Abstract
Background
Impaired endothelial function is thought to contribute to the increased cardiovascular risk associated with above‐normal blood pressure (BP). However, the association between endothelial function and BP classified by 2017 American College of Cardiology/American Heart Association guidelines is unknown. Our objective was to determine if endothelial function decreases in midlife/older adults across the 2017 American College of Cardiology/American Heart Association guidelines BP classifications and identify associated mechanisms of action.
Methods and Results
A retrospective analysis of endothelial function (brachial artery flow‐mediated dilation) from 988 midlife/older adults (aged 50+ years) stratified by BP status (normal BP; elevated BP; stage 1 hypertension; stage 2 hypertension) was performed. Endothelium‐independent dilation (sublingual nitroglycerin), reactive oxygen species–mediated suppression of endothelial function (∆brachial artery flow‐mediated dilation with vitamin C infusion), and endothelial cell and plasma markers of oxidative stress and inflammation were assessed in subgroups. Compared with normal BP (n=411), brachial artery flow‐mediated dilation was 12% (P=0.04), 15% (P<0.01) and 20% (P<0.01) lower with elevated BP (n=173), stage 1 hypertension (n=248) and stage 2 hypertension (n=156), respectively, whereas endothelium‐independent dilation did not differ (P=0.14). Vitamin C infusion increased brachial artery flow‐mediated dilation in those with above‐normal BP (P≤0.02) but not normal BP (P=0.11). Endothelial cell p47phox (P<0.01), a marker of superoxide/reactive oxygen species–generating nicotinamide adenine dinucleotide phosphate oxidase, and circulating interleukin‐6 concentrations (P=0.01) were higher in individuals with above‐normal BP.
Conclusions
Vascular endothelial function is progressively impaired with increasing BP in otherwise healthy adults classified by 2017 American College of Cardiology/American Heart Association guidelines. Impaired endothelial function with above‐normal BP is mediated by excessive reactive oxygen species signaling associated with increased endothelial expression of nicotinamide adenine dinucleotide phosphate oxidase and circulating interleukin‐6.
Keywords: hypertension, inflammation, NADPH oxidase, oxidative stress
Subject Categories: High Blood Pressure, Hypertension, Inflammation, Oxidant Stress
Nonstandard Abbreviations and Acronyms
- ACC
American College of Cardiology
- AHA
American Heart Association
- BMI
body mass index
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- FMDBA
brachial artery flow‐mediated dilation
- NADPH
nicotinamide adenine dinucleotide phosphate
- ROS
reactive oxygen species
- SBP
systolic blood pressure
Clinical Perspective
What Is New?
This study assessed the relation between endothelial function and blood pressure (BP) in a large cohort of midlife and older adults classified according to the 2017 American College of Cardiology/American Heart Association BP guidelines.
Compared with adults with normal BP, vascular endothelial function was progressively impaired in those with elevated BP and stage 1 and 2 hypertension.
Multiple translational mechanistic approaches, including “pharmaco‐dissection,” endovascular biopsies of endothelial cells, and circulating biomarkers, show that the impaired endothelial function of groups with above‐normal BP was associated with increased reactive oxygen species bioactivity, endothelial cell nicotinamide adenine dinucleotide phosphate oxidase p47phox, and plasma interleukin‐6.
What Are the Clinical Implications?
Understanding the impact of above‐normal BP based on current American College of Cardiology/American Heart Association guidelines, but below previous clinical cutoffs for hypertension, on vascular endothelial function in adults is important for optimal management of cardiovascular disease risk in the ever‐growing population of midlife and older adults.
New insight into the cellular and molecular mechanisms that may contribute to impaired endothelial function in groups with above‐normal BP may help identify potential therapeutic targets for novel therapies.
Our results support the need for healthy lifestyle‐based strategies, in particular, to lower BP and improve endothelial function in midlife and older adults in all above‐normal BP classifications according to the 2017 American College of Cardiology/American Heart Association guidelines.
above‐normal resting (casual) blood pressure (BP), defined as systolic BP (SBP) ≥120 mm Hg or diastolic BP (DBP) ≥80 mm Hg, is associated with increased risk of cardiovascular disease (CVD), kidney disease, cognitive impairment, and other chronic disorders. 1 , 2 , 3 One of the nontraditional risk factors linking above‐normal BP to these conditions is believed to be vascular endothelial dysfunction, most commonly characterized by impaired endothelium‐dependent dilation. 4 , 5 , 6 , 7 Consistent with this notion, patients with hypertension, defined previously as SBP/DBP ≥140/90 mm Hg, 8 , 9 often demonstrate impaired endothelium‐dependent dilation, 10 , 11 which, in turn, predicts incident risk of CVD. 1 , 2 , 12 , 13 , 14
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and other professional societies updated the Guidelines for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. 15 This update created an “elevated” BP classification (SBP 120–129 and DBP <80 mm Hg) while revising downward the BP levels defining stage 1 (SBP 130–139 or DBP 80–89 mm Hg) and stage 2 (SBP ≥140 or DBP ≥90 mm Hg) hypertension. 15 These changes in BP guidelines were based on new epidemiologic and clinical information suggesting BP that is above normal, but below the previously defined threshold for hypertension (ie, SBP 120–139/DBP 80–89 mm Hg), is associated with increased cardiovascular risk and mortality. 16 On the basis of these revised guidelines, more than half of adults >50 years of age have hypertension, whereas less than a third have normal BP. 17
Although understanding the inherent pathophysiological processes that underlie this recent reclassification is essential to develop optimal strategies for clinical BP management, no information presently is available related to these new guidelines. In this context, vascular endothelial dysfunction is a likely pathophysiological mechanism that contributes to the progressively greater cardiovascular risk of increasing BP across the 2017 ACC/AHA classifications. However, the relation between BP stratification in these current guidelines and endothelial function, as well as the potential underlying mechanisms, have not been established. Accordingly, the primary aim of this study was to determine, among otherwise healthy midlife/older adults with no known presence of other chronic clinical diseases, if endothelial function decreases with increasing BP status based on the 2017 ACC/AHA guidelines. If so, our secondary aim was to gain initial insight into the potential clinical factors and physiological mechanisms through which endothelial function is associated with BP status per these new guidelines.
To address our primary aim, we first determined the association between brachial artery flow‐mediated dilation (FMDBA), a well‐established measure of endothelium‐dependent dilation and vascular endothelial function, and BP across the 2017 ACC/AHA BP classifications in a large sample of adults ≥50 years of age from our laboratory database. To address our secondary aim, we then determined if differences in other clinical characteristics, such as age or blood lipids, explained, at least in part, BP‐based group differences in endothelial function. Because antihypertensive medications influence vascular endothelial function, 18 , 19 we also assessed whether BP‐related group differences in endothelial function were apparent when examining only unmedicated individuals. Next, we determined if differences in vascular smooth muscle sensitivity to NO, that is, endothelium‐independent dilation, might explain group differences in endothelial function (FMDBA). Finally, we determined if reactive oxygen species (ROS)‐associated suppression of endothelial function (ie, change in FMDBA in response to acute supratherapeutic infusion of the antioxidant ascorbic acid [vitamin C]) or endothelial cell or circulating markers of oxidative stress and inflammation were linked to differences in endothelial function across BP classifications.
