Abstract
Background
The incidence and mortality of out‐of‐hospital cardiac arrest (OHCA) remains high, but predicting outcomes is challenging. Being able to better assess prognosis of hospitalized patients after return of spontaneous circulation would enable improved management of survival expectations. In this study, we assessed the predictive value of ECG indexes in hospitalized patients with OHCA.
Methods and Results
PR interval and QT interval corrected by the Bazett formula (QTc) for all leads were calculated from standard 12‐lead ECGs 24 hours after return of spontaneous circulation in 93 patients who were hospitalized following OHCA. PR interval and QT and QTc duration did not differentiate OHCA survivors and nonsurvivors. However, QT and QTc dispersion was significantly increased in patients who died during hospitalization compared with survivors discharged from the hospital (P<0.01). Logistic regression indicated a strong association between increased QT dispersion and in‐hospital mortality (P<0.0001; area under the curve, 0.8918 for QT dispersion and 0.8673 for QTc dispersion). Multinomial logistic regression indicated that the increase of QTc dispersion correlated with worse Cerebral Performance Category scores at discharge (P<0.001; likelihood ratio, 51.42). There was also significant correlation between dispersion measures and serum potassium at the time of measurement and between dispersion measures and cumulative epinephrine administration. No difference existed regarding the number of measurable leads.
Conclusions
Lesser QT and QTc dispersion at 24 hours after return of spontaneous circulation was significantly associated with survival and neurologic status at discharge. Routine evaluation of QT and QTc dispersion during hospitalization following return of spontaneous circulation might improve outcome prognostication for patients hospitalized for OHCA.
Keywords: cardiac arrest, cardiopulmonary resuscitation, ECG, ECMO, QT interval electrocardiography
Subject Categories: Cardiopulmonary Resuscitation and Emergency Cardiac Care, Sudden Cardiac Death
Nonstandard Abbreviations and Acronyms
- CPC
Cerebral Performance Category
- CT
computed tomography
- NSE
neuron‐specific enolase
- OHCA
out‐of‐hospital cardiac arrest
- ROSC
return of spontaneous circulation
- VA‐ECMO
venoarterial extracorporeal membrane oxygenation
Clinical Perspective
What Is New?
The 12‐lead ECG is an inexpensive and readily available tool with unexplored utility for management of patients with cardiac arrest.
What Are the Clinical Implications?
QT dispersion calculated in the 24‐hour 12‐lead ECG following resuscitation can significantly improve the neuroprognostication of patients with cardiac arrest treated with extracorporeal cardiopulmonary membrane oxygenation.
The 2018 American Heart Association Heart and Stroke Statistics indicate that >300 000 people in the United States had out‐of‐hospital cardiac arrest (OHCA), with 250 000 reported deaths. 1 Over the past decade, venoarterial extracorporeal cardiopulmonary membrane oxygenation (VA‐ECMO) has emerged for the management of patients with OHCA. 2 Nonetheless, despite improvements in outcomes, mortality remains high, exceeding 85%.
Patients with OHCA exhibit multiorgan dysfunction secondary to the global ischemia associated with cardiac arrest. 3 This severe organ dysfunction adversely affects the conventional prognosticating tools that have been developed for assessment of intensive care patients. 4 Serum markers, such as neuron‐specific enolase (NSE), have also been used, but their widespread use is limited by uncertainty regarding the optimal time of measurement, the optimal cutoff points, and the cost. 5 Despite acute multiorgan dysfunction, the majority of deaths are ultimately attributed to cardiovascular morbidity or brain death. 6 Therefore, a successful prognosticating tool should respond to both cardiac and cerebral insults.
Recently, Endoh et al reported that heart rate variability following return of spontaneous circulation (ROSC) differed between survivors and deceased patients with OHCA and strongly correlated to neurologic outcome, indicating that electrical activity of the heart could contribute to survival prognostication after cardiac arrest. 7 The 12‐lead ECG is an inexpensive and widely available tool that provides not only information regarding the status of intrinsic cardiac electrical function but also the input of extracardiac systems, particularly the central nervous system, to the electrical activity of the heart. Among other measurements derived from ECG recordings, the dispersion of the QT interval provides a readily accessible marker of repolarization abnormalities that is affected by alterations of both heart and brain activity. Consequently, we decided to assess whether QT and corrected QT (QTc) dispersion measures obtained at approximately 24 hours following hospital admission would be useful for prognostication of hospital survival in patients with OHCA.
Materials and Methods
This study was approved by University of Minnesota institutional review board (STUDY00004017). The study population included patients who achieved ROSC after out‐of‐hospital refractory ventricular fibrillation and who were admitted for further care between December 2015 and August 2019. Given the retrospective nature of the study and in accordance with Health Insurance Portability and Accountability Act (HIPAA) regulations, consent for participation was waived. Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to Marinos Kosmopoulos at marinos.kosmopoulos@gmail.com. This population has been described in detail 8 and consists of patients between 18 and 75 years old who were transferred from the scene of the arrest to the cardiac catheterization laboratory in <30 minutes. These patients underwent coronary angiography with primary percutaneous coronary intervention if either significant obstructive coronary artery disease or an acute coronary syndrome was identified.
If the patient had not achieved ROSC by the time of arrival at the cardiac catheterization laboratory, the treating interventional cardiologist would institute percutaneous VA‐ECMO support. At 12 to 36 hours after cannulation, patients underwent ECG assessment with standard 12‐lead ECG. Patients received therapeutic hypothermia under standard protocol with rapid intra‐arrest infusion of ice‐cold saline with a target temperature of 34°C, which could be allowed to reach 35°C if bleeding complications occurred. Patients were maintained at this temperature for approximately 24 hours. 8 , 9 Moreover, acute physiologic derangements after the arrest were also assessed through collection of plasma and arterial blood gas, and patients underwent computed tomography (CT) of the head at admission to assess for the presence of early brain injury. Patients’ last available ECG before death or hospital discharge was also assessed. Patients who died in <72 hours after cannulation were excluded from the secondary ECG assessment.
