (70, 90) | 70 (50, 80) | 70 (50, 80) | <0.0001 |
| ΔEQ‐5D, n (%) | |||||
| Decrease | 1 (3.6) | 22 (24.5) | 46 (38.0) | 28 (27.2) | <0.0001 |
| No change | 25 (89.3) | 47 (52.2) | 45 (37.2) | 38 (36.9) | |
| Increase | 2 (7.1) | 21 (23.3) | 30 (24.8) | 37 (35.9) | |
CSS indicates KCCQ clinical summary score; EQ‐5D, EuroQOL 5 dimensions questionnaire; FACT‐An, Functional Assessment of Cancer Therapy—Anemia; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HRQoL, health‐related quality of life; IQR, interquartile range; KCCQ, Kansas City Cardiomyopathy Questionnaire; N, number; OSS, KCCQ overall summary score; SD, standard deviation; and Δ, change.
Data on availability of HRQoL measurements is provided in Table S4.
Change of NYHA and HRQoL Over Time in Patients With HF
Respectively, 30.5% and 32.4% of patients with HF experienced at least 5 points improvement or worsening in KCCQ OSS at 12 months (Table 1 and Figure 1), and this did not differ between HFpEF and HFrEF subgroups (Figure S1, Table 1). Similar findings were observed for the physical limitation score of the KCCQ (Figure 1), and there was no difference between study subgroups in terms of change of FACT‐An or EQ‐5D at 12 months (Table 1). Improved NYHA functional class was reported in 43 patients with HF (15.7%) at 12 months (Table S5); this did not differ between populations with HFpEF and HFrEF.
Figure 1. The change in KCCQ overall summary score (A) and physical limitations score (B) over 12‐month follow‐up in patients from different Alberta HEART (Heart Failure Aetiology and Analysis Team) subgroups (N=392).
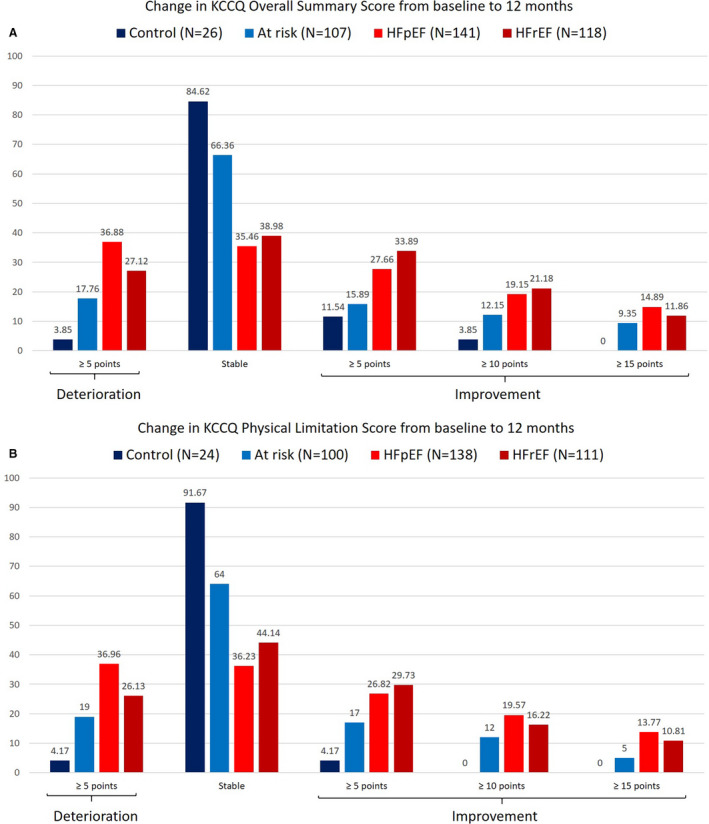
HFpEF indicates heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; KCCQ, Kansas City Cardiomyopathy Questionnaire; and N, number.
Patient Characteristics and Change of HRQoL
Among all Alberta HEART participants, patient characteristics varied between those with changed KCCQ over 12 months compared with those with stable KCCQ over the same time frame (Table 2). Patients with decreased KCCQ over 12 months period were older; had a higher rate of comorbidities such as coronary artery disease, chronic obstructive pulmonary disease, or atrial fibrillation, higher body mass index; lower hemoglobin levels; higher brain‐type natriuretic peptide and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) levels; lower left ventricular ejection fraction (LVEF); higher left ventricular mass index; higher right ventricular systolic pressure; and higher E/e' ratio compared with those with no change in the quality of life in the same follow‐up period (Table 2).
Table 2.
