Abstract
Background
Gait speed is a reliable measure of physical function and frailty in patients with aortic stenosis undergoing transcatheter aortic valve replacement (TAVR). Slow gait speed pre‐TAVR predicts worse clinical outcomes post‐TAVR. The consequences of improved versus worsened physical function post‐TAVR are unknown.
Methods and Results
The REPRISE III (Repositionable Percutaneous Replacement of Stenotic Aortic Valve Through Implantation of Lotus Valve System–Randomized Clinical Evaluation) trial randomized high/extreme risk patients to receive a mechanically‐expanded or self‐expanding transcatheter heart valve. Of 874 patients who underwent TAVR, 576 with complete data at baseline and 1 year were included in this analysis. Slow gait speed in the 5‐m walk test was defined as <0.83 m/s. A clinically meaningful improvement (≥0.1 m/s) in gait speed 1 year after TAVR occurred in 39% of patients, 35% exhibited no change, and 26% declined (≥0.1 m/s). Among groups defined by baseline/1‐year post‐TAVR gait speeds, 1‐ to 2‐year mortality or hospitalization rates were as follows: 6.6% (normal/normal), 8.0% (slow/normal), 20.9% (normal/slow), and 21.5% (slow/slow). After adjustment, slow gait speed at 1 year (regardless of baseline speed) was associated with a 3.5‐fold increase in death/hospitalization between 1 and 2 years compared with those with normal baseline/1‐year gait speed. Patients whose slow gait speed normalized at 1 year had no increased risk. One‐year, but not baseline, gait speed was associated with death or hospitalization between 1 and 2 years (adjusted hazard ratio, 0.83 per 0.1 m/s faster gait; 95% CI, 0.74–0.93, P=0.001).
Conclusions
Marked heterogeneity exists in the trajectory of physical function after TAVR and this, more than baseline function, has clinical consequences. Identifying and optimizing factors associated with physical resilience after TAVR may improve outcomes.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02202434.
Keywords: aortic valve stenosis, frailty, gait speed, outcomes, physical function, transcatheter aortic valve replacement
Subject Categories: Aging, Mortality/Survival, Heart Failure, Valvular Heart Disease
Nonstandard Abbreviations and Acronyms
- AS
aortic stenosis
- BMI
body mass index
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- TAVR
transcatheter aortic valve replacement
Clinical Perspective
What Is New?
After transcatheter aortic valve replacement, there is marked heterogeneity in the magnitude and direction of changes in physical function of patients; these trajectories have implications for subsequent survival, hospitalizations, and quality of life.
What Are the Clinical Implications?
Additional studies are needed to understand which patients are vulnerable to a decline in physical function after transcatheter aortic valve replacement as well as which and how interventions can be successfully applied to improve physical resilience after transcatheter aortic valve replacement.
Over the past decade, several trials have compared transcatheter and surgical approaches to valve replacement for patients with severe symptomatic aortic stenosis (AS). 1 , 2 , 3 , 4 , 5 , 6 Each of these trials has enrolled patients in a specific surgical risk category: extreme, high, intermediate, or low. Recognizing that an objective assessment of frailty and physical function is essential to properly determine procedural risk, several objective tests have been incorporated into these trials to assess and characterize this aspect of a patient's presentation. 7 , 8 , 9 Chief and simplest among these measures has been the 5‐m gait speed test, which has been recommended for clinical and research use to characterize physical function and frailty and has been used in epidemiology studies to predict survival in older adults. 10 , 11
Slow gait speed before transcatheter aortic valve replacement (TAVR) or surgical valve replacement is associated with increased postprocedure mortality and an overall poor outcome. 12 , 13 , 14 , 15 Given its simplicity and predictive value, assessment of gait speed is now routinely performed in all patients considered for TAVR and is a required data element in the TVT (Transcatheter Valve Therapies) registry. However, while its value in preprocedure risk assessment and planning has been demonstrated, what happens to physical function after TAVR is less clear. Accordingly, after the stressor of TAVR, we aimed to characterize the frequency of physical resilience (improvement in physical function as measured by gait speed) versus vulnerability (worsening of physical function) and the clinical implications of each of these trajectories. 16 , 17 Specifically, we evaluated gait speed at baseline and 1 year after TAVR, the predictors of change in gait speed, and the associations with mortality and hospitalizations between 1 and 2 years. Given the high proportion of patients undergoing TAVR with impaired physical function, knowing these trajectories of physical function after TAVR, predictors of them, and their clinical implications could inform patient selection and identify opportunities for adjunctive interventions targeting impaired physical function to promote resilience and optimize patient outcomes.
Methods
Study Population
The REPRISE III (Repositionable Percutaneous Replacement of Stenotic Aortic Valve Through Implantation of Lotus Valve System–Randomized Clinical Evaluation) randomized clinical trial compared treatment with a mechanically expanded versus a self‐expanding valve in 912 high or extreme risk patients with severe symptomatic AS. The study design, definitions for clinical characteristics, and results have been published previously. 18 For the current analysis, patients who had a transcatheter valve implanted with gait speed recorded both before and 1 year after TAVR were included. The study was approved by the institutional review board at each participating center, and all subjects gave informed consent. The data and study protocol for this clinical trial may be made available to other researchers in accordance with the Boston Scientific Data Sharing Policy (http://www.bostonscientific.com/en-US/data-sharing-requests.html).
