Abstract
Approximately 50% of patients with heart failure have preserved ejection fraction. Although a wide variety of conditions cause or contribute to heart failure with preserved ejection fraction, elevated left ventricular filling pressures, particularly during exercise, are common to all causes. Acute elevation in left‐sided filling pressures promotes lung congestion and symptoms of dyspnea, while chronic elevations often lead to pulmonary vascular remodeling, right heart failure, and increased risk of mortality. Pharmacologic therapies, including neurohormonal modulation and drugs that modify the nitric oxide/cyclic GMP‐protein kinase G pathway have thus far been limited in reducing symptoms or improving outcomes in patients with heart failure with preserved ejection fraction. Hence, alternative means of reducing the detrimental rise in left‐sided heart pressures are being explored. One proposed method of achieving this is to create an interatrial shunt, thus unloading the left heart at rest and during exercise. Currently available studies have shown 3‐ to 5‐mm Hg decreases of pulmonary capillary wedge pressure during exercise despite increased workload. The mechanisms underlying the hemodynamic changes are just starting to be understood. In this review we summarize results of recent studies aimed at elucidating the potential mechanisms of improved hemodynamics during exercise tolerance following interatrial shunt implantation and the current interatrial shunt devices under investigation.
Keywords: exercise, exercise capacity, interatrial, shunt
Subject Categories: Heart Failure, Clinical Studies, Hemodynamics, Mechanisms, Physiology
Nonstandard Abbreviations and Acronyms
- 6MWT
6-minute walk test
- CVP
central venous pressure
- EF
ejection fraction
- HF
heart failure
- HFmrEF
heart failure with midrange ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HR
heart rate
- IASD
interatrial shunt device
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- LA
left atrial
- LAP
left atrial pressure
- LV
left ventricular
- MAGGIC
Meta‐Analysis Global Group in Chronic Heart Failure
- MLWHF
Minnesota Living With Heart Failure
- NYHA
New York Heart Association
- PA
pulmonary artery
- PAC
pulmonary artery compliance
- PCWP
pulmonary capillary wedge pressure
- PVR
pulmonary vascular resistance
- Qp:Qs
ratio of pulmonary to systemic blood flow
- RA
right atrial
- RAP
right atrial pressure
- RV
right ventricular
- VO2
peak oxygen consumption
Over 50% of patients with heart failure (HF) have a left ventricular (LV) ejection fraction (EF) >40%. HF with an EF of 40% to 50% has been termed HF with midrange EF (HFmrEF), and in the setting of EF >50% the condition is referred to as HF with preserved EF (HFpEF). These conditions are present in a heterogenous group of patients with a broad spectrum of underlying conditions and disorders. Nevertheless, patients with HFpEF/HFmrEF share common findings of elevated left atrial (LA) and LV filling pressures during exercise, which are associated with acute development of pulmonary congestion and considered to be largely responsible for the hallmark symptom of dyspnea on exertion. 1 , 2 Although theoretically attractive, therapies designed to attenuate neurohormonal activation have been met with limited success in this HF subgroup. 3 , 4 Therefore, attention has turned to other means of reducing the detrimental rise in left‐sided pressures during exercise. One method of achieving this goal is by creation of an interatrial shunt, which may be particularly effective at reducing pulmonary venous and LA pressures (LAPs) during exercise, even if patients are not overtly volume overloaded or have normal LAP at rest. Several recent clinical studies of interatrial shunt devices (IASDs) have shed new light on the pathophysiology of exercise intolerance and the mechanisms by which IASDs may provide benefit.
In this review, we summarize factors contributing to effort intolerance in HFpEF/HFmrEF, the current IASDs under investigation, and the results of studies aimed at elucidating the potential mechanisms of improved exercise tolerance after IASD implantation.
Hemodynamics and Exercise Intolerance in HFmrEF or HFpEF
In healthy patients, cardiac output is augmented by increases in stroke volume, and heart rate (HR) without excessive increases in ventricular filling pressures. 5 , 6 Stroke volume has been shown to increase by ≈40% in healthy volunteers during exercise as a result of both an increase in LV end‐diastolic volume and decrease of LV end‐systolic volume, resulting in an ≈15% absolute increase in LVEF, partially resulting from a marked ≈60% reduction in systemic vascular resistance. 7 The increase in LV end‐diastolic volume requires a compliant ventricle and enhanced early diastolic relaxation to facilitate filling at higher HRs in order to avoid a significant rise in pressure. Diastolic relaxation and enhancements of atrial contractility may also be important for increasing LV end‐diastolic volume at high HRs. In healthy patients, pulmonary capillary wedge pressure (PCWP) at peak supine exercise is generally <20 mm Hg and LV end‐diastolic pressure is generally <25 mm Hg. 8 , 9 , 10
In contrast, marked elevations of pulmonary arterial and venous pressures are observed during exercise in patients with HFpEF and these have been considered to significantly contribute to exercise intolerance. 6 Borlaug et al 11 showed in patients with HFpEF who had normal resting hemodynamics that increases of HR, decreases of systemic vascular resistance, and increases of cardiac output were all blunted compared with healthy controls during exercise. They also showed that, in comparison to control patients, PCWP and LV end‐diastolic pressure significantly increased in patients with HFpEF simply after raising their legs into the pedals of a supine bicycle and even more dramatically after 1 minute of initiating exercise. Additional increases were found at peak exercise (Figure 1), with PCWP increasing to >25 mm Hg in 88% of patients with HFpEF and on average reaching ≈35 mm Hg. 11 A more specific link between these increases of PCWP and exercise intolerance was recently demonstrated by its association with the development of pulmonary congestion as evidenced by B‐line artefacts seen on lung ultrasound. 2 Elevation in PCWP during exercise is associated with reductions in aerobic capacity in patients with HFpEF, even after accounting for other determinants such as cardiac output and peripheral oxygen extraction. 12
Figure 1. Changes in pulmonary capillary wedge pressure (PCWP) during exercise in patients with heart failure with preserved ejection fraction (red squares) vs controls (black circles).
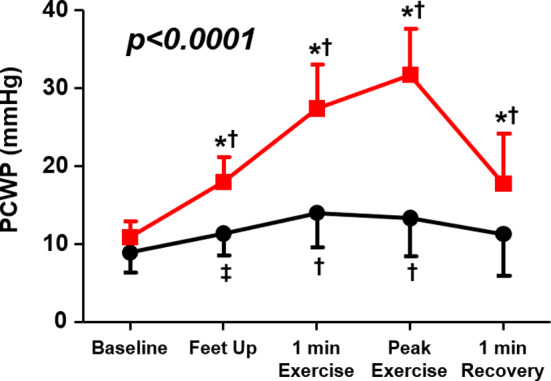
*P<0.001 for change in PCWP (vs control); †P<0.001 vs baseline (within group); ‡P<0.01 vs baseline (within group). Reproduced in part from Borlaug et al 11 with permission. Copyright ©2010, Wolters Kluwer Health, Inc.
Chronic elevations of pulmonary pressures also lead to pulmonary vascular remodeling including decreased compliance 13 , 14 and blunted reductions in pulmonary vascular resistance (PVR) and pulmonary arterial elastance during exercise. 14 Such changes in pulmonary properties can cause detrimental effects on right ventricular (RV) function, further compounding the ability of the left ventricle to fill adequately during exercise.
In addition to its association with exercise intolerance, elevated estimated diastolic pulmonary artery (PA) pressures or PCWPs at rest 15 , 16 and during exercise 17 have been associated with increased mortality. Regarding the interpretation of PCWP during exercise, it has been shown to be important to normalize this parameter for workload. This approach takes into account clinical improvements that may allow patients to reach higher levels of exercise before the PCWP rises to levels that produce symptoms 17 (discussed further below). Lower values of work‐normalized PCWP are associated with increased survival. 17 In addition, development of RV dysfunction has been shown to be associated with an almost 2‐fold increased risk of death in patients with HFpEF. 18
Given the above‐noted associations, approaches designed to prevent or reduce elevated left‐sided filling pressures during exercise have become a potential target of treatment. Since many patients with HFpEF have normal left‐sided filling pressures at rest, a therapy that does not reduce resting preload may be beneficial. Diuretics, which are currently the mainstay of therapy, cause frequent problems with orthostatic hypotension, prerenal azotemia, and electrolyte imbalances. A therapy without these undesirable side effects would be very helpful.
