Abstract
Background
Differences in the impact of contrast medium on the development of contrast‐induced acute kidney injury (CI‐AKI) in patients undergoing transcatheter aortic valve implantation (TAVI) or a coronary angiography/percutaneous coronary intervention (CA/PCI) have not been previously investigated.
Methods and Results
Patients treated with TAVI or elective CA/PCI were retrospectively analyzed in terms of baseline and procedural characteristics, including preprocedural and postprocedural kidney function. CI‐AKI was defined as a relative increase in serum creatinine concentration of at least 0.3 mg/dL within 72 hours of contrast‐medium administration compared with baseline. The incidence of CI‐AKI in the TAVI versus CA/PCI group was compared. After the exclusion of patients in dialysis and emergency procedures, 977 patients were analyzed; there were 489 patients who had undergone TAVI (50.1%) and 488 patients who had undergone CA/PCI (49.9%). Patients treated by TAVI were older, presenting a higher rate of anemia and chronic kidney disease (P<0.001 for all comparisons). Consistently, they also had a significantly lower glomerular filtration rate and higher serum creatinine concentration (P<0.001 for all). However, the occurrence of CI‐AKI was significantly lower in these patients compared with patients treated by a CA/PCI (6.7% versus 14.5%, P<0.001). At multivariate analysis, the TAVI procedure had an independent protective effect on CI‐AKI incidence among total population (odds ratio, 0.334; 95% CI, 0.193–0.579; P<0.001). This observation was confirmed after propensity score matching among 360 patients (180 by TAVI and 180 by CA/PCI; P=0.002).
Conclusions
CI‐AKI occurred less frequently in patients undergoing TAVI than in patients undergoing a CA/PCI, despite a worse‐risk profile. The impact of contrast administration on kidney function in patients who had undergone TAVI may be better tolerated because of the hemodynamic changes following aortic valve replacement.
Keywords: contrast‐induced nephropathy, contrast‐induced acute kidney injury, coronary angiography, percutaneous coronary intervention, transcatheter aortic valve implantation
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Percutaneous Coronary Intervention
Nonstandard Abbreviations and Acronyms
- CA
coronary angiography
- CI‐AKI
contrast‐induced acute kidney injury
- eGFR
estimated glomerular filtration rate
- OR
odds ratio
- PCI
percutaneous coronary intervention
- Scr
serum creatinine
- TAVI
transcatheter aortic valve implantation
Clinical Perspective
What Is New?
The risk of contrast‐induced acute kidney injury is significantly lower in patients undergoing transcatheter aortic valve implantation compared with coronary angiography/percutaneous interventions, despite a much higher baseline risk profile.
What Are the Clinical Implications?
The awareness of a limited nephrotoxicity of contrast media in patients undergoing transcatheter aortic valve implantation could help clinicians when estimating the risk–benefit balance of aortic valve replacement, in particular among patients with chronic kidney disease.
contrast‐induced acute kidney injury (CI‐AKI) is a common complication of interventional procedures; it is conventionally defined as an acute impairment of renal function after iodinated‐contrast‐medium administration in the absence of other related causes. The reported incidence of CI‐AKI presents significant variations among different studies. 1 , 2
For coronary angiography (CA) and percutaneous interventions (PCIs), CI‐AKI incidence has been reported from less than 5% in low‐risk patients up to 50% in high‐risk populations 3 , 4 with well‐characterized patients and procedural‐related CI‐AKI predictors. 3 , 5
Unarguably, CI‐AKI may also occur in patients undergoing transcatheter aortic valve implantation (TAVI); it has been observed in 10% to 20% of cases. 6 In addition, for patients undergoing TAVI, clinical and procedural CI‐AKI predictors have been identified. 7 , 8
Nevertheless, to date, no studies have specifically compared the occurrence of CI‐AKI between patients undergoing TAVI or a CA/PCI. Besides, in CI‐AKI predictive models, the type of procedure (TAVI or CA/PCI) has never been considered. 9
Patients undergoing TAVI are older, often frail, and usually present baseline characteristics that make them more prone to CI‐AKI compared with patients undergoing a CA/PCI. 10 , 11 Conversely, beneficial hemodynamic changes occur immediately after valve replacement, potentially mitigating the toxic effects of contrast medium on the glomerular filtration rate. 12 , 13
This study assessed the impact of the type of endovascular procedure (TAVI or CA/PCI), on the occurrence and severity of CI‐AKI in a real‐world population of elective patients.
