Abstract
Background
Current noninvasive modalities to diagnose coronary artery disease (CAD) have several limitations. We sought to derive and externally validate a hs‐cTn (high‐sensitivity cardiac troponin)–based proteomic model to diagnose obstructive coronary artery disease.
Methods and Results
In a derivation cohort of 636 patients referred for coronary angiography, predictors of ≥70% coronary stenosis were identified from 6 clinical variables and 109 biomarkers. The final model was first internally validated on a separate cohort (n=275) and then externally validated on a cohort of 241 patients presenting to the ED with suspected acute myocardial infarction where ≥50% coronary stenosis was considered significant. The resulting model consisted of 3 clinical variables (male sex, age, and previous percutaneous coronary intervention) and 3 biomarkers (hs‐cTnI [high‐sensitivity cardiac troponin I], adiponectin, and kidney injury molecule‐1). In the internal validation cohort, the model yielded an area under the receiver operating characteristic curve of 0.85 for coronary stenosis ≥70% (P<0.001). At the optimal cutoff, we observed 80% sensitivity, 71% specificity, a positive predictive value of 83%, and negative predictive value of 66% for ≥70% stenosis. Partitioning the score result into 5 levels resulted in a positive predictive value of 97% and a negative predictive value of 89% at the highest and lowest levels, respectively. In the external validation cohort, the score performed similarly well. Notably, in patients who had myocardial infarction neither ruled in nor ruled out via hs‐cTnI testing (“indeterminate zone,” n=65), the score had an area under the receiver operating characteristic curve of 0.88 (P<0.001).
Conclusions
A model including hs‐cTnI can predict the presence of obstructive coronary artery disease with high accuracy including in those with indeterminate hs‐cTnI concentrations.
Keywords: high‐sensitivity cardiac troponin, obstructive coronary artery disease, proteomic model
Subject Categories: Biomarkers
Nonstandard Abbreviations and Acronyms
- AUC
area under the receiver operating characteristic curve
- BACC
Biomarkers in Acute Cardiac Care
- CAD
coronary artery disease
- CASABLANCA
Catheter Sampled Blood Archive in Cardiovascular Diseases
- CT
computed tomography
- ED
emergency department
- hs‐cTn
high‐sensitivity cardiac troponin
- ISCHEMIA
International Study of Comparative Health Effectiveness with Medical and Invasive Approaches
- KIM‐1
kidney injury molecule‐1
- MI
myocardial infarction
- NPV
negative predictive value
- PPV
positive predictive value
Clinical Perspective
What Is New?
A model inclusive of 3 clinical variables (male sex, age, and previous percutaneous coronary intervention) and 3 biomarkers (hs‐cTn [high‐sensitivity cardiac troponin], adiponectin, and kidney injury molecule‐1) can accurately predict the presence of obstructive coronary artery disease.
The model predicted obstructive coronary artery disease in a diverse population of patients undergoing coronary angiography for both acute and elective indications.
What Are the Clinical Implications?
A clinical/biomarker model inclusive of hs‐cTn may be useful for the evaluation of acute chest pain in the emergency department (including patients who had acute myocardial infarction neither ruled in nor ruled out) and in outpatients presenting for evaluation of stable angina including those with renal injury.
The prevalence of atherosclerotic coronary artery disease (CAD) remains high. In the United States, ≈7 million patients visit the emergency department (ED) each year for chest pain 1 ; almost half will be found to have noncardiac causes. 2 Furthermore, the burden of chest pain is not limited to the ED, with 9.4 million outpatient adults in the United States experiencing angina pectoris. 3
In symptomatic patients, the standard evaluation of chest pain is evolving. In the ED, the introduction of hs‐cTn (high‐sensitivity cardiac troponin) assays has facilitated earlier detection of myocardial injury while also facilitating safe discharge of a large proportion of patients. 4 , 5 Yet, approximately one third of patients presenting to the ED will have myocardial infarction (MI) neither ruled in nor ruled out using hs‐cTn–based accelerated diagnostic pathways. These patients in the “indeterminate zone” frequently require further evaluation with noninvasive testing for risk stratification. 6 Unfortunately, current noninvasive tests to evaluate for CAD are not without drawbacks, including cost, limited availability in off hours, and need for contrast or ionizing radiation.
