Abstract
Background
Mathematical optimization of automated external defibrillator (AED) placement may improve AED accessibility and out‐of‐hospital cardiac arrest (OHCA) outcomes compared with American Heart Association (AHA) and European Resuscitation Council (ERC) placement guidelines. We conducted an in silico trial (simulated prospective cohort study) comparing mathematically optimized placements with placements derived from current AHA and ERC guidelines, which recommend placement in locations where OHCAs are usually witnessed.
Methods and Results
We identified all public OHCAs of presumed cardiac cause from 2008 to 2016 in Copenhagen, Denmark. For the control, we computationally simulated placing 24/7‐accessible AEDs at every unique, public, witnessed OHCA location at monthly intervals over the study period. The intervention consisted of an equal number of simulated AEDs placements, deployed monthly, at mathematically optimized locations, using a model that analyzed historical OHCAs before that month. For each approach, we calculated the number of OHCAs in the study period that occurred within a 100‐m route distance based on Copenhagen’s road network of an available AED after it was placed (“OHCA coverage”). Estimated impact on bystander defibrillation and 30‐day survival was calculated by multivariate logistic regression. The control scenario involved 393 AEDs at historical, public, witnessed OHCA locations, covering 15.8% of the 653 public OHCAs from 2008 to 2016. The optimized locations provided significantly higher coverage (24.2%; P<0.001). Estimated bystander defibrillation and 30‐day survival rates increased from 15.6% to 18.2% (P<0.05) and from 32.6% to 34.0% (P<0.05), respectively. As a baseline, the 1573 real AEDs in Copenhagen covered 14.4% of the OHCAs.
Conclusions
Mathematical optimization can significantly improve OHCA coverage and estimated clinical outcomes compared with a guidelines‐based approach to AED placement.
Keywords: automated external defibrillator, guidelines, optimization, out‐of‐hospital cardiac arrest, public access defibrillation
Subject Categories: Cardiopulmonary Arrest, Cardiopulmonary Resuscitation and Emergency Cardiac Care, Statements and Guidelines
Nonstandard Abbreviations and Acronyms
- AED
automated external defibrillator
- EMS
emergency medical service
- OHCA
out‐of‐hospital cardiac arrest
Clinical Perspective
What Is New?
We conducted the first comparison of optimized automated external defibrillator (AED) placement and AED placement derived from the American Heart Association and European Resuscitation Council guidelines (placement at every public bystander‐witnessed location of out‐of‐hospital cardiac arrest [OHCA]) in an 8‐year in silico trial (simulated prospective cohort study).
AEDs placed by optimization covered a significantly larger number of OHCAs, increasing estimated bystander defibrillation and 30‐day survival compared with the guidelines‐based approach.
With optimized placement, 15 times fewer AEDs could provide the same coverage of OHCAs as the 1573 existing AEDs in Copenhagen.
What Are the Clinical Implications?
Optimization models may improve the accessibility and the cost‐effectiveness of AED placements for public OHCAs compared with guidelines‐based approaches and existing AED placements
AED placement strategies can benefit by targeting areas of high historical OHCA incidence, regardless of witnessed status, and accounting for the coverage and utilization of existing AEDs placements to effectively allocate resources.
A centralized decision maker that coordinates an optimized public access defibrillator program can outperform local initiatives following guidelines or unguided approaches in a cost‐effective manner.
Automated external defibrillator (AED) placement in public locations can significantly increase the likelihood of survival from out‐of‐hospital cardiac arrest (OHCA) by facilitating rapid bystander defibrillation. 1 , 2 , 3 , 4 , 5 , 6 , 7 Recognizing the need for a consistent approach to deploying AEDs, the American Heart Association (AHA) and the European Resuscitation Council (ERC) developed guidelines for AED placement. Current AHA guidelines recommend, for example, AED placement in locations with a “relatively high likelihood of witnessed cardiac arrest.” 8 , 9 Current ERC guidelines recommend placement “in public places with a high density and movement of citizens … where cardiac arrests are usually witnessed” and notes that placement in “areas where one cardiac arrest per 5 years can be expected is considered cost‐effective.” 10 Past and present recommendations focused on high‐traffic public locations and historical OHCA incidence. This focus is based on prior research establishing the importance of AED availability in high‐risk public areas to improve patient outcomes and studies demonstrating cost‐effectiveness of AED deployment. 6 , 7 , 11 , 12 , 13 , 14 , 15 , 16 By design, such guidelines aim for generalizability instead of offering location‐specific placement recommendations. A downside of this approach is that the guidelines are left to local interpretation, which may lead to uneven or no implementation, given the lack of prescriptiveness.
At the other end of the spectrum, mathematical optimization models utilize historical OHCA data to identify specific and tailored locations for simultaneous AED deployment, accounting for existing AED placements, that maximize coverage of OHCAs for any given geography. 17 , 18 , 19 , 20 , 21 Optimization approaches have demonstrated superiority over population‐guided AED placement heuristics 17 and actual AED placement decisions. 22 Although location recommendations provided by an optimization model in a specific geographic setting may not generalize to other geographic settings because of differences in spatiotemporal OHCA risk and city infrastructure, the optimization methodology itself is generalizable and can be applied in any setting. 23 In other words, optimization is both prescriptive and generalizable. As a result, optimization is being introduced in policy statements regarding AED placement. 24 However, the literature lacks a rigorous head‐to‐head comparison of optimization methods against placement approaches based on established international guidelines, the current gold standard for evidence‐based AED placement.
This article leverages a novel, recently developed, in silico trial framework 22 to compare the effectiveness of AED placements determined by an optimization model with a guidelines‐based approach using data from Copenhagen, Denmark. We compare the performance of the 2 approaches to AED placements using OHCA coverage—a metric that quantifies the proximity of OHCAs to AEDs—retrospectively but in a rigorous out‐of‐sample manner, and we estimate the potential impact on bystander defibrillation and 30‐day survival using logistic regression.
Methods
This study was approved by the Danish Data Protection Agency. In Denmark, informed consent is not required for registry‐based studies. The analytic methods (ie, code) that support the findings of this study are available from the corresponding author on reasonable request. However, because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols should be sent to Copenhagen Emergency Medical Services (EMS).
Study Setting
Copenhagen has a population of 600 000 and covers roughly 97 km2. Copenhagen EMS consists of paramedic‐staffed basic life support ambulances and physician‐staffed mobile emergency care units that dispatch simultaneously in a 2‐tiered system.
Study Design
We conducted a retrospective in silico trial (simulated prospective cohort study) to compare the public OHCA coverage provided by simulated AED placements at mathematically optimized AED locations (intervention) with simulated AED placements based on international guidelines (control). In this article, we use “simulate” to refer to the hypothetical comparison of 2 AED placement schemes conducted using a computer model and not a method that involves random‐number generation. Described conceptually, we went back in time twice, placing AEDs first according to our optimization model and second according to the guidelines‐based placement strategy and compared their performance. The control and intervention were also compared with the real AED placements in Copenhagen as a baseline.
Study Population and Data Sources
We used retrospective, registry‐based OHCA data collected by the mobile emergency care unit physicians in Copenhagen, Denmark. 25 All public, bystander‐witnessed and unwitnessed OHCAs of presumed cardiac cause with attempted resuscitation by either bystanders or EMS personnel in Copenhagen were included in the study. We excluded OHCAs related to trauma, drug overdose, attempted suicide, or violent assault; those with obvious signs of death (eg, rigor mortis); and EMS‐witnessed cases. The study period comprised OHCA data from January 2008 to December 2016; outcomes were measured using these data. OHCA data from the prestudy period from January 1994 to December 2007 were used as part of the training data for the optimization model.