Methods
Study Population and Exclusion Criteria
The data that support the findings of this study are available from the corresponding author upon reasonable request.
This is a retrospective observational single‐center study based on a consecutive series of elective patients who underwent CA/PCI or TAVI (January 2012–December 2019) at the University Hospital of Verona (Verona, Italy).
For patients undergoing multiple contrast‐media exposures (staged procedures), only the first one (index procedure) was considered for CI‐AKI assessment, whether it was a diagnostic CA, a CA followed by “ad hoc” PCI, or a TAVI alone or with CA in the same procedure. Patients needing urgent/emergent procedures and those unable to receive preventive hydration when needed according to baseline risk of CI‐AKI as detailed below, were excluded. Similarly, patients for whom serum creatinine (SCr) values at 24 and 72 hours after the procedure were not available (eg, patients with same‐day procedure discharge or patients that returned back to the referral hospital) and all patients in dialytic treatment were not included in the study. Biochemical variables (including preprocedural SCr and at 24 and 72 hours postprocedure), baseline clinical (sex, age, weight, height, body mass index, left ventricular ejection fraction, mean transaortic gradient, hypertension, diabetes mellitus (DM), dyslipidemia, anemia, dialysis), and procedural information (eg, procedure type, amount of contrast) were retrieved by electronic query of the institutional database.
All patients included in the analysis provided their informed consent to the anonymous elaboration of their data.
Creatinine and Glomerular Filtration Rate Determination
SCr determinations were centralized in the same laboratory and quantified with the kinetic Jaffe method (Dimension, Dade Behring; reference intervals: male, 0.8–1.3 mg/dL; female, 0.6–1.0 mg/dL). The SCr measurements were recalibrated to standardized measurements obtained at the Cleveland Clinic Research Laboratories (Cleveland, OH). 14 The estimated glomerular filtration rate (eGFR) was calculated for each patient using the Cockcroft–Gault formula. 15 , 16 , 17
Procedures
The CAs/PCIs were performed by either a standard femoral or radial percutaneous approach.
TAVI procedures were all performed electively by either percutaneous transfemoral access or by surgical transapical access. The Edwards SAPIEN‐XT, S3, or ULTRA (Edwards Lifesciences, Irvine, CA); the Medtronic CoreValve, Evolut‐R, or Evolut‐Pro (Medtronic, Inc., Minneapolis, MN); or the Accurate Neo (Boston Scientific, Marlborough, MA) were used, according to the patient’s anatomical characteristics as assessed by ECG‐gated computed tomography.
When patients undergoing TAVI had CA performed during the same procedure, the reported contrast volumes included the total administered amount.
Contrast Medium and Preventive Measures
In all cases, patients were administered intra‐arterial iso‐osmolar contrast medium (iodixanol) or low‐osmolar‐contrast medium (iohexol, iopromide). Standard measures to prevent CI‐AKI were adopted based on the risk profile of each patient, according to the available guidelines at the moment of the procedure. 18 , 19 , 20 , 21 Metformin was suspended in all patients with impaired baseline renal function before contrast‐medium administration and restarted 48 hours after the procedure, except in the cases that developed CI‐AKI. 18 , 22 Similarly, all patients with abnormal baseline renal function started isotonic saline hydration 12 hours before the procedure, and the infusion rate was standardized at 1 mL/kg per hour of 0.9% saline, except in cases of severe left ventricular dysfunction (left ventricular ejection fraction<35%) when the infusion rate was reduced to 0.5 mL/kg per hour. Intravenous hydration was given for at least 24 hours after the procedure, and was continued if any increment of SCr compared with baseline was detected. 21 Therefore, patients treated under emergency conditions or on outpatient modality were not included in the present analysis.