Gait Speed
All patients included in the current analysis completed a 5‐m walk test at baseline (pre‐TAVR) and 1 year after TAVR. Participants were asked to stand stationary with their feet behind a starting line marked with tape and then, after the examiner's command of "Go," to walk at their usual pace over a 5‐m course and to stop just past the finish line. Timing was started with the first foot fall and stopped when the participant's first foot completely crossed the 5‐m end line. 19 Gait speed was calculated in m/s based on the 5‐m walk time. Slow walker was defined as those with a gait speed <0.83 m/s, and normal walker was defined as a gait speed ≥0.83 m/s. 12 , 13 Four groups were identified based on their gait speed at baseline and 1 year: Normal/Normal; Slow/Normal; Normal/Slow; and Slow/Slow. A clinically meaningful difference in gait speed is considered to be 0.1 m/s, which informed our categorization of the magnitude and direction of change in gait speed. 10
Clinical End Points
The end points and outcomes of this trial were based on the Valve Academic Research Consortium (VARC) end points and definitions and were adjudicated by an independent clinical events committee. 18 , 20 Our primary end point for this analysis was the composite of all‐cause death or hospitalization between 1 and 2 years. Hospitalizations were counted in this trial if they were for valve‐related symptoms or worsening congestive heart failure. The Kansas City Cardiomyopathy Questionnaire (KCCQ) was used to assess quality of life. 21
Statistical Analysis
Continuous variables were summarized as mean (SD) and compared using the Student t test or ANOVA as appropriate. Discrete variables were reported as counts and percent, and differences were assessed using χ2 or Fisher exact tests. Gait speeds for the whole population are shown as a box and whisker plot (whiskers at 2.5 and 97.5 percentiles and box from 25th to 75th percentiles with line at median). To identify factors associated with 1‐year gait speed, univariable linear regression models evaluated the association between baseline and 30‐day clinical and echocardiographic parameters and 1‐year gait speed. Factors with a univariable association (P≤0.10) were considered for a multivariable model using backward selection with entry/exit criteria of 0.10. Survival curves for time‐to‐event variables all‐cause death and hospitalization, based on all available follow‐up data, were prepared for events occurring between 1 and 2 years with the use of Kaplan–Meier estimates. Cox proportional hazards models were used to estimate hazard ratios. To filter and reduce the number of adjustment variables, based on their known or hypothesized association with adverse outcomes after TAVR, we considered the following variables in a selection model for their association with the composite of all‐cause death or hospitalization between 1 and 2 years: age, sex, body mass index, chronic obstructive pulmonary disease, Society of Thoracic Surgeons (STS) predicted risk of mortality score, immunosuppressive therapy, diabetes mellitus, New York Heart Association III/IV versus I/II, coronary artery disease, history of cerebrovascular disease, history of atrial fibrillation or flutter, baseline mean transvalvular gradient, baseline moderate or severe mitral regurgitation, the following events between the procedure and 1 year (stroke, life‐threatening or disabling bleeding, major bleeding, acute kidney injury, major vascular complications), and moderate or severe total aortic regurgitation on the 30‐day echocardiogram. Factors with a univariable association (P≤0.20) were considered for a multivariable model using stepwise selection with entry/exit criteria of 0.10. In addition to age and sex (both forced into the model), this process identified the following adjustment factors for all multivariable Cox models examining clinical events: immunosuppressive therapy, history of chronic obstructive pulmonary disease, diabetes mellitus, baseline mean transvalvular gradient, and stroke through 1‐year post‐TAVR. KCCQ overall summary scores were compared by using ANCOVA to adjust for baseline differences in KCCQ scores between groups. All statistical analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Patient Population and Baseline Characteristics
A total of 874 patients underwent TAVR in the REPRISE III clinical trial. For the current analysis, 58 patients were excluded because of missing gait speed (n=6) or inability to complete the gait speed test (n=52) at baseline. Additionally, 240 patients were excluded because of death within 1 year (n=88) or missing gait speed (n=77) or inability to complete the gait speed test (n=75) at 1 year. After these exclusions, 576 patients had complete data and were included in the final analysis (Figure 1). A comparison of those included in the analytic cohort, those unable to complete the walk test at baseline or 1 year, and those with missing walk data is shown in Table S1.
Figure 1. Patient selection flowchart for the current study.
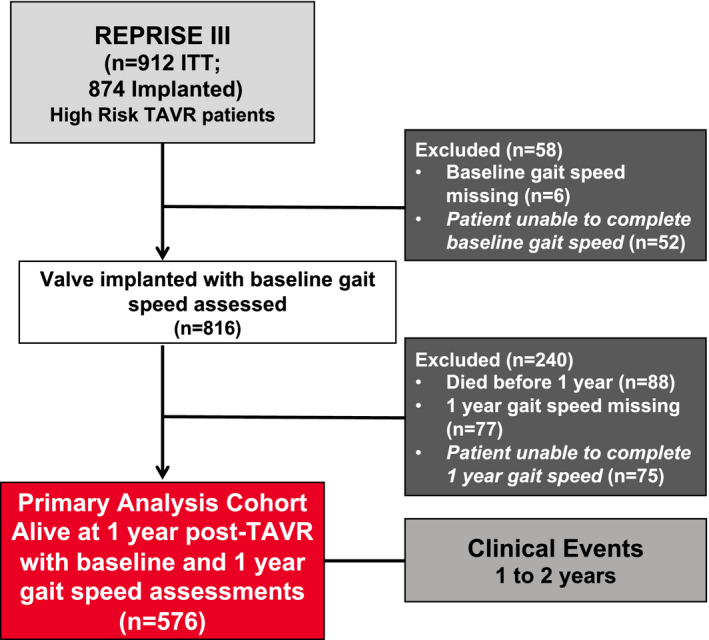
ITT indicates intent‐to‐treat; and TAVR, transcatheter aortic valve replacement.
Table 1 shows the baseline demographics, clinical and frailty characteristics, baseline and 30‐day echocardiographic characteristics, and early post‐TAVR complications according to 4 groups based on slow versus normal gait speed at baseline and 1 year. Age, sex, measures of frailty, some comorbidities, and STS score differed across the groups, but echocardiographic characteristics were similar. Rates of 30‐day complications tended to be similar across the groups.
Table 1.
