Abstract
Background
Left ventricular assist devices (LVADs) generate electromagnetic interference that causes high‐frequency noise artifacts on 12‐lead ECGs. We describe the causes of this interference and potential solutions to aid ECG interpretation in patients with LVAD.
Methods and Results
Waveform data from ECGs performed before and after LVAD implantation were passed through a fast Fourier transform to identify LVAD‐related changes in the spectral profile. ECGs recorded in 9 patients with HeartMate II, HeartMate 3, and HeartWare LVADs were analyzed to identify the LVAD model‐specific spectral patterns. Waveform data were then passed through digital low‐pass and bandstop filters and redisplayed to evaluate the effect of filtering on LVAD‐related electromagnetic interference. The spectral profile of patients with HeartMate II and HeartMate 3 LVADs demonstrated a prominent signal at the device‐specific frequency of impeller rotation. In patients with the HeartMate 3 LVAD, 2 additional peaks were observed at the frequencies equivalent to the LVAD's artificial pulsatility rotational speeds. Patients with HeartWare devices demonstrated a prominent signal peak at a frequency equal to double their LVAD's set rotational speed. Applying a low‐pass filter to a value below the observed frequency peak from the LVAD significantly improved the waveform tracing and quality of the ECG. Applying a speed‐specific bandstop filter to remove the observed LVAD frequency peak also improved the clarity of the ECG without compromising physiological high‐frequency signal components.
Conclusions
LVADs create impeller rotational speed‐specific electromagnetic interference that can be ameliorated by application of low‐pass or bandstop filters to improve ECG clarity.
Keywords: signal processing, ECG, left ventricular assist device
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- EMI
electromagnetic interference
- HPF
high‐pass filter
- LPF
low-pass filter
- LVAD
left ventricular assist device
- MOMENTUM 3
Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate
- S-ICD
subcutaneous implantable cardioverter-defibrillator
Clinical Perspective
What Is New?
Left ventricular assist devices produce electromagnetic interference on ECGs in predictable ways.
Left ventricular assist device electromagnetic interference is primarily driven by the impeller rotational speed; however, different left ventricular assist device models exhibit different minor electromagnetic interference components as a consequence of differences in their designs.
Use of low-pass and bandstop filters can reduce this electromagnetic interference and improve the clarity of ECGs.
What Are the Clinical Implications?
Application of filters tailored to individual left ventricular assist device models and impeller rotational speed can improve ECG clarity without sacrificing physiological high‐frequency signal components.
Arrhythmias are common in patients with left ventricular assist devices (LVADs), with atrial fibrillation occurring in up to 32% of patients. 1 However, continuous‐flow LVADs generate electromagnetic interference (EMI), which manifests as high‐frequency noise artifacts on the surface 12‐lead ECG and impedes rhythm identification. 2 A study comparing ECGs before and after LVAD implantation showed that electrical artifact in all leads was the most common change seen after LVAD, found in 98% of included patients. 3 In addition to hindering 12‐lead ECG clinical interpretation, this EMI may impact screening tests for subcutaneous implantable cardioverter‐defibrillators (S‐ICDs), interfere with catheter ablation procedures, and carry a risk for inappropriate implantable cardioverter‐defibrillator therapy delivery. 4 , 5 , 6 A study comparing ECG findings of 415 patients before and after LVAD implantation found significant shortening of the QRS duration, reduction in the amplitudes of the R and S waves in some leads, and an alteration of the R:T ratio, which may impact the ability of implantable cardioverter‐defibrillators to accurately identify dangerous cardiac arrhythmias. 4
ECGs are susceptible to interference from noncardiac sources of electromagnetic activity. Bandpass filters eliminate signal components that arise from external sources and may interfere with ECG interpretation. ECGs typically focus on signals within the 0.04 to 150 Hz range in order to capture both low‐frequency (eg, the T wave) and high‐frequency (eg, the QRS complex) components that are clinically important. This focus is achieved by applying high‐pass filters (HPFs) and low‐pass filters (LPFs). HPFs set the lower frequency limit for which signals will be included in the displayed ECG waveform (ie, only signals higher than this threshold will be included). Conversely, LPFs demarcate the upper frequency limit. Electromagnetic waveforms from power lines can contribute to ECG noise, so an additional “notch” filter is often applied at the typical utility frequency (60 Hz in the Americas and parts of Asia, 50 Hz in much of the rest of the world). The application of HPF, LPF, and notch filters allows ECG machines to display the signal frequencies within the region of interest while minimizing EMI. 7
Currently available continuous‐flow LVADs typically run at impeller rotational speeds in the range of 2400 to 3200 rpm (HeartWare by Medtronic), 5000 to 6000 rpm (HeartMate 3 by Abbott), and 8800 to 10 000 rpm (HeartMate II by Abbott). These correspond to oscillating frequencies of 40 to 53.3 Hz, 83.3 to 100 Hz, and 146.7 to 166.7 Hz, respectively. These high‐frequency oscillations have been hypothesized to be a source of noise artifacts seen on ECGs in patients with LVADs. 8 In this study, we sought to confirm that the LVAD impeller rotational speed corresponded to the dominant noise frequency on the 12‐lead ECG and to demonstrate how adjustments in filtering may help ameliorate this interference.
