Abstract
Background
The optimal antithrombotic therapy for patients with atrial fibrillation undergoing percutaneous coronary intervention is a topic of debate. We aimed at defining the efficacy and safety of double antithrombotic therapy with single antiplatelet therapy (SAPT) plus a non–vitamin K antagonist oral anticoagulant (NOAC) against triple antithrombotic therapy with dual antiplatelet therapy (DAPT) added to a vitamin K antagonist (VKA), illustrating the pooled cumulative distribution of events, the ranking of different NOACs tested in NOAC+SAPT combination strategies, and the state of the current evidence in the field.
Methods and Results
Randomized controlled trials meeting the inclusion criteria were identified. The primary efficacy end point was the composite of trial‐defined major adverse cardiac events. The primary safety end point was clinically significant bleeding. Secondary end points were the components of primary end points. Trial‐level pairwise and Bayesian network meta‐analyses, reconstructed Kaplan–Meier analyses, and trial sequential analysis were performed. Four randomized controlled trials (10 969 patients) were included. No differences were found in terms of major adverse cardiac events (hazard ratio [HR], 1.07; 95% CI, 0.94–1.22), and the NOAC+SAPT strategy showed a lower rate of clinically significant bleeding compared with VKA + DAPT (HR, 0.56; 95% CI, 0.39–0.80). These results were consistent in reconstructed Kaplan–Meier analyses. In the Bayesian network meta‐analysis, different NOACs displayed diverse risk–benefit profiles. Trial sequential analyses suggest that the evidence for the similarity in major adverse cardiac events compared with VKA + DAPT and the bleeding risk reduction observed with NOAC+SAPT is likely to be conclusive.
Conclusions
NOAC+SAPT does not increase the risk of major adverse cardiac events and reduces the risk of bleeding compared with VKA + DAPT in AF patients undergoing percutaneous coronary intervention. Various NOACs may have different risk–benefit profiles in combination strategies.
Registration
URL: https://www.crd.york.ac.uk/prospero/; Unique identifier: CRD42020151089.
Keywords: acute coronary syndrome, anticoagulant therapy, antiplatelet therapy, antithrombotic therapy, atrial fibrillation, percutaneous coronary intervention
Subject Categories: Anticoagulants, Pharmacology, Stent
Nonstandard Abbreviations and Acronyms
- ACS
acute coronary syndrome
- AF
atrial fibrillation
- CHA2DS2‐VASc
Congestive heart failure, Hypertension, Age ≥75 years, Diabetes, Prior stroke or transient ischemic attack, Vascular disease, Sex class
- DAPT
dual antiplatelet therapy
- HAS‐BLED
Hypertension, Abnormal liver/renal function, Stroke history, Bleeding history or predisposition, Labile INR, Elderly, Drug/alcohol usage
- HR
hazard ratio
- MACE
major adverse cardiovascular event
- MI
myocardial infarction
- NOAC
non–vitamin K antagonist oral anticoagulant
- PCI
percutaneous coronary intervention
- PROSPERO
International prospective register of systematic reviews
- RCT
randomized controlled trial
- SAPT
single antiplatelet therapy
- ST
stent thrombosis
- VKA
vitamin K antagonist
Clinical Perspective
What Is New?
This meta‐analysis of more than 10 000 patients provides robust evidence that the incidence of major adverse cardiac events does not differ between the non–vitamin K antagonist oral anticoagulant + single antiplatelet therapy and the vitamin K antagonist + dual antiplatelet therapy strategies, whereas a non–vitamin K antagonist oral anticoagulant + single antiplatelet therapy strategy reduces bleeding compared with a vitamin K antagonist + dual antiplatelet therapy regimen.
What Are the Clinical Implications?
A strategy of double antithrombotic therapy with a non–vitamin K antagonist oral anticoagulant + single antiplatelet therapy, with a periprocedural period of aspirin, should be the first‐line approach in patients with atrial fibrillation undergoing percutaneous coronary intervention.
Percutaneous coronary intervention (PCI) is the standard of care for patients with acute coronary syndrome (ACS) and a treatment option for those with stable ischemic heart disease. 1 , 2 , 3 Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor is mandatory after PCI to prevent ischemic events, including stent thrombosis (ST), but this comes at the price of an increased risk of bleeding complications. 4 , 5 , 6 , 7 , 8 The trade‐off of thrombotic and bleeding complications is even more challenging when a patient undergoing PCI has a requirement for long‐term oral anticoagulation therapy, such as atrial fibrillation (AF). 9 , 10 It is estimated that ≈20% to 30% of patients with AF presents with SIHD, and AF coexists in up to 7% to 10% of those undergoing PCI. 11 Because the mechanisms underpinning coronary ischemic events and ST are largely different from those responsible for cardioembolic stroke in patients with AF, both antiplatelet and anticoagulant therapy are indicated in the context of AF‐PCI. 2 , 3 , 12 , 13 , 14 Unfortunately, the combination of DAPT and oral anticoagulation, also known as triple antithrombotic therapy, is associated with a high rate of fatal and nonfatal bleeding complications. 15
Although non–vitamin K antagonist oral anticoagulants (NOAC) should be preferred to vitamin K antagonists (VKA) for stroke prevention in patients with AF, 16 , 17 , 18 , 19 triple therapy with VKA is still broadly used in clinical practice. 20 , 21 Four randomized controlled trials (RCTs) conducted in AF patients with ACS and/or undergoing PCI compared double antithrombotic therapy with a NOAC plus single antiplatelet therapy (SAPT) to triple antithrombotic therapy with VKA plus DAPT. 22 , 23 , 24 , 25 A post hoc analysis of the AUGUSTUS (A Study of Apixaban in Patients With Atrial Fibrillation, Not Caused by a Heart Valve Problem, Who Are at Risk for Thrombosis [Blood Clots] Due to Having Had a Recent Coronary Event, Such as a Heart Attack or a Procedure to Open the Vessels of the Heart) trial was also published providing more details on ST for the comparison between NOAC+SAPT and VKA+DAPT. 26 To date, meta‐analyses including these trials showed that a NOAC+SAPT strategy significantly reduces the risk of bleeding complications compared with a VKA+DAPT strategy. Cumulatively, there was no apparent greater risk for hard ischemic events but an increase in ST, although the power for such comparisons, even in the setting of a meta‐analysis, was limited. 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 Importantly, these meta‐analyses included data from NOAC+SAPT versus VKA+DAPT for all but the AUGUSTUS trial. For the latter, only data from triple versus double antithrombotic therapy (and not specifically NOAC+SAPT versus VKA+DAPT) were used, causing heterogeneity in the compared groups. It is also noteworthy that the available meta‐analyses typically used standard frequentist methodologies and lacked a Bayesian approach to investigate the relative merits of the different NOAC+SAPT strategies. In addition, the summary estimates were pooled at the study level without taking into account any time‐related effect, and no subgroup analyses were performed. Finally, whether the comparison of NOAC+SAPT versus VKA+DAPT regarding bleeding and thrombotic outcomes are conclusive or susceptible to change according to future data remains unclear.
On this background, we conducted an up‐to‐date comprehensive meta‐analysis of AF‐PCI trials of NOACs using state‐of‐the‐art frequentist and Bayesian approaches. 35 Specifically, the aims of this meta‐analysis were to (1) define the treatment effect of NOAC+SAPT with respect to efficacy and safety in the overall population and in subgroups of interest; (2) illustrate the time‐dependent pooled cumulative distribution of events across trials; (3) use a Bayesian approach to rank the merits of different NOAC+SAPT strategies; (4) perform a trial‐sequential analysis to define the need for future studies in the field and explore whether the current evidence on efficacy of a NOAC+SAPT regimen is sufficient and conclusive.
METHODS
This meta‐analysis is registered in PROSPERO (international prospective register of systematic reviews; CRD42020151089) and was designed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines (Table S1). 36 , 37 , 38 . Methods used in the analysis, including the search string, are available from the corresponding author to any researcher for purposes of reproducing the results or replicating it.
Study Selection Criteria and Information Sources
For the purpose of the present meta‐analysis, RCTs comparing NOAC+SAPT versus VKA+DAPT in patients with AF undergoing PCI were considered. To assess study eligibility and to perform data extraction, 2 authors (M.D.M., A.G.) independently performed a systematic review of the current literature and disagreements were discussed by the whole authorship group. A comprehensive literature exploration was undertaken using PubMed, SCOPUS, and Web of Science as searching tools from inception up to the final search date of February 1, 2020. The following keywords were used to search all the relevant studies: ("AF" or "atrial fibrillation") AND (“coronary stenting” or “coronary angioplasty” or “PCI” or “percutaneous coronary intervention” or “stenting” or “stent” or “drug‐eluting stent” or “DES” or “BMS” or “bare metal stent” or “acute coronary syndrome”) AND (“antithrombotic therapy” or “DAPT” or “dual antiplatelet therapy” or “clopidogrel” or “ticagrelor” or “prasugrel” or “P2Y12 inhibitor” or “triple therapy” or “antithrombotic drugs” or “antiplatelets” or "oral anticoagulant" or "VKA" or "NOAC" or “DOAC” or "dabigatran" or "apixaban" or "edoxaban" or "rivaroxaban”). Search terms were combined using the Boolean operators “AND” and “OR.”