Baseline Characteristics of the Alberta HEART Patients With Available Baseline and 12‐Months KCCQ data (N=392) Categorized by the Change of KCCQ Overall Summary Score Over 12‐Month Period
| Change in KCCQ OSS at 12‐mo Compared to Baseline | P Value | |||
|---|---|---|---|---|
| Decrease (≥5 Pts) | No Change (<5 Pts) | Increase (≥5 Pts) | ||
| N | 104 | 189 | 99 | |
| Male | 68 (65.4) | 116 (61.4) | 58 (58.6) | 0.6028 |
| Age, median (IQR) | 71.5 (64.0, 80.0) | 65.0 (56.0, 72.0) | 68.0 (57.0, 77.0) | <0.0001 |
| NYHA functional classification, N | 81 | 94 | 78 | |
| Class I | 13 (16.0) | 32 (34.0) | 21 (26.9) | 0.1148 |
| Class II | 46 (56.8) | 45 (47.9) | 37 (47.4) | |
| Class III | 22 (27.2) | 17 (18.1) | 19 (24.4) | |
| Class IV | 0 (0.0) | 0 (0.0) | 1 (1.3) | |
| Medical comorbidity | ||||
| Atrial fibrillation | 53 (51.0) | 48 (25.4) | 42 (42.4) | <0.0001 |
| Coronary artery disease | 26 (25.0) | 17 (9.0) | 16 (16.2) | 0.0011 |
| Diabetes mellitus | 40 (38.5) | 59 (31.2) | 34 (34.3) | 0.4537 |
| COPD | 52 (50.0) | 64 (33.9) | 32 (32.3) | 0.0106 |
| Laboratory measurements, median (IQR) | ||||
| Hemoglobin, g/dL (N=277/333) | 134.0 (124.5, 145.0) | 142.0 (131.0, 151.0) | 140.0 (130.0, 147.0) | 0.0071 |
| BNP, pg/mL (N=298/353) | 138.0 (51.0, 264.0) | 45.0 (20.0, 131.0) | 109.0 (43.0, 271.0) | <0.0001 |
| NT‐proBNP, pg/mL (N=298/353) | 664.7 (207.2, 1788.7) | 145.5 (46.5, 631.7) | 553.9 (157.3, 1252.5) | <0.0001 |
| BMI (N=332/392) | 31.3 (27.5, 34.9) | 28.9 (25.8, 33.2) | 29.6 (26.4, 34.6) | 0.0223 |
| Signs and symptoms | ||||
| Leg edema | 44 (42.3) | 43 (22.8) | 39 (39.4) | 0.0006 |
| Shortness of breath | 64 (61.5) | 75 (39.7) | 57 (57.6) | 0.0004 |
| Fatigue | 64 (61.5) | 71 (37.6) | 65 (65.7) | <0.0001 |
| Heart sounds S3 | 11 (10.6) | 13 (6.9) | 11 (11.1) | 0.3859 |
| JVD | 46 (44.2) | 40 (21.2) | 27 (27.3) | 0.0002 |
| PND | 8 (7.7) | 7 (3.7) | 9 (9.1) | 0.1432 |
| PHJR | 18 (17.3) | 23 (12.2) | 17 (17.2) | 0.3682 |
| Echocardiographic parameters, median (IQR) | ||||
| LVEF, % (N=302/357) | 55.8 (42.2, 64.0) | 59.0 (48.4, 66.0) | 52.1 (40.7, 61.3) | 0.0028 |
| LVEDVI, mL/m2 (N=301/355) | 52.6 (40.6, 66.3) | 50.5 (39.6, 70.9) | 55.9 (39.8, 73.4) | 0.7289 |
| LVMI, g/m2 (N=319/372) | 93.5 (78.4, 114.9) | 85.2 (66.5, 111.5) | 92.1 (72.5, 116.8) | 0.0247 |
| RVSP, mm Hg (N=182/211) | 34.0 (27.0, 39.8) | 29.6 (24.6, 36.3) | 33.8 (28.8, 42.7) | 0.0315 |
| E/e' average (N=276/326) | 12.8 (9.6, 16.1) | 9.9 (7.7, 12.7) | 9.9 (8.2, 13.3) | 0.0004 |
| Medications | ||||
| ACEi/ARB | 82 (78.8) | 141 (74.6) | 77 (77.8) | 0.6747 |
| Beta blocker | 90 (86.5) | 106 (56.1) | 75 (75.8) | <0.0001 |
| Loop diuretic | 62 (59.6) | 62 (32.8) | 50 (50.5) | <0.0001 |
| MRA (spironolactone) | 21 (20.2) | 37 (19.6) | 27 (27.3) | 0.2936 |
| Digoxin | 14 (13.5) | 10 (5.3) | 13 (13.1) | 0.0253 |
All comparisons are across 3 groups for available data, with the exception of NYHA Functional Classification that is compared among 3 groups for patients with HF only. ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, brain‐type natriuretic peptide; COPD, chronic obstructive pulmonary disease; E/e', the ratio of transmitral Doppler early filling velocity to tissue Doppler early diastolic mitral annular velocity; F/U, follow‐up; IQR, interquartile range; JVD, jugular vein distension; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEDVI, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; MRA, mineralocorticoid receptor antagonist; N, number; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; OSS, KCCQ overall summary score; PHJR, positive hepatojugular reflex; PND, paroxysmal nocturnal dyspnea; and RVSP, right ventricle systolic pressure.