Definitions
CI‐AKI was defined as a relative increase in SCr concentration of at least 0.3 mg/dL as measured 24 hours and 72 hours after contrast‐medium administration compared with baseline. 2 , 23 The choice of this definition was based on a previous report from our center that demonstrated its superiority as a clinical cut‐off for CI‐AKI. 24 Chronic kidney disease was defined by basal eGFR<60 mL/min per 1.73 m2.
End Points
The primary end point was the incidence of CI‐AKI in patients who underwent TAVI compared with patients who underwent CA and or PCI in a single procedure.
Statistical Methods
Continuous variables are presented as mean and SD if normally distributed and compared with an unpaired t test. Categorical data are reported as a percentage and compared with the chi‐square test or Fisher exact test as appropriate. Univariate and multivariate logistic regression analyses were performed to identify independent predictors of CI‐AKI. The 95% CIs and odds ratios (ORs) are provided in the text and tables. Variables, associated with CI‐AKI at univariate logistic regression analysis with a P<0.1, were used in the multivariate regression model.
Propensity score matching 1:1 was performed to compare CI‐AKI in patients undergoing TAVI or CA/PCI, irrespective of different baseline characteristics that may lead to biased estimates of treatment effect. The variables included in the propensity score were age, eGFR, and contrast‐medium volume. We chose these variables because they have been reported as the most relevant in the literature on CI‐AKI. 25 , 26 Propensity scores were then matched using a greedy 5‐to‐1 digit‐matching algorithm. The distribution of patient characteristics in the matched sample was compared.
A probability value of P<0.05 was considered statistically significant. All statistical analyses were performed using SPSS 20.0 (IBM, Inc., Armonk, NY).
Results
Study Population
After application of the exclusion criteria, 977 patients were considered for the present study: 489 patients (50.1%) who had undergone TAVI; 488 patients (49.9%) who had undergone CA/PCI. Of the 488 patients, 339 patients (69.5%) underwent diagnostic CA, whereas 144 (30.5%) had a CA together with ad hoc PCI.
Clinical and Procedural Characteristics of TAVI Group Versus CA/PCI
Patients in the TAVI group were older, less frequently male, with lower body mass index, and showed a higher prevalence of anemia and chronic kidney disease (P<0.001; Table 1). Consistently, they also had a significantly lower eGFR and higher SCr values (P<0.001). Diabetes mellitus rate and dyslipidemia did not differ between the 2 groups (P=0.484 and P=0.791, respectively). Also, the left ventricular ejection fraction and the amount of contrast medium did not differ between the 2 groups (P=0.054 and P=0.353, respectively).
Table 1.
Clinical and Procedural Characteristics of the Overall Population: TAVI Versus CA/PCI
| Baseline Characteristics | Total Population (N=977) | TAVI (n=489; 50.1%) | CA/PCI (n=488; 49.9%) | P Value |
|---|---|---|---|---|
| Sex (% male) | 57.60% | 47.60% | 58.60% | <0.001 |
| Hypertension | 82.10% | 51.50% | 48.50% | 0.351 |
| Diabetes mellitus | 31.40% | 30.30% | 32.50% | 0.484 |
| Dyslipidemia | 59.20% | 59.60% | 58.70% | 0.785 |
| Anemia | 34.30% | 50.10% | 18.40% | <0.001 |
| eGFR<60 mL/min | 56.20% | 73.70% | 39.80% | <0.001 |
| Age, y | 75.63±11.53 | 81.41±7.31 | 69.93±12.09 | <0.001 |
| BMI | 26.43±4.60 | 25.80±4.51 | 27.05±4.62 | 0.927 |
| Basal creatinine, mg/dL | 1.15±0.49 | 1.21±0.54 | 1.09±0.42 | <0.001 |
| Basal eGFR, mL/min | 63.14±32.11 | 50.21±23.34 | 75.25±34.41 | <0.001 |
| LVEF, % | 52.63±12.34 | 53.50±13.12 | 51.44±11.10 | 0.54 |
| Contrast medium, mL | 143.91±81.76 | 140.63±68.44 | 146.12±89.60 | 0.353 |
BMI indicates body mass index; CA, coronary angiography; CA/PCI, coronary angiography/percutaneous coronary intervention; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; and TAVI, transcatheter aortic valve implantation.