Methods
All procedures were reviewed and approved by the Institutional Review Board at the University of Colorado Boulder. The nature, benefits, and risks of all study procedures were explained to volunteers, and their written informed consent was obtained before study participation. The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Subjects
A retrospective analysis of data collected on 988 midlife/older adults (50–79 years) who underwent testing for casual (resting) BP and vascular endothelial function by our laboratory with well‐standardized procedures at the University of Colorado Boulder between 2008 and 2019 was performed. Subjects were included if they were in the appropriate age range and had undergone standardized measurements of casual BP, vascular endothelial function, and clinical characteristics. Individuals from all racial, ethnic, and socioeconomic backgrounds were included, but the majority of subjects were White and of higher education and income status. No subjects were current smokers, and all subjects were free of overt clinical disease, including CVD, based on a medical history, physical examination, resting and maximal exercise BP and ECG, and standard blood chemistries. 20 , 21 , 22 , 23 , 24 Individuals taking prescription antihypertensive medications and other classes of prescription medications (eg, statins) were included provided their drug regimen was stable for the previous ≥3 months. Subjects were separated into 4 groups according to 2017 ACC/AHA guidelines: normal BP (SBP <120 and DBP <80 mm Hg; n=411); elevated BP (SBP 120–129 and DBP <80 mm Hg; n=173); stage 1 hypertension (SBP 130–139 or DBP 80–89 mm Hg; n=248); and stage 2 hypertension (SBP ≥140 or DBP ≥90 mm Hg; n=156).
Measurements
All measurements were taken following an overnight fast and abstaining from caffeine for 12 hours, and alcohol and vigorous exercise for 24 hours. Casual BP was determined as seated brachial artery BP measured in triplicate after ≥5 minutes of quiet rest according to World Health Organization, Joint National Committee 7, and ACC/AHA guidelines. 8 , 9 , 15 Measurements were either taken manually by a trained investigator via brachial auscultation or by an automated oscillometric sphygmomanometer. These 2 methods have been shown to yield similar BP measurements. 25 , 26 The average of the 3 BP measurements was taken and used for group allocation.
Endothelium‐dependent Dilation
FMDBA was assessed using high‐resolution ultrasonography in response to a 5‐minute period of blood flow occlusion with a cuff positioned on the upper forearm, as described previously by our laboratory 27 , 28 , 29 , 30 and others. 31 , 32 For more detail, see Data S1. FMDBA was quantified as percentage change from baseline diameter, calculated as FMDBA(∆%)=((Peak Diameter−Baseline Diameter)/Baseline Diameter)×100. 31 , 33 , 34
Endothelium‐Independent Dilation
A subset of subjects underwent testing for endothelium‐independent dilation (n=259), that is, brachial artery dilation in response to 0.4 mg of sublingual nitroglycerin, to assess vascular smooth muscle sensitivity to NO. 27 , 28 , 35 Ultrasound images of the brachial artery were captured at baseline and for 10 minutes following administration. The maximal brachial artery dilation to nitroglycerin was calculated as a percentage change in diameter from baseline. 31 , 33 , 34
ROS‐Associated Suppression of Endothelium‐dependent Dilation
To gain causal mechanistic insight, tonic suppression of endothelial function by ROS was assessed in a subset of subjects (n=234) using a supratherapeutic systemic infusion of vitamin C (ascorbic acid), as described previously by our laboratory. 27 , 36 The change in FMDBA from baseline (preinfusion) in response to vitamin C was interpreted as the magnitude of tonic ROS‐associated suppression of endothelium‐dependent dilation (vascular endothelial function). 27 , 36 Additional details are available in Data S1.
Endothelial Cell Analysis via Endovascular Biopsy
The abundance of protein markers of oxidative stress and inflammation was assessed in biopsied endothelial cells collected on J‐wires advanced into an antecubital vein via immunofluorescent staining, as previously described 29 , 37 , 38 and as presented in detail in Data S1. Our laboratory established previously that protein markers assessed in venous endothelial cells correlate well with the same markers assessed in arterial endothelial cells obtained by invasive brachial artery catheterization. 29 , 37 For analysis, endothelial cells were stained with primary antibodies for nitrotyrosine (posttranslational oxidative modification; n=240), nicotinamide adenine dinucleotide phosphate (NADPH) oxidase subunit p47phox (pro‐oxidant enzyme marker; n=187), manganese superoxide dismutase (antioxidant enzyme; n=96), and nuclear factor kappa B p65 (proinflammatory transcription factor; n=197), as well as with an AlexaFluor fluorescent secondary antibody (Invitrogen, Carlsbad, CA). Cells were viewed with a fluorescence microscope and analyzed with Metamorph Software. Endothelial cells were identified by cell staining for von Willebrand factor and nuclear integrity was confirmed via 4′,6′‐diamidino‐2‐phenylindole hydrochloride staining. 29 , 30 , 37 Values are expressed as ratios of endothelial cell protein expression to human umbilical vein endothelial cells to minimize the effects of differences in staining intensities across multiple staining sessions. 29 , 30 , 37
Markers of Systemic Oxidative Stress and Inflammation
Circulating plasma markers of inflammation (interleukin‐6, n=331; tumor necrosis factor alpha, n=225; high‐sensitivity C‐reactive protein, n=432), oxidative stress (oxidized low‐density lipoprotein [LDL], n=452), and antioxidant defenses (total antioxidant status, n=278) were analyzed using ELISA (interleukin‐6; tumor necrosis factor alpha; oxidized LDL), immunoturbidimetry (high‐sensitivity C‐reactive protein), and the oxidative method (total antioxidant status). 22
Statistical Analysis
Statistical comparisons were made in SPSS version 25 using the General Linear Model. Sidak post hoc tests were used to assess between‐group mean differences for continuous variables. Linear regression was used to determine how endothelial function was related to ACC/AHA BP classifications, ROS‐associated suppression of endothelial function, endothelial cell NADPH oxidase p47phox, and circulating interleukin‐6 concentration. Multiple linear regression was used to account for additional clinical characteristics (eg, age) explaining differences in endothelial function. The Mixed Linear Model was used to determine group by condition (saline versus vitamin C) interaction for ROS‐associated suppression of FMDBA; the Sidak post hoc test was used to assess the within‐group effect of vitamin C. Because of the smaller sample sizes available as a result of the highly technical nature of measurements, for the endothelial cell markers of inflammation and oxidative stress, subjects were classified as having either normal (SBP <120 mm Hg and DBP <80 mm Hg) or above‐normal BP (SBP ≥120 mm Hg or DBP ≥80 mm Hg) based on the 2017 ACC/AHA guidelines, and group comparisons were made with the General Linear Model. Subject characteristics for each subset analysis presented in the results were not different from the characteristics of the whole group. Categorical variables were compared with the chi‐square test. Unless otherwise specified, continuous variables in the text and table are expressed as mean±SD while categorical variables are expressed as percentages. Statistical significance was set a priori at α=0.05.