Methods
Study Population
Patients with an implanted LVAD and an ECG performed at Duke University Medical Center between January 2019 and February 2020 were considered for analysis. One patient was identified in whom ECGs were obtained 2 days before and directly following their LVAD implantation allowing for direct comparison of pre‐ and post‐LVAD ECGs. Additionally, for each currently available LVAD model type (HeartMate 3, HeartMate II, and HeartWare), 3 representative patients were chosen, with their LVADs set at different impeller rotational speeds. Demographic information including age, sex, height, and weight, as well as LVAD information (speed and implantation date), were abstracted from the patients' electronic medical records. The study was determined exempt by Duke University's institutional review board and no informed consent was required. The authors had full access to the data and the first author (Z.L.) attests to the integrity of the data analysis. Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the corresponding author at zak.loring@duke.edu.
ECG Analysis
The ECGs for each of the included patients were first evaluated using the default HPF (0.04 Hz), LPF (150 Hz), and notch filters (60 Hz) in the MUSE Cardiology Information System version 8.0.2.10132 with 12SL analysis software version 241 (General Electric Healthcare). Waveform data from each of the 8 independent ECG leads (I, II, V1, V2, V3, V4, V5, and V6 [leads III, aVR, aVL, and aVF are derived from these primary data]) were extracted from the ECG XML file as a time series (millivolts per 0.002‐second interval). A fast Fourier transform was applied to this time series data to display the component frequencies that comprise each lead's waveform data as a spectral profile using R (R Core Team, 2014) and the spectral package (Seilmayer, 2019; https://CRAN.R-project.org/package=spectral). Observed peaks on an individual's spectral profile occurring above the typical frequencies for the major ECG waves (P waves 0.67–5 Hz, QRS complexes 10–50 Hz, and T waves 1–7 Hz) were hypothesized to be coming from the LVAD. For each patient, the frequency of the observed peak was compared with the impeller rotational speed. The LPF was adjusted in the MUSE system to exclude the high‐frequency peak, and the filtered and unfiltered ECGs were compared.
Proposed Bandstop Filter
Waveform data from example ECGs from one of the representative patients from each LVAD model type (HeartWare, HeartMate 3, and HeartMate II) were evaluated as described above for high‐frequency spectral peaks thought to be coming from the LVAD. The waveform data were then imported into MATLAB (version R2019b; The MathWorks, Inc.) to facilitate application of additional postprocessing filters. A second order Butterworth infinite impulse response bandstop filter 9 was applied that excluded frequencies around the hypothesized LVAD principal oscillation frequency. Additionally, a second order Butterworth infinite impulse response LPF was constructed with a cutoff frequency of 40 Hz and applied to the ECG data. The filtered ECG was then displayed and reevaluated.
Results
ECG Before and After LVAD Implantation
The pre‐LVAD ECG for one of the patients is displayed in Figure 1A. The patient was a 73‐year‐old man with end‐stage ischemic cardiomyopathy admitted for planned LVAD implantation (Table 1, patient 1). His admission ECG demonstrated an atriobiventricular paced rhythm with ventricular premature complexes and minimal artifact. The spectral profile of this ECG demonstrated that the majority of the signal data were composed of frequencies <40 Hz (Figure 1B).
Figure 1. Pre– and post–left ventricular assist device (LVAD) ECGs.
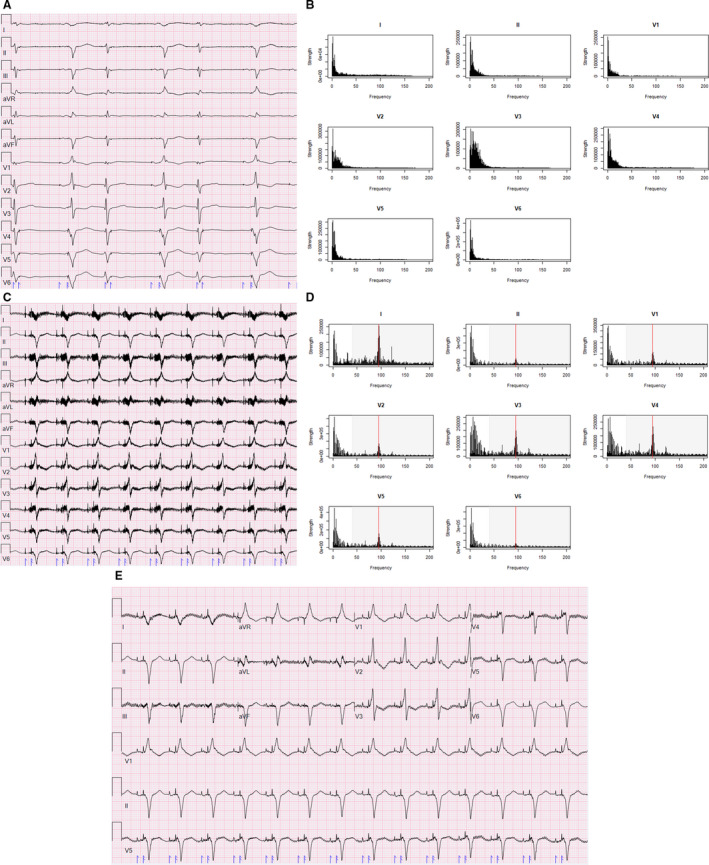
A, ECG of a patient before LVAD implantation demonstrating atriobiventricular pacing with ventricular premature complexes and minimal artifact. B, Spectral profile from this patient's pre‐LVAD ECG demonstrating frequency vs strength. C, ECG of the same patient after HeartMate 3 LVAD implantation demonstrating substantial electromagnetic interference artifact. D, Spectral profile of Post‐LVAD ECG with red line indicating LVAD rotational speed (5700 rpm/95 Hz). E, Post‐LVAD ECG with low‐pass filter reduced to 40 Hz (shaded signals on [D] excluded).