It is not just left‐sided pressures that rise dramatically with exercise in HFpEF. Figure 2A shows individual patient data summarizing the relationship between central venous pressure (CVP) and PCWP at rest in patients with HFpEF (solid blue circles) in comparison to a group of healthy controls (open red circles; age matched to patients with HFpEF, shown by the black dots). Control data are from the HemReX (Effect of Age on the Hemodynamic Response During Rest and Exercise in Healthy Humans) study. 19 Only approximately one third of patients with HFpEF have reasonably normal resting values of both CVP and PCWP, whereas in nearly all patients without HF they are within the normal range. As shown in Figure 2B, there are marked elevations of both PCWP and CVP during exercise in patients with HFpEF, which was the case in only a minority of control patients. The difference between controls and patients with HFpEF was especially apparent when approximately matching the groups for age (mean age of 69 years in both groups) and workload (43±18 watts [W] in patients with HFpEF versus 63±23 W in controls) (Figure 2B). Even comparing CVP and PCWP values in patients with HFpEF at the average of 43 W (which represented peak exercise in this group) with values achieved by healthy controls at peak exercise (100±35 W in the age‐matched subgroup), PCWP in patients with HFpEF was still significantly elevated (Figure 2C). The potential independent prognostic significance of an increase in CVP has not been specifically studied in HFpEF, although it has been shown to be important in HF with reduced EF (HFrEF). 20 There is increased evidence that elevated CVP interferes with end organ function, particularly renal and hepatic function. 21 Indeed, it has been shown that elevated CVP portends a worse prognosis, being independently associated with mortality. 22 As such, increases in CVP are relevant to the potential efficacy of IASDs.
Figure 2. Relationship between central venous pressure (CVP) and pulmonary capillary wedge pressure (PCWP).
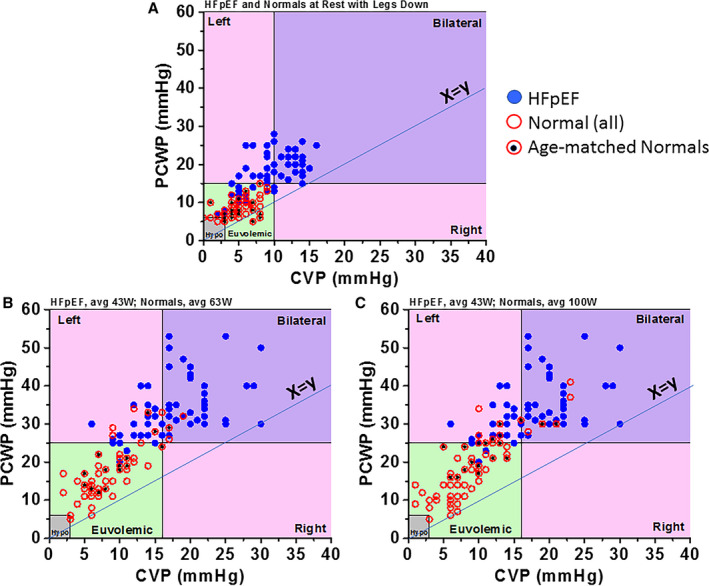
Patients with heart failure with preserved ejection fraction (HFpEF; blue dots), controls (open red circles), and age‐matched controls (red circles with black dots). A, Resting data. B, Data at peak exercise for patients with HFpEF and at submaximal exercise for controls. C, Data at peak exercise for both patients with HFpEF and controls. Bilateral refers to left‐ and right‐sided congestion, Left refers to left‐sided congestion only, and Right refers to right‐sided congestion only. Hypo indicates hypovolemic. Adapted from Wessler et al 31 .
Finally, it is noted that atrial fibrillation, which is present in 30% to 50% of patients with HFpEF, has a significant adverse impact on exercise tolerance, 23 reducing the ability to increase stroke volume by almost 50% compared with patients in sinus rhythm. Aside from loss of atrial kick, 1 additional factor potentially contributing to elevations in PCWP are decreased atrial and pulmonary venous compliances in HFpEF. 24
Extracardiac Factors Contribute to Exercise Intolerance in HF
A multitude of comorbidities are associated with HFpEF, including advanced age, obesity, diabetes mellitus, kidney dysfunction, sleep‐disordered breathing, chronic obstructive pulmonary disease, anemia, skeletal muscle dysfunction, and chronotropic incompetence. It is therefore not surprising that noncardiac factors contribute to exercise intolerance. 25 It has been shown that peak oxygen consumption (VO2) in HFpEF is determined by both cardiac and extracardiac factors, each contributing about 50%. 26 . To explore this hypothesis in the context of data available from IASD‐treated patients, Wolsk et al 27 compared baseline characteristics, exercise performance, and exercise hemodynamics of patients with HFpEF/HFmrEF enrolled in 2 IASD studies (Corvia Medical, Inc.) to those of healthy controls in the HemReX trial. 19 Mean peak workload was markedly lower in the HF group (45 W versus 137 W, P<0.0001). Consistent with the studies noted above, there were significantly blunted increases in HR of patients with HF (29 versus 64 beats per min), cardiac index (1.5 versus 5.8 L/min per m2), and stroke volume index (4 versus 26 mL/m2) at peak exercise. At reasonably matched workloads, these and other characteristics differed between healthy controls and patients with HF (Figure 2). In multiregression analysis, higher PCWPs (47%) and reduced stroke volume (12%) accounted for 59% of the difference in exercise tolerance between groups using ergometer exercise in the supine position. Of all other parameters examined, higher body mass index contributed to 31% of the difference between the groups. Higher body mass index may be a marker for a multitude of other comorbidities (eg, diabetes mellitus, hypertension, and sedentary lifestyle) that affect the ability of peripheral vascular, musculature, and metabolic machinery to accommodate increased workloads. In a separate study, exercise PCWP was found to inversely correlate with peak VO2 in patients with HFpEF, whether performed in the supine or upright position. Remarkably, exercise PCWP remained associated with peak VO2 even after accounting for other determinants of oxygen transport including cardiac output and arterial‐venous oxygen content difference, 12 but the latter variables were also strongly correlated with exercise capacity. Thus, extracardiac factors contribute to exercise intolerance, and it should be recognized that an IASD can only impact the hemodynamic components contributing to exercise intolerance.
Theoretical Foundation for Hemodynamic Effects of IASDs
Given the lack of evidence‐based medical therapies for the treatment of HFpEF/HFmrEF to date, efforts have turned to directly targeting exercise‐induced elevations of LAP and PCWP with IASDs. By creating a conduit in the interatrial septum, the pressure gradient between the left and right atrium should allow for decompression of the left heart when PCWP is high, as occurs especially during exercise. A detailed theoretical foundation to support this approach was first provided by a previously developed cardiovascular model used to simulate hemodynamic changes typically seen in patients with HFpEF during exercise. 28 A complete recounting of the equations underlying this simulation is beyond the scope of this review. In brief, the contractile properties of each heart chamber were modeled by time‐varying elastances and the systemic and pulmonary vascular beds were modeled by series of resistance and capacitance elements. Model parameters were adjusted to match, as closely as possible, the average hemodynamic data from 2 published studies involving 39 patients with HFpEF, both at rest and during exercise. 11 , 29 Then, an interatrial shunt was introduced into the model by allowing flow between the right and left atria as determined by the equation for flow through a thin orifice (which depends on the area of the shunt and the pressure gradient between the chambers); results were obtained while varying the shunt diameter between 0 to 12 mm to predict the potential effects on resting and exercise hemodynamics. The impact of the interatrial shunt on right atrial (RA) pressure (RAP) and LAP for this average patient are summarized in Figure 3. The pressure gradient between chambers decreases as the interatrial shunt diameter increases, and this effect plateaus at ≈10 mm. There was a relatively steep increase in the pressure gradient with smaller shunt sizes.
Figure 3. Hemodynamic effects of interatrial shunt device (IASD) based on cardiovascular simulation models.
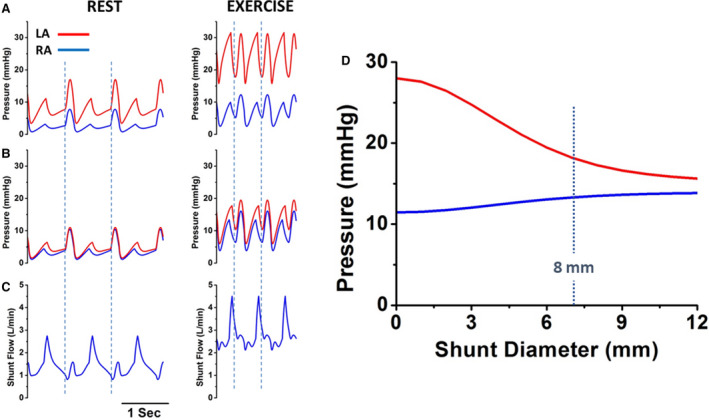
A, Mean right (blue) and left (red) atrial pressures as a function of shunt diameter based on hemodynamics of an average patient with heart failure with preserved ejection fraction (HFpEF). B, Simulations of right and left atrial pressure waves at rest (left) and during exercise (right) from the average patient with HFpEF before simulated insertion of an IASD. C, Right and left atrial pressure waves following simulated insertion of an IASD. D, Blood flow waveform across the IASD at rest and during exercise showing continuous left‐to‐right flow. LA indicates left atrium; and RA, right atrium. Reproduced in part from Kaye et al 28 with permission. Copyright ©2014, Elsevier.