The OHCA data included standard Utstein predictors of outcome. Information on bystander‐witnessed collapse, bystander cardiopulmonary resuscitation, and bystander defibrillation before EMS arrival was available for OHCAs starting January 2008. Public locations were defined as areas accessible to the public, including outdoor locations, public transportation areas, commercial buildings, and civic buildings. Hospitals and residential locations (eg, nursing, private, and senior homes) were excluded.
Publicly available AEDs registered as of 2016 from the Danish AED Network in Copenhagen and AEDs outside the city near historical OHCAs were included in the study. The AED registry, which is routinely validated, contains the registration date, location, and available times of all registered AEDs. AED registration is recommended by the National Board of Health in Denmark and most AED vendors but is voluntary. Approximately two‐thirds of all AEDs sold in Denmark are registered in the Danish AED Network. 26 Registered AEDs are typically placed in a decentralized fashion, with individual stakeholders independently placing AEDs in an uncoordinated manner. 25
We generated a grid system in which each grid point was a candidate AED location. The grid system comprised a square grid with 15 m between points, augmented with grid points centered on each historical OHCA event and a large set of businesses and public locations of interest across the city (n=2138). The addresses of the businesses and public locations were collected from a previous study. 23
We used ArcMap 10.5 to calculate the true route distance (100‐m walking distance according to the road network) between each grid point and historical cardiac arrest using pedestrian‐accessible paths, roads, and trail networks, accounting for buildings and city infrastructure.
Development of Trial Groups
Control
The control was formed by placing a 24/7‐available AED at the location of every historical, public, bystander‐witnessed OHCA during the study period. bystander‐witnessed OHCAs that occurred within 10 m of each other, based on the EMS‐recorded pick‐up address, were treated as the same arrest location and not as 2 unique arrest locations. Consequently, if a bystander‐witnessed OHCA occurred within 10 m of a prior bystander‐witnessed OHCA, a second AED would not be placed at that location.
To simulate AED placement over the study period, we divided it into consecutive and disjoint 30‐day time periods. In each time period, the numbers and locations of the bystander‐witnessed OHCAs were identified. Then, an AED was placed at each location (notwithstanding the 10‐m rule) at the end of the time period. The total number of AEDs placed during time period t was denoted as nt. Implementation of the control resulted in a gradual placement of AEDs over time (Figure 1).
Figure 1. An illustration of the optimization (intervention) and guidelines‐based (control) approaches to AED placement.
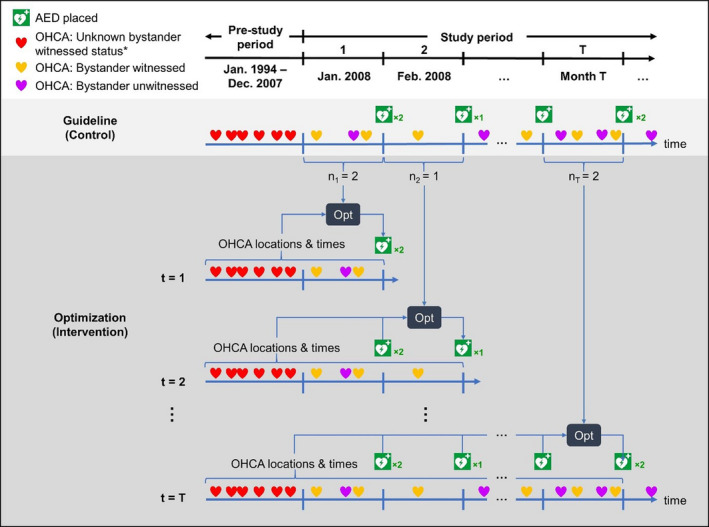
In the control, the AEDs (green AED symbol) are placed at the end of each time period at the locations of the historical, bystander‐witnessed OHCAs (orange heart symbol). The optimization model placed an equal number of AEDs as the control at the same point in time but considering all previous OHCAs (all heart symbols) and placed AEDs. *bystander‐witnessed status of an OHCA was available starting January 2008. Therefore, OHCAs that occurred before this date could not be used in the control. AED indicates automated external defibrillator; and OHCA, out‐of‐hospital cardiac arrest.
Note that all available bystander‐witnessed OHCAs in Copenhagen, consisting of data from January 2008 onward, were used to inform the AED placement decisions in the control. Before this date, information on bystander‐witnessed status was not collected for inclusion in the OHCA database; therefore, a guidelines‐based approach requiring knowledge of bystander‐witnessed status would not be implementable. Although the control utilizes bystander‐witnessed OHCAs to guide AED placement, the simulated placements are evaluated on all OHCAs (bystander witnessed and unwitnessed) meeting the inclusion criteria (see Primary Outcome for further details).
Intervention
A previously developed and validated optimization model 17 (Data S1) was used to place an equal number of optimized 24/7‐available AEDs in each 30‐day time period as the number placed in the control group. In each time period, the optimization model determined AED locations that maximized coverage of all OHCAs, witnessed and unwitnessed, that occurred before the end of that time period, while accounting for previously placed AEDs and previous OHCAs already covered by those AEDs. That is, for the first 30‐day time period (t 1), the model examined all OHCAs from January 1994 to January 2008 to optimize n1 AED placements. In the second time period (t 2), n2 AED placements were optimized considering all OHCAs from January 1994 to February 2008, excluding those already covered by the n1 AEDs placed in the first time period. This procedure was repeated for all subsequent 30‐day time periods until the end of the study period (Figure 1).
Primary Outcome
We used OHCA coverage to evaluate the performance of the control and intervention. OHCA coverage was defined as the number of all OHCAs, both bystander witnessed and unwitnessed, that occurred within 100‐m walking distance according to the road network (ie, route distance) from an AED after it was placed. A distance of 100 m was chosen to represent the farthest roundtrip distance an individual can travel on foot from an OHCA victim to a nearby AED, based on previous AHA guidelines advising defibrillation within 3 minutes of collapse 27 and previous studies that also used a 100‐m distance.
Primary Analysis
Comparing OHCA Coverage
Significant differences in OHCA coverage between the control and intervention were determined using the McNemar test for paired data (2 approaches were compared over the same OHCA population). A χ2 test (1‐way ANOVA for age‐related variables) was used to test for significant differences between the characteristics of the OHCAs covered in the intervention and the control. P<0.05 was considered statistically significant. Coverage gain was computed as the percentage increase in OHCA coverage of the intervention over the control. As a measure of efficiency, the number of AEDs placed per covered OHCA was calculated as the total number of AEDs placed divided by the total OHCA coverage at the end of the study period. The percentage of all OHCAs covered, defined as OHCA coverage divided by the total number of public OHCAs in the study, was also calculated for each approach. We verified the robustness of our results on OHCA coverage by randomly permuting the sequence of OHCA incidents over the study period and repeating the analysis (Data S2). In addition, the cumulative distribution of covered OHCAs based on the route distances between each OHCA and the nearest AED, up to 300 m, was visualized for the control and intervention approaches to further characterize the differences in proximity to an AED.