CI‐AKI in TAVI Versus CA/PCI
CI‐AKI occurred in 104 patients (9.6% of the overall population): in 33 patients who underwent TAVI (6.7% of TAVI population) and in 71 patients who underwent CA/PCI (14.5% of CA/PCI population) (P<0.001). The mean basal‐to‐peak delta creatinine value in patients developing CI‐AKI was 0.70±0.67 mg/dL. It was significantly higher for patients who underwent TAVI (1.07±0.94 mg/dL) than for patients who underwent CA/PCI (0.64±0.47 mg/dL) (P=0.002). However, the mean delta SCr value calculated from baseline to discharge did not differ between the TAVI group (0.18±0.42 mg/dL) and the CA/PCI group (0.16±0.65 mg/dL) (P=0.889).
In the multivariate analysis, TAVI was associated with a lower incidence of CI‐AKI (OR, 0.350; 95% CI, 0.207–0.593; P<0.001). Complete univariate and multivariate analyses are presented in Table 2.
Table 2.
Univariate and Multivariate Regression Analyses
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | CI 95% | P Value | OR | CI 95% | P Value | |
| Procedure type | 2.353 | 1.525–3.360 | <0.001 | 0.350 | 0.207–0.593 | <0.001 |
| Contrast medium amount | 1.003 | 1.000–1.005 | 0.024 | 1.003 | 1.000–1.005 | 0.023 |
| Baseline eGFR | 0.993 | 0.986–1.000 | 0.062 | 0.986 | 0.977–0.994 | 0.001 |
| Age | 1.004 | 0.987–1.023 | 0.627 | |||
| Diabetes mellitus | 1.324 | 0.860–2.039 | 0.202 | |||
| Baseline LVEF % | 0.995 | 0.974–1.017 | 0.678 | |||
| Sex (female) | 0.797 | 0.524–1.211 | 0.288 | |||
eGFR indicates the estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; and OR, odds ratio.
The probability of CI‐AKI, when adjusted for age, contrast‐medium amount, and eGFR, was proportional to the severity of kidney disease and considerably lower for TAVI than for CA/PCI at a decremental value of eGFR (P for interaction 0.01; Figure).
Figure 1. Probability of CI‐AKI adjusted for sex, age, basal eGFR, and contrast‐medium amount: TAVI vs CA/PCI (P for interaction 0.01).
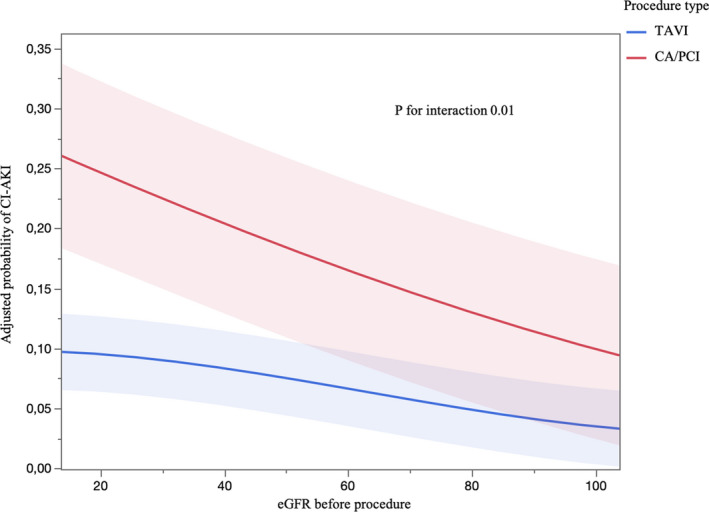
CA/PCI indicates coronary angiography/percutaneous coronary intervention; CI‐AKI, contrast‐induced acute kidney injury; eGFR, estimated glomerular filtration rate; and TAVI, transcatheter aortic valve implantation.