Simulated effects of an IASD are further illustrated for an example of an 8‐mm shunt (the diameter of the Corvia Atrial Shunt described below). RAP and LAP tracings at rest and during exercise before shunt placement are shown in Figure 3B. Following introduction of the shunt, RAP and LAP gradients decreased (Figure 3C). At peak exercise, a decrease from ≈17 to ≈5 mm Hg was seen in the presence of the shunt, the result of an ≈8‐mm Hg decrease in LAP and an ≈3‐mm Hg increase in RAP. Under resting conditions, the average interatrial shunt flow was ≈1.4 L/min, which corresponded to a ratio of pulmonary to systemic blood flow (Qp:Qs) of 1.3 (Figure 3D). In addition, there was continuous flow across the shunt from left to right (and no right‐to‐left shunting). During exercise, there was also continuous, unidirectional left‐to‐right shunt flow, resulting in a similar Qp:Qs (1.4) and an average flow of 2.8 L/min. Of note, because of the reduction in LAP, peak and mean PA pressures did not increase as a consequence of the increased pulmonary flow. Furthermore, as noted above, it was predicted that an 8‐mm shunt diameter would yield a Qp:Qs of ≈1.3, which is significant in that prior literature and congenital heart guidelines indicate that congenital shunts of this magnitude are unlikely to have long‐term detrimental effects on pulmonary pressures, RV size, or function. 30
Finally, while these results provided a foundation for the concept of the use of an IASD to reduce PCWP and LAP, this study represented a simulation based on 1 average patient. The viability of the IASD approach relies on there being a meaningful pressure gradient between the left and right atria both at rest and during exercise in each patient treated. As illustrated in Figure 2, both at rest and during exercise, the CVP‐PCWP point of each patient falls above the line of identity (x=y), satisfying this criterion. 31 However, the distance from the line of identity is variable among patients, indicating considerable variability of the pressure gradient for shunting.
Clinical Data Related to IASDs
There are currently 12 reports summarizing the results of clinical studies with 5 different devices (Table 1). The first and most widely studied IASD has been developed by Corvia Medical, Inc. This device, the Corvia Atrial Shunt, is composed of a nitinol frame with an 8‐mm central channel (Figure 4A). It is implanted percutaneously via the femoral vein under fluoroscopic and echocardiographic guidance. Deployment is by transseptal puncture into the left atrium and advancement of the system in the left atrium. The LA side is opened then the system is retracted against the septum with subsequent deployment of the RA side to secure the device in place.
Table 1.
Summary of Completed Clinical Trials Performed With IASDs
| Device | Study | Study Design (Key Inclusion Criteria) | No. | Author | Year Published | Time Frame | Main Efficacy Findings* |
|---|---|---|---|---|---|---|---|
| Corvia Atrial Shunt | Feasibility | Single arm (EF >45%, NYHA class II–IV, PCWP >15 mm Hg at rest or >25 mm Hg during exercise) | 11 | Søndergaard et al 32 | 2014 | 1 mo | PCWP at rest† ↓5 mm Hg |
| Malek et al 33 | 2015 | 12 mo | NYHA class ↓0.5; MLWHF ↓29; 6MWT ↑32 m | ||||
| REDUCE LAP‐HF 34 |
Single arm (EF >40%, NYHA II–IV, PCWP >15 mm Hg at rest or >25 mm Hg during exercise) |
64 | Hasenfuß et al 35 | 2016 | 6 mo |
Qp:Qs 1.27 Peak exercise PCWP/W per kg 15 mm Hg/W per kg |
|
| Kaye et al 36 | 2016 | 12 mo | NYHA median by 1 class; 6MWT ↑32 m; MLWHF questionnaire ↓15; patency confirmed in 98.5% | ||||
| Kaye et al 40 | 2018 | 36 mo | Observed mortality was 3.4/100 pt‐y; lower than 10.2/100 pt‐y predicted by baseline MAGGIC score (P=0.02) | ||||
| REDUCE LAP‐HF I mechanistic study 38 | Randomized, sham‐controlled, double‐blinded (NYHA III/IV, EF ≥40%, exercise PCWP ≥15 mm Hg) | 44 | Feldman et al 37 | 2018 | 1 mo |
PCWP at peak exercise † ↓3 mm Hg Peak exercise PCWP/W per kg 16 mm Hg/W per kg |
|
| Shah et al 39 | 2018 | 12 mo | Yearly HFH rate ↓67% (P=0.06); NYHA median by 1 class; 6MWT no difference; KCCQ no difference. 100% shunt patency | ||||
| V‐Wave Gen1 | Special access pilot (Canada) | Single arm (NYHA III/IV, EF ≤40%, PCWP ≥15 mm Hg) | 10 | Del Trigo et al 47 | 2016 | 3 mo | N=8/9 showed improved NYHA; Duke activity status 11; KCCQ 35; 6MWT 74 m; PCWP ↓6 mm Hg |
| Pilot | Single arm (NYHA III/IV; EF >15%; ≥1 HFHs within 1 y) | 38 | Rodes‐Cabau et al 48 | 2018 | 12 mo | NYHA class I or II in 62%; KCCQ 73% improved ≥5‐point improvement; 6MWT ↑28 m; no significant change in PCWP, RAP, or PAP; Qp:Qs 1.1; 14% of valves occluded and 36% stenotic by 12 mo; better hemodynamic responses in patients with patent shunt | |
|
V‐Wave Gen2 |
Pilot | Single arm (NYHA III or IV) | 6 | Guimaraes et al 49 | 2020 | 12 mo | NYHA class I or II in 83%; Duke activity status ↑15; KCCQ ↑32; 6MWT ↑64 m; Qp:Qs 1.16. |
| Occlutech AFR | AFR‐PRELIEVE | Single arm (NYHA III or IV; EF ≥15%) | 36 | Paitazoglou et al 51 | 2019 | 3 mo | In HFrEF/HFpEF: NYHA class ‐1.4/‐1.1; KCCQ ↑16/↑20; 6MWT 30/↑26 m; PCWP ↓2/↓5 mm Hg; Qp:Qs 1.3/1.1 |
| NoYa | Pilot | Single arm (EF >35%) | 10 | Lotan 53 | 2019 | 1–3 mo | Shunt diameter 3‐4 mm; 6MWT 56 m; BNP 1878 pg/mL |
6MWT indicates 6‐minute walk test; AFR, atrial flow regulator; BNP, B‐type natriuretic peptide; EF, ejection fraction; HFH, heart failure hospitalization; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IASD, interatrial shunt device; KCCQ, Kansas City Cardiomyopathy Questionnaire; MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; MLWHF, Minnesota Living With Heart Failure; NYHA, New York Heart Association; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PRELIEVE, Pilot Study to Assess Safety and Efficacy of a Novel Atrial Flow Regulator (AFR) in Heart Failure Patients; REDUCE LAP‐HF, Reduce Elevated Left Atrial Pressure in Patients With Heart Failure; Qp:Qs, ratio of pulmonary to systemic blood flow; RAP, right atrial pressure; and W, watts.
For single‐arm studies, values are changes relative to patients’ baseline values; for randomized studies, changes are relative to the control group.
Indicates primary end point when one was declared prospectively.
Figure 4. Interatrial shunt devices (IASDs).
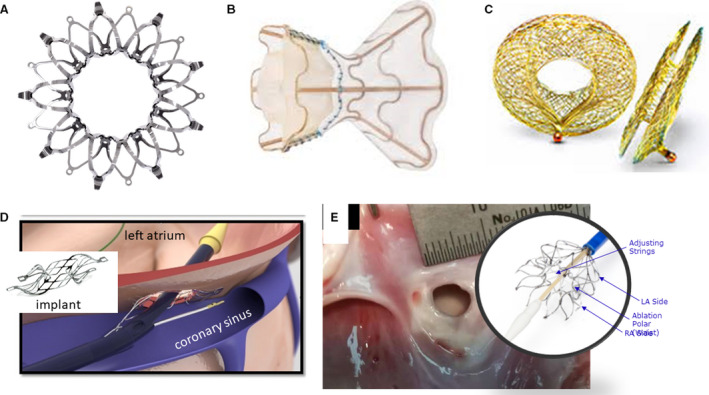
A, IASD II system (Corvia Medical, Inc.); B, V‐Wave device (V‐Wave Ltd.); C, Atrial flow regulator (AFR) Occlutech; D, Edwards Lifesciences Corporation transcatheter atrial shunt system; and E, NoYA adjustable interatrial shunt system (NoYA Global). LA indicates left atrial; and RA, right atrial.