Initially, each article of potential interest was screened by reading the title and abstract; subsequently, articles with chances of inclusion underwent a full‐text appraisal. Only the studies that met our predefined inclusion criteria were included in the final analysis: (1) RCTs with a comparison between double and triple therapy regimens; (2) study population of AF patients with ACS and/or undergoing PCI either for SIHD or ACS; (3) at least an antithrombotic regimen including a P2Y12 inhibitor in association with a NOAC at a standard or reduced dose approved for prevention of cardioembolic stroke; (4) reported major bleeding and major adverse cardiovascular event (MACE) according to validated definitions; (5) follow‐up period of at least 6 months. No language or publication date restrictions were applied. In addition, the reference lists of prior systematic reviews and meta‐analyses were screened to find further potentially relevant studies, but no additional trials meeting our inclusion criteria called for attention.
Outcome Measures
The primary efficacy outcome was the composite of trial‐defined MACE (Table S2), which was usually defined as a combination of either all‐cause or cardiovascular death, myocardial infarction (MI), stroke, and ST. Secondary efficacy outcomes were the individual components of the primary efficacy outcome.
The primary safety outcome was trial‐defined clinically significant bleeding (Table S3), typically the composite of major bleeding or clinically relevant nonmajor bleeding (Table S2). Secondary safety outcomes were major bleeding (according to the Thrombolysis in Myocardial Infarction or the International Society on Thrombosis and Haemostasis criteria) clinically relevant nonmajor bleeding, and intracranial haemorrhage.
Quality Assessment and Publication Bias
Two independent reviewers (M.D.M., A.G.) performed the trial‐level qualitative assessment using the 7‐domain Cochrane Collaboration tool. The risk of bias was classified as high, low or unclear. We assessed the reliability of the results for each outcome according to Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach. 38 , 39 Funnel plots for both the primary outcomes were used to evaluate the presence of publication bias, heterogeneity of studies, or data irregularities. The significance of asymmetry was explored using visual inspection and tested by a rank correlation test based on Kendall’s τ. 40
Statistical Analysis
Full details about the statistical methodology are given in Data S1. In brief, trial‐level and pooled estimates are reported as event rates (per 100 patient‐years), hazard ratios (HRs), and 95% CIs. Both fixed‐effects and random‐effects were used in pairwise meta‐analyses first. Heterogeneity was assessed using I2 statistics and Cochran's Q tests. Subgroups analyses were performed to investigate the consistency of the effect sizes across subsets of interest. Reconstructed Kaplan–Meier analyses were performed extracting survival data from the published Kaplan–Meier curves of each study using the WebPlotDigitizer software 41 (4.2 version) and combining them. Landmark analyses at 30 and 180 days were performed for the primary bleeding end point. A network meta‐analysis was fitted to simultaneously compare and rank multiple regimens. For the purpose of the network meta‐analysis, we used the Bayesian approach, with noninformative priors, which is a conservative and commonly used method. Furthermore, the state of the current evidence was tested through the trial sequential analyses. A sensitivity analysis was performed with leave‐one‐out method; this technique consists in reanalyzing the results after removing each of the trials included, in order to verify whether the main result is influenced by a particular trial.
RESULTS
The preliminary search yielded a total of 2698 articles, reduced to 1567 after duplicates removal. After title and abstract screening, 1561 articles were excluded. The remaining 6 articles were read full text and 4 were found to be eligible for inclusion in our meta‐analysis: PIONEER AF‐PCI 22 (A Study Exploring Two Strategies of Rivaroxaban and One of Oral Vitamin K Antagonist in Patients With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention), RE‐DUAL PCI 23 (Randomized Evaluation of Dual Antithrombotic Therapy with Dabigatran versus Triple Therapy with Warfarin in Patients with Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention), AUGUSTUS, 24 and ENTRUST‐AF PCI 25 (Edoxaban Treatment Versus Vitamin K Antagonist in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention). The flow diagram of the study selection process is shown in Figure S1. The trials’ design and inclusion/exclusion criteria are summarized in Tables S2 and S4 and Figure S2. The follow‐up ranged from 6 months (AUGUSTUS) to a mean of 14 months (RE‐DUAL PCI). One of the arms in PIONEER AF‐PCI was excluded because it used DAPT in addition to a very low dose (2.5 mg bid) of rivaroxaban, which is not approved for cardioembolic risk prevention in AF and not endorsed by any guideline or consensus recommendation. Because AUGUSTUS had a factorial randomization (double versus triple therapy and apixaban versus VKA), for the purpose of this meta‐analysis and consistency with the other trials, we prioritized comparative data of apixaban+SAPT and VKA+DAPT, if available. Where only data concerning double versus triple therapy regimens were available (ie, for patient baseline characteristics and the subgroup analyses of primary end points), the same were used, as detailed later.
A total of 10 969 patients were included in the 4 trials. The baseline characteristics of the study populations are reported in Table S5. The mean age ranged between 69.9 and 70.8 years. Male sex represented between 71.0% (AUGUSTUS) and 76.0% (RE‐DUAL PCI) of patients. The overall prevalence of ACS ranged from 50.5% (AUGUSTUS) to 60.9% (RE‐DUAL PCI) and all patients underwent PCI (except in AUGUSTUS, where 23.9% of cases were medically managed ACS). The mean time in the therapeutic range among patients in the warfarin groups varied from 58.6% (AUGUSTUS) to 65% (PIONEER AF‐PCI). The prevalence of various comorbidities was relatively high, as well as the thromboembolic and bleeding risks, with a mild degree of variation among RCTs (CHA2DS2‐VASc [Congestive heart failure, Hypertension, Age ≥75 years, Diabetes, Prior stroke or transient ischemic attack, Vascular disease, Sex class] from 3.8–4.0 and HAS‐BLED [Hypertension, Abnormal liver/renal function, Stroke history, Bleeding history or predisposition, Labile INR, Elderly, Drug/alcohol usage] from 2.8–3.0). Clopidogrel was administered in 90.8% of patients, ticagrelor was used in 7.0%, and prasugrel in 0.8% of cases. In all the trials, aspirin was used in the peri‐PCI period potentially allowing for a period of triple therapy before randomization (mean time to randomization 1.9–6.6 days, with minimum 1 day and maximum 14 days).
Primary Efficacy Outcome
The incidences of MACE are plotted in the Figure 1 and Figure S3. No significant differences were found in MACE between the NOAC+SAPT and VKA+DAPT strategies, both by random‐effects (HR, 1.07; 95% CI, 0.94–1.22) and by fixed‐effects (HR, 1.07; 95% CI, 0.94–1.22) models (Figure 2A). 22 , 23 , 24 , 25 The RE‐DUAL PCI trial had the highest relative weight. There was no evidence of heterogeneity (I2=0%, P=0.60 in the fixed‐effects model). At the reconstructed Kaplan–Meier analysis, the AUGUSTUS trial could not be included because the survival curve for this end point was not reported in the trial. The reconstructed Kaplan–Meier analysis from the other 3 trials showed the overlap between the event‐free survival curves of the 2 treatments over time (Figure 1), with an event rate of 10.6 and 9.8 per 100 patient‐years, respectively. The number of MACE caused per 1000 patients treated with NOAC+SAPT versus VKA+DAPT was 5 (Figure 1). The sensitivity analysis demonstrated that the result was not affected by any specific trial (Table S6). The trial sequential analysis demonstrated that in light of the available data, significant differences in terms of MACE between the NOAC+SAPT and VKA+DAPT regimens are not likely to occur because the Z‐values line was in the area of futility (Figure 3A). Thus, even though the required sample size was not achieved, it is unlikely that any eventual future study could demonstrate a significant difference in term of MACE between the 2 treatments. The subgroup analysis showed that the effect was consistent in all the investigated subsets of patients, without significant interaction with the main baseline variables (Figures S4–S9).
Figure 1. Incidences (%) of efficacy and safety outcomes (upper left panel), respective effects of NOAC+SAPT regimens vs VKA+DAPT (forest plot in the upper central panel), and number of events prevented or caused per 100 patients treated (upper right panel).