Table 1.
Age, Sex, and LVAD Settings for Included Patients
| Patient No. | Age, y | Sex | LVAD | Speed, rpm |
|---|---|---|---|---|
| 1 | 73 | Male | HeartMate 3 | 5700 |
| 2 | 66 | Male | HeartMate 3 | 5200 |
| 3 | 46 | Male | HeartMate 3 | 6200 |
| 4 | 51 | Male | HeartMate II | 9600 |
| 5 | 70 | Male | HeartMate II | 9800 |
| 6 | 65 | Male | HeartMate II | 9600 |
| 7 | 55 | Male | HeartWare | 2780 |
| 8 | 18 | Male | HeartWare | 2500 |
| 9 | 68 | Male | HeartWare | 3000 |
LVAD indicates left ventricular assist device.
Two days following admission, the patient underwent implantation of a HeartMate 3 LVAD set to 5700 rpm. A repeat ECG (Figure 1C) demonstrated substantial EMI artifact impeding accurate assessment of waveform durations and identification of an isoelectric baseline. The spectral profile analysis demonstrated a substantial peak at 95 Hz (a frequency equivalent to the set speed of 5700 rpm) on all recorded leads, but most prominent in lead I, V3, and V4 (which, in addition to the leads derived from I, demonstrate the most artifact on the displayed ECG) (Figure 1D). Reducing the LPF to 40 (above the threshold where the majority of the pre‐LVAD ECG signal was, below the observed peak frequency at 95 Hz, shaded region on Figure 1D) resulted in an ECG with less artifact, more easily discernable waveform onsets/offsets, and a more clear isoelectric baseline (Figure 1E).
HeartMate 3 ECGs
Three patients with HeartMate 3 LVADs set to different rotational speeds were included (Table 1, patients 1–3). Figure 2A demonstrates the ECG and associated spectral profile of a 66‐year‐old man with ischemic cardiomyopathy with a HeartMate 3 LVAD running at 5200 rpm seen for routine follow‐up in an LVAD clinic. The ECG displayed on the left was recorded using the default HPF (0.16 Hz), LPF (150 Hz), and notch filter (60 Hz). Similar to the prior patient, the spectral profile exhibited a spectral peak identical to the LVAD impeller rotational speed (5200 rpm or 86.7 Hz, red line). HeartMate 3 devices also employ a software algorithm to generate an “artificial pulse” by accelerating and decelerating the pump speed by 2000 rpm once every 2 seconds in order to reduce stasis and thrombus formation in the pump. 10 This corresponds to rotational speeds of 3200 rpm and 7200 rpm in this patient (53.3 Hz and 120 Hz, respectively). Minor peaks near these frequencies are visible on the spectral profile (green lines). Reducing the LPF to 40 Hz (a value >10 Hz below the principal and minor oscillation frequencies of this patient's LVAD) eliminated the majority of the electrical artifact (right panel). This normalized the isoelectric baseline and supported the clinical interpretation of atrial fibrillation on the ECG (Figure 2A).
Figure 2. ECGs and spectral profiles of all left ventricular assist device (LVAD) models.
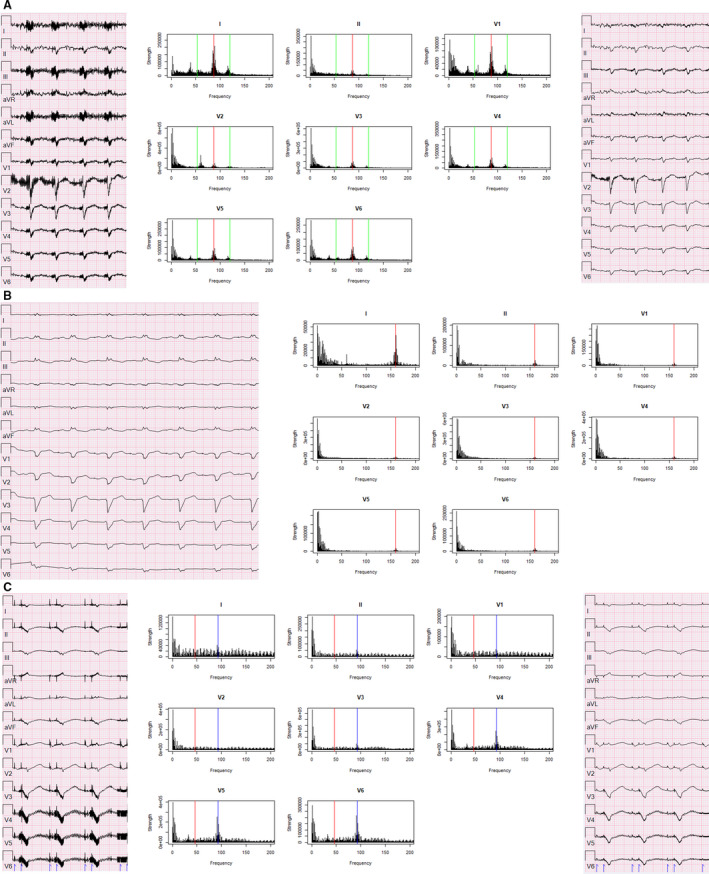
ECG (left) spectral profiles (middle) and ECGs with low‐pass filter adjusted to 10 Hz below rotational speed (right) from patients with LVADs of different models and rotational speeds. Set rotational speed marked with a red line on spectral profile, green line is ±2000 rpm for HeartMate 3 (artificial pulse), and blue line is double the set rotational speed for HeartWare. A, HeartMate 3 set at 5200 rpm (86.7 Hz, green lines at 53.4 Hz, 120 Hz). B, HeartMate II set at 9600 rpm (160 Hz). C, HeartWare set at 2780 rpm (46.3 Hz, blue line=92.6 Hz).