As a performance baseline, we calculated OHCA coverage of the real AED placements in Copenhagen over the study period. We used the location, date, and hours of availability of each AED registered in the Danish AED network with respect to the time and dates of historical OHCAs to calculate spatiotemporal OHCA coverage using the same framework described earlier. Spatiotemporal coverage accounts for the limited temporal availability of the real AEDs by considering an OHCA as covered only if it occurred during the hours of availability of an AED within 100 m. The final AED placements in the control and intervention and a heatmap of the OHCAs were visualized using QGIS v2.18.4.
Estimating Patient Outcomes
We developed separate multivariate logistic regression models to estimate the effect of OHCA coverage on bystander defibrillation and 30‐day survival, adjusting for standard Utstein variables (age, sex, response time, bystander‐witnessed arrest, and bystander cardiopulmonary resuscitation). 22 Each model was trained using the OHCAs from the study period. Values of the Utstein variables and outcome (bystander defibrillation or 30‐day survival) were taken from each OHCA directly. The value of the OHCA coverage variable (1=covered, 0=uncovered) was based on whether the OHCA was covered by a real AED during the study period. We measured model fit using the area under the curve of the receiver operating characteristic curve. We performed 10‐fold cross‐validation to calculate a mean area under the curve and 95% CIs. Implementation details, including additional information about the models, data preprocessing, and missing data imputation are provided in Data S3.
The fitted models were then applied to the OHCA coverage values from the simulated AEDs in the intervention and control to estimate the potential impact on bystander defibrillation and 30‐day survival. Specifically, the models predicted the probability of bystander defibrillation or 30‐day survival for each OHCA in the study period, using the OHCA coverage values corresponding to the intervention or control placements while adjusting for the Utstein variables. The exact values of the Utstein variables from each historical OHCA were used in the model to appropriately account for the actual circumstances of the response to the OHCA. In other words, our predictions implicitly account for factors that influence patient outcomes, such as regional variation of bystander response rates to OHCAs.
As is standard with logistic regression, we transformed the predicted probabilities to a binary outcome using a discriminant threshold: probabilities above the threshold are rounded up to 1 (ie, positive outcome), and probabilities below the threshold are rounded down to 0 (ie, negative outcome). The discriminant threshold value for each model was derived from the historical OHCAs and real AED placements. The threshold value was calibrated such that when the fitted model was applied to the OHCA coverage values from the real AED placements, the predicted proportion of positive outcomes matched the actual historical proportion.
We calculated the percentage of OHCAs with positive predicted outcomes over all OHCAs in the study period and the percentage increase of the intervention over the control. Significant differences in estimated outcomes were measured using the McNemar test. Patient outcomes were also estimated in the sensitivity analysis assessing OHCA coverage following the random permutation of OHCA incidents, as outlined in Data S2.
To examine the sensitivity of the estimated outcomes to different modeling approaches, we repeated this process using the route distance to the nearest AED, in place of OHCA coverage, as a predictive variable and cubic spline regression models (described in Data S4).
Secondary Analysis
To examine the effect of limited resources on AED network design and the resulting performance difference between the control and intervention approaches, we repeated the primary analysis with a limit on the total number of AEDs that could be placed during the study period. Specifically, both the control and intervention proceeded as described previously, until the number of AEDs placed reached the limited total number of AEDs, after which no additional AEDs were placed for the remainder of the study period. We considered AED network sizes from 50 up to the number of AEDs placed in the primary analysis, in increments of 50 (eg, 50, 100, 150). For each limited total number of AEDs, we calculated OHCA coverage, bystander defibrillation, and 30‐day survival, as described previously. Differences in these values between control and intervention were measured using the McNemar test. The number of AEDs required to reach the level of OHCA coverage provided by the real AED placements for each approach was also determined by comparing the coverage of each AED network of reduced size against the coverage from the real AEDs.
Sensitivity Analysis of the Control Placement Strategy
We examined the sensitivity of our primary result by altering the definition of the control and repeating the in silico trial. In this analysis, instead of placing an AED at every bystander‐witnessed arrest, the control placed an AED at every “hotspot” composed of 2 bystander‐witnessed OHCAs during the study period. That is, an AED was placed at every bystander‐witnessed OHCA that had a previous bystander‐witnessed OHCA occurring within 100 m of it; as before, OHCAs occurring within 10 m were not counted as a second distinct arrest location. The intervention was unchanged, except that the number of optimized AEDs placed in every time period was reduced to match the number of AEDs placed in the control. As before, the control and intervention were evaluated on coverage of witnessed and unwitnessed OHCAs. We repeated the regression analyses to estimate impact on bystander defibrillation and 30‐day survival.
Results
Table 1 summarizes the characteristics of the 653 public OHCAs that occurred during the study period (January 2008 to December 2016). Approximately 13.4 public OHCAs occurred per year per 100 000 people in Copenhagen.
Table 1.
Baseline Characteristics of All Public OHCAs in Copenhagen During the Study Period (January 2008 to December 2016)
| OHCA Characteristics | Study Period: January 2008 to December 2016 (n=653)* |
|---|---|
| Age, median (IQR), y | 64 (53–75) |
| Men, n (%) | 62 (52–72) |
| Women, n (%) | 75 (60–83) |
| Male sex, n (%) | 484 (76.5) |
| Shockable initial heart rhythm, n (%) | 283 (43.3) |
| bystander‐witnessed arrest, n (%) | 438 (69.9) |
| Received bystander CPR, n (%) | 440 (70.4) |
| Received bystander defibrillation, n (%) | 94 (14.6) |
| 30‐d survival, n (%) | 192 (32.3) |
CPR indicates cardiopulmonary resuscitation; IQR, interquartile range; and OHCA, out‐of‐hospital cardiac arrest.
Number of missing for variables available and described for the OHCAs during the study period: age (n=34), sex (n=20), bystander‐witnessed arrest (n=26), received bystander CPR (n=28), received bystander defibrillation (n=8), and 30‐day survival (n=59).
Primary Analysis
Comparing OHCA Coverage
The control placed 393 AEDs that covered 15.8% (103 of 653) of all OHCAs in the study period. The 393 optimized AED placements in the intervention covered 24.2% (158 of 653) of all OHCAs, which was significantly higher than the control (P<0.001) and corresponded to a relative coverage gain of 53.4%. The number of AEDs placed per covered OHCA was 3.8 for the control and 2.5 for the intervention. The coverage gain due to optimization remained statistically significant when the sequence of OHCAs was permuted, indicating robustness of the results (Figure S1). Figure 2 illustrates the progression of OHCA coverage from the start of the study period.
Figure 2. OHCA coverage over the study period for the optimization approach (intervention) and the guidelines‐based approach (control) in the primary analysis and the real AED placements.