In the outpatient setting, management strategies for obstructive CAD are changing. ISCHEMIA (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches) recently found that an invasive management strategy did not improve a composite end point of acute MI, cardiovascular death, hospitalization for unstable angina or heart failure, or cardiac arrest when added to optimal medical therapy in patients with stable CAD and moderate or severe ischemia. 7 On the other hand, earlier and more confident recognition of CAD in asymptomatic patients would improve application of secondary prevention therapies. 7 Thus, a noninvasive approach keyed to identify obstructive CAD across all venues where chest pain is evaluated might improve diagnostic accuracy, triage throughput, and secondary prevention.
A novel noninvasive approach to predicting obstructive CAD is the use of biomarkers in combination with clinical variables. Using proteomics and artificial intelligence, we previously described a clinical/proteomic biomarker panel to predict prevalent obstructive CAD in patients undergoing diagnostic coronary angiography. 8 However, hs‐cTn, which has been associated with the presence of CAD, was not evaluated in the model. 9 , 10 As hs‐cTn is frequently measured in symptomatic patients, it was logical to derive and validate a newer biomarker panel inclusive of hs‐cTn for the diagnosis of obstructive CAD.
Methods
All study procedures were approved by Partners HealthCare's institutional review board. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results.
Study Population: Derivation and Internal Validation Cohort
The clinical/proteomic panel was first derived using patients enrolled in the CASABLANCA (Catheter Sampled Blood Archive in Cardiovascular Diseases) study (ClinicalTrials.Gov NCT00842868). 11 The CASABLANCA study was a prospective, single‐center, investigator‐initiated, observational cohort study performed at the Massachusetts General Hospital in Boston, MA. In this study, 1251 patients undergoing coronary and/or peripheral angiography (with or without intervention) were enrolled between 2008 and 2011. Informed consent was provided by the participants. Patients were referred for these procedures for numerous reasons including acute MI, unstable angina pectoris, and heart failure, and for the elective diagnostic evaluation of stable chest pain. 11 For the patients included in the CASABLANCA study, 15 mL of blood was obtained immediately before angiography through a centrally placed vascular access sheath. 11
For the purpose of this analysis, 911 patients enrolled in the study who underwent coronary angiography were randomly divided into a derivation cohort of 649 patients (70%) and a separate internal validation cohort of 278 patients (30%). After excluding patients with missing data from the derivation cohort (n=13) and the interval validation cohort (n=3), the final CASABLANCA study cohorts were composed of 636 patients (derivation) and 275 patients (internal validation) (Figure S1).
For biomarker analysis, 200 μL of plasma was analyzed for a panel of 108 biomarkers reflecting acute phase reactants, inflammation, and atherosclerosis pathophysiology on a Luminex 100/200 xMAP technology platform (Luminex Corporation) (Table S1 and Table 1). This technology utilizes multiplexed, microsphere‐based assays in a single reaction vessel where the assay‐specific capture antibody on each microsphere binds to the protein of interest. hs‐cTnI (high‐sensitivity cardiac troponin I) was measured in the CASABLANCA study cohort using the Siemens Atellica hs‐cTn I assay (Siemens Healthcare GmbH). This assay has a limit of detection of 1.27 ng/L, a limit of quantification of 2.51 ng/L, and an upper reference limit 99th percentile of 34 ng/L in women and 53 ng/L in men. For this study, the assay had a between‐run coefficient of variation <10% (8.2% and 6.7%) at these 99th percentile values.
Table 1.
Baseline Characteristics of Patients in the Derivation Cohort With and Without Obstructive CAD
|
With Obstructive CAD (≥70% Stenosis) (n=421) |
Without Obstructive CAD (70% Stenosis) (n=215) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age, y (SD) | 67.4 (11.5) | 64.2 (11.7) | 0.001 |
| Men, No. (%) | 330 (78.4) | 125 (58.1) | <0.001 |
| Body mass index (SD) | 29.2 (5.4) | 28.8 (6.3) | 0.41 |
| Medical history, No. (%) | |||
| Hypertension | 331 (78.6) | 143 (66.5) | 0.001 |
| Type 1/type 2 diabetes mellitus | 132 (31.4) | 32 (14.9) | <0.001 |
| Previous coronary percutaneous intervention | 180 (42.8) | 6 (2.8) | <0.001 |
| Laboratory tests | |||
| Adiponectin, μg/mL | 3.4 (2.2–5.1) | 4.5 (2.9–7.2) | <0.001 |
| KIM‐1, ng/mL | 0.04 (0.01–0.07) | 0.03 (0.01–0.05) | <0.001 |
| hs‐cTnI, ng/L | 9.7 (4–35.7) | 5.9 (3–13.6) | <0.001 |
Values are expressed as mean and standard deviation unless otherwise indicated. CAD indicates coronary artery disease; hs‐cTnI, high‐sensitivity cardiac troponin I; and KIM‐1; kidney injury molecule‐1.