In the bottom left (for MACE) and right (for clinically significant bleeding) panels, the reconstructed Kaplan–Meier curves represent the probability of events in the 2 strategy groups of the population included in all the trials. AUGUSTUS indicates A Study of Apixaban in Patients With Atrial Fibrillation, Not Caused by a Heart Valve Problem, Who Are at Risk for Thrombosis (Blood Clots) Due to Having Had a Recent Coronary Event, Such as a Heart Attack or a Procedure to Open the Vessels of the Heart; DAPT, dual antiplatelet therapy; ENTRUST‐AF PCI, Edoxaban Treatment vs Vitamin K Antagonist in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention; HR, hazard ratio (CI between squared brackets); MACE, major adverse cardiovascular event; NOAC, non–vitamin K antagonist oral anticoagulant; PIONEER AF‐PCI, A Study Exploring Two Strategies of Rivaroxaban and One of Oral Vitamin K Antagonist in Patients With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention; RE‐DUAL PCI, Evaluation of Dual Therapy With Dabigatran vs Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting; SAPT, single antiplatelet therapy; and VKA, vitamin K antagonist.
Figure 2. Forest plot for MACE (A) and clinically significant bleeding (B) end points.
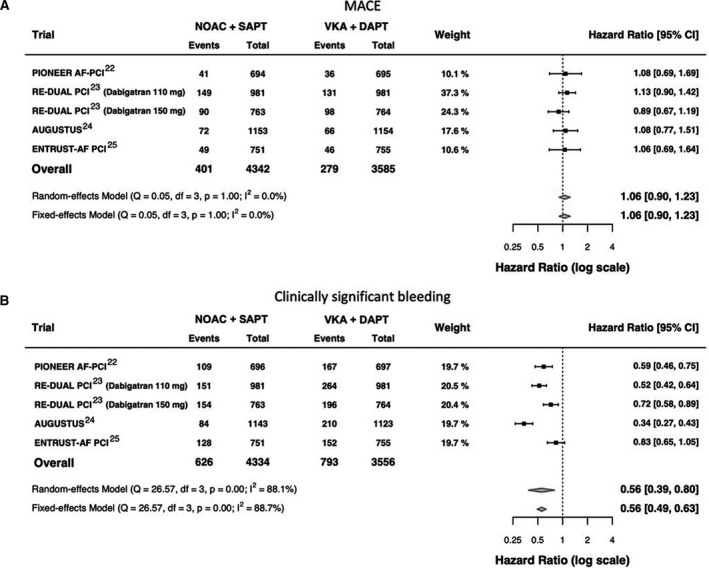
In the analysis of the overall population, the number of the patients included in DAPT+VKA arms of the RE‐DUAL PCI trial were not summed because the group of 764 patients compared with dabigatran 110 mg were a subset of the group of 981 patients compared with dabigatran 150 mg. Thus, only a total of 981 patients were included in the overall analysis. AUGUSTUS indicates A Study of Apixaban in Patients With Atrial Fibrillation, Not Caused by a Heart Valve Problem, Who Are at Risk for Thrombosis (Blood Clots) Due to Having Had a Recent Coronary Event, Such as a Heart Attack or a Procedure to Open the Vessels of the Heart; DAPT, dual antiplatelet therapy; df, degrees of freedom; ENTRUST‐AF PCI, Edoxaban Treatment vs Vitamin K Antagonist in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention; MACE, major adverse cardiovascular event; NOAC, non–vitamin K antagonist oral anticoagulant; PIONEER AF‐PCI, A Study Exploring Two Strategies of Rivaroxaban and One of Oral Vitamin K Antagonist in Patients With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention; Q, Cochran's Q test; RE‐DUAL PCI, Evaluation of Dual Therapy With Dabigatran vs Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting; SAPT, Single Antiplatelet Therapy; TAT, triple antithrombotic therapy; and VKA, vitamin K antagonist.
Figure 3. Trial sequential analysis for MACE (A) and clinically significant bleeding (B) end points.
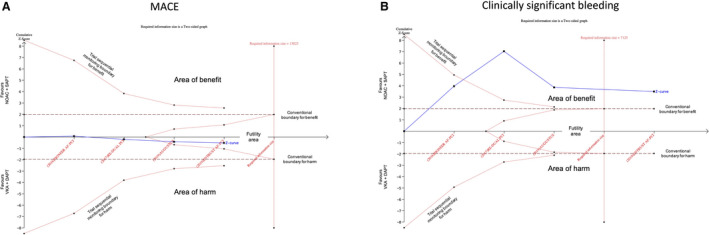
The vertical red dotted line represents required information size (ie, the number of patients required) to definitely demonstrate the risk difference (alpha 5%, power 80%). The horizontal axis represents the number of patients included in the meta‐analysis and is linear scaled, hence the distance of a new trial from the previous one on the axis represents the new trial population. The vertical axis represents the cumulative z‐score. The red dotted lines represent the trial sequential monitoring boundaries (inward sloping) and the futility boundaries (outward sloping). The solid blue line represents the cumulative z‐curve. According to the trial sequential analysis methodology, crossing the monitoring boundaries for the z‐curve indicates a clinically meaningful effect of a specific intervention that is also supported by statistical significance; crossing the required information size line indicates that the evidence is conclusive, whereas being in the futility area suggest that the effect size is neither clinically nor statistically meaningful and it is improbable that with further trials the cumulative evidence could demonstrate a significance in the effect size. In panel A, the required information size to demonstrate or reject a 35% relative risk reduction with an incidence in the control group of 22.6% is 7125 patients (required information size line). With the ENTRUST AF‐PCI trial the z‐curve crossed the required information size line. In panel B, the required information size to demonstrate or reject a 20% relative risk reduction with an incidence in the control group of 7% is 13 023 patients. With the AUGUSTUS and ENTRUST AF‐PCI trial the z‐curve entered the futility area. AUGUSTUS indicates A Study of Apixaban in Patients With Atrial Fibrillation, Not Caused by a Heart Valve Problem, Who Are at Risk for Thrombosis (Blood Clots) Due to Having Had a Recent Coronary Event, Such as a Heart Attack or a Procedure to Open the Vessels of the Heart; DAPT, dual antiplatelet therapy; ENTRUST‐AF PCI, Edoxaban Treatment vs Vitamin K Antagonist in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention; MACE, major adverse cardiovascular event; NOAC, non–vitamin K antagonist oral anticoagulant; SAPT, single antiplatelet therapy; and VKA, vitamin K antagonist.
At the Bayesian network meta‐analysis, the following 6 treatments were compared: DAPT plus VKA, apixaban 5 mg plus P2Y12 inhibitor, dabigatran 110 mg plus P2Y12 inhibitor, dabigatran 150 mg plus P2Y12 inhibitor, rivaroxaban 15 mg plus P2Y12 inhibitor, and edoxaban 60 mg plus P2Y12 inhibitor. The network of treatment regimens used in the analysis is displayed in Figure 4. Pairwise comparisons for the primary efficacy end point among regimens are displayed in the Table for the fixed effect model and in Table S7 for the random‐effects model. There was no significant difference between the NOAC+SAPT and VKA+DAPT regimens in terms of MACE. All NOAC+SAPT regimens were similar to each other. The treatment ranking is represented in Figure 5A and in Figure S10 for the fixed‐effect model and in Figures S11A and S12 for the random‐effects model, respectively.
Figure 4. Network of treatments.
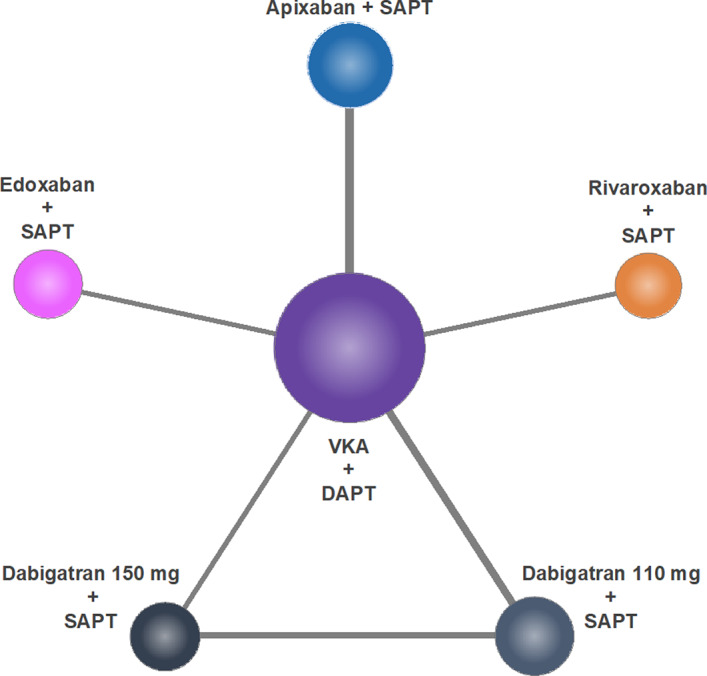
DAPT indicates dual antiplatelet therapy; SAPT, single antiplatelet therapy; and VKA, vitamin K antagonist.