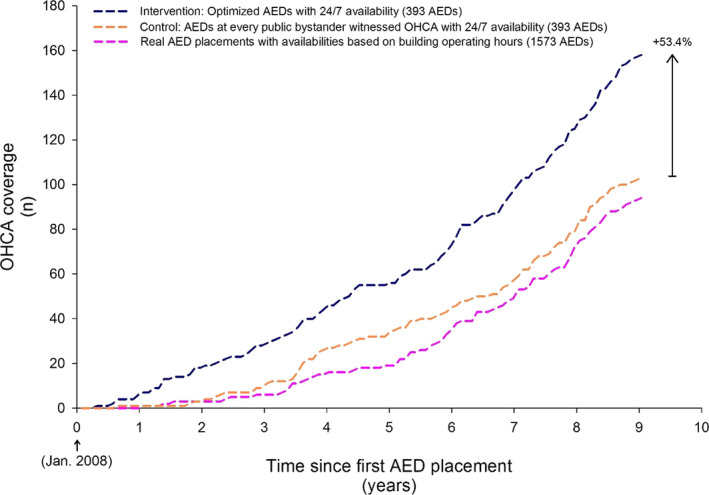
Of the 653 OHCAs that occurred during the study period, the control covered 103 OHCAs (15.8%), the intervention covered 158 OHCAs (24.2%) using 393 AEDs, and the real AEDs covered 94 OHCAs (14.4%) with 1573 AEDs. The total coverage of the intervention was significantly greater than the control (P<0.001). AED indicates automated external defibrillator; and OHCA, out‐of‐hospital cardiac arrest.
Figure S2 shows that the geographical distribution of optimized AEDs matches with the OHCA distribution better than the guidelines‐based AEDs. This alignment is particularly noticeable in the large OHCA hotspot north of the central core of Copenhagen, where optimized AEDs were placed directly in the hotspot, as opposed to around the periphery as in the control.
The 1573 real AEDs that were placed in Copenhagen during the study period covered 14.4% (94 of 653) of all OHCAs, corresponding to 16.7 AEDs per covered OHCA (Figure 2). OHCA coverage from the real AED placements was significantly lower than the intervention (P<0.001) but was not significantly different from the control (P=0.498). The number of real AEDs placed was greater than the number of AEDs placed by the intervention and control in each time period (Figure S3). Figure 3 shows the cumulative distribution of the number of covered OHCAs as a function of the distance from OHCAs and the nearest AED, up to 300 m, at the end the study period. The control had a larger number of OHCAs in close proximity to an AED (<20 m), but the cumulative count lagged behind the intervention once the distance reached ≈70 m. The number of covered arrests in the intervention increased much more rapidly than the control as the distance approached the 100‐m coverage radius.
Figure 3. The cumulative count of covered OHCAs based on the distance of the OHCA to the nearest AED for the optimization approach (intervention) and guidelines‐based approach (control).
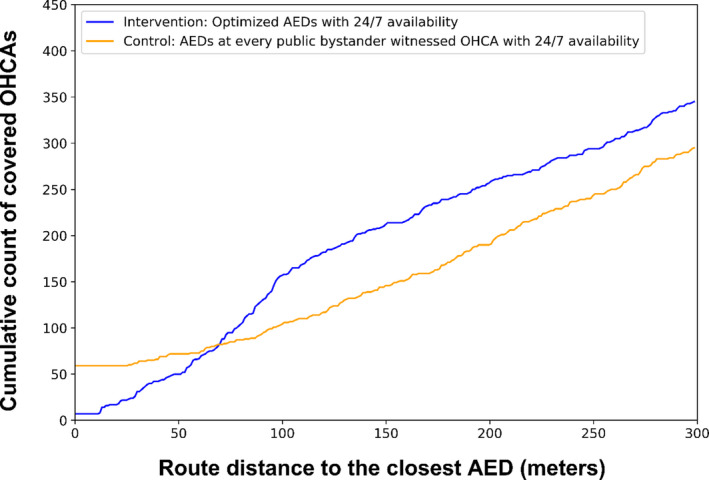
AED indicates automated external defibrillator; and OHCA, out‐of‐hospital cardiac arrest.
Estimating Patient Outcomes
The bystander defibrillation model had an average area under the curve of 0.69 (95% CI, 0.64–0.73) over the 10 folds. The 30‐day survival model had an average area under the curve of 0.74 (95% CI, 0.67–0.81). The OHCA data used to fit the model are further described in Data S3. The models predicted outcomes within 0.1 percentage point of actual outcomes, indicating that the discriminant thresholds were well calibrated (see Table S1). The specific threshold values used are given in Data S3.
Applying the fitted bystander defibrillation model to OHCA coverage values from the control and intervention over the study period demonstrated a significant increase in the estimated bystander defibrillation rate from 15.6% in the control to 18.2% in the intervention (16.7% relative increase; P<0.05) across all 653 OHCAs. Estimated 30‐day survival demonstrated a significant increase from 32.6% in the control to 34.0% in the intervention (4.2% relative increase; P<0.05). The characteristics of the covered OHCAs are given in Table S2. For reference, the historical bystander defibrillation and 30‐day survival rates corresponding to the real AED placements in Copenhagen are shown in Table 1. The directions of the estimated outcomes were consistent with these results when using the cubic spline regressions (Data S4) and when the sequence of OHCAs was permuted (Figure S1), further demonstrating robustness of the results.
Secondary Analysis
Figure 4 summarizes OHCA coverage and predicted outcomes as a function of the number of AEDs placed during the study period. Coverage was significantly higher (P<0.05) in the intervention for all AED network sizes. Accordingly, fewer AEDs were placed for each covered OHCA in the intervention (0.8–2.5 AEDs placed per covered OHCA) compared with the control (1.7–3.8 AEDs placed per covered OHCA) for each AED network size. The increase in bystander defibrillation was significant for network sizes of 50, 300, 350, and 393 AEDs (P<0.05). The increase in 30‐day survival was significant for AED network sizes of 50, 150, 250, 300, 350, and 393 AEDs (P<0.05).
Figure 4. The OHCA coverage (A), estimated bystander defibrillation (B), and estimated 30‐day survival (C) over the 653 OHCAs during the study period for networks with between 50 and 393 AEDs placed in accordance with the optimization approach (intervention) and guidelines‐based approach (control).
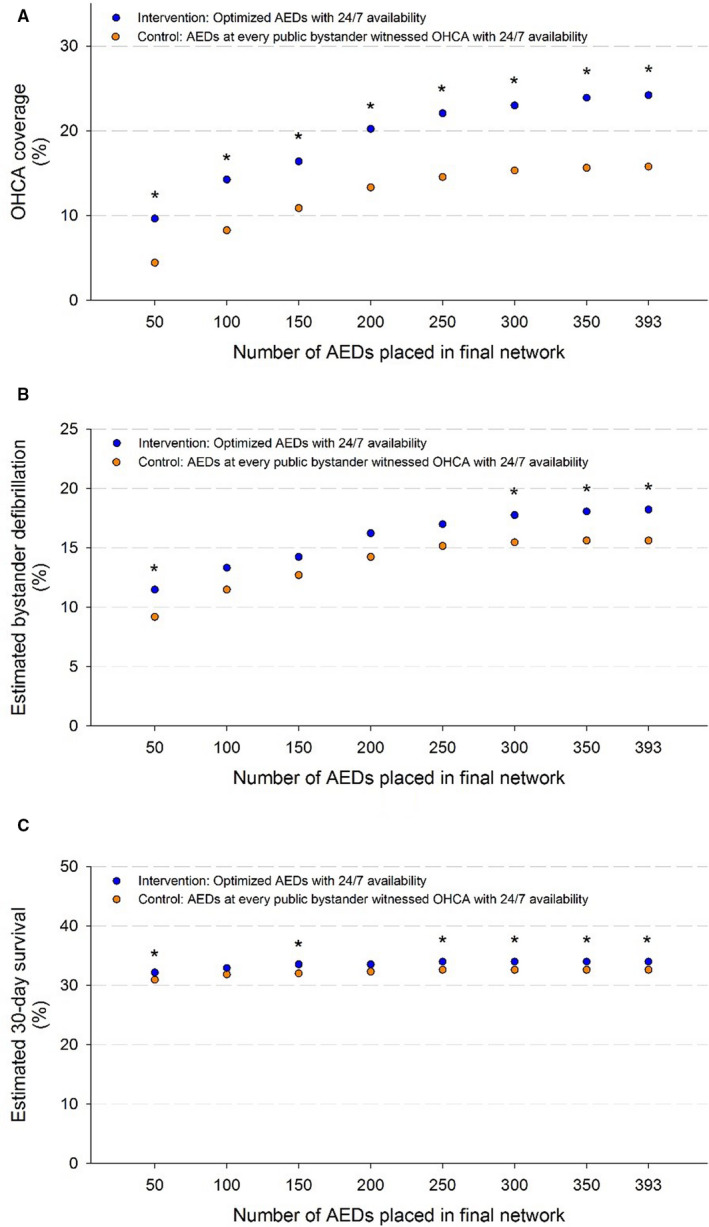
Asterisks indicate network sizes where the corresponding outcome measure was significantly higher in the intervention than the control. AED indicates automated external defibrillator; and OHCA, out‐of‐hospital cardiac arrest.