Comorbidities such as coronary artery disease and chronic obstructive pulmonary disease, health status at baseline and the change of NT‐proBNP and LVEF from baseline to 12 months were shown to be predictors of change in KCCQ over 12 months in patients with HF (Table S6).
HRQoL and Clinical Outcomes
After the 12‐month HRQoL assessment, we followed patients for a median follow‐up period of 3 (IQR, 2, 4) years, during which 60 (9.8%) participants died and 254 (41.5%) were hospitalized. Death or rehospitalization after the 12‐month assessment was higher in patients who had changed (increased or decreased) KCCQ in the first 12 months as compared with those with stable KCCQ (Figure 2). Patients with increased KCCQ over 12 months still had a lower KCCQ at 12 months compared to those with unchanged KCCQ (Tables S7 through S9). However, when adjusted for the baseline KCCQ level and clinical variables incorporated in the Meta‐Analysis Global Group In Chronic heart failurerisk score, only decrease in KCCQ compared with no change over time was associated with adverse clinical outcomes. The composite outcome data were mainly driven by rehospitalizations as the deaths were few in number. In the subgroup analysis, a decrease in KCCQ was a significant predictor of clinical events in patients with HFpEF. Similar pattern was found in the HFrEF subgroup; however, this was underpowered to demonstrate a statistical difference (Table 3).
Figure 2. Kaplan–Meier curves for death and rehospitalization after 12‐month assessment for patients with KCCQ overall summary score available at baseline and at 12 months: (A) in total Alberta HEART (Heart Failure Aetiology and Analysis Team) cohort (N=392); (B) in patients with HFpEF (N=141); (C) in patients with HFrEF (N=118); and (D) the adjusted hazard ratios in patients with HF and those with HFrEF and HFpEF.
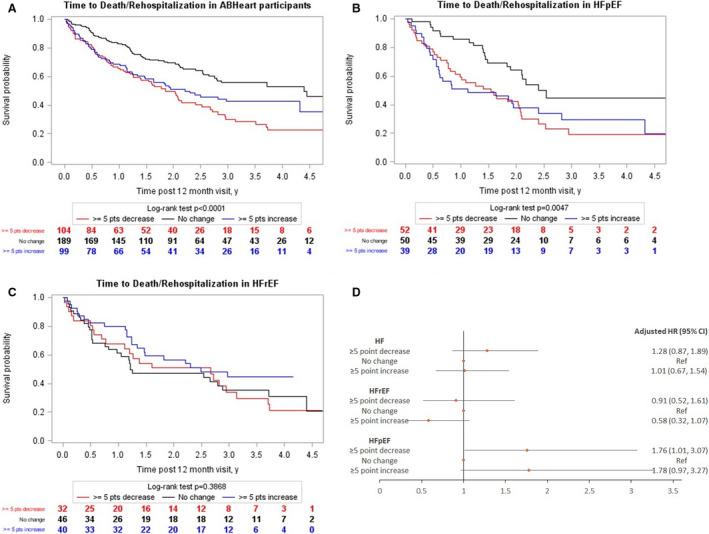
HF indicates heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; KCCQ, Kansas City Cardiomyopathy Questionnaire; and Ref, reference.
Table 3.