External Validation Cohort
The clinical/proteomic panel was externally validated in a cohort of patients enrolled in the BACC (Biomarkers in Acute Cardiac Care) study (ClinicalTrials.Gov NCT02355457). 12 The BACC study is a prospective observational study that enrolled patients presenting with acute chest pain in the ED of the University Medical Center Hamburg‐Eppendorf in Germany beginning in July 19, 2013. 12 Informed consent was provided by the participants. For the purpose of this analysis, the first 303 patients who underwent a coronary angiogram for evaluation of their symptoms were included in this cohort. After excluding those with ST‐segment–elevation MI (n=46) and those with missing protein concentrations (n=16), we identified 241 patients for inclusion in our analysis (Figure S1). Enrollees in the BACC study had blood drawn immediately on presentation to the ED with their symptoms as described. 12 In the BACC study validation cohort, the ARCHITECT i1000SR (Abbott Diagnostics) hs‐cTnI was used. The limit of detection for the assay is 1.2 ng/L and the 99th percentile is 34 ng/L for men and 16 ng/L for women.
Outcome: Obstructive CAD
In the derivation and internal validation cohort from the CASABLANCA study, “significant” obstructive coronary artery stenosis was defined as ≥70% luminal obstruction in at least 1 coronary vessel, determined by invasive coronary angiography. Study investigators adjudicating angiographic severity of CAD were blinded to results of all biomarker testing.
In the external validation BACC study cohort, obstructive CAD was recorded as a ≥50% luminal obstruction in at least 1 coronary artery. This was determined by invasive coronary angiography and adjudicated by the BACC study investigators.
Statistical Analysis
Baseline characteristics were compared in the CASABLANCA study derivation cohort between those with and without ≥70% coronary stenosis in at least 1 major coronary artery. The dichotomous variables were compared using 2‐sided Fisher exact tests, and continuous variables were compared using 2‐sided 2‐sample Student t tests. The biomarkers were tested with the Wilcoxon rank sum test because their concentrations were not normally distributed. For any biomarker result that was unmeasurable, a standard approach of imputing concentrations 50% below the limit of detection was used.
For this analysis, we utilized machine learning, a subset of artificial intelligence, to identify predictors of significant CAD. The concentration values for all proteins underwent the following transformations: (1) they were log‐transformed to achieve a normal distribution; (2) outliers were clipped at the value of 3 times the median absolute deviation; and (3) the values were rescaled to a distribution with zero mean and unit variance. The starting sets of variables consisted of all 109 proteins as well as 6 clinical factors (sex, age, body mass index, history of type 2 diabetes mellitus, history of hypertension, and prior percutaneous coronary intervention) in the CASABLANCA study data set. Candidate panels of proteins and clinical features were generated via least‐angle regression. 13 In this method, factors were included in the model one at a time, with their coefficients determined by their correlation with the outcome. This was repeated until all factors were included in the model, and the step at which the performance plateaued resulted in our initial panel of interest.
We then conducted backwards elimination, starting with this initial panel of interest and iteratively removing the factor that contributed the least, as determined by its P value. This process concluded when all features had P<0.05 for their contribution to the model, resulting in our final panel. All predictive analyses during backwards elimination were run using the least absolute shrinkage and selection operator with logistic regression, 14 via 400 iterations of Monte Carlo cross‐validation, predicting the outcome of obstructive CAD. Once our final panel was selected, we trained the final diagnostic model from the entire training set using least absolute shrinkage and selection operator with logistic regression, predicting the outcome of obstructive CAD from the final panel. We then assessed the discrimination and calibration of this model's contributing features with the Akaike and Bayesian information criteria, and the goodness of fit with Hosmer‐Lemeshow testing.