Figure 5 highlights the improved efficiency of AEDs placed according to the control and intervention approaches compared with the real AEDs in Copenhagen. The 1573 real AEDs (262.2 AEDs per 100 000 inhabitants) in Copenhagen covered 95 of the 653 OHCAs in the study period. In contrast, 100 AEDs with 24/7 accessibility (16.7 AEDs per 100 000 inhabitants) that were placed by optimization generated a similar coverage level (93 OHCAs covered). When placed by guidelines, 250 AEDs with 24/7 accessibility (41.7 AEDs per 100 000 inhabitants) were needed to reach a similar coverage level (95 OHCAs covered). These results equate to a 15‐fold reduction in the number of AEDs needed for optimization compared with the real AEDs (relative decrease of 93.6%) and a 6‐fold reduction for AEDs placed by guidelines (relative decrease of 84.1%).
Figure 5. The number of AEDs placed to cover at least 93 (14.2%) of 653 OHCAs for the optimization approach (intervention) and guidelines‐based approach (control) in the secondary analysis and the real AED placements.
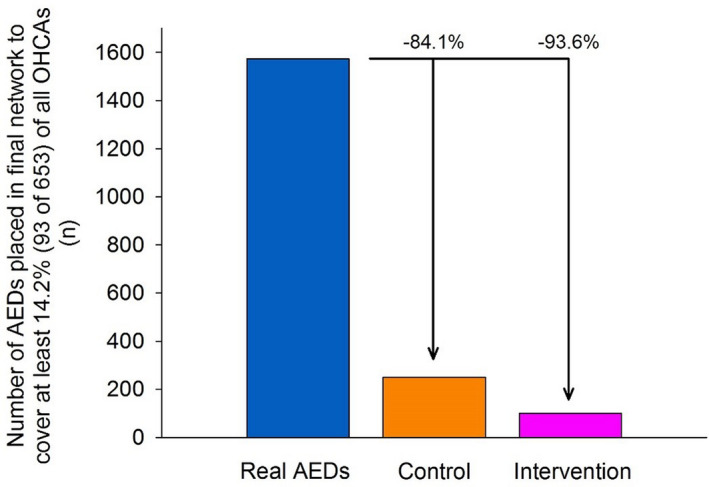
The specified level of coverage was reached with 1573 of the real AEDs in Copenhagen (262.2 AEDs per 100 000 inhabitants), 100 optimally located AEDs that were 24/7 accessible (16.7 AEDs per 100 000 inhabitants), and 250 AEDs placed by guidelines and that were 24/7 accessible (41.7 AEDs per 100 000 inhabitants). AED indicates automated external defibrillator; and OHCA, out‐of‐hospital cardiac arrest.
Sensitivity Analysis of the Control Placement Strategy
Comparing OHCA Coverage for the Modified Control Placement Strategy
In total, 131 AEDs were placed at hotspots of ≥2 bystander‐witnessed OHCAs and covered 5.1% (33 of 653) of all OHCAs, corresponding to 4.0 AEDs placed per covered OHCA. The corresponding intervention covered 9.2% (60 of 653) of OHCAs, corresponding to 2.2 AEDs per covered OHCA (Figure 2). The difference in coverage was significant (P<0.001) and corresponded to a relative coverage gain of the intervention over the control of 81.8%. OHCA coverage of the intervention remained significantly higher than the control after permuting the sequence of OHCAs (Table S3), again confirming robustness of the results. Figure S4 shows that the optimized AEDs were better aligned with the distribution of OHCAs, especially near the city center, compared with the AEDs in the control. As a result of placing far fewer AEDs, the control and intervention in the sensitivity analysis provided significantly lower coverage than the control and intervention in the primary analysis.
Estimating Patient Outcomes for the Modified Control Placement Strategy
The intervention was associated with small but statistically insignificant improvements in estimated patient outcomes. Estimated bystander defibrillation increased from 9.8% in the control to 11.0% in the intervention (P=0.17), corresponding to a relative increase of 12.5%. Similarly, estimated 30‐day survival increased from 31.2% in the control to 31.7% in the intervention (P=0.45), corresponding to a relative increase of 1.5%. The characteristics of the covered OHCAs are given in Table S4.
Discussion
This article presents the first rigorous comparison of optimized AED placements and placements based on AHA and ERC guidelines, 8 , 9 , 10 using a novel in silico trial framework developed previously for evaluating AED placement decisions. 22 Our first main finding is that optimized AED placements significantly increased OHCA coverage, which can result in significant estimated increases in bystander defibrillation and 30‐day survival compared with a guidelines‐based placement strategy that placed an AED at every public, bystander‐witnessed, OHCA location. Second, 6 to 15 times fewer AEDs (guidelines and optimization strategy, respectively) are needed to achieve the same level of coverage as existing deployed AEDs in Copenhagen.
Our primary finding is that optimized AED placements significantly increased OHCA coverage compared with a guidelines‐based placement strategy (15.8% in the control versus 24.2% in the intervention). In other words, if we went back in time twice, first placing AEDs according to our optimization model and then, second, placing them according to the guidelines‐based placement strategy, 3 OHCAs would have been covered in the optimized placement strategy for every 2 covered in the guidelines‐based placement strategy. The significant increase in OHCA coverage corresponded to significant estimated increases in bystander defibrillation (15.6% in the control versus 18.2% in the intervention) and 30‐day survival (32.6% in the control versus 34.0% in the intervention).
Furthermore, the increase in OHCA coverage due to optimization over guidelines remained statistically significant even as the number of AEDs placed during the study period was reduced. Just more than half of the smaller AED network sizes had estimated bystander defibrillation and 30‐day survival rates that were statistically larger in the optimization intervention. For the range of AED network sizes considered in our secondary analysis, optimization consistently outperformed guidelines on the larger network sizes. In comparison with real AEDs, our results are also consistent with previous studies that demonstrated that optimization and guidelines‐based approaches outperform real placement decisions. 12 , 22
We considered the placement of a 24/7‐available AED at every historical, unique, witnessed OHCA location as a reasonable implementation strategy for international placement guidelines because it accurately reflects the AHA and ERC recommendations 8 , 9 , 10 and provides explicit guidance that is easy to understand and implement. We modeled 24/7‐available AEDs because it is well known that increased temporal AED accessibility improves OHCA coverage and is positively associated with bystander defibrillation and survival. 18 , 26 , 28 The use of historical OHCA data to guide placement decisions is appropriate because the spatial distribution of OHCAs has been shown to be stable over time 19 , 29 and is in line with previous AHA and ERC guidelines and studies focused on developing AED placement strategies 12 , 27 , 30 , 31 , 32 that recommended placement at historical OHCA locations. Our guidelines‐based approach was shown to provide at least as much coverage as the real AEDs placed in Copenhagen with only one‐sixth the number of AEDs (Figure 5), validating its effectiveness as a placement strategy.