Similar patterns were seen in all 3 patients with HeartMate 3 LVADs with a prominent spectral peak at the patient's specific principal oscillation frequency and minor peaks near the frequencies corresponding to the “artificial pulse” (Figure S1). Reducing the LPF to 40 Hz reduced the noise from the electrical artifact and improved the legibility of all of the ECGs.
HeartMate II ECGs
There were 3 patients with HeartMate II LVADs included in this study, also with different set rotational speeds (Table 1, patients 4–6). Figure 2B shows the ECG and associated spectral profile of a 51‐year‐old man with a HeartMate II LVAD running at 9600 rpm who was admitted to the hospital with a driveline infection. The ECG on the left was recorded using default filtering settings (HPF=0.16 Hz, LPF=150 Hz, notch=60 Hz) and demonstrates an atrial paced rhythm with complete left bundle branch block. Analysis of the waveform spectral profile demonstrated a peak frequency identical to the LVAD's rotational speed (9600 rpm or 160 Hz, red line). However, unlike the HeartMate 3 device, the faster rotational speed of the HeartMate II places this peak frequency above the default LPF of the ECG display. As such, the unfiltered ECG demonstrates little to no electrical artifact that impedes its interpretation.
The typical rotational speeds of HeartMate II devices range from 8800 to 10 000 rpm, corresponding to frequencies of 146.7 to 166.7 Hz. These frequencies are often above the default LPF settings of ECG display systems and thus do not generate substantial electrical artifact. In all 3 HeartMate II patients included in this study, the principal oscillating frequency exceeded the LPF and the ECGs were largely free of electrical artifact (Figure S2).
HeartWare ECGs
Three patients with HeartWare LVADs were also included in this study (Table 1, patients 7–9). Figure 2C shows the ECG and spectral profile of a 55‐year‐old man with a HeartWare device set at 2780 rpm admitted to the hospital for presyncope caused by recurrent ventricular tachycardia. The ECG on the left was recorded with default filtering settings (HPF=0.16 Hz, LPF=150 Hz, notch=60 Hz) and demonstrates an atrioventricular paced rhythm. The hypothesized spectral peak for this patient was 46.3 Hz (red line); however, the most prominent peak was observed at a value double this principal oscillating frequency (92.7 Hz, blue line). Additionally, the spectral profile demonstrated low amplitude spikes across the entire frequency range. Adjusting the LPF to 40 Hz (a value >10 Hz below the observed peak but very near the principal oscillation frequency) resulted in a clearer ECG with much of the electrical artifact removed (right panel).
This observation of the most prominent spectral peak occurring at twice the principal oscillation frequency was consistent across all 3 patients with HeartWare devices (Figure S3). While the HeartWare device does not employ the same “artificial pulse” algorithm used in the HeartMate 3 device, it does exhibit intermittent pulsatility by using the Lavare cycle. 11 The pump accelerates and decelerates by 200 rpm across a 3‐second interval every minute. The infrequency of this change in rotational speed as well as the small magnitude of change (200 rpm=3.3 Hz) makes it unlikely that this algorithm would generate additional frequency peaks on the spectral profile from a 10‐second ECG. Indeed, there did not appear to be other distinct frequency peaks on the spectral profiles of the 3 patients with HeartWare.
Bandstop Filtering
While adjusting the LPF on patients with LVADs can facilitate rhythm identification, this comes with the tradeoff of discarding high‐frequency components, which may also carry clinical value. The typical frequencies for the major ECG waves are on the lower end of the bandwidth; however, high‐frequency components do contribute to the QRS morphology, particularly in patients with pathology such as scar or hypertrophy. 12 Additionally pacemaker spikes are high‐frequency signals that are better visualized when high‐frequency components are included in the ECG display. 13 The addition of an adjustable bandstop style filter for patients with LVADs that excludes a band of frequencies around the principal oscillation frequency causing the EMI allows for a more selective, speed‐specific exclusion of LVAD‐related interference with preservation of the high‐frequency components of the ECG.