Standard ECG was assessed for the number of leads with a measurable QT interval. If U waves were present, they were not included in the measured interval. The end of the QT interval was determined by intersection of the tangent with the T‐wave downslope and isoelectric line. QT was corrected using the Bazett formula. Each trace was measured by the same individuals (M.K., T.G.) to minimize error. In case of interobserver variability >25 mm, a third observer (D.Y.) determined QT duration. To confirm that the observed trends did not result from the overestimation and underestimation of maximum and minimum QT, respectively, we also calculated the relative QT dispersion, which utilizes QT intervals from all leads and thus corrects for outlying measurements. If the presenting rhythm was atrial fibrillation, QT correction was done using the Fridericia formula, which is widely accepted to exhibit the best accuracy and reproducibility for these cases among the available methods. In contrast to the Bazett formula, in which corrected QT is calculated as the ratio of absolute QT to the square root of RR interval in seconds, the Fridericia formula uses the third root of RR. Although the Bazett formula is the most widely used, the Fridericia formula is considered to be the most accurate, so we decided to use both for our calculations. 10
Calculation of absolute and relative dispersion measures was done as recommended by Priori et al. 11 In brief, absolute dispersion was calculated as the difference between the longest and shortest QT intervals, and relative QT dispersion was calculated as the ratio of the standard deviation of the QT interval in the 12 ECG leads to the average QT duration. The primary end points for this study were survival at discharge and neurologic status. Neurologic status at discharge was evaluated by Cerebral Performance Category (CPC) score, a widely used system for interpretation of neurocognitive outcomes in patients with cardiac arrest. 12 , 13 A score of 1 or 2 was treated as neurologically intact survival, scores 3 and 4 identified survivors with disabling neurologic impairment, and patients who did not survive were assigned a score of 5.
Statistical Analysis
Data analysis was done with STATA software (v15; StataCorp). The Student t test was used to assess for differences in ECG indexes between survivors and deceased patients. ANOVA was used to compare the ECG indexes among the various neurologic status measures at discharge. Binomial regression was used to assess predictions regarding survival, and multinomial regression was chosen to assess predictions regarding neurologic status at discharge. Ordinal logistic regression was done to assess the correlation between dispersion measures and neurologic outcome.
Results
ECG Findings
Between January 2015 and August 2019, 192 patients with out‐of‐hospital ventricular tachycardia/ventricular fibrillation were admitted. None of the patients that received VA‐ECMO support had achieved ROSC before the institution of percutaneous VA‐ECMO support. All 93 patients in this investigation obtained ROSC within the first 24 hours of admission. ECG recordings at 12 to 36 hours after ROSC (mean time, 20 hours and 45 minutes; 95% CI, 19 hours and 18 minutes to 22 hours and 10 minutes) were available for 93 patients. Baseline characteristics and outcomes are shown in Table 1. Of the 93 patients, 68 had angiographically confirmed coronary artery disease and 25 had no evidence of coronary artery disease. In addition, 27 patients presented with acute thrombotic lesions, 34 had ischemic cardiomyopathy, and 18 had underlying nonischemic cardiomyopathy. One patient had arrest secondary to opiate overdose; for the remaining 13, the underlying cause was unidentified. Nine patients had ventricular rhythm, 12 patients had junctional rhythm, and 1 developed atrial fibrillation. Overall, 71 patients had sinus rhythm on their ECG recordings.
Table 1.
Patient Baseline Characteristics
| Patient Population | n | Age, Mean±SD | Male, n (%) |
|---|---|---|---|
| Survivors | 41 | 54.93±1.91 | 25 (61.0) |
| Neurologically intact | 30 | 55.45±2.38 | 20 (66.7) |
| Residual neurologic instability | 11 | 53.58±3.18 | 5 (44.4) |
| Deceased | 52 | 57.79±1.79 | 47 (90.4) |
| Total | 93 | 57.38±2.99 | 72 (77.4) |
ECG indexes at 24 hours are summarized in Table 2. The interquartile ranges of ECG indexes at 24 hours are presented in Table 3, and those of discharge are summarized in Table 4. Deceased patients and survivors did not differ with regard to PR‐interval duration. There was a trend of increased QT interval (P=0.07) in deceased patients compared with survivors, whereas no difference was present regarding QTc.
Table 2.
Summary of Measured ECG Variables in Patients With Cardiac Arrest 24 Hours After ROSC
| Marker | Total Population | Survivors | Neurologically Intact Survivors | Residual Neurologic Disability | Deceased |
|---|---|---|---|---|---|
| QT, ms | 553.47±107.55 | 536.96±112.79 | 532.32±35.43 | 549.61±111.59 | 568.83±94.08 |
| QTc, ms | 557.54±91.49 | 545.20±91.78 | 534.14±97.08 | 574.38±71.92 | 569.71±90.11 |
| PR, ms | 175.85±38.75 | 171.01±36.99 | 175.63±38.30 | 153.32±27.08 | 179.98±40.28 |
| QRS, ms | 105.44±34.42 | 100.33±29.06 | 104.12±30.65 | 90.34±22.64 | 108.86±38.05 |
| QT dispersion, ms | 135.01±57.83 | 95.93±34.24 | 93.31±35.44 | 103.09±31.18 | 166.31±54.29 |
| Bazett QTc dispersion, ms | 139.18±57.74 | 104.62±40.33 | 103.37±43.33 | 108.04±32.31 | 167.53±54.75 |
| Fridericia QTc dispersion, ms | 135.33±55.61 | 98.31±33.13 | 95.09±33.66 | 106.8±31.59 | 165.26±52.74 |
| Relative QT dispersion, ms | 7.86±3.54 | 5.91±2.11 | 5.71±1.84 | 6.51±2.74 | 9.11±3.04 |
Data are shown as mean±SD. Neurologically intact: CPC 1–2 at discharge. Residual neurologic disability: CPC 3–4 at discharge. CPC indicates Cerebral Performance Category; QTc, corrected QT interval; and ROSC, return of spontaneous circulation.
Table 3.