Although guidelines are designed to be general, subjectivity in their interpretation can be a shortcoming. The lack of prescriptiveness in current guidelines can, for example, lead to different interpretations and varying levels of effectiveness depending on how the guidelines are implemented. In fact, our sensitivity analysis identifies the exact change in the performance due because of an alternative interpretation of the guidelines text. Instead of placing an AED at every witnessed OHCA, the sensitivity analysis considered only hotspots of ≥2 witnessed OHCAs for AED placement. This seemingly minor change resulted in a much smaller number of AED placements (an amount that had previously been shown to be inadequate in the study setting 12 ) and a dramatically lower level of OHCA coverage than the control (guidelines‐based placement strategy correctly implemented) in the primary analysis. This result suggests that placing AEDs only in hotspots of multiple OHCAs is less effective than placing them at each historical witnessed OHCA location, even if other historical OHCAs and AEDs are within 100 m.
In contrast to guidelines, optimization prescribes specific locations for AED placement that maximize OHCA coverage. An optimization model can support the design of tailored AED networks that consider resource and data constraints specific to the individual community 17 , 20 , 23 while being generalizable to different geographic locations. 23 For example, if resources are insufficient to place AEDs in all high‐risk locations, optimization can prioritize placement within these locations. In addition, optimization models can effectively utilize OHCA data of varying levels of granularity (eg, witnessed status being known or not known) to inform placement decisions. In comparison, guidelines focus on bystander‐witnessed OHCAs only, but these data are not always collected. Without optimization, communities that do not have bystander‐witnessed status for their OHCAs may need to rely on older and less effective guidelines based on general OHCA incidence.
The benefits of optimization can be realized through centralized decision makers that develop and maintain public access defibrillator programs. However, implementation of an optimization model requires additional technical expertise compared with following a simple guidelines approach. As an alternative to optimization, some benefits can be realized through a modified guidelines‐based approach, placing AEDs in areas of high historical OHCA incidence, regardless of bystander‐witnessed status, and accounting for the coverage and utilization of existing AEDs placements.
Coincidentally, the suboptimal placement strategy defined by the control in the sensitivity analysis is roughly equivalent to placing an AED in a location with at least 1 witnessed OHCA every 5 years, a strategy deemed cost‐effective in previous guidelines. 10 , 15 , 27 If such an approach is deemed cost‐effective, and our sensitivity analysis demonstrates that optimization outperforms this approach, it suggests that optimization can be an even more cost‐effective approach to deploying AEDs. In particular, our results demonstrated that ≈100 optimized AED placements can provide a level of coverage equivalent to the 1573 AEDs in Copenhagen (Figure 5), corresponding to a 15‐fold reduction in the number of AED placements. Our modeling approach focused on 24/7‐accessible AEDs because we believe it is the most appropriate way to interpret the AHA and ERC guidelines, which do not mention temporal accessibility. An ideal AED network should have no temporal accessibility barriers to AED use. Although 24/7 placements may be associated with higher costs compared with standard indoor placements that comprise the majority of real AED networks, 6 , 25 , 26 , 33 , 34 , 35 innovative colocation venues such as vending machines, bank machines, and advertisement boards may reduce costs compared with outdoor stand‐alone cabinets. 36 , 37 , 38 Housing AEDs in commercial locations that are typically open 24/7, such as bank vestibules or certain food outlets, may also serve as an effective means to improve AED accessibility in an affordable manner. 39
This study has several limitations. First, our results are dependent on how our control was defined, and there may be other possible ways to implement a guidelines‐based AED placement strategy beyond the definitions we considered. Second, our in silico trial is essentially a counterfactual analysis with causal certainty, which requires an end point that can be measured unambiguously on the computer. As such, we can estimate the potential impact of optimized AEDs on only clinical outcomes using our primary end point of OHCA coverage. Third, optimization models rely on accurate historical OHCA data to identify optimal AED placements. Limited or low‐quality data can negatively affect the performance of the model. Although the spatial distribution of historical OHCA incidents has been shown to be stable over time, 19 , 29 potential differences between the historical OHCA data and future OHCAs may affect the effectiveness of data‐driven AED placement strategies. Utilizing up‐to‐date data in AED placement models will help minimize potential bias in the historical data. Fourth, the set of candidate locations selected for AED placement were based on a grid system that included locations unattached to a specific building. In practice, suitability of locations for placing 24/7‐accessible AEDs will depend on infrastructure and population flows. Fifth, although our distance measurement reflects realistic pedestrian travel between AEDs and OHCAs, it does not consider the vertical dimension. However, we believe this limitation to be minor because there are very few high rises in Copenhagen and because AED placement models can be extended to account for the vertical dimension. 40
Parallel efforts to increase the probability of use of available AEDs will increase the benefit of optimal AED placement on patient outcomes. 41 For example, bystander use of AEDs may be increased by interventions geared toward improving public awareness of AEDs, signage and visibility, AED registries, dispatcher‐guided AED retrieval, and smartphone applications to facilitate crowdsourced AED delivery to the scene. 42 , 43 Furthermore, establishing centralized decision‐making systems that consider OHCA prevention, prediction, and response, in addition to AED placement, can aid in increasing survival from OHCA. 41 , 44 , 45 , 46 These systems may also facilitate the integration of novel technologies, such as drone delivery of AEDs 47 , 48 and the aforementioned smartphone applications that support bystander responders, 42 , 43 to further improve OHCA outcomes in combination with static AED placement; there is a fundamental limit on the OHCA coverage that can be provided by static AEDs. 49 This article contributes to the development of such a system by demonstrating that optimization is an effective means of designing AED networks. Next steps should be to develop and evaluate real‐world implementation of optimization‐based placement strategies.
Conclusions
Optimized AED placements increased OHCA coverage from 15.8% to 24.2% of all OHCAs (53.4% relative increase) compared with the same number of AEDs placed according to a guidelines‐based placement strategy in a computer‐simulated clinical trial in Copenhagen, Denmark. This increase in OHCA coverage was associated with statistically significant increases in estimated bystander defibrillation, from 15.6% to 18.2%, and 30‐day survival, from 32.6% to 34.0%. Optimization also has the potential to increase cost‐effectiveness of AED placements because our findings suggest that an equivalent coverage level provided by the real AEDs in Copenhagen could have been achieved with a 15‐times reduction in AEDs placed by optimization.
Sources of Funding
This work was supported by the Danish Foundation TrygFonden with no commercial interest in the field of cardiac arrest.
Disclosures
None.
Supporting information
Data S1–S4
Tables S1–S4
Figures S1–S4
Acknowledgments
The authors acknowledge the Danish AED Network (http://www.hjertestarter.dk) for sharing information on the automated external defibrillators registered in their network and to the emergency medical service personnel that report and support the out‐of‐hospital cardiac arrest database.