Association Between Changes in KCCQ Overall Summary Score From Baseline to 12 Months With Clinical Outcomes in Patients With HFpEF and HFrEF
| HF Group (N=259) | HFpEF (N=141) | HFrEF (N=118) | P Value† | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Events in Total N of Group | Unadjusted | Adjusted for Baseline KCCQ | Adjusted for Clinical Variables* | Number of Events in Total N of Group | Unadjusted | Adjusted for Baseline KCCQ | Adjusted for Clinical Variables* | Number of events in Total N of Group | Unadjusted | Adjusted for Baseline KCCQ | Adjusted for Clinical Variables* | ||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||||
| All‐cause death | |||||||||||||
| ≥5 points decrease in KCCQ OSS | 12/84 | 0.79 (0.38, 1.66) | 0.8 (0.38, 1.67) | 0.59 (0.28, 1.27) | 5/52 | 0.47 (0.16, 1.39) | 0.47 (0.16, 1.40) | 0.35 (0.11, 1.06) | 7/32 | 1.35 (0.49, 3.74) | 1.39 (0.50, 3.84) | 1.06 (0.37, 3.04) | 0.1563 |
| No change in KCCQ OSS | 17/96 | Ref | Ref | Ref | 9/50 | Ref | Ref | Ref | 8/46 | Ref | Ref | Ref | |
| ≥5 points increase in KCCQ OSS | 11/79 | 0.74 (0.35, 1.58) | 0.72 (0.33, 1.57) | 0.74 (0.34, 1.62) | 5/39 | 0.62 (0.21, 1.84) | 0.62 (0.19, 2.04) | 0.62 (0.19, 1.99) | 6/40 | 0.88 (0.30, 2.55) | 0.85 (0.29, 2.48) | 0.9 (0.31, 2.65) | 0.4927 |
| All‐cause death and/or rehospitalization‡ | |||||||||||||
| ≥5 points decrease in KCCQ OSS | 59/84 | 1.52 (1.04, 2.21) | 1.46 (1.00, 2.13) | 1.29 (0.87, 1.9) | 37/52 | 2.17 (1.27, 3.71) | 2.11 (1.23, 3.61) | 1.76 (1.01, 3.08) | 22/32 | 1.03 (0.59, 1.79) | 1.01 (0.58, 1.77) | 0.91 (0.52, 1.61) | 0.0848 |
| No change in KCCQ OSS | 50/96 | Ref | Ref | Ref | 21/50 | Ref | Ref | Ref | 29/46 | Ref | Ref | Ref | |
| ≥5 points increase in KCCQ OSS | 48/79 | 1.17 (1.14, 1.74) | 0.96 (0.64, 1.46) | 0.99 (0.65, 1.5) | 27/39 | 2.02 (1.14, 3.58) | 1.79 (0.97, 3.28) | 1.77 (0.97, 3.26) | 21/40 | 0.7 (0.4, 1.24) | 0.54 (0.3, 0.97) | 0.56 (0.31, 1.02) | 0.0272 |
| All‐cause death/cardiovascular hospitalization | |||||||||||||
| ≥5 points decrease in KCCQ OSS | 43/84 | 1.41 (0.91, 2.19) | 1.41 (0.91, 2.2) | 1.21 (0.77, 1.91) | 22/52 | 1.61 (0.82, 3.15) | 1.6 (0.82, 3.13) | 1.23 (0.62, 2.45) | 21/32 | 1.4 (0.77, 2.53) | 1.45 (0.8, 2.62) | 1.34 (0.73, 2.46) | 0.8466 |
| No change in KCCQ OSS | 37/96 | Ref | Ref | Ref | 14/50 | Ref | Ref | Ref | 23/46 | Ref | Ref | Ref | |
| ≥5 points increase in KCCQ OSS | 30/79 | 0.95 (0.59, 1.53) | 0.84 (0.51, 1.39) | 0.88 (0.53, 1.45) | 15/39 | 1.36 (0.66, 2.83) | 1.23 (0.57, 2.68) | 1.2 (0.56, 2.58) | 15/40 | 0.7 (0.36, 1.35) | 0.62 (0.32, 1.21) | 0.67 (0.34, 1.31) | 0.3618 |
| Cardiovascular hospitalization§ | |||||||||||||
| ≥5 points decrease in KCCQ OSS | 40/84 | 1.71 (1.07, 2.75) | 1.73 (1.08, 2.79) | 1.54 (0.95, 2.5) | 21/52 | 2.85 (1.26, 6.42) | 2.83 (1.26, 6.37) | 2.24 (0.98, 5.1) | 19/32 | 1.35 (0.74, 2.46) | 1.44 (0.78, 2.66) | 1.39 (0.75, 2.59) | 0.1758 |
| No change in KCCQ | 29/96 | Ref | Ref | Ref | 8/50 | Ref | Ref | Ref | 21/46 | Ref | Ref | Ref | |
| ≥5 points increase in KCCQ OSS | 28/79 | 1.16 (0.69, 1.96) | 0.99 (0.56, 1.74) | 1.02 (0.58, 1.79) | 14/39 | 2.35 (0.997, 5.55) | 2.08 (0.85, 5.11) | 2.04 (0.85, 4.93) | 14/40 | 0.72 (0.36, 1.43) | 0.61 (0.29, 1.29) | 0.63 (0.29, 1.36) | 0.0781 |
HFpEF indicates heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; KCCQ, Kansas City Cardiomyopathy Questionnaire; and OSS, overall summary score.