Of note, after dividing the TAVI population in 4 groups based on the date of procedure, a reduction in the CI‐AKI incidence throughout the different years was observed: 13.3% in the first biennium (2012–2013), 9.9% in the second (2014–2015), and 3.9% both in the third (2015–2017) and the last biennium (2017–2019). The difference was statistically significant between the first biennium and the third and/or fourth biennium (13.3% versus 3.9%, P=0.012).
Propensity Score Matching
Among the study population, a propensity score match was obtained for 180 patients undergoing TAVI and 180 patients undergoing CA/PCI. As shown in Table 3, patients who had undergone TAVI had a significantly higher rate of DM, anemia, and dyslipidemia (P=0.031, 0.005, and<0.001, respectively). All other characteristics did not differ significantly between the 2 groups. In this population, 46 patients developed CI‐AKI: 13 patients who had undergone TAVI (7.2%) versus 33 patients who had undergone CA/PCI (18.3%). Therefore, TAVI was confirmed to be significantly associated with a lower incidence of CI‐AKI (OR, 0.347; 95% CI, 1.176–0.684; P=0.002) even after propensity matching.
Table 3.
Clinical and Procedural Characteristics of the Propensity Score Matching Population: TAVI Versus CA/PCI
| Baseline Characteristics | Total Population (N=360) | TAVI (n=180; 50%) | CGF/PTCA (n=180; 50%) | P Value |
|---|---|---|---|---|
| Sex (% male) | 55.3% | 53.3% | 57.2% | 0.458 |
| Hypertension | 84.6% | 83.2% | 85.9% | 0.492 |
| Diabetes mellitus | 30.7% | 36.0% | 25.4% | 0.031 |
| Dyslipidemia | 62.4% | 69.7% | 55.1% | 0.005 |
| Anemia | 38.6% | 50.6% | 26.7% | <0.001 |
| eGFR<60 mL/min | 64.7% | 61.7% | 67.8% | 0.225 |
| Age, y | 79.84±6.86 | 79.89±6.91 | 79.79±6.82 | 0.890 |
| BMI | 25.95±4.13 | 26.04±4.23 | 25.86±4.03 | 0.688 |
| Basal creatinine, mg/dL | 1.15±0.47 | 1.12±0.50 | 1.19±0.43 | 0.203 |
| Basal eGFR, mL/min | 55.29±22.13 | 55.95±23.09 | 54.62±21.16 | 0.570 |
| LVEF% | 51.26±13.15 | 52.48±13.19 | 49.61±12.99 | 0.139 |
| Contrast medium, m | 139.55±71.89 | 141.18±67.47 | 137.92±76.20 | 0.668 |
BMI indicates body mass index; eGFR, estimated glomerular filtration rate; and LVEF, left ventricular ejection fraction.
Discussion
This is the first study comparing the occurrence of CI‐AKI in patients undergoing TAVI versus patients undergoing CA/PCI.
The main findings of the study are:
The occurrence of CI‐AKI was significantly lower in patients who underwent TAVI compared with patients who underwent CA/PCI, despite a much higher baseline risk profile.
For patients developing CI‐AKI, the mean basal‐to‐peak increase in SCr value was significantly higher among the patients who underwent TAVI compared with patients who underwent CA/PCI; however, the mean delta SCr value calculated from baseline to discharge did not differ between the 2 groups.
At present, no studies have assessed if different angiographic procedures could have a different impact on renal function. In addition, in predictive models of CI‐AKI, the type of procedure (TAVI or CA/PCI) has never been considered. 9
In the context of coronary interventions, CI‐AKI affects morbidity and mortality, 27 , 28 raising concerns among clinicians about the real benefit of some CA/PCI procedures, particularly in nephropathic patients, and their best periprocedural management. 29 Similarly, CI‐AKI is associated with worse short‐ and long‐term outcomes after TAVI with a significant negative impact on mortality during the first year after the procedure. 8
As expected, patients who underwent TAVI in our study presented baseline characteristics configuring much higher CI‐AKI risk compared with patients who underwent elective CA/PCI, in line with results reported in the available literature. 10 , 11 Conversely, they reported a significantly lower rate of CI‐AKI.