Interquartile Range of ECG Indexes 24 Hours After ROSC
| Patient Population | Quartile, % | QT, ms | QTc, ms | PR, ms | QRS, ms | QTd, ms | Bazett QTcd, ms | Fridericia QTcd, ms | Relative QTd, ms | Temperature, °C, Mean±SD |
|---|---|---|---|---|---|---|---|---|---|---|
| Total population | 25 | 476 | 509 | 142 | 83 | 94 | 100 | 101 | 5.03 | 34.35±1.2 |
| 50 | 568 | 561 | 170 | 95 | 125 | 126 | 125 | 6.81 | ||
| 75 | 704 | 613 | 204 | 114 | 164 | 159 | 157 | 9.57 | ||
| 99 | 816 | 816 | 262 | 254 | 301 | 335 | 331 | 20.83 | ||
| Survivors | 25 | 466 | 503 | 137 | 81 | 74 | 76 | 77 | 4.32 | 34.6±1.1 |
| 50 | 513 | 543 | 164 | 91 | 88 | 94 | 100 | 5.25 | ||
| 75 | 616 | 608 | 201 | 111 | 107 | 111 | 112 | 6.59 | ||
| 99 | 772 | 692 | 244 | 200 | 206 | 197 | 185 | 13.32 | ||
| Neurologically intact | 25 | 476 | 503 | 140 | 78 | 72 | 62 | 76 | 4.17 | 34.75±1.2 |
| 50 | 518 | 538 | 164 | 87 | 77 | 85 | 87 | 5.13 | ||
| 75 | 600 | 583 | 206 | 106 | 107 | 99 | 112 | 6.33 | ||
| 99 | 772 | 692 | 244 | 136 | 172 | 158 | 159 | 10.52 | ||
| Residual neurologic disability | 25 | 406 | 490 | 128 | 85 | 82 | 93 | 94 | 5.03 | 34.27±0.6 |
| 50 | 498 | 570 | 140 | 97 | 91 | 105 | 102 | 6.11 | ||
| 75 | 647 | 631 | 166 | 111 | 112 | 115 | 120 | 7.66 | ||
| 99 | 703 | 666 | 195 | 200 | 206 | 197 | 186 | 13.32 | ||
| Deceased | 25 | 520 | 538 | 155 | 85 | 126 | 126 | 126 | 6.35 | 34.1±1.3 |
| 50 | 588 | 593 | 181 | 96 | 150 | 150 | 148 | 8.45 | ||
| 75 | 644 | 639 | 204 | 125 | 209 | 210 | 202 | 11.74 | ||
| 99 | 816 | 816 | 262 | 254 | 321 | 335 | 331 | 20.83 |
Neurologically intact: CPC 1–2 at discharge. Residual neurologic disability: CPC 3–4 at discharge. CPC indicates Cerebral Performance Category; QTc, corrected QT interval; QTcd, corrected QT dispersion; QTd, QT dispersion; and ROSC, return of spontaneous circulation.
Table 4.
Interquartile Range of ECG Indexes at Discharge
| Patient Population | Quartile, % | QT, ms | QTc, ms | PR, ms | QRS, ms | QTd, ms | Bazett QTcd, ms | Fridericia QTcd, ms | Relative QTd, ms |
|---|---|---|---|---|---|---|---|---|---|
| Total population | 25 | 346 | 514 | 142 | 99 | 75 | 108 | 89 | 6.13 |
| 50 | 389 | 578 | 167 | 109 | 105 | 138 | 110 | 7.87 | |
| 75 | 446 | 625 | 187 | 126 | 130 | 186 | 145 | 10.6 | |
| 99 | 771 | 813 | 288 | 231 | 226 | 254 | 236 | 18.28 | |
| Survivors | 25 | 350 | 503 | 137 | 96 | 75 | 104 | 78 | 6.13 |
| 50 | 382 | 550 | 147 | 107 | 88 | 123 | 95 | 7.98 | |
| 75 | 438 | 612 | 171 | 112 | 122 | 177 | 137 | 9.66 | |
| 99 | 501 | 685 | 208 | 186 | 191 | 226 | 202 | 18.28 | |
| Neurologically intact | 25 | 359 | 503 | 136 | 99 | 74 | 105 | 76 | 5.84 |
| 50 | 384 | 543 | 145 | 109 | 85 | 117 | 94 | 7.46 | |
| 75 | 439 | 596 | 175 | 114 | 122 | 172 | 137 | 9.00 | |
| 99 | 499 | 692 | 208 | 183 | 191 | 226 | 202 | 14.52 | |
| Residual neurologic disability | 25 | 340 | 530 | 139 | 82 | 91 | 121 | 83 | 7.75 |
| 50 | 363 | 595 | 154 | 89 | 107 | 164 | 129 | 10.14 | |
| 75 | 444 | 508 | 166 | 99 | 134 | 191 | 154 | 14.68 | |
| 99 | 502 | 666 | 195 | 107 | 159 | 196 | 154 | 18.28 | |
| Deceased | 25 | 342 | 542 | 153 | 103 | 126 | 76 | 94 | 6.05 |
| 50 | 417 | 586 | 180 | 116 | 150 | 109 | 128 | 7.87 | |
| 75 | 492 | 634 | 219 | 131 | 209 | 134 | 145 | 10.61 | |
| 99 | 771 | 816 | 288 | 231 | 321 | 226 | 237 | 14.47 |
Neurologically intact: CPC 1–2 at discharge. Residual neurologic disability: CPC 3–4 at discharge. CPC indicates Cerebral Performance Category; QTc, corrected QT interval; QTcd, corrected QT dispersion; and QTd, QT dispersion.
Regarding dispersion measures, there was no significant difference when assessed for PR‐interval dispersion (P=0.1383). When assessing for QT dispersion indexes, deceased patients had markedly increased QT dispersion, QTc dispersion, and relative QT and QTc dispersion at 24‐hour ECG (P<0.01).