Adjusted for baseline KCCQ OSS and Meta‐Analysis Global Group In Chronic heart failure risk score.
P value for interaction between HF subtype and change in KCCQ OSS adjusted for baseline KCCQ OSS and MAGGIC risk score.
There were only 3 patients with death and without all‐cause hospitalization. Modeling of all‐cause hospitalization in the presence of death as competing risk was not attempted.
The hazard of cardiovascular hospitalization subdistribution was modelled. Fine and Gray models were used.
Discussion
Patients with HFpEF are shown to have both similarities and differences in the pathophysiology and clinical outcomes compared with HFrEF. In this study, we identified 3 key findings worthy of further exploration. First, patients with HFpEF had numerically lower KCCQ scores compared with patients with HFrEF at baseline and 12 months. The number of patients with either an improvement or deterioration in KCCQ was not distinguishable by ejection fraction alone, but patients with HFpEF were starting at a worse HRQoL. Second, although HFrEF or HFpEF was not a predictor of KCCQ change among patients with HF, the study showed differential patterns of relationship between the change of KCCQ levels and subsequent clinical outcomes with a stronger relationship of change in HRQoL to clinical outcomes (death and/or rehospitalization) in patients with HFpEF (P for interaction after adjustment for baseline KCCQ and clinical variables=0.0272).
In the Alberta HEART cohort study, KCCQ, FACT‐An, and EQ‐5D were used to assess and follow the patient‐reported health status among study participants. EQ‐5D was similarly reduced in the at‐risk patients who had symptoms of other diseases when compared with those with HF (data not shown). Similarly, there was no difference between Alberta Heart subgroups in terms of the change of FACT‐An over 6 and 12 months. Numerous studies have shown that disease‐specific measures of QoL are more accurate as compared with generic ones in assessing the health status in patients with HF. 21 Prior literature evaluating the HRQoL measures demonstrated the KCCQ to have the best performance among all disease‐specific HRQoL instruments in terms of validity and sensitivity to change. 22 , 23
We have confirmed the findings of other studies that both the baseline results 1 , 6 , 24 , 25 , 26 , 27 , 28 and changes over time 26 , 27 in the KCCQ are predictors of cardiovascular mortality and morbidity. In previous studies, for every 5‐point decrease in the KCCQ score, there was a 6% to 11% increase in the risk of cardiovascular death and HF hospitalization in patients with HFrEF. 10 , 14 , 26 , 29 There is less information about this from the HFpEF setting. Data from the TOPCATE (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) trial showed the increase in KCCQ‐OSS between visits was associated with a lower risk of cardiovascular death or first HF hospitalization in patients with HFpEF. 10 In our study, we compared the subsequent clinical outcomes in patients with increased, unchanged, and reduced KCCQ in their first 12 months of follow‐up and showed the composite outcomes of death and rehospitalization were more common in patients with HFpEF who had exhibited decreases in their KCCQ as compared with those with stable KCCQ.
Previous studies purported a lack of correlation between HRQoL and changes in biomarker levels such as NT‐proBNP over time 30 , 31 or the lack of correlation between HRQoL and LVEF at baseline. 5 , 32 , 33 , 34 In this study, higher natriuretic peptide levels, lower LVEF, and higher right ventricle systolic pressure levels at baseline were associated with higher likelihood of change (increase or decrease) in KCCQ over 12 months, and changes in NT‐proBNP and LVEF were independent predictors of KCCQ change over 12 months.
Several strengths and limitations are noteworthy. The Alberta HEART cohort included a broad spectrum of subjects from healthy controls to at‐risk patients and those with HFpEF and HFrEF. We recruited from the community via contact with clinicians, patient groups, and the media and had excellent female participation in all groups with the exception of patients with HFrEF—further work is needed on this front. We had missing data on HRQoL measurements and some other clinical variables. Although there were differences between patients with available and missing HRQoL data in terms of clinical characteristics, the rate of missing data was negligible in the HF subgroups, and the results remained consistent when the missing data were imputed in a sensitivity analysis. Similar to other observational studies lacking the randomization, this study might also be limited by the possibility of unmeasured or unknown confounders; however, given the extensive amount of data collected in the Alberta HEART cohort from clinical features to imaging etc, we believe that the likelihood of having a major effect on findings is probably low. The KCCQ is designed for evaluating HRQoL in patients with HF and had not been tested previously in at‐risk population or controls, although there was no difficulty in completing the KCCQ in those subgroups. Finally, all measures have variability and some of the observed differences may have reflected variations in the measures rather than true clinical changes. Also, roughly one eighth of patients with HF were subject to a ceiling effect; however, this proportion was higher as expected in the at‐risk and control populations. The lower variability and the observed ceiling effect in the at‐risk and control groups point to the fact that the changes in HF subgroups are more likely owing to variability of HRQoL over time.