In an initial safety and feasibility study of the Corvia Atrial Shunt in an open‐label pilot study of patients with an EF >45%, Søndergaard et al 32 showed that PCWP decreased from a mean baseline (rest) value of 19 to 14 mm Hg in 10 of the 11 patients at 30 days following implantation. Compared with baseline preimplantation values, 30‐meter improvements in median 6‐minute walk test (6MWT) and a 29‐point improvement in median Minnesota Living With Heart Failure (MLWHF) questionnaire score were reported at 1‐year of follow‐up. 33
That pilot study with the Corvia Atrial Shunt was followed by the REDUCE LAP‐HF (Reduce Elevated Left Atrial Pressure in Patients With Heart Failure) trial, 34 an open‐label study in which 64 patients with EF ≥40% received an IASD following exercise hemodynamic evaluation. Qp:Qs at rest averaged 1.27 at the 6‐month follow‐up. Compared with baseline, at peak exercise 6 months following implantation, there was an average ≈3 mm Hg reduction in PCWP (from 35 to 31 mm Hg) and a 2‐mm Hg increase in RAP (from 9 to 11 mm Hg), resulting in an average 5‐mm Hg reduction in the average gradient between PCWP to RAP (from 17 to 12 mm Hg). 35
Concomitantly, patients exercised ≈1 minute more, achieving 6.5 W more at 6 months compared with baseline (discussed further below). At 1 year, PCWP reductions and Qp:Qs values were sustained, and RAP was stable compared with that at 6 months. 36
Wessler et al 31 stratified these patients into 4 groups based on values of CVP and PCWP (Figure 5): both parameters normal (euvolemic); elevated CVP/normal PCWP (right‐sided congestion); normal CVP/elevated PCWP (left‐sided congestion); and elevated CVP and PCWP (bilateral congestion). At baseline (pre‐IASD) during exercise, a majority of patients (59%) fell into the elevated CVP/PCWP group and 33% into the elevated PCWP/normal CVP group, while only 8% had normal CVP and PCWP (Figure 5A). At 6 months post‐lASD during exercise (Figure 5B), 17% of patients were in the normal hemodynamic group and only 22% were in the elevated PCWP/normal CVP group, with similar percentages of patients in the other groups. Overall, the relationship between PCWP and CVP shifted downward by ≈4 mm Hg. It was also demonstrated that the larger the pressure gradient between the left and right atria at baseline, the greater the shunt flow, which correlated with a greater reduction in peak exercise PCWP (Figure 6A). 31 Corresponding to these hemodynamic changes, peak exercise tolerance increased at 6 months post‐IASD, from 42.5 W to 49.0 W (P=0.002), exercise duration increased by 12.5% from 7.3 to 8.2 minutes (P=0.03), and work‐normalized PCWP decreased significantly from 89.1 to 70.5 mm Hg/W per kg. Importantly, the study showed that systemic blood flow quantified by Fick principle was not decreased at rest (4.8 versus 5.1 L/min). Finally, there were statistically significant improvements at 6 and 12 months following IASD implantation in New York Heart Association (NYHA) class (from a median NYHA class III at baseline to II at 6 and 12 months), quality of life (MLWHF score decreased by 13 points at 6 months and 15 points at 12 months), and 6MWT (increasing by 32 meters at both 6 and 12 months). 35 , 36 An independent echocardiography core laboratory documented patency at 1 year in all but 1 patient and at 1 year and beyond in several patients.
Figure 5. Relationship between central venous pressure (CVP) and pulmonary capillary wedge pressure (PCWP) in patients with heart failure with preserved ejection fraction at peak exercise (A) at baseline and (B) at 6 months following interatrial shunt device implantation.
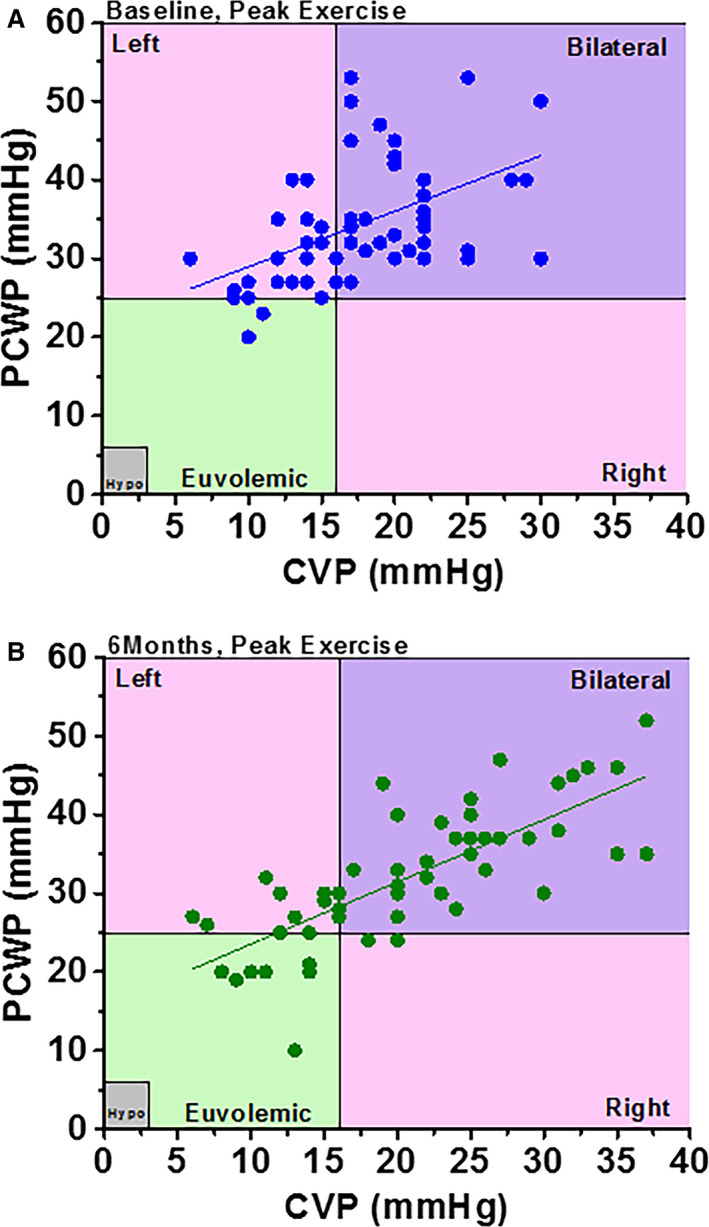
Bilateral refers to left‐ and right‐sided congestion, Left refers to left‐sided congestion only, and Right refers to right‐sided congestion only. Hypo indicates hypovolemic. Reproduced in part from Wessler et al 13 with permission. Copyright ©2018, Wolters Kluwer Health, Inc.
Figure 6. Correlates of interatrial shunt efficacy.
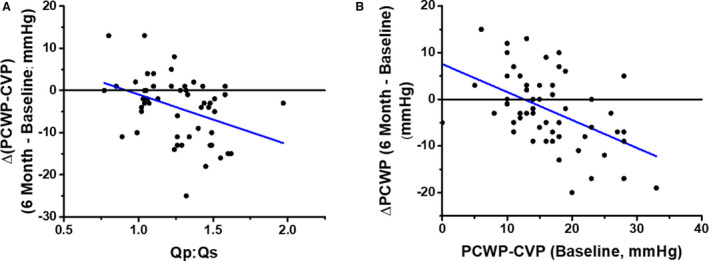
A, Dependence of reduction in pressure gradient on the magnitude of shunt flow ratio of pulmonary to systemic blood flow (Qp:Qs). B, Dependence of reduction in pulmonary capillary wedge pressure (PCWP) on baseline PCWP‐central venous pressure (CVP), which is the initial driving pressure for shunt flow. Reproduced in part from Wessler et al 13 with permission. Copyright ©2018, Wolters Kluwer Health, Inc.
To confirm these effects on hemodynamics and exercise tolerance identified in the open‐label study, the multicenter, randomized, double‐blind, sham‐controlled REDUCE LAP‐HF I mechanistic trial was conducted in patients with HF who had EF ≥40% and remained symptomatic despite medical therapy. 37 , 38 Patients were included if they had NYHA class III or ambulatory class IV HF with an exercise PCWP ≥25 mm Hg and a PCWP‐to‐RAP gradient ≥5 mm Hg. The primary end point was exercise PCWP at 1‐month postrandomization. All hemodynamic tracings were read in a blinded core laboratory by a single reader. Forty‐four patients were randomized 1:1. At 1 month, there was a statistically significant 3.2±5.2‐mm Hg reduction in exercise PCWP in the IASD group compared with a 0.9±5.1‐mm Hg increase in the sham control group (primary end point, P=0.03) (Figure 7). The study showed trends for improved delta exercise duration between baseline and 1 month (1.2±3.7 versus 0.4±3.5 minutes), increased workload (1.5±14.6 versus –1.9±10.8 W), workload‐corrected PCWP (–5.7±27.3 versus 10.3±45.9), and reductions in PVR index (–0.29±1.22 versus 0.31±1.64 Wood unit, P=0.051). At 1‐year follow‐up there were no significant differences in major adverse cardiac, cerebral, or renal events, with trends in favor of the IASD for improvement in NYHA class and reduction in HF hospitalization. 39 An independent echocardiography core laboratory documented patency of the shunt at 1 year in all patients.