A possible explanation of the reduced CI‐AKI risk for patients who had undergone TAVI is the immediate hemodynamic impact that follows the removal of the aortic valve obstruction after TAVI, with the subsequent increase of the cardiac output. 30 The improvement in kidney function that follows is known as acute kidney recovery. 12 , 13 In addition, aortic valve stenosis is associated with a chronic left ventricular pressure overload, which leads to high left ventricular filling pressures and systemic venous congestion. The systemic venous congestion, through a type 2 cardiorenal syndrome, is associated with impaired renal function. 31 , 32 Such additional hemodynamic effects may further mitigate the negative effect of the contrast medium, a beneficial mechanism that does not occur in ischemic patients undergoing elective CA/PCI exposed to the toxic effects of similar doses of contrast media without a concomitant increment of the renal perfusion rate.
The awareness of a limited nephrotoxicity of contrast media in patients who underwent TAVI could help clinicians when estimating the risk–benefit balance of aortic valve replacement in general, and among patients with chronic kidney disease in particular. Furthermore, this beneficial effect on renal function may play an additional role in favor of TAVI over a surgical approach to aortic valve replacement because the extracorporeal circulation required for surgery is a well‐known risk factor for further renal deterioration. 33 , 34
Of note, because of the development of procedural TAVI experience and its extension to lower risk patients, both the Society of Thoracic Surgeons (STS) score and the amount of contrast‐media administration have significantly decreased in parallel over time. Therefore, the absolute CI‐AKI incidence dropped from more than 14% in the early years of TAVI, to values below 4% after 2015, and has stabilized thereafter. Nevertheless, such changes in the baseline and procedural‐related risks did not influence the effect of the type of procedure (TAVI or CA/PCI) as a strong and independent predictor of contrast‐related acute renal injury.
Among patients who developed CI‐AKI, the SCr peak was significantly higher in patients who underwent TAVI than in patients who underwent CA/PCI. This is likely related to the much higher baseline risk characteristics for CI‐AKI among patients who had undergone TAVI, which certainly expose them more to the toxic effects of the contrast medium. However, after the SCr peak, patients who had undergone TAVI yielded a rapid reduction of SCr to baseline values that, at the time of hospital discharge, were similar to those of ischemic patients. Because SCr is an indicator of the GFR, the observed dynamics of SCr is likely indicative of the previously mentioned acute kidney recovery that follows the removal of the aortic stenosis. Nevertheless, such a favorable outcome is limited to elective patients who could benefit from guidelines‐recommended preventive measures to minimize the renal toxic effects of a contrast medium.
Limitations
This is a single‐center, retrospective observational study; therefore, the observations derived from this experience need further evaluation.
Sources of Funding
None.
Disclosures
None.
Acknowledgments
All authors contributed significantly to the completion of the study and the writing of this article, including reading and approving of this article in its final form.
(J Am Heart Assoc. 2020;9:e017194 DOI: 10.1161/JAHA.120.017194.)
For Sources of Funding and Disclosures, see page 6.