In conclusion, patients with HFpEF had numerically lower KCCQ scores compared with patients with HFrEF at baseline and 12 months. Although the HRQoL change was not distinguishable by ejection fraction alone, after including key prognostic factors such as natriuretic peptides, HRQoL change remained an important predictor of further clinical events in patients with HFpEF. This lends weight to trial design as HRQoL could be an early marker of therapeutic interventions that hold promise, or given the observed variability and prognostication in different patient trajectories, it could be a valuable potential measure for quantifying the quality of care in healthcare systems.
Appendix
Alberta Heart Investigators
Jason Dyck, PhD, Mazankowski Alberta Heart Institute, Department of Pediatrics, University of Alberta, Edmonton, AB, Canada; Todd Anderson, MD, FRCPC, Libin Cardiovascular Institute of Alberta, Department of Cardiac Sciences, University of Calgary, Calgary, AB, Canada; Harald Becher, MD, PhD, FRCPC, Mazankowski Alberta Heart Institute, Department of Medicine, University of Alberta, Edmonton, AB, Canada; Israel Belenkie, MD, FRCPC, Libin Cardiovascular Institute of Alberta, Department of Cardiac Sciences, University of Calgary, Calgary, AB, Canada; Alexander Clark, PhD, Mazankowski Alberta Heart Institute, Faculty of Nursing, University of Alberta, Edmonton, AB, Canada; Henry Duff, MD, FRCPC, Libin Cardiovascular Institute of Alberta, Department of Cardiac Sciences, University of Calgary, Calgary, AB, Canada; Justin Ezekowitz, MBBCh, MSc, Mazankowski Alberta Heart Institute, Department of Medicine, University of Alberta, Edmonton, AB, Canada; Matthias Friedrich, MD, FRCPC, Montreal Heart Institute, Departments of Cardiology and Radiology, Université de Montréal, Montréal, QC, Canada; Mark Haykowsky, PhD, Mazankowski Alberta Heart Institute, Faculty of Rehabilitation Medicine, University of Alberta, Edmonton, AB, Canada; Jonathan Howlett, MD, FRCPC, Libin Cardiovascular Institute of Alberta, Department of Cardiac Sciences, University of Calgary, Calgary, AB, Canada; Zamaneh Kassiri, PhD, Mazankowski Alberta Heart Institute, Department of Physiology, University of Alberta, Edmonton, AB, Canada; Padma Kaul, PhD, Department of Medicine, University of Alberta, Edmonton, AB, Canada; Daniel Kim, MD, FRCPC, Mazankowski Alberta Heart Institute, Department of Medicine, University of Alberta, Edmonton, AB, Canada; Merril Knudtson, MD, FRCPC, Libin Cardiovascular Institute of Alberta, Department of Cardiac Sciences, University of Calgary, Calgary, AB, Canada; Peter Light, PhD, Mazankowski Alberta Heart Institute, Alberta Diabetes Institute, Department of Pharmacology, University of Alberta, Edmonton, AB, Canada; Gary Lopaschuk, PhD, Mazankowski Alberta Heart Institute, Department of Pediatrics, University of Alberta, Edmonton, AB, Canada; Finlay McAlister, MD, FRCPC, Mazankowski Alberta Heart Institute, Department of Medicine, University of Alberta, Edmonton, AB, Canada; Michelle Noga, MD, FRCPC, Mazankowski Alberta Heart Institute, Department of Radiology and Diagnostic Imaging, University of Alberta, Edmonton, AB, Canada; Gavin Oudit, MD, PhD, FRCPC, Mazankowski Alberta Heart Institute, Department of Medicine, University of Alberta, Edmonton, AB, Canada; Ian Paterson, MD, FRCPC, Mazankowski Alberta Heart Institute, Department of Medicine, University of Alberta, Edmonton, AB, Canada; Hude Quan, PhD, Libin Cardiovascular Institute of Alberta, Department of Cardiac Sciences, University of Calgary, Calgary, AB, Canada; Richard Schulz, PhD, Departments of Pediatrics and Pharmacology, University of Alberta, Edmonton, AB, Canada; Richard Thompson, PhD, Mazankowski Alberta Heart Institute, Department of Biomedical Engineering, University of Alberta, Edmonton, AB, Canada; Sarah Weeks, MD, FRCPC, Libin Cardiovascular Institute of Alberta, Department of Cardiac Sciences, University of Calgary, Calgary, AB, Canada; James White, MD, FRCPC, Libin Cardiovascular Institute of Alberta, Department of Cardiac Sciences, University of Calgary, Calgary, AB, Canada.