(J Am Heart Assoc. 2020;9:e016701 DOI: 10.1161/JAHA.120.016701.)
For Sources of Funding and Disclosures, see page 12.
References
- 1. Caffrey SL, Willoughby PJ, Pepe PE, Becker LB. Public use of automated external defibrillators. N Engl J Med. 2002;347:1242–1247. [DOI] [PubMed] [Google Scholar]
- 2. Page RL, Joglar JA, Kowal RC, Zagrodzky JD, Nelson LL, Ramaswamy K, Barbera SJ, Hamdan MH, McKenas DK. Use of automated external defibrillators by a U.S. Airline. New Engl J Med. 2000;343:1210–1216. [DOI] [PubMed] [Google Scholar]
- 3. Valenzuela TD, Roe DJ, Nichol G, Clark LL, Spaite DW, Hardman RG. Outcomes of rapid defibrillation by security officers after cardiac arrest in casinos. N Engl J Med 2000;343:1206–1209. [DOI] [PubMed] [Google Scholar]
- 4. Robertson RM. Sudden death from cardiac arrest–improving the odds. N Engl J Med. 2000;343:1259–1260. [DOI] [PubMed] [Google Scholar]
- 5. Baekgaard JS, Viereck S, Moller TP, Ersboll AK, Lippert F, Folke F. The effects of public access defibrillation on survival after out‐of‐hospital cardiac arrest: a systematic review of observational studies. Circulation. 2017;136:954–965. [DOI] [PubMed] [Google Scholar]
- 6. Kitamura T, Iwami T, Kawamura T, Nagao K, Tanaka H, Hiraide A. Implementation Working Group for the All-Japan Utstein Registry of the F, Disaster Management A. Nationwide public-access defibrillation in Japan. N Engl J Med. 2010;362:994–1004. [DOI] [PubMed] [Google Scholar]
- 7. Hallstrom AP, Ornato JP, Weisfeldt M, Travers A, Christenson J, McBurnie MA, Zalenski R, Becker LB, Schron EB, Proschan M, et al. Public-access defibrillation and survival after out‐of‐hospital cardiac arrest. N Engl J Med. 2004;351:637–646. [DOI] [PubMed] [Google Scholar]
- 8. American Heart Association . Part 4: systems of care and continuous quality improvement - ECC guidelines. 2015. Available at: https://eccguidelines.heart.org/index.php/circulation/cpr-ecc-guidelines-2/part-4-systems-of-care-and-continuous-quality-improvement/?Strue=1&id=1. Accessed January 24, 2019. [DOI] [PubMed]
- 9. Kronick SL, Kurz MC, Lin S, Edelson DP, Berg RA, Billi JE, Cabanas JG, Cone DC, Diercks DB, Foster JJ, et al. Part 4: systems of care and continuous quality improvement: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S397–413. [DOI] [PubMed] [Google Scholar]
- 10. Perkins GD, Handley AJ, Koster RW, Castren M, Smyth MA, Olasveengen T, Monsieurs KG, Raffay V, Grasner JT, Wenzel V, et al. European resuscitation council guidelines for resuscitation 2015: section 2. Adult basic life support and automated external defibrillation. Resuscitation. 2015;95:81–99. [DOI] [PubMed] [Google Scholar]
- 11. Nichol G, Valenzuela T, Roe D, Clark L, Huszti E, Wells GA. Cost effectiveness of defibrillation by targeted responders in public settings. Circulation. 2003;108:697–703. [DOI] [PubMed] [Google Scholar]
- 12. Folke F, Lippert FK, Nielsen SL, Gislason GH, Hansen ML, Schramm TK, Sorensen R, Fosbol EL, Andersen SS, Rasmussen S, et al. Location of cardiac arrest in a city center: strategic placement of automated external defibrillators in public locations. Circulation. 2009;120:510–517. [DOI] [PubMed] [Google Scholar]
- 13. Weisfeldt ML, Sitlani CM, Ornato JP, Rea T, Aufderheide TP, Davis D, Dreyer J, Hess EP, Jui J, Maloney J, et al. Survival after application of automatic external defibrillators before arrival of the emergency medical system: evaluation in the resuscitation outcomes consortium population of 21 million. J Am Coll Cardiol. 2010;55:1713–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cram P, Vijan S, Fendrick AM. Cost‐effectiveness of automated external defibrillator deployment in selected public locations. J Gen Intern Med. 2003;18:745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Link MS, Atkins DL, Passman RS, Halperin HR, Samson RA, White RD, Cudnik MT, Berg MD, Kudenchuk PJ, Kerber RE. Part 6: electrical therapies: automated external defibrillators, defibrillation, cardioversion, and pacing: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S706–S719. [DOI] [PubMed] [Google Scholar]
- 16. Koster RW, Baubin MA, Bossaert LL, Caballero A, Cassan P, Castrén M, Granja C, Handley AJ, Monsieurs KG, Perkins GD. European resuscitation council guidelines for resuscitation 2010 section 2. Adult basic life support and use of automated external defibrillators. Resuscitation. 2010;81:1277–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan TC, Li H, Lebovic G, Tang SK, Chan JY, Cheng HC, Morrison LJ, Brooks SC. Identifying locations for public access defibrillators using mathematical optimization. Circulation. 2013;127:1801–1809. [DOI] [PubMed] [Google Scholar]
- 18. Sun CL, Demirtas D, Brooks SC, Morrison LJ, Chan TC. Overcoming spatial and temporal barriers to public access defibrillators via optimization. J Am Coll Cardiol. 2016;68:836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan TCY, Demirtas D, Kwon RH. Optimizing the deployment of public access defibrillators. Manage Sci. 2016;62:3617–3635. [Google Scholar]
- 20. Tierney NJ, Reinhold HJ, Mira A, Weiser M, Burkart R, Benvenuti C, Auricchio A. Novel relocation methods for automatic external defibrillator improve out‐of‐hospital cardiac arrest coverage under limited resources. Resuscitation. 2018;125:83–89. [DOI] [PubMed] [Google Scholar]
- 21. Tsai Y-S, Ko PC-I, Huang C-Y, Wen T-H. Optimizing locations for the installation of automated external defibrillators (AEDS) in urban public streets through the use of spatial and temporal weighting schemes. Appl Geogr. 2012;35:394–404. [Google Scholar]
- 22. Sun CLF, Karlsson L, Torp-Pedersen C, Morrison LJ, Brooks SC, Folke F, Chan TCY. In silico trial of optimized versus real public defibrillator locations. J Am Coll Cardiol. 2019;74:1557–1567. [DOI] [PubMed] [Google Scholar]
- 23. Sun CLF, Karlsson L, Torp-Pedersen C, Morrison LJ, Folke F, Chan TCY. Spatiotemporal AED optimization is generalizable. Resuscitation. 2018;131:101–107. [DOI] [PubMed] [Google Scholar]
- 24. heartandstroke.ca . Addressing cardiac arrest in Canada. 2019. Available at: https://www.heartandstroke.ca/-/media/pdf-files/canada/2017-position-statements/final-en-addressingcardiacarreststatement-nov-2019.Ashx?Rev=388eeef4069747dcb4ab6353d36b3f7b&hash=9e27a3232e8f908e45e115b0b9dcc9d5. Accessed March 16, 2019.