Figure 7. Pulmonary capillary wedge pressure (PCWP) at different stages of exercise in (A) patients who underwent sham procedure (control group) and (B) patients who received an interatrial shunt device (IASD).
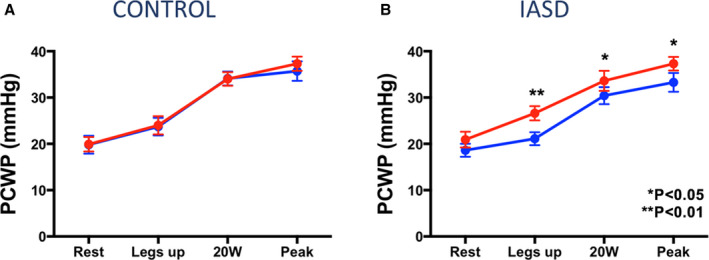
Comparison time points: baseline (red) and 1‐month postprocedure (blue). Reproduced in part from Feldman et al 37 with permission. Copyright ©2017, Wolters Kluwer Health, Inc.
In the 1‐year outcomes report for the REDUCE LAP‐HF open label study, Kaye et al 36 reported no significant changes in RA or LA size, small reductions in LV end‐diastolic volume, small increases in RV end‐diastolic volume, no changes in LVEF, and increases in RVEF. Noted changes were evident by 6 months following IASD implantation and did not change significantly from 6 to 12 months. Three‐year follow‐up on these patients compared observed mortality with that predicted by the Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC) score. While MAGGIC predicted 10.2 deaths per 100 patient‐years, the observed rate was 3.4 per 100 patient‐years (P=0.02). 40 Interestingly, there were no baseline characteristics that correlated with mortality or HF hospitalizations in the HFpEF cohort.
To explore the impact on LA size, which provides important prognostic information in HFpEF, 41 Hanff et al 42 noted that while group‐averaged LA volume did not change, there was a wide distribution of response to IASD, ranging between 50% reductions and 50% increases in LA size. It was further shown that patients with greater LA compliance (defined as the ratio between maximal LA volume and PCWP) and greater RA reservoir strain at baseline were each independently associated with greater reductions in LA volume at 6‐month follow‐up. Whether these findings provide a noninvasive means of identifying patients more likely to respond clinically to IASDs requires further study. The potential clinical implications of this finding include: (1) patients with a stiff, fibrotic left atrium (in whom LA compliance is low) may not be able to decompress their left atrium with IASD implantation, and (2) a relatively healthy right atrium, as evidenced by a higher RA reservoir strain (which means the right atrium is able to accept an increased volume of blood from the left atrium via the IASD during ventricular systole) is required for adequate decompression of the left atrium in response to IASD implantation. This latter finding suggests that in addition to avoiding IASD implantation in patients with HFpEF or HFmrEF who have significant RV failure, it may be important to avoid the IASD in patients with significant RV dysfunction.
Currently ongoing is the 608‐patient randomized, sham‐controlled REDUCE LAP‐HF II study (NCT03088033). This is a pivotal study evaluating the safety and clinical efficacy of the Corvia Atrial Shunt being conducted globally to attain regulatory approval in the United States. The primary end point is the composite of: (1) incidence of and time to cardiovascular mortality or first nonfatal ischemic stroke through 12 months; (2) total rate (first plus recurrent) per patient‐year of HF admissions or healthcare facility visits for intravenous diuresis for HF up to 24 months, analyzed when the last randomized patient completes 12 months of follow‐up, and time to first HF event; and (3) change in baseline Kansas City Cardiomyopathy Questionnaire (KCCQ) total summary score at 12 months assessed by the composite of cardiovascular mortality, nonfatal ischemic stroke, HF events, and quality of life.
Pulmonary Factors Contributing To Improved Exercise Tolerance by an IASD
The studies noted above have shown improvement in exercise capacity after implantation of the Corvia Atrial Shunt associated with reductions in PCWP and workload‐corrected PCWP. Additional analyses of the data from these studies have explored correlations between hemodynamic effects and other factors potentially influencing functional capacity and quality of life. It has previously been shown that in patients with HFpEF, but not controls, there is an inverse relationship between PA oxygen content and PVR, suggesting a possible role for hypoxic vasoconstriction as a contributor to pulmonary hypertension in this cohort. Recently, Obokata et al 43 hypothesized that the presence of the IASD may impact PA function at rest and during exercise by increasing the delivery of more richly oxygenated blood to the lungs without impacting systemic blood flow or oxygen delivery. Using data from the REDUCE LAP‐HF and REDUCE LAP‐HF I studies they found, before IASD implantation, blunted exercise‐induced reductions in PVR and effective pulmonary arterial elastance in addition to reduced pulmonary arterial compliance (PAC), in keeping with findings from prior studies. 13 , 14
After implantation of the IASD and establishment of an average Qp:Qs of 1.3, there were reductions in PCWP and the PCWP‐RAP gradient and a small increase in RAP. Notably, there was a 17% reduction in PVR, a 12% reduction in effective pulmonary arterial elastance, and a 24% increase in PAC (Figure 8A). Both the changes in PA elastance and compliance were directly correlated with the increase in pulmonary flow. Furthermore, patients who had a higher PA oxygen content had a lower PVR (Figure 8B). Patients whose PAC increased following IASD had better improvements in exercise duration compared with those in whom PAC did not increase. When exercise hemodynamics were evaluated post‐IASD, a 14% increase in exercise duration (7.4 to 8.4 minutes, P=0.006) and 12% increase in workload (43 to 48 W, P=0.003) were achieved. Importantly, despite the left‐to‐right shunt during exercise, systemic blood flow and oxygen delivery was higher when compared with baseline with septum intact. Similar to at rest, pulmonary compliance was higher and effective pulmonary arterial elastance was lower during exercise; although PVR trended lower by the same percentage, this was not statistically significant.
Figure 8. Impact of interatrial shunt on pulmonary vascular properties.
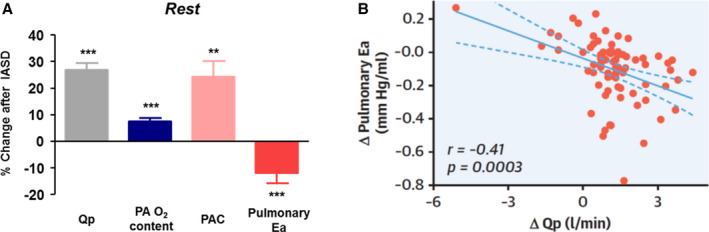
(A) Changes in pulmonary hemodynamics and mechanics 6 months following interatrial shunt device (IASD) implantation. (B) Relationship between changes in pulmonary effective arterial elastance (Ea) to the change of pulmonary flow (ΔQp) in response to the IASD. **P<0.01 and ***P<0.001 baseline vs follow‐up visit. PAO2 indicates pulmonary arterial oxygen concentration; PAC, pulmonary arterial compliance; and Qp, pulmonary blood flow. Reproduced in part from Obokata et al 43 with permission. Copyright ©2019, Elsevier.
Overall, it was demonstrated that increases in pulmonary blood flow and PA oxygen saturation following Corvia Atrial Shunt implantation were associated with improvements in PVR, PAC, and effective pulmonary arterial elastance during exercise without negatively affecting systemic blood flow or oxygen delivery. As such, in addition to the beneficial effect of lowering PCWP during exercise, the presence of an IASD positively impacted pulmonary hemodynamics, which may independently contribute to improved exercise capacity. It was postulated that 2 possible mechanisms may contribute to improved pulmonary vascular properties. First, increased oxygen content of pulmonary blood attributable to the shunt may oppose hypoxic vasoconstriction in the lungs, resulting in pulmonary vasodilation. Another possibility is that the increase in blood flow in the pulmonary bed may recruit underperfused zones of the lung. It should be noted that patients with significant pulmonary vascular disease (elevated PVR) were excluded from the trials that contributed to these analyses.
Characteristics of Patients Who May Benefit From an IASD
In view of the limited clinical outcome data, criteria for optimal patient selection for IASD therapy are unknown. Available data have thus far shown only weak correlations between hemodynamic effects and changes in exercise tolerance. 31 Nevertheless, hemodynamic factors would appear to be at the foundation of any clinical effects that can be provided by IASDs. Specifically, a left‐to‐right pressure gradient is required for the device to exert its hemodynamic effects. Accordingly, patients with a small gradient may benefit less. 28 Indeed, it has been shown that the greater the pressure gradient during exercise at baseline the greater the decrease in PCWP during exercise following IASD implantation (Figure 6B). Accordingly, this leads to the hypothesis that requiring a certain pressure gradient may increase the likelihood of the clinical effectiveness of the IASD.