Predictive Value
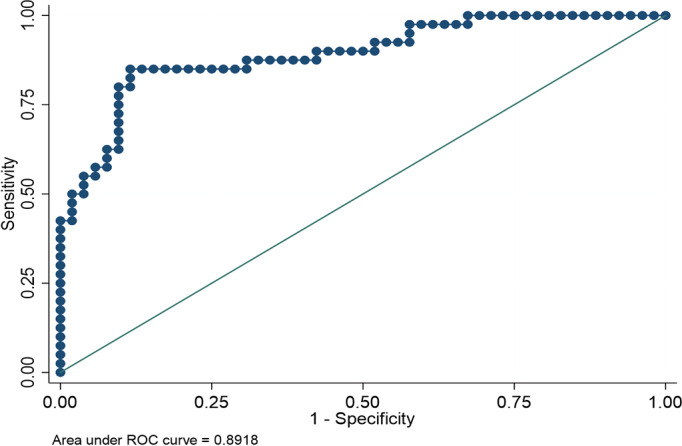
Logistic regression was done to assess the predictive value of QT dispersion indexes with respect to survival (Table 5). Among the tested QT dispersion indexes, corrected dispersion yielded the highest accuracy, followed by absolute QT dispersion and relative QT dispersion. Among the 2 different methods for the calculation of QTc, the measurement derived by the Fridericia formula appeared to outperform the one derived from the Bazett formula, with better receiver operating characteristic area under the curve (0.89 compared with 0.86). Regarding specificity and sensitivity, 118 ms for QT dispersion, 120.7 ms for Bazett QTc dispersion, and 114.7 for Fridericia QT dispersion were identified as the most favorable indexes, with 85.19% sensitivity and 81.4% specificity for absolute QT dispersion and 89.8% sensitivity and 80% specificity for Fridericia QT dispersion. The detailed results of the sensitivity analysis can be found in Table S1. The receiver operating characteristic curve for the association between QT dispersion of the 24‐hour 12‐lead ECG and probability of survival of patients with OHCA is depicted in Figure 1.
Table 5.
Predictive Value of QT Dispersion Indexes 24 Hours After Arrest Regarding Survival After Refractory Ventricular Tachycardia/Ventricular Fibrillation
| ECG Index | P Value | Likelihood Ratio | Area Under the Curve | Predicted Outcome |
|---|---|---|---|---|
| QTd | <0.00001 | 52.54 | 0.8918 | Survival |
| QTc dispersion | <0.00001 | 36.74 | 0.8673 | Survival |
| Relative QTd | 0.0022 | 32.46 | 0.82 | Survival |
| Absolute QT | 0.05 | 3.7 | 0.6128 | Survival |
| QTc | 0.1883 | 1.73 | 0.5714 | Survival |
| Fridericia QTcd | <0.00001 | 47.66 | 0.8913 | Survival |
| Admission NSE | 0.0768 | 3.13 | 0.65 | Survival |
| 24‐h NSE | <0.00001 | 22.56 | 0.8942 | Survival |
| QTd | <0.00001 | 55.4 | Status at discharge | |
| QTcd | <0.00001 | 36.36 | Status at discharge | |
| Relative QTd | <0.00001 | 35.47 | Status at discharge | |
| Absolute QT | 0.1358 | 3.99 | Status at discharge | |
| QTc | 0.4155 | 1.76 | Status at discharge | |
| Fridericia QTcd | <0.0001 | 47.4 | Status at discharge | |
| Admission NSE | 0.04 | 6.14 | Status at discharge | |
| 24‐h NSE | <0.00001 | 22.65 | Status at discharge |
Status at discharge: neurologic outcome as assessed by CPC score. CPC indicates Cerebral Performance Category; NSE, neuron‐specific enolase; QTc, corrected QT interval; QTcd, corrected QT dispersion; and QTd, QT dispersion.
Figure 1. Receiver operating characteristic (ROC) curve for the association between QT dispersion of the 24‐hour 12‐lead ECG and survival of patients with out‐of‐hospital cardiac arrest.
NSE levels at admission were not significantly different between survivors and nonsurvivors. However, at 24 hours, survivors’ levels were markedly lower than those of deceased patients. NSE levels at 24 hours could reliably predict survival, with an area under the curve of 0.89. Both NSE and QT dispersion were independently associated with discharge outcomes in a multivariate model. Patients without a sinus rhythm had a higher nonsurvival probability trend. However, the difference in survival was not significant (P=0.15). Adjusting for sinus rhythm presence and the time elapsed from ROSC did not affect the association between QT dispersion and survival.
When patients were assessed according to neurologic status at discharge, ANOVA indicated no statistically significant differences among patients with different neurologic discharge status for QT, QTc, and PR intervals. However, when dispersion measures were assessed, significant differences were evident among QT dispersion indexes for patients with different outcomes, most importantly, QT dispersion, QTc dispersion, and relative QT dispersion, which are summarized in Table 5. Specifically, patients who were neurologically intact at discharge had significantly lower QT dispersion measurements compared with patients who had residual neurologic disability. Both groups had significantly lower QT dispersion than deceased patients.
Multinomial logistic regression indicated that QT and QTc dispersion could reliably predict neurologic status at discharge (Table 5 and Figure 2). The tested ECG values were independent of sex and age of the patients with cardiac arrest. Association between neurologic outcomes at discharge and QT dispersion and NSE measured at 24 hours remained significant for both variables when they were assessed together. ECGs at discharge were also analyzed.
Figure 2. Probability of neurologic outcomes according to QT dispersion derived from 12‐lead ECG 24 hours after return of spontaneous circulation in patients with out‐of‐hospital cardiac arrest (calculated by ordinal logistic regression).