Results
Clinical Characteristics
Clinical characteristics are presented in Table1. By design, BP differed between all groups, with SBP and DBP increasing across ACC/AHA BP classifications. Excluding BP status in those with above‐normal BP, subjects were free of clinical metabolic or cardiovascular diseases. Although selective group differences existed, mean values were all within clinical norms. All groups with above‐normal BP were 2 to 3 years older (P<0.01), had a slightly higher body mass index (BMI) (P≤0.02) and modestly lower high‐density lipoprotein cholesterol (P<0.01), were more likely to be men (P<0.05), and were more likely to be taking antihypertensive medications (P<0.05) than subjects with normal BP. Those with stage 1 hypertension had higher fasting glucose than normotensive subjects (P=0.01). Subjects with stage 1 or stage 2 hypertension had higher triglycerides than normotensive subjects (P<0.01). Finally, those with stage 1 and stage 2 hypertension also had a modestly higher BMI than the group with elevated BP (P<0.03).
Table 1.
Subject Characteristics
|
Normal N=411 |
Elevated N=173 |
Stage 1 N=248 |
Stage 2 N=156 |
|
|---|---|---|---|---|
| SBP, mm Hg | 108±8 | 124±3* | 131±7 * | 147±9 * |
| DBP, mm Hg | 67±6 | 72±5* | 79±6 * | 85±9 * |
| Age, y | 62±6 | 65±7* | 64±7* | 64±7* |
| Women, % | 62 | 49* | 38* | 47* |
| BMI, kg/m2 | 24.1±3.9 | 25.1±3.9* | 26.2±3.9 * | 26.5±4.1 * , † |
| Triglycerides, mg/dL | 94±50 | 104±52 | 115±58* | 113±58* |
| HDL‐C, mg/dL | 63±18 | 57±16* | 57±18* | 57±18* |
| LDL‐C, mg/dL | 118±30 | 114±27 | 121±29 | 119±33 |
| Glucose, mg/dL | 88±9 | 89±8 | 90±10* | 89±11 |
| BP medication, % | 3.2 | 9.8* | 12.6* | 15.6* |
| Other prescription medications, % | 28.9 | 32.9 | 32.9 | 38.7 |
Continuous variables are means±SD. Categorical variables are percentages. BMI indicates body mass index; DBP, diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; and SBP, systolic blood pressure.
P<0.05 compared with normal BP.
P<0.05 compared with elevated BP.
P<0.05 compared with stage 1 hypertension.
Endothelial Function
Overall Cohort
On the basis of FMDBA, endothelial function was significantly impaired in midlife/older adults with elevated BP (5.2±2.6∆%, P=0.04), stage 1 hypertension (5.1±2.7∆%, P<0.01), and stage 2 hypertension (4.7±2.4∆%, P<0.01) compared with midlife/older adults with normal BP (5.9±2.9∆%) (Figure 1). Although there was no significant difference in mean FMDBA among the 3 groups with above‐normal BP, there was a significant inverse relation between FMDBA and increasing BP status (elevated BP, −12%; stage 1 hypertension, −15%; stage 2 hypertension, −20% versus normal BP group; r=−0.157; P<0.01).
Figure 1. Vascular endothelial function (endothelium‐dependent dilation).
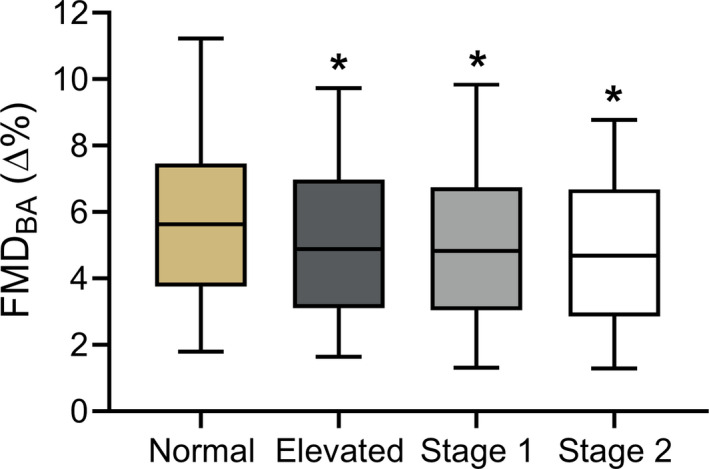
Brachial artery flow‐mediated dilation (FMDBA) in midlife/older adults classified according to 2017 American College of Cardiology/American Heart Association blood pressure (BP) guidelines. Box indicates interquartile range and median, whiskers indicate 5th to 95th percentiles. Normal BP: n=411; elevated BP: n=173; stage 1 hypertension: n=248; stage 2 hypertension: n=156. *P<0.05 compared with normal BP.
Influence of Clinical Factors
BP classification, along with age, sex, BMI, serum triglycerides, high‐density lipoprotein cholesterol, LDL cholesterol, and fasting blood glucose were entered into the linear model. Fit for the whole model was R 2=0.05. Within the model, BP classification was related to FMDBA (β=−0.325; 95% CI, −0.481 to −0.161; P<0.01; partial R 2=0.02), such that those individuals in the above‐normal BP classifications were more likely to have lower values of FMDBA. Age was also related to FMDBA (β=−0.051; 95% CI, −0.077 to −0.026; P<0.01; partial R 2=0.016), such that being of older age was associated with lower values of FMDBA. Finally, sex was related to FMDBA (β=−0.420; 95% CI, −0.796 to −0.043; P=0.03; partial R 2=0.005) with men tending to have lower values of endothelial function than women. The other variables in the model, including BMI (P=0.95), triglycerides (P=0.53), high‐density lipoprotein cholesterol (P=0.92), LDL cholesterol (P=0.56), and fasting glucose (P=0.66), were not significantly related to FMDBA.
Nonmedicated Individuals
FMDBA also was determined in the subgroup of the overall cohort who were not prescribed BP‐lowering medications to assess the influence of BP status on endothelial function independent of the effects of antihypertensive pharmacotherapy. Compared with those with normal BP (n=398; FMDBA 5.9±2.9∆%), FMDBA tended to be lower in adults with elevated BP (n=156; FMDBA 5.3±2.6∆%; P=0.09) and was significantly lower in adults with stage 1 hypertension (n=217; FMDBA 5.1±2.7∆%; P<0.01) or stage 2 hypertension (n=132; FMDBA 4.8±2.3∆%; P<0.01). Thus, the associations between FMDBA and BP across the 2017 ACC/AHA BP classifications were similar in the overall cohort and in the subgroup not taking antihypertensive medications.
Endothelium‐independent Dilation
Endothelium‐independent dilation, that is, vascular smooth muscle sensitivity to NO, was assessed by brachial artery dilation in response to sublingual nitroglycerin in a subset of subjects within the overall cohort. As in the overall cohort, FMDBA was lower in the subgroups with above‐normal BP versus the subgroup with normal BP (range, −15% to −21%). Brachial artery dilation in response to sublingual nitroglycerin did not differ across the normal (24.6±7.1∆%), elevated BP (22.5±4.7∆%), stage 1 hypertension (23.0±7.2∆%) and stage 2 hypertension (25.0±6.2∆%) subgroups (P=0.14), indicating no BP status‐related differences in vascular smooth muscle sensitivity to NO (Figure 2).
Figure 2. Endothelium‐independent dilation.