Regarding other factors investigated thus far, data indicate that patients with HFmrEF (40 to 49%) appear to benefit similarly to those with HFpEF (>50%), at least as it relates to the hemodynamic effects of the Corvia Atrial Shyunt. 31 Other ongoing studies noted below are including patients with reduced LV function (NCT03499236), and further study is required to evaluate the potential role in patients with valvular heart disease.
Several other factors that may identify ideal candidates for IASD therapy have not yet been investigated. First, since IASDs increase right‐sided blood flow, patients with existing RV dysfunction and pulmonary hypertension (PVR >4 Wood units) have thus far been excluded from Corvia Atrial Shunt studies. In addition, patients with hypertrophic obstructive cardiomyopathy and restrictive and amyloid cardiomyopathies have also not been studied.
Addressing Potential Long‐Term Concerns
There are several theoretical safety concerns that need to be considered following IASD implantation. First, the development of right HF as a result of left‐to‐right shunting is a consideration. While small increases in RV size have been noted at 6 months, without further increase at 1 year, there has not been any indication of deterioration of RV function or clinical signs of right HF in Corvia Atrial Shunt studies. 36 Relevant to this concern, congenital heart disease guidelines suggest that a small shunt is not typically associated with deterioration of RV function and need only be closed in the presence of impaired functional capacity or RA and/or RV enlargement with a Qp:Qs >1.5; 30 the Corvia Atrial Shunt typically has an average clinically assessed Qp:Qs of ≈1.3.
Second, resting CVP tends to increase, albeit minimally (≈1 to 2 mm Hg), 6 months post‐IASD implantation, 31 without further increase at 1 year. However, the long‐term effects on liver and renal function should be evaluated.
Third, there was initial concern that there could be a significant reduction in systemic blood flow and delivery of oxygen following IASD implantation. However, as noted above, this is not the case as has been shown by measurements obtained 1, 6, and 12 months following implantation in Corvia studies. 35 , 36 , 37 We have speculated that rapid reflex‐mediated homeostatic mechanisms adjust systemic properties such that systemic flow is maintained. Acute reductions in PVR via the mechanisms described above may also be contributory.
Last, peripheral venous emboli may cross to the left side in the presence of an atrial septal defect. As such, creation of a shunt may increase the risk of paradoxical stroke, which is the cause of ischemic stroke in 25% to 40% of cases. One study showed that 14% of patients who underwent atrial septal defect closure presented with a paradoxical embolus and those patients tended to have a mean left‐to‐right shunt (Qp:Qs) of 1.4. 44 Nevertheless, despite simulation results and echocardiographic Doppler measurements showing continuous left‐to‐right shunting at rest and during exercise, right‐to‐left flow could be expected during certain maneuvers, such as Valsalva strain. Accordingly, longer‐term follow‐up in a larger number of patients is required to allay concerns regarding the risk of stroke.
Other IASD Devices
The data summarized above were obtained from studies of the Corvia Atrial Shunt. There are several other interatrial shunts currently under development and investigation. The V‐Wave device (V‐Wave Ltd.) initially introduced an hourglass‐shaped nitinol frame that is partially covered with expanded polytetrafluoroethylene (Figure 3B). The inner diameter of this device is 5 mm. The first generation of the device contained a porcine trileaflet pericardial valve, designed to avoid flow reversal through the shunt. 45 Following a preclinical study, 46 a first‐in‐human study reported on 38 patients with successful implantation of the device (30 with HFrEF and 8 with HFpEF). At 3 and 12 months of follow‐up there were improvements in NYHA class, quality of life, and 6MWT distance. However, at 12 months, 5 of 36 (14%) shunts occluded and 13 (36%) were stenotic. Patients with patent shunts showed encouraging clinical results in addition to a reduction in PCWP (23.3 to 18 mm Hg, P=0.011). 47 , 48 A second‐generation device has been developed by removing the 1‐way bioprosthetic valve to overcome pannus thickening, which was reportedly associated with device occlusion. This has been shown to remain patent at 6 months of follow‐up. 49 A 400‐patient randomized trial, the RELIEVE‐HF (Reducing Lung Congestion Symptoms in Advanced Heart Failure, NCT03499236), is currently enrolling in the United States, Canada, Europe, and Israel. Notably, this trial includes patients with HF across the EF spectrum (including HFrEF) and does not require invasive hemodynamic exercise testing for enrollment into the trial.
Another device, the Atrial Flow Regulator has been developed by Occlutech. Composed of a nitinol mesh, it has 2 flat discs connected by a 1‐ to 2‐mm neck with a central fenestration allowing communication between the atria. It is produced in 3 different fenestration sizes (6, 8, and 10 mm). This has been implanted in 12 patients with severe pulmonary arterial hypertension, all of whom had relief of syncope and improvement in 6MWT distance in addition to increase in cardiac index (2.36 to 2.89 L/min per m2) and systemic oxygen transport (367.5 to 428.0 mL/min per m2). 50 An open‐label nonrandomized clinical trial (PRELIEVE [Pilot Study to Assess Safety and Efficacy of a Novel Atrial Flow Regulator (AFR) in Heart Failure Patients], NCT03030274) is currently recruiting patients with symptomatic HFpEF or HFrEF and an HF admission in the past 12 months. A recent report of results from 36 patients (16 with EF <40% and 20 with EF ≥40%) followed for 3 months showed statistically nonsignificant 1‐ to 2‐mm Hg increases in RAP and 2‐ to 5‐mm Hg decreases in PCWP; resting cardiac output did not change. 51 Statistically nonsignificant improvements in NYHA, quality of life, and 6MWT have also been reported.
Another device currently under study is the coronary sinus to LA shunt (Edwards Lifesciences Corporation, Figure 3D). The coronary sinus is accessed via the right internal jugular vein; a puncture is then created between the coronary sinus and the left atrium with subsequent deployment of the shunt device. The shunt flow is alongside the natural coronary sinus flow, which preserves the interatrial septum and coronary sinus for the possibility of future interventions. In a first‐in‐human study, 11 patients with NYHA III or IV despite maximal tolerated HF therapy were included. 52 At rest, PCWP was reported to decrease by 10 mm Hg at 30‐day follow‐up from a baseline value of 21 mm Hg, with no significant change in RAP. Improvements in NYHA functional class were also reported.
The NoYA Global adjustable shunting system (Figure 4E) differs from the devices described above in that it consists of a radioablation catheter to create a persistent hole in the interatrial septum and has no device implant. 53 The diameter of the hole can, in principle, be adjusted between 4 and 12 mm. The catheter is removed after the procedure so that after creation of the atrial septal defect, no foreign body remains and there is no requirement for anticoagulation after the procedure. A pilot study including 10 patients reported orifice sizes of 5 mm at baseline, which decreased to 4 mm by 30‐day follow‐up, and neither LAP (21.9 versus 20.3, P=0.36) nor RAP (11.9 versus 13.0, P=0.13) changed significantly. In contrast, N‐terminal pro‐B‐type natriuretic peptide and 6MWT distance were each reported to improve significantly.
Finally, Alleviant Medical, Inc. is currently developing a transcatheter system that creates a left‐to‐right interatrial shunt without a permanent implant by cutting and removing septal tissue. The technology has been evaluated in animal studies and may enter human clinical trials in 2020.
Summary and Future Directions
Clinical studies support the notion that the abnormal, rapid, and marked increases of PCWP experienced by patients with HF is a therapeutic target for improving their exercise tolerance, quality of life, and clinical outcomes. Significant effort is being invested into the development and clinical evaluation of IASDs for this purpose. Current understanding of the effects of IASDs and the main studies that have contributed to this understanding are summarized in Figure 9. Much has already been learned regarding the hemodynamic effects of the 8‐mm Corvia Atrial Shunt and the relationship between hemodynamics and exercise tolerance. PCWP reductions amounting to ≈4 mm Hg during exercise have been identified in 2 Corvia Atrial Shunt clinical trials that employed a blinded hemodynamic core laboratory. These PCWP reductions have been associated with increases in exercise duration and Watts, which emphasizes the need to account for workload when interpreting the findings and comparing different IASDs. 17 The finding of beneficial effects on the pulmonary vasculature postulated to be mediated by increased flow and oxygen tension of blood perfusing the lung represents an additional effect that may also contribute to improved exercise tolerance and clinical outcomes. Additional unforeseen secondary factors may be identified (be they beneficial or detrimental) as more information is collected. Although currently limited in number of patients and duration of follow‐up, the midterm safety profile of IASDs appears to be acceptable.
Figure 9.