Figure 5. Rankograms according to fixed‐effects model analysis for MACE (A) and clinically significant bleeding (B) end points.
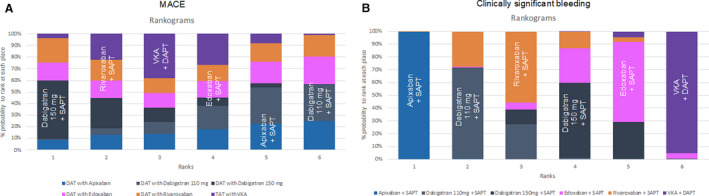
In these rankograms, the probability to be ranked in each position (from the first in the left to the sixth in the right) is plotted for all NOAC+SAPT strategies. DAPT indicates dual antiplatelet therapy; MACE, major adverse clinical event; NOAC, non–vitamin K antagonist oral anticoagulant; SAPT, single antiplatelet therapy; and VKA, vitamin K antagonist.
Primary Safety Outcome
The incidences of clinically significant bleedings are plotted in the Figure 1 and Figure S13. All NOAC+SAPT strategies (except edoxaban+SAPT) showed a significantly lower rate of clinically significant bleeding compared with VKA+DAPT, with a significant pooled effect both by random‐effects (HR, 0.56; 95% CI, 0.39–0.80) and by fixed‐effects (HR, 0.56; 95% CI, 0.49–0.63) models (Figure 2B). The RE‐DUAL PCI trial had the highest relative weights. There was a significant degree of heterogeneity (I2=88.7%, P<0.01 in the fixed‐effects model). Reconstructed Kaplan–Meier analysis confirmed the significant lower rate of clinically significant bleedings in the NOAC+SAPT versus VKA+DAPT groups over time and showed early separation of the curves within the first 6 months (Figure 1). The event rates were 17.8 per 100 patient‐years in the NOAC+SAPT group and 32.8 per 100 patient‐years in the VKA+DAPT group. The number of clinically significant bleedings prevented per 1000 patients treated with NOAC+SAPT versus VKA+DAPT was 58 (Figure 1), with a number needed to treat to avoid an event of 17 patients. Based on landmark analyses, most of the bleeding reduction was concentrated in the first 6 months; after this time frame no significant further effect was detected until 720 days (Figure S14). The sensitivity analysis demonstrated that the result was not affected by any specific trial (Table S6). The trial sequential analysis demonstrated that the results provided from the available data were in favor of NOAC+SAPT (versus VKA+DAPT) and conclusive, because the Z‐values line was in the area of significant benefit and the required sample size was achieved (Figure 3B). Subgroup analyses showed that the effect size was consistent in different subsets of patients, including male or female, elderly or nonelderly, SIHD or ACS, high or low thromboembolic risk as defined by the CHA2DS2‐VASc score, and high or low bleeding risk as defined by the HAS‐BLED score, without any significant interaction with the explored baseline variables (Figures S4 through S9).
At the Bayesian meta‐analysis, the network of treatment regimens compared was the same of the primary efficacy end point (Figure 4). Pairwise comparisons for the primary safety end point among regimens are displayed in Table 1 for the fixed‐effects model and in Table S7 for the random‐effects model. Consistently with the frequentist approach, the NOAC+SAPT regimens resulted in a lower rate of the primary safety end point when compared with VKA+DAPT. Among NOAC+SAPT regimens, the one with apixaban demonstrated a lower risk of the primary bleeding end point. However, all these findings were no longer significant using the random‐effects model. The treatment ranking is represented in Figure 5B and in Figure S10A for the fixed‐effects model and in Figures S11 and S12 for the random‐effects model, respectively.
Table 1.
Relative Effect Tables for MACE and Clinically Significant Bleeding End Points From Fixed Effect Model Analysis
| Apixaban+SAPT |
Dabigatran 110 mg+SAPT |
Dabigatran 150 mg+SAPT |
Edoxaban+SAPT | Rivaroxaban+SAPT | VKA+DAPT | ||
|---|---|---|---|---|---|---|---|
| MACE | Apixaban+SAPT | … |
1.05 (0.7, 1.58) |
0.81 (0.53, 1.24) |
0.98 (0.58, 1.66) |
0.94 (0.55, 1.59) |
0.92 (0.66, 1.29) |
|
Dabigatran 110 mg+SAPT |
0.95 (0.63, 1.43) |
… |
0.78 (0.6, 1) |
0.93 (0.59, 1.48) |
0.89 (0.56, 1.44) |
0.88 (0.7, 1.11) |
|
|
Dabigatran 150 mg+SAPT |
1.23 (0.8, 1.89) |
1.29 (1, 1.68) |
… |
1.2 (0.74, 1.96) |
1.15 (0.71, 1.89) |
1.14 (0.87, 1.49) |
|
| Edoxaban+SAPT |
1.03 (0.6, 1.73) |
1.07 (0.67, 1.71) |
0.83 (0.51, 1.35) |
… |
0.96 (0.54, 1.7) |
0.95 (0.63, 1.41) |
|
| Rivaroxaban+SAPT |
1.07 (0.63, 1.82) |
1.12 (0.7, 1.8) |
0.87 (0.53, 1.42) |
1.04 (0.59, 1.86) |
… |
0.99 (0.65, 1.49) |
|
| VKA+DAPT |
1.08 (0.77, 1.51) |
1.13 (0.9, 1.44) |
0.88 (0.67, 1.15) |
1.06 (0.71, 1.58) |
1.01 (0.67, 1.53) |
… | |
| Clinically significant bleeding | Apixaban+SAPT | … |
1.68 (1.22, 2.32) |
2.2 (1.6, 3.04) |
2.38 (1.69, 3.38) |
1.84 (1.3, 2.61) |
2.92 (2.29, 3.78) |
|
Dabigatran 110 mg+SAPT |
0.6 (0.43, 0.82) |
… |
1.31 (1.05, 1.65) |
1.42 (1.04, 1.95) |
1.1 (0.8, 1.5) |
1.75 (1.43, 2.14) |
|
|
Dabigatran 150 mg+SAPT |
0.46 (0.33, 0.62) |
0.76 (0.61, 0.95) |
… |
1.08 (0.79, 1.48) |
0.84 (0.61, 1.14) |
1.33 (1.09, 1.62) |
|
| Edoxaban+SAPT |
0.42 (0.3, 0.59) |
0.7 (0.51, 0.96) |
0.92 (0.68, 1.26) |
… |
0.77 (0.55, 1.09) |
1.23 (0.96, 1.57) |
|
| Rivaroxaban+SAPT |
0.54 (0.38, 0.77) |
0.91 (0.67, 1.25) |
1.2 (0.88, 1.64) |
1.29 (0.92, 1.83) |
… |
1.59 (1.25, 2.03) |
|
| VKA+DAPT |
0.34 (0.26, 0.44) |
0.57 (0.47, 0.7) |
0.75 (0.62, 0.91) |
0.81 (0.64, 1.04) |
0.63 (0.49, 0.8) |
… |
DAPT indicates dual antiplatelet therapy; MACE, major adverse clinical event; SAPT, single antiplatelet therapy; VKA, vitamin K antagonist.
Bivariate End Point and Secondary Outcomes
A plot with a bivariate outcome is presented in Figure 6. In this plot, the primary efficacy and safety end points are plotted together, visually confirming that despite a similar effect on the primary ischemic end point as compared with VKA+DAPT, the tendency toward a reduction in the primary safety end point is heterogeneous, with a more pronounced effect for apixaban+SAPT and a more modest effect for edoxaban+SAPT.
Figure 6. Bivariate end point plot for clinically significant bleeding and MACE end points.