Baseline and 30‐Day Characteristics According to Groups Defined by Gait Speed at Baseline and 1 Year
|
Normal/Normal (n=150) |
Slow/Normal (n=114) |
Normal/Slow (n=59) |
Slow/Slow (n=253) |
P Value (Overall) |
|
|---|---|---|---|---|---|
| Baseline clinical characteristics | |||||
| Age, mean (SD), y | 81 (8.3) | 82 (7.1) | 82 (8.0) | 84 (6.6) | 0.0003 |
| Female sex, % | 45 (30) | 51 (45) | 20 (34) | 161 (64) | <0.0001 |
| Body mass index, mean (SD), kg/m2 | 28.4 (5.3) | 28.5 (6.8) | 28.4 (6.5) | 29.8 (6.5) | 0.06 |
| Extreme risk, % | 30 (20) | 21 (18) | 12 (20) | 54 (21) | 0.93 |
| Society of Thoracic Surgeons score, mean (SD) | 5.46 (2.93) | 6.19 (3.38) | 5.99 (4.13) | 7.42 (4.24) | <0.0001 |
| Orthopedic disease, % | 8/119 (6.7) | 11/89 (12) | 6/44 (13.6) | 30/166 (18) | 0.049 |
| Frailty, % | 32/119 (27) | 81/89 (91) | 17/44 (39) | 158/166 (95) | <0.0001 |
| Gait speed, mean to walk 5 m (SD), s | 5.09 (0.82) | 8.65 (3.08) | 5.46 (0.84) | 10.08 (4.70) | <0.0001 |
| Maximal grip strength, mean (SD), kg | 27.31 (11.12) | 20.65 (8.10) | 25.71 (9.63) | 18.17 (7.89) | <0.0001 |
| Katz Index Activities of Daily Living Score mean (SD)* | 5.88 (0.45) | 5.81 (0.46) | 5.83 (0.53) | 5.54 (0.98) | <0.0001 |
| Mini‐Cognitive Assessment for Dementia Score (SD)† | 3.82 (1.34) | 3.85 (1.27) | 3.67 (1.42) | 3.53 (1.42) | 0.10 |
| New York Heart Association Class III/IV, % | 79 (53) | 82 (72) | 41 (70) | 188 (74) | 0.33 |
| Medically treated diabetes mellitus, % | 51 (35) | 36 (34) | 15 (25) | 76 (30) | 0.52 |
| Currently taking immunosuppressive therapy, % | 14 (9.3) | 9 (8.0) | 6 (10) | 21 (8.3) | 0.95 |
| History of coronary artery disease, % | 121 (81) | 92 (81) | 42 (71) | 171 (68) | 0.008 |
| History of myocardial infarction, % | 31 (21) | 22 (19) | 14 (24) | 38 (15) | 0.33 |
| History of cerebrovascular accident, % | 15 (10) | 7 (6.2) | 5 (8.5) | 35 (14) | 0.14 |
| History of peripheral vascular disease, % | 52 (35) | 36 (32) | 15 (26) | 78 (31) | 0.61 |
| History of chronic obstructive pulmonary disease, % | 32 (22) | 34 (30) | 15 (25) | 96 (38) | 0.004 |
| Chronic obstructive pulmonary disease: Supplemental oxygen dependent, % | 5 (3.4) | 4 (3.5) | 2 (3.4) | 18 (7.2) | 0.32 |
| History of atrial fibrillation, % | 40 (27) | 34 (30) | 12 (20) | 103 (41) | 0.003 |
| Prior pacemaker implant, % | 22 (15) | 18 (16) | 10 (17) | 53 (21) | 0.38 |
| Baseline echocardiography | |||||
| Aorticߚvalve area, mean (SD), cm2 | 0.74 (0.20) | 0.66 (0.17) | 0.72 (0.19) | 0.69 (0.19) | 0.005 |
| Mean aortic‐valve gradient, mean (SD), mm Hg | 43.28 (11.31) | 46.87 (15.37) | 43.74 (13.94) | 44.45 (12.16) | 0.15 |
| Left ventricular ejection fraction (SD), % | 55.47 (12.00) | 55.24 (12.27) | 57.03 (11.79) | 55.07 (10.93) | 0.44 |
| Moderate or greater aortic regurgitation, % | 12 (8.3) | 7 (6.4) | 3 (5.5) | 13 (5.6) | 0.75 |
| Moderate or greater mitral regurgitation, % | 14 (10) | 12 (11) | 3 (5.8) | 24 (11) | 0.74 |
| 30‐d echocardiography | |||||
| Effective orifice area, mean (SD), cm2 | 1.73 (0.52) | 1.69 (0.47) | 1.69 (0.50) | 1.71 (0.52) | 0.93 |
| Mean aortic‐valve gradient, mean (SD), mm Hg | 10.27 (5.17) | 11.04 (6.05) | 10.75 (5.54) | 10.57 (6.62) | 0.79 |
| Left ventricular ejection fraction (SD), % | 53.91 (11.37) | 54.01 (11.48) | 53.85 (11.54) | 55.49 (10.41) | 0.52 |
| Moderate or greater aortic regurgitation, % | 5 (3.4) | 3 (2.8) | 0 (0) | 10 (4.1) | 0.53 |
| 30‐d clinical findings | |||||
| New York Heart Association Class III/IV, % | 6 (4.0) | 11 (9.8) | 2 (3.6) | 27 (11) | 0.04 |
| Major vascular complications, % | 7 (4.7) | 5 (4.4) | 0 (0) | 11 (4.3) | 0.43 |
| Bleeding (life‐threatening or disabling), % | 7 (4.7) | 8 (7.0) | 4 (6.8) | 11 (4.3) | 0.64 |
| Hospitalization for valve‐related symptoms or worsening congestive heart failure, % | 0 (0) | 3 (2.6) | 1 (1.7) | 7 (2.8) | 0.13 |
| New pacemaker, % | 52 (35) | 35 (31) | 11 (19) | 84 (33) | 0.13 |
| New‐onset atrial fibrillation or flutter, % | 4 (2.7) | 4 (3.5) | 8 (13.6) | 19 (7.5) | 0.01 |
Values are mean (SD) or No. (%) in the implanted patient population.
Scores independence in performance of bathing, dressing, toileting, transferring, continence, and feeding (1 point each); a score of 6 indicates full function, ≤2 indicates severe functional impairment.
A score to differentiate patients with dementia based on a clock drawing distractor test (0–2 points) and recall of words (0–3 points); scores above 3 (out of 5) are considered negative for dementia.
Gait Speed at Baseline and 1 Year
The mean gait speed was 0.72±0.26 m/s at baseline and increased to 0.77±0.27 m/s at 1 year (P<0.001). At baseline, 64% of patients were slow walkers, which decreased to 54% at 1 year (P=0.047). An overall increase in gait speed for the whole population was observed despite the same median gait speed at baseline and 1 year because those with gait speeds above the median tended to be faster at 1 year than at baseline (Figure 2A and Table S2). Figure 2B shows the magnitude and direction of change in gait speed between baseline and 1 year for the whole population and for those who were slow versus normal speed walkers at baseline. Among all patients, 39% patients had a clinically meaningful improvement (≥0.10 m/s) in their gait speed at 1 year. A marked improvement of ≥0.30 m/s was noted in 14% of the patients. No change (±0.10 m/s) was noted in 35% of the patients, and a clinically meaningful decline was observed in 26% of patients at 1 year (Figure 2B and Table S3). Among those who were slow walkers at baseline, almost half (47%) experienced a clinically meaningful improvement in gait speed at 1 year (Figure 2B). Additional details for the data presented in Figure 2 can be found in Tables S2 and S3.