References
- 1. Bangalore S, Vlachos HA, Selzer F, Wilensky RL, Kip KE, Williams DO, Faxon DP. Percutaneous coronary intervention of moderate to severe calcified coronary lesions: insights from the National Heart, Lung, and Blood Institute Dynamic Registry. Catheter Cardiovasc Interv. 2011;77:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fliser D, Laville M, Covic A, Fouque D, Vanholder R, Juillard L, Van Biesen W. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast‐induced nephropathy. Nephrol Dial Transplant. 2012;27:4263–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, et al. A simple risk score for prediction of contrast‐induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. [DOI] [PubMed] [Google Scholar]
- 4. Ribichini F, Gambaro G, Graziani MS, Pighi M, Pesarini G, Pasoli P, Anselmi M, Ferrero V, Yabarek T, Sorio A, et al. Comparison of serum creatinine and cystatin C for early diagnosis of contrast‐induced nephropathy after coronary angiography and interventions. Clin Chem. 2012;58:458–464. [DOI] [PubMed] [Google Scholar]
- 5. Maioli M, Toso A, Gallopin M, Leoncini M, Tedeschi D, Micheletti C, Bellandi F. Preprocedural score for risk of contrast‐induced nephropathy in elective coronary angiography and intervention. J Cardiovasc Med. 2010;11:444–449. [DOI] [PubMed] [Google Scholar]
- 6. Saad M, Karam B, Faddoul G, El Douaihy Y, Yacoub H, Baydoun H, Boumitri C, Barakat I, Saifan C, El-Charabaty E, et al. Is kidney function affecting the management of myocardial infarction? A retrospective cohort study in patients with normal kidney function, chronic kidney disease stage III–V, and ESRD. Int J Nephrol Renovasc Dis. 2016;9:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bagur R, Webb JG, Nietlispach F, Dumont É, De Larochellire R, Doyle D, Masson JB, Gutiérrez MJ, Clavel MA, Bertrand OF, et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J. 2010;31:865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nunes Filho ACB, Katz M, Campos CM, Carvalho LA, Siqueira DA, Tumelero RT, Portella ALF, Esteves V, Perin MA, Sarmento-Leite R, et al. Impact of acute kidney injury on short‐ and long‐term outcomes after transcatheter aortic valve implantation. Rev Esp Cardiol. 2019;72:21–29. [DOI] [PubMed] [Google Scholar]
- 9. Silver SA, Shah PM, Chertow GM, Harel S, Wald R, Harel ZIV. Risk prediction models for contrast induced nephropathy: systematic review. BMJ. 2015;351h:4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pighi M, Piazza N, Martucci G, Lachapelle K, Perrault LP, Asgar AW, Lauck S, Webb JG, Popma JJ, Kim DH, et al. Sex-specific determinants of outcomes after transcatheter aortic valve replacement. Circ Cardiovasc Qual Outcomes. 2019;12:e005363. [DOI] [PubMed] [Google Scholar]
- 11. Gilard M, Schlüter M, Snow TM, Dall’Ara G, Eltchaninoff H, Moat N, Goicolea J, Ussia GP, Kala P, Wenaweser P, et al. The 2011–2012 pilot European society of cardiology sentinel registry of transcatheter aortic valve implantation: 12-month clinical outcomes. EuroIntervention. 2016;12:79–87. [DOI] [PubMed] [Google Scholar]
- 12. Azarbal A, Leadholm KL, Ashikaga T, Solomon RJ, Dauerman HL. Frequency and prognostic significance of acute kidney recovery in patients who underwent transcatheter aortic valve implantation. Am J Cardiol. 2018;121:634–641. [DOI] [PubMed] [Google Scholar]
- 13. Azarbal A, Malenka DJ, Huang YL, Ross CS, Solomon RJ, DeVries JT, Flynn JM, Butzel D, McKay M, Dauerman HL. Recovery of kidney dysfunction after transcatheter aortic valve implantation (from the Northern New England Cardiovascular Disease Study Group). Am J Cardiol. 2019;123:426–433. [DOI] [PubMed] [Google Scholar]
- 14. Selvin E, Manzi J, Stevens LA, Van Lente F, Lacher DA, Levey AS, Coresh J. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50:918–926. [DOI] [PubMed] [Google Scholar]
- 15. Sokoll LJ, Russell RM, Sadowski JA, Morrow FD. Establishment of creatinine clearance reference values for older women. Clin Chem. 1994;40:2276–2281. [PubMed] [Google Scholar]
- 16. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 17. Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function — measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. [DOI] [PubMed] [Google Scholar]