This final model was then evaluated to predict obstructive CAD in the internal validation cohort. We generated a receiver operating characteristic curve to calculate the area under the receiver operating characteristic curve (AUC), and, using a cutoff determined by the optimal Youden index with the training set, we calculated sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
In addition, the predictive score generated by the diagnostic model was linearly rescaled to the range of 0 to 10, to facilitate interpretation, and we stratified this score into a 5‐level risk score corresponding to multiple levels of CAD risk. The boundaries of these risk levels were determined from the model's performance in the training set, with level 5 (highest risk) being >95% PPV, level 4 being >90% PPV, level 1 (lowest risk) being >90% NPV, and level 2 being >80% NPV. PPVs were calculated for the 2 high‐risk levels, and NPVs were calculated for the 2 low‐risk levels.
Since computed tomography (CT) coronary angiography is contraindicated in patients with severe renal failure, in a subgroup analysis, we sought to determine how this model performed on patients with renal injury. Our criterion for renal injury was defined as creatinine ≥1.5 mg/dL, and since the number of patients meeting this criterion was small on the CASABLANCA study derivation and internal validation sets separately, we combined them both to get a larger population (n=122). In addition, we assessed the performance of the model in lower‐risk patients without acute MI.
We then assessed the performance of the model in the exertional validation cohort (BACC study patients): we calculated the model's AUC, sensitivity, specificity, PPV, and NPV at the same optimal cutoff. In subgroup analyses of the BACC study external validation cohort, we assessed the performance of the model among: (1) patients who had acute MI neither “ruled in” nor “ruled out” using the European Society of Cardiology 0/3‐hour hs‐cTnI–based accelerated diagnostic pathway (Table S2), 15 ie, indeterminate zone (n=65); (2) patients with renal injury (defined as a creatinine level ≥1.5 mg/dL); and (3) patients without acute MI.
All statistics were performed using R software, version 3.4 (R Foundation for Statistical Computing). P values were 2‐sided, with a value <0.05 considered significant.
Results
Patient Characteristics of the Derivation Cohort
Of the 636 patients included in the derivation cohort, 421 (66.2%) had obstructive CAD. Patients with obstructive CAD were older, more likely to be male, and had a higher prevalence of hypertension, prior percutaneous coronary intervention, and diabetes mellitus (Table 1). Patients with obstructive CAD had higher concentrations of kidney injury molecule‐1 (KIM‐1) and hs‐cTnI, and lower concentrations of adiponectin (Table 1).
Final Panel and Diagnostic Model
After the machine learning model‐building process, the final panel consisted of 3 clinical variables (age, sex, and prior percutaneous coronary intervention) and 3 biomarkers (adiponectin, KIM‐1, and hs‐cTnI). The final model trained on these features yielded an in‐sample AUC of 0.83 (95% CI, 0.80–0.87; P<0.001). Iterative model fitting performed on the derivation cohort (Table S3) shows that adding the individual biomarkers to the clinical variables improves the model's discrimination and calibration, as evidenced by the increasing AUC, the decreasing of the Akaike or Bayesian information criteria, and the goodness of fit in Hosmer‐Lemeshow testing. When compared with commonly used biomarkers in clinical practice such as C‐reactive protein, N‐terminal pro‐B‐type natriuretic peptide, and hs‐cTnI alone or in combination with the clinical variables, the model outperformed these variables (Table S4). With the data represented in the 0 to 10 scale, the optimal cutoff for the score was determined to be 2.97, using the optimal Youden index. This cutoff was also used with the CASABLANCA study internal validation and BACC study external validation cohorts.