Figure 2. Gait speed at baseline and 1 year and changes in between those time points.
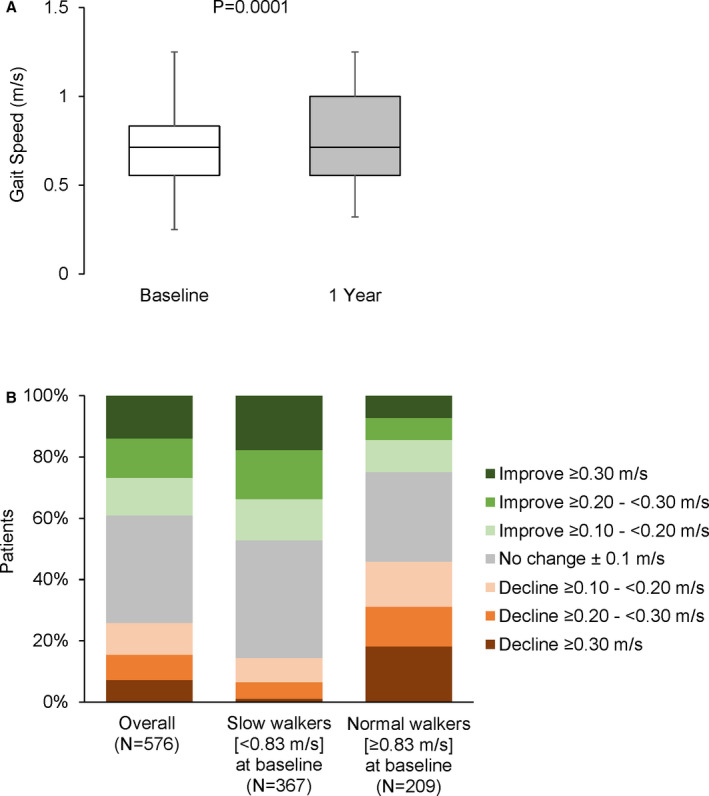
Gait speeds at baseline and 1 year for the whole population are shown (whiskers at 2.5 and 97.5 percentiles and box from 25th to 75th percentiles with line at median) (A). The magnitude and direction of change in gait speed from baseline to 1 year is shown for the whole population and then according to whether baseline gait speed was slow or normal (B).
Predictors of Gait Speed at 1 Year
As gait speed at 1 year was associated with improvement in clinical outcomes, we assessed the predictors of gait speed at 1 year. Table 2 outlines the univariate and multivariate baseline and postprocedure predictors of gait speed at 1 year. Slower baseline gait speed, female sex, higher STS score, greater dependence for activities of daily living, New York Heart Association class III/IV at baseline, orthopedic disease, absence of coronary disease, history of chronic obstructive pulmonary disease, and worse cognitive function were each independent predictors of slower gait speed at 1 year. No echocardiographic parameters were associated with gait speed. The associations between baseline factors alone and gait speed at 1 year are shown in Table S4.
Table 2.
Baseline and Postprocedural Predictors of 1‐Year Gait Speed
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
|
β Estimate (95% CI) |
P Value |
β Estimate (95% CI) |
P Value | ||
| Clinical factors (baseline) | |||||
| Gait speed at baseline (per 0.1 m/s increase) | 0.49 (0.41 to 0.56) | <0.0001 | 0.38 (0.30 to 0.46) | <0.0001 | |
| Max grip strength (per 1 kg increase) | 0.09 (0.07 to 0.11) | <0.0001 | |||
| Female sex | −1.47 (−1.90 to −1.04) | <0.0001 | −0.51 (−0.93 to −0.09) | 0.02 | |
| STS score (per increase of 1) | −0.17 (−0.23 to −0.12) | <0.0001 | −0.09 (−0.14 to −0.04) | 0.0007 | |
| Katz ADLs (per 1 increase) | 0.78 (0.49 to 1.06) | <0.0001 | 0.28 (0.02 to 0.54) | 0.03 | |
| NYHA III/IV at baseline | −1.18 (−1.65 to −0.71) | <0.0001 | −0.49 (−0.90 to −0.07) | 0.02 | |
| Orthopedic disease | −1.47 (−2.14 to −0.81) | <0.0001 | −0.85 (−1.45 to −0.25) | 0.005 | |
| Age (per 1‐y increase) | −0.06 (−0.09 to −0.03) | <0.0001 | |||
| History of coronary disease | 0.85 (0.35 to 1.35) | 0.001 | 0.50 (0.05 to 0.95) | 0.03 | |
| History of COPD | −0.77 (−1.25 to −0.28) | 0.002 | −0.40 (−0.82 to 0.02) | 0.06 | |
| Mini‐COG (per 1‐point increase) | 0.21 (0.05 to 0.37) | 0.01 | 0.16 (0.01 to 0.30) | 0.03 | |
| NYHA III/IV at 30 d | −0.90 (−1.70 to −0.09) | 0.03 | |||
| New onset Afib between the procedure and 1 y | −0.89 (−1.83 to 0.05) | 0.06 | |||
| History of Afib or flutter | −0.44 (−0.91 to 0.03) | 0.06 | |||
| Stroke (any) between procedure and 1 y | −0.92 (−1.99 to 0.14) | 0.09 | |||
| BMI (per increase of 1) | −0.02 (−0.06 to 0.01) | 0.21 | |||
| Bleeding (life‐threatening, disabling, or major) between the procedure and 1 y | −0.32 (−0.97 to 0.34) | 0.35 | |||
| Hospitalization for valve‐related symptoms or worsening CHF between the procedure and 1 y | −0.36 (−1.16 to 0.44) | 0.37 | |||
| Moderate or severe mitral regurgitation at baseline | −0.29 (−1.06 to 0.48) | 0.46 | |||
| History of PVD | 0.17 (−0.31 to 0.66) | 0.49 | |||
| History of CVA | −0.25 (−0.97 to 0.47) | 0.49 | |||
| Major vascular complication between the procedure and 1 y | −0.32 (−1.37 to 0.73) | 0.55 | |||
| EF at 30 d (per 5% increase in EF) | −0.02 (−0.13 to 0.09) | 0.71 | |||
| Diabetes mellitus (medically treated) | −0.06 (−0.54 to 0.41) | 0.79 | |||
| New pacemaker between the procedure and 1 y | 0.06 (−0.41 to 0.54) | 0.80 | |||
| Immunosuppressive therapy | −0.08 (−0.88 to 0.72) | 0.85 | |||
| Mean pressure gradient (MPG) on 30‐d echo (per 5‐mm Hg increase) | −0.02 (−0.21 to 0.17) | 0.85 | |||
| Mean pressure gradient (MPG) at baseline (per 5 mm Hg increase) | 0.01 (−0.08 to 0.09) | 0.89 | |||
| EF at baseline (per 5% increase in EF) | 0.00 (−0.10 to 0.11) | 0.96 | |||
| Moderate or severe aortic regurgitation on 30‐d echo | 0.02 (−1.28 to 1.32) | 0.98 | |||
Multivariable model R 2=0.29. Afib indicates atrial fibrillation; BMI, body mass index; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; EF, ejection fraction; Katz ADLs, Katz Index Activities of Daily Living Score; Mini‐COG, Mini‐Cognitive Assessment for Dementia Score; MPG, mean pressure gradient; NYHA, New York Heart Association; PVD, peripheral vascular disease; and STS, Society of Thoracic Surgeons score.