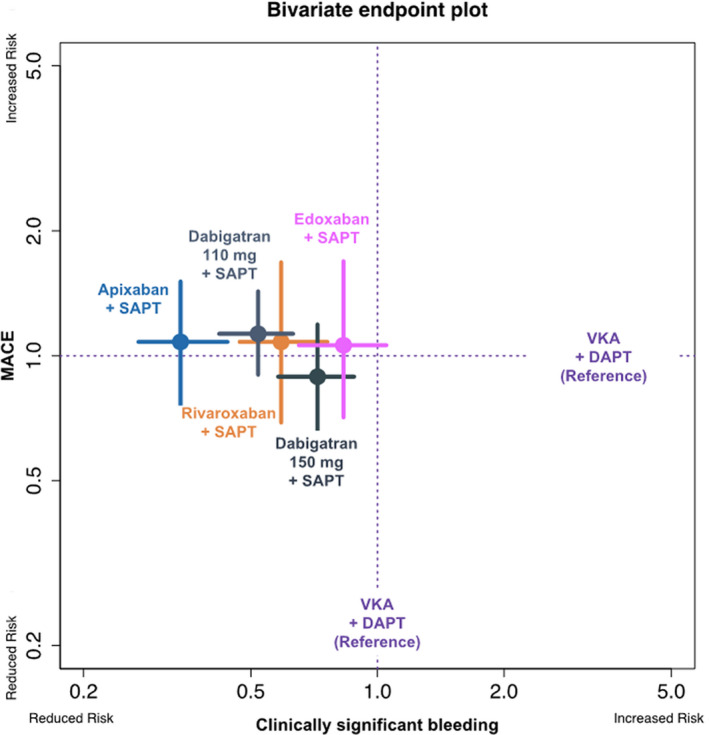
In this plot, the relative effects of different NOAC+SAPT regimens vs VKA+DAPT (set as reference, dotted lines) both in terms of MACE (vertical axis) and clinically significant bleeding (horizontal axis) are contemporary plotted. The colored points indicate the hazard ratios, whereas the colored lines indicate the CIs. DAPT indicates dual antiplatelet therapy; MACE, major adverse clinical event; NOAC, non–vitamin K antagonist oral anticoagulant; SAPT, single antiplatelet therapy; and VKA, vitamin K antagonist.
The incidences of secondary end points are plotted in the Figure 1 and Figures S3 and S13. The forest plots for secondary outcomes are displayed in Figures 7 and 8. 22 , 23 , 24 , 25 Among single components of MACE, data on apixaban+SAPT and VKA+DAPT were not uniformly available for stroke and MI end points in the AUGUSTUS trial. Thus, for death and ST, the apixaban+SAPT and VKA+DAPT groups where used, whereas the entire double and triple therapy groups were considered for stroke and MI. No significant difference in terms of death (HR, 1.07; 95% CI, 0.87–1.33), stroke (HR, 0.89; 95% CI, 0.58–1.36), MI (HR, 1.18; 95% CI, 0.92–1.52) and ST (HR, 1.38; 95% CI, 0.86–2.20) were detected between the 2 groups. All NOAC+SAPT strategies showed a lower incidence of major bleeding (HR, 0.71; 95% CI, 0.53–0.97), clinically relevant nonmajor bleeding (HR, 0.66; 95% CI, 0.49–0.88), and intracranial haemorrhage (HR, 0.46; 95% CI, 0.22–0.98) compared with the VKA+DAPT strategy. The numbers of events prevented or caused per 1000 patients treated, for all the secondary end points, are plotted in the Figure 1. The sensitivity analysis for secondary end points is shown in Table S8, again showing substantial consistency in treatment effects.
Figure 7. Forest plots for single components of MACEs.

Stent thrombosis was definite plus probable in AUGUSTUS and ENTRUST‐AF PCI, definite only in RE‐DUAL PCI, and it was not specified in PIONEER AF‐PCI. AUGUSTUS indicates A Study of Apixaban in Patients With Atrial Fibrillation, Not Caused by a Heart Valve Problem, Who Are at Risk for Thrombosis (Blood Clots) Due to Having Had a Recent Coronary Event, Such as a Heart Attack or a Procedure to Open the Vessels of the Heart; DAPT, dual antiplatelet therapy; df, degrees of freedom; ENTRUST‐AF PCI, Edoxaban Treatment vs Vitamin K Antagonist in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention; MACE, major adverse cardiovascular event; NOAC, non–vitamin K antagonist oral anticoagulant; PIONEER AF‐PCI, A Study Exploring Two Strategies of Rivaroxaban and One of Oral Vitamin K Antagonist in Patients With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention; Q, Cochran's Q test; RE‐DUAL PCI, Evaluation of Dual Therapy With Dabigatran vs Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting; SAPT, single antiplatelet therapy; and VKA, vitamin K antagonist.
Figure 8. Forest plots for secondary bleedings end points.
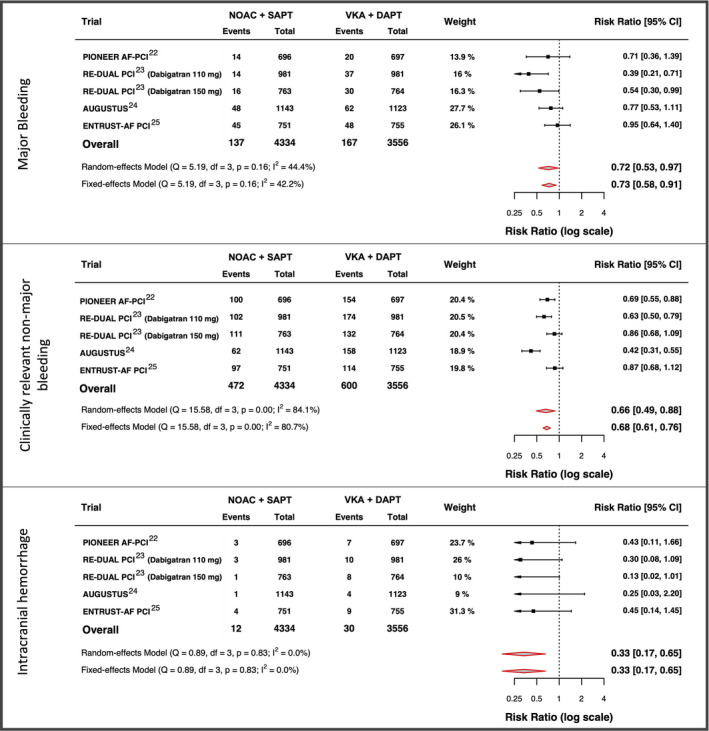
AUGUSTUS indicates A Study of Apixaban in Patients With Atrial Fibrillation, Not Caused by a Heart Valve Problem, Who Are at Risk for Thrombosis (Blood Clots) Due to Having Had a Recent Coronary Event, Such as a Heart Attack or a Procedure to Open the Vessels of the Heart; DAPT, dual antiplatelet therapy; df, degrees of freedom; ENTRUST‐AF PCI, Edoxaban Treatment vs Vitamin K Antagonist in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention; NOAC, non–vitamin K antagonist oral anticoagulant; PIONEER AF‐PCI, A Study Exploring Two Strategies of Rivaroxaban and One of Oral Vitamin K Antagonist in Patients With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention; Q, Cochran's Q test; RE‐DUAL PCI, Evaluation of Dual Therapy With Dabigatran vs Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting; SAPT, single antiplatelet therapy; and VKA, vitamin K antagonist.
Quality Assessment and Publication Bias
The judgments of the risk of bias for every single study and as percentages across all included studies are reported in Figures S15 and S16, respectively. Visual inspection of funnel plots and the rank correlation test showed the absence of significant asymmetry both for MACE and clinically significant bleeding end points (Kendall's tau: −0.67 and 0.33, P: 0.333 and 0.750, respectively; Figure S17).
DISCUSSION
The main findings of the present meta‐analysis, including 4 RCTs, are as follows. First, in patients undergoing PCI, the incidence of trial‐defined MACE is not different between the NOAC+SAPT and the VKA+DAPT strategies, a finding unlikely to change with hypothetical further trials. Second, a NOAC+SAPT strategy reduces bleeding by 44% compared with a VKA+DAPT regimen, and this evidence can be considered conclusive. This finding is quantitatively heterogeneous as the result of the different magnitudes of treatment effect detected in the 4 trials, with AUGUSTUS showing the largest bleeding risk reduction in the apixaban+SAPT arm.