Model Performance in the Internal Validation Cohort
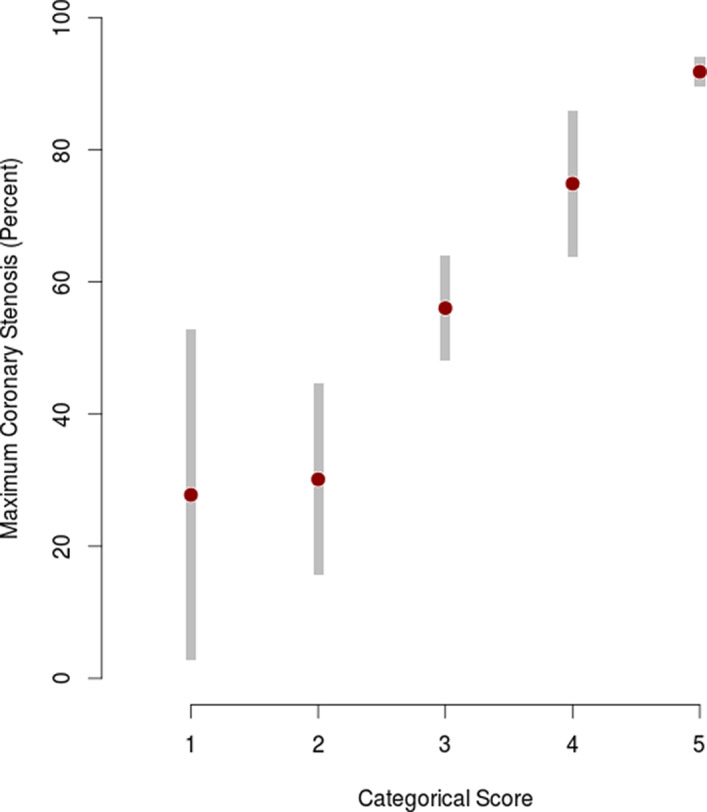
Of the 275 patients in the CASABLANCA study internal validation cohort, 177 (64.4%) had obstructive CAD. Evaluating the model on this cohort yielded an AUC of 0.85 (95% CI, 0.80–0.90). At the optimal cutoff, the score had a sensitivity of 80% (95% CI, 74%–86%), specificity of 71% (95% CI, 62%–80%), PPV of 83% (95% CI, 79%–89%), and NPV of 66% (95% CI, 57%–75%) (Figure 1). We found a higher prevalence of obstructive CAD in patients with higher scores and lower prevalence among patients with lower scores (Figure S2). Partitioning the score into 5 risk levels yielded a PPV of 97% for level 5 (highest risk, n=99) and an NPV of 89% for level 1 (lowest risk, n=9) (Table 2). These 5 risk levels correlate with an increasing degree of CAD stenosis (Figure 2).
Figure 1. Receiver operating characteristic curve for (A) the internal validation cohort (n=275) and (B) the external validation cohort (n=241).
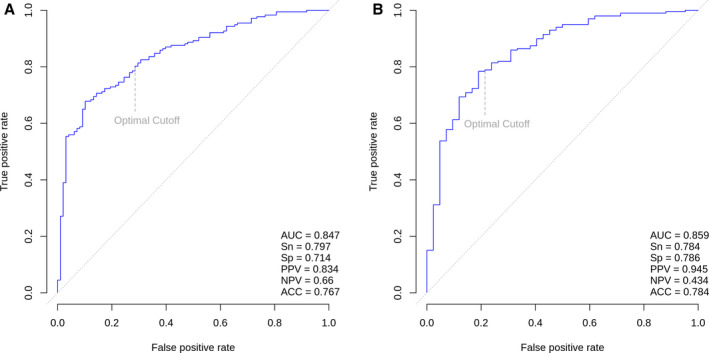
ACC indicates predictive accuracy; AUC, area under the receiver operating characteristic curve; NPV, negative predictive value; PPV, positive predictive value; Sn, sensitivity; and Sp, specificity.
Table 2.
Predictive Performance as a 5‐Level Risk Score for the Internal Validation Cohort (n=275)
| Score | No. of Patients | PPV | NPV |
|---|---|---|---|
| 5 | 99 | 0.97 | … |
| 4 | 43 | 0.70 | … |
| 3 | 95 | N/A | N/A |
| 2 | 29 | … | 0.76 |
| 1 | 9 | … | 0.89 |
N/A indicates not available; NPV, negative predictive value; and PPV, positive predictive value.
Figure 2. Stenosis associated with 5‐level score for the internal validation cohort (n=275), shown as the sample mean with 95% CIs for the population mean.
Among all CASABLANCA study patients with renal injury (n=122; defined as a creatinine level ≥1.5 mg/dL), the model yielded an AUC of 0.79 (95% CI, 0.69–0.89) with this cohort, and using the optimum cutoff, it performed with a sensitivity of 85% (95% CI, 77%–92%), specificity of 60% (95% CI, 42%–78%), PPV of 87% (95% CI, 80%–94%), and NPV of 56% (95% CI, 39%–73%) (Figure S3).
In a subgroup analysis of patients in the CASABLANCA study internal validation set without acute MI (n=245), the score had an AUC of 0.84 (95% CI, 0.79–0.90).