Gait Speed and Clinical Outcomes
Between 1 and 2 years, 47 (8.2%) patients died and 45 (7.8%) were hospitalized. Compared with those with normal gait speed at baseline and 1 year, the rate of all‐cause death or hospitalization between 1 and 2 years was higher among those with slow gait speed at 1 year regardless of their baseline gait speed, but not higher for those whose slow gait speed at baseline normalized at 1 year (Figure 3). Similar relationships were observed for all‐cause mortality and hospitalizations when each was examined individually (Figure 3). After adjustment, compared with those with normal gait speed at baseline and 1 year, those with slow gait speed at both time points had an adjusted hazard ratio of 3.40 (95% CI, 1.61–7.18, P=0.001) and those with normal gait speed at baseline but slow gait speed at 1 year had an adjusted hazard ratio of 3.64 (95% CI, 1.53–8.68, P=0.004) for all‐cause death or hospitalization between 1 and 2 years (Figure 3 and Table 3). Similar adjusted relationships were observed when these end points were examined individually. There was no increased hazard observed for those with a slow baseline gait speed that normalized at 1 year for any end point. Adjusted analyses are also shown in Table 3 for each end point based on the magnitude and direction of change in gait speed between baseline and 1 year. When both baseline and 1‐year gait speed were included in a multivariable model with adjustment for other clinical factors, only 1‐year gait speed was associated with clinical outcomes between 1 and 2 years. A faster 1‐year gait speed was associated with a lower adjusted hazard of mortality or hospitalization between 1 and 2 years (adjusted hazard ratio, 0.83 for 0.1 m/s increase in gait speed, 95% CI, 0.74–0.93, P=0.001) (Table 3). This association was driven by the association with rates of hospitalization.
Figure 3. Clinical outcomes between 1 and 2 years based on gait speed at baseline and 1 year.
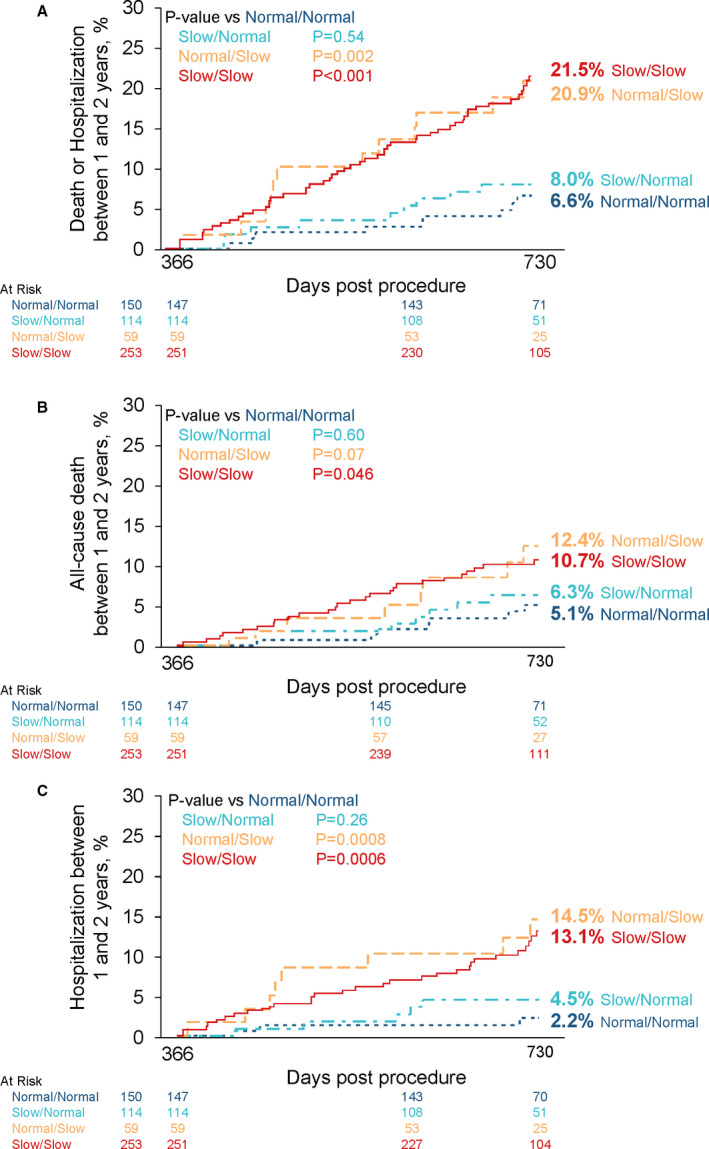
Kaplan–Meier curves are shown based on slow vs normal gait speed at baseline and 1 year for 1‐ to 2‐year death or hospitalization (A), death (B), and hospitalization (C).
Table 3.