An example of this filtering is shown in Figure 3. Figure 3A shows an unfiltered ECG of a patient with a HeartMate 3 LVAD running at 5700 rpm, as well as the spectral profile demonstrating the principal oscillation frequency and minor peaks at the “artificial pulse” frequencies. Figure 3B depicts the ECG after applying a bandstop filter for frequencies between 85 to 105 Hz (95 Hz principal oscillation frequency ±10 Hz), which reduces the electrical artifact and improves the clarity of the ECG. Applying a wider bandstop filter for frequencies 0.67 Hz to 50 Hz (Figure 3C) that excludes both the principal oscillation frequency peak and the minor peaks from the artificial pulse generates an ECG with even less artifact. Figure 3D depicts the same ECG with a 40‐Hz LPF applied. While this ECG does demonstrate little electrical artifact, the high‐frequency pacemaker spikes are less well visualized than on the bandstop‐filtered ECG in Figure 3C. A similar pattern was seen when these filters were applied to a patient with a HeartWare LVAD (Figure S4). A summary of the effect of each of the LVAD model–specific artifacts and effects of filtering are shown in Table 2.
Figure 3. Filtering solutions to left ventricular assist device (LVAD) artifact in HeartMate 3.
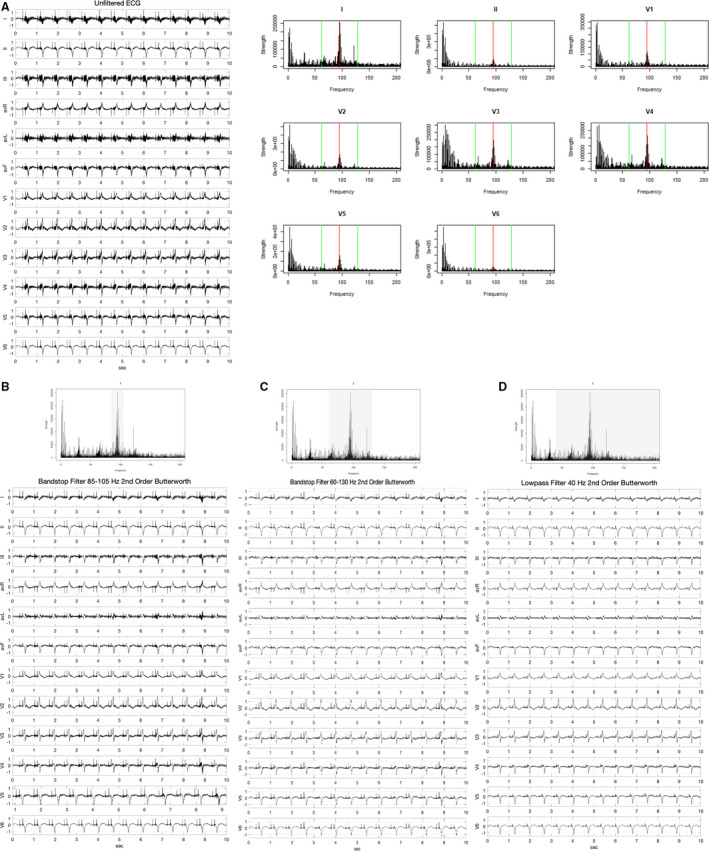
A, ECG and spectral profile of a patient with a HeartMate 3 LVAD with red line indicating LVAD rotational speed (5700 rpm/95 Hz) and green lines indicating artificial pulse frequencies (61.7 and 128.3 Hz). B, ECG after signal filtered through an 85 to 105 Hz bandstop filter. C, ECG after signal filtered through a 65 to 125 Hz bandstop filter. D, ECG after signal filtered through a 40 Hz low‐pass filter.
Table 2.
Summary of LVAD Model‐Specific Artifacts and Filtering Solutions
| LVAD Model | Typical Impeller Rotational Speed, rpm | Principal EMI Frequency, Hz | Additional EMI Frequencies | Effect of LPF | Effect of Bandstop Filter |
|---|---|---|---|---|---|
| HeartWare | 2400–3200 | 40–53.3 | ±33.3 Hz | Excludes high‐frequency EMI when at least 10 Hz below the lowest EMI frequencies | Selectively excludes EMI from LVAD while preserving high‐frequency signals |
| HeartMate 3 | 5000–6000 | 83.3–100 | +2x Principal frequency | Excludes high‐frequency EMI when at least 10 Hz below principal EMI frequency | Selectively excludes EMI from LVAD while preserving high‐frequency signals |
| HeartMate II | 8800–10 000 | 146.7–166.7 | None | No effect | No effect |
EMI indicates electromagnetic interference; LPF, low‐pass filter; and LVAD, left ventricular assist device.
Discussion
In this study we describe the effect of continuous‐flow LVADs on 12‐lead ECGs, identify the mechanistic basis for the observed artifacts, and propose filtering solutions that ameliorate interference to improve clinical interpretation of ECGs in patients with LVADs. By identifying the principal frequency related to the mechanical oscillation of the LVADs, one can employ speed‐specific filtering techniques to remove nonphysiologic EMI while preserving physiologic body surface potentials. Improving the clarity of ECGs in patients with LVADs may aid in their clinical interpretation and improve the diagnostic yield of this test. These findings also have important clinical implications for S‐ICD sensing and programming in patients with LVADs.