In patients with AF undergoing PCI, the general goal of antithrombotic therapy should be to minimize both the coronary ischemic risk due to PCI (with antiplatelet drugs) and the cerebral and systemic thromboembolic risk due to AF (with anticoagulant drugs). The other side of the coin is to limit the increased risk of bleeding associated with stacking of multiple antithrombotic drugs. Although the prevalence of AF‐PCI is relatively low (about 7%–10%), this proportion may vary across geographies and is likely to increase in the future as the consequence of more elderly patients being offered PCI and the availability of more sensitive methods to make diagnosis of AF. 11 In the WOEST (What is the Optimal Antiplatelet & Anticoagulant Therapy in Patients With Oral Anticoagulation and Coronary Stenting) and ISAR‐TRIPLE (Triple Therapy in Patients on Oral Anticoagulation After Drug Eluting Stent Implantation) trials, simplification of the reference VKA+DAPT strategy was attempted by aspirin withdrawal or shortening DAPT duration by stopping clopidogrel, respectively. 42 , 43 In the WOEST trial, double antithrombotic therapy with clopidogrel was associated with a significant reduction in bleeding complications and no increase in the rate of thrombotic events compared with triple therapy. 42 In the ISAR‐TRIPLE trial, the primary end point, comprising a combination of ischemic and bleeding events, did not differ at 9 months between the two groups; in a landmark analysis of events between 6 weeks and 6 months, the risk of bleeding was higher in the group where clopidogrel was used longer (for 6 months), supporting the safety benefit of double versus triple antithrombotic therapy. 43 Importantly, both WOEST and ISAR‐TRIPLE were relatively small and underpowered to detect significant differences in ischemic end points. Recently, the SAFE‐A (Safety and Effectiveness Trial of Apixaban Use in Association with Dual Antiplatelet Therapy in Atrial Fibrillation Patients Undergoing Percutaneous Coronary Intervention) study compared 1‐ to 6‐month P2Y12 inhibitor‐therapy on top of aspirin and apixaban in patients with AF who undergo PCI in terms of bleeding: the trial had not enough statistical power because it was prematurely terminated due to slow enrolment. 44
Subsequently, the PIONEER AF‐PCI and RE‐DUAL PCI trials demonstrated that a NOAC+SAPT regimen (rivaroxaban 15 mg and dabigatran 110/150 mg, respectively) reduced clinically significant bleedings against VKA+DAPT, without any significant increase in ischemic events. 22 , 23 Interestingly, the design of both trials does not allow us to discriminate the effect of NOAC versus VKA from the effect of double versus triple therapy. The AUGUSTUS trial, with its 2×2 factorial design, demonstrated both a superiority of the double versus triple therapy and of the apixaban versus VKA regimens in terms of clinically significant bleedings, without significant differences in the incidence of ischemic events. 24 Closing the quartet of trials, the ENTRUST‐AF PCI trial recently demonstrated the noninferiority (but not the superiority) of edoxaban+SAPT against VKA+DAPT in terms of significant bleedings, without significant differences in ischemic events. 25 It should be noted that none of these trials was powered for the ischemic end point. Interestingly, in these trials, being the randomization performed several days after the index PCI, nearly all the patients likely had aspirin (hence some brief duration of triple therapy) before randomization.
The recent 2019 European Society of Cardiology guidelines on chronic coronary syndromes recommend a NOAC in preference to VKA for combination with antiplatelet therapy in patients with AF who are eligible for a NOAC (class of recommendation I). Moreover, an early cessation (≤1 week) of aspirin and continuation of double antithrombotic therapy with an oral anticoagulant and clopidogrel should be considered if the risk of ST is low or if concerns about bleeding risk prevail over the risk of ST (class of recommendation IIa). 3 On the other hand, the same class of recommendation is given for aspirin continuation up to 6 months in patients where the risk of thrombotic complications is perceived as higher than the risk of bleeding. As such, the European perspective so far is to consider both the double and triple antithrombotic therapy strategies as viable approaches to be selected depending on net benefit considerations. This is different from the North American approach, which currently recommends double therapy as the default strategy, with the triple therapy strategy restricted to very selected patients at high ischemic and low bleeding risk. 10
Our meta‐analysis confirms that a NOAC+SAPT strategy, implemented after a brief period of aspirin in the peri‐PCI period does not significantly increase the combined ischemic risk and is safer than VKA+DAPT with respect to major or clinically relevant nonmajor bleedings. The trial sequential analyses suggested that further trials are not required both for primary efficacy (because it is improbable that the cumulative evidence could become clinically and statistically significant) and primary safety end points (because the required sample size to demonstrate the superiority is already achieved).
Recently, an analysis from the AUGUSTUS trial demonstrated nonsignificantly higher ST rates with placebo compared to aspirin among patients with AF with recent PCI. 26 However, it is also important to note that the overall incidence of ST was low and mostly occurring early after PCI. Importantly, in this sub‐analysis, data regarding apixaban+SAPT and VKA+DAPT regimens were disclosed. Furthermore, a previous meta‐analysis revealed a significant increase in the risk of ST with aspirin discontinuation compared with VKA+DAPT. 45 This evidence was not clearly visible in the 4 trials taken individually given that they were underpowered for this end point. The results of our analysis are slightly different from previous meta‐analysis given that the difference in ST rates were nonsignificant (HR, 1.38; 95% CI, 0.86–2.20). This difference becomes even weaker after removing the dabigatran 110 mg arm at the sensitivity analysis (HR, 1.22; 95% CI, 0.74–2.03). 3 , 45 , 46 This is attributable to the availability of new data from AUGUSTUS, comparing the NOAC+SAPT versus VKA+DAPT groups similar to others trials, which were not included in other meta‐analyses.
The pooled analysis with reconstructed patient‐level data corroborates the evidence from the trial‐level meta‐analyses and gives insights on the distribution of the bleeding reduction with NOAC+SAPT. Understandably, bleeding was mostly reduced during the first 6 months, when the proportion of triple therapy patients in the control group was higher than in the subsequent period. Trial‐level subgroup analyses demonstrated that the effect of NOAC+SAPT versus VKA+DAPT was consistent in different settings, including presence or absence of ACS. Moreover, the trial sequential analyses demonstrated that the evidence about the absence of significant differences in the composite ischemic outcome, even though not conclusive, are not likely to change with further studies and those supporting the superiority in terms of clinically significant bleedings of NOAC+SAPT against VKA+DAPT could be considered conclusive. These results strengthen new guidelines recommendations.
About antiplatelet drugs selection, the 2019 European Society of Cardiology Chronic Coronary Syndromes guidelines recommend (class IIb) that double therapy with more potent P2Y12 inhibitors may be considered as an alternative to triple therapy with clopidogrel in patients with a moderate or high risk of ST. 3 A North American consensus document indicates that ticagrelor, but not prasugrel, may be considered in patients at high thrombotic but low bleeding risk and only in the context of a double therapy regimen. 10 Our subgroup analysis showed that the kind of P2Y12 inhibitor did not affect significantly the efficacy and the safety of NOAC+SAPT against VKA+DAPT. However, only 7.4% of patients were treated with more potent antiplatelet drugs; this justifies the weak recommendation of ticagrelor and prasugrel from the guidelines and its limitation (due to their known stronger antiplatelet effect) to patients with higher risk of ST. Studies are warranted to better understand the safety and efficacy profiles of prasugrel and ticagrelor in a NOAC+SAPT regimen.
Finally, the risk–benefit profiles of various NOACs have been previously analyzed in patients with AF with heterogeneous results in different settings. 47 , 48 , 49 , 50 , 51 In our meta‐analysis, heterogeneity among different trials in the reduction of clinically significant bleeding risk could reflect a difference in individual NOACs profile. The Bayesian network meta‐analysis, indirectly comparing various NOACs in double therapy regimens, revealed a trend toward a better bleeding profile of apixaban against other NOACs, which was significant in the fixed‐effects model but not significant in the random‐effects model. These results should be interpreted with caution. In fact, various confounders (primarily the trial design) could affect this analysis. On the other hand, these data could suggest that beyond a strategy effect (double versus triple therapy) and a class effect (NOAC versus VKA), a specific drug effect could be hypothesized. On the basis of these and other previous evidence, further investigation comparing different NOACs may be justified to directly assess the different risk–benefit profiles of all NOACs in order to select the appropriate drug for each patient rather than attempting to identify the best in class for all patients.
Some limitations of this meta‐analysis should be acknowledged, which are in common with other meta‐analyses. The different characteristics of the trials included could generate a certain degree of heterogeneity that cannot be adequately controlled for. These differences include the timing from the index event to randomization, inclusion and exclusion criteria, patients' characteristics, time in therapeutic range in VKA group, treatment protocol, length of treatment/follow‐up, and definition of end points. This heterogeneity could potentially also affect the indirect comparisons at the network analysis. In particular, in two out of the four included trials, the MACE definition included revascularization (which is a softer end point compared with cardiovascular death, MI, stroke or ST). Notwithstanding this limitation, the heterogeneity for the MACE outcome was 0% in the fixed‐effects model.
Another potential caveat is that, according to the PIONEER AF‐PCI trial design, we included in our analyses data on NOAC+SAPT with rivaroxaban 15 mg, which is not approved for stroke prevention in AF. In addition, the AUGUSTUS trial had a factorial design. Our nonfactorial analysis of its results does not respect the primary aim of the trial. However, nonfactorial analysis of factorial trials is a feasible and used technique, both in context of trials and meta‐analyses. 30 , 52 , 53
It is also notable that details on timing of ST were not fully available, thus limiting the current analysis from drawing final conclusions on the optimal duration of aspirin in combination with NOAC and a P2Y12 inhibitor in the double therapy group. Finally, reconstructed individual patient data were obtained from digitized curve reconstructions through a dedicated software, therefore our work should not be viewed as a traditional patient‐level meta‐analysis.