Adjusted Association Between Gait Speed at 1 Year or Change From Baseline With Clinical Outcomes Between 1 and 2 Years Post‐TAVR
| Mortality or Hospitalization | Mortality | Hospitalization | ||||
|---|---|---|---|---|---|---|
| aHR (95% CI) | P Value | aHR (95% CI) | P Value | aHR (95% CI) | P Value | |
| Adjusted models for normal vs slow gait speed at baseline and 1 y* | ||||||
| Normal baseline/normal 1 y (referent) | 1.0 | … | 1.0 | … | 1.0 | … |
| Slow baseline/normal 1 y | 1.38 [0.54–3.50] | 0.50 | 1.48 [0.51–4.28] | 0.47 | 2.01 [0.48–8.48] | 0.34 |
| Normal baseline/slow 1 y | 3.64 [1.53–8.68] | 0.004 | 2.53 [0.88–7.27] | 0.09 | 7.05 [1.86–26.69] | 0.004 |
| Slow baseline/slow 1 y | 3.40 [1.61–7.18] | 0.001 | 2.69 [1.11–6.51] | 0.03 | 4.04 [1.18–13.92] | 0.03 |
| Adjusted models for magnitude and direction of change in gait speed between baseline and 1 y† | ||||||
| Large worsening in gait speed from baseline to 1 y | 2.05 [1.06–3.99] | 0.03 | 1.66 [0.67–4.10] | 0.27 | 2.68 [1.15–6.27] | 0.02 |
| Small worsening in gait speed from baseline to 1 y | 1.05 [0.48–2.30] | 0.91 | 1.25 [0.48–3.25] | 0.64 | 0.80 [0.23–2.76] | 0.73 |
| No change in gait speed from baseline to 1 y | 1.0 | … | 1.0 | … | 1.0 | … |
| Small improvement in gait speed from baseline to 1 y | 0.50 [0.21–1.19] | 0.12 | 0.47 [0.14–1.63] | 0.24 | 0.45 [0.13–1.53] | 0.20 |
| Large improvement in gait speed from baseline to 1 y | 0.65 [0.35–1.20] | 0.17 | 0.92 [0.43–1.98] | 0.84 | 0.52 [0.21–1.25] | 0.14 |
| Adjusted models for 1 y gait speed‡ | ||||||
| 1‐y gait speed (per 0.1 increase) | 0.83 [0.74–0.93] | 0.001 | 0.91 [0.80–1.04] | 0.18 | 0.76 [0.65–0.89] | 0.0007 |
| Baseline gait speed (per 0.1 increase) | 0.96 [0.87–1.07] | 0.48 | 0.97 [0.84–1.10] | 0.61 | 0.99 [0.86–1.14] | 0.85 |
aHR indicates adjusted hazard ratio; and TAVR, transcatheter aortic valve replacement.
Each model adjusted for the variables in the selection model: age (at time of consent), subject currently taking immunosuppressive therapy, female sex, history of chronic obstructive pulmonary disease, medically treated diabetes mellitus, mean aortic valve gradient at baseline, and stroke through 365 days.
Each model adjusted for variables in the selection model+baseline gait speed.
Each model adjusted for variables in the selection and included both baseline and 1 year gait speed.
Gait Speed and Quality of Life
After adjustment for baseline KCCQ and baseline gait speed, faster gait speed at 1 year was associated with better quality of life at 1 year (+1.95 KCCQ points per increase of 0.1 m/s in gait speed, 95% CI, 1.32–2.56, P<0.001). KCCQ scores at baseline differed across the 4 groups based on slow versus normal gait speed at baseline and 1 year (Table S5). Compared with those with normal gait speed at both time points, those with slow gait speed at 1 year had lower baseline KCCQ regardless of baseline gait speed. After adjustment for baseline KCCQ, 1‐year KCCQ also differed between the groups and pairwise comparisons followed a similar pattern as at baseline (Table S5).
Inability to Perform the Gait Speed Test and Outcomes
Of the 874 patients with implanted valves, 110 unique patients were unable to complete the gait speed test at baseline or at 1 year: 75 patients were able to perform gait speed at baseline, but “became unable” at 1 year; 12 patients were unable to complete gait speed at baseline, but “became able” at 1 year; and 23 patients were unable to perform the gait speed test at baseline and at 1 year. Table S6 shows the rates of mortality or hospitalizations and each outcome individually between 1 and 2 years for each of these groups of patients. While the numbers are small, higher mortality and hospitalization rates were observed among those who were able to perform the gait speed test at baseline but became unable at 1 year and no events were observed among those who were unable to perform the test at baseline but became able at 1 year.
Discussion
Among high or extreme risk patients with severe symptomatic AS treated with TAVR, the magnitude and direction of change in physical function at 1 year after TAVR varied greatly, with 39% demonstrating a resilient trajectory characterized by faster gait speed, 26% demonstrating a vulnerable trajectory characterized by slower gait speed, and 35% experiencing no change. There was variability in these trajectories regardless of whether baseline gait speed was slow or normal. Compared with patients with normal gait speed at baseline and 1 year, having a slow gait speed at 1 year was associated with an adjusted hazard ratio of 3.5 for mortality or hospitalization between 1 and 2 years, regardless of whether a patient had normal or slow gait speed before TAVR. With the same comparator group, those with a slow gait speed before TAVR that normalized at 1 year had no increased hazard for clinical events between 1 and 2 years. In line with this, when both baseline and 1‐year gait speed were included in a model, 1 year but not baseline gait speed was associated with subsequent clinical outcomes. Associations with 1‐year quality of life were similar to those for mortality or hospitalization. Collectively, these data reveal there is marked heterogeneity in the trajectory of physical function after TAVR and that this trajectory—more so than baseline physical function—is clinically consequential. Indeed, both directions of change—normal to slow and slow to normal gait speeds—were consequential. Identifying and optimizing factors associated with physical resilience after TAVR may improve patient outcomes.