- 25. Hansen CM, Lippert FK, Wissenberg M, Weeke P, Zinckernagel L, Ruwald MH, Karlsson L, Gislason GH, Nielsen SL, Kober L, et al. Temporal trends in coverage of historical cardiac arrests using a volunteer‐based network of automated external defibrillators accessible to laypersons and emergency dispatch centers. Circulation. 2014;130:1859–1867. [DOI] [PubMed] [Google Scholar]
- 26. Karlsson L, Malta Hansen C, Wissenberg M, Moller Hansen S, Lippert FK, Rajan S, Kragholm K, Moller SG, Bach Sondergaard K, Gislason GH, et al. Automated external defibrillator accessibility is crucial for bystander defibrillation and survival: a registry‐based study. Resuscitation. 2019;136:30–37. [DOI] [PubMed] [Google Scholar]
- 27. Aufderheide T, Hazinski MF, Nichol G, Steffens SS, Buroker A, McCune R, Stapleton E, Nadkarni V, Potts J, Ramirez RR, et al. Community lay rescuer automated external defibrillation programs: key state legislative components and implementation strategies: a summary of a decade of experience for healthcare providers, policymakers, legislators, employers, and community leaders from the American Heart Association emergency cardiovascular care committee, council on clinical cardiology, and office of state advocacy. Circulation. 2006;113:1260–1270. [DOI] [PubMed] [Google Scholar]
- 28. Hansen CM, Wissenberg M, Weeke P, Ruwald MH, Lamberts M, Lippert FK, Gislason GH, Nielsen SL, Kober L, Torp-Pedersen C, et al. Automated external defibrillators inaccessible to more than half of nearby cardiac arrests in public locations during evening, nighttime, and weekends. Circulation. 2013;128:2224–2231. [DOI] [PubMed] [Google Scholar]
- 29. Sasson C, Keirns CC, Smith D, Sayre M, Macy M, Meurer W, McNally BF, Kellermann AL, Iwashyna TJ; CARES Study Group . Small area variations in out‐of‐hospital cardiac arrest: does the neighborhood matter? Ann Intern Med. 2010;153:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Handley AJ, Koster R, Monsieurs K, Perkins GD, Davies S, Bossaert L; European Resuscitation C . European resuscitation council guidelines for resuscitation 2005. Section 2. Adult basic life support and use of automated external defibrillators. Resuscitation. 2005;67(suppl 1):S7–S23. [DOI] [PubMed] [Google Scholar]
- 31. Becker L, Eisenberg M, Fahrenbruch C, Cobb L. Public locations of cardiac arrest. Implications for public access defibrillation. Circulation. 1998;97:2106–2109. [DOI] [PubMed] [Google Scholar]
- 32. Gratton M, Lindholm DJ, Campbell JP. Public-access defibrillation: where do we place the AEDS? Prehosp Emerg Care. 1999;3:303–305. [DOI] [PubMed] [Google Scholar]
- 33. Berdowski J, Blom MT, Bardai A, Tan HL, Tijssen JG, Koster RW. Impact of onsite or dispatched automated external defibrillator use on survival after out‐of‐hospital cardiac arrest. Circulation. 2011;124:2225–2232. [DOI] [PubMed] [Google Scholar]
- 34. Hansen SM, Hansen CM, Folke F, Rajan S, Kragholm K, Ejlskov L, Gislason G, Kober L, Gerds TA, Hjortshoj S, et al. Bystander defibrillation for out‐of‐hospital cardiac arrest in public vs residential locations. JAMA Cardiol. 2017;2:507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wissenberg M, Lippert FK, Folke F, Weeke P, Hansen CM, Christensen EF, Jans H, Hansen PA, Lang-Jensen T, Olesen JB, et al. Association of national initiatives to improve cardiac arrest management with rates of bystander intervention and patient survival after out‐of‐hospital cardiac arrest. JAMA. 2013;310:1377–1384. [DOI] [PubMed] [Google Scholar]
- 36. Krammel M, Weidenauer D, Ettl F, Orlob S, Knogler T, van Tulder R, Schreiber W. Public access defibrillation (PAD) in Vienna: a new approach in technique and funding. Resuscitation. 2013;84:S71. [Google Scholar]
- 37. wien.at . More defibrillators for Vienna - every second counts. 2016. Available at: https://www.wien.gv.at/english/health-socialservices/defibrillators.html. Accessed March 16, 2019.
- 38. Mitamura H. Public access defibrillation: advances from japan. Nat Clin Pract Cardiovasc Med. 2008;5:690–692. [DOI] [PubMed] [Google Scholar]
- 39. Sun CL, Brooks SC, Morrison LJ, Chan TC, Rescu EI. Ranking businesses and municipal locations by spatiotemporal cardiac arrest risk to guide public defibrillator placement. Circulation. 2017;135:1104–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chan TCY. Rise and shock: optimal defibrillator placement in a high‐rise building. Prehosp Emerg Care. 2017;21:309–314. [DOI] [PubMed] [Google Scholar]
- 41. Smith CM, Lim Choi Keung SN, Khan MO, Arvanitis TN, Fothergill R, Hartley-Sharpe C, Wilson MH, Perkins GD. Barriers and facilitators to public access defibrillation in out‐of‐hospital cardiac arrest: a systematic review. Eur Heart J Qual Care Clin outcomes. 2017;3:264–273. [DOI] [PubMed] [Google Scholar]
- 42. Ringh M, Rosenqvist M, Hollenberg J, Jonsson M, Fredman D, Nordberg P, Jarnbert-Pettersson H, Hasselqvist-Ax I, Riva G, Svensson L. Mobile-phone dispatch of laypersons for CPR in out‐of‐hospital cardiac arrest. N Engl J Med. 2015;372:2316–2325. [DOI] [PubMed] [Google Scholar]
- 43. Smith CM, Wilson MH, Ghorbangholi A, Hartley-Sharpe C, Gwinnutt C, Dicker B, Perkins GD. The use of trained volunteers in the response to out‐of‐hospital cardiac arrest - the GoodSAM experience. Resuscitation. 2017;121:123–126. [DOI] [PubMed] [Google Scholar]
- 44. Narayan SM, Wang PJ, Daubert JP. New concepts in sudden cardiac arrest to address an intractable epidemic: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:70–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Johnson KW, Torres Soto J, Glicksberg BS, Shameer K, Miotto R, Ali M, Ashley E, Dudley JT. Artificial intelligence in cardiology. J Am Coll Cardiol. 2018;71:2668–2679. [DOI] [PubMed] [Google Scholar]
- 46. Naylor CD. On the prospects for a (deep) learning health care system. JAMA. 2018;320:1099–1100. [DOI] [PubMed] [Google Scholar]
- 47. Boutilier JJ, Brooks SC, Janmohamed A, Byers A, Buick JE, Zhan C, Schoellig AP, Cheskes S, Morrison LJ, Chan TCY, et al. Optimizing a drone network to deliver automated external defibrillators. Circulation. 2017;135:2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Claesson A, Backman A, Ringh M, Svensson L, Nordberg P, Djarv T, Hollenberg J. Time to delivery of an automated external defibrillator using a drone for simulated out‐of‐hospital cardiac arrests vs emergency medical services. JAMA. 2017;317:2332–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Siddiq AA, Brooks SC, Chan TC. Modeling the impact of public access defibrillator range on public location cardiac arrest coverage. Resuscitation. 2013;84:904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1–S4
Tables S1–S4
Figures S1–S4