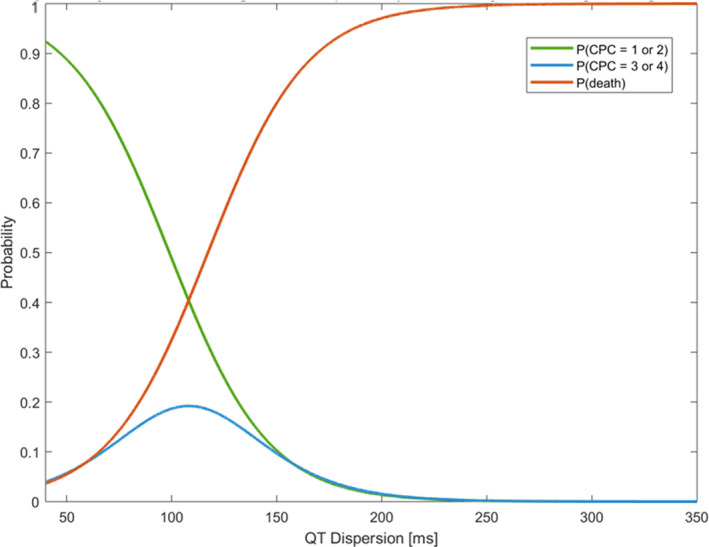
The yellow line indicates the probability of discharge with a Cerebral Performance Category (CPC) score between 1 and 2. The red line indicates the probability of discharge with a CPC score between 3 and 4. The blue line indicates the probability of death. This figure shows that as QT dispersion increases, the probability of neurologically intact survival decreases while the probability of death increases. Survival with neurologic disability follows a distinct sigmoid curve.
In contrast to admission ECG, no association was found between QT dispersion indexes and survival on the discharge ECG. No difference was identified in average QRS intervals for both admission and discharge (Table 5). Moreover, we assessed the association between QT dispersion and findings on admission head CT. Patients with abnormal CT, defined as presence of brain herniation, loss of gray‐white matter differentiation, or cerebral edema, had increased QT dispersion compared with patients without identified intracranial pathology (P=0.04). Nevertheless, regression analysis did not confirm this finding, although a nonsignificant trend was observed (P=0.09).
Sensitivity Analyses
To identify potential confounders for the relationship between QT dispersion and status at discharge, we assessed the relationship of QT dispersion with electrolytes at the time of measurement and with the administration of inotropes and catecholamines. Linear regression analysis indicated a significant negative correlation between QT dispersion and serum potassium at the time of the ECG being obtained (P=0.03) and a positive correlation between QT dispersion and epinephrine (P=0.001) and total dose of catecholamines, calculated as the sum of the dose of epinephrine, norepinephrine, and dopamine (P=0.04). QT dispersion’s association with survival and neurologic status at discharge remained significant after adjustment for potassium and catecholamines No correlation was found between QT dispersion and dose of amiodarone, pH at the time of measurement, or temperature.
Discussion
In our study, the associations of QT dispersion with neurologic status at discharge and with survival at discharge were assessed. Both QTc dispersion and uncorrected QT dispersion at 24 hours after ROSC was significantly associated with survival and with CPC at discharge in patients with OHCA. NSE provided a similar outcome, supporting our ECG observations, but at much higher cost.
OHCA is a highly morbid condition with an annual burden of >300 000 hospitalizations and 250 000 deaths. 1 Despite the improvement of outcomes with VA‐ECMO hemodynamic support 3 and application of therapeutic hypothermia, 14 mortality is still high, and there is a need for development of novel prognostication techniques that will guide supportive care and permit establishment of reasonable treatment expectations. Furthermore, because most patients with OHCA die from either brain death or heart failure, 15 the prognostic indexes should focus on assessment of cerebral and cardiac injury in the first hours after the insult. In contrast, patients who have OHCA and are able to regain consciousness before hospital admission have a more positive neurologic prognosis than patients who are unable to regain consciousness in the field. 16 The highly specialized refractory ventricular tachycardia/ventricular fibrillation OHCA population in this investigation represents a difficult clinical scenario 8 in which novel prognostic markers of neurologic function may be of use. This is because obtaining ROSC in these patients suggests the possibility of a positive cardiac prognosis, 6 but the neurologic prognosis is often still difficult to address by the time ROSC has been obtained. Consequently, we think that QTc dispersion is a metric that might aid neurologic prognosis. Specifically, a QT dispersion cutoff value of 118 to 122 ms seems the most suitable for determining survival. However, like all the markers, QT dispersion is imperfect and should never be used without evaluation of other clinical and biochemical information.
QT Dispersion
Cardiac Injury
QT dispersion for assessment of brain injury has been performed previously in cases of stroke and subarachnoid hemorrhage. 17 Correct interpretation requires consideration of the timing of ECG recording and knowledge of other parameters that might interfere with repolarization such as electrolytes and temperature. 18 , 19 To address this issue, we included only ECGs performed 12 to 36 hours after ROSC and correlated the findings to electrolytes, temperature, and pH at the time at which the ECG was obtained and to the administered medications. We also used NSE as a separate measure to assess the validity of ECG findings.
QT dispersion was significantly affected by serum potassium and cumulative dose of epinephrine. However, the relationship between QT dispersion and survival remained significant in the multivariate analysis. No association was drawn between QT dispersion and body temperature; however, a temperature effect is something that should be expected. In our study, all patients were treated with therapeutic hypothermia, and thus there were minimal deviations in temperature at the time of measurement.
The connection between QT dispersion measures and heart and brain injury probably lies in the sympathetic innervation of the left ventricle. Sympathetic afferent fibers are distributed across the inferoposterior and anterior wall of the left ventricle. 20 Following myocardial ischemia, which occurs globally during cardiac arrest, sympathetic afferent fibers have been demonstrated to be activated 21 and consequently result in activation of the cardiac sympathetic efferent fibers. 22 QT dispersion is documented to be elevated in states of sympathetic activation such as hyperthyroidism 23 and alcoholism. 24 Even more important, acute physiologic activation of the sympathetic nervous system was shown to increase QT dispersion in a head‐up tilt test of healthy individuals without any underlying disease. 25 In our study, we identified that the dose of epinephrine and the total dose of the composite of epinephrine, dobutamine, and norepinephrine were positively correlated to QT dispersion.