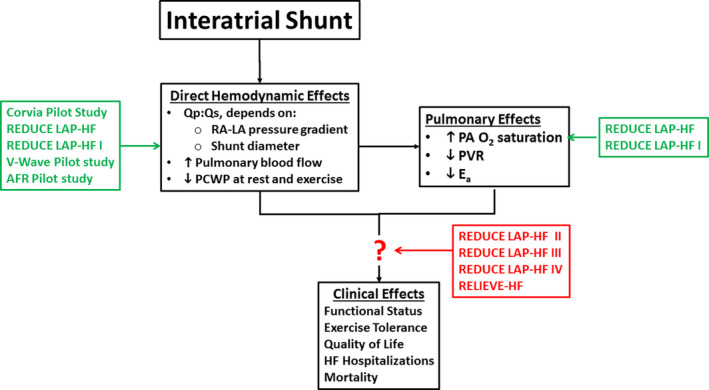
Summary of the current understanding of the mechanisms of interatrial shunts and the studies that generated data to support these findings.AFR indicates atrial flow regulator; HF, heart failure; LA, left atrial; O2, oxygen; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; REDUCE LAP‐HF, Reduce Elevated Left Atrial Pressure in Patients With Heart Failure; Qp:Qs, ratio of pulmonary to systemic blood flow; RAP, right atrial; and RELIEVE‐HF, Reducing Lung Congestion Symptoms in Advanced Heart Failure. Refer to Table 1 for study details and references.
Several important questions remain to be addressed, including:
Is the PCWP‐RAP pressure gradient an important determinant of IASD clinical effectiveness?
Are results of invasive exercise hemodynamics at baseline beneficial in selecting patients more likely to respond to IASDs?
Should the size of the IASD be optimized for individual patients, and, if so, what factors are critical for such a determination?
Are there upper or lower bounds of RVEF and LVEF where this form of therapy provides clinical benefits?
Does atrial fibrillation or LA or RA myopathy impact the effectiveness of IASDs?
Aside from hemodynamic factors, what other clinical characteristics are important for identifying patients most likely to benefit from IASDs?
Will IASDs serve as a viable treatment option for patients with HFrEF or valvular heart disease?
Will any safety issues arise during long‐term follow‐up (eg, impact on RV and RA size and function, impact on pulmonary vasculature, right‐to‐left shunting with paradoxical stroke,and atrial arrhythmias)?
Conclusions
Several randomized pivotal clinical outcome studies are currently underway that will provide critical information regarding several of these questions. Primarily, however, such studies aim to generate definitive proof that IASDs provide meaningful improvements in clinical outcomes and quality of life.
Sources of Funding
Studies reported on in this article have been supported by Corvia Medical, Inc.
Disclosures
D.B. reports hemodynamic core laboratory fees from Corvia Medical, Inc. J.K. is an employee of Corvia Medical. Inc. F.G. reports consulting fees from Carmat, Abbott, Pfizer, and Boehringer‐Ingelheim, and speakers’ fees from Astra‐Zeneca, Orion Pharma. and Novartis. S.J.S. reports receiving grants from the National Institutes of Health (R01 HL140731, R01 HL120728, R01 HL107577, and R01 HL149423), the American Heart Association (#16SFRN28780016 and #15CVGPSD27260148), Actelion, AstraZeneca, Corvia, and Novartis; and has received consulting fees from Actelion, Amgen, AstraZeneca, Bayer, Boehringer‐Ingelheim, Cardiora, Eisai, Ionis, Ironwood, Merck, Novartis, Pfizer, Sanofi, and United Therapeutics. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2020;9:e016760 DOI: 10.1161/JAHA.120.016760.)
For Sources of Funding and Disclosures, see page 14.
References
- 1. Obokata M, Olson TP, Reddy YNV, Melenovsky V, Kane GC, Borlaug BA. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J. 2018;39:2810–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reddy YNV, Obokata M, Wiley B, Koepp KE, Jorgenson CC, Egbe A, Melenovsky V, Carter RE, Borlaug BA. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J. 2019;40:3721–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124:1598–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Triposkiadis F, Butler J, Abboud FM, Armstrong PW, Adamopoulos S, Atherton JJ, Backs J, Bauersachs J, Burkhoff D, Bonow RO, et al. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J. 2019;40:2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shibata S, Hastings JL, Prasad A, Fu Q, Bhella PS, Pacini E, Krainski F, Palmer MD, Zhang R, Levine BD. Congestive heart failure with preserved ejection fraction is associated with severely impaired dynamic Starling mechanism. J Appl Physiol. 1985;2011:964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Higginbotham M, Morris K, Williams R, McHale P, Coleman R, Cobb F. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58:281–291. [DOI] [PubMed] [Google Scholar]
- 8. Thadani U, Parker JO. Hemodynamics at rest and during supine and sitting bicycle exercise in normal subjects. Am J Cardiol. 1978;41:52–59. [DOI] [PubMed] [Google Scholar]
- 9. Parker JO, Thadani U. Cardiac performance at rest and during exercise in normal subjects. Bull Eur Physiopathol Respir. 1979;15:935–949. [PubMed] [Google Scholar]
- 10. Yoshida AK, Kambara H, Tamaki S, Suzuki Y, Kawai C, Tamaki N, Torizuka K. Left ventricular responses to supine bicycle exercise assessed by radionuclide angiocardiography and a Swan-Ganz catheter. Jpn Circ J. 1985;49:661–671. [DOI] [PubMed] [Google Scholar]
- 11. Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reddy YN, Olson TP, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic correlates and diagnostic role of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37:3293–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gorter TM, Obokata M, Reddy YN, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J. 2018;39:2825–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mascherbauer J, Zotter-Tufaro C, Duca F, Binder C, Koschutnik M, Kammerlander AA, Aschauer S, Bonderman D. Wedge pressure rather than left ventricular end‐diastolic pressure predicts outcome in heart failure with preserved ejection fraction. JACC Heart Fail. 2017;5:795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zile MR, Bennett TD, El Hajj S, Kueffer FJ, Baicu CF, Abraham WT, Bourge RC, Warner SL. Intracardiac pressures measured using an implantable hemodynamic monitor: relationship to mortality in patients with chronic heart failure. Circ Heart Fail. 2017;10:e003594 10.1161/CIRCHEARTFAILURE.116.003594. [DOI] [PubMed] [Google Scholar]
- 17. Dorfs S, Zeh W, Hochholzer W, Jander N, Kienzle RP, Pieske B, Neumann FJ. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3103–3112. [DOI] [PubMed] [Google Scholar]
- 18. Obokata M, Reddy YNV, Melenovsky V, Pislaru S, Borlaug BA. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J. 2019;40:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolsk E, Bakkestrom R, Thomsen JH, Balling L, Andersen MJ, Dahl JS, Hassager C, Moller JE, Gustafsson F. The influence of age on hemodynamic parameters during rest and exercise in healthy individuals. JACC Heart Fail. 2017;5:337–346. [DOI] [PubMed] [Google Scholar]
- 20. Cooper LB, Mentz RJ, Stevens SR, Felker GM, Lombardi C, Metra M, Stevenson LW, O'Connor CM, Milano CA, Patel CB, et al. Hemodynamic predictors of heart failure morbidity and mortality: fluid or flow? J Card Fail. 2016;22:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. [DOI] [PubMed] [Google Scholar]
- 23. Kaye DM, Silvestry FE, Gustafsson F, Cleland JG, van Veldhuisen DJ, Ponikowski P, Komtebedde J, Nanayakkara S, Burkhoff D, Shah SJ. Impact of atrial fibrillation on rest and exercise haemodynamics in heart failure with mid-range and preserved ejection fraction. Eur J Heart Fail. 2017;19:1690–1697. [DOI] [PubMed] [Google Scholar]
- 24. Reddy YNV, Obokata M, Egbe A, Yang JH, Pislaru S, Lin G, Carter R, Borlaug BA. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur J Heart Fail. 2019;21:891–900. [DOI] [PubMed] [Google Scholar]
- 25. Butler J, Hamo CE, Udelson JE, Pitt B, Yancy C, Shah SJ, Desvigne-Nickens P, Bernstein HS, Clark RL, Depre C, et al. Exploring new endpoints for patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9:e003358 10.1161/CIRCHEARTFAILURE.116.003358. [DOI] [PubMed] [Google Scholar]
- 26. Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, Houstis NE, Eisman AS, Hough SS, Lewis GD. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015;8:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wolsk E, Kaye D, Komtebedde J, Shah SJ, Borlaug BA, Burkhoff D, Kitzman DW, Lam CS, van Veldhuisen DJ, Ponikowski P, et al. Central and peripheral determinants of exercise capacity in heart failure patients with preserved ejection fraction. JACC Heart Fail. 2019;7:321–332. [DOI] [PubMed] [Google Scholar]