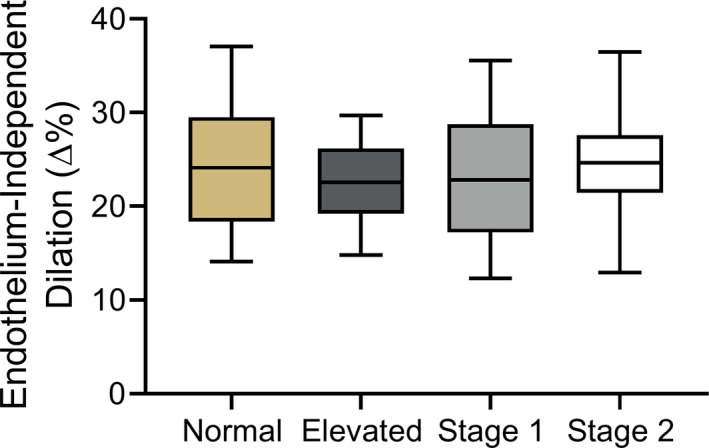
Brachial artery dilation in response to sublingual nitroglycerin in midlife/older adults classified according to 2017 American College of Cardiology/American Heart Association guidelines. Box indicates interquartile range and median, whiskers indicate 5th to 95th percentiles. Normal blood pressure (BP): n=96; elevated BP: n=42; stage 1 hypertension: n=84; stage 2 hypertension: n=37.
Association With Oxidative Stress and Inflammation
ROS‐Associated Suppression of Endothelial Function
In subjects in whom supratherapeutic concentrations of the antioxidant vitamin C were infused to determine tonic ROS‐associated suppression of endothelium‐dependent dilation, vitamin C significantly increased FMDBA above baseline (saline control) in those with elevated BP (saline, 4.6±2.4∆%; vitamin C, 5.5±2.3∆%; P=0.02), stage 1 hypertension (saline, 5.0±2.6∆%; vitamin C, 5.7±3.0∆%; P=0.01), and stage 2 hypertension (saline; 4.6±2.2∆%; vitamin C, 5.6±2.7∆%; P<0.01), but not in those with normal BP (saline, 5.4±3.0∆%, vitamin C, 5.8±3.1∆%; P=0.12) (Figure 3). There was a modest but statistically significant inverse correlation between the change in FMDBA with vitamin C infusion and baseline FMDBA (r=−0.14; P=0.04).
Figure 3. Tonic reactive oxygen species‐mediated suppression of endothelial function.
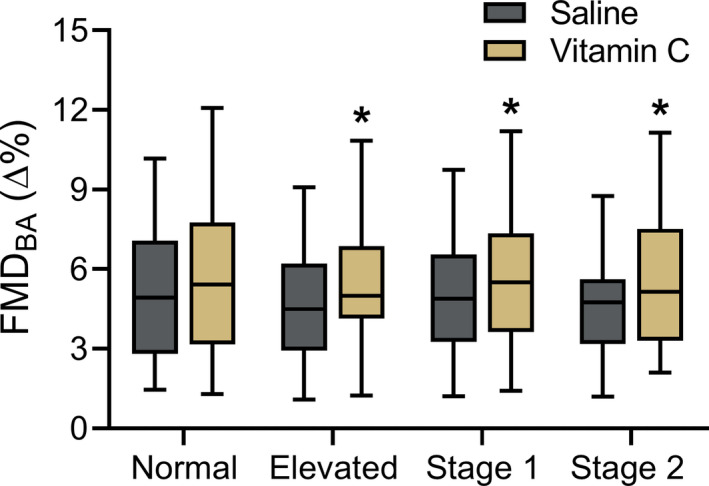
Brachial artery flow‐mediated dilation (FMDBA) following intravenous infusion of saline and vitamin C in midlife/older adults classified according to 2017 American College of Cardiology/American Heart Association guidelines. Data are expressed as percent change (∆%) from baseline diameter. Box indicates interquartile range and median, whiskers indicate 5th to 95th percentiles. Normal blood pressure (BP): n=100; elevated BP: n=34; stage 1 hypertension: n=64; stage 2 hypertension: n=36. *P<0.05 compared with saline condition.
Molecular Characteristics of Endothelial Cells Obtained via Endovascular Biopsy
To assess potential molecular mechanisms underlying impairments in vascular endothelial function in the groups with above‐normal compared with normal BP, the abundance of protein markers associated with oxidative stress and inflammation was measured in endothelial cells obtained from subsets of subjects via endovascular biopsy (Figure 4). Expression of p47phox, a subunit of selective isoforms of the superoxide/ROS‐generating enzyme NADPH oxidase, was higher in the groups with above‐normal BP (0.75±0.44 AU) compared with the group with normal BP (0.55±0.26 AU; P<0.01). The abundance of nitrotyrosine (normal, 0.69±0.68 AU; above‐normal, 0.67±0.56 AU; P=0.78), manganese superoxide dismutase (normal, 0.49±0.26 AU; above‐normal, 0.48±0.36 AU; P=0.89), and manganese superoxide dismutase (normal, 0.39±0.35 AU; above‐normal, 0.44±0.31 AU; P=0.37) were not different between the groups. There were no significant relations between any endothelial cell marker and FMDBA (all P>0.05).
Figure 4. Endothelial cell protein markers of oxidative stress and inflammation.
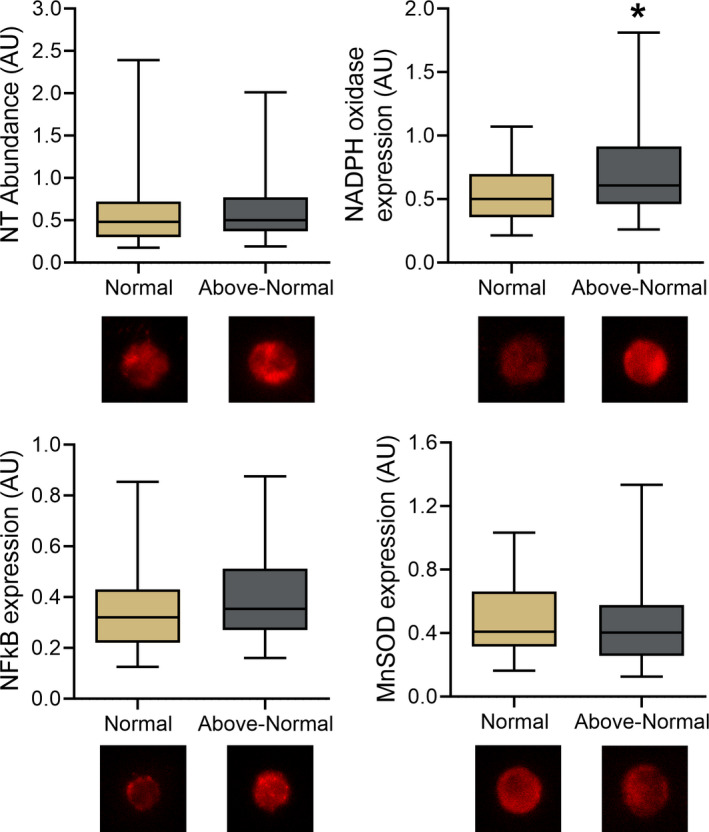
Abundance of nitrotyrosine (NT; oxidative modification of proteins) and expression of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (oxidant‐producing enzyme), manganese superoxide dismutase (MnSOD; antioxidant defenses) and nuclear factor κ B (NFκB; proinflammatory transcription factor) in biopsied venous endothelial cells from midlife/older adults with normal blood pressure (BP; <120/80 mm Hg systolic BP [SBP]/diastolic BP [DBP]) vs above‐normal BP (≥120 mm Hg SBP or ≥80 mm Hg DBP) with example immunofluorescent images of individual endothelial cells below. Data are normalized to human umbilical vein endothelial cell protein expression via immunofluorescence. Box indicates interquartile range and median, whiskers indicate 5th to 95th percentiles. *P<0.05 compared with normal BP.