The prevalence of frailty in patients considered for and undergoing TAVR or surgical valve aortic replacement is high, ranging from 26% to 68%, depending on the assessment tool used. 9 Including objective assessment of frailty in the evaluation of patients with AS has been helpful to identify patients who may not benefit from the procedure and to appropriately characterize procedural risk for clinical trials and shared decision making. 22 Indeed, the presence and severity of frailty is associated with worse outcomes after valve replacement. 9
While the components and definition of frailty are debated, impaired physical function is widely accepted as a core feature. 8 Assessment of gait speed is perhaps the most commonly utilized single test of physical function because of the ease of administration and prognostic value. 10 As reported recently from the STS/American College of Cardiology TVT Registry, the median gait speed of patients undergoing TAVR is 0.63 m/s, with only 24% able to walk at what is considered a normal pace (>0.83 m/s). 13 Slower walkers had increased mortality, longer hospital stays, and lower probability of discharge to home. 13 In a recent analysis of the STS database for patients undergoing cardiac surgery, slower gait speed before surgery was associated with lower survival at early (<30 days), intermediate (30 days–1 year), and longer (>1 year) timeframes after surgery. 23
However, little is known about the degree to which frailty or, more specifically, physical function is responsive to TAVR and the implications of its potential reversibility. This could have important ramifications for shared decision making and anticipated clinical benefit of TAVR for patients with significant impairment of physical function before TAVR. Some insights come from recent reports. Kim et al reported on functional status after TAVR by conducting serial phone interviews with patients or their proxy to assess the patient's ability to perform 22 daily activities and physical tasks. 24 They identified a spectrum of trajectories for functional status as measured by disability burden after TAVR, and although baseline status influenced the trajectory it did not determine it. The implications of these trajectories for subsequent clinical events were not examined. Schoenenberger et al also reported rates of decline in activities of daily living after TAVR and predictors of it. 25 Abdul‐Jawad Altisent et al reported on changes in 6‐minute walk distance, a marker of aerobic fitness, at 6 months after TAVR and the clinical implications of these changes in functional capacity. 26 They, too, showed a heterogenous response to TAVR in terms of magnitude and direction of change in walk distance and observed that these changes were associated with subsequent clinical outcomes. We extend these prior studies by showing for the first time specifically how physical function as measured by the gait speed test changes after TAVR and the clinical consequences of these changes. Although many factors associated with slower gait speed (Table 2) are also markers of worse survival, we adjusted for multiple factors indicative of poor health status and showed that slower gait speed was independently associated with worse clinical outcomes. As such, slower gait speed is not merely a marker of underlying comorbidities and known risk factors.
These findings highlight the opportunity for and importance of interventions to target impaired physical function as an adjunct to TAVR. We identified several factors associated with 1‐year gait speed, which may help identify patients at risk for a decline in physical function and may provide clues as to what interventions may promote improvements in physical function. However, many of them such as sex, STS risk score, orthopedic disease, history of lung disease, and underlying cognitive status are less modifiable. There is a need for additional studies to identify factors that are modifiable—behaviors, nutrition, and biological pathways—associated with a resilient physical function trajectory after TAVR or other cardiovascular procedures.
While cardiac rehabilitation is known to improve clinical outcomes after cardiovascular procedures, only a small minority of patients participate for a variety of reasons that are magnified in patients treated with TAVR who tend to be older with a higher burden of frailty. 27 Accordingly, there is a push to consider alternative models of cardiac rehabilitation, including in‐home or hybrid strategies. 28 Reeves et al showed success in a pilot study employing a novel rehabilitation intervention in older adults with heart failure and a high burden of frailty, and a larger study is under way. 29 There are also ongoing studies testing novel rehabilitation strategies in patients undergoing TAVR, including ACTIVE AFTER TAVR (NCT03270124) and PERFORM‐TAVR (NCT03522454). Other strategies that may improve postprocedure resilience and outcomes include prehabilitation (preprocedure conditioning), less procedural sedation, early postprocedure ambulation, and shorter hospital stays.
Limitations
Our study has several limitations to consider. This was a post hoc secondary analysis of a device trial that was not focused on elucidating trajectories of physical function, so we lacked 30‐day and 6‐month gait speed data, which would have allowed us to look at trajectories of physical function at earlier postprocedure time points and their clinical consequences. Patients who died in the first year were excluded (n=88), introducing a survivor bias, and gait speed data were missing on 83 patients at baseline or 1 year who were excluded from our analysis. These analyses were based on data that were collected in the context of a clinical trial, which may vary from a real‐world analysis in some ways. However, the median gait speed in our study was 0.71 m/s and 34% had a normal gait speed, which is comparable to the nationally representative study from the STS/TVT database, which reported a median gait speed of 0.63 m/s and 24% of patients with a normal gait speed. 13
Conclusions
In conclusion, frailty and, more specifically, impaired physical function are common in older adults with AS being considering for valve replacement. Further, after the stressor of TAVR, there is marked heterogeneity in the magnitude and direction of changes in physical function, and these trajectories have implications for subsequent survival, hospitalizations, and quality of life. Additional studies are needed to clarify which patients are most vulnerable to a decline in physical function and what interventions can be successfully and widely implemented to facilitate physical resilience and optimize patient outcomes after TAVR.
Sources of Funding
The REPRISE III Pivotal trial and this analysis were supported by Boston Scientific (Marlborough, MA).
Disclosures
Dr Barker is on the medical advisory board for Boston Scientific. Dr Rajagopal is on the scientific advisory board for Boston Scientific and is a speaker/proctor for Medtronic, Edwards, and Abbott vascular; Dr Makkar receives research grants from Boston Scientific and Medtronic. Dr Bajwa is a consultant for Medtronic. Dr Kleiman provides educational services for Medtronic. Dr Linke has received speaker honoraria or served as a consultant for the following companies: Medtronic Inc., St. Jude Medical Inc., Claret Medical Inc., Boston Scientific, Edwards Lifesciences, Symetis as well as Bard, and owns stock option from Claret Medical Inc and receives grants and consultant fees from Medtronic, Edwards, Boston Scientific, and Abbott. Dr Kereiakes is a consultant and is on the Scientific Advisory Board, Boston Scientific, Inc and receives research grants from Edwards Lifesciences. Dr Waksman receives grant/research support or consulting fees/honoraria from Boston Scientific, Biotronik Biosensors, Medtronic Vascular, Abbott Vascular, Symetis, Med Alliance, LifeTech, Amgen, and Volcano/Philips. Dr Allocco is a full‐time employee and shareholder of Boston Scientific. Dr Reardon receives research grants from Boston Scientific and Medtronic. Dr Lindman has served on the scientific advisory board for Roche Diagnostics, has received research grants from Edwards Lifesciences and Roche Diagnostics, and has consulted for Medtronic. The remaining authors have no disclosures to report.