CONCLUSIONS
In patients with AF undergoing PCI, no significant differences were found between NOAC+SAPT and VKA+DAPT strategies in terms of MACE and single ischemic end points in an updated meta‐analysis now encompassing ≈10 000 patients. On the other hand, a strategy of NOAC+SAPT is associated with a significantly lower incidence of both all clinically relevant bleedings and major bleedings compared with a strategy of VKA+DAPT. Finally, various NOACs showed a variable benefit–risk profile, suggesting the opportunity for tailored choices based on individual patients’ profiles, which warrants future investigation.
Sources of Funding
None.
Disclosures
Capodanno discloses the following relationships: Advisory Board: Bayer, Daiichi Sankyo. Lecture fees: AstraZeneca, Bayer, Daiichi Sankyo, Sanofi Aventis. Dr Bhatt discloses the following relationships—Advisory Board: Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice‐Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE‐DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS‐II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co‐leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co‐Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda. Gibson reported grants and personal fees from Portola, Bayer, Janssen, Johnson and Johnson, Portola, Bayer, Janssen, Johnson and Johnson and grants from Bristol‐Myers Squibb outside the submitted work. Goette has received honoraria and speaker fees from AstraZeneca, Bayer Health Care, Berlin‐Chemie, Bristol‐Myers Squibb, Pfizer, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Medtronic, Novartis, and Omeicos. AG’s research has been supported by Josef‐Freitag‐Stiftung and Deutsche Herzstiftung outside the submitted work. Lopes reported grants and personal fees from Bristol‐Myers Squibb and Pfizer, personal fees from Boehringer Ingelheim and Bayer AG and grants from Amgen Inc, GlaxoSmithKline, Medtronic PLC, and Sanofi Aventis outside the submitted work. Mehran reported grants from AstraZeneca, Bayer, Beth Israel Deaconess, Bristol Myers Squibb/Sanofi, CSL Behring, DSI, Medtronic, Novartis Pharmaceuticals, and OrbusNeich; personal fees from Boston Scientific, Medscape, Siemens Medical Solutions, Regeneron Pharmaceuticals, Roivant Sciences, and Sanofi; grants and personal fees from Abbott Vascular; other support from Abbott Laboratories, Spectranetics/ Philips/Volcano, Janssen, BMS, Watermark Research, Medtelligence/Janssen, Claret Medical, and Elixir Medical; personal fees and other support from PLx Opco/PLx Pharma outside the submitted work. Vranckx has received personal fees from Daiichi Sankyo, AstraZeneca, Bayer, and Terumo outside the submitted work. Angiolillo declares that he has received consulting fees or honoraria from Abbott, Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol‐Myers Squibb, Chiesi, Daiichi‐Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company and has received payments for participation in review activities from CeloNova and St Jude Medical. D.J.A. also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi‐Sankyo, Eisai, Eli Lilly, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions and the Scott R. MacKenzie Foundation. The remaining authors have no disclosures to report.
Supporting information
(J Am Heart Assoc. 2020;9:e017212 DOI: 10.1161/JAHA.120.017212.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017212
For Sources of Funding and Disclosures, see page 14.
References
- 1. Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, Smith PK. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease. J Am Coll Cardiol. 2017;69:2212–2241. [DOI] [PubMed] [Google Scholar]
- 2. Neumann F‐J, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J‐P, Falk V, Head SJ, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2018;40:87–165. [DOI] [PubMed] [Google Scholar]
- 3. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck‐Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes the Task Force for the diagnosis and management of chronic. Eur Heart J. 2019;00:1–71. [DOI] [PubMed] [Google Scholar]
- 4. Vranckx P, Valgimigli M, Jüni P, Hamm C, Steg PG, Heg D, van Es GA, McFadden EP, Onuma Y, van Meijeren C, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug‐eluting stent: a multicentre, open‐label, randomised superiority trial. Lancet. 2018;392:940–949. [DOI] [PubMed] [Google Scholar]
- 5. Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, Ohya M, Suwa S, Takagi K, Nanasato M, et al. Effect of 1‐month dual antiplatelet therapy followed by clopidogrel vs 12‐month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT‐2 randomized clinical trial. JAMA. 2019;321:2414–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hahn J‐Y, Bin SY, Oh J‐H, Chun WJ, Park YH, Jang WJ, Im E‐S, Jeong J‐O, Cho BR, Oh SK, et al. Effect of P2Y12 inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: the SMART‐CHOICE randomized clinical trial. JAMA. 2019;321:2428–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ. Dual antiplatelet therapy: appraisal of the ACC/AHA and ESC focused updates. J Am Coll Cardiol. 2018;72:103–119. [DOI] [PubMed] [Google Scholar]
- 8. Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, Cha JY, Collier T, Dangas G, Dudek D, et al. Ticagrelor with or without aspirin in high‐risk patients after PCI. N Engl J Med. 2019;381:2032–2042. [DOI] [PubMed] [Google Scholar]
- 9. Capodanno D, Huber K, Mehran R, Lip GYH, Faxon DP, Granger CB, Vranckx P, Lopes RD, Montalescot G, Cannon CP, et al. Management of antithrombotic therapy in atrial fibrillation patients undergoing PCI. J Am Coll Cardiol. 2019;74:83–99. [DOI] [PubMed] [Google Scholar]
- 10. Angiolillo DJ, Goodman SG, Bhatt DL, Eikelboom JW, Price MJ, Moliterno DJ, Cannon CP, Tanguay J‐FF, Granger CB, Mauri L, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention. Circulation. 2018;138:527–536. [DOI] [PubMed] [Google Scholar]
- 11. Capodanno D, Lip GYH, Windecker S, Huber K, Kirchhof P, Boriani G, Lane D, Gilard M, Collet JP, Valgimigli M, et al. Triple antithrombotic therapy in atrial fibrillation patients with acute coronary syndromes or undergoing percutaneous coronary intervention or transcatheter aortic valve replacement. EuroIntervention. 2015;10:1015–1021. [DOI] [PubMed] [Google Scholar]
- 12. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 13. Capodanno D. Triple antithrombotic therapy after ACS and PCI in patients on chronic oral anticoagulation: update. Heart. 2018;104:1976–1983. [DOI] [PubMed] [Google Scholar]
- 14. Valgimigli M, Bueno H, Byrne RA, Collet J‐P, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur Heart J. 2018;39:213–260.28886622 [Google Scholar]
- 15. van Rein N, Heide‐Jørgensen U, Lijfering WM, Dekkers OM, Sørensen HT, Cannegieter SC. Major bleeding rates in atrial fibrillation patients on single, dual, or triple antithrombotic therapy. Circulation. 2019;139:775–786. [DOI] [PubMed] [Google Scholar]
- 16. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 17. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 18. Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 19. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 20. Lane DA, Dagres N, Dan G‐A, García Seara J, Iliodromitis K, Lenarczyk R, Lip GYH, Mansourati J, Marín F, Scherr D, et al. Antithrombotic treatment in patients with atrial fibrillation and acute coronary syndromes: results of the European Heart Rhythm Association survey. Europace. 2019;21:1116–1125. [DOI] [PubMed] [Google Scholar]
- 21. Kontos MC; American College of Cardiology . Poll results: anticoagulation in AF with PCI and stent. Published on American College of Cardiology site. Available at: https://www.acc.org/latest‐in‐cardiology/articles/2019/09/06/14/31/poll‐results‐anticoagulation‐in‐af‐with‐pci‐and‐stent. September 9, 2019. Accessed April 15, 2020.