Circulating Markers of Systemic Oxidative Stress and Inflammation
To gain insight into whether systemic inflammation and oxidative stress differed between groups with normal and above‐normal BP, circulating plasma markers of inflammation, oxidative stress, and antioxidant defenses were measured in subsets of subjects (Figure 5). Interleukin‐6, a proinflammatory cytokine, was higher in those with above‐normal BP (1.81±2.54 pg/mL) compared with normotensive subjects (1.25±1.16 pg/mL; P=0.01), and there was a modest but statistically significant inverse relation between interleukin‐6 and FMDBA (r=−0.14; P=0.01; n=331). There were no significant differences between groups in plasma oxidized LDL (normal, 56.49±18.77 U/L; above‐normal, 58.50±18.55 U/L; P=0.29), total antioxidant status (normal, 1.25±0.21 mmol/L; above‐normal, 1.30±0.22 mmol/L; P=0.06), high‐sensitivity C‐reactive protein (normal, 1.08±1.29 mg/L; above‐normal, 1.20±1.57 mg/L; P=0.41), or tumor necrosis factor alpha (normal, 1.41±1.25 pg/L; above‐normal, 1.51±1.27 pg/L; P=0.47).
Figure 5. Circulating concentrations of inflammatory, oxidative stress and antioxidant markers.
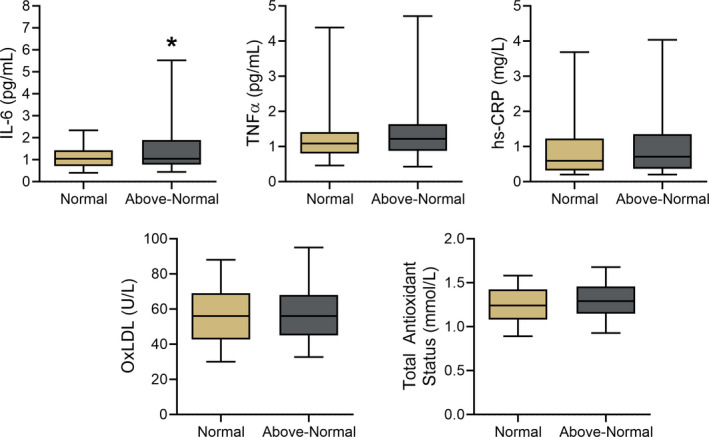
Plasma concentrations of inflammatory (IL‐6 [interleukin‐6]; TNFα [tumor necrosis factor alpha]; hsCRP [high‐sensitivity C‐reactive protein]), oxidative stress (oxidized LDL [OxLDL]), and antioxidant (total antioxidant status) markers in midlife/older adults with normal blood pressure (BP; <120/80 mm Hg systolic BP [SBP]/diastolic BP [DBP]) vs above‐normal BP (≥120 mm Hg SBP or ≥80 mm Hg DBP). Box indicates interquartile range and median, whiskers indicate 5th to 95th percentiles. *P<0.05 compared with normal BP.
Discussion
In this investigation, we assessed the relation between vascular endothelial function and BP status in almost 1000 men and women ≥50 years of age. Our primary finding was that vascular endothelial function, assessed using FMDBA, is progressively impaired in otherwise healthy midlife/older adults with elevated BP, stage 1 hypertension and stage 2 hypertension compared with their counterparts with normal BP based on the 2017 ACC/AHA guidelines. 15 At the extremes, FMDBA was 20% lower in subjects with stage 2 hypertension compared with individuals with normal BP. Importantly, after controlling for other clinical characteristics (eg, age, BMI), BP classification remained independently related to FMDBA. The lower FMDBA in individuals in the above‐normal BP classifications was not related to reduced smooth muscle sensitivity to NO, as brachial artery dilation in response to sublingual nitroglycerin did not differ across groups on the basis of BP classification, indicating an impairment in the vascular endothelium per se. Administration of the ROS‐scavenging antioxidant molecule vitamin C improved FMDBA only in individuals in the above‐normal BP groups, suggesting that the impaired endothelial function in these groups was mediated by excessive ROS‐associated suppression of endothelium‐dependent dilation. This ROS‐mediated inhibition of FMDBA in individuals in the above‐normal BP classifications was, in turn, associated with evidence of greater abundance of the ROS (superoxide)‐producing enzyme, NADPH oxidase, in biopsied vascular endothelial cells. Finally, individuals in the above‐normal BP groups demonstrated higher circulating concentrations of the proinflammatory marker interleukin‐6 than their peers with normal BP, and interleukin‐6 was inversely related to FMDBA in the overall cohort.
Potential Clinical Implications
In the present analysis, there was a statistically significant trend for progressively lower FMDBA among individuals classified with elevated BP, stage 1 hypertension and stage 2 hypertension, respectively. Meta‐analyses show an inverse relation between FMDBA and future CVD events, with a 1% (∆% units) decrease in FMDBA associated with an ≈8% to 13% increase in risk of future CVD events. 39 , 40 , 41 , 42 , 43 Compared with individuals with normal BP, we found that FMDBA was 0.7 to 1.2 ∆% units lower in the groups with above‐normal BP. These results suggest that the magnitude of the impairments in FMDBA observed in midlife/older adults in the above‐normal BP classifications of the 2017 ACC/AHA guidelines may be clinically significant.
Other than the higher SBP of those individuals in the above‐normal BP groups, the midlife/older adults included in this analysis were healthy. On the one hand, our cohort affords the unique experimental advantage of isolating, as much as is possible in free‐living humans, the effects of differing BP status per se on endothelial function. On the other hand, it is possible, perhaps likely, that our results underestimate differences in endothelial function in the broader population of adults differing in BP status, particularly those with greater CVD risk factor burden or of lower socioeconomic status.
It may be noteworthy that the midlife/older adults in our analysis with BP in the “elevated” and “stage 1 hypertension” classifications based on the 2017 ACC/AHA BP cutoffs would not have been recommended for treatment under previous guidelines, despite having reduced FMDBA (ie, impaired endothelial function). In this context, we and others have shown that healthy lifestyle‐based interventions, including those recommended in ACC/AHA guidelines (eg, exercise, low‐salt diet, weight loss), improve endothelial function in midlife/older adults with BP in the current elevated and stage 1 hypertension classifications. 20 , 44 , 45 As such, the present findings support the concept that evidence‐based healthy lifestyle strategies should be encouraged across all stages of above‐normal BP status, not only for their antihypertensive effects but also for their ability to improve vascular endothelial function.