Supporting information
Table S1–S6
Acknowledgments
We thank Kristine Roy, PhD and Hong Wang, MS, both employees of Boston Scientific, for editorial and statistical support, respectively.
(J Am Heart Assoc. 2020;9:e017075 DOI: 10.1161/JAHA.120.017075.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al. Transcatheter versus surgical aortic‐valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 2. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J Jr, Kleiman NS, et al. Transcatheter aortic‐valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–1798. [DOI] [PubMed] [Google Scholar]
- 3. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, et al. Transcatheter or surgical aortic‐valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 4. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, et al. Surgical or transcatheter aortic‐valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]
- 5. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. Transcatheter aortic‐valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 6. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, et al. Transcatheter aortic‐valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–1715. [DOI] [PubMed] [Google Scholar]
- 7. Afilalo J, Alexander KP, Mack MJ, Maurer MS, Green P, Allen LA, Popma JJ, Ferrucci L, Forman DE. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 9. Afilalo J, Lauck S, Kim DH, Lefevre T, Piazza N, Lachapelle K, Martucci G, Lamy A, Labinaz M, Peterson MD, et al. Frailty in older adults undergoing aortic valve replacement: the FRAILTY-AVR study. J Am Coll Cardiol. 2017;70:689–700. [DOI] [PubMed] [Google Scholar]
- 10. Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–889. [DOI] [PubMed] [Google Scholar]
- 12. Afilalo J, Eisenberg MJ, Morin JF, Bergman H, Monette J, Noiseux N, Perrault LP, Alexander KP, Langlois Y, Dendukuri N, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–1676. [DOI] [PubMed] [Google Scholar]
- 13. Alfredsson J, Stebbins A, Brennan JM, Matsouaka R, Afilalo J, Peterson ED, Vemulapalli S, Rumsfeld JS, Shahian D, Mack MJ, et al. Gait speed predicts 30‐day mortality after transcatheter aortic valve replacement: results from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circulation. 2016;133:1351–1359. [DOI] [PubMed] [Google Scholar]
- 14. Afilalo J, Kim S, O'Brien S, Brennan JM, Edwards FH, Mack MJ, McClurken JB, Cleveland JC Jr, Smith PK, Shahian DM, et al. Gait speed and operative mortality in older adults following cardiac surgery. JAMA Cardiol. 2016;1:314–321. [DOI] [PubMed] [Google Scholar]
- 15. Arnold SV, Afilalo J, Spertus JA, Tang Y, Baron SJ, Jones PG, Reardon MJ, Yakubov SJ, Adams DH, Cohen DJ, et al. Prediction of poor outcome after transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68:1868–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hadley EC, Kuchel GA, Newman AB, Workshop S and Participants . Report: NIA workshop on measures of physiologic resiliencies in human aging. J Gerontol A Biol Sci Med Sci. 2017;72:980–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ, Colon-Emeric CS. Physical resilience in older adults: systematic review and development of an emerging construct. J Gerontol A Biol Sci Med Sci. 2016;71:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feldman TE, Reardon MJ, Rajagopal V, Makkar RR, Bajwa TK, Kleiman NS, Linke A, Kereiakes DJ, Waksman R, Thourani VH, et al. Effect of mechanically expanded vs self-expanding transcatheter aortic valve replacement on mortality and major adverse clinical events in high-risk patients with aortic stenosis: the REPRISE III randomized clinical trial. JAMA. 2018;319:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, Tylavsky FA, Brach JS, Satterfield S, Bauer DC, et al. Prognostic value of usual gait speed in well-functioning older people–results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. [DOI] [PubMed] [Google Scholar]
- 20. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. [DOI] [PubMed] [Google Scholar]
- 21. Arnold SV, Spertus JA, Lei Y, Allen KB, Chhatriwalla AK, Leon MB, Smith CR, Reynolds MR, Webb JG, Svensson LG, et al. Use of the kansas city cardiomyopathy questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6:61–67. [DOI] [PubMed] [Google Scholar]
- 22. Lindman BR, Alexander KP, O'Gara PT, Afilalo J. Futility, benefit, and transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2014;7:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Afilalo J, Sharma A, Zhang S, Brennan JM, Edwards FH, Mack MJ, McClurken JB, Cleveland JC Jr, Smith PK, Shahian DM, et al. Gait speed and 1‐year mortality following cardiac surgery: a landmark analysis from the society of thoracic surgeons adult cardiac surgery database. J Am Heart Assoc. 2018;7:e010139 DOI: 10.1161/JAHA.118.010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim DH, Afilalo J, Shi SM, Popma JJ, Khabbaz KR, Laham RJ, Grodstein F, Guibone K, Lux E, Lipsitz LA. Evaluation of changes in functional status in the year after aortic valve replacement. JAMA Intern Med. 2019;179:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schoenenberger AW, Stortecky S, Neumann S, Moser A, Juni P, Carrel T, Huber C, Gandon M, Bischoff S, Schoenenberger CM, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur Heart J. 2013;34:684–692. [DOI] [PubMed] [Google Scholar]
- 26. Abdul-Jawad Altisent O, Puri R, Regueiro A, Chamandi C, Rodriguez-Gabella T, Del Trigo M, Campelo-Parada F, Couture T, Marsal JR, Cote M, et al. Predictors and association with clinical outcomes of the changes in exercise capacity after transcatheter aortic valve replacement. Circulation. 2017;136:632–643. [DOI] [PubMed] [Google Scholar]
- 27. Beatty AL, Truong M, Schopfer DW, Shen H, Bachmann JM, Whooley MA. Geographic variation in cardiac rehabilitation participation in medicare and veterans affairs populations: opportunity for improvement. Circulation. 2018;137:1899–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, Franklin BA, Keteyian SJ, Kitzman DW, Regensteiner JG, et al. Home-based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation. 2019;140:e69–e89. [DOI] [PubMed] [Google Scholar]
- 29. Reeves GR, Whellan DJ, O'Connor CM, Duncan P, Eggebeen JD, Morgan TM, Hewston LA, Pastva A, Patel MJ, Kitzman DW. A novel rehabilitation intervention for older patients with acute decompensated heart failure: the REHAB-HF Pilot Study. JACC Heart Fail. 2017;5:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S6