- 22. Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels M, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–2434. [DOI] [PubMed] [Google Scholar]
- 23. Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, Maeng M, Merkely B, Zeymer U, Gropper S, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513–1524. [DOI] [PubMed] [Google Scholar]
- 24. Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, Goodman SG, Windecker S, Darius H, Li J, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380:1509–1524. [DOI] [PubMed] [Google Scholar]
- 25. Vranckx P, Valgimigli M, Eckardt L, Tijssen J, Lewalter T, Gargiulo G, Batushkin V, Campo G, Lysak Z, Vakaliuk I, et al. Edoxaban‐based versus vitamin K antagonist‐based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST‐AF PCI): a randomised, open‐label, phase 3b trial. Lancet. 2019;394:1335–1343. [DOI] [PubMed] [Google Scholar]
- 26. Lopes RD, Leonardi S, Wojdyla DM, Vora AN, Thomas L, Storey RF, Vinereanu D, Granger CB, Goodman SG, Aronson R, et al. Stent thrombosis in patients with atrial fibrillation undergoing coronary stenting in the AUGUSTUS trial. Circulation. 2019;141:781–783. [DOI] [PubMed] [Google Scholar]
- 27. Golwala HB, Cannon CP, Steg PG, Doros G, Qamar A, Ellis SG, Oldgren J, Ten Berg JM, Kimura T, Hohnloser SH, et al. Safety and efficacy of dual vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta‐analysis of randomized clinical trials. Eur Heart J. 2018;39:1726–1735a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lopes RD, Hong H, Harskamp RE, Bhatt DL, Mehran R, Cannon CP, Granger CB, Verheugt FWA, Li J, Ten Berg JM, et al. Safety and efficacy of antithrombotic strategies in patients with atrial fibrillation undergoing percutaneous coronary intervention: a network meta‐analysis of randomized controlled trials. JAMA Cardiol. 2019;4:747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galli M, Andreotti F, Porto I, Crea F. Intracranial haemorrhages vs. stent thromboses with direct oral anticoagulant plus single antiplatelet agent or triple antithrombotic therapy: a meta‐analysis of randomized trials in atrial fibrillation and percutaneous coronary intervention/acute coronary syndrome patients. Europace. 2020;22:538–546. [DOI] [PubMed] [Google Scholar]
- 30. Gargiulo G, Goette A, Tijssen J, Eckardt L, Lewalter T, Vranckx P, Valgimigli M. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta‐analysis of non‐vitamin K antagonist oral anticoagulant‐based randomized clinical trials. Eur Heart J. 2019;40:3757–3767. [DOI] [PubMed] [Google Scholar]
- 31. Sullivan A, Nanna M, Rao S, Cantrell S, Gibson CM, Verheugt F, Peterson E, Yee M, Kong D. A systematic review of randomized and non‐randomized studies comparing dual therapy with triple therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2018;72:B309. [Google Scholar]
- 32. Andò G, Costa F. Double or triple antithrombotic therapy after coronary stenting and atrial fibrillation: a systematic review and meta‐analysis of randomized clinical trials. Int J Cardiol. 2020;302:95–102. [DOI] [PubMed] [Google Scholar]
- 33. Gao X, Ge Z, Kong X, Wang Z, Zuo G, Wang F, Chen S, Zhang J. Clinical outcomes of antithrombotic strategies for patients with atrial fibrillation after percutaneous coronary intervention evidence from a network meta‐analysis. Int Heart J. 2019;60:546–553. [DOI] [PubMed] [Google Scholar]
- 34. Lopes RD, Hong H, Harskamp RE, Bhatt DL, Mehran R, Cannon CP, Granger CB, Verheugt FWA, Li J, ten Berg JM, et al. Optimal antithrombotic regimens for patients with atrial fibrillation undergoing percutaneous coronary intervention. JAMA Cardiol. 2020;5:582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sjölander A, Vansteelandt S. Frequentist versus Bayesian approaches to multiple testing. Eur J Epidemiol. 2019;34:809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement—flow of information through the different phases of a systematic review (downloadable template document for researchers to re‐use). PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JPA, Straus S, Thorlund K, Jansen JP, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. [DOI] [PubMed] [Google Scholar]
- 38. Cochrane Training . Cochrane Handbook for Systematic Reviews of Interventions 6. http://www.Handbook.Cochrane.Org. Updated 2019. Available at: https://training.cochrane.org/handbook/current. Accessed October 09, 2019.
- 39. Holger S, Jan B, Gordon G, Andrew O. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Available at: https://gdt.gradepro.org/app/handbook/handbook.html. Accessed October 09, 2019.
- 40. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088. [PubMed] [Google Scholar]
- 41. Drevon D, Fursa SR, Malcolm AL. Intercoder reliability and validity of WebPlotDigitizer in extracting graphed data. Behav Modif. 2017;41:323–339. [DOI] [PubMed] [Google Scholar]
- 42. Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman J‐P, Adriaenssens T, Vrolix M, Heestermans AACM, Vis MM, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open‐label, randomised, controlled trial. Lancet. 2013;381:1107–1115. [DOI] [PubMed] [Google Scholar]
- 43. Fiedler KA, Maeng M, Mehilli J, Schulz‐Schüpke S, Byrne RA, Sibbing D, Hoppmann P, Schneider S, Fusaro M, Ott I, et al. Duration of triple therapy in patients requiring oral anticoagulation after drug‐eluting stent implantation: the ISAR‐TRIPLE trial. J Am Coll Cardiol. 2015;65:1619–1629. [DOI] [PubMed] [Google Scholar]
- 44. Hoshi T, Sato A, Hiraya D, Watabe H, Takeyasu N, Nogami A, Ohigashi T, Gosho M, Ieda M, Anoumi K. Short‐duration triple antithrombotic therapy for atrial fibrillation patients who require coronary stenting: results of the SAFE‐A study. EuroIntervention. 2020;16:e164–e172. [DOI] [PubMed] [Google Scholar]
- 45. Galli M, Andreotti F, D’Amario D, Vergallo R, Montone RA, Porto I, Crea F. Dual therapy with direct oral anticoagulants significantly increases the risk of stent thrombosis compared to triple therapy. Eur Heart J Cardiovasc Pharmacother. 2019;6:128–129. [DOI] [PubMed] [Google Scholar]
- 46. Gargiulo G, Valgimigli M. Antithrombotic therapy after transcatheter aortic valve implantation: a new piece of the still unresolved puzzle. J Thorac Dis. 2017;9:4260–4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang J, Tang J, Cui X, Wang B, Bu M, Bai Y, Wang K, Guo J, Shen D, Zhang J. Indirect comparison of novel oral anticoagulants among Asians with non‐valvular atrial fibrillation in the real world setting: a network meta‐analysis. BMC Cardiovasc Disord. 2019;19:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cohen AT, Hill NR, Luo X, Masseria C, Abariga SA, Ashaye AO. A systematic review of network meta‐analyses among patients with nonvalvular atrial fibrillation: a comparison of efficacy and safety following treatment with direct oral anticoagulants. Int J Cardiol. 2018;269:174–181. [DOI] [PubMed] [Google Scholar]
- 49. Baker WL, Phung OJ. Systematic review and adjusted indirect comparison meta‐analysis of oral anticoagulants in atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012;5:711–719. [DOI] [PubMed] [Google Scholar]
- 50. Sherrill B, Fernandez M, Wang J, Ye X, Kwong W, Sherif B, Hogue S. Network meta‐analysis of relative efficacy and safety of edoxaban versus other novel oral anticoagulants (NOACs) among atrial fibrillation patients with CHADS2 score ≥ 2. J Am Coll Cardiol. 2015;65:A346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Silverio A, Di Maio M, Prota C, De Angelis E, Radano I, Citro R, Carrizzo A, Ciccarelli M, Vecchione C, Capodanno D, et al. Safety and efficacy of non‐vitamin K antagonist oral anticoagulants in elderly patients with atrial fibrillation. Eur Heart J Cardiovasc Pharmacother. 2019:pvz073 [Epub ahead of print] 10.1093/ehjcvp/pvz073 [DOI] [PubMed] [Google Scholar]
- 52. Randomised trial of intravenous strptokinase, oral aspirin, both, or neither among 17 187 cases of suspected acute myocardial infarction: ISIS‐2. Lancet. 1988;332:349–360. [PubMed] [Google Scholar]
- 53. Korn EL, Freidlin B. Non‐factorial analyses of two‐by‐two factorial trial designs. Clin Trials. 2016;13:651–659. [DOI] [PubMed] [Google Scholar]
- 54. Guyot P, Ades AE, Ouwens MJNM, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan‐Meier survival curves. BMC Med Res Methodol. 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Stat Sci. 1992;7:457–472. [Google Scholar]
- 56. Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. [DOI] [PubMed] [Google Scholar]
- 57. Andò G, Capodanno D. Radial access reduces mortality in patients with acute coronary syndromes results from an updated trial sequential analysis of randomized trials. JACC Cardiovasc Interv. 2016;9:660–670. [DOI] [PubMed] [Google Scholar]
- 58. Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive—trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. Int J Epidemiol. 2009;38:287–298. [DOI] [PubMed] [Google Scholar]
- 59. Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JPA, Thabane L, Gluud LL, Als‐Nielsen B, Gluud C. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses? Int J Epidemiol. 2009;38:276–286. [DOI] [PubMed] [Google Scholar]
- 60. Bangalore S, Kumar S, Wetterslev J, Messerli FH. Angiotensin receptor blockers and risk of myocardial infarction: meta‐analyses and trial sequential analyses of 147 020 patients from randomised trials. BMJ. 2011;342:d2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. J Clin Epidemiol. 2008;61:64–75. [DOI] [PubMed] [Google Scholar]
- 62. de Jonge SW, Atema JJ, Solomkin JS, Boermeester MA. Meta‐analysis and trial sequential analysis of triclosan‐coated sutures for the prevention of surgical‐site infection. Br J Surg. 2017;104:e118–e133. [DOI] [PubMed] [Google Scholar]
- 63. Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta‐analysis. BMC Med Res Methodol. 2017;17:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