Role of Clinical Factors
As anticipated, age and sex were also related to vascular endothelial function (FMDBA). However, BP classification remained significantly related to FMDBA after accounting for these and other clinical characteristics. Moreover, our results were largely unchanged when examining only those subjects not prescribed antihypertensive medications. As such, our findings suggest that having above‐normal BP per se according to the 2017 ACC/AHA guidelines is associated with impaired endothelial function in midlife/older men and women without other chronic clinical disorders.
Vascular Smooth Muscle Sensitivity to NO
Our assessments of endothelium‐independent dilation (brachial artery dilation in response to sublingual nitroglycerin) indicate that vascular smooth muscle sensitivity to NO does not differ among midlife/older adults across the 2017 ACC/AHA BP classifications (Figure 2). These findings indicate that group differences in FMDBA are not explained by this mechanism but rather likely reflect impaired endothelial NO production or bioavailability, consistent with the results of previous reports based on smaller cohorts than the present study. 11 , 20 , 46 It is possible, however, that decreased responsiveness to NO contributes to impaired FMDBA in midlife/older adults with a more adverse CVD risk factor profile than the healthier individuals included in our analysis. 5 Indeed, impaired endothelium‐independent dilation has been reported in subjects with above‐normal BP and increased adiposity, 47 chronic kidney disease, 48 and coronary heart disease. 49
ROS‐Related Suppression of Endothelial Function
Excess ROS, particularly superoxide, can impair endothelium‐dependent dilation (FMDBA) via direct reaction with NO and by inhibiting NO production by the enzyme endothelial NO synthase. 5 , 50 Vitamin C (ascorbic acid) infusion is a well‐established model for gaining causal insight into the role of tonic ROS‐related suppression of endothelial function in humans. 27 , 36 , 51 , 52 With this approach, vitamin C, a potent antioxidant, is infused to attain supratherapeutic/physiological circulating levels to temporarily (reversibly) reduce ROS bioactivity. The increase in endothelium‐dependent dilation in response to vitamin C infusion is interpreted as reflecting the degree of tonic ROS‐dependent suppression of vascular endothelial function. 27 , 36 , 51 , 52 In our analysis, intravenous infusion of vitamin C increased FMDBA in individuals in the above‐normal BP classifications but not in those with normal BP (Figure 3). Consistent with this observation, among individuals, baseline FMDBA and the degree of tonic ROS‐associated suppression of FMDBA were inversely related. These experimental findings provide direct evidence suggesting that increased ROS bioactivity contributes to impaired vascular endothelial function in midlife/older adults with above‐normal BP based on the 2017 ACC/AHA guidelines.
Endothelial Molecular Mechanisms
To gain insight into potential molecular mechanisms underlying ROS‐mediated suppression of FMDBA in individuals classified with above‐normal BP, we analyzed data obtained from endovascular biopsies of endothelial cells in a subset of our subjects. We found that expression of the p47phox subunit of NADPH oxidase, a major superoxide/ROS‐producing enzyme in the vasculature, 53 , 54 was higher in endothelial cells from individuals with above‐normal versus normal BP (Figure 4). This finding suggests that increased expression of NADPH oxidase may have contributed to increased ROS bioactivity and its tonic suppression of NO‐mediated endothelial function in adults with above‐normal BP. Of note, manganese superoxide dismutase, the mitochondrial isoform of superoxide dismutase, an important endogenous antioxidant enzyme for regulating superoxide/ROS in arteries, 55 , 56 was similar in individuals with normal and above‐normal BP (Figure 4). This observation suggests a lack of an appropriate compensatory increase in endogenous antioxidant defenses in the face of increased superoxide/ROS bioactivity in the above‐normal BP groups. Finally, nitrotyrosine, a posttranslational marker of oxidant modification of tyrosine residues on proteins, did not differ between the normal and above‐normal BP groups. This may indicate that altered ROS signaling in individuals with above‐normal BP, although functionally significant, did not attain levels necessary to induce a measurable increase in this indirect marker of oxidative stress.
Circulating Markers of Oxidative Stress and Inflammation
We found no differences in plasma oxidized LDL or total antioxidant status, indirect markers of systemic oxidative stress and antioxidant defenses, respectively, across the normal and above‐normal BP classifications. Although circulating concentrations of high‐sensitivity C‐reactive protein and tumor necrosis factor alpha also did not differ among the groups, plasma interleukin‐6, a major proinflammatory cytokine, was 46% greater in subjects with above‐normal compared with normal BP (Figure 5) and was inversely related to FMDBA among individuals in the overall cohort. By increasing angiotensin type 1 receptor expression, interleukin‐6 stimulates production of ROS from vascular smooth muscle cells and induces endothelial dysfunction in mice. 57 , 58 , 59 Circulating concentrations of interleukin‐6 also correlate inversely with FMDBA and other measures of endothelial dysfunction among healthy midlife/older men 60 and participants of the Framingham Offspring Study, 61 which is positively associated with future cardiovascular events. 62 , 63 Thus, the greater circulating interleukin‐6 in individuals with above‐normal BP may have contributed to their lower FMDBA in the present study.
Finally, we wish to emphasize that there are well‐established links in hypertension between NADPH oxidase‐stimulated superoxide production and systemic inflammatory signaling, as indicated by elevated levels of interleukin‐6. For example, NADPH oxidase‐associated oxidative stress is observed in antigen‐presenting cells of hypertensive animals and humans and can stimulate release of interleukin‐6 from mononuclear cells. 64 , 65 These events could, in turn, contribute to an imbalance in pro‐ versus anti‐inflammatory T‐cell regulation, thus linking NADPH oxidase activity to oxidative stress, inflammation, and endothelial dysfunction in the setting of hypertension.
Study Limitations
Mechanistic outcomes were measured in different subsets of the overall cohort, including subgroups of subjects who underwent the technically demanding vascular endothelial cell analyses via endovascular biopsy. Given the retrospective design of this analysis, it was not possible to assess all mechanistic outcomes in the same subjects as not all measurements were included in each prior investigation. When combined with inherent measurement variability, this limitation likely masked group differences in some protein markers or interindividual relations between those markers, such as p47phox abundance and FMDBA. Moreover, because of the cross‐sectional nature of our analysis, we cannot determine whether changes in endothelial function, oxidative stress, and inflammation are a cause or consequence of above‐normal BP. Multiple investigators collected the vascular function data in this analysis, which may have increased variability; however, our laboratory used standardized data collection and analysis procedures for all of the measurements, which minimized variability as much as is possible. Finally, because of the small number of subjects prescribed antihypertensive medications, we were not able to perform direct comparisons of those receiving and not receiving BP medication.
Conclusions
In summary, our findings indicate that vascular endothelial function, as assessed by FMDBA, is progressively impaired in otherwise healthy midlife/older adults with elevated BP, stage 1 hypertension, or stage 2 hypertension based on 2017 ACC/AHA guidelines compared with their peers with normal BP. We present evidence that the lower FMDBA in individuals with above‐normal BP may be mediated by tonic ROS‐related suppression of endothelium‐dependent dilation associated with increased endothelial cell expression of the superoxide‐generating enzyme, NADPH oxidase. Higher circulating concentrations of the proinflammatory cytokine interleukin‐6 also may contribute to impaired endothelial function in the subjects with above‐normal BP.