Model Performance in the External Validation Cohort
Of the 241 patients included in the external validation cohort, the majority had obstructive CAD (n=199). Patients with obstructive CAD were older, more likely to be male, and had a higher prevalence of hypertension, hyperlipidemia, and history of MI (Table S5). In this cohort (n=241), the model predicted ≥50% stenosis with an AUC of 0.86 (95% CI, 0.79–0.93, P<0.001), sensitivity of 78% (95% CI, 73%–84%), specificity of 79% (95% CI, 66%–91%), PPV of 95% (95% CI, 91%–98%), and NPV of 43% (95% CI, 32%–55%) at the optimal cut‐off (Figure 1). Partitioning the score into the five risk levels yielded a PPV of 97% for level 5 (highest risk, n=112) and an NPV of 67% for level 1 (lowest risk, n=3) (Table S6).
In a subgroup of patients in the BACC study external validation set who had acute MI neither ruled out nor ruled in, ie, patients in the indeterminate zone (n=65), the majority had obstructive CAD (n=53; 81.5%). When evaluated in this cohort, the score had an AUC of 0.88 (95% CI, 0.75–1.0; P<0.001), sensitivity of 81% (95% CI, 71%–92%), specificity of 75% (95% CI, 51%–100%), PPV of 93% (95% CI, 86%–100%), and NPV of 47% (95% CI, 25%–70%).
In a subgroup of BACC study patients with renal injury (n=30; defined as a creatinine level ≥1.5 mg/dL), the score had an AUC of 0.92 (95% CI, 0.76–1.00), and using the optimal cutoff, it had a sensitivity of 88% (95% CI, 74%–100%), specificity of 67% (95% CI, 29%–100%), PPV of 91% (95% CI, 80%–100%), and NPV of 57% (95% CI, 20%–94%) (Figure S4).
Last, in patients in the BACC study without acute MI (n=138), the score had an AUC of 0.87 (95% CI, 0.79–0.95) as compared with an hs‐cTnI AUC of 0.57 (95% CI, 0.45–0.68) for CAD assessment.
Discussion
We describe a novel clinical/proteomic model inclusive of hs‐cTnI capable of predicting severe CAD across a wide variety of patient types that had excellent performance in the internal (AUC 0.85) and external (AUC 0.86) validation cohorts. The performance of the diagnostic model was far superior to hs‐cTnI alone. Notably, among patients with acute chest discomfort but nondiagnostic (but abnormal) troponin concentrations—the patient in the common indeterminate zone—the panel had consistent discrimination, specifically up‐classifying nearly two thirds of patients to a higher risk category.
Derived from more than 100 biomarkers in a targeted proteomics approach, the final panel contains 3 biomarkers (adiponectin, KIM‐1, and hs‐cTnI) previously associated with atherosclerotic disease. Low concentrations of adiponectin have been previously associated with the presence of CAD and predicted progression of carotid artery disease, 16 , 17 and, similarly, higher adiponectin concentrations have a negative influence in our final diagnostic model. KIM‐1 is a proximal renal tubular biomarker whose concentrations have been linked to the presence of CAD and predicts major adverse cardiovascular events. 8 , 18 Last, in angiographic studies, we reported that hs‐cTnI was associated with a higher prevalence of CAD and prediction of incident MI, cardiovascular death, and all‐cause mortality. 9 Although hs‐cTnI is commonly used in clinical practice, KIM‐1 and adiponectin are not. Addition of these biomarkers did lead to the model's diagnostic performance, with improvement in discrimination, calibration, and reclassification (Table S3). However, further evaluation of their cost benefit is needed.
Our results may have clinical utility in several important clinical settings. In the acute evaluation of chest pain in the ED, the clinical/biomarker algorithm could be useful for triage. In patients presenting to the ED for evaluation of chest pain, we found excellent performance, including among patients with “indeterminate” hs‐cTnI results. Such patients with nondiagnostic hs‐cTnI frequently require further observation with serial troponin measurements over several hours (which often requires a stay in an observation unit) or further testing with noninvasive imaging such as CT coronary angiography or stress testing. However, these tests have limitations including restricted availability (including off hours), cost, and need for exposure to radiation. For these patients with indeterminate hs‐cTnI alone, our model may be identifying those who require admission for invasive coronary angiography rather than further noninvasive testing. A prospective evaluation with a score‐based decision on invasive or noninvasive strategy should be considered in the future.