- 28. Kaye D, Shah SJ, Borlaug BA, Gustafsson F, Komtebedde J, Kubo S, Magnin C, Maurer MS, Feldman T, Burkhoff D. Effects of an interatrial shunt on rest and exercise hemodynamics: results of a computer simulation in heart failure. J Card Fail. 2014;20:212–221. [DOI] [PubMed] [Google Scholar]
- 29. Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–863. [DOI] [PubMed] [Google Scholar]
- 30. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139:e698–e800. [DOI] [PubMed] [Google Scholar]
- 31. Wessler J, Kaye D, Gustafsson F, Petrie MC, Hasenfuβ G, Lam CSP, Borlaug BA, Komtebedde J, Feldman T, Shah SJ, et al. Impact of baseline hemodynamics on the effects of a transcatheter interatrial shunt device in heart failure with preserved ejection fraction. Circ Heart Fail. 2018;11:e004540 10.1161/CIRCHEARTFAILURE.116.003358. [DOI] [PubMed] [Google Scholar]
- 32. Søndergaard L, Reddy V, Kaye D, Malek F, Walton A, Mates M, Franzen O, Neuzil P, Ihlemann N, Gustafsson F. Transcatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel interatrial implant to lower left atrial pressure. Eur J Heart Fail. 2014;16:796–801. [DOI] [PubMed] [Google Scholar]
- 33. Malek F, Neuzil P, Gustafsson F, Kaye DM, Walton A, Mates M, Søndergaard L, Ihlemann N, Mariani JA, Reddy V. Clinical outcome of transcatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel implant. Int J Cardiol. 2015;187:227–228. [DOI] [PubMed] [Google Scholar]
- 34. Hasenfuss G, Gustafsson F, Kaye D, Shah SJ, Burkhoff D, Reymond MC, Komtebedde J, Hunlich M, Reduce LAPHFTI. Rationale and design of the reduce elevated left atrial pressure in patients with heart failure (Reduce LAP‐HF) trial. J Card Fail. 2015;21:594–600. [DOI] [PubMed] [Google Scholar]
- 35. Hasenfuß G, Hayward C, Burkhoff D, Silvestry FE, McKenzie S, Gustafsson F, Malek F, Van der Heyden J, Lang I, Petrie MC, et al. A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP‐HF): a multicentre, open-label, single-arm, phase 1 trial. Lancet. 2016;387:1298–1304. [DOI] [PubMed] [Google Scholar]
- 36. Kaye DM, Hasenfuss G, Neuzil P, Post MC, Doughty R, Trochu JN, Kolodziej A, Westenfeld R, Penicka M, Rosenberg M, et al. One-year outcomes after transcatheter insertion of an interatrial shunt device for the management of heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9:e003662 10.1161/CIRCHEARTFAILURE.116.003662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feldman T, Mauri L, Kahwash R, Litwin SE, Ricciardi MJ, van der Harst P, Penicka M, Fail PS, Kaye DM, Petrie MC, et al. Transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction (REDUCE LAP‐HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]): A Phase 2, Randomized. Sham-Controlled Trial. Circulation. 2018;137:364–375. [DOI] [PubMed] [Google Scholar]
- 38. Feldman T, Komtebedde J, Burkhoff D, Massaro J, Maurer MS, Leon MB, Kaye D, Silvestry FE, Cleland JG, Kitzman D, et al. Transcatheter interatrial shunt device for the treatment of heart failure: rationale and design of the randomized trial to REDUCE elevated left atrial pressure in heart failure (REDUCE LAP‐HF I). Circ Heart Fail. 2016;9. [DOI] [PubMed] [Google Scholar]
- 39. Shah SJ, Feldman T, Ricciardi MJ, Kahwash R, Lilly S, Litwin S, Nielsen CD, Van der Harst P, Hoendermis E, Penicka M, et al. One-year safety and clinical outcomes of a transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction in the reduce elevated left atrial pressure in patients with heart failure (REDUCE LAP‐HF I) trial. JAMA Cardiol. 2018;3:968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaye DM, Petrie MC, McKenzie S, Hasenfuβ G, Malek F, Post M, Doughty RN, Trochu JN, Gustafsson F, Lang I, et al. Impact of an interatrial shunt device on survival and heart failure hospitalization in patients with preserved ejection fraction. ESC Heart Fail. 2019;6:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, Klein DA, Dixon D, Baldridge A, Rasmussen-Torvik LJ, et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction. Circ Cardiovasc Imaging. 2016;9:e003754 DOI: 10.1161/CIRCIMAGING.115.003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hanff TC, Kaye DM, Hayward CS, Post MC, Malek F, Hasenfuβ G, Gustafsson F, Burkhoff D, Shah SJ, Litwin SE, et al. Assessment of predictors of left atrial volume response to a transcatheter interatrial shunt device (from the REDUCE LAP‐HF Trial). Am J Cardiol. 2019;124:1912–1917. [DOI] [PubMed] [Google Scholar]
- 43. Obokata M, Reddy YN, Shah SJ, Kaye DM, Gustafsson F, Hasenfuβ G, Hoendermis E, Litwin SE, Komtebedde J, Lam C, et al. Effects of interatrial shunt on pulmonary vascular function in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2019;74:2539–2550. [DOI] [PubMed] [Google Scholar]
- 44. Bannan A, Shen R, Silvestry FE, Herrmann HC. Characteristics of adult patients with atrial septal defects presenting with paradoxical embolism. Catheter Cardiovasc Interv. 2009;74:1066–1069. [DOI] [PubMed] [Google Scholar]
- 45. Amat-Santos IJ, Bergeron S, Bernier M, Allende R, Barbosa Ribeiro H, Urena M, Pibarot P, Verheye S, Keren G, Yaacoby M, et al. Left atrial decompression through unidirectional left-to‐right interatrial shunt for the treatment of left heart failure: first-in-man experience with the V‐Wave device. EuroIntervention. 2015;10:1127–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eigler NL, del Rio CL, Verheye S, McConnell PI, Lilly SM, George R, Hamlin RL, Ueyama Y, Youngblood BL, Shkurovich S, et al. Cardiac unloading with an implantable interatrial shunt in heart failure: serial observations in an ovine model of ischemic cardiomyopathy. Structural Heart. 2017;1:40–48. [Google Scholar]
- 47. Del Trigo M, Bergeron S, Bernier M, Amat-Santos IJ, Puri R, Campelo‐Parada F, Altisent OA, Regueiro A, Eigler N, Rozenfeld E, et al. Unidirectional left-to‐right interatrial shunting for treatment of patients with heart failure with reduced ejection fraction: a safety and proof-of-principle cohort study. Lancet. 2016;387:1290–1297. [DOI] [PubMed] [Google Scholar]
- 48. Rodes-Cabau J, Bernier M, Amat-Santos IJ, Ben Gal T, Nombela-Franco L, Garcia Del Blanco B, Kerner A, Bergeron S, Del Trigo M, Pibarot P, et al. Interatrial shunting for heart failure: early and late results from the first-in-human experience with the V‐Wave system. JACC Cardiovasc Interv. 2018;11:2300–2310. [DOI] [PubMed] [Google Scholar]
- 49. Guimaraes L, Bergeron S, Bernier M, Rodriguez-Gabella T, Del Val D, Pibarot P, Eigler N, Abraham WT, Rodes-Cabau J. Interatrial shunt with the second-generation V‐Wave system for patients with advanced chronic heart failure. EuroIntervention. 2020;15:1426–1428. [DOI] [PubMed] [Google Scholar]
- 50. Rajeshkumar R, Pavithran S, Sivakumar K, Vettukattil JJ. Atrial septostomy with a predefined diameter using a novel Occlutech Atrial Flow Regulator improves symptoms and cardiac index in patients with severe pulmonary arterial hypertension. Catheter Cardiovasc Interv. 2017;90:1145–1153. [DOI] [PubMed] [Google Scholar]
- 51. Paitazoglou C, Ozdemir R, Pfister R, Bergmann MW, Bartunek J, Kilic T, Lauten A, Schmeisser A, Zoghi M, Anker S, et al. The AFR-PRELIEVE trial: a prospective, non-randomised, pilot study to assess the Atrial Flow Regulator (AFR) in heart failure patients with either preserved or reduced ejection fraction. EuroIntervention. 2019;15:403–410. [DOI] [PubMed] [Google Scholar]
- 52. Simard T, Labinaz M, Zahr F, Nazer B, Gray W, Hermiller J, Chaudhry SP, Guimaraes L, Philippon F, Eckman P, et al. TCT-87 levoatrial to coronary sinus shunting as a novel strategy for symptomatic heart failure: first-in-human experience. J Am Coll Cardiol. 2019;74:B87. [DOI] [PubMed] [Google Scholar]
- 53. Lotan C. A Novel, Stentless RF-Based Shunt Solution for Advanced Heart Failure: The NoYATM System; 2019:2019.