There has been a steady increase in the use of LVADs in the United States. 14 , 15 The American Heart Association issued a statement in 2019 that raised the issue of LVADs causing high‐frequency noise on the surface ECG that can be improved by adjustment of the LPF; however, they acknowledge that this comes at a cost of losing some fractionation of amplitude of the QRS signal. 2 This study highlights that the particular wavelength of the high‐frequency noise generated by the LVAD can be deduced from the readily available rotational speed, and speed‐specific filtering can be applied to remove the interference without compromising high‐frequency signal components. This more tailored approach to filtering allows clinicians to improve the signal‐to‐noise ratio of their ECG recordings without unnecessarily discarding clinically important signal data.
Each of the 3 most commonly implanted continuous‐flow LVAD models demonstrated unique signal profile characteristics that appear to result from their designs. The typical rotational speeds of HeartMate 3 LVADs (5000–6000 rpm, 83.3–100 Hz) create principal oscillating frequencies in the middle of the typical frequency spectrum of ECGs (0.04–150 Hz). Additionally, the device's “artificial pulse” creates 2 additional minor frequency peaks in this spectrum. In this study, the ECGs from these patients demonstrated the largest amount of electrical artifact. Since publication of MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3), 16 HeartMate 3 devices have become an increasingly popular choice for LVAD implantation, highlighting the clinical importance of this interference. 17 The proposed bandstop filter significantly reduces the interference generated by HeartMate 3 LVADs while preserving high‐frequency signal components. Adjustment of the LPF is a simple alternative solution that comes at a cost of high‐frequency signal components, but can be easily performed at the time of ECG acquisition or during postprocessing in most ECG reading software. Of note, neither filtering technique significantly distorts the morphology of the P, QRS, or T waves. These waveforms fall on the lower end of the ECG bandwidth (P waves 0.67–5 Hz, QRS complexes 10–50 Hz, and T waves 1–7 Hz); however, high‐frequency components do contribute to the QRS morphology particularly in patients with pathologies such as scar or hypertrophy, which may be excluded by application of LPFs. 12
The high rotational speed of HeartMate II devices (8800–10 000 rpm, 146.7–166.7 Hz) place the principal oscillating frequency above the typical ECG frequency spectrum. Consequently, in this study, none of the ECGs from these patients demonstrated significant electrical artifact when displayed with the default filtering settings (HPF=0.16 Hz, LPF=150 Hz, notch=60 Hz). The spectral analysis of the raw ECG waveforms in these patients did demonstrate a clear peak at the principal oscillation frequency, illustrating that these devices similarly create electrical interference signals at the set rotational speed despite their markedly different design (use of an axial pump, mechanical bearings, and extrathoracic implantation location compared with the HeartMate 3's centrifugal pump, magnetically levitated bearings, and intrapericardial implantation location). 18 However, while the typical speed ranges make this interference largely inconsequential, these results lend corroborating insight into the mechanistic basis of the EMI observed on traditional 12‐lead ECGs.
The HeartWare devices were unique in that the principal frequency peak observed was approximately double the set rotational speed of their LVAD. Like the HeartMate 3, the HeartWare is a magnetically levitated, centrifugal pump implanted in the pericardium. 19 A unique aspect of the HeartWare device is the inclusion of the Lavare cycle, which accelerates and decelerates the pump speed by 200 rpm across a 3‐second interval every minute. 11 This infrequent and minor (3.3 Hz) oscillation does not substantially impact the spectral profile of ECGs in these patients. One notable difference between the HeartWare and HeartMate devices is the design of the brushless motor. Both types of pumps utilize 3 sets of electromagnetic coils arranged around the impeller and, by alternating the current direction through these coils to switch the polarity of the magnetic fields, they exert torque on the impeller (which is imbedded with permanent magnets). While some pumps (such as the HeartMate II) have permanent magnets with 2 poles imbedded in the impeller, the impeller in the HeartWare device is imbedded with permanent magnets with 4 poles. 20 For each rotation of an impeller with a 4‐pole magnet, the magnetic field changes twice as frequently as in an impeller with a 2‐pole magnet. This changing magnetic field at twice the frequency of impeller rotation may be the source of artifact seen in HeartWare devices. Further work is needed to delineate which design features of the HeartWare LVAD lead to this consistent pattern. Nonetheless, adjusting the LPF to below the principal frequency peak or applying a “bandstop” filter results in an ECG with less electrical artifact that is easier to interpret.