Importantly, the panel performed well in patients with renal impairment, a cohort where CT coronary angiography is contraindicated. As patients with acute and chronic renal disease commonly have elevated troponin concentrations through various mechanisms, hs‐cTn at first draw has reduced utility to predict the presence of obstructive CAD. It should be noted, however, that the number of patients with renal impairment in these cohorts is small so these results may exhibit some bias as a result of their small sample sizes.
With respect to the clinical role of this clinical/biomarker‐based diagnostic model, in addition to testing those patients who are presenting more acutely, this model might have a role in lesser‐acute patients. The CASABLANCA study predominantly included patients undergoing elective coronary angiography. 11 Indeed, after excluding patients with acute MI, the panel performed similarly well. Thus, it appears that the model is well‐validated for the evaluation of outpatients presenting with symptoms concerning stable angina. In this cohort, the score may be useful for identifying patients presenting with chest pain who have a high likelihood of obstructive CAD, thereby reducing the need for stress testing or CT coronary angiography.
Study Limitations
Although novel, our study has limitations. Biomarkers were measured at a single point in time, which may not reflect levels at future time periods. As the rates of stress testing and CT coronary angiography were variable in both cohorts (CASABLANCA and BACC studies), comparisons of their diagnostic performance cannot be made. However, the score performs well when compared with the gold standard, invasive coronary angiography. Last, the definition of obstructive CAD differed in each cohort as a result of differences in adjudication in the CASABLANCA and BACC study cohorts.
Conclusions
We have derived and externally validated a clinical/proteomic panel that can predict the presence of obstructive CAD with high accuracy. The score performs similarly well in the evaluation of acute chest pain in the ED (including patients who had MI neither ruled in nor ruled out) and in outpatients presenting for evaluation of stable angina including those with renal injury.
Sources of Funding
Dr Januzzi is supported in part by the Hutter Family Professorship in Cardiology. Dr Gaggin is supported in part by the Ruth and James Clark Fund for Cardiac Research Innovation. Dr Neumann was supported by a grant from the German Research Foundation, the German Heart Foundation/German Foundation of Heart Research, the Else Kröner Fresenius Stiftung, and the DZHK; and is the recipient of a research fellowship by the Deutsche Forschungsgemeinschaft (NE 2165/1‐1). The BACC study was supported by the German Center of Cardiovascular Research and an unrestricted grant from Abbott Diagnostics. This work was supported by a grant from Prevencio, Inc.
Disclosures
Dr Neumann received honoraria from Abbott Diagnostics, Siemens, and Prevencio. Dr Westermann reports personal fees from Bayer, Boehringer‐Ingelheim, Berlin Chemie, Astra Zeneca, Biotronik, and Novartis. Dr Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; has received grant support from Novartis Pharmaceuticals, Roche Diagnostics, Abbott, Singulex, and Prevencio, and consulting income from Abbott, Janssen, Novartis, Pfizer, Merck, and Roche Diagnostics; and participates in clinical end point committees/data safety monitoring boards for Abbott, AbbVie, Amgen, Boehringer‐Ingelheim, Janssen, and Takeda. Mr Magaret is a consultant to Prevencio, Inc. Dr Gaggin has received grant support from Roche and Portola; consulting income from Roche Diagnostics, American Regent, Amgen, Boston Heart Diagnostics, and Critical Diagnostics; and research payments for clinical end point committees for EchoSense. Ms Rhyne and Dr Barnes are employees of Prevencio, Inc. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S6
Figures S1–S4
(J Am Heart Assoc. 2020;9:e017221 DOI: 10.1161/JAHA.120.017221.)
For Sources of Funding and Disclosures, see page 7.
References
- 1. Centers for Disease Control and Prevention, Ambulatory and Hospital Care Statistics Branch . National Hospital Ambulatory Medical Care Survey: 2010 Emergency Department Summary Tables. Available at: http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2010_ed_web_tables.pdf. Accessed December 10, 2019.