Our results provide evidence that impaired endothelial function is apparent in midlife/older adults with above‐normal BP per current ACC/AHA guidelines in the absence of other cardiometabolic clinical disorders. As such, differences in endothelial function may constitute an important pathophysiological “substrate” contributing to the increased risk of CVD across these recent BP reclassifications. Overall, our findings support the implementation of healthy lifestyle practices and, possibly, pharmacological strategies to improve vascular endothelial function in individuals with BP in the elevated and stage 1 and 2 hypertension ranges based on the 2017 ACC/AHA guidelines.
Sources of Funding
This research was funded by an American Heart Association Post‐Doctoral Fellowship 18POST33990034 (Craighead).
Disclosures
None.
Supporting information
Acknowledgments
The authors would like to thank the laboratory personnel who contributed to data acquisition for this retrospective analysis. The authors would also like to thank Dr Zhiying You for his statistical consultation.
(J Am Heart Assoc. 2020;9:e016625 DOI: 10.1161/JAHA.120.016625.)
For Sources of Funding and Disclosures, see page 11.
References
- 1. Huang Y, Wang S, Cai X, Mai W, Hu Y, Tang H, Xu D. Prehypertension and incidence of cardiovascular disease: a meta-analysis. BMC Med. 2013;11:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang Y, Cai X, Zhang J, Mai W, Wang S, Hu Y, Ren H, Xu D. Prehypertension and incidence of ESRD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;63:76–83. [DOI] [PubMed] [Google Scholar]
- 3. Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction. Circulation. 2007;115:1285–1295. [DOI] [PubMed] [Google Scholar]
- 5. Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci. 2011;120:357–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Virdis A, Ghiadoni L, Giannarelli C, Taddei S. Endothelial dysfunction and vascular disease in later life. Maturitas. 2010;67:20–24. [DOI] [PubMed] [Google Scholar]
- 7. Vita JA, Keaney JF. Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640–642. [DOI] [PubMed] [Google Scholar]
- 8. Chalmers J, MacMahon S, Mancia G, Whitworth J, Beilin L, Hansson L, Neal B, Rodgers A, Ni Mhurchu C, Clark T. 1999 World Health Organization-International Society of Hypertension guidelines for the management of hypertension. Guidelines sub-committee of the World Health Organization. Clin Exp Hypertens. 1999;21:1009–1060. [DOI] [PubMed] [Google Scholar]
- 9. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 10. Panza JA, Quyyumi AA, Brush JE, Epstein SE. Abnormal endothelium‐dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. [DOI] [PubMed] [Google Scholar]
- 11. Li J, Zhao SP, Li XP, Zhuo QC, Gao M, Lu SK. Non-invasive detection of endothelial dysfunction in patients with essential hypertension. Int J Cardiol. 1997;61:165–169. [DOI] [PubMed] [Google Scholar]
- 12. Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium‐dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. [DOI] [PubMed] [Google Scholar]
- 13. Yeboah J, Crouse JR, Hsu F-C, Burke GL, Herrington DM. Brachial flow‐mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. [DOI] [PubMed] [Google Scholar]
- 14. Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y, Shechter A, Feinberg MS. Long-term association of brachial artery flow‐mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol. 2009;134:52–58. [DOI] [PubMed] [Google Scholar]
- 15. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 16. Bundy JD, Li C, Stuchlik P, Bu X, Kelly TN, Mills KT, He H, Chen J, Whelton PK, He J. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 18. Craighead DH, Smith CJ, Alexander LM. Blood pressure normalization via pharmacotherapy improves cutaneous microvascular function through NO‐dependent and –independent mechanisms. Microcirculation. 2017;24:e12382. https://doi.org/10.1111.micc.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, Iwamoto A, Kajikawa M, Matsumoto T, Oda N, et al. Endothelial function is impaired in patients receiving antihypertensive drug treatment regardless of blood pressure level: FMD-J Study (flow‐Mediated Dilation Japan). Hypertension. 2017;70:790–797. [DOI] [PubMed] [Google Scholar]
- 20. Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol. 2013;61:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaplon RE, Hill SD, Bispham NZ, Santos-Parker JR, Nowlan MJ, Snyder LL, Chonchol M, LaRocca TJ, McQueen MB, Seals DR. Oral trehalose supplementation improves resistance artery endothelial function in healthy middle-aged and older adults. Aging. 2016;8:1167–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Santos-Parker JR, Strahler TR, Bassett CJ, Bispham NZ, Chonchol MB, Seals DR. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging. 2017;9:187–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rossman MJ, Santos-Parker JR, Steward CAC, Bispham NZ, Cuevas LM, Rosenberg HL, Woodward KA, Chonchol M, Gioscia-Ryan RA, Murphy MP, et al. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension. 2018;71:1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, Chonchol M, Seals DR. Chronic nicotinamide riboside supplementation is well‐tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun. 2018;9:1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heinemann M, Sellick K, Rickard C, Reynolds P, McGrail M. Automated versus manual blood pressure measurement: a randomized crossover trial. Int J Nurs Pract. 2008;14:296–302. [DOI] [PubMed] [Google Scholar]
- 26. Skirton H, Chamberlain W, Lawson C, Ryan H, Young E. A systematic review of variability and reliability of manual and automated blood pressure readings. J Clin Nurs. 2011;20:602–614. [DOI] [PubMed] [Google Scholar]
- 27. Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow‐mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium‐dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium‐dependent dilatation with ageing. J Physiol. 2006;571:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow‐mediated dilation with age is not explained by L-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol. 2007;102:63–71. [DOI] [PubMed] [Google Scholar]
- 31. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. [DOI] [PubMed] [Google Scholar]
- 32. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, et al. Guidelines for the ultrasound assessment of endothelial‐dependent flow‐mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. [DOI] [PubMed] [Google Scholar]
- 33. Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow‐mediated dilation. Hypertension. 2010;55:1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thijssen DHJ, Bruno RM, van Mil ACCM, Holder SM, Faita F, Greyling A, Zock PL, Taddei S, Deanfield JE, Luscher T, et al. Expert consensus and evidence‐based recommendations for the assessment of flow‐mediated dilation in humans. Eur Heart J. 2019;40:2534–2547. [DOI] [PubMed] [Google Scholar]
- 35. Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A. 1996;93:3704–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eskurza I, Monahan KD, Robinson JA, Seals DR. Ascorbic acid does not affect large elastic artery compliance or central blood pressure in young and older men. Am J Physiol Heart Circ Physiol. 2004;286:H1528–H1534. [DOI] [PubMed] [Google Scholar]
- 37. Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans. Circ Res. 2007;100:1659–1666. [DOI] [PubMed] [Google Scholar]