- 18. Stacul F, Van Der Molen AJ, Reimer P, Webb JAW, Thomsen HS, Morcos SK, Almén T, Aspelin P, Bellin MF, Clement O, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;21:2527–2541. [DOI] [PubMed] [Google Scholar]
- 19. van der Molen AJ, Reimer P, Dekkers IA, Bongartz G, Bellin M-F, Bertolotto M, Clement O, Heinz-Peer G, Stacul F, Webb JAW, et al. Post-contrast acute kidney injury - Part 1: definition, clinical features, incidence, role of contrast medium and risk factors: recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol. 2018;28:2845–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van der Molen AJ, Reimer P, Dekkers IA, Bongartz G, Bellin M-F, Bertolotto M, Clement O, Heinz-Peer G, Stacul F, Webb JAW, et al. Post-contrast acute kidney injury. Part 2: risk stratification, role of hydration and other prophylactic measures, patients taking metformin and chronic dialysis patients recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol. 2018;28:2856–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 22. Brown JR, Robb JF, Block CA, Schoolwerth AC, Kaplan AV, O’Connor GT, Solomon RJ, Malenka DJ. Does safe dosing of iodinated contrast prevent contrast‐induced acute kidney injury? Circ Cardiovasc Interv. 2010;3:346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thomas ME, Blaine C, Dawnay A, Devonald MAJ, Ftouh S, Laing C, Latchem S, Lewington A, Milford DV, Ostermann M. The definition of acute kidney injury and its use in practice. Kidney Int. 2015;87:62–73. [DOI] [PubMed] [Google Scholar]
- 24. Pesarini G, Lunardi M, Ederle F, Zivelonghi C, Scarsini R, Gambaro A, Lupo A, Vassanelli C, Ribichini F. Long-term (3 Years) prognosis of contrast‐induced acute kidney injury after coronary angiography. Am J Cardiol. 2016;117:1741–1746. [DOI] [PubMed] [Google Scholar]
- 25. Mehran R, Dangas GD, Weisbord SD. Contrast-associated acute kidney injury. N Engl J Med. 2019;380:2146–2155. [DOI] [PubMed] [Google Scholar]
- 26. Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, Kosiborod M, Amin AP, Messenger JC, Rumsfeld JS, et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR cath-PCI registry. JACC Cardiovasc Interv. 2014;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown JR, Malenka DJ, DeVries JT, Robb JF, Jayne JE, Friedman BJ, Hettleman BD, Niles NW, Kaplan AV, Schoolwerth AC, et al. Transient and persistent renal dysfunction are predictors of survival after percutaneous coronary intervention: insights from the Dartmouth Dynamic Registry. Catheter Cardiovasc Interv. 2008;72:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crimi G, Leonardi S, Costa F, Ariotti S, Tebaldi M, Biscaglia S, Valgimigli M. Incidence, prognostic impact, and optimal definition of contrast‐induced acute kidney injury in consecutive patients with stable or unstable coronary artery disease undergoing percutaneous coronary intervention. Insights from the all-comer PRODIGY trial. Catheter Cardiovasc Interv. 2015;86:E19–E27. [DOI] [PubMed] [Google Scholar]
- 29. Szummer K, Lundman P, Jacobson SH, Schön S, Lindbäck J, Stenestrand U, Wallentin L, Jernberg T. Relation between renal function, presentation, use of therapies and in-hospital complications in acute coronary syndrome: data from the SWEDEHEART register. J Intern Med. 2010;268:40–49. [DOI] [PubMed] [Google Scholar]
- 30. Voigtländer L, Schewel J, Martin J, Schewel D, Frerker C, Wohlmuth P, Thielsen T, Kuck KH, Schäfer U. Impact of kidney function on mortality after transcatheter valve implantation in patients with severe aortic valvular stenosis. Int J Cardiol. 2015;178:275–281. [DOI] [PubMed] [Google Scholar]
- 31. Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. [DOI] [PubMed] [Google Scholar]
- 32. Valvular OCM. Aortic stenosis. disease severity and timing of intervention. J Am Coll Cardiol. 2006;47:2141–2151. [DOI] [PubMed] [Google Scholar]
- 33. Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. [DOI] [PubMed] [Google Scholar]
- 34. Chertow GM. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–348. [DOI] [PubMed] [Google Scholar]