- 2. Hsia RY, Hale Z, Tabas JA. A national study of the prevalence of life‐threatening diagnoses in patients with chest pain. JAMA Intern Med. 2016;176:1029–1032. [DOI] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 4. Neumann JT, Twerenbold R, Ojeda F, Sörensen NA, Chapman AR, Shah ASV, Anand A, Boeddinghaus J, Nestelberger T, Badertscher P, et al. Application of high‐sensitivity troponin in suspected myocardial infarction. N Engl J Med. 2019;380:2529–2540. [DOI] [PubMed] [Google Scholar]
- 5. Twerenbold R, Costabel JP, Nestelberger T, Campos R, Wussler D, Arbucci R, Cortes M, Boeddinghaus J, Baumgartner B, Nickel CH, et al. Outcome of applying the ESC 0/1‐hour algorithm in patients with suspected myocardial infarction. J Am Coll Cardiol. 2019;74:483–494. [DOI] [PubMed] [Google Scholar]
- 6. Twerenbold R, Boeddinghaus J, Nestelberger T, Wildi K, Rubini Gimenez M, Badertscher P, Mueller C. Clinical use of high‐sensitivity cardiac troponin in patients with suspected myocardial infarction. J Am Coll Cardiol. 2017;70:996–1012. [DOI] [PubMed] [Google Scholar]
- 7. Maron DJ, Hochman JS, O'Brien SM, Reynolds HR, Boden WE, Stone GW, Bangalore S, Spertus JA, Mark DB, Alexander KP, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: rationale and design. Am Heart J. 2018;201:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ibrahim NE, Januzzi JL Jr, Magaret CA, Gaggin HK, Rhyne RF, Gandhi PU, Kelly N, Simon ML, Motiwala SR, Belcher AM, et al. A clinical and biomarker scoring system to predict the presence of obstructive coronary artery disease. J Am Coll Cardiol. 2017;69:1147–1156. [DOI] [PubMed] [Google Scholar]
- 9. McCarthy CP, Ibrahim NE, Lyass A, Li Y, Gaggin HK, Simon ML, Mukai R, Gandhi P, Kelly N, Motiwala SR, et al. Single‐molecule counting of high‐sensitivity troponin I in patients referred for diagnostic angiography: results from the CASABLANCA (Catheter Sampled Blood Archive in Cardiovascular Diseases) study. J Am Heart Assoc. 2018;7:e007975 DOI: 10.1161/JAHA.117.007975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Januzzi JL Jr, Suchindran S, Coles A, Ferencik M, Patel MR, Hoffmann U, Ginsburg GS, Douglas PS. High‐sensitivity troponin I and coronary computed tomography in symptomatic outpatients with suspected CAD: insights from the PROMISE trial. JACC Cardiovasc Imaging. 2019;12:1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaggin HK, Bhardwaj A, Belcher AM, Motiwala SR, Gandhi PU, Simon ML, Kelly NP, Anderson AM, Garasic JM, Danik SB, et al. Design, methods, baseline characteristics and interim results of the Catheter Sampled Blood Archive in Cardiovascular Diseases (CASABLANCA) study. IJC Metab Endocr. 2014;5:11–18. [Google Scholar]
- 12. clinicaltrials.gov . Biomarkers in Acute Cardiac Care (BACC). NCT02355457. Available at: https://clinicaltrials.gov/ct2/show/NCT02355457. Accessed December 10, 2019.
- 13. Efron B, Hastie T, Johnstone I, Tibshirani R. Least angle regression. Ann Stat. 2004;32:407–499. [Google Scholar]
- 14. Tibshirani R. Regression shrinkage and selection via the lasso: a retrospective. J R Stat Soc Series B Stat Methodol. 2011;73:273–282. [Google Scholar]
- 15. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 16. Rizza S, Gigli F, Galli A, Micchelini B, Lauro D, Lauro R, Federici M. Adiponectin isoforms in elderly patients with or without coronary artery disease. J Am Geriatr Soc. 2010;58:702–706. [DOI] [PubMed] [Google Scholar]
- 17. Persson J, Strawbridge RJ, McLeod O, Gertow K, Silveira A, Baldassarre D, Van Zuydam N, Shah S, Fava C, Gustafsson S, et al. Sex‐specific effects of adiponectin on carotid intima‐media thickness and incident cardiovascular disease. J Am Heart Assoc. 2015;4:e001853 DOI: 10.1161/JAHA.115.001853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCarthy CP, van Kimmenade RR, Gaggin HK, Simon ML, Ibrahim NE, Gandhi P, Kelly N, Motiwala SR, Belcher AM, Harisiades J, et al. Usefulness of multiple biomarkers for predicting incident major adverse cardiac events in patients who underwent diagnostic coronary angiography (from the Catheter Sampled Blood Archive in Cardiovascular Diseases [CASABLANCA] study). Am J Cardiol. 2017;120:25–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6
Figures S1–S4