Source of Funding
Funding was provided by an Alberta Innovates – Health Solutions Interdisciplinary Team Grant to Alberta Heart Failure Etiology and Analysis Research Team (Alberta HEART), grant no. AHFMR ITG 200801018.
Disclosures
None.
Supporting information
Tables S1–S9
Figure S1
(J Am Heart Assoc. 2020;9:e017278 DOI: 10.1161/JAHA.120.017278.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017278
For Sources of Funding and Disclosures, see page 11.
References
- 1. Joseph SM, Novak E, Arnold SV, Jones PG, Khattak H, Platts AE, Davila‐Roman VG, Mann DL, Spertus JA. Comparable performance of the Kansas City Cardiomyopathy Questionnaire in patients with heart failure with preserved and reduced ejection fraction. Circ Heart Fail. 2013;1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rector TS, Tschumperlin LK, Kubo SH, Bank AJ, Francis GS, McDonald KM, Keeler CA, Silver MA. Use of the living with heart failure questionnaire to ascertain patients' perspectives on improvement in quality of life versus risk of drug‐induced death. J Card Fail. 1995;201–206. [DOI] [PubMed] [Google Scholar]
- 3. Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;1016–1024. [DOI] [PubMed] [Google Scholar]
- 4. Flynn KE, Lin L, Moe GW, Howlett JG, Fine LJ, Spertus JA, McConnell TR, Pina IL, Weinfurt KP. Relationships between changes in patient‐reported health status and functional capacity in outpatients with heart failure. Am Heart J. 2012;88–94.e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Myers J, Zaheer N, Quaglietti S, Madhavan R, Froelicher V, Heidenreich P. Association of functional and health status measures in heart failure. J Card Fail. 2006;439–445. [DOI] [PubMed] [Google Scholar]
- 6. Parissis JT, Nikolaou M, Farmakis D, Paraskevaidis IA, Bistola V, Venetsanou K, Katsaras D, Filippatos G, Kremastinos DT. Self‐assessment of health status is associated with inflammatory activation and predicts long‐term outcomes in chronic heart failure. Eur J Heart Fail. 2009;163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoekstra T, Jaarsma T, van Veldhuisen DJ, Hillege HL, Sanderman R, Lesman‐Leegte I. Quality of life and survival in patients with heart failure. Eur J Heart Fail. 2013;94–102. [DOI] [PubMed] [Google Scholar]
- 8. Hoekstra T, Lesman‐Leegte I, van Veldhuisen DJ, Sanderman R, Jaarsma T. Quality of life is impaired similarly in heart failure patients with preserved and reduced ejection fraction. Eur J Heart Fail. 2011;1013–1018. [DOI] [PubMed] [Google Scholar]
- 9. Lewis EF, Lamas GA, O'Meara E, Granger CB, Dunlap ME, McKelvie RS, Probstfield JL, Young JB, Michelson EL, Halling K, et al. Characterization of health‐related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur J Heart Fail. 2007;83–91. [DOI] [PubMed] [Google Scholar]
- 10. Pokharel Y, Khariton Y, Tang Y, Nassif ME, Chan PS, Arnold SV, Jones PG, Spertus JA. Association of serial Kansas City Cardiomyopathy Questionnaire assessments with death and hospitalization in patients with heart failure with preserved and reduced ejection fraction: a secondary analysis of 2 randomized clinical trials. JAMA Cardiol. 2017;1315–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ezekowitz JA, Becher H, Belenkie I, Clark AM, Duff HJ, Friedrich MG, Haykowsky MJ, Howlett JG, Kassiri Z, Kaul P, et al. The Alberta heart failure etiology and analysis research team (HEART) study. BMC Cardiovasc Disord. 2014;91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ezekowitz JA, McAlister FA, Howlett J, Alemayehu W, Paterson I, Belenkie I, Oudit GY, Kaul P, Dyck JR, Anderson T. A prospective evaluation of the established criteria for heart failure with preserved ejection fraction using the Alberta HEART cohort. ESC Heart Fail. 2018;19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;1245–1255. [DOI] [PubMed] [Google Scholar]
- 14. Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;707–715. [DOI] [PubMed] [Google Scholar]
- 15. Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE, Mc‐Guire DK, Pitt B, Scirica BM, Austin B, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE‐HF trial. Circulation. 2019;1463–1476. [DOI] [PubMed] [Google Scholar]
- 16. Herdman M, Gudex C, Lloyd A, Janssen MF, Kind P, Parkin D, Bonsel G, Badia X. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D-5L). Qual Life Res. 2011;1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoehle LP, Phillips KM, Speth MM, Caradonna DS, Gray ST, Sedaghat AR. Responsiveness and minimal clinically important difference for the EQ‐5D in chronic rhinosinusitis. Rhinology. 2019;110–116. [DOI] [PubMed] [Google Scholar]