An increasing number of patients who undergo LVAD implant have a preexisting S‐ICD. Because the primary and secondary sensing vectors of the S‐ICD encompass the LVAD, numerous cases of inappropriate S‐ICD shocks caused by EMI have been reported. 21 This has been more frequently observed in the HeartWare and HeartMate 3 than in the HeartMate II, likely because of the high rotational speed and intraperitoneal location of the HeartMate II. 22 The second‐generation S‐ICD incorporated an additional 9‐Hz HPF (SMART Pass filter, Boston Scientific) to reduce inappropriate therapy caused by oversensing of low‐frequency T waves. However, no specific programming exists to prevent shocks caused by EMI. Modification of the built‐in S‐ICD notch filter from 60 Hz (US settings) to 50 Hz (UK settings) was used to prevent inappropriate therapy attributable to EMI in a patient with the S‐ICD and a HeartWare set at 3000 rpm and resulting dominant frequency of 50 Hz. 23 We believe that our findings suggest that incorporation of programmable bandstop filters and/or LPFs into the S‐ICD may be applied to patients with LVADs that operate at a broader range of frequencies and may help reduce the incidence of inappropriate therapy in the growing population of patients with an LVAD and S‐ICD.
Limitations
This study has several important limitations. Only 9 patients were included in this analysis; however, the patterns reported were consistent within each LVAD manufacturer group. The use of bandstop filters and LPFs reduced electrical artifact in all included patients, but it did not completely eliminate it. The mechanical nature of the LVAD pump creates reverberation at frequencies above and below the principal oscillation frequency, which hampers precise exclusion of all interference. The use of these lower order filters improves the legibility of the ECG to aid clinical interpretation; however, in some cases, residual artifact may still preclude accurate reading. Future application of more sophisticated digital filter designs may be able to further ameliorate LVAD‐associated EMI while preserving physiologically important frequency components. This was a retrospective review of clinically obtained ECGs, which may have been obtained in suboptimal recording environments; however, these ECGs are representative of tracings obtained during routine clinical care of patients with LVADs.
Conclusions
ContinuouS‐flow LVADs generate electrical artifacts on 12‐lead ECGs that are fundamentally related to their set impeller rotational speed. Adjusting filtering parameters such as the LPF or the addition of a bandstop filter can exclude many of the signals responsible for this artifact and improve the clarity and legibility of the ECG. Application of this technology to the ECG at the time of acquisition or during postprocessing can improve the signal‐to‐noise ratio, may improve the diagnostic yield of this test in patients with LVADs, and has important implications for patients with S‐ICDs.
Sources of Funding
This work was supported in part by a National Institutes of Health (NIH) T32 training grant (#5T32HL069749).
Disclosures
Dr Loring is supported in part by an NIH T32 training grant (#5T32HL069749), receives grant support for clinical research from Boston Scientific, and serves as a consultant for Huxley Medical Inc. Dr Sen has no disclosures. Dr Black‐Maier has no disclosures. Dr Atwater receives grants for clinical research from Abbott and Boston Scientific and serves as a consultant to Abbott, Biotronik, Boston Scientific, Medtronic, and Siemens. Dr Russell serves as a consultant for Medtronic and is on the Data Safety Monitoring Board for an Abbott clinical trial. Dr DeVore reports research funding through his institution from the American Heart Association; Amgen; AstraZeneca; Bayer; Intra‐Cellular Therapies; American Regent, Inc; the National Heart, Lung, and Blood Institute (NHLBI); Novartis; and PCORI. He also provides consulting services for Amgen, AstraZeneca, Bayer, CareDx, InnaMed, LivaNova, Mardil Medical, Novartis, Procyrion, scPharmaceuticals, and Zoll. He has also received nonfinancial support from Abbott for educational activities. Dr Piccini is supported by R01HL128595 from the NHLBI and receives grants for clinical research from Abbott, the Association for the Advancement of Medical Instrumentation, the American Heart Association, Bayer, Boston Scientific, Gilead, Janssen Pharmaceuticals, and the NHLBI. He also serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, LivaNova, Medtronic, Milestone, Sanofi, Philips, and Up‐to‐Date.