- 38. Colombo PC, Ashton AW, Celaj S, Talreja A, Banchs JE, Dubois NB, Marinaccio M, Malla S, Lachmann J, Ware JA, et al. Biopsy coupled to quantitative immunofluorescence: a new method to study the human vascular endothelium. J Appl Physiol. 2002;92:1331–1338. [DOI] [PubMed] [Google Scholar]
- 39. Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow‐mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26:631–640. [DOI] [PubMed] [Google Scholar]
- 40. Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. flow‐mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011;57:363–369. [DOI] [PubMed] [Google Scholar]
- 41. Ras RT, Streppel MT, Draijer R, Zock PL. flow‐mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. 2013;168:344–351. [DOI] [PubMed] [Google Scholar]
- 42. Xu Y, Arora RC, Hiebert BM, Lerner B, Szwajcer A, McDonald K, Rigatto C, Komenda P, Sood MM, Tangri N. Non-invasive endothelial function testing and the risk of adverse outcomes: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. 2014;15:736–746. [DOI] [PubMed] [Google Scholar]
- 43. Matsuzawa Y, Kwon T-G, Lennon RJ, Lerman LO, Lerman A. Prognostic value of flow‐mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta-analysis. J Am Heart Assoc. 2015;4:e002270 DOI: 10.1161/JAHA.115.002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension. 2008;52:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feairheller DL, Diaz KM, Kashem MA, Thakkar SR, Veerabhadrappa P, Sturgeon KM, Ling C, Williamson ST, Kretzschmar J, Lee H, et al. Effects of moderate aerobic exercise training on vascular health and blood pressure in African Americans. J Clin Hypertens. 2014;16:504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jablonski KL, Gates PE, Pierce GL, Seals DR. Low dietary sodium intake is associated with enhanced vascular endothelial function in middle-aged and older adults with elevated systolic blood pressure. Ther Adv Cardiovasc Dis. 2009;3:347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Christou DD, Pierce GL, Walker AE, Hwang M-H, Yoo J-K, Luttrell M, Meade TH, English M, Seals DR. Vascular smooth muscle responsiveness to nitric oxide is reduced in healthy adults with increased adiposity. Am J Physiol Heart Circ Physiol. 2012;303:H743–H750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kopel T, Kaufman JS, Hamburg N, Sampalis JS, Vita JA, Dember LM. Endothelium‐dependent and ‐independent vascular function in advanced chronic kidney disease. Clin J Am Soc Nephrol. 2017;12:1588–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang X, Zhao S-P, Li X-P, Gao M, Zhou Q-C. Endothelium‐dependent and ‐independent functions are impaired in patients with coronary heart disease. Atherosclerosis. 2000;149:19–24. [DOI] [PubMed] [Google Scholar]
- 50. Herrera MD, Mingorance C, Rodríguez-Rodríguez R, Alvarez de Sotomayor M. Endothelial dysfunction and aging: an update. Ageing Res Rev. 2010;9:142–152. [DOI] [PubMed] [Google Scholar]
- 51. Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ. high‐dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol. 2007;103:1715–1721. [DOI] [PubMed] [Google Scholar]
- 52. Monahan KD, Eskurza I, Seals DR. Ascorbic acid increases cardiovagal baroreflex sensitivity in healthy older men. Am J Physiol Heart Circ Physiol. 2004;286:H2113–H2117. [DOI] [PubMed] [Google Scholar]
- 53. Bedard K, Krause K-H. The NOX family of ROS‐generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. [DOI] [PubMed] [Google Scholar]
- 54. Konior A, Schramm A, Czesnikiewicz-Guzik M, Guzik TJ. NADPH oxidases in vascular pathology. Antioxid Redox Signal. 2014;20:2794–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. 2004;24:1367–1373. [DOI] [PubMed] [Google Scholar]
- 57. Ikeda U, Ikeda M, Oohara T, Oguchi A, Kamitani T, Tsuruya Y, Kano S. Interleukin 6 stimulates growth of vascular smooth muscle cells in a PDGF‐dependent manner. Am J Physiol. 1991;260:H1713–1717. [DOI] [PubMed] [Google Scholar]
- 58. Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol. 2007;27:2576–2581. [DOI] [PubMed] [Google Scholar]
- 59. Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Böhm M, Nickenig G. Interleukin‐6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res. 2004;94:534–541. [DOI] [PubMed] [Google Scholar]
- 60. Esteve E, Castro A, López-Bermejo A, Vendrell J, Ricart W, Fernández-Real J-M. Serum interleukin‐6 correlates with endothelial dysfunction in healthy men independently of insulin sensitivity. Diabetes Care. 2007;30:939–945. [DOI] [PubMed] [Google Scholar]
- 61. Vita JA, Keaney JF, Larson MG, Keyes MJ, Massaro JM, Lipinska I, Lehman BT, Fan S, Osypiuk E, Wilson PWF, et al. Brachial artery vasodilator function and systemic inflammation in the Framingham Offspring Study. Circulation. 2004;110:3604–3609. [DOI] [PubMed] [Google Scholar]
- 62. Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin‐6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. [DOI] [PubMed] [Google Scholar]
- 63. Ridker PM, Libby P, MacFadyen JG, Thuren T, Ballantyne C, Fonseca F, Koenig W, Shimokawa H, Everett BM, Glynn RJ. Modulation of the interleukin‐6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J. 2018;39:3499–3507. [DOI] [PubMed] [Google Scholar]
- 64. Crowley SD. The cooperative roles of inflammation and oxidative stress in the pathogenesis of hypertension. Antioxid Redox Signal. 2014;20:102–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Loperena R, Harrison DG. Oxidative stress and hypertensive diseases. Med Clin North Am. 2017;101:169–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kaplon RE, Chung E, Reese L, Cox-York K, Seals DR, Gentile CL. Activation of the unfolded protein response in vascular endothelial cells of nondiabetic obese adults. J Clin Endocrinol Metab. 2013;98:E1505–E1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rossman MJ, Kaplon RE, Hill SD, McNamara MN, Santos-Parker JR, Pierce GL, Seals DR, Donato AJ. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am J Physiol Heart Circ Physiol. 2017;313:H890–H895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Silver AE, Christou DD, Donato AJ, Beske SD, Moreau KL, Magerko KA, Seals DR. Protein expression in vascular endothelial cells obtained from human peripheral arteries and veins. J Vasc Res. 2010;47:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Colombo PC, Banchs JE, Celaj S, Talreja A, Lachmann J, Shailesh M, DuBois NB, Ashton AE, Latif F, Jorde UP, et al. Endothelial cell activation in patients with decompensated heart failure. Circulation. 2005;111:58–62. [DOI] [PubMed] [Google Scholar]
- 70. Yoo J-K, Hwang M-H, Luttrell MJ, Kim H, Meade TH, English M, Segal MS, Christou DD. Higher levels of adiponectin in vascular endothelial cells are associated with greater brachial artery flow‐mediated dilation in older adults. Exp Gerontol. 2015;63:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jalal DI, Decker E, Perrenoud L, Nowak KL, Bispham N, Mehta T, Smits G, You Z, Seals DR, Chonchol M, et al. Vascular function and uric acid-lowering in stage 3 CKD. J Am Soc Nephrol. 2017;28:943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nowak KL, Chonchol M, Ikizler TA, Farmer-Bailey H, Salas N, Chaudhry R, Wang W, Smits G, Tengesdal I, Dinarello CA, et al. IL-1 inhibition and vascular function in CKD. J Am Soc Nephrol. 2017;28:971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