In accordance with previous reports, QT dispersion has been documented increasing in cases of myocardial ischemia, and successful reperfusion of ischemic areas is able to reverse that increase. 26 Compared with patients with unstable angina, patients presenting with ST‐segment–elevation myocardial infarction had increased QT dispersion at the time of presentation. 27 Moreover, the increase of QT dispersion correlates with tissue perfusion as assessed by Myocardial Blush Grade. 28 Studies have also correlated QT dispersion with the magnitude of viable left ventricle. 29 Counterintuitively, QT dispersion at 72 hours after the onset of ST‐segment–elevation myocardial infarction was not associated with survival. 30 However, this analysis was done before the development of coronary angioplasty, and deep Q waves were equally present in both survivors and nonsurvivors. This result indicated that necrosis had already occurred in both groups, and therefore repolarization abnormalities should be universally expected.
Brain Injury
A second mechanism that might explain the strong association between neurologically intact survival and QT dispersion of the 24‐hour ECG is its correlation with brain injury. QT dispersion has been noted to increase in cases of stroke when calculated at the first day after the event. Involvement of the insular cortex might be a crucial determinant for the observed increase in QT dispersion. 31 , 32 In particular, involvement of the insula is associated with alteration of sympathovagal balance, which results from a concomitant increase in circulating norepinephrine levels. 33 The autonomic instability that results from brain injury potentiates regional repolarization differences, 34 which might be represented by QT dispersion in patients with cardiac arrest treated with ECMO. 35 , 36 The hippocampus is another brain region that regulates the autonomic nervous system. 37 Temporal lobe seizures arising from the hippocampus have been reported to alter cardiac repolarization without affecting the heart rate 38 , 39 Whether these effects are carried through autonomic regulation or another pathway connecting the hippocampus and the left ventricle has yet to be elucidated. Finally, brainstem stimulation has been noted to alter cardiac repolarization. 40 Taken together, these findings point toward a potential brain injury effect on QT dispersion. In our study, there was a trend for association between abnormal findings on the head CT scan and increase in QT dispersion. However, the sensitivity of the CT scan of the head at admission of patients with cardiac arrest is uncertain 41 ; therefore, the nonsignificant trend that was observed should be interpreted with caution.
Correlation of QT Dispersion and Clinical Outcomes
In our population, neurologically intact patients who survived did not have a decrease of QT dispersion in the discharge ECG. Bartos et al previously demonstrated that although the left ventricle recovers in the first 2 weeks following the arrest, neurologic recovery and rehabilitation is prolonged; the delayed recovery of the latter may account for the persistently elevated QT dispersion. 6 Moreover, despite their independence and intact consciousness, neurologically intact survivors still have significant deficits in memory and hippocampal atrophy. 42 The hippocampus is a potent regulator of the sympathetic nervous system, and thus lesions at that location may lead to autonomic instability 43 and potentially explain the increased QT dispersion seen in survivors. In deceased patients, QT dispersion significantly decreased in the discharge ECG. However, compared with the QT dispersion of the discharge ECG in OHCA survivors, it was still elevated (P=0.008). In patients with prolonged coma duration, normal electrical signals do not correlate with outcomes and do not seem to affect prognosis. 44 The absence of sinus rhythm was found more frequently in deceased patients; however, there was no statistical significance, and thus reassessment with a larger sample is necessary. The underlying heart disease also did not affect QT dispersion. The lack of association probably derives from the power of the effect of the global ischemic injury that results from cardiac arrest regardless of the underlying cause and affects both the heart and the brain.
Limitations
The findings in this report are subject to several important limitations. First, the study was retrospective and comprised a relatively small population of OHCA victims. Second, despite the established paradigms, QT interval utilization has important methodologic limitations. It has been argued that QT dispersion derives mostly from projection differences rather than true depolarization abnormalities. However, the data to support this criticism derive mostly from healthy individuals, and the projection effect has not been tested in patients with structural heart disease. 45 Moreover, the reproducibility of QT intervals in all leads is poor. Consequently, the absolute QT dispersion, which is derived from the difference between the minimum and the maximum QT interval, might be inaccurate and depends on the accuracy of the measurement. 46 To address these procedural limitations, we assessed the digitized ECGs twice with blinding to patient outcomes. Given the subjective nature of the measurements, each trace was measured by the same individuals to minimize error. To confirm that the observed trends did not result from the overestimation and underestimation of maximum and minimum QT, respectively, we also calculated the relative QT dispersion, which utilizes QT intervals from all leads and thus corrects for outlying measurements. Relative QT dispersion also yielded strong associations with both survival and neurologic outcome. It is possible that patients with OHCA who are resuscitated in the field and are conscious on arrival may not have as marked, if any, QTc dispersion. This group of patients, by definition, exhibits a more positive neurologic prognosis than the patients with refractory ventricular tachycardia/ventricular fibrillation OHCA in this cohort because none of the refractory VT/VT patients with OHCA had regained spontaneous circulation or consciousness before institution of VA‐ECMO support. We were unable to directly compare the QTc dispersion metric for the patients who had regained consciousness before arriving at the hospital and the patients with ventricular tachycardia/ventricular fibrillation OHCA in this cohort.
Conclusions
Cardiac arrest is a complex and highly morbid condition. Development of tools that can aid in the rapid assessment of neurologic injury is necessary. QT dispersion in the 24‐hour ECG is a relatively simple and low‐cost technique. If desired, it can be used in conjunction with other markers, significantly aiding in the prognostication of survival at discharge. Prospective validation in other cardiac arrest cohorts and application of techniques for automated calculation will determine its potential for wider clinical use.
Sources of Funding
This work was supported by a grant from the Helmsley Charitable Trust and the Robert Eddy Endowment for Resuscitation Medicine to Dr Yannopoulos. Dr Benditt was supported in part by a grant from the Dr Earl E Bakken family in support of heart‐brain research.
Disclosures
None.
Supporting information
Table S1
(J Am Heart Assoc. 2020;9:e05336 DOI: 10.1161/JAHA.120.016485.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.016485
For Sources of Funding and Disclosures, see page 9.