- 18. Della Patrona S, Zanini A, Aiello M, Adamo D, Casale S, Cherubino F, Raimondi E, Zampogna E, Chetta A, Spanevello A. Estimation of minimum clinically important difference in EQ‐VAS score after pulmonary rehabilitation in COPD patients. Eur Respir J. 2014;P3669. [DOI] [PubMed] [Google Scholar]
- 19. Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution‐based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;547–561. [DOI] [PubMed] [Google Scholar]
- 20. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;1404–1413. [DOI] [PubMed] [Google Scholar]
- 21. Schowalter M, Gelbrich G, Stork S, Langguth JP, Morbach C, Ertl G, Faller H, Angermann CE. Generic and disease‐specific health‐related quality of life in patients with chronic systolic heart failure: impact of depression. Clin Res Cardiol. 2013;269–278. [DOI] [PubMed] [Google Scholar]
- 22. Garin O, Herdman M, Vilagut G, Ferrer M, Ribera A, Rajmil L, Valderas JM, Guillemin F, Revicki D, Alonso J. Assessing health‐related quality of life in patients with heart failure: a systematic, standardized comparison of available measures. Heart Fail Rev. 2014;359–367. [DOI] [PubMed] [Google Scholar]
- 23. Eurich DT, Johnson JA, Reid KJ, Spertus JA. Assessing responsiveness of generic and specific health related quality of life measures in heart failure. Health Qual Life Outcomes. 2006;89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hawwa N, Vest AR, Kumar R, Lahoud R, Young JB, Wu Y, Gorodeski EZ, Cho L. Comparison between the Kansas City Cardiomyopathy Questionnaire and New York Heart Association in assessing functional capacity and clinical outcomes. J Card Fail. 2017;280–285. [DOI] [PubMed] [Google Scholar]
- 25. Heidenreich PA, Spertus JA, Jones PG, Weintraub WS, Rumsfeld JS, Rathore SS, Peterson ED, Masoudi FA, Krumholz HM, Havranek EP, et al. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol. 2006;752–756. [DOI] [PubMed] [Google Scholar]
- 26. Kosiborod M, Soto GE, Jones PG, Krumholz HM, Weintraub WS, Deedwania P, Spertus JA. Identifying heart failure patients at high risk for near‐term cardiovascular events with serial health status assessments. Circulation. 2007;1975–1981. [DOI] [PubMed] [Google Scholar]
- 27. Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation. 2004;546–551. [DOI] [PubMed] [Google Scholar]
- 28. Arnold SV, Spertus JA, Vemulapalli S, Dai D, O'Brien SM, Baron SJ, Kirtane AJ, Mack MJ, Green P, Reynolds MR, et al. Association of patient‐reported health status with long‐term mortality after transcatheter aortic valve replacement: report from the STS/ACC TVT Registry. Circ Cardiovasc Interv. 2015;9:e002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luo N, O'Connor CM, Cooper LB, Sun JL, Coles A, Reed SD, Whellan DJ, Pina IL, Kraus WE, Mentz RJ. Relationship between changing patient‐reported outcomes and subsequent clinical events in patients with chronic heart failure: insights from HF‐ACTION. Eur J Heart Fail. 2019;63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Napier R, McNulty SE, Eton DT, Redfield MM, AbouEzzeddine O, Dunlay SM. Comparing measures to assess health‐related quality of life in heart failure with preserved ejection fraction. JACC Heart Fail. 2018;552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luther SA, McCullough PA, Havranek EP, Rumsfeld JS, Jones PG, Heidenreich PA, Peterson ED, Rathore SS, Krumholz HM, Weintraub WS, et al. The relationship between B‐type natriuretic peptide and health status in patients with heart failure. J Card Fail. 2005;414–421. [DOI] [PubMed] [Google Scholar]
- 32. Juenger J, Schellberg D, Kraemer S, Haunstetter A, Zugck C, Herzog W, Haass M. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart. 2002;235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gorkin L, Norvell NK, Rosen RC, Charles E, Shumaker SA, McIntyre KM, Capone RJ, Kostis J, Niaura R, Woods P, et al. Assessment of quality of life as observed from the baseline data of the studies of left ventricular dysfunction (SOLVD) trial quality‐of-life substudy. Am J Cardiol. 1993;1069–1073. [DOI] [PubMed] [Google Scholar]
- 34. Dracup K, Walden JA, Stevenson LW, Brecht ML. Quality of life in patients with advanced heart failure. J Heart Lung Transplant. 1992;273–279. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S9
Figure S1