Supporting information
Figures S1–S4
(J Am Heart Assoc. 2020;9:e017563 DOI: 10.1161/JAHA.120.017563.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Hickey KT, Garan H, Mancini DM, Colombo PC, Naka Y, Sciacca RR, Abrams MP, Solove M, Zeoli N, Flannery M, et al. Atrial fibrillation in patients with left ventricular assist devices: incidence, predictors, and clinical outcomes. JACC Clin Electrophysiol. 2016;2:793–798. [DOI] [PubMed] [Google Scholar]
- 2. Gopinathannair R, Cornwell WK, Dukes JW, Ellis CR, Hickey KT, Joglar JA, Pagani FD, Roukoz H, Slaughter MS, Patton KK. Device therapy and arrhythmia management in left ventricular assist device recipients: a scientific statement from the American Heart Association. Circulation. 2019;139:e967–e989. [DOI] [PubMed] [Google Scholar]
- 3. Martinez SC, Fansler D, Lau J, Novak EL, Joseph SM, Kleiger RE. Characteristics of the electrocardiogram in patients with continuouS‐flow left ventricular assist devices. Ann Noninvasive Electrocardiol. 2015;20:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zormpas C, Mueller-Leisse J, Koenig T, Schmitto JD, Veltmann C, Duncker D. Electrocardiographic changes after implantation of a left ventricular assist device—potential implications for subcutaneous defibrillator therapy. J Electrocardiol. 2019;52:29–34. [DOI] [PubMed] [Google Scholar]
- 5. Miller MA, Dukkipati SR, Koruth JS, d'Avila A, Reddy VY. How to perform ventricular tachycardia ablation with a percutaneous left ventricular assist device. Heart Rhythm. 2012;9:1168–1176. [DOI] [PubMed] [Google Scholar]
- 6. Yalcin YC, Kooij C, Theuns D, Constantinescu AA, Brugts JJ, Manintveld OC, Yap SC, Szili-Torok T, Bogers A, Caliskan K. Emerging electromagnetic interferences between implantable cardioverter-defibrillators and left ventricular assist devices. Europace. 2020;22:584–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Venkatachalam KL, Herbrandson JE, Asirvatham SJ. Signals and signal processing for the electrophysiologist. Circ Arrhythm Electrophysiol. 2011;4:965–973. [DOI] [PubMed] [Google Scholar]
- 8. Schettle S, Kassi M, Asleh R, Pereira N, Maltais S, Stulak J, Boilson B. LVAD ECG artifact reflecting HeartWare pump speed. J Am Coll Cardiol. 2018;71:A816. [Google Scholar]
- 9. Butterworth S. On the theory of filter amplifiers. Wireless Engineer. 1930;7:536–541. [Google Scholar]
- 10. Schmitto JD, Hanke JS, Rojas SV, Avsar M, Haverich A. First implantation in man of a new magnetically levitated left ventricular assist device (HeartMate III). J Heart Lung Transplant. 2015;34:858–860. [DOI] [PubMed] [Google Scholar]
- 11. Kumar J, Elhassan A, Dimitrova G, Essandoh M. The Lavare Cycle: a novel pulsatile feature of the HVAD continuouS‐flow left ventricular assist device. J Cardiothorac Vasc Anesth. 2019;33:1170–1171. [DOI] [PubMed] [Google Scholar]
- 12. Tereshchenko LG, Josephson ME. Frequency content and characteristics of ventricular conduction. J Electrocardiol. 2015;48:933–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun J, Lu QF, Zhao Y, Zhang PP, Wang J, Wang QS, Liu XH, Li YG. A low-pass filter of 300 Hz improved the detection of pacemaker spike on remote and bedside electrocardiogram. Chin Med J (Engl). 2019;132:534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Briasoulis A, Inampudi C, Akintoye E, Adegbala O, Alvarez P, Bhama J. Trends in utilization, mortality, major complications, and cost after left ventricular assist device implantation in the United States (2009 to 2014). Am J Cardiol. 2018;121:1214–1218. [DOI] [PubMed] [Google Scholar]
- 15. Kormos RL, Cowger J, Pagani FD, Teuteberg JJ, Goldstein DJ, Jacobs JP, Higgins RS, Stevenson LW, Stehlik J, Atluri P, et al. The Society of Thoracic Surgeons Intermacs database annual report: evolving indications, outcomes, and scientific partnerships. J Heart Lung Transplant. 2019;38:114–126. [DOI] [PubMed] [Google Scholar]
- 16. Mehra MR, Uriel N, Naka Y, Cleveland JC, Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, et al. A fully magnetically levitated left ventricular assist device—final report. N Engl J Med. 2019;380:1618–1627. [DOI] [PubMed] [Google Scholar]
- 17. Barac YD, Wojnarski CM, Junpaparp P, Jawitz OK, Billard H, Daneshmand MA, Agrawal R, Devore A, Patel CB, Schroder JN, et al. Early outcomes with durable left ventricular assist device replacement using the HeartMate 3. J Thorac Cardiovasc Surg. 2020;160:132–139.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heatley G, Sood P, Goldstein D, Uriel N, Cleveland J, Middlebrook D, Mehra MR. Clinical trial design and rationale of the Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3 (MOMENTUM 3) investigational device exemption clinical study protocol. J Heart Lung Transplant. 2016;35:528–536. [DOI] [PubMed] [Google Scholar]
- 19. Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, Boyce SW, Najjar SS, Jeevanandam V, Anderson AS, et al. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med. 2017;376:451–460. [DOI] [PubMed] [Google Scholar]
- 20. Asgari SS, Bonde P. Implantable physiologic controller for left ventricular assist devices with telemetry capability. J Thorac Cardiovasc Surg. 2014;147:192–202. [DOI] [PubMed] [Google Scholar]
- 21. Lopez-Gil M, Fontenla A, Delgado JF, Rodriguez-Munoz D. Subcutaneous implantable cardioverter defibrillators in patients with left ventricular assist devices: case report and comprehensive review. Eur Heart J Case Rep. 2019;3:ytz057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raman AS, Shabari FR, Kar B, Loyalka P, Hariharan R. No electromagnetic interference occurred in a patient with a HeartMate II left ventricular assist system and a subcutaneous implantable cardioverter-defibrillator. Tex Heart Inst J. 2016;43:183–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahmed AS, Patel PJ, Bagga S, Gilge JL, Schleeter T, Lakhani BA, Ravichandran AK, Donnelley S, Allavatam V, Prystowsky EN, et al. Troubleshooting electromagnetic interference in a patient with centrifugal flow left ventricular assist device and subcutaneous implantable cardioverter defibrillator. J Cardiovasc Electrophysiol. 2018;29:477–481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S4