References
- 1. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, et al. Heart disease and stroke statistics‐2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2. Rao P, Khalpey Z, Smith R, Burkhoff D, Kociol RD. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest. Circ Heart Fail. 2018;11:e004905. [DOI] [PubMed] [Google Scholar]
- 3. Nobile L, Taccone FS, Szakmany T, Sakr Y, Jakob SM, Pellis T, Antonelli M, Leone M, Wittebole X, Pickkers P, et al. The impact of extracerebral organ failure on outcome of patients after cardiac arrest: an observational study from the ICON database. Crit Care. 2016;20:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wengenmayer T, Duerschmied D, Graf E, Chiabudini M, Benk C, Muhlschlegel S, Philipp A, Lubnow M, Bode C, Staudacher DL. Development and validation of a prognostic model for survival in patients treated with venoarterial extracorporeal membrane oxygenation: the PREDICT VA‐ECMO score. Eur Heart J Acute Cardiovasc Care. 2019;8:350–359. [DOI] [PubMed] [Google Scholar]
- 5. Tiainen M, Roine RO, Pettila V, Takkunen O. Serum neuron‐specific enolase and S‐ 100B protein in cardiac arrest patients treated with hypothermia. Stroke. 2003;34:2881–2886. [DOI] [PubMed] [Google Scholar]
- 6. Bartos JA, Carlson K, Carlson C, Raveendran G, John R, Aufderheide TP, Yannopoulos D. Surviving refractory out‐of‐hospital ventricular fibrillation cardiac arrest: critical care and extracorporeal membrane oxygenation management. Resuscitation. 2018;132:47–55. [DOI] [PubMed] [Google Scholar]
- 7. Endoh H, Kamimura N, Honda H, Nitta M. Early prognostication of neurological outcome by heart rate variability in adult patients with out‐of‐hospital sudden cardiac arrest. Crit Care. 2019;23:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yannopoulos D, Bartos JA, Martin C, Raveedran G, Missov E, Conterato M, Frascone RJ, Trembley A, Sipprell K, John R, et al. Minnesota Resuscitation Consortium’s advanced perfusion and reperfusion cardiac life support strategy for out‐of‐ hospital refractory ventricular fibrillation. J Am Heart Assoc. 2016;5:e003732 Available at: 10.1161/JAHA.116.003732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stub D, Bernard S, Pellegrino V, Smith K, Walker T, Sheldrake J, Hockings L, Shaw J, Duffy SJ, Burrell A, et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation. 2015;86:88–94. [DOI] [PubMed] [Google Scholar]
- 10. Musat DL, Adhaduk M, Preminger MW, Arshad A, Sichrovsky T, Steinberg JS, Mittal S. Correlation of QT interval correction methods during atrial fibrillation and sinus rhythm. Am J Cardiol. 2013;112:1379–1383. [DOI] [PubMed] [Google Scholar]
- 11. Priori SG, Napolitano C, Diehl L, Schwartz PJ. Dispersion of the QT interval. A marker of therapeutic efficacy in the idiopathic long QT syndrome. Circulation. 1994;89:1681–1689. [DOI] [PubMed] [Google Scholar]
- 12. Hsu CH, Li J, Cinousis MJ, Sheak KR, Gaieski DF, Abella BS, Leary M. Cerebral performance category at hospital discharge predicts long‐term survival of cardiac arrest survivors receiving targeted temperature management*. Crit Care Med. 2014;42:2575–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. [DOI] [PubMed] [Google Scholar]
- 14. Donnino MW, Andersen LW, Berg KM, Reynolds JC, Nolan JP, Morley PT, Lang E, Cocchi MN, Xanthos T, Callaway CW, et al. Temperature management after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Resuscitation. 2016;98:97–104. [DOI] [PubMed] [Google Scholar]
- 15. Witten L, Gardner R, Holmberg MJ, Wiberg S, Moskowitz A, Mehta S, Grossestreuer AV, Yankama T, Donnino MW, Berg KM. Reasons for death in patients successfully resuscitated from out‐of‐hospital and in‐hospital cardiac arrest. Resuscitation. 2019;136:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eva S, Martin R, Marko N, Peter R. Abstract 20034: outcome of conscious survivors of out‐of‐hospital cardiac arrest. Circulation. 2017;136:A20034. [Google Scholar]
- 17. Randell T, Tanskanen P, Scheinin M, Kytta J, Ohman J, Lindgren L. QT dispersion after subarachnoid hemorrhage. J Neurosurg Anesthesiol. 1999;11:163–166. [DOI] [PubMed] [Google Scholar]
- 18. Howse M, Sastry S, Bell GM. Changes in the corrected QT interval and corrected QT dispersion during haemodialysis. Postgrad Med J. 2002;78:273–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guill A, Trapero I, Roses E, Millet J, Tormos A, Pelechano F, Such‐Miquel LM, Martinez‐Climent A, Such L, Chorro FJ. QT dispersion induced by local temperature variations. Comput Cardiol. 2008;2008:697–700. [Google Scholar]
- 20. Minisi AJ, Thames MD. Distribution of left ventricular sympathetic afferents demonstrated by reflex responses to transmural myocardial ischemia and to intracoronary and epicardial bradykinin. Circulation. 1993;87:240–246. [DOI] [PubMed] [Google Scholar]
- 21. Minisi AJ, Thames MD. Activation of cardiac sympathetic afferents during coronary occlusion. Evidence for reflex activation of sympathetic nervous system during transmural myocardial ischemia in the dog. Circulation. 1991;84:357–367. [DOI] [PubMed] [Google Scholar]
- 22. Malik R, Minisi AJ. Simultaneous cardiac and renal sympathetic neural responses to activation of left ventricular sympathetic afferents. J Auton Nerv Syst. 1997;65:10–16. [DOI] [PubMed] [Google Scholar]
- 23. Cai Z, Dai M, Zhang Y, Zhong H, Tan T, Bao M. Imbalance of cardiac autonomic nervous activity and increase of ventricular repolarization dynamicity induced by thyroid hormones in hyperthyroidism. Auton Neurosci. 2018;213:86–91. [DOI] [PubMed] [Google Scholar]
- 24. Johnson RH, Eisenhofer G, Lambie DG. The effects of acute and chronic ingestion of ethanol on the autonomic nervous system. Drug Alcohol Depend. 1986;18:319–328. [DOI] [PubMed] [Google Scholar]
- 25. Nakagawa M, Takahashi N, Iwao T, Yonemochi H, Ooie T, Hara M, Saikawa T, Ito M. Evaluation of autonomic influences on QT dispersion using the head‐up tilt test in healthy subjects. Pacing Clin Electrophysiol. 1999;22:1158–1163. [DOI] [PubMed] [Google Scholar]
- 26. Glancy JM, Garratt CJ, de Bono DP. Dynamics of QT dispersion during myocardial infarction and ischaemia. Int J Cardiol. 1996;57:55–60. [DOI] [PubMed] [Google Scholar]
- 27. Higham PD, Furniss SS, Campbell RW. QT dispersion and components of the QT interval in ischaemia and infarction. Br Heart J. 1995;73:32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fukushima N, Tsurumi Y, Jujo K, Fukushima K, Sekiguchi H, Honda A, Yumino D, Kawana M, Hagiwara N. Impact of myocardial reperfusion status on QT dispersion after successful recanalization of the infarct‐related artery in acute myocardial infarction. J Interv Cardiol. 2014;27:252–259. [DOI] [PubMed] [Google Scholar]
- 29. Schneider CA, Voth E, Baer FM, Horst M, Wagner R, Sechtem U. QT dispersion is determined by the extent of viable myocardium in patients with chronic Q‐wave myocardial infarction. Circulation. 1997;96:3913–3920. [DOI] [PubMed] [Google Scholar]
- 30. Glancy JM, Garratt CJ, Woods KL, de Bono DP. QT dispersion and mortality after myocardial infarction. Lancet. 1995;345:945–948. [DOI] [PubMed] [Google Scholar]
- 31. Alabd AA, Fouad A, Abdel‐Nasser R, Nammas W. QT interval dispersion pattern in patients with acute ischemic stroke: Does the site of infarction matter? Int J Angiol. 2009;18:177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rahar KK, Pahadiya HR, Barupal KG, Mathur CP, Lakhotia M. The QT dispersion and QTc dispersion in patients presenting with acute neurological events and its impact on early prognosis. J Neurosci Rural Pract. 2016;7:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sander D, Klingelhofer J. Changes of circadian blood pressure patterns after hemodynamic and thromboembolic brain infarction. Stroke. 1994;25:1730–1737. [DOI] [PubMed] [Google Scholar]
- 34. Kralios FA, Martin L, Burgess MJ, Millar K. Local ventricular repolarization changes due to sympathetic nerve‐branch stimulation. Am J Physiol. 1975;228:1621–1626. [DOI] [PubMed] [Google Scholar]
- 35. Pasquini M, Laurent C, Kroumova M, Masse I, Deplanque D, Leclerc X, Bordet R, Leys D. Insular infarcts and electrocardiographic changes at admission: results of the PRognostic of Insular CErebral infarctS Study (PRINCESS). J Neurol. 2006;253:618–624. [DOI] [PubMed] [Google Scholar]
- 36. Oppenheimer S, Cechetto D. The insular cortex and the regulation of cardiac function. Compr Physiol. 2016;6:1081–1133. [DOI] [PubMed] [Google Scholar]
- 37. Castle M, Comoli E, Loewy AD. Autonomic brainstem nuclei are linked to the hippocampus. Neuroscience. 2005;134:657–669. [DOI] [PubMed] [Google Scholar]
- 38. Surges R, Jordan A, Elger CE. Ictal modulation of cardiac repolarization, but not of heart rate, is lateralized in mesial temporal lobe epilepsy. PLoS One. 2013;8:e64765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brotherstone R, Blackhall B, McLellan A. Lengthening of corrected QT during epileptic seizures. Epilepsia. 2010;51:221–232. [DOI] [PubMed] [Google Scholar]
- 40. Shusterman V, Jannetta PJ, Aysin B, Beigel A, Glukhovskoy M, Usiene I. Direct mechanical stimulation of brainstem modulates cardiac rhythm and repolarization in humans. J Electrocardiol. 2002; 35(suppl):247–256. [DOI] [PubMed] [Google Scholar]
- 41. Cocchi MN, Lucas JM, Salciccioli J, Carney E, Herman S, Zimetbaum P, Donnino MW. The role of cranial computed tomography in the immediate post‐cardiac arrest period. Intern Emerg Med. 2010;5:533–538. [DOI] [PubMed] [Google Scholar]
- 42. Stamenova V, Nicola R, Aharon‐Peretz J, Goldsher D, Kapeliovich M, Gilboa A. Long‐term effects of brief hypoxia due to cardiac arrest: hippocampal reductions and memory deficits. Resuscitation. 2018;126:65–71. [DOI] [PubMed] [Google Scholar]
- 43. Scheiderer CL, McCutchen E, Thacker EE, Kolasa K, Ward MK, Parsons D, Harrell LE, Dobrunz LE, McMahon LL. Sympathetic sprouting drives hippocampal cholinergic reinnervation that prevents loss of a muscarinic receptor‐dependent long‐term depression at CA3‐CA1 synapses. J Neurosci. 2006;26:3745–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koenig MA, Kaplan PW, Thakor NV. Clinical neurophysiologic monitoring and brain injury from cardiac arrest. Neurol Clin. 2006;24:89–106. [DOI] [PubMed] [Google Scholar]
- 45. di Bernardo D, Langley P, Murray A. Dispersion of QT intervals: a measure of dispersion of repolarization or simply a projection effect? Pacing Clin Electrophysiol. 2000;23:1392–1396. [DOI] [PubMed] [Google Scholar]
- 46. Coumel P, Maison‐Blanche P, Badilini F. Dispersion of ventricular repolarization: reality? Illusion? Significance? Circulation. 1998;97:2491–2493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


